Abstract
One of the most pressing issues confronting modern society is carbon dioxide pollution (CO2). The reliance of social progress on CO2-producing technologies such as power generation, automobiles, and specialized industrial processes exacerbates the problem. Due to this reliance, it is critical to develop solutions to reduce CO2 emissions from these sources. One such solution is carbon capture and sequestration (CCS), which employs chemical methods to prevent CO2 emissions. The irreversibility of current CCS technology is its primary problem. Chitin, chitosan, and their derivatives, which were recovered from local seafood waste, are studied as reversible CO2 capture materials in this study in an effort to lessen this issue. Polysulfone (PSF) blends were employed to lessen chitosan edema, as chitosan’s hydrophilicity reduces its active sorption surface. Blends with only 20% chitosan have the same high sorption capacity as pure chitosan due to decreased swelling. Hydrolysis was used to boost the chitin sorption abilities. The CO2 sorption data were analyzed using an Intelligent Gravimetric Analyzer (IGA), Fourier-Transform Infrared (FTIR) spectroscopy, and Matrix-Assisted Laser Desorption/Ionization-Time of Flight (MALDI-TOF) spectroscopy. This study reveals that shrimp shells were the best source of chitin. This research led to the creation of eco-friendly, reversible, and reusable carbon sequestration sorbents.
1. Introduction
Increased quantities of greenhouse gases, especially CO2, are the cause of climate change and sea level rise, which endangers life on earth due to major changes in the temperature and ecology [1]. By 2050, the concentration of CO2 in the atmosphere is predicted to increase to 450 parts per million [2]. If steps are not made to start effectively preventing CO2 pollution in the first place, this figure will only increase as emerging countries in Africa, West Asia, and South Asia start to imitate Western societies in terms of economic and social behaviors.
The main emphasis of attempts to reduce greenhouse gas emissions is to use materials that can bind CO2 and lower its amount in the atmosphere. With each power plant capable of emitting several million tons of CO2 each year, the use of fossil fuels to generate electricity accounts for a lot of CO2 emissions caused by human activity. Instead of releasing CO2 into the environment, CCS absorbs and stores it from large point sources like fossil fuel power plants [3]. The utilization of liquid-based absorbents, silica-based absorbents, and reversible absorbents has been undertaken in various CO2 absorption techniques. Amines have filled the role of CO2 capturing group since the conception of carbon capture by Bottoms in the 1930s [4]. All types of amine solutions have been used as liquid CO2 absorbents. The selective removal of CO2 from gas streams was made possible by these absorbents.
According to Robinson et al. (2012), the incorporation of post-combustion CO2 capture (PCC) technology into both existing and new coal-fired power plants is crucial to reducing CO2 emissions in coal-fired power plants [5]. The most common type of PCC is aqueous amine-based compounds such as aqueous monoethanolamine (MEA) and diethanolamine (DEA). With absorbents like MEA, which have been used for decades to extract CO2 from gas streams in small-scale industrial activities like ammonia production and natural gas processing, PCC has demonstrated its worth in the industry [6,7].
However, the use of liquid-based CO2 absorbents has several disadvantages. Some of these amines decompose under process conditions, and MEA and DEA are not very stable. This leads to increased viscosity, excessive foaming, and a decrease in scrubbing efficiency. Decomposed amines may be discharged as pollutants into the atmosphere during the scrubbing process.
Amines have also been grafted onto mesoporous silicas during the capturing/absorbing process by silica-based absorbents. The reaction between carbon molecules and amines aids in the absorption of CO2 since it is based on the silica surface. Consequently, the process becomes irreversible as CO2 forms a carbonate bond with the silica absorbent. This leads indefinitely to the accumulation of solid absorbent waste that is incapable of breaking down [8]. As demonstrated by the literature, irreversible materials have been used in earlier research. Solid waste accumulates as a result of the inability to release CO2 during sorption.
Because of the high energy consumption and corrosive qualities of liquid amines, as well as the irreversible nature of silica sorbents, reversible and reusable sorbents are required for successful and realistic carbon sequestration. Amine groups in chitin and chitosan help them adsorb CO2.
Chitin is a high-molecular-weight natural polymer with incredible properties such as biocompatibility, non-toxicity, biodegradability, and antimicrobial properties. Chitosan, the most popular derivative of chitin, has numerous applications, and the market for a wide range of products is expanding. As a result, experts worldwide are researching new sources and technologies for chitin and chitosan from various raw materials [9,10,11]. According to Silva et al. (2017) [12], it was shown that chitin has a strong enough bonding potential to capture CO2, yet the process is reversible, and the sorbent can be reused.
Chitosan is obtained through the deacetylation of chitin, and the degree of deacetylation is at least 50 percent [13,14,15,16,17]. Chitosan can be processed chemically and industrially considerably more easily than chitin because of these different degrees of deacetylation (DDAs). Chitosan has an amino pKa of 6.3 and acts as a strong base as a result of the primary amines that are created during the deacetylation of chitin [18].
Numerous studies have been conducted on chitin and chitosan. For example, the CO2 sorption properties of amino-functionalized activated carbon (AC) were explored recently [19]. Tetraethylenepentamine (TEPA), pentaethylenehexamine (PEHA), and polyethylenimine (PEI) have been utilized by several researchers for CO2 sorption [20,21,22,23]. By chemically altering amino groups, Yamada et al. [24] presented a unique method to improve both adsorption and desorption capabilities. It was discovered that replacing TEPA with large hydrocarbon groups increased adsorption capacity and decreased regeneration heat. The amine loading greatly increased the CO2 sorption capacity of the materials despite reducing the mean pore size and diameter [25]. The attempt to use chitosan as a solid-state CO2 sorbent has been explored by Gao et al. (2015) [26]. Pohako (2016) [27] prepared chitosan ion gels for the preparation of inverse supported ionic liquid-phase (SILP) materials for CO2 absorption. Several attempts were made to create silica-based or composite materials with different types of matrices covered by chitosan [28].
Lopes et al. (2022) [29] combined ionic liquids (ILs) with chitin and chitosan biopolymers for CO2 sorption. ILs have received a lot of interest as more environmentally friendly alternatives to conventional solvents in various fields [30,31,32]. In comparison to volatile organic solvents, one of their key characteristics that contributes to less air pollution is their low vapor pressure [33]. Moreover, ILs are significantly effective at dissolving biopolymers like chitin and chitosan [34,35,36,37,38]. Despite the increased interest in using biopolymers to capture CO2, the regeneration of the materials is still an energy-intensive process, and their limited solubility is still a deal-breaker for the majority of CO2 capture methods [39,40,41].
An ideal sorbent in this context should be recycled with minimal energy expenditure, preferably at room temperature, in order to generate a competitive material. This study involves developing a reversible and affordable CO2 sorbent using chitin, chitosan, and its derivatives that were extracted from local seafood waste. Several methods to effectively evaluate the efficacy of a particular sorbent, including spectroscopic studies such as FTIR and IGA by Hidden Isochema, have been used to study the gravimetric mass transfer in materials as a function of time and/or temperature during the sorption and desorption processes.
In earlier tests by our research group in 2012, it was demonstrated that chitosan with a molecular weight of approximately 11,000 Daltons is biocompatible, biodegradable, and most importantly, a reversible sorbent [42]. By using an IGA, it was shown that pure chitosan has a CO2 capacity of up to 0.66 g CO2/g chitosan. The moist reaction conditions, like fossil gases in power plants, showed a fast loss of sorption capacity due to swelling in water vapors and a reduction in the sorption surface over time. In an attempt to solve the problem, this study probes PSF blends that contain both pure and modified chitosan with functional groups in reversible carbon sequestration.
Additionally, chitin’s capacity to capture CO2 was enhanced through hydrolysis. Seafood waste products offer a substantial untapped source of chitin because the Delmarva Peninsula generates the most seafood waste. Utilizing this waste as a potential source of sorbent would resolve additional ecological challenges. A comprehensive analysis was also carried out to evaluate and pinpoint the most concentrated sources of chitin in this region. Chitin can be recovered from shrimp shells and other crustaceans using alkaline reagents [16,43,44,45]. Pure chitin was separated from these biological structures through a standard procedure consisting of decalcification and deproteinization.
2. Materials and Methods
General procedures. Polysulfone with a molecular weight of 35,000 Dalton and chitosan with a low molecular weight of 11,000 Dalton (75–85 percent deacetylated) were obtained from Sigma-Aldrich and dried in a vacuum at 120 °C for 8 h before usage. Airgas (USA) received an order for high-quality nitrogen and carbon dioxide gas tanks. All of the remaining reagents and solvents were obtained from Fisher Scientific and utilized as-is. All sorption and desorption investigations were conducted using the Hidden Isochema IGA-003 (Edinburgh, UK) with a seven-channel MS gas analyzer and a combination of nitrogen and moist CO2 at various ratios. To make blends containing 2.5–5 wt.% chitosan, low-molecular-weight chitosan was dissolved in a minimum amount of 1% acetic acid water solution.
Preparation of polymeric blends with chitosan. Every blend was made in India in accordance with the synthesis description given below, which has already been detailed in the literature [46].
A homogeneous solution was obtained by dissolving 3.9 g of PSF in 15.6 mL of N-Methyl-2-pyrrolidone (NMP) at 60 °C. Subsequently, 5 mL of the chitosan solution (11,000 Dalton) and a further 5 mL of NMP were added while vigorously stirring at the same temperature. The product precipitated in the homogenous PSF solution after each addition. For 12 h, the resulting turbid solution was agitated at 70 °C to create a homogenous solution. After filtering the homogenous viscous solution with a G4 sand filter at about 50 °C, it was cast over a glass plate and placed in a coagulation bath with distilled water at 20 °C. After repeatedly washing the prepared sample with distilled water, it was dried for 24 h at 30 °C.
The same procedure was used to create a sample with greater chitosan concentrations, but a larger volume of chitosan solution was added to the NMP solution (instead of using 5 mL of the chitosan solution to create a sample with 5% chitosan, 10 mL volumes were used).
Chemical modification of chitosan. Due to poor solubility in only a limited number of organic solvents, chitosan needs chemical modifications to be blended with PSF.
Synthesis of N-succinyl chitosan (NSCS). NSCS is an amphiprotic derivative obtained from the N-acylation of chitosan. The synthesis was performed as described in the literature [47]. An amount of 1 g of chitosan was dissolved under magnetic stirring and at room temperature in 100 mL of a 1% (w/w) glacial acetic acid solution. Subsequently, a solution of succinic anhydride (1.8 g) in 99.9% acetone (20 mL) was added dropwise and under stirring. The mixture was then exposed to an ultrasonic bath at 50 °C for 60 min. The resulting solution was then cooled to room temperature; 95% hydrated ethyl alcohol (100 mL) was added, and the mixture was transferred to a freezer (−20 °C), where it remained for 24 h. After this period, a 1 mol/ L aqueous sodium hydroxide solution was added until pH 10. Then, acetone was added until precipitation occurred. The mixture was again placed in the freezer for 48 h. After this period, the product was vacuum-filtered using 95% ethyl alcohol (about 1000 mL) to wash the retained solid. The final product was obtained as a yellowish-white powder and dried in a vacuum [48].
Synthesis of N-propyl-phosphonic chitosan (NPPCS). An amount of 2 g of chitosan was mixed with 100 mL of deionized water at 30 °C; then, propyl-phosphonic anhydride (0.5 mL, 50% solution in EtOAc) was added dropwise over a period of 10 min at the same temperature, and the clear homogeneous solution was observed once the addition was completed. The reaction mass was stirred at 30 °C for 6 h. The product was precipitated by adding an excess of acetone, filtered to remove the solvent, and then washed with 80% acetone in water, 90% acetone in water, and with pure acetone, respectively. Finally, the product was dried at 40 °C under vacuum for 24 h to obtain the desired N-propyl-phosphonic chitosan as a white powder. This yielded approximately 2.4 g of product [49].
IGA analysis. A typical TGA (thermogravimetric analyzer) instrument consists of an analytical balance, a furnace, a way of controlling the gaseous environment of the sample, and a computerized control unit for the whole system and for data collection. The advanced modification of TGA instruments that has been proven useful for CO2 sorption studies is the Intelligent Gravimetric Analyzer (IGA). This instrument allows us to control flow, the mixture of gas composition and wetness, pressure, and temperature inside the reactor with the TGA balance built in, while also measuring the intensity of mass spectrometer channels tuned for up to seven different gases, along with a general ESI-MS function.
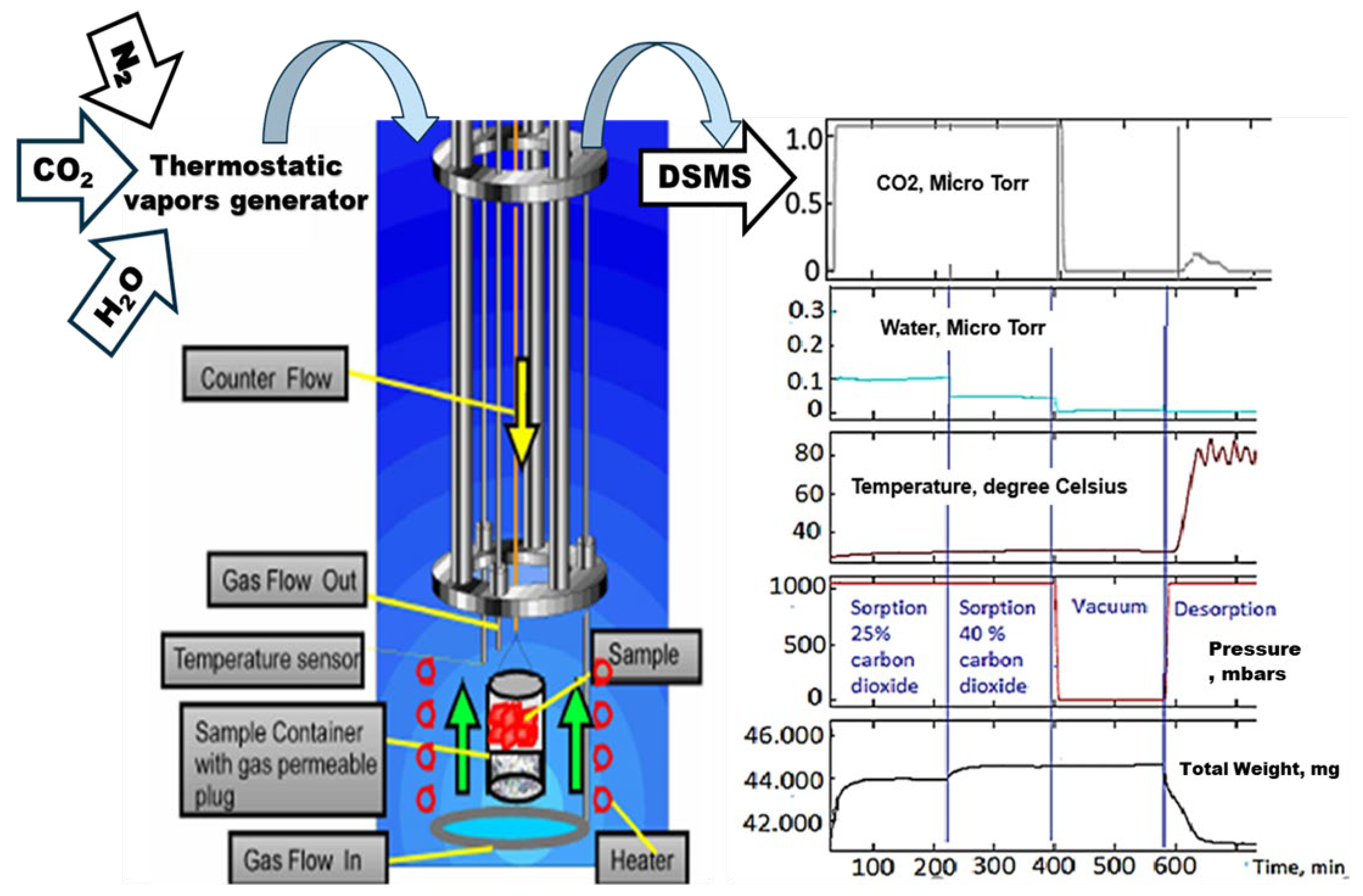
The IGA design incorporates precise computer control and measurement of weight change, pressure, and temperature to enable the fully automatic and reproducible determination of gas adsorption–desorption isotherms and isobars. The fully automated instrument designed for this study employs the Hiden IGA (Hiden Isochema Ltd., IGA-003, Edinburgh, UK) gravimetric method [50,51] to continuously weigh the sample in the presence of a carrier gas flow that is regulated upstream in the balance chamber at a constant total pressure. The vapors are introduced from a thermostatic vapor generator and fed into a reactor with a separate isothermal jacket that houses the sample (Figure 1). An independent heating element at the sample position is used for the controlled ramp desorption rate. A Hiden HPR-20 series DSMS mass spectrometer (Hiden Isochema., Edinburg, UK) was used for gas analysis. Figure 1 illustrates the general scheme of the IGA and an example of output.
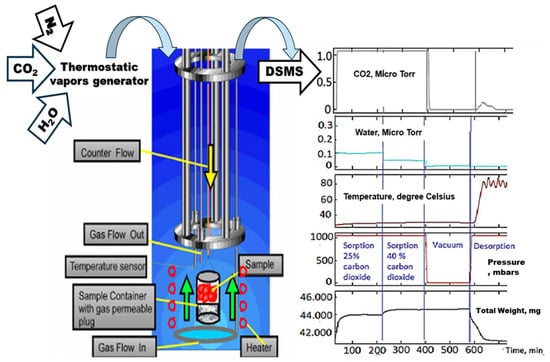
Figure 1.
Scheme of the IGA machine and an example of the output.
To imitate the fossil gases of the power plant, a mixture of nitrogen and carbon dioxide gases was flown through the system at two ratios: 25% of carbon dioxide for the first 220 min. of the experiment and 40% of carbon dioxide for the next 220 min. The carbon dioxide was kept moist by passing it through a vapor generator filled with water. A sample of solid sorbent in the form of powder was placed on the sample holder of a built-in precise balance. Then, the reactor was sealed, and the initial weight of the sample was recorded after the balance was steady for at least 2 min with the flow of nitrogen through the reactor at the same flow as during the experiment.
All experiments were carried out at atmospheric pressure and room temperature, with a gas mixture kept at roughly 15% humidity. The flow of gas was 250 mL/min, while the composition of gases varied at different steps of the experiment, as described above.
FTIR analysis of chitosan samples. Each sample for FTIR measurement was dried under vacuum in a round-bottom flask at room temperature for approximately 24 h or until the mass of the sample became constant. The spectrum of each sample was then measured using a Cary 630 FTIR (Philadelphia, PA, USA) spectrometer with a diamond ATR attachment. The 32 scans of spectra were compared with a chitosan standard obtained from Tokyo Chemical Industries (TCI) and dried similarly to the samples above.
Carbon sorption–desorption experiments with chitin and chitosan samples.
The aim of this procedure was to characterize the ability of pure chitin to react with atmospheric carbon dioxide, since it also has amine groups to capture CO2. This experiment was conducted for pure chitin at atmospheric pressure and room temperature. Also, chitin was purchased from TCI. Samples were treated at 24, 30, 48, and 72 h and dried under vacuum for 10–12 h. An FTIR scan (Agilent, Santa Clara, CA, USA) was taken of the samples before and after CO2 exposure.
Since chitin is a polymer with a high molecular weight (200,000 Dalton), it is insoluble in the majority of solvents. The long-chain polymer was then treated with an acid to initiate a hydrolysis reaction [52], which resulted in the formation of smaller oligomers and short chain structures with more exposed amine binding sites and greater solubility. Reagent-grade chitin obtained from TCI and chitin extracted from shell waste were used for hydrolysis. This reaction is usually catalyzed by a weak acid or a base [53].
Hydrolysis of chitin.
In a conical flask, 20 g of chitin was dissolved in 350 mL of 11M HCl. It was stirred with a magnetic stir bar for one to one and a half hours at 4 °C. Secondly, the chitin paste was heated in HCl constantly for two hours at 40 °C in a water bath. Third, an ice bath was used to chill the hydrolysate to 4 °C. Next, while stirring, 50% NaOH was added dropwise to the hydrolysis solution until the acid was neutralized (confirmed with a pH paper). Ultimately, the precipitate was extracted from the solution using a Buchner funnel and Whatman filter paper [51]. The hydrolysis process was carried out at various temperatures and periods of time, and the outcomes were evaluated using MALDI-TOF.
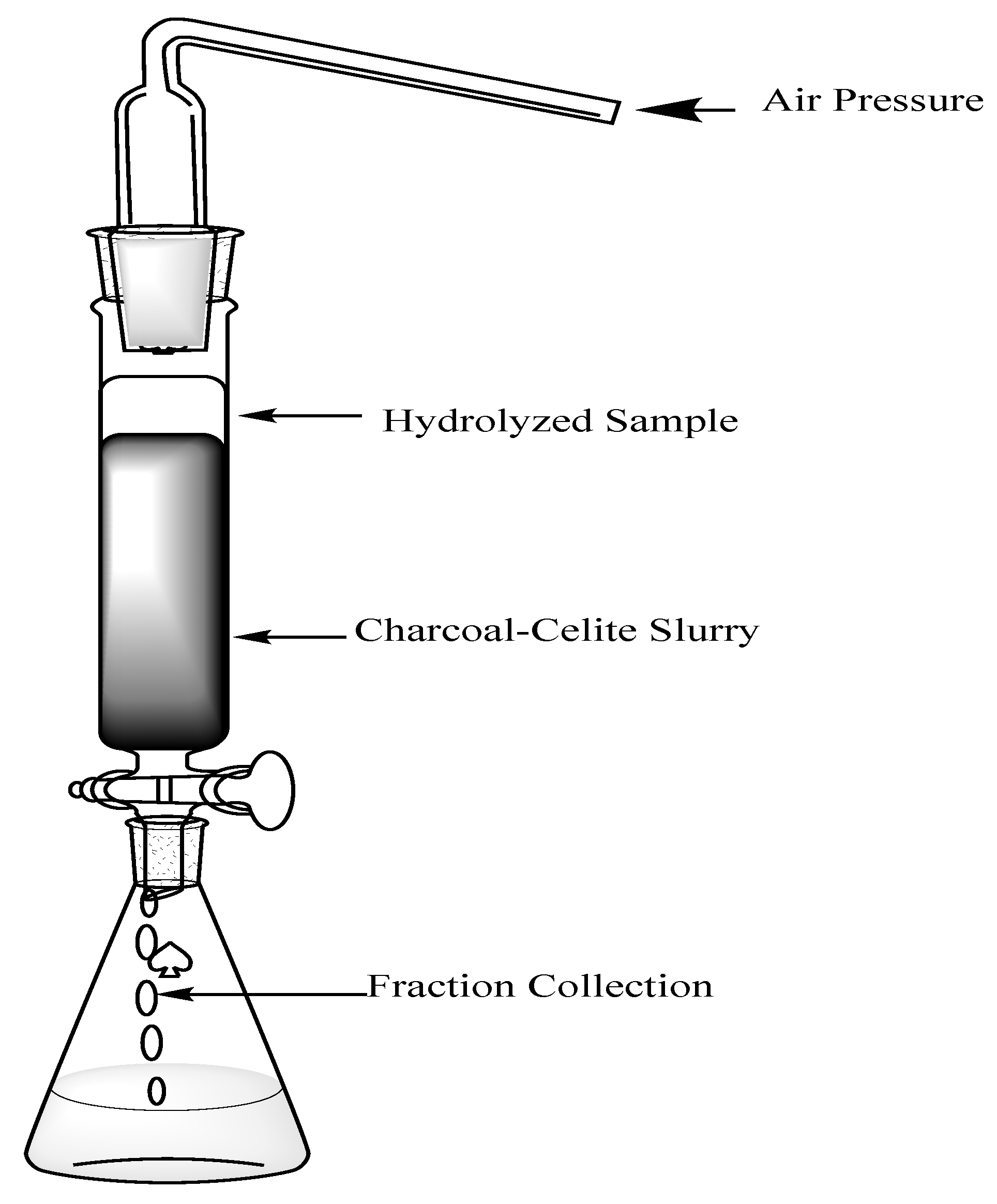
Preparation of charcoal–celite column.
To prepare the slurry, 300 g of activated charcoal was mixed with 300 g of celite into 2500 mL of deionized water. This solution was boiled until thickening occurred, giving the slurry consistency. The slurry was cooled in a flask covered with parafilm at room temperature until it was able to be added to the column. Two inches of sand was added to the bottom of the column (5 cm × 70 cm). Then, the slurry was added into the column in 5 parts to allow even layers of the slurry throughout. Water was continuously running throughout to prevent cracking of the column (Figure 2).

Figure 2.
Charcoal–celite column to separate hydrolysis products under pressure.
Separation of chitin oligomers on Charcoal–Celite column.
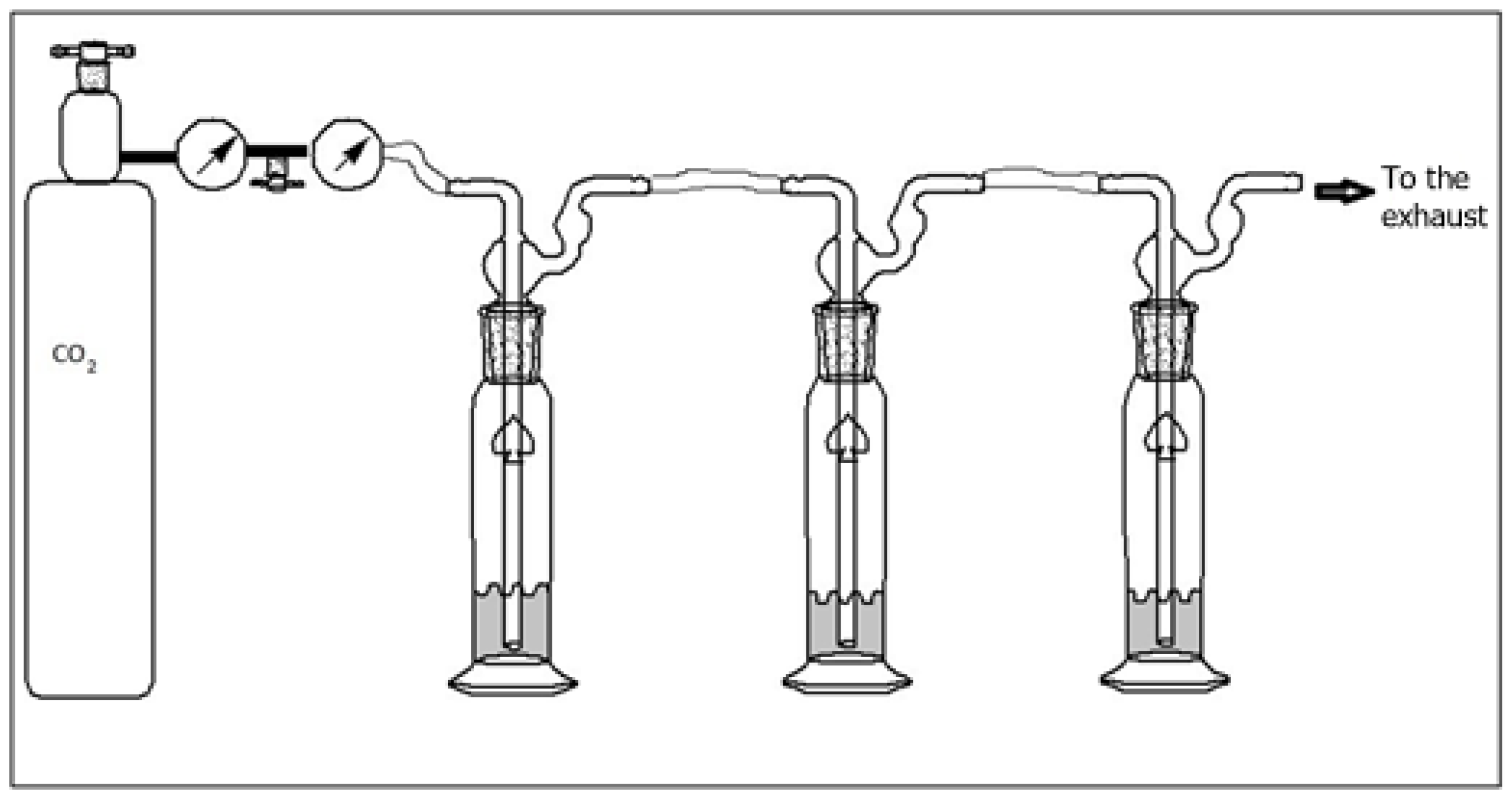
To isolate the individual pieces and assess their respective sorbance capacities, the hydrolysate was filtered through a charcoal–celite column using 5%, 10%, 15%, and 20% ethanol in water solutions. Then, 50 mL fractions were collected and concentrated using a rotary evaporator to ~25 mL and freeze-dried. The dry fraction of chitin was used for MALDI. Samples for CO2 sorption (1 g) were dissolved in deionized water with a gas flow rate of 5 PSI, as shown in Figure 3. The 10 and 20% fractions were utilized for FTIR spectroscopy and CO2 sorption because they produced a larger yield of hydrolyzed chitin.
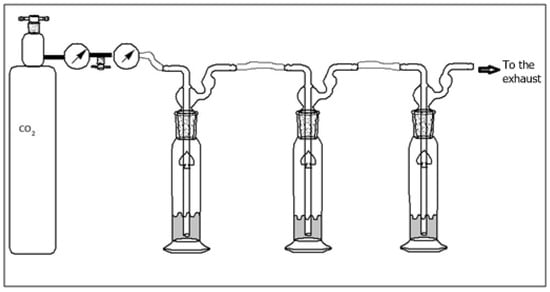
Figure 3.
Apparatus for CO2 sorption.
Isolation of raw chitin from samples of typical Delmarva Peninsula waste.
In this study, the method used 25% HCl and 20% KOH for demineralization [54] and deproteination [55], respectively. Seven types of shells were tested in this project, as these were all readily available products in the Delmarva region at the time of testing: whelk (1), hard clam (2), blue crab (3), eastern oyster (4), scallop shell (5), shrimp shell (6), and horseshoe shell (7) Reagent-grade chitin was obtained from TCI for comparison.
Decalcification of shell samples.
The purpose of the decalcification procedure was to remove the calcium carbonate layer by adding 25% (v/v) HCl, thus isolating the underlying chitin and protein matrix, which can be filtered from the suspension, dried, and calculated as a weight fraction of the original raw shell. Samples were prepared by drying about 5 g of the raw material in the oven at 50 °C until constant mass measurements were achieved, indicating that all water had been desorbed. This small aliquot of known mass was then transferred to a 400 mL Erlenmeyer flask with about 10 mL of deionized water. The shell portions were then treated with 25% (v/v) HCl until visible effervescence ceased. After this, the base solution was added until a neutral pH was reached, as indicated by phenolphthalein. The suspension was then vacuum-filtered and dried in an incubator at 50 °C until a constant mass was observed.
Deproteination of shell samples.
Decalcification by the above procedure simply removed the inorganic CaCO3 layers in the shell samples while leaving the organic material intact—namely, a mixture of chitin, proteins, and a small amount of pigments. Additional treatment of this organic material with a diluted base (20% w/v KOH) decomposes the amino acid polymers while leaving the chitin in the sample [56]. For this procedure, approximately 1 g of dry, decalcified sample was measured into a 250 mL conical flask and combined with 20% (w/v) KOH to determine optimal conditions. These mixtures were stirred for 1 day and then filtered under vacuum. The resulting filter cake was dried in an incubator at 50 °C until constant mass was attained.
3. Results
Polymeric blends with chitosan.
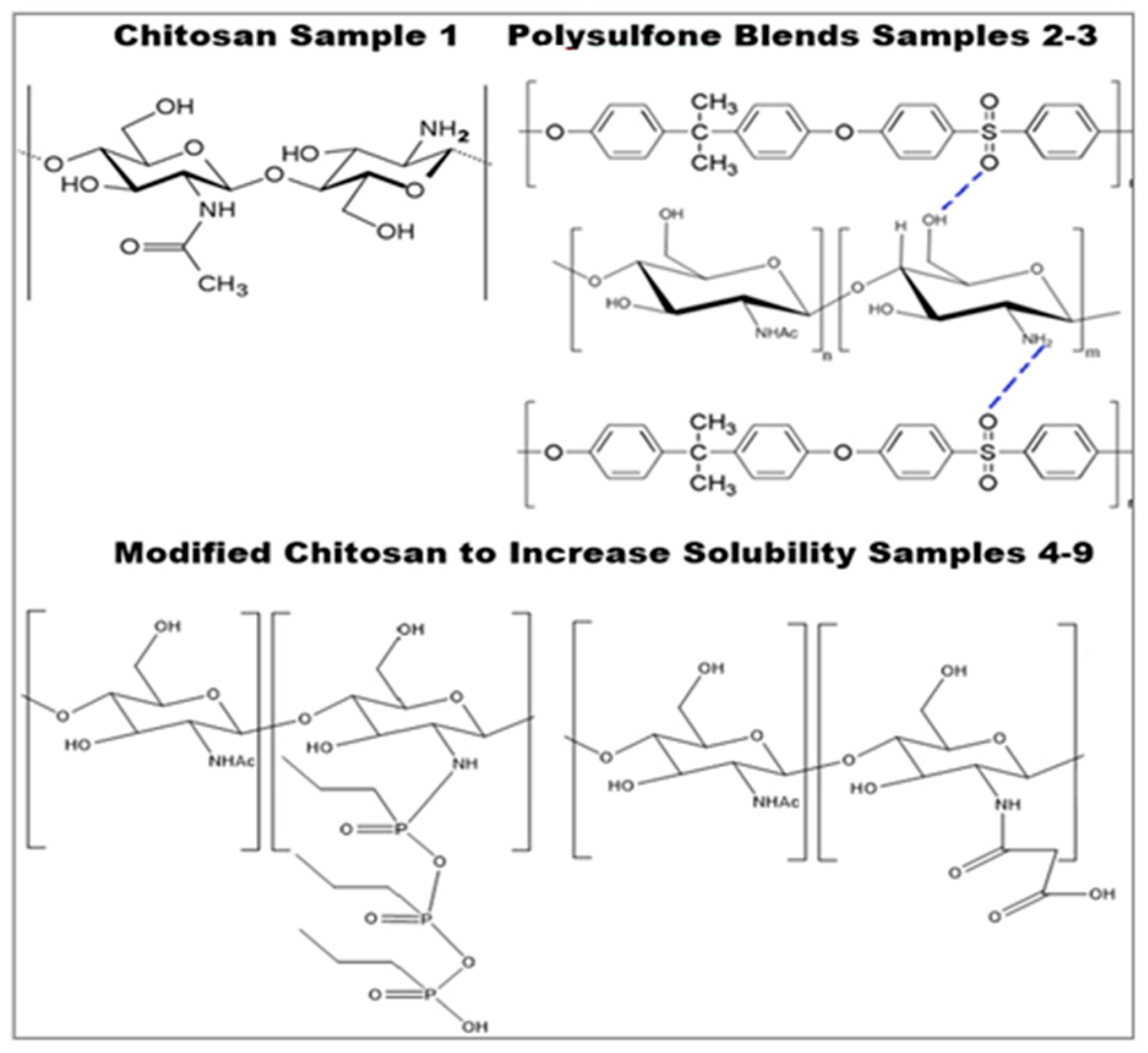
When pure chitosan was employed for CO2 sorption, it demonstrated up to 0.66 g CO2/g chitosan sorption capacity. Moisture-rich reaction circumstances, such as those seen in fossil gas power plants, have shown a rapid loss of sorption ability due to swelling in water vapors and a reduction in sorption surface with time. In order to address the issue, nonpolar polymer PSF was combined with both pure and modified chitosan with functional groups developed. Chitosan has amino groups capable of CO2 capture, whereas PSF does not. For the blends, the capacity increases with the increasing concentration of chitosan in the blend. In pure chitosan, the concentration of amino groups per weight unit of the sample is the highest, and as a result, the sorption capacity is the best. However, due to solubility restrictions, only a small amount (only 5%) of chitosan can be mixed with PSF. Figure 4 displays hydrogen bonding between chitosan and PSF blends of up to 5% (sample 2 with 2.5% of chitosan and sample 3 with 5% of chitosan).
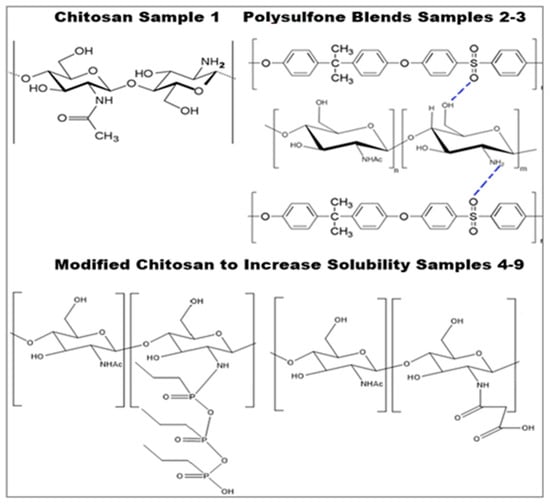
Figure 4.
Chemical modification of chitosan [46,48,49].
Chemically modified chitosan. Enhancing chitosan’s solubility at a neutral pH was the purpose of this modification. Figure 4 displays the reaction product. The cyclic anhydride group in propyl-phosphonic anhydride instantly cleaves in water to produce an open hydrated intermediate with a phosphonic acid group. As a result, water becomes somewhat acidic instead of neutral, which increases the solubility of more chitosan [49]. In the case of NSCS, succinylation occurred by substituting the H from the amino group, and an amidic link was generated with the opening of the anhydride ring [48,57]. With chitosan modified into NPPCS and NSCS, the solubility increases, allowing for the incorporation of up to 20% of chitosan into the blend (samples 4–9).
IGA analysis of chitosan and blend samples. An overview of the research literature has revealed that there are two different types of measurements that are typically made when studying gas sorption with an IGA apparatus: these are sorption cycling and isotherm modelling. Sorption cycling experiments are conducted to determine the stability of a sorbent over a specific lifetime by measuring sorption and desorption as a function of time. This second function was utilized in this research [50,51,58,59].
The example of IGA output for sample 1 of pure chitosan is presented in Figure 1. The amount of chitosan was roughly 42 mg, as seen in the first channel. An external heater regulated the temperature in the IGA system. Using MS channels with signal intensities of m/z = 18 for water and 44 for CO2, the experiment’s emissions of carbon dioxide and water were measured. To clean the image for gases of interest, the nitrogen signal at m/z = 28 was automatically eliminated from the spectrum.
As a mixture of CO2 and nitrogen passes through the sorbent, the experiment was designed to begin at room temperature and atmospheric pressure. During the whole experiment, the mass gain and loss as a function of time were recorded, and at the same time, the intensity of CO2 and water peaks in the gas analyzed was recorded as well. The weight of the sample increases as 25% CO2 flows through it, and it increases once more when the concentration is raised to 40%. The replica fossil gas mixture was allowed to flow for 400 min before the flow was stopped, and a room-temperature vacuum was used to clear the system of gases and ensure that there was no more CO2 in the pipes.
Throughout the whole vacuumizing process, the mass of the sorbent containing the adsorbed CO2 remained constant, suggesting that the sorbent retained all of the CO2 that had been adsorbed and that the bonds between the CO2 and chitosan were stable and chemical in nature. The system was only filled with dry nitrogen following the application of the vacuum, and the nitrogen flow continued into the stage of desorption at atmospheric pressure.
The experiment’s desorption process involved raising the system’s temperature from ambient temperature to about 90 °C at a rate of 2 °C per minute. While the total release of all adsorbed CO2 was recorded at about 80 °C, a point at which the weight of sorbent decreased, the majority of the sorbent samples began releasing CO2 above 50 °C, as demonstrated by increasing the intensity of the MS channel at m/z = 44. After being taken out of the instrument sample holder, the sorbent was vacuum-dried in an oven once more, making it reusable.
Carbon dioxide sorption and desorption results for the pure chitosan (sample 1) and its blends with PSF (sample 2 with 2.5% of chitosan and sample 3 with 5% of chitosan) are shown in Table 1. The sorption capacity is the best for pure chitosan (0.66 gCO2, sample 1). Swelling caused the loss of surface, and the sorption stopped after the first 20 min of the run. Blending of the pure chitosan with PSF solved the problem of swelling, yet, because no more than 5% of chitosan can be incorporated, the sorption capacity was reduced (0.8 gCO2 and 0.23 gCO2). However, when 5% of chitosan was added to PSF sample 3, the concentration of the chitosan was reduced 20 times as compared to the pure chitosan (compare entries 1 and 3 of Table 1), and the sorption capacity was only reduced by about 3.2 times due to a larger sorption surface with no swelling.

Table 1.
Carbon dioxide sorption–desorption data for pure chitosan and its blends with polysulfone.
Next, chemical alteration was carried out to make chitosan more soluble in PSF. In the same table, sample 4 is a modified chitosan into NPPCS, followed by samples 5 and 6 with 10 and 20% w/w of NPPCS in polysulfone, respectively. Sample 7 is another type of modified chitosan into NSCS, followed by samples 8 and 9 with 10 and 20% w/w of NSCS in PSF, respectively. As a result, the sorption capacity increases to 0.53 g CO2 (sample 9), almost matching that of pure chitosan and without the swelling problem. The amount of CO2 adsorbed per gram of sample per hour was used to determine the sorption capacity for both 25% and 40% of CO2.
FTIR analysis of chitosan.
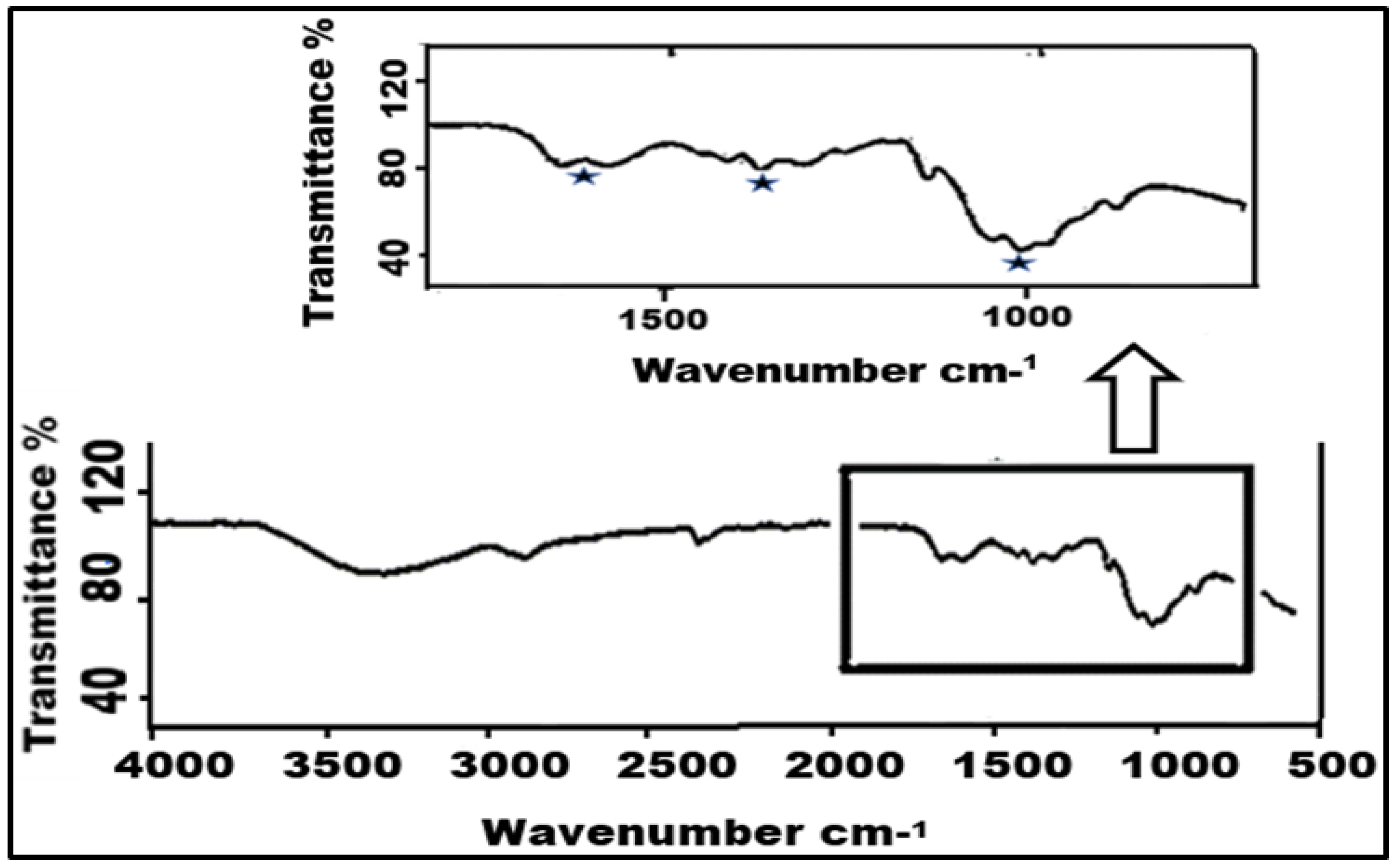
As has been shown in the literature, two products are possible when the primary amine of chitosan reacts with carbon dioxide. The formation of carbamate is an indicator for reversible carbon sequestration, while the formation of bicarbonate or carbonate points to irreversible capture [42,60]. Smit et al. (2014) [61], who used pure water as a model for the kinetics of carbon capture, showed that CO2 reacts with water to form carbonic acid as the rate-determining step and then further dissociates to form the carbamate and bicarbonate forms. The FTIR difference between a carbamate and bicarbonate adsorption product from the literature, which was studied for liquid amines, shows a clear difference between bicarbonate peaks at ≈1350 and 1450 cm−1, and carbamate peaks at ≈1050, 1410, and 1550 cm−1 can be seen [62].
The presence of amino groups of NH2 was not detected by the FTIR analysis of the chitosan during extended CO2 capture. Additionally, Figure 5 displays FTIR peaks at around ≈1050, 1410, and 1550 cm−1. As a result, it matches the maxima of carbamate production during CO2 sorption found in the literature. Because the chitosan sample was tested in transmittance rather than absorbance, the peaks are inverted. Following CO2 sorption, the FTIR results for the chitosan samples verify the reversibility and carbamate production.
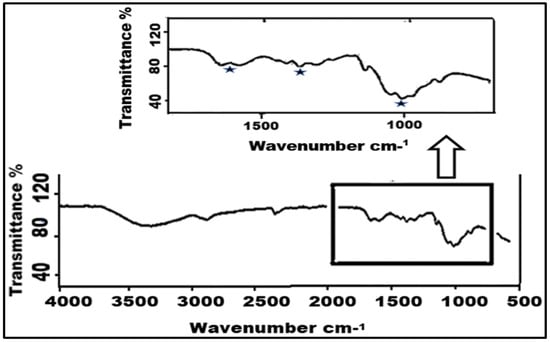
Figure 5.
FTIR of chitosan after CO2 absorption.
FTIR Analysis of Chitin.
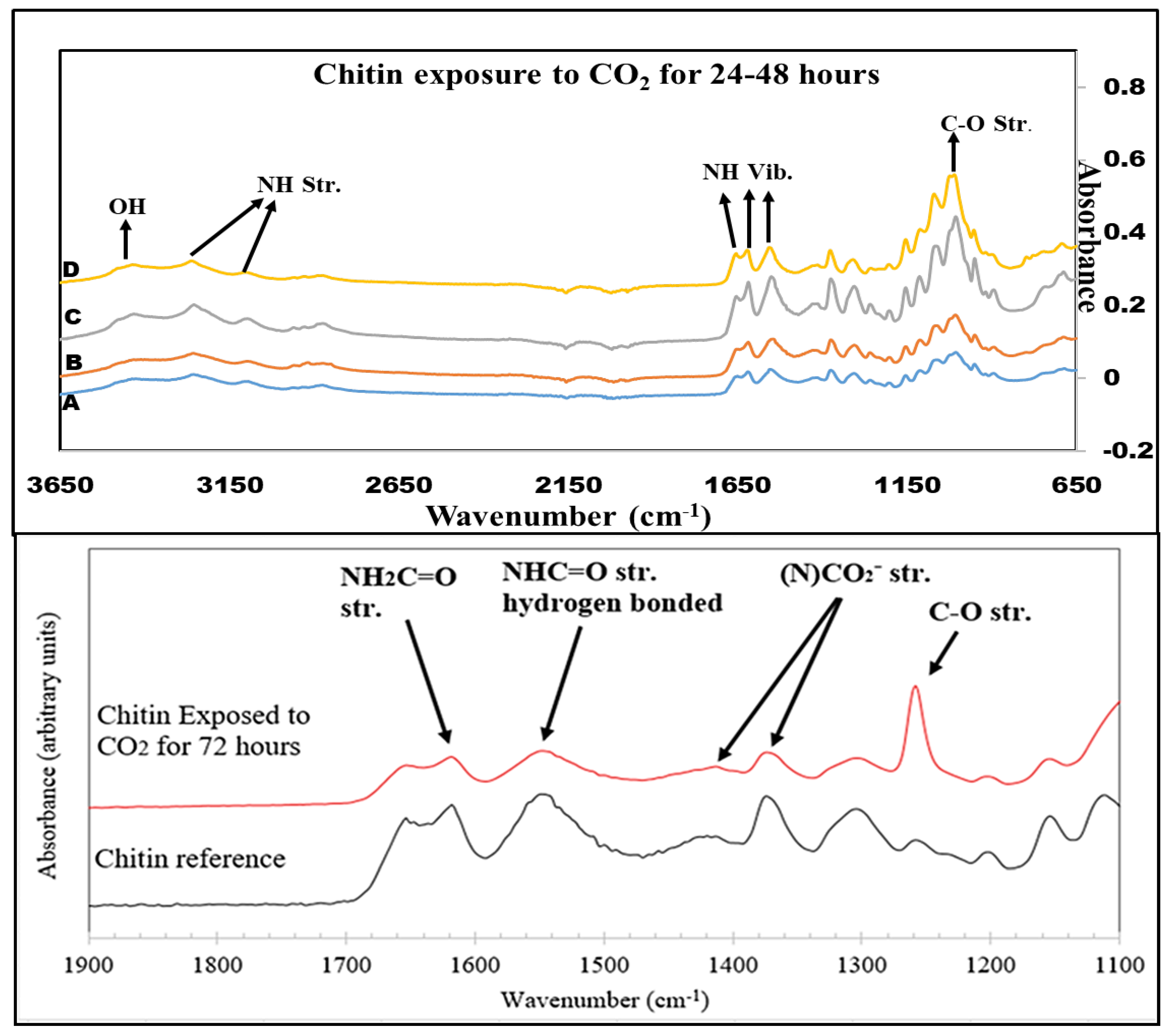
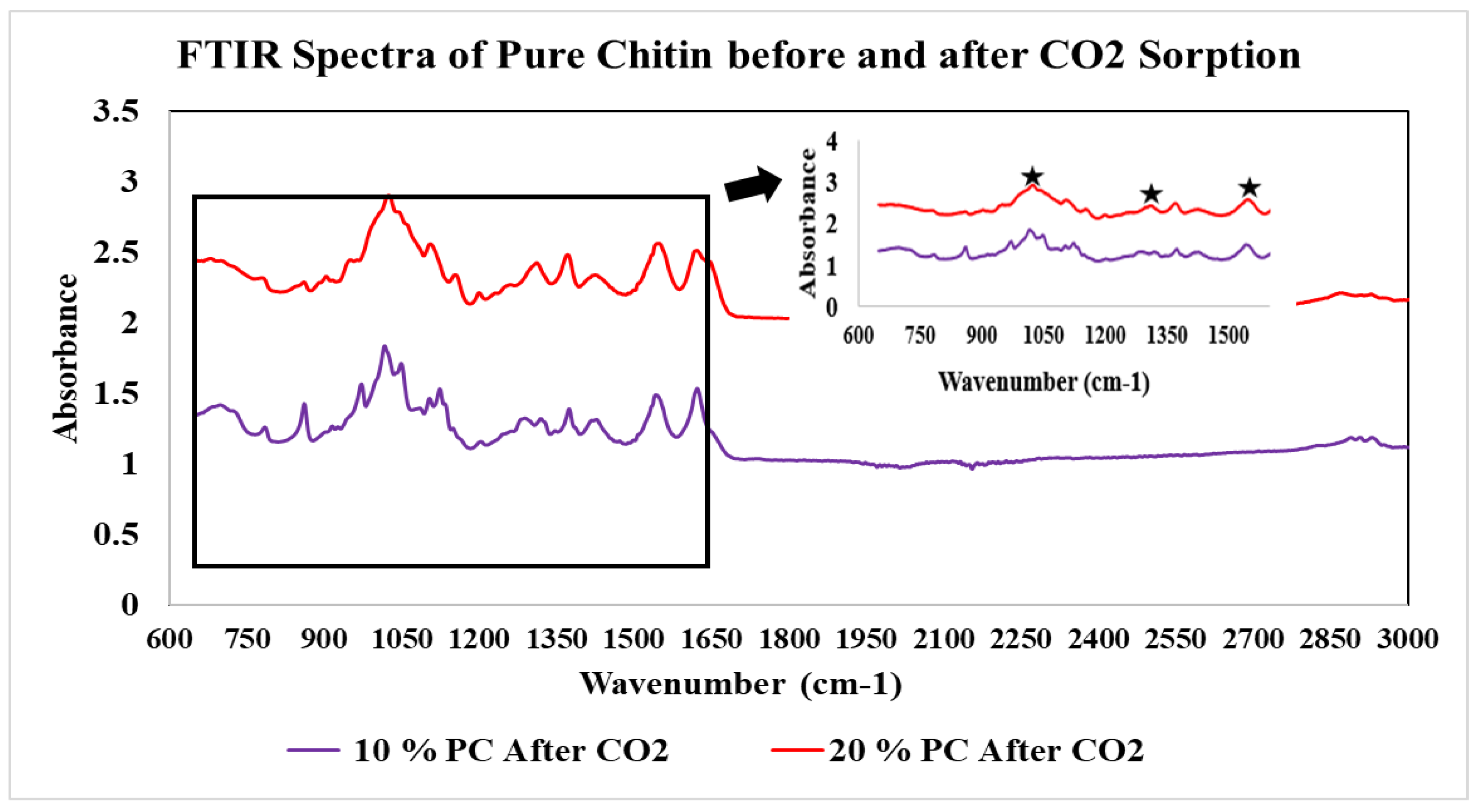
The FTIR spectra of the pure chitin samples exposed to CO2 for varying time lengths can be seen in Figure 6. These reveal that no change occurs over the course of 48 h. During exposure for a longer time, at 72 h, a more noticeable change began to take place in the lower wavenumber region between 650 and 1650 cm−1. Carbon–oxygen stretching vibration was observed in 1260–1300 cm−1. A NCOO− moiety was found at 1350–1450 cm−1. Double-bonded carbon and oxygen were present at 1550–1600 cm−1. Also, NH stretching occurred between 1600 and 1650 cm−1. To overcome this drawback, hydrolysis was performed to boost the solubility and CO2 sorption capability of chitin.
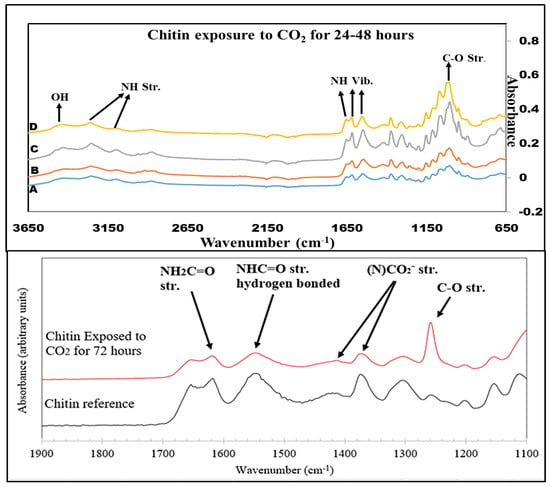
Figure 6.
FTIR spectra of Chitin exposure to CO2 for (A) 0 h, (B) 24 h, (C) 36 h, (D) 48 h, and 72 h.
MALDI Analysis of 30, 40, and 50 degrees Celsius.
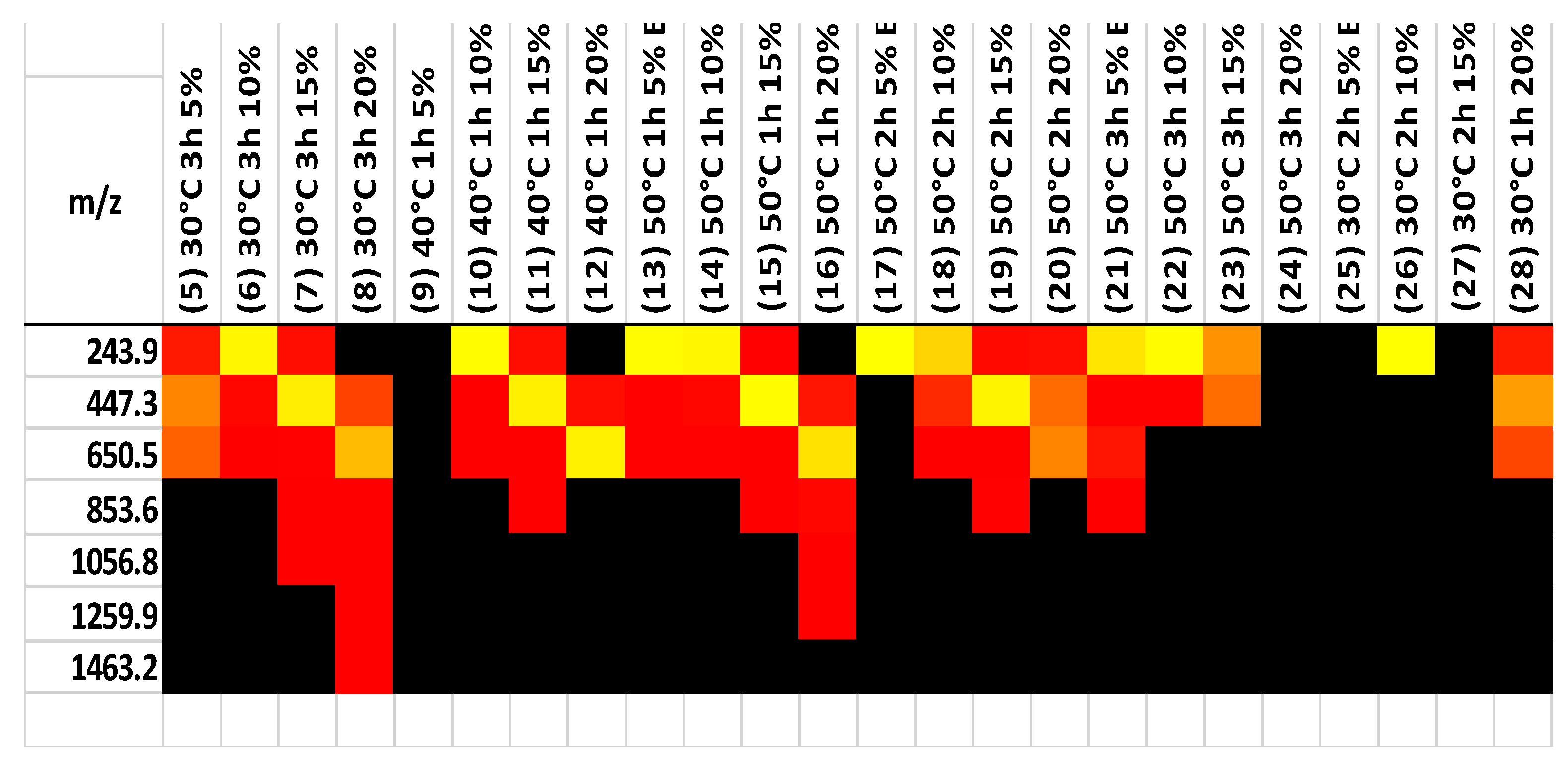
Partial hydrolysis is typically completed with hydrochloric acid. The products consist of chitin monomers and small oligomers. So, the acid hydrolysis of chitin leads to the cleavage of glycoside bonds. Previous research [43,44,45] has shown that these fragments are often more soluble and provide additional bonding opportunities for the relevant molecules by removing size limits. MALDI was used to test samples of hydrolysis and separation products, obtained at various temperatures and times, and the results are shown in Figure 7. The majority of the samples have higher concentrations of monomers, dimers, and trimers. Only a 20% fraction at 30 degrees Celsius for 3 h and 50 degrees Celsius for 1 h had a higher chain polymer of chitin. Based on the MALDI data, it was confirmed that chitin was broken down into small oligomers, which increased its solubility and CO2 sorption capacity, as shown in Table 2.
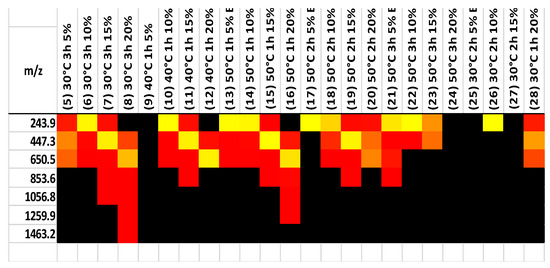
Figure 7.
MALDI results for 30, 40, and 50 degrees Celsius.

Table 2.
Adsorption capacity of chitin samples.
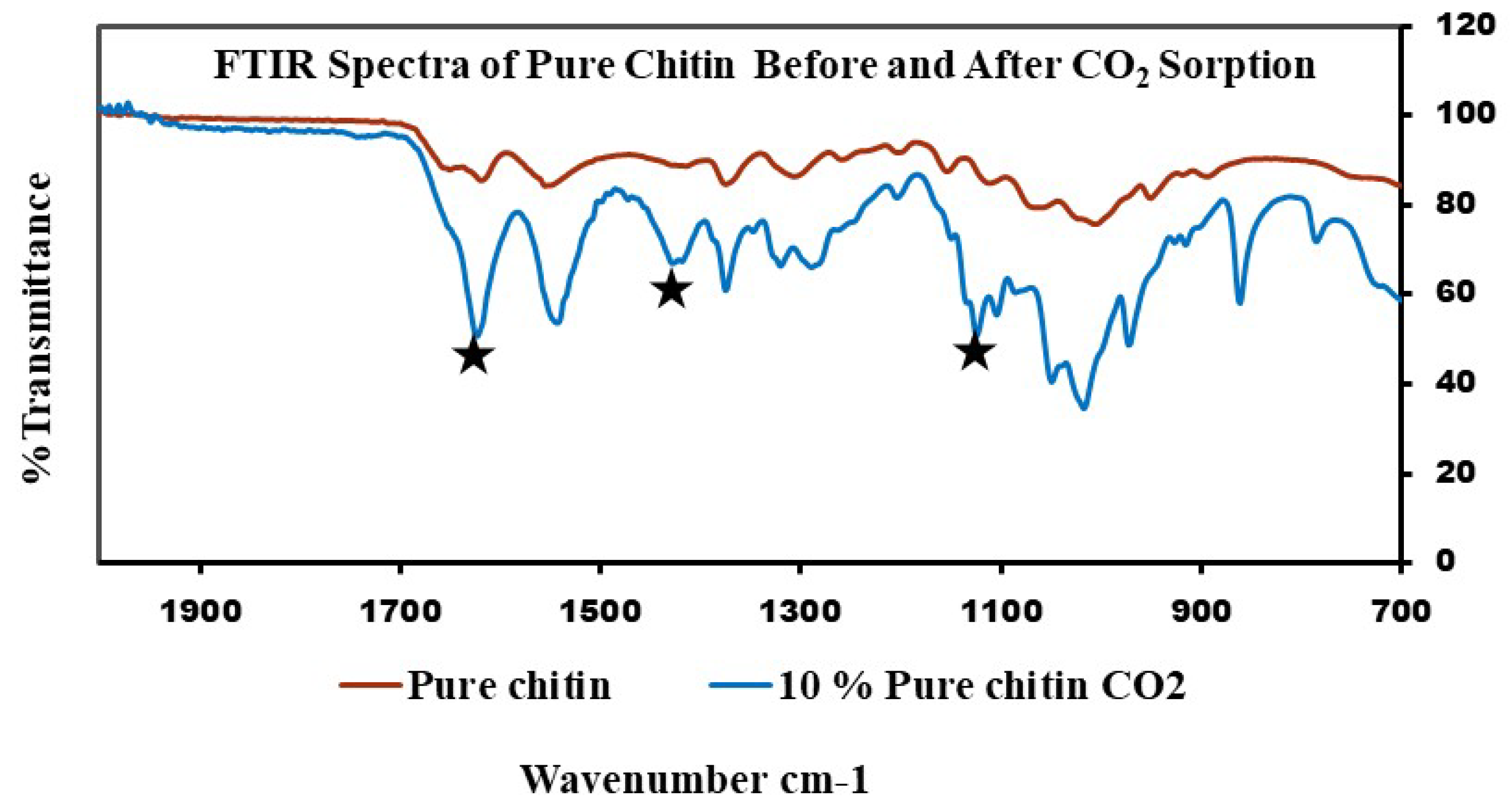
FTIR spectra for this experiment and the adsorption capacity of samples can be seen below in Figure 8 and Figure 9 and Table 2. According to the literature, the formation of carbamate shows peaks between the 1000 and 1600 cm−1 region. The pure chitin spectra of the 10% fraction after CO2 sorption confirms the formation of carbamate (Figure 8). The peaks were upside down to match the peaks from the chitosan sample, which was measured in transmittance instead of absorbance. The same results were obtained for all 10 and 20% fractions of the shrimp and crab chitin; however, the spectra were measured in absorbance to match the literature (Figure 9).
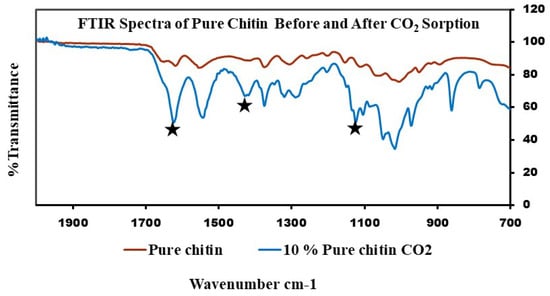
Figure 8.
FTIR spectra of pure chitin (before exposure to CO2) and 10% fraction after exposure to CO2.
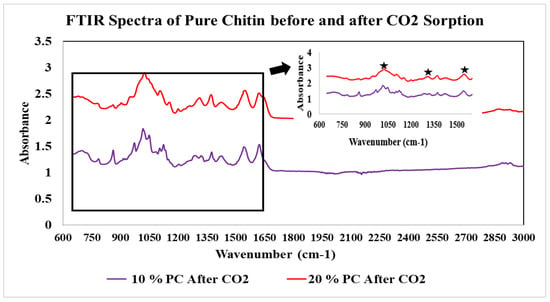
Figure 9.
FTIR spectra of hydrolyzed chitin exposed to CO2.
Furthermore, Table 2 shows the sorption capacity of 10 and 20% fractions of pure, shrimp, and crab chitin samples. This indicates that chitin, after hydrolysis, can capture CO2 almost as effectively as chitosan. Chitin has benefits over chitosan, which swells after absorbing water vapors. On the other hand, chitin can be dissolved in water, so CO2 can be bubbled through it.
Percent chitin content from shell waste of Delmarva Peninsula.
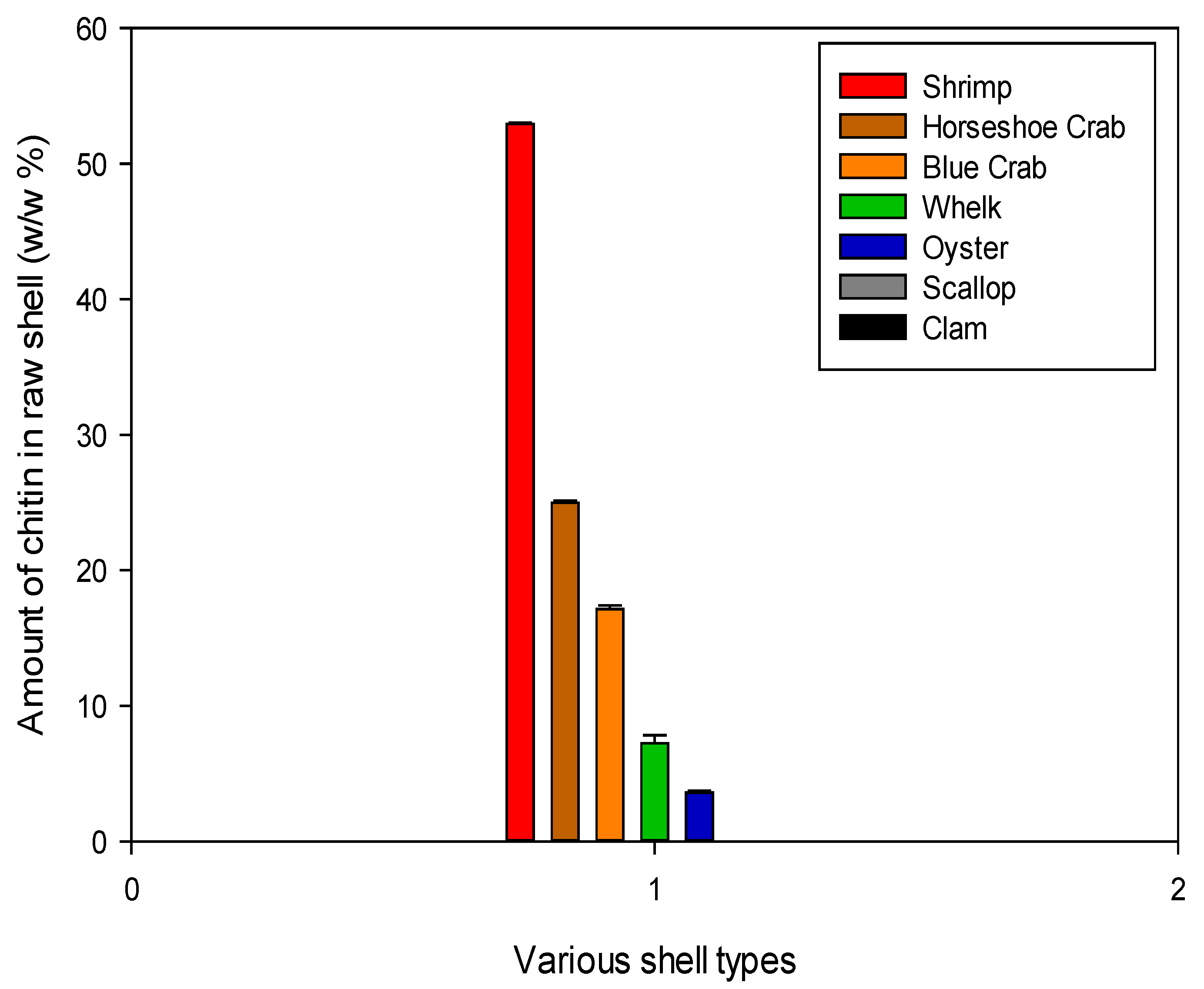
The protein and mineral content were used to calculate the total chitin concentration in each shell type as a final metric of the shell’s industrial feasibility. Below are the concentrations of chitin in the dry raw shell. The highest concentration was found in shrimp shells at about 50% of the dry shell mass because these shells are soft and do not contain many minerals (CaCO3), as shown in Figure 10. This was largely anticipated, as shrimp shells are currently the primary source of chitin in industrial settings and have received the most attention in chitin literature. The other arthropods, blue crab and horseshoe crab, had mutually comparable chitin concentrations of around 20%.
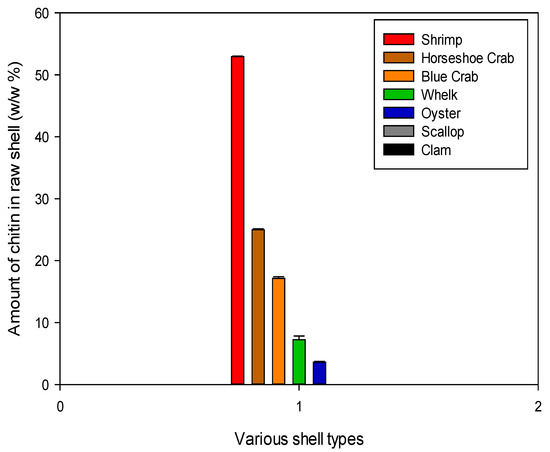
Figure 10.
Chitin content of each shell type. These values represent the mass remaining after demineralization and deproteinization treatments.
4. Conclusions
Chitin, chitosan, and their derivatives were studied as a reversible CO2 capture sorbent. Qualitative carbon sorption experiments were conducted in this study by subjecting pure and modified chitosan and its blends with PSF to a steady flow of a wet CO2 and nitrogen mixture, imitating compositions of fossil gases and recording changes in the infrared spectrum. This study revealed that chitosan, the conversion product of chitin, is a successful carbon capture material. The moist reaction conditions, however, produced problems by causing the chitosan sample to swell, thus reducing its capture efficiency over time. The problem was largely mitigated by blending PSF, a nonpolar polymer, with the chitosan sample, reducing its overall attractiveness to water but restoring its CO2 capture capacity. According to the statistical analysis, shrimp shells were found to be the best source of chitin in terms of simplicity of processing, purity, and yield. The MALDI analysis proved that the hydrolysis method decreases the molecular weight of the polymer by breaking some of the C-O-C links and shortening the chitin chains, boosting the number of carbon sorption reaction sites. The FTIR spectra revealed the synthesis of carbamate, a reversible carbon dioxide sorbent according to published research. The sorption was reversible in all chitosan samples, and CO2 may be easily released at ambient temperatures utilizing more sophisticated instruments such as IGA/MS. Therefore, this research is crucial to the development of reusable, reversible, and environmentally acceptable sorbents for efficient carbon sequestration. For future research, novel modifications are required to raise the chitosan’s solubility by 25% to 30%. To compare its capacity to capture carbon, chitin can be hydrolyzed at room temperature and at zero degrees to produce more tetramers and pentamers.
Author Contributions
P.S.: major researcher, performed experiments and wrote original draft; V.V.V.: principal investigator, review, editing, and supervision; B.B.: undergrad research assistant, methodology, helped in data collection; R.J.: undergrad research assistant, methodology, helped in data collection. All authors have read and agreed to the published version of the manuscript.
Funding
A Title III grant at the University of Maryland, Eastern Shore, supports this work.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank Isloor’s group for manufacturing all polymer blends and chemically modifying chitosan. They provided samples to us as part of a collaborative effort. Preeti Sharma would like to thank the Department of Natural Sciences at the University of Maryland Eastern Shore for supporting this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kawahata, H.; Fujita, K.; Iguchi, A.; Inoue, M.; Iwasaki, S.; Kuroyanagi, A.; Maeda, A.; Manaka, T.; Moriya, K.; Takagi, H.; et al. Perspective on the Response of Marine Calcifiers to Global Warming and Ocean Acidification—Behavior of Corals and Foraminifera in a High CO2 World “Hot House”. Prog. Earth Planet. Sci. 2019, 6, 5. [Google Scholar] [CrossRef]
- Keith, D.W. Why Capture CO2 from the Atmosphere? Science 2009, 325, 1654–1655. [Google Scholar] [CrossRef]
- Felder, F.A. Carbon Capture and Storage: Technologies, Policies, Economics, and Implementation Strategies; CRC: Boca Raton, FL, USA, 2013. [Google Scholar]
- Bottoms, R.R. Process for Separating Acidic Gases. U.S. Patent Application No. US1783901A, 2 December 1930. [Google Scholar]
- Robinson, K.; McCluskey, A.; Attalla, M.I. An ATR-FTIR Study on the Effect of Molecular Structural Variations on the CO2 Absorption Characteristics of Heterocyclic Amines, Part II. ChemPhysChem 2012, 13, 2331–2341. [Google Scholar] [CrossRef] [PubMed]
- Gielen, D.; Podkanski, J.; Unander, F. International Energy Agency. In Prospects for CO2 Capture and Storage; Organization for Economic Co-Operation and Development (OECD): Paris, France, 2004. [Google Scholar]
- Blomen, E.; Hendriks, C.; Neele, F. Capture Technologies: Improvements and Promising Developments. Energy Procedia 2009, 1, 1505–1512. [Google Scholar] [CrossRef]
- Gray, M.L.; Fauth, J.P.; Baltrus, H. Henry Pennline. Int. J. Green House Gas Control 2008, 2, 3–8. [Google Scholar] [CrossRef]
- Dhanabalan, V.; Xavier, K.A.M.; Eppen, S.; Joy, A.; Balange, A.; Asha, K.K.; Murthy, L.N.; Nayak, B.B. Characterization of Chitin Extracted from Enzymatically Deproteinized Acetes Shell Residue with Varying Degree of Hydrolysis. Carbohydr. Polym. 2021, 253, 117203. [Google Scholar] [CrossRef] [PubMed]
- Dayakar, B.; Xavier, K.A.M.; Das, O.; Porayil, L.; Balange, A.K.; Nayak, B.B. Application of Extreme Halophilic Archaea as Biocatalyst for Chitin Isolation from Shrimp Shell Waste. Carbohydr. Polym. 2021, 2, 100093. [Google Scholar] [CrossRef]
- Rasweefali, M.; Sabu, S.; Sunooj, K.; Sasidharan, A.; Xavier, K.M. Consequences of chemical deacetylation on physicochemical, structural and functional characteristics of chitosan extracted from deep-sea mud shrimp. Carbohydr. Polym. 2021, 2, 100032. [Google Scholar] [CrossRef]
- Silva, N.C.M.; De Sá, L.F.R.; Oliveira, E.A.G.; Costa, M.N.; Ferreira, A.T.S.; Perales, J.; Fernandes, K.V.S.; Xavier-Filho, J.; Oliveira, A.E.A. Albizia lebbeck Seed Coat Proteins Bind to Chitin and Act as a Defense against Cowpea Weevil Callosobruchus maculatus. J. Agric. Food Chem. 2016, 64, 3514–3522. [Google Scholar] [CrossRef]
- Maliki, S.; Sharma, G.; Kumar, A.; Moral-Zamorano, M.; Moradi, O.; Baselga, J.; Stadler, F.J.; García-Peñas, A. Chitosan as a Tool for Sustainable Development: A Mini Review. Polymers 2022, 14, 1475. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and Chitosan: Properties and Applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Mukhtar Ahmed, K.B.; Khan, M.M.A.; Siddiqui, H.; Jahan, A. Chitosan and Its Oligosaccharides, a Promising Option for Sustainable Crop Production—A Review. Carbohydr. Polym. 2020, 227, 115331. [Google Scholar] [CrossRef]
- Hammi, N.; Chen, S.; Dumeignil, F.; Royer, S.; El Kadib, A. Chitosan as a Sustainable Precursor for Nitrogen-Containing Carbon Nanomaterials: Synthesis and Uses. Mater. Today Sustain. 2020, 10, 100053. [Google Scholar] [CrossRef]
- Özel, N.; Elibol, M. A Review on the Potential Uses of Deep Eutectic Solvents in Chitin and Chitosan Related Processes. Carbohydr. Polym. 2021, 262, 117942. [Google Scholar] [CrossRef] [PubMed]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and Chitosan Polymers: Chemistry, Solubility and Fiber Formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Foungchuen, J.; Pairin, N.; Phalakornkule, C. Impregnation of Chitosan onto Activated Carbon for Adsorption Selectivity towards CO2: Biohydrogen Purification. Appl. Sci. Eng. Prog. 2016, 9, 197–209. [Google Scholar] [CrossRef]
- Guo, L.P.; Hu, X.; Hu, G.S.; Chen, J.; Li, Z.M.; Dai, W.; Dacosta, H.F.M.; Fan, M.H. Tetraethylenepentamine modified protonated titanate nanotubes for CO2 capture. Fuel Process. Technol. 2015, 138, 663–669. [Google Scholar] [CrossRef]
- Jo, D.H.; Jung, H.; Shin, D.K.; Lee, C.H.; Kim, S.H. Effect of Amine Structure on CO2 Adsorption over Tetraethylenepentamine Impregnated Poly Methyl Methacrylate Supports. Sep. Purif. Technol. 2014, 125, 187–193. [Google Scholar] [CrossRef]
- Prud’homme, A.; Nabki, F. Comparison between Linear and Branched Polyethylenimine and Reduced Graphene Oxide Coatings as a Capture Layer for Micro Resonant CO2 Gas Concentration Sensors. Sensors 2020, 20, 1824. [Google Scholar] [CrossRef]
- Fisher, J.C.; Tanthana, J.; Chuang, S.S.C. Oxide-Supported Tetraethylenepentamine for CO2 Capture. Environ. Prog. Sustain. Energy 2009, 28, 589–598. [Google Scholar] [CrossRef]
- Yamada, H.; Chowdhury, F.A.; Fujiki, J.; Yogo, K. Enhancement Mechanism of the CO2 Adsorption-Desorption Efficiency of Silica-Supported Tetraethylenepentamine by Chemical Modification of Amino Groups. ACS Sustain. Chem. Eng. 2019, 7, 9574–9581. [Google Scholar] [CrossRef]
- Keramati, M.; Ghoreyshi, A.A. Improving CO2 Adsorption onto Activated Carbon through Functionalization by Chitosan and Triethylenetetramine. Phys. E Low Dimens. Syst. Nanostruct. 2014, 57, 161–168. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, X.; Zhang, J.; Yan, N. Chitin-Derived Mesoporous, Nitrogen-Containing Carbon for Heavy-Metal Removal and Styrene Epoxidation. ChemPlusChem 2015, 80, 1556–1564. [Google Scholar] [CrossRef] [PubMed]
- Pohako-Esko, K.; Bahlmann, M.; Schulz, P.S.; Wasserscheid, P. Chitosan Containing Supported Ionic Liquid Phase Materials for CO2 Absorption. Ind. Eng. Chem. Res. 2016, 55, 7052–7059. [Google Scholar] [CrossRef]
- Wen, J.; Li, Y.; Wang, L.; Chen, X.; Cao, Q.; He, N. Carbon Dioxide Smart Materials Based on Chitosan. Progr. Chem. 2020, 32, 417–422. [Google Scholar] [CrossRef]
- Lopes, M.; Cecílio, A.; Zanatta, M.; Corvo, M.C. From Biopolymer Dissolution to CO2 Capture under Atmospheric Pressure—A Molecular View on biopolymer@Ionic Liquid Materials. J. Clean. Prod. 2022, 367, 132977. [Google Scholar] [CrossRef]
- Paiva, T.; Echeverria, C.; Godinho, M.H.; Almeida, P.L.; Corvo, M.C. On the Influence of Imidazolium Ionic Liquids on Cellulose Derived Polymers. Eur. Polym. J. 2019, 114, 353–360. [Google Scholar] [CrossRef]
- Trivedi, T.J.; Rao, K.S.; Kumar, A. Facile Preparation of Agarose–Chitosan Hybrid Materials and Nanocomposite Ionogels Using an Ionic Liquid via Dissolution, Regeneration and Sol–Gel Transition. Green Chem. 2014, 16, 320–330. [Google Scholar] [CrossRef]
- Yang, X.; Qiao, C.; Li, Y.; Li, T. Dissolution and Resourcfulization of Biopolymers in Ionic Liquids. React. Funct. Polym. 2016, 100, 181–190. [Google Scholar] [CrossRef]
- Mallakpour, S.; Dinari, M. Ionic Liquids as Green Solvents: Progress and Prospects. In Green Solvents II; Springer: Dordrecht, The Netherlands, 2012; pp. 1–32. [Google Scholar]
- Feng, J.; Zang, H.; Yan, Q.; Li, M.; Jiang, X.; Cheng, B. Dissolution and Utilization of Chitosan in a 1-carboxymethyl-3-methylimidazolium Hydrochloride Ionic Salt Aqueous Solution. J. Appl. Polym. Sci. 2015, 132, 41964. [Google Scholar] [CrossRef]
- Ferreira, I.C.; Araújo, D.; Voisin, P.; Alves, V.D.; Rosatella, A.A.; Afonso, C.A.M.; Freitas, F.; Neves, L.A. Chitin-Glucan Complex—Based Biopolymeric Structures Using Biocompatible Ionic Liquids. Carbohydr. Polym. 2020, 247, 116679. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yuan, B.; Liu, S.-W.; Yu, S.-T.; Xie, C.-X.; Liu, F.-S.; Shan, L.-J. Clean Preparation Process of Chitosan Oligomers in Gly Series Ionic Liquids Homogeneous System. J. Polym. Environ. 2012, 20, 388–394. [Google Scholar] [CrossRef]
- Liu, D.; Chen, Q.; Li, M.; Lou, B.; Yu, R.; Li, Z.; Zhang, Y. Influence of Carboxyl Anion on the Dissolution of Chitosan in Cholinium-Based Ionic Liquids. AIP Conf. Proc. 2018, 1971, 050015. [Google Scholar] [CrossRef]
- Shamshina, J.L. Chitin in Ionic Liquids: Historical Insights into the Polymer’s Dissolution and Isolation. A Review. Green Chem. 2019, 21, 3974–3993. [Google Scholar] [CrossRef]
- Eftaiha, A.F.; Alsoubani, F.; Assaf, K.I.; Troll, C.; Rieger, B.; Khaled, A.H.; Qaroush, A.K. An Investigation of Carbon Dioxide Capture by Chitin Acetate/DMSO Binary System. Carbohydr. Polym. 2016, 152, 163–169. [Google Scholar] [CrossRef]
- Osman, A.I.; Hefny, M.; Abdel Maksoud, M.I.A.; Elgarahy, A.M.; Rooney, D.W. Recent Advances in Carbon Capture Storage and Utilisation Technologies: A Review. Environ. Chem. Lett. 2021, 19, 797–849. [Google Scholar] [CrossRef]
- Elfving, J.; Kauppinen, J.; Jegoroff, M.; Ruuskanen, V.; Järvinen, L.; Sainio, T. Experimental Comparison of Regeneration Methods for CO2 Concentration from Air Using Amine-Based Adsorbent. Chem. Eng. J. 2021, 404, 126337. [Google Scholar] [CrossRef]
- Volkis, V.; Kumar, R.; Isloor, A.; Jiru, F. Biocompatible Polymeric Blends for the Reversible Capturing of Carbon Dioxide. Polym. Prepr. 2012, PMCE-409. [Google Scholar]
- Suginta, W.; Khunkaewla, P.; Schulte, A. Electrochemical Biosensor Applications of Polysaccharides Chitin and Chitosan. Chem. Rev. 2013, 113, 5458–5479. [Google Scholar] [CrossRef]
- Jayakumar, R.; Menon, D.; Manzoor, K.; Nair, S.V.; Tamura, H. Biomedical Applications of Chitin and Chitosan Based Nanomaterials—A Short Review. Carbohydr. Polym. 2010, 82, 227–232. [Google Scholar] [CrossRef]
- Dutta, K.; Dutta, J.; Tripathi, S. Chitin and chitosan: Chemistry, Properties and Applications. J. Sci. Ind. Res. 2004, 63, 20–31. [Google Scholar]
- Padaki, M.; Isloor, A.M.; Wanichapichart, P. Polysulfone/N-Phthaloylchitosan Novel Composite Membranes for Salt Rejection Application. Desalination 2011, 279, 409–414. [Google Scholar] [CrossRef]
- Bashir, S.; Teo, Y.Y.; Ramesh, S.; Ramesh, K.; Khan, A.A. N-Succinyl Chitosan Preparation, Characterization, Properties and Biomedical Applications: A State of the Art Review. Rev. Chem. Eng. 2015, 31, 563–597. [Google Scholar] [CrossRef]
- Kumar, R.; Isloor, A.M.; Ismail, A.F.; Matsuura, T. Performance Improvement of Polysulfone Ultrafiltration Membrane Using N-Succinyl Chitosan as Additive. Desalination 2013, 318, 1–8. [Google Scholar] [CrossRef]
- Kumar, R.; Isloor, A.M.; Ismail, A.F.; Matsuura, T. Synthesis and Characterization of Novel Water Soluble Derivative of Chitosan as an Additive for Polysulfone Ultrafiltration Membrane. J. Membr. Sci. 2013, 440, 140–147. [Google Scholar] [CrossRef]
- Benham, M.J.; Ross, D.K. Experimental Determination of Absorption-Desorption Isotherms by Computer-Controlled Gravimetric Analysis. Z. Phys. Chem. 1989, 163, 25–32. [Google Scholar] [CrossRef]
- Shiflett, M.B.; Corbin, D.R.; Yokozeki, A. Comparison of the Sorption of Trifluoromethane (R-23) on Zeolites and in an Ionic Liquid. Adsorp. Sci. Technol. 2013, 31, 59–83. [Google Scholar] [CrossRef]
- Rupley, J.A. The Hydrolysis of Chitin by Concentrated Hydrochloric Acid, and the Preparation of Low-Molecular-Weight Substrates for Lysozyme. Biochim. Biophys. Acta 1964, 83, 245–255. [Google Scholar] [CrossRef]
- Dutta, P.K. Chitin and Chitosan for Regenerative Medicine; Springer: New Delhi, India, 2016. [Google Scholar]
- Weiss, I.M.; Schönitzer, V. The Distribution of Chitin in Larval Shells of the Bivalve Mollusk Mytilus Galloprovincialis. J. Struct. Biol. 2006, 153, 264–277. [Google Scholar] [CrossRef]
- Irani, M.; Jacobson, A.T.; Gasem, K.A.M.; Fan, M. Modified carbon nanotubes/tetraethylenepentamine for CO2 capture. Fuel 2017, 206, 10–18. [Google Scholar] [CrossRef]
- Roberts, G.A.F. Progress on Chemistry and Application of Chitin and Its Derivatives. Pol. Chitin Soc. 2008, 13, 7–15. [Google Scholar]
- Bashir, S.; Teo, Y.Y.; Ramesh, S.; Ramesh, K.; Rizwan, M.; Rizwan, M. Synthesis and Characterization of pH-Sensitive N-Succinyl Chitosan Hydrogel and Its Properties for Biomedical Applications. J. Chil. Chem. Soc. 2019, 64, 4571–4574. [Google Scholar] [CrossRef]
- Fletcher, A.J.; Benham, M.J.; Thomas, K.M. Multicomponent Vapor Sorption on Active Carbon by Combined Microgravimetry and Dynamic Sampling Mass Spectrometry. J. Phys. Chem. B 2002, 106, 7474–7482. [Google Scholar] [CrossRef]
- Du, X.; Guang, W.; Cheng, Y.; Hou, Z.; Liu, Z.; Yin, H.; Huo, L.; Lei, R.; Shu, C. Thermodynamics Analysis of the Adsorption of CH4 and CO2 on Montmorillonite. Appl. Clay Sci. 2020, 192, 105631. [Google Scholar] [CrossRef]
- Epling, W.S.; Peden, C.; Szanyi, J. Carbonate Formation and Stability on a Pt/BaO/γ-Al2O3 NOX Storage/Reduction Catalyst. J. Phys. Chem. C 2008, 112, 10952–10959. [Google Scholar] [CrossRef]
- Smit, B.; Reimer, J.A.; Oldenburg, C.M. Introduction to Carbon Capture and Sequestration; Imperial College Press: London, UK, 2014. [Google Scholar]
- Park, H.; Jung, Y.M.; You, J.K.; Hong, W.H.; Kim, J.-N. Analysis of the CO2 and NH3 Reaction in an Aqueous Solution by 2D IR COS: Formation of Bicarbonate and Carbamate. J. Phys. Chem. A 2008, 112, 6558–6562. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).