Synthesis of Heterocyclic Compounds with a Cineole Fragment in Reactions of α-Pinene-Derived Diol and Monoterpenoid Aldehydes
Abstract
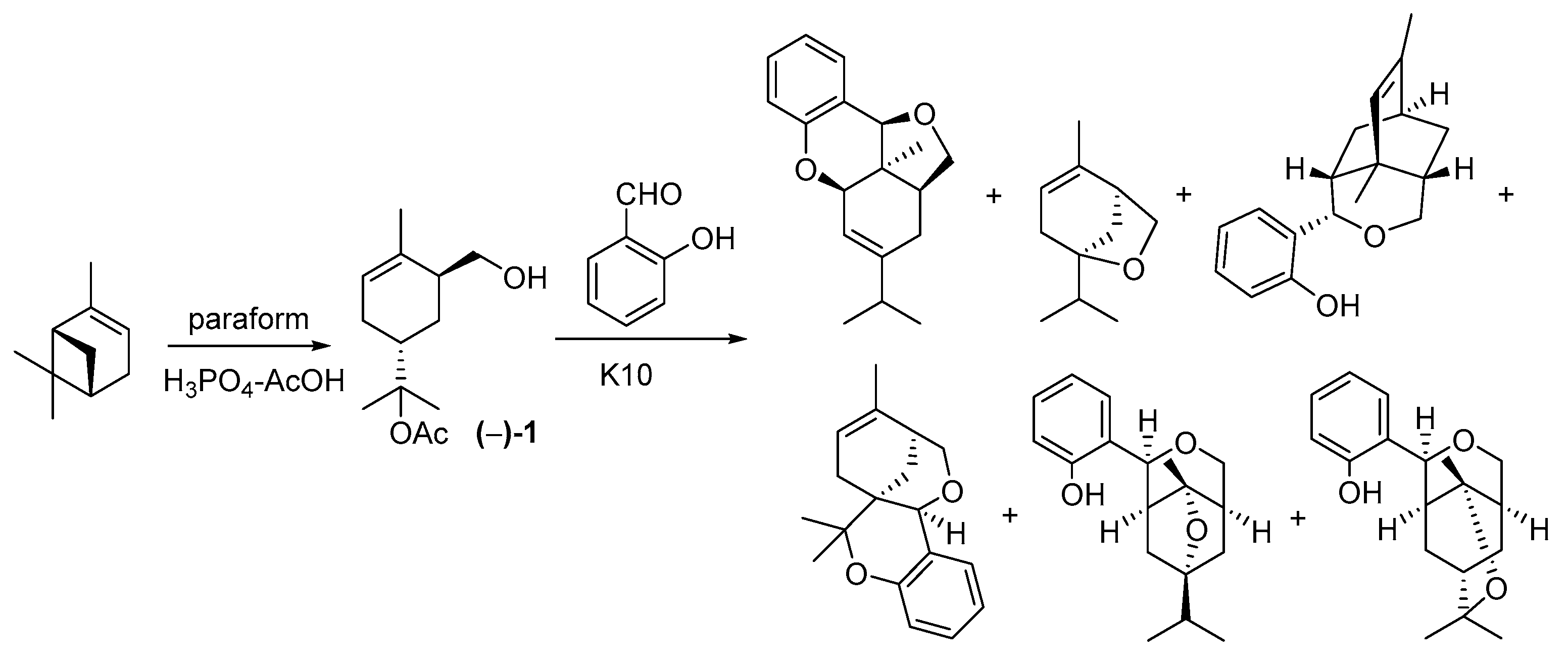
1. Introduction
2. Materials and Methods
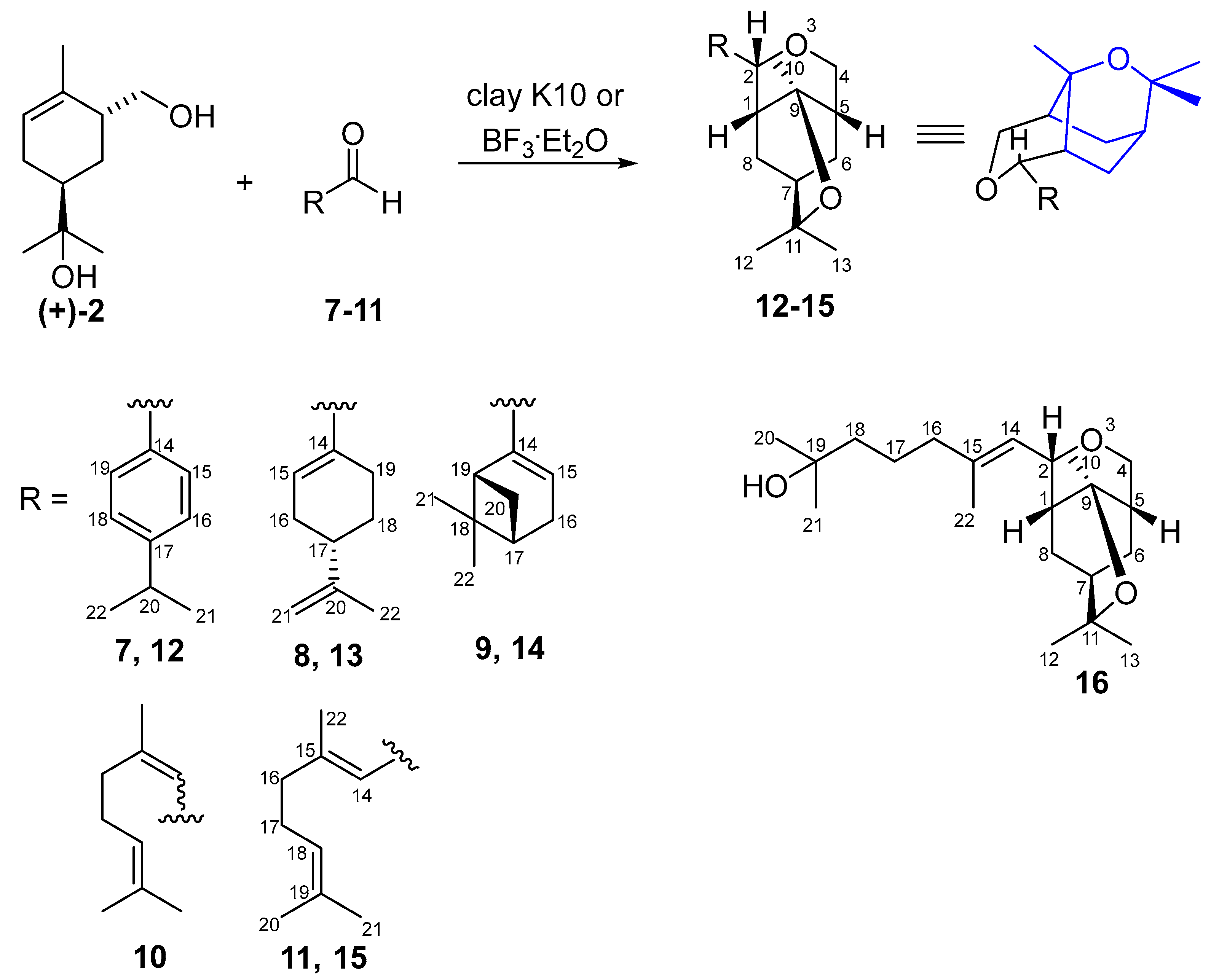
2.1. General Procedure 1 (GP1)—Reaction (+)-8-Hydroxy-6-hydroxymethyllimonene (+)-2 and Aldehydes 7–11 in the Presence of Montmorillonite K10
2.2. Reaction of (+)-8-Hydroxy-6-hydroxymethyllimonene 2 and Cuminaldehyde 7 in the Presence of Montmorillonite K10
2.3. Reaction of (+)-8-Hydroxy-6-hydroxymethyllimonene 2 and Perillaldehyde 8 in the Presence of Montmorillonite K10
2.4. Reaction of (+)-8-Hydroxy-6-hydroxymethyllimonene (+)-2 and Myrtenal 9 in the Presence of Montmorillonite K10
2.5. Reaction of 8-Hydroxy-6-hydroxymethyllimonene (+)-2 and Citral 10 in the Presence of Montmorillonite K10
2.6. Reaction of (+)-8-Hydroxy-6-hydroxymethyllimonene (+)-2 and Geranial 11 in the Presence of Montmorillonite K10
2.7. General Procedure 2 (GP2)—Reaction (+)-8-Hydroxy-6-hydroxymethyllimonene (+)-2 and Aldehydes 7–9, 11 in the Presence of BF3·Et2O
2.8. Reaction of (+)-8-Hydroxy-6-hydroxymethyllimonene (+)-2 and Cuminaldehyde 7 in the Presence of BF3·Et2O
2.9. Reaction of (+)-8-Hydroxy-6-hydroxymethyllimonene (+)-2 and Perillaldehyde 8 in the Presence of BF3·Et2O
2.10. Reaction of (+)-8-Hydroxy-6-hydroxymethyllimonene (+)-2 and Myrtenal 9 in the Presence of BF3·Et2O
2.11. Reaction of (+)-8-Hydroxy-6-hydroxymethyllimonene (+)-2 and Citral 10 in the Presence of BF3·Et2O
2.12. Reaction of (+)-8-Hydroxy-6-hydroxymethyllimonene (+)-2 and Geranial 11 in the Presence of BF3·Et2O
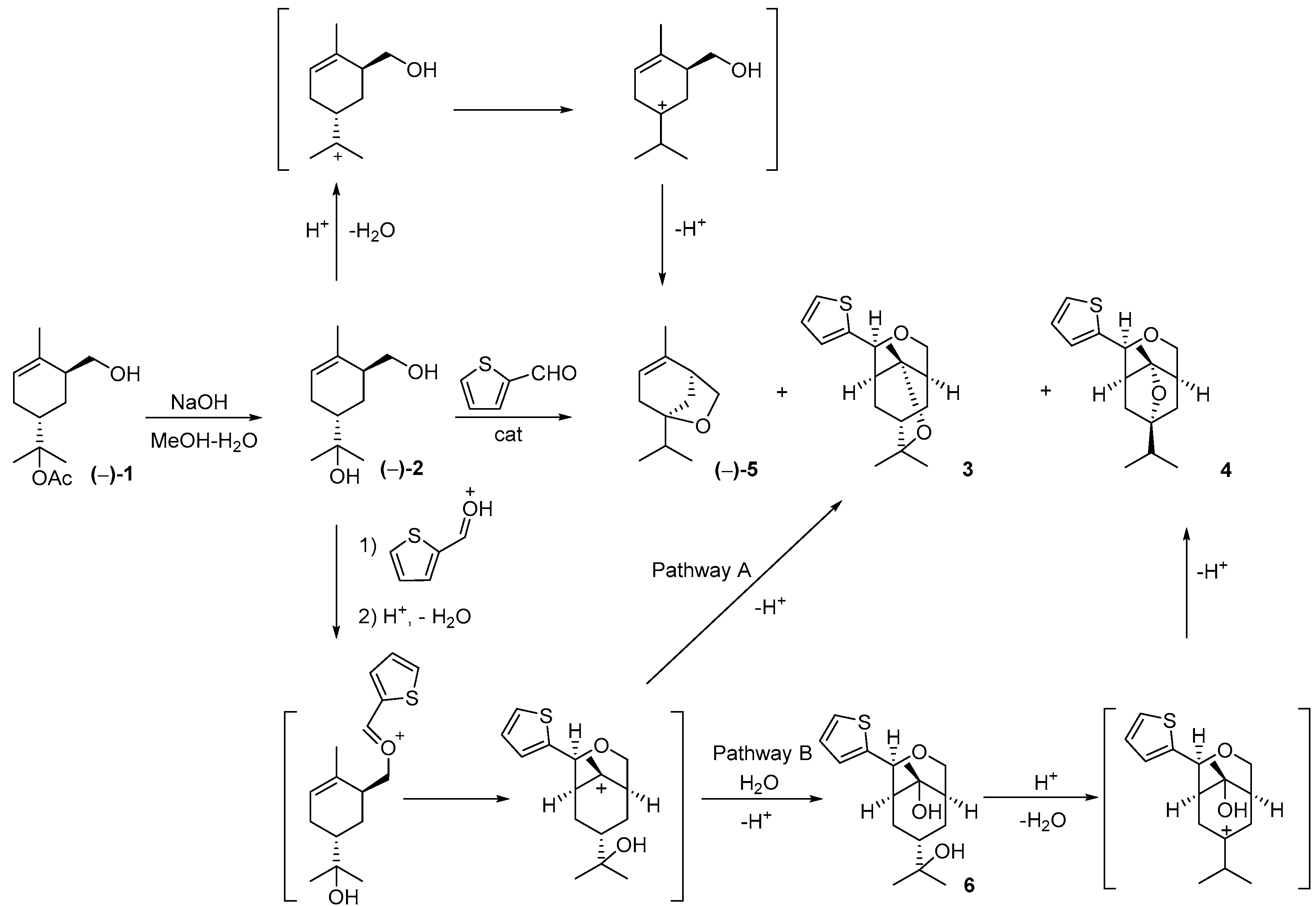
3. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Sousa, D.P.; de Assis Oliveira, F.; Arcanjo, D.D.R.; da Fonsêca, D.V.; Duarte, A.B.S.; de Oliveira Barbosa, C.; Ong, T.P.; Brocksom, T.J. Essential Oils: Chemistry and Pharmacological Activities—Part II. Biomedicines 2024, 12, 1185. [Google Scholar] [CrossRef] [PubMed]
- de Alvarenga, J.F.R.; Genaro, B.; Costa, B.L.; Purgatto, E.; Manach, C.; Fiamoncini, J. Monoterpenes: Current knowledge on food source, metabolism, and health effects. Crit. Rev. Food Sci. Nutr. 2023, 63, 1352–1389. [Google Scholar] [CrossRef] [PubMed]
- Mołdoch, J.; Agacka-Mołdoch, M.; Jóźwiak, G.; Wojtunik-Kulesza, K. Biological Activity of Monoterpene-Based Scaffolds: A Natural Toolbox for Drug Discovery. Molecules 2025, 30, 1480. [Google Scholar] [CrossRef]
- Barras, B.J.; Ling, T.; Rivas, F. Recent Advances in Chemistry and Antioxidant/Anticancer Biology of Monoterpene and Meroterpenoid Natural Product. Molecules 2024, 29, 279. [Google Scholar] [CrossRef]
- Zielińska-Błajet, M.; Feder-Kubis, J. Monoterpenes and their derivatives—Recent development in biological and medical applications. Int. J. Mol. Sci. 2020, 21, 7078. [Google Scholar] [CrossRef]
- Le, T.M.; Szakonyi, Z. Enantiomeric Isopulegol as the Chiral Pool in the Total Synthesis of Bioactive Agents. Chem. Rec. 2022, 22, e202100194. [Google Scholar] [CrossRef]
- Chukicheva, I.Y.; Buravlev, E.V.; Dvornikova, I.A.; Fedorova, I.V.; Zarubaev, V.V.; Slita, A.V.; Esaulkova, Y.L.; Kutchin, A.V. Evaluation of antiviral activity of terpenophenols and some of their N- and O-derivatives. Russ. Chem. Bull. 2022, 71, 2473–2481. [Google Scholar] [CrossRef]
- Valdivia, A.; Rocha, M.; Luque, F.J. Mining Druggable Sites in Influenza A Hemagglutinin: Binding of the Pinanamine-Based Inhibitor M090. ACS Med. Chem. Lett. 2025, 16, 126–135. [Google Scholar] [CrossRef]
- Dong, J.; Xiao, M.; Ma, Q.; Zhang, G.; Zhao, W.; Kong, M.; Zhang, Y.; Qiu, L.; Hu, W. Design and synthesis of pinane oxime derivatives as novel anti-influenza agents. Bioorganic Chem. 2020, 102, 104106. [Google Scholar] [CrossRef]
- Zhao, X.; Li, R.; Zhou, Y.; Xiao, M.; Ma, C.; Yang, Z.; Zeng, S.; Du, Q.; Yang, C.; Jiang, H.; et al. Discovery of Highly Potent Pinanamine-Based Inhibitors against Amantadine- and Oseltamivir-Resistant Influenza A Viruses. J. Med. Chem. 2018, 61, 5187–5198. [Google Scholar] [CrossRef]
- Anjaneyulu, B.; Sangeeta; Saini, N. A Study on Camphor Derivatives and Its Applications: A Review. Curr. Org. Chem. 2021, 25, 1404–1428. [Google Scholar] [CrossRef]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial Activity of Terpenes and Terpenoids Present in Essential Oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef]
- Fajdek-Bieda, A.; Pawlińska, J.; Wróblewska, A.; Łuś, A. Evaluation of the Antimicrobial Activity of Geraniol and Selected Geraniol Transformation Products against Gram-Positive Bacteria. Molecules 2024, 29, 950. [Google Scholar] [CrossRef]
- Khabibrakhmanova, A.M.; Faizova, R.G.; Lodochnikova, O.A.; Zamalieva, R.R.; Latypova, L.Z.; Trizna, E.Y.; Porfiryev, A.G.; Tanaka, K.; Sachenkov, O.A.; Kayumov, A.R.; et al. The Novel Chiral 2(5H)-Furanone Sulfones Possessing Terpene Moiety: Synthesis and Biological Activity. Molecules 2023, 28, 2543. [Google Scholar] [CrossRef]
- Nikitina, L.E.; Lisovskaya, S.A.; Startseva, V.A.; Frolova, L.L.; Kutchin, A.V.; Shevchenko, O.G.; Ostolopovskaya, O.V.; Pavelyev, R.S.; Khelkhal, M.A.; Gilfanov, I.R.; et al. Biological Activity of Bicyclic Monoterpene Alcohols. BioNanoSci 2021, 11, 970–976. [Google Scholar] [CrossRef]
- Moustafa, R.; Remete, A.M.; Szakonyi, Z.; Szemerédi, N.; Spengler, G.; Le, T.M. Synthesis and Antimicrobial Evaluation of (+)-Neoisopulegol-Based Amino and Thiol Adducts. Int. J. Mol. Sci. 2025, 26, 4791. [Google Scholar] [CrossRef]
- Zielińska-Błajet, M.; Pietrusiak, P.; Feder-Kubis, J. Selected Monocyclic Monoterpenes and Their Derivatives as Effective Anticancer Therapeutic Agents. Int. J. Mol. Sci. 2021, 22, 4763. [Google Scholar] [CrossRef] [PubMed]
- Le, T.M.; Njangiru, I.K.; Vincze, A.; Zupkó, I.; Balogh, G.T.; Szakonyi, Z. Synthesis and medicinal chemical characterisation of antiproliferative O,N-functionalised isopulegol derivatives. RSC Adv. 2024, 14, 18508–18518. [Google Scholar] [CrossRef]
- Sueishi, Y.; Nii, R. Monoterpene’s multiple free radical scavenging capacity as compared with the radioprotective agent cysteamine and amifostine. Bioorganic Med. Chem. Lett. 2018, 28, 3031–3033. [Google Scholar] [CrossRef]
- Nyamwihura, R.J.; Ogungbe, I.V. The pinene scaffold: Its occurrence, chemistry, synthetic utility, and pharmacological importance. RSC Adv. 2022, 12, 11346–11375. [Google Scholar] [CrossRef]
- Patrusheva, O.S.; Volcho, K.P.; Salakhutdinov, N.F. Approaches to the synthesis of oxygen-containing heterocyclic compounds based on monoterpenoids. Russ. Chem. Rev. 2018, 87, 771–796. [Google Scholar] [CrossRef]
- Ahammed, S.; Chowdhury, R.; Al Hasan, M.S.; Mia, E.; Akbor, M.S.; Islam, M.T.; Chowdhury, R.I.; Hossain, M.S.; Ansari, I.A.; Ansari, S.A.; et al. Anti-arthritic potential of linalool: In vitro, in vivo, and in silico mechanistic insights for safer therapeutic applications. Naunyn Schmiedebergs Arch. Pharmacol. 2025; online ahead of print. [Google Scholar] [CrossRef]
- Al Kury, L.T.; Abdoh, A.; Ikbariah, K.; Sadek, B.; Mahgoub, M. In vitro and in vivo antidiabetic potential of monoterpenoids: An update. Molecules 2022, 27, 182. [Google Scholar] [CrossRef] [PubMed]
- Juergens, U.R. Anti-inflammatory properties of the monoterpene 1.8-cineole: Current evidence for co-medication in inflammatory airway diseases. Drug Res. 2014, 64, 638–646. [Google Scholar] [CrossRef]
- Pries, R.; Jeschke, S.; Leichtle, A.; Bruchhage, K.-L. Modes of Action of 1,8-Cineol in Infections and Inflammation. Metabolites 2023, 13, 751. [Google Scholar] [CrossRef]
- Hoch, C.C.; Petry, J.; Griesbaum, L.; Weiser, T.; Werner, K.; Ploch, M.; Verschoor, A.; Multhoff, G.; Bashiri Dezfouli, A.; Wollenberg, B. 1,8-Cineole (eucalyptol): A versatile phytochemical with therapeutic applications across multiple diseases. Biomed. Pharmacother. 2023, 167, 115467. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.-M.; Peng, J.-Q.; Chen, Y.; Tao, L.; Zhang, Y.-Y.; Fu, L.-Y.; Long, Q.-D.; Shen, X.-C. 1,8-Cineole: A review of source, biological activities, and application. J. Asian Nat. Prod. Res. 2020, 23, 938–954. [Google Scholar] [CrossRef]
- Bruchhage, K.L.; Koennecke, M.; Drenckhan, M.; Plötze-Martin, K.; Pries, R.; Wollenberg, B. 1,8-cineol inhibits the Wnt/β-catenin signaling pathway through GSK-3 dephosphorylation in nasal polyps of chronic rhinosinusitis patients. Eur. J. Pharmacol. 2018, 835, 140–146. [Google Scholar] [CrossRef]
- Costa, A.O.C.; Rego, R.I.A.; Andrade, H.H.N.; Costa, T.K.V.L.; Salvadori, M.G.S.S.; Almeida, R.N.; Castro, R.D. Evaluation of the antiniciceptive effect generated by citronellal monoterpene isomers. Braz. J. Biol. 2023, 83, e271781. [Google Scholar] [CrossRef]
- Petitjean, H.; Héberlé, E.; Hilfiger, L.; Łapieś, O.; Rodrigue, G.; Charlet, A. TRP channels and monoterpenes: Past and current leads on analgesic properties. Front. Mol. Neurosci. 2022, 15, 945450. [Google Scholar] [CrossRef]
- Perri, F.; Coricello, A.; Adams, J.D. Monoterpenoids: The Next Frontier in the Treatment of Chronic Pain? J 2020, 3, 195–214. [Google Scholar] [CrossRef]
- Lataliza-Carvalho, N.L.; Cotta, R.F.; Martins, R.A.; Da Silva Rocha, K.A.; Kozhevnikova, E.F.; Kozhevnikov, I.V.; Gusevskaya, E.V. Heteropoly acid catalysis in the valorization of bio-renewables: Acetylation of 1,4-cineole and 1,8-cineole (eucalyptol) in green solvents. Mol. Catal. 2024, 554, 113863. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, H.; Xu, S.; Jiang, J.; Chen, Y.; Zhao, Z. Synthesis of 1,8-cineole derivatives from 3-Carene. Chem. Select. 2021, 6, 8640–8644. [Google Scholar] [CrossRef]
- Azerad, R. 1,8-Cineole: Chemical and biological oxidation reactions and products. ChemPlusChem 2014, 79, 634–655. [Google Scholar] [CrossRef]
- Sidorenko, A.Y.; Kurban, Y.M.; Il’ina, I.V.; Li-Zhulanov, N.S.; Patrusheva, O.S.; Goltsova, V.V.; Bei, M.P.; Aho, A.; Wärnå, J.; Heinmaa, I.; et al. Catalytic condensation of α-pinene with formaldehyde. J. Catal. 2024, 430, 115306. [Google Scholar] [CrossRef]
- Ilyina, I.V.; Patrusheva, O.S.; Goltsova, V.V.; Christopher, K.M.; Gatilov, Y.V.; Sidorenko, A.Y.; Agabekov, V.E.; Salakhutdinov, N.F.; Alabugin, I.V.; Volcho, K.P. Unusual cascade reactions of 8-acetoxy-6-hydroxymethyllimonene with salicylic aldehydes: Diverse oxygen heterocycles from common precursors. J. Org. Chem. 2024, 89, 11593–11606. [Google Scholar] [CrossRef]
- Sidorenko, A.Y.; Khalimonyuka, T.V.; Kurbana, Y.M.; Ilyina, I.V.; Li-Zhulanov, N.S.; Patrusheva, O.S.; Aho, A.; Heinmaa, I.; Volcho, K.P.; Salakhutdinov, N.F.; et al. Catalytic synthesis of heterocyclic compounds with a cineole moiety based on α-pinene. Appl. Catal. A General 2025, 691, 120070. [Google Scholar] [CrossRef]
- Il’ina, I.V.; Volcho, K.P.; Korchagina, D.V.; Salakhutdinov, N.F. The convenient way for obtaining geranial by acid-catalyzed kinetic resolution of citral. Helv. Chim. Acta 2016, 99, 373–377. [Google Scholar] [CrossRef]
- Sowbhagya, H.B. Chemistry, technology, and nutraceutical functions of cumin (Cuminum cyminum L.): An overview, Crit. Rev. Food Sci. Nutr. 2012, 53, 1–10. [Google Scholar] [CrossRef]
- Erhunmwunsee, F.; Pan, C.; Yang, K.; Li, Y.; Liu, M.; Tian, J. Recent development in biological activities and safety concerns of perillaldehyde from perilla plants: A review. Crit. Rev. Food Sci. Nutr. 2021, 62, 6328–6340. [Google Scholar] [CrossRef]
- Dragomanova, S.; Andonova, V.; Volcho, K.; Salakhutdinov, N.; Kalfin, R.; Tancheva, L. Therapeutic Potential of Myrtenal and Its Derivatives—A Review. Life 2023, 13, 2086. [Google Scholar] [CrossRef]
- Sharma, S.; Habib, S.; Sahu, D.; Gupta, J. Chemical Properties and Therapeutic Potential of Citral, a Monoterpene Isolated from Lemongrass. Med. Chem. 2021, 17, 2–12. [Google Scholar] [CrossRef] [PubMed]
| Aldehyde | K10, CH2Cl2, 24 h | BF3·Et2O, CH2Cl2, 5 °C, 1 h | ||
|---|---|---|---|---|
| Diol (+)-2 Conversion | Yield 1 | Diol (+)-2 Conversion | Yield | |
| 7 | 87% | 12 77% | 100% | 12 92% |
| 8 | 80% | 13 35% | 100% | 13 56% |
| 9 | 80% | 14 37% | 100% | 14 54% |
| 10 | 77% | 15 35% | 100% | 15 76% |
| 11 | 80% | 15 32% | 100% | 15 76% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patrusheva, O.S.; Ilyina, I.V.; Salakhutdinov, N.F.; Dragomanova, S.T.; Volcho, K.P. Synthesis of Heterocyclic Compounds with a Cineole Fragment in Reactions of α-Pinene-Derived Diol and Monoterpenoid Aldehydes. Compounds 2025, 5, 25. https://doi.org/10.3390/compounds5030025
Patrusheva OS, Ilyina IV, Salakhutdinov NF, Dragomanova ST, Volcho KP. Synthesis of Heterocyclic Compounds with a Cineole Fragment in Reactions of α-Pinene-Derived Diol and Monoterpenoid Aldehydes. Compounds. 2025; 5(3):25. https://doi.org/10.3390/compounds5030025
Chicago/Turabian StylePatrusheva, Oksana S., Irina V. Ilyina, Nariman F. Salakhutdinov, Stela T. Dragomanova, and Konstantin P. Volcho. 2025. "Synthesis of Heterocyclic Compounds with a Cineole Fragment in Reactions of α-Pinene-Derived Diol and Monoterpenoid Aldehydes" Compounds 5, no. 3: 25. https://doi.org/10.3390/compounds5030025
APA StylePatrusheva, O. S., Ilyina, I. V., Salakhutdinov, N. F., Dragomanova, S. T., & Volcho, K. P. (2025). Synthesis of Heterocyclic Compounds with a Cineole Fragment in Reactions of α-Pinene-Derived Diol and Monoterpenoid Aldehydes. Compounds, 5(3), 25. https://doi.org/10.3390/compounds5030025









