1. Introduction
The World Health Organization recommends a daily intake of 400 g (five portions) of fruits and vegetables to aid a diverse and nutritious diet [
1]. Fruit juices are a convenient way to help meet this nutritional goal, offering not only appealing flavors but also a rich source of bioactive compounds, such as polyphenols and vitamins, which provide antioxidative, anti-inflammatory, antitumor, and antimicrobial benefits [
2]. Recent studies indicate that bioactive components in juices may be absorbed more efficiently than those in whole fruits, emphasizing their role in promoting health [
3].
Free radicals, or reactive species, are by-products of aerobic cell metabolism. They can cause oxidative damage if their production is unregulated, potentially leading to lipid, protein, and DNA damage and increasing disease risk. Antioxidants, including phenolic compounds and carotenoids from fruits and vegetables, help neutralize these radicals and protect against oxidative stress, which is associated with reduced risks of chronic diseases like cardiovascular conditions, cancers, and neurodegenerative disorders [
4,
5].
While inflammation is part of the body’s healing response, chronic inflammation can worsen health issues such as heart disease, diabetes, and cancer. Thus, managing chronic inflammation is essential for preventing chronic diseases [
6]. The antioxidant potential of phenolic compounds, which neutralize free radicals, suggests a promising therapeutic approach for managing inflammatory diseases [
7].
Bioavailability, in pharmacology, refers to the proportion of an administered dose that reaches the systemic circulation, while in nutritional science, it relates to the intake and absorption of nutrients and dietary compounds. Unlike pharmacology, defining bioavailability for dietary supplements is challenging due to the individual variability in nutritional and physiological conditions [
8,
9].
Fruit juices are rich in bioactive compounds that must undergo chemical changes to become “bioaccessible” for intestinal absorption and subsequent “bioavailability” in the bloodstream, where they can benefit metabolic functions [
10,
11]. Juices may enhance bioavailability by facilitating compound release from the fruit matrix, unlike whole fruits that require digestion to release these compounds for absorption [
12,
13]. Soluble dietary fiber in juices affects the bioavailability of phenolics, as fiber can form gels, increase viscosity, or entrap compounds, though pectin may have a positive impact. The effects of fiber on phenolic bioavailability range from positive to negative, indicating the importance of studying fiber’s role in bioactive compound absorption [
10].
Digestive stages affect the quantity and bioactivity of absorbed antioxidants. During digestion, interactions with macronutrients like fiber and proteins can reduce the bioaccessibility of antioxidant compounds [
12]. In vitro, gastrointestinal models simulate human digestion and provide a controlled, cost-effective method to study the bioavailability and stability of antioxidants, like ascorbic acid and polyphenols, under digestive conditions [
11,
14]. These models help evaluate bioavailability in a non-invasive way but should be validated against human trials to ensure accuracy in real-world absorption and effectiveness assessments [
10,
14].
In juice production, the degree of cloudiness, often due to pectin, is carefully monitored, as it may be either desired or undesirable depending on the product [
15]. Cloudiness involves suspended particles (0.4–5 μm), while pulp typically settles. Pectin-rich fruit, when crushed, creates a high-viscosity juice with a jelly-like texture that is difficult to extract mechanically [
16]. The addition of pectinases can reduce juice viscosity, enhance pulp processability, break down the gel structure, and improve juice extraction [
16,
17,
18]. The enzymatic depectinization may increase yield, as well as improve juice clarity and the bioavailability of antioxidants, due to the modification of the compound structure. Antioxidants in juices are highly susceptible to degradation during processing and storage, affecting quality and nutritional value. However, moderate processing, like gentle heating or enzymatic treatments, may increase antioxidant levels and bioavailability. The use of immobilized enzymes in juice bioprocessing aims to maximize enzyme retention and reuse as well as the enhancement of the beneficial compounds on juice composition and antioxidant activity [
11].
Peach nectar, known for its high turbidity, antioxidants, and anti-inflammatory properties, was selected as the focus of this work. Peaches contain phenolic compounds, such as flavan-3-ols, flavonols, and anthocyanins, which contribute to their antioxidants, and anti-inflammatory activities and global health benefits. Additionally, peaches are rich in both soluble and insoluble fiber, promoting gut health, aiding digestion, and supporting cholesterol and blood sugar regulation. Overall, peaches are a nutrient-rich addition to a balanced diet [
19,
20].
Pectin is a crucial component in the middle lamella of plant cells, contributing to cell cohesion and the structural integrity of plant cell walls by forming a complex network with cellulose and hemicellulose [
21,
22]. In its natural form, pectin is insoluble (protopectin) and strengthens cell walls by interacting with other polysaccharides and proteins. During fruit ripening, enzymatic activity degrades pectin, increasing its solubility, which softens the tissue and affects texture [
23].
Chemically, pectin is a complex polysaccharide with a backbone of α-(1–4)-linked D-galacturonic acid, often branching with sugars like D-xylose and D-apiose. It includes two main forms, rhamnogalacturonan I and II, known as the “hairy regions”, which vary by plant origin [
17]. Around 80% of its galacturonic acid groups are methylated, and depending on the degree of methylation, pectin is classified as high methoxyl (HM) or low methoxyl (LM) pectin, each with distinct gelling properties suited for different food applications [
24,
25].
Pectin’s applications are broad, spanning biodegradable films, adhesives, pharmaceuticals, and biomedical materials. In the food industry, it acts as an emulsifier, gelling agent, and stabilizer, while in pharmaceuticals, it is used in colon-specific drug delivery and controlled-release tablets [
25]. It also offers health benefits, such as lowering cholesterol and glucose levels and potentially inhibiting cancer cell growth. However, pectin can cause undesirable haze in some beverages, which is mitigated by pectinase enzymes [
25].
Pectinases, or pectinolytic enzymes, are a diverse group that degrades pectic substances in plants and microbes, playing roles in plant growth, fruit ripening, cell wall metabolism, and pathogenesis [
21,
26,
27]. A primary focus in pectinase research is endo-polygalacturonase (endo-PG), which breaks down pectic acid, but there are many other types of pectinases, including pectin methyl esterases, polygalacturonases, and lyases, which are classified by substrate preference, action mechanism, and cleavage site [
17,
27].
Pectinases are categorized as hydrolases, catalyzing hydrolysis reactions, or lyases, using β-elimination to cleave bonds. For example, polygalacturonases (PGs) hydrolyze α-1,4 glycosidic bonds in pectin, reducing viscosity, while lyases like PGL break bonds by trans-elimination, producing unsaturated products [
28,
29,
30,
31,
32,
33]. Protopectinases release soluble pectin from protopectin, which varies in molecular weight depending on enzyme type [
34,
35,
36,
37,
38].
Fungal species, especially
Aspergillus niger, are key sources of industrial pectinase production, and they are widely applied in the food industry, particularly in fruit juice clarification, yield improvement, and stability enhancement [
39,
40]. Pectinases are also used in textile retting, waste treatment, coffee and tea fermentation, oil extraction, and biomass conversion. These enzymes, especially those from
A. niger, work optimally in acidic conditions, making them well suited for use in fruit juice processing. Pectinases now represent about 25% of the enzyme market due to their broad utility across industries [
41].
Biocatalysis is increasingly used across industries due to its specific substrate targeting and eco-friendly nature. However, using free enzymes presents challenges like low stability, lack of reusability, and difficulties in process control, making enzyme immobilization essential in large-scale applications for cost-efficiency. Immobilized enzymes offer multiple advantages, including enhanced stability, reusability, and simplified separation from reaction media, reducing contamination [
41,
42,
43,
44]. Supports for immobilization can be inorganic (e.g., silica, ceramics), organic (e.g., chitosan, alginate), or hybrid (e.g., magnetic nanoparticles) with the choice of support based on properties like stability, biocompatibility, and low toxicity [
45].
Enzyme immobilization methods include adsorption, covalent binding, affinity immobilization, and entrapment/encapsulation. Entrapment confines enzymes within a polymer network, allowing substrate and product passage while retaining the enzyme. This method is simple and minimizes enzyme structural changes but has drawbacks like mass transfer resistance and possible enzyme leakage [
44]. Despite its simplicity, entrapment can face challenges with low enzyme loading, potential support damage, and reduced stability compared to covalent immobilization, making it suitable only under specific conditions [
25].
In this work, the entrapment method of encapsulation in sol–gel has been selected. The sol–gel process enables the formation of metal oxide, silica, and organosiloxane matrices with defined porosity through the reaction of organic precursors at room temperature [
44]. TMOS (tetramethoxysilane), a compound consisting of a silicon atom with four attached methoxyl groups, was used as the key reactant. This approach was chosen for its potential benefits, including optical transparency, opportunities for chemical modifications, pore size tuning, and the potential for increased enzyme stability [
45,
46].
2. Materials and Methods
2.1. Enzymes and Reagents
The enzymes, α-amylase from Aspergillus oryzae, pectinase from Aspergillus niger (E.C. 3.2.1.15), and pancreatic from porcine pancreas, were from Sigma-Aldrich, St. Louis, MI, USA, as well the substrates, pectin from citrus fruits and pepsin from porcine gastric mucosa.
The reagents albumin bovine serum (BSA), fraction V, ≥96%, Bradford reagent for 0.1–1.4 mg/mL protein, D-(+)-glucose monohydrate, iron (II, III) oxide, tetraethyl orthosilicate (TMOS), 98% were from Sigma-Aldrich. 1,1-Diphenyl-2-hydroxy-3,5-dinitrobenzoic acid, ethanol, glycerol, sodium acetate trihydrate, and sodium hydroxide were from Merck, Rahway, NJ, USA. Acetic acid glacial, hydrochloric acid, 36.5–38%, and potassium-sodium tartrate tetrahydrate were from Scharlau, Barcelona, Spain. Picrylhydrazyl free radical was from Tokyo Chemical Industry Co., Boereveldseweg, Haven, Zwijndrecht, Belgium and Trolox®, 97% from Acros Organics, Janssen-Pharmaceuticalaan, Geel, Belgium. Peach nectar juice was from Sumol + Compal, S.A. Carnaxide, Oeiras, Portugal.
2.2. Analytical Methods
The protein content in the medium was determined by employing the Bradford method, using a microscale approach adapted to a micromethod [
47,
48] for faster multiple sample processing. In this adapted method, 50 µL of Bradford reagent (Coomassie Brilliant Blue dye) was added to 100 µL of the sample to be tested in a microplate (Thermo Scientific Nunc
TM 96-well microplates, Thermo Scientific, Essex, UK). The reaction developed for 5 min, and absorbance was read at 595 nm in a microplate reader (BMG LABTACH Fluostar Omega, Biogen Cientifica S.L., Madrid, Spain). The calibration curve for protein quantification was obtained using BSA standard solutions.
The reducing sugar content was determined based on the 3,5-dinitrosalicylic acid (DNS) method described by Miller [
49]. A microscale approach developed by Nunes et al. [
50] was adopted to conduct a microassay in a 96-well microtiter plate. This method is based on the oxidation of the carbonyl group of a sugar by DNS. Consequently, the yellow compound (DNS) is reduced to 3-amino,5-nitrosalicylic acid (ANS), which has a reddish-brown color and can be detected by measuring absorbance at 575 nm [
51]. A glucose calibration curve was attained for reducing sugar quantification.
The antioxidant activity was determined by employing the DPPH method [
52]. The DPPH method is based on the color change in the radical species 2,2-difenil-1-picrilhidrazil (DPPH) from purple to pale yellow when reduced. This reaction’s velocity is directly proportional to the antioxidant activity of the compounds present. The absorbance was measured at 517 nm [
53]. To evaluate the antioxidant activity, 100 µL of the sample was added to 1.4 mL of 100 µM DPPH stock solution in an Eppendorf tube (2 mL). The absorbances at 517 nm were then read at times 0, 10, 20, and 30 min against the blank (ethanol). The samples were protected from the light while the reaction took place. Subsequently, the antioxidant activity was assessed by the scavenging percentage of the DPPH radical calculated according to Equation (1):
Equation (1)—DPPH radical scavenging activity, where
A0 is the time 0 min absorbance and
At is the absorbance at a given time,
t min, adapted from [
54].
The anti-inflammatory activity of a sample was determined based on its capacity to inhibit albumin thermal denaturation as described by Mizushima and Kobayashi [
55]. The chosen method was an adaptation of the one illustrated by Chandra et al. [
56].
A 1% BSA solution (25 mL) in distilled water was prepared. For juice sample analysis, 1 mL of the BSA solution was added to 200 µL of the sample in an Eppendorf tube. This mixture was then incubated at 37 °C for 15 min followed by a water bath at 70 °C for 5 min. Additionally, a blank was prepared with distilled water instead of the sample extract. Finally, absorbances were read at 660 nm against the blank. The control assay was conducted accordingly with 1 mL of the albumin solution, and the absorbance was read against distilled water. Subsequently, the anti-inflammatory activity was assessed by the inhibition percentage of protein denaturation calculated through Equation (2).
Equation (2)—Inhibition of protein denaturation, where
A0 is the control assay absorbance and
A1 is the sample absorbance [
57].
2.3. Enzyme Immobilization
The enzyme immobilization was achieved by encapsulation in lens-shaped sol–gel matrices.
Sol–gel bio-immobilisates are generally prepared in three main steps: sol preparation, gel formation, and aging [
45,
46]. The sol preparation involves hydrolysis of the precursor followed by condensation and polycondensation, and in the case of a tetra-alkoxysilane, complete hydrolysis [
45]. The route involves the following 2 steps. First, suspension or dissolution of the metal alkoxide precursor, such as tetramethoxysilane or methyltrimethoxysilane, in an appropriate liquid (acidic pH in the presence of water) for hydrolysis to produce silanol groups (Si-OH). Then, the hydrolyzed precursor is activated by the addition of a base to induce condensation reactions between the silanol moieties, resulting in the formation of a gel of siloxane (Si-O-Si) polymers [
44]. The aging step involves solvent removal and continuous condensation reactions. As the network grows and ages over time, the viscosity of the liquid increases exponentially until gelation occurs, resulting in a matrix in which the enzyme molecules are encapsulated, leading to the formation of a xerogel [
44,
45].
The production of the hydrogel followed the methods described in [
58]. Each batch of silica sol mixture was prepared according to [
58]. Briefly, glycerol (0.1920 g), distilled water (140 µL), HCl 80 mM (diluted from HCl 1 M) (30 µL) and tetramethoxysilane (TMOS) (600 µL) were added to an Eppendorf tube (1.5 mL) and then sonicated for 25 min in an ice bath. The hydrogel capsules were prepared in a 96-well microplate adopting a 1:1 ratio of the previous sol mixture (25 µL) and a pectinase solution (25 µL). The microplates were then left to dry at room temperature for 2–3 days to promote sol–gel aging. Afterward, the capsules were separated and weighed to be used in further enzymatic reactions.
To obtain magnetic enzymatic capsules, the same general procedure was followed. Additionally, after sonication, 0.01 g of iron oxide was added to the solution and mixed in the vortex.
Two different types of microplates were used in order to study the morphology of the obtained capsules. In this way, 96-well plates with round bottoms (U-well) and flat bottoms (F-well) were employed. The average weight of capsules obtained from U-well plates was 0.015 g with a standard deviation of ±0.003 g.
2.4. Enzymatic Activity
The enzymatic activity of the immobilized pectinase was evaluated following the enzymatic hydrolysis of the substrate pectin in acetate buffer (20.0 mM) pH 4.0 for 3 h at 45 °C and 200 rpm in the incubator shaker. In each reaction, 100 µL samples of medium was taken for reducing sugar quantification at times 0, 1, 2, and 3 h. Additionally, both at the initial and final stamps, a 20 µL sample was taken for protein quantification. All samples were placed in Eppendorf tubes in ice to stop the reaction, until the end of the 3 h, when the analytical methods were performed.
The product obtained from immobilization was then used to perform the enzymatic reaction adopting a ratio of 1:10–immobilized pectinase (g): 0.15 mg mL−1 pectin in buffer. To optimize this process, multiple assays were performed where various parameters were studied: namely, A—microplate well morphology used in enzyme immobilization; B—concentration of pectinase solutions used in enzyme immobilization and C—recipient vessel where the reaction took place. In assays 1 and 3, additional similar reactions were performed with magnetized lenses.
After optimization, the hydrolysis reaction was performed with the following optimum parameters: (A) U-well; (B) 10.0 mg mL−1; (C) 24-well microplate and 0.15 g of pectinase capsules. Various pectin solutions of concentrations 0.1, 0.2, 0.3, 0.4 concentrations and 0.5 mg mL−1 were tested (1.5 mL). To evaluate the magnetization effect on the enzymatic activity, identical assays with both regular and magnetized capsules were performed.
2.5. Operational Stability
To understand the reusability of the immobilized enzyme, the pectinase capsules used in assay 1 were reused to perform several other enzymatic reactions and to evaluate residual activity. The general procedure consisted of first recovering and storing (0–4 °C) the medium of the previous reaction. In an attempt to fully dry the capsules, filtration paper was used to absorb the remained medium by capillarity. Afterward, the same amount of 0.150 mg mL−1 from pectin was used in each original assay, and 1.3 mL was added. The enzymatic reaction was then performed in identical conditions overnight. To monitor the reaction, samples were taken for both reducing sugar (100 µL) and protein quantification (20 µL) at times 0, 1 and 3 h. At the end of the 24 h, samples were removed for reducing sugar and protein quantification. Each batch of enzymatic capsules was used in the first original assay and re-used in 4 subsequent 24 h reactions.
2.6. Enzymatic Juice Processing
The peach nectar juice went through enzymatic bioprocessing and in vitro digestion whose effect was assessed through the assessment of antioxidant and anti-inflammatory activities. The DNS method was also performed on the unprocessed original juice to quantify reducing sugars. It was necessary to dilute the samples since the juice sugar content was initially already above the absorbance limit. Dilutions of 1/50, 1/100 and 1/200 of the juice were tested, arriving at 1/200 as the optimal condition to measure absorbance with the DNS method on further assessments.
The encapsulated pectinase capacity to degrade the pectin present in a fruit juice with reducing cloudiness was assessed with peach nectar juice. To enzymatically process the aforementioned juice, the technique, optimized in
Section 2.6, was adopted. So, reactions with 0.150 g of both regular and magnetized pectinase (10.0 mg mL
−1) lenses and 1.5 mL of the juice were run.
To monitor the reaction, samples were taken for protein quantification and antioxidant activity as described in
Section 2.2. For reducing sugar quantification, the DNS method was performed. As previously mentioned, the juice samples ought to be diluted 200 times. This way, in each reaction, 10 µL samples of medium were taken at times 0, 1, 2 and 3 h and added to 1990 µL of distilled water in an Eppendorf tube. Additionally, samples of the bioprocessed juice were taken for both antioxidant and anti-inflammatory activity assessment of the bioprocessed juice.
2.7. Juice Clarification Efficiency
To evaluate the pectin processing efficacy, changes in turbidity were examined. The Optical Density (OD) of the juice was measured at 600 nm both before and after the bioprocessing reactions, in a Biophotometer, Eppendorf Model 6131. Due to the initial heavy cloudiness of the juice, it was necessary to dilute the samples 5 times.
2.8. Juice Bioavailability: In Vitro Digestion
To study the bioavailability of both the bioprocessed and original juices, an in vitro enzymatic digestion simulation was conducted adapted from the methods described by Gião et al. [
59]. In this procedure, the oral, gastric and intestinal phases of digestion physiological conditions were mimicked employing enzymes. In vitro digestion was conducted on the original juice and bioprocessed juice with both regular and magnetized lenses. For each sample, 4 assays were performed each on 800 µL extracts placed in Falcon tubes (15 mL). Three of them simulated each digestion phase individually as described further on. Additionally, a fourth assay consisted of the phases performed in series to model a complete digestion. At the end, samples of the obtained juice of each digestion step were extracted for both antioxidant and anti-inflammatory activity evaluation of the digested juice.
2.8.1. Oral Phase
After adding 22 µL of CaCl2 2% and 43 µL of concentrated amylase solution (50 µL) to the 800 µL sample, the pH was adjusted to 7 with NaOH 1 M. After being incubated at 37 °C for 2 min, the tubes were placed in ice to stop the reaction. The tube corresponding to the complete digestion assay proceeded to the next stage.
2.8.2. Gastric Phase
The pH was adjusted to 2 with a solution of HCl 1 M. Next, 0.04 mL of a 25 mg mL−1 pepsin solution in HCl 0.1 M was added to each Falcon tube (0.05 mL per mL of the initial sample). The tubes were then wrapped in tin foil and incubated at 37 °C and 100 rpm for 2 h. Once again, after incubation, the reactions were stopped by placing the Falcon tubes in ice, and complete digestion assays went on to the last digestion phase.
2.8.3. Intestinal Phase
In this last step, NaHCO3 1 M was used to adjust the pH to 6. Next, to each Falcon tube was added 0.20 mL of a 2 mg mL−1 pancreatin solution in 0.1 M NaHCO3 (0.25 mL per mL of initial sample). After being incubated at 37 °C and 45 rpm and wrapped in tin foil for an hour, all tubes were placed in ice.
2.9. Statistical Analysis
All data assays were repeated at least 3 times. The representativeness of these data was presented by the mean ± standard deviation. Statistical analyses were performed using IBM SPSS Statistics, version 24.0 55, considering a significance level, α, of 0.05.
3. Results and Discussion
3.1. Pectinase Immobilization: Efficiency and Activity
In this study, pectinase was immobilized using a microscale sol–gel technique, where the type of microplate well influenced capsule morphology. Capsules formed in F-well microplates began as fragmented particles, which were unsuitable for the desired applications. However, switching to U-well microplates yielded lens-shaped capsules with an average mass of 0.0150 g. These lenses required two days to fully dry, becoming ready for bioprocessing. Immobilization efficiency was nearly 100%, as the entire sol–enzyme mixture solidified without any enzyme loss. Adding iron oxide to create magnetized capsules had no adverse effect on lens integrity.
After immobilization, the pectinase lenses were evaluated using a standard substrate to confirm enzymatic activity, optimizing reaction parameters to accurately determine kinetic properties. Enzymatic assays tracked product formation through reducing sugar measurements, providing insights essential for potential applications like juice clarification.
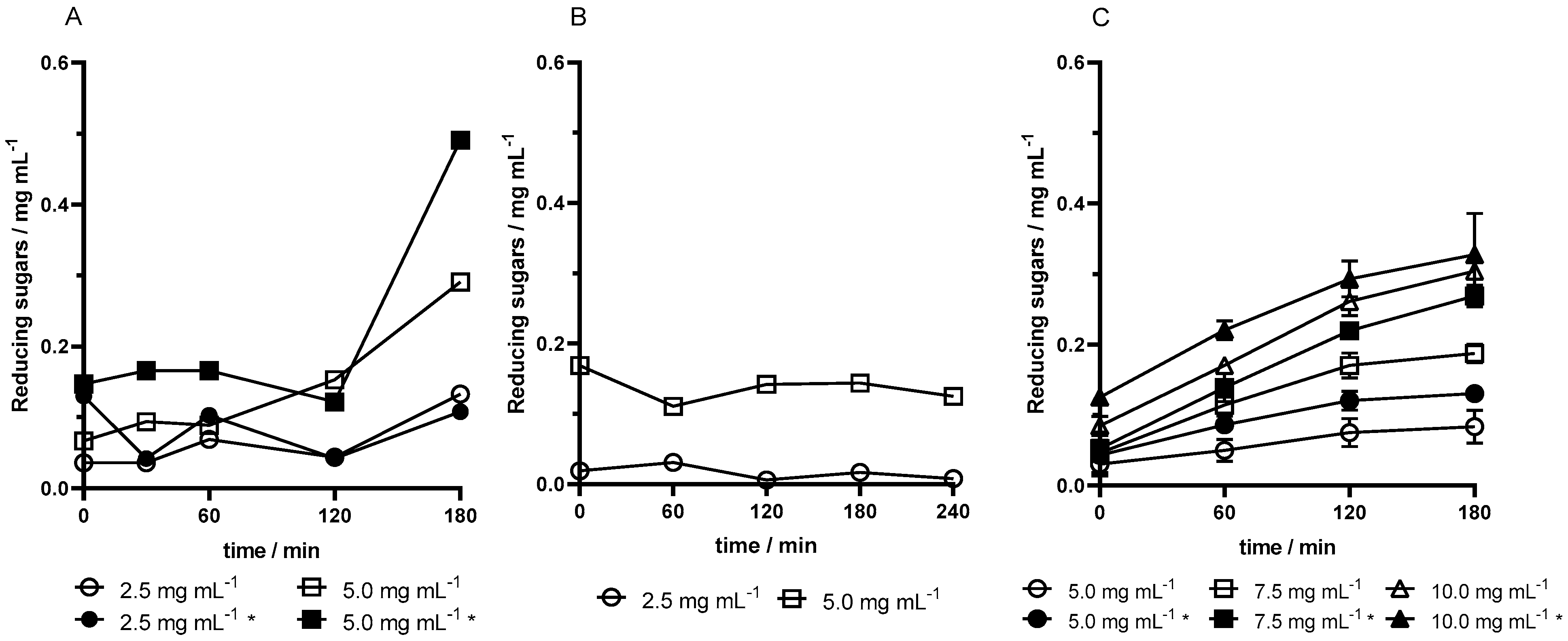
The enzymatic activity of sol–gel pectinase capsules from F-well microplates was tested in assays using different microreactor configurations. In assay 1, using 1.5 mL Eppendorf tubes, a significant increase in reducing sugars was observed at 180 min. In assay 2, 15 mL of Falcon tubes provided a larger contact area for capsules in the medium; however, this setup did not yield higher activity and reducing sugar production remained similar (
Figure 1A,B). In assay 3, U-well microplate capsules combined with 24-well microplate reactors significantly increased activity due to an improved contact area, yielding a hyperbolic reaction curve that stabilized after an initial rapid increase (
Figure 1C).
Higher pectinase concentrations in the capsules resulted in proportionally higher product formation across all assays. Additionally, magnetized capsules exhibited greater activity than non-magnetized ones at equivalent enzyme concentrations, producing higher reducing sugars quantities, as verified in assay 3 (
Figure 1C). Thus, both high enzyme concentration and magnetization improved pectinase activity.
To evaluate enzyme release from the capsules into the medium, the protein content was determined by the Bradford method. In this process, the difference between the protein content in the medium at the start and end of the reaction did not undergo a large difference. In fact, in the working range of pectinase concentration, 2.5 to 10.0 mg mL−1, both the presence of iron oxide and the concentration of enzyme do not directly affect the loss of protein from capsules. The capsules obtained from F-well microplates were highly fragmented (assays 1 and 2), so a higher release of the enzyme could be expected than in the lenses obtained from U-well microplates (assay 3). Assay 3 showed a slight loss (0.5–≈2%), then assay 1 (2.5–5.5%), and assay 2 had a small increase (0–≈1%).
To conclude, the most favorable activity curves were obtained in assay 3 as they are the ones that resemble Michaelis–Menten kinetics. The lenses with 10.0 mg mL−1 pectinase have a steeper slope and higher activity and initial velocity. The values of enzyme release from capsules also did not prove to be concerning in this assay. All variables considered sufficient conditions for determining kinetic parameters were achieved. The optimized reaction with the enzyme primary substrate comprises 0.15 g of 10.0 mg mL−1 pectinase lenses obtained from U-well microplates in a 24-well microplate as a microreactor vessel.
3.2. Immobilized Pectinase Kinetics Parameters
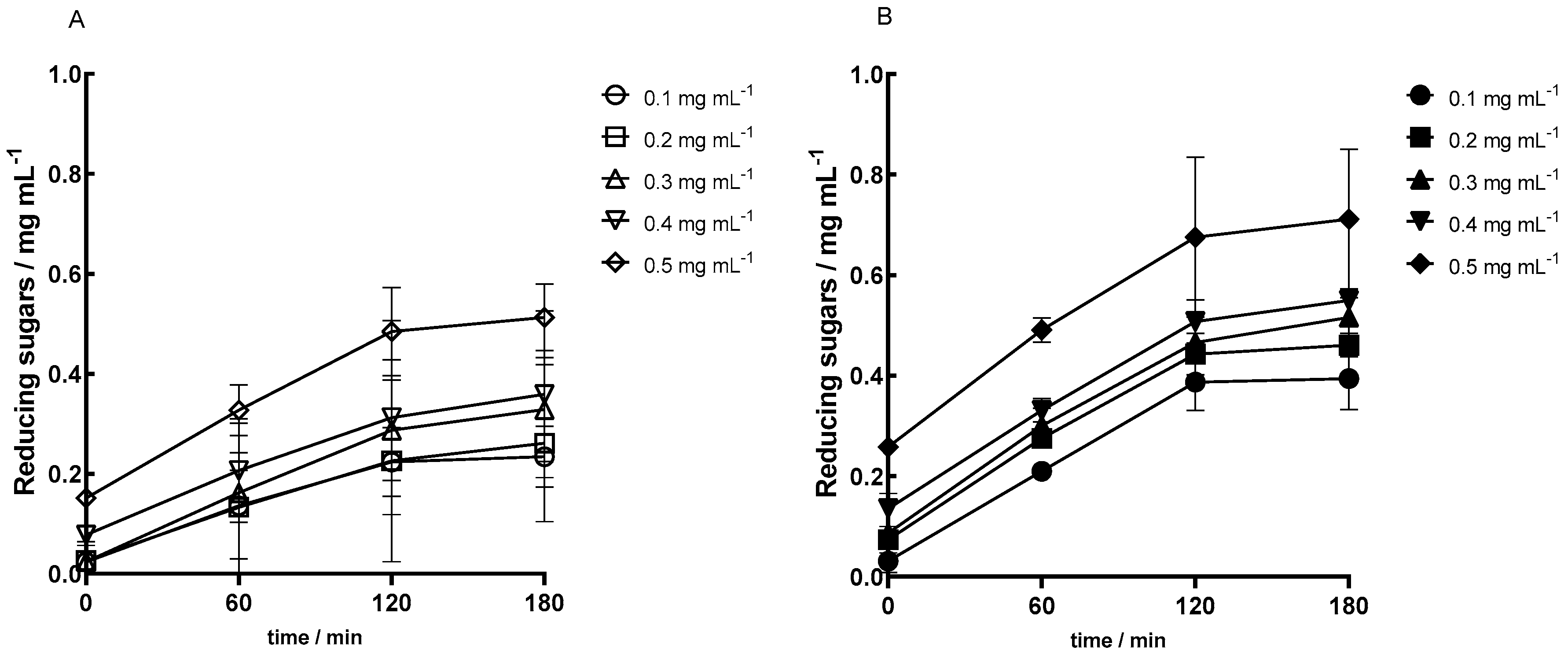
Enzymatic assays were carried out based on the optimum conditions determined in the previous section. Assays with different concentrations of the primary substrate, pectin, were conducted to plot the initial velocity versus substrate concentration and thereafter obtain the kinetic parameters for the immobilized enzyme.
The obtained activity curves in
Figure 2 show first a highlighted increase in reducing sugars followed by a stabilization period. Furthermore, higher concentrations of substrate, pectin, led to higher values of the product, reducing sugars. This is confirmed for both assays with regular lenses and magnetized ones. In the curves presented in
Figure 2, reactions performed with magnetized lenses the enzyme had a higher activity.
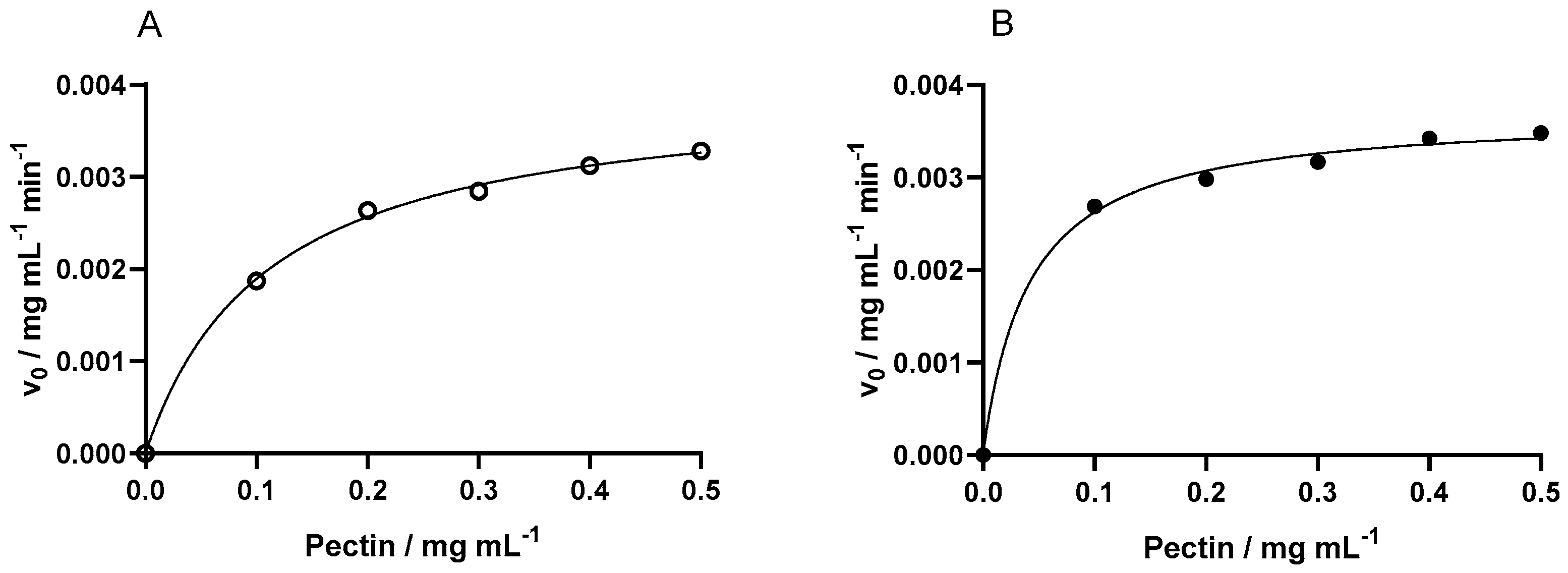
To calculate the kinetic parameters, the first derivate of each curve was calculated to find the initial velocity for each concentration of substrate. Using the software GraphPad Prism 8.0.2, a non-linear analysis was performed on the Michaelis–Menten fit (
Figure 3). Both the fits for the enzyme immobilized in regular and magnetized lenses were considered accurate as the R-squared values were 0.9986 and 0.9969, respectively. Thereafter, the kinetic parameters maximum reaction rate (V
max) and Michaelis–Menten constant (K
m) were obtained, and are the results presented in
Table 1 with their respective R-squared.
The effect of magnetization in immobilization on catalytic activity was assessed when comparing the kinetics of regular and magnetized lenses. The parameter Vmax was quite similar, while the regular ones were slightly more efficient, meaning they converted the substrate into product slightly more rapidly. Magnetized lenses showed an advantage by showing a more than two and half times smaller Km, confirming a higher substrate affinity and thus requiring lower substrate concentration for efficient catalysis compared with the regular ones.
Moreover, the effect of immobilization itself on kinetics can be evaluated by comparing the same mentioned parameters with the ones from the enzyme in its free state. The K
m obtained in [
40] for the free Endo-PG was 0.44 mg mL
−1. Notably upon immobilization in regular lenses, the K
m value decreased 4 times to 0.1105, suggesting an enhancement in substrate affinity compared to the free enzyme. This reduction in K
m could be attributed to the microenvironment within the capsules, which may shield the enzyme from environmental factors and alter its conformational dynamics, influencing substrate-binding affinity. Furthermore, the immobilization employing magnetized lenses resulted in a further reduction in the K
m, which was 10 times smaller than the one for the free enzyme (0.0412 mg mL
−1). This further indicates that the presence of iron might induce spatial constraints on the enzyme molecules, promoting a more favorable orientation for substrate binding or facilitating the diffusion of substrates toward the active sites.
In conclusion, while both regular and magnetized capsules exhibited lower maximum velocity values compared to the free enzyme, their values were not significantly different. This suggests that magnetization did not substantially affect the catalytic activity beyond the effects induced by immobilization. The main advantage of the magnetized capsules lies in their significant reduction in the Km value, highlighting their more efficient enzyme–substrate interactions compared to both the free enzyme and the regular capsules and thus offering promising prospects for the advancement of enzyme-based biocatalysis in various biotechnological applications.
3.3. Enzymatic Operational Stability
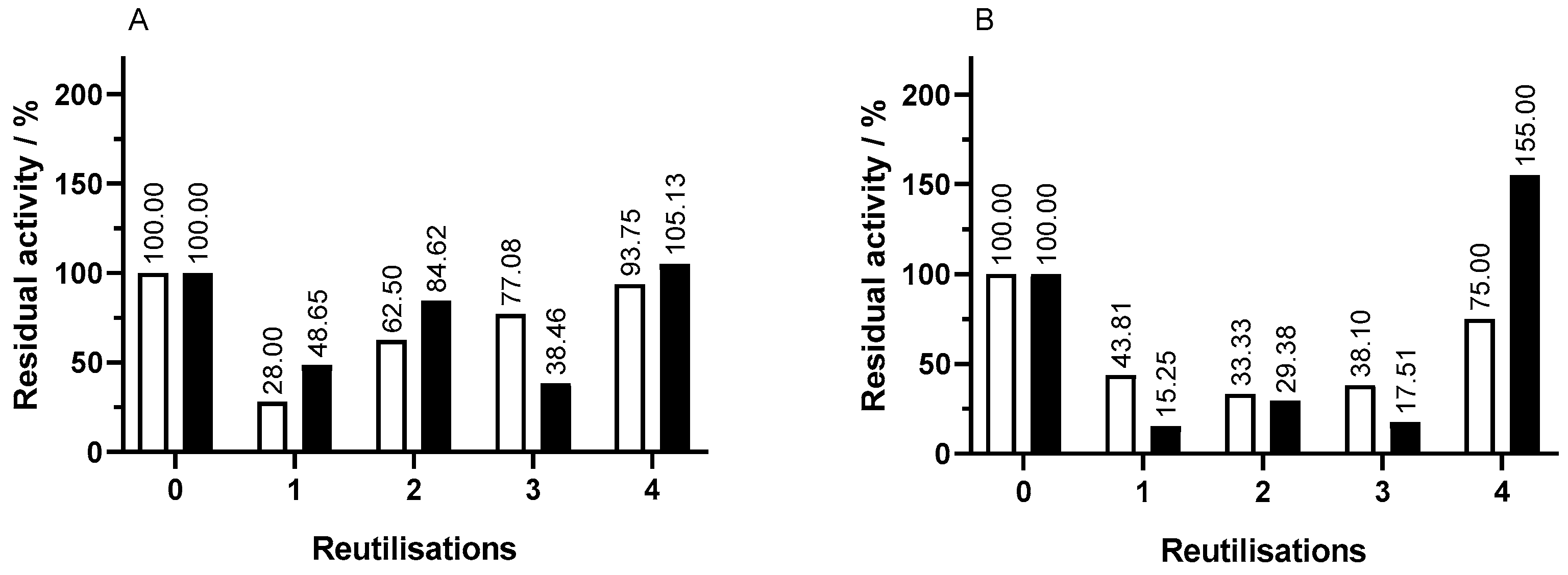
The operational stability of capsules used in assay 1 was tested, performing 24 h assays in the same conditions as in the original assay. It was aimed to assess the release and residual activity of the immobilized enzyme after each reutilization.
The activity of the enzymatic capsules in each reutilization was assessed by reducing sugar evolution over time. The magnetization proved to be beneficial in terms of product formation. In reutilization assays of capsules with the same concentration of pectinase, the iron-containing ones lead to higher concentrations of reducing sugars showing higher activity. In addition, it was further confirmed that higher concentrations of pectinase led to higher activity. This was suitable for all reutilizations of capsules.
The residual activity of the immobilized enzyme throughout capsule reuses was calculated with the maximum concentration of reducing sugars until 180 min in each reutilization in comparison to the equivalent value in the original assay, which is shown in
Figure 4. In regular capsules, throughout reutilizations, there is a decrease in residual activity to around 30.0% followed by an increase in activity. Capsules with 2.5 mg mL
−1 pectinase showed a decrease in activity in the first reutilization to 26.0% followed by a gradual increase through the second, third, and fourth reutilizations. The results for capsules with 5.0 mg mL
−1 pectinase were in alignment but with a less pronounced decrease in the second reutilization. The fourth reutilization showed a residual activity of 75.0%.
In comparison to regular capsules, the 2.5 mg mL−1 pectinase magnetized ones tend to have higher residual activity as well as the ones with 5.0 mg mL−1 of pectinase, which showed a residual activity more than double in the fourth reutilization. The magnetized capsules stand out for having above 100% residual activity in the fourth reutilization, while regular ones do not.
3.4. Juice Bioprocessing
3.4.1. Bioprocessing Enzymatic Reaction
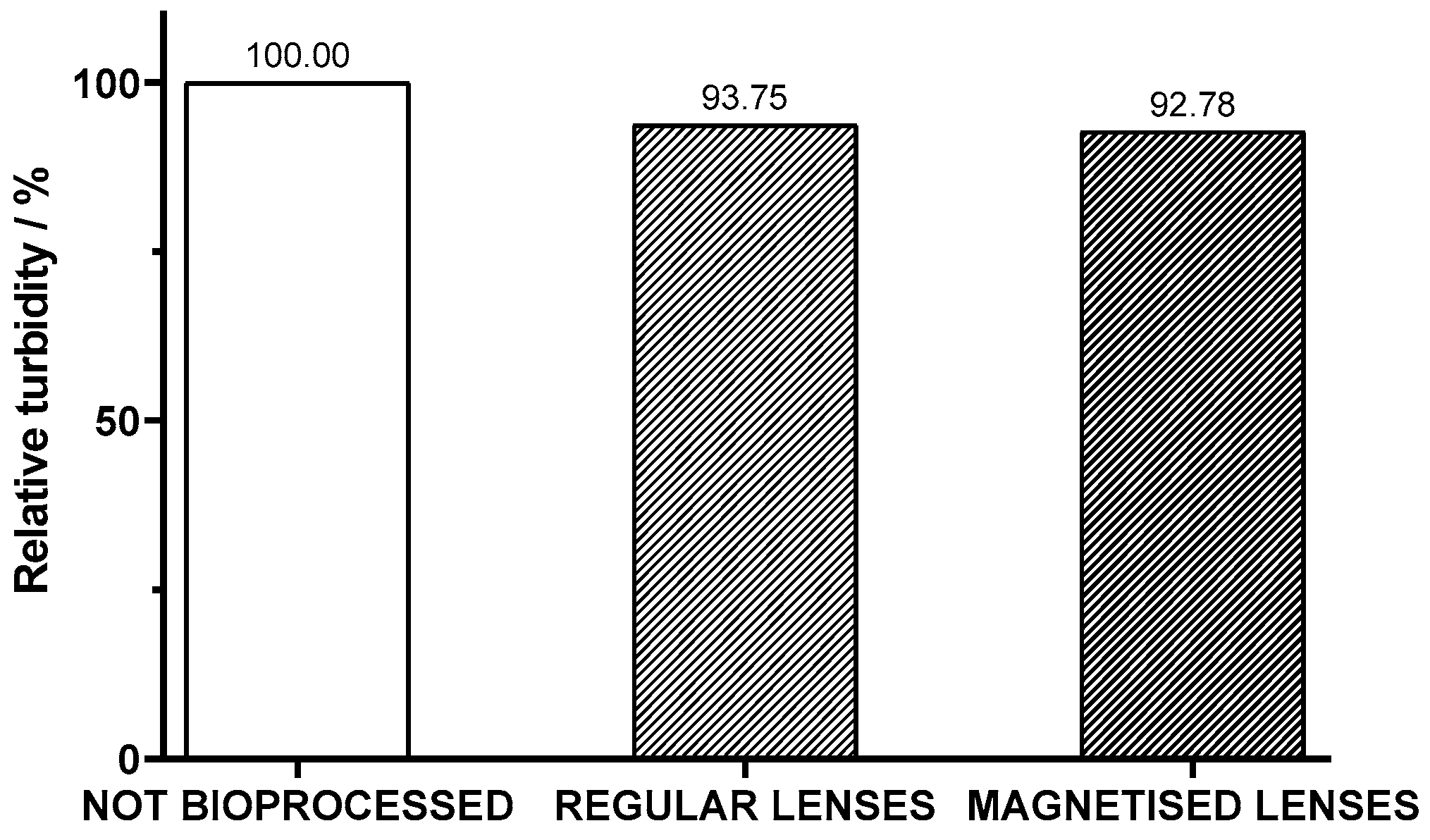
After the assessment of the enzymatic activity in standard solutions of pectin and operational stability of the lenses, the juice bioprocessing with the encapsulated pectinase in sol–gel was conducted. Since one of the goals of this process was to diminish the cloudiness of the juice, to evaluate its efficacy, the turbidity of the juice was measured before and after the bioprocess with both regular and magnetized lenses. The percentage of turbidity of the bioprocessed juice samples relative to the original juice was evaluated. As shown in
Figure 5, there was a decrease in the cloudiness of the juice after three hours of bioprocessing. It is also to be noted that magnetized lenses were 1% more effective than regular ones.
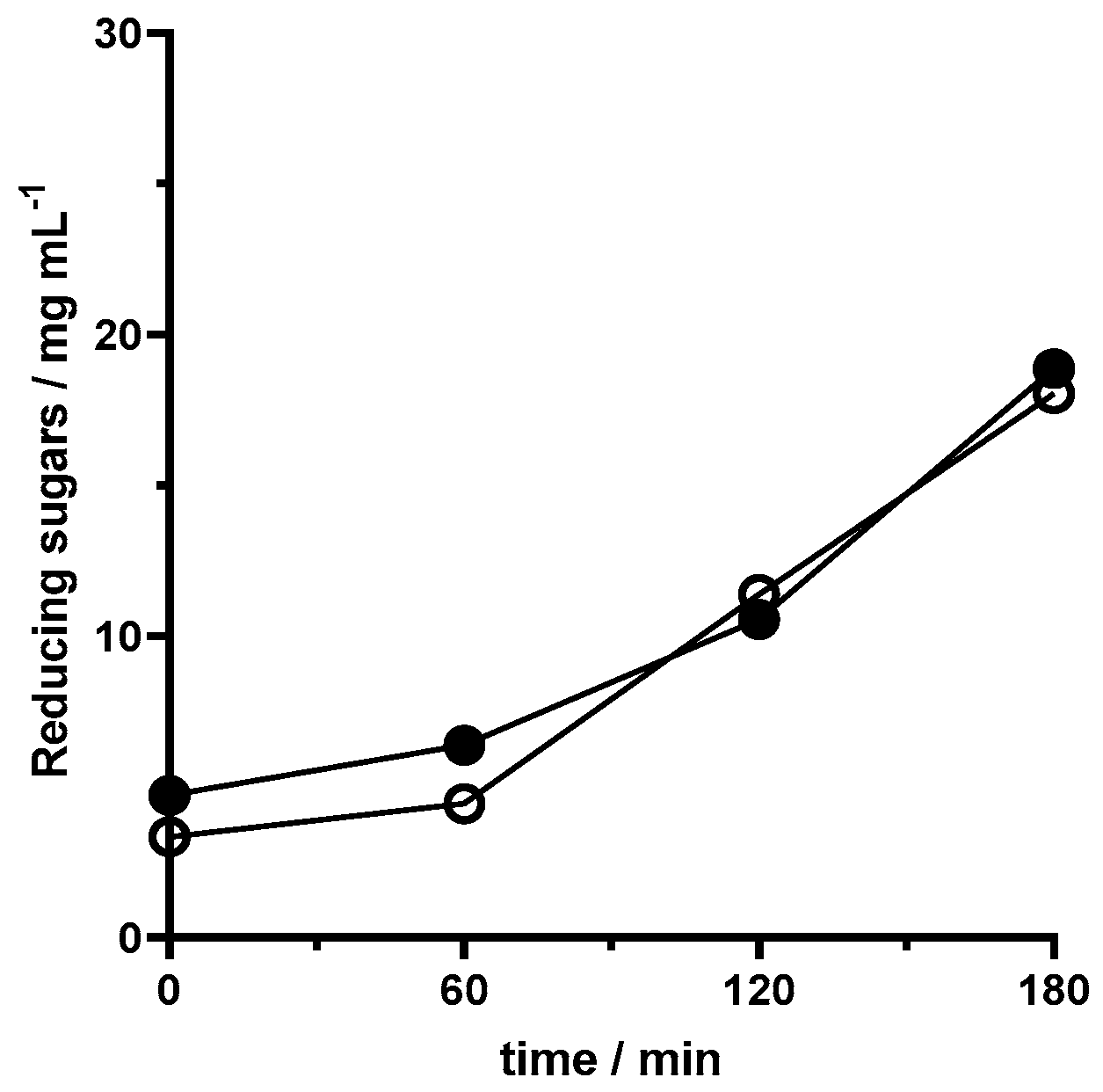
The activity of the pectinase lenses on the juice was assessed by the evolution of reducing sugar concentration over time, as presented in
Figure 6. The fact that there was a significant increase in the product of the breakdown of pectin catalyzed by the immobilized pectinase further confirms the assumed initial presence of pectin. In comparison to assays with exclusively the primary substrate in the medium but the same amount of 10.0 mg mL
−1 pectinase capsules, this reaction exhibited a small increase in the initial product formation. This could be attributed to the potential lower accessibility of pectin in the juice; on the other hand, after 60 min, there was a higher rate of the enzymatic reaction (
Figure 6). The two types of lenses, regular and magnetized, led to similar results. Regular lenses tend to reach slightly smaller values of product and initially have a higher rate of biocatalysis.
In the bioprocess of juice, enzyme release was small, respectively, 1.26% and 0.63% of enzyme content from regular and magnetized capsules respectively.
3.4.2. Effects of Bioprocessing on Juice Antioxidant and Anti-Inflammatory Activity
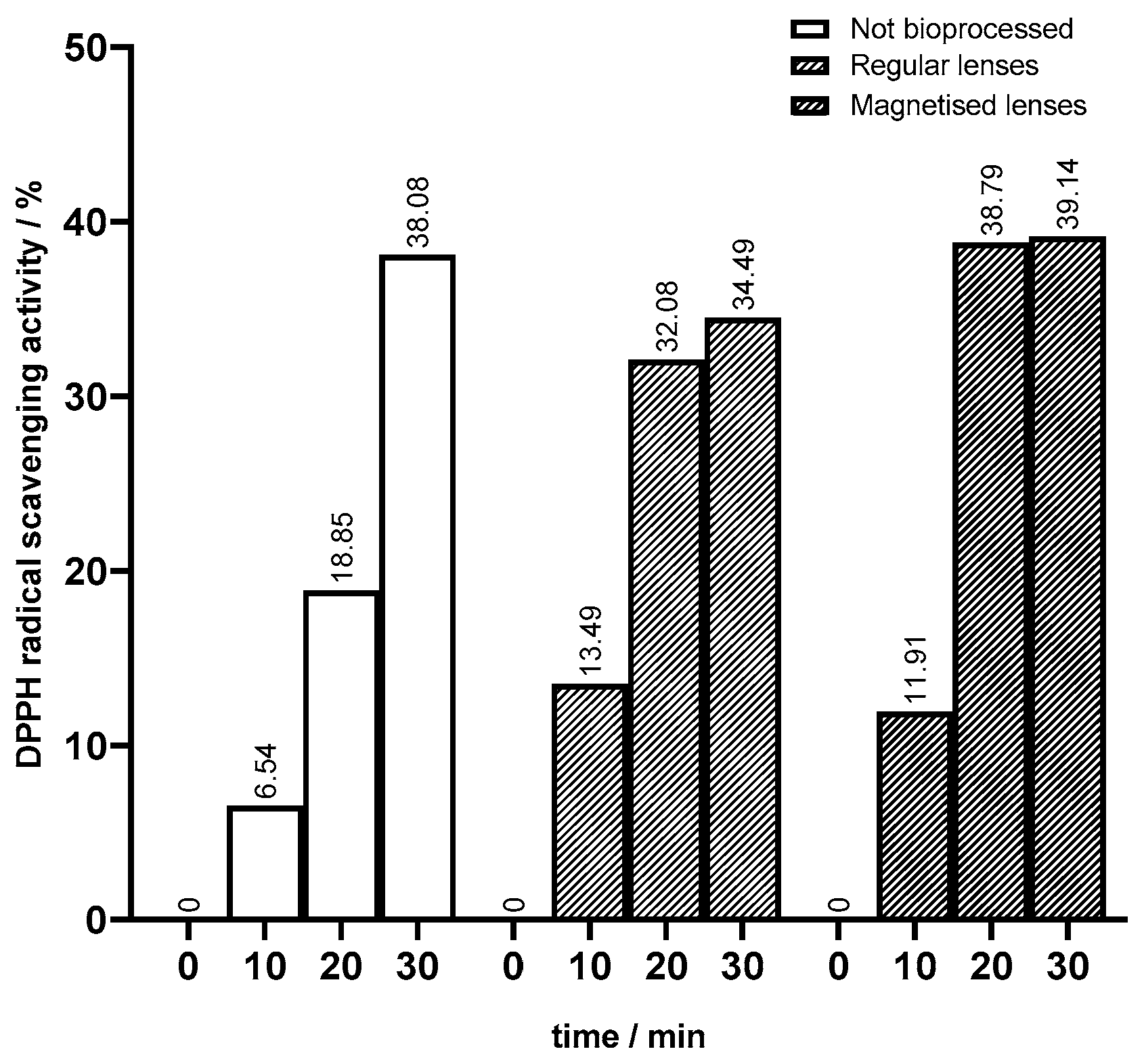
The juice bioprocess was successful in diminishing texture as aimed. To assess the effects of the bioprocess on the antioxidant and anti-inflammatory activities of peach juice, it was important to perform the DPPH method on unbioprocessed juice samples as a control. The percentage of DPPH radical scavenging activity at different times is presented in
Figure 7 in comparison with bioprocessed juices. The differences suggest that the bioprocessing of juice employing both regular and magnetized lenses impacts antioxidant activity. The unbioprocessed juice sample exhibited a gradual increase over time, reaching 38% after 30 min. This progressive increase suggested a natural antioxidant activity inherent to the juice, which was likely attributed to its composition of various bioactive compounds. Upon bioprocessing with regular lenses, the juice demonstrated a more pronounced enhancement in antioxidant activity when compared to the unbioprocessed juice: at 10 min, the DPPH scavenging activity was doubled, and at 20 min, it was 1.7 times higher (
Figure 7). Similarly, bioprocessed juice with magnetized lenses induced a substantial increase in antioxidant activity compared to unbioprocessed juice as well as juice bioprocessed with regular lenses. The highest increase in DPPH scavenging activity was after 20 min with 39%, which is more than double the unbioprocessed juice’s value and almost 7% higher than the bioprocessed juice with regular lenses. The improvement of antioxidant activity after bioprocessing in general was attributed to the action of immobilized pectinase in sol–gel lenses. The breakdown of pectin might lead to the release of previously bound bioactive compounds. Consequently, the interaction of these compounds responsible for scavenging DPPH radicals will be facilitated. As mentioned in the previous topic, in bioprocessing with magnetized lenses, there was a higher enzymatic activity entailing a greater pectin breakdown compared to regular lenses. This way, there was a more release of bioactive compounds which aligned with the enhancement in antioxidant activity observed in juice bioprocessed with magnetized lenses.
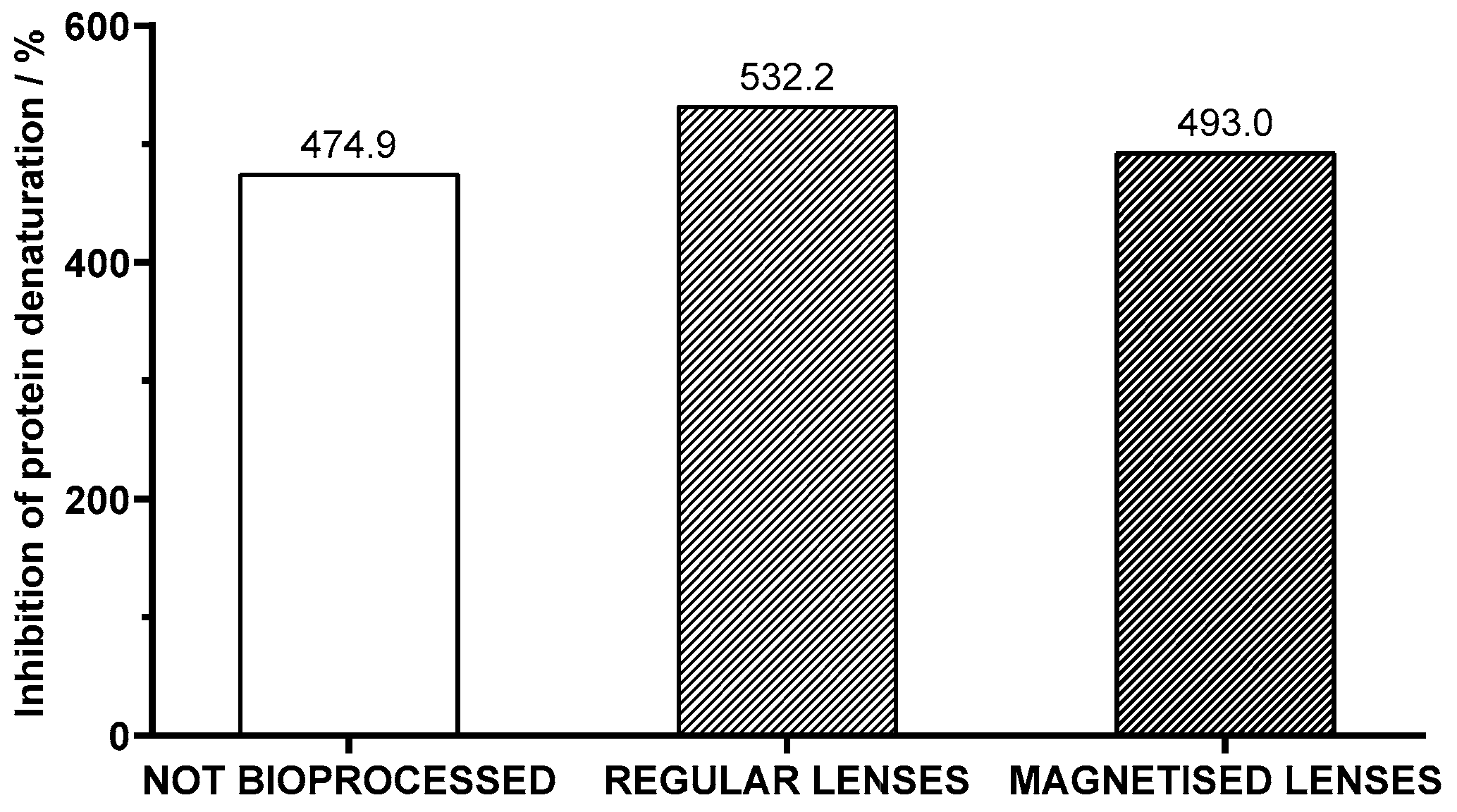
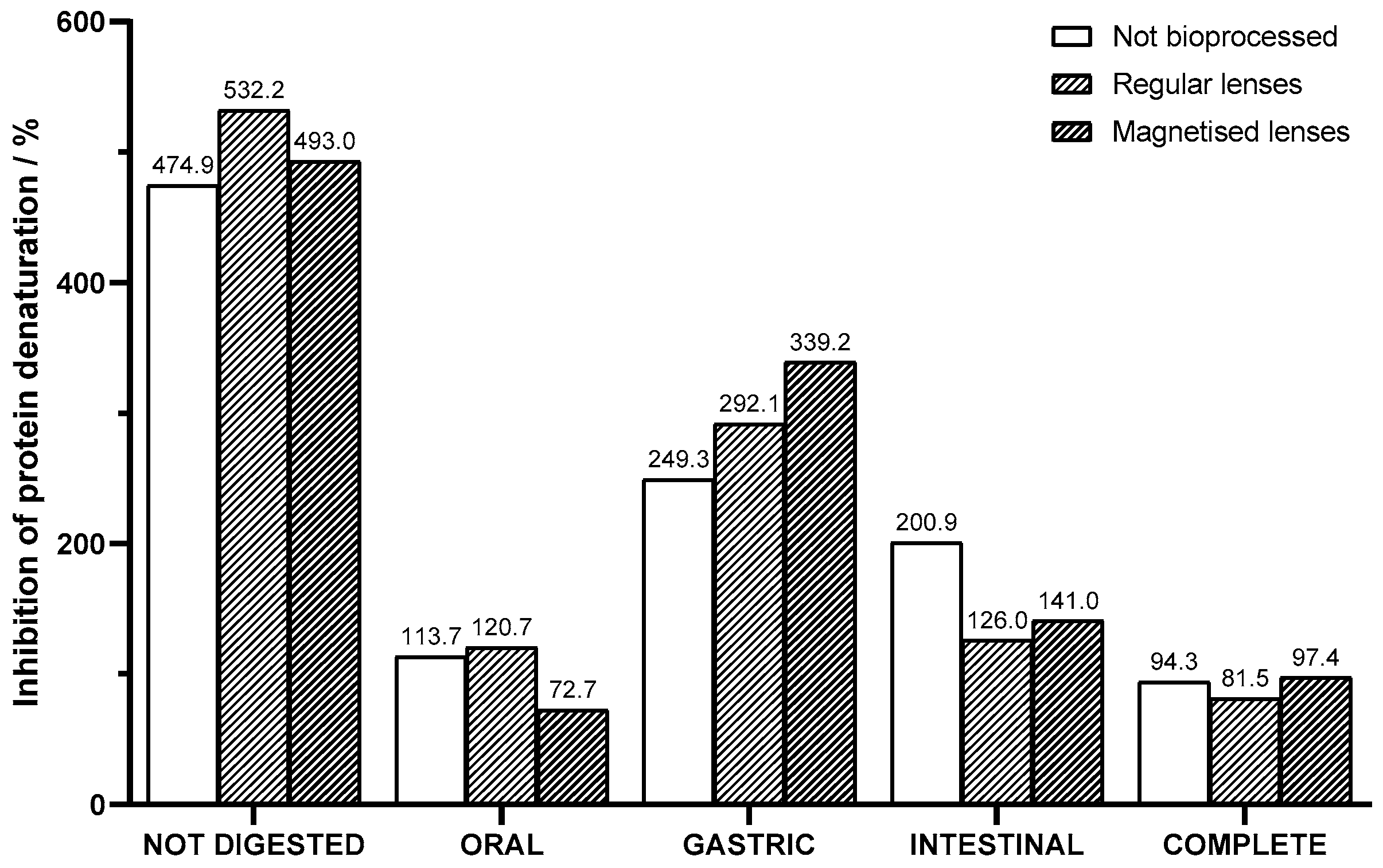
The anti-inflammatory activity correlates to the percentage of inhibition of protein denaturation assessed through the albumin method. The data presented in
Figure 8 indicate that the not bioprocessed juice shows anti-inflammatory activity with a 474.9% inhibition of protein denaturation. Upon bioprocessing the juice with regular lenses containing immobilized pectinase, an increase in anti-inflammatory activity was observed, with the percentual inhibition rising to 532.2%. This enhancement suggests that the enzymatic treatment may have facilitated the release or activation of bioactive compounds within the juice, resulting in an augmented anti-inflammatory response. Samples of juice bioprocessed with magnetized lenses, although not as pronounced, also showed significant improvement in anti-inflammatory activity, reaching 493.0%.
The enhancement of anti-inflammatory activity can be partly attributed to the augmented availability of the bioactive compounds caused by the same factors as mentioned in the antioxidant activity analysis. The heat in the albumin method not only directly promotes protein denaturation but also accelerates oxidative reactions that contribute to protein damage. This way, bioactive species are important for scavenging reactive oxygen species generated during heating, reducing oxidative damage to proteins and preserving their native structure. Samples processed with magnetized lenses were outperformed in terms of anti-inflammatory activity by juice bioprocessed with regular lenses, whereas the opposite happened in antioxidant activity. This suggests that either there was some thermal degradation of bioactive compounds which was detrimental to their activity or there are other factors concerning anti-inflammatory activity besides bioactive compounds’ availability.
3.5. Bioavailability: In Vitro Digestion
The bioavailability of bioprocessed juice was assessed through in vitro digestion. To perform it, was necessary to conduct an identical assay of bioprocessing with the pectinase sol–gel lenses.
Reducing sugar content was assessed at the beginning and end to monitor the bioreaction. There was an increase from 0 to 180 min in assays with both regular and magnetized capsules. Enzyme release was also monitored as in previous assays. Overall, the bioprocess was successful and samples were fitted to be digested.
3.5.1. Effects of In Vitro Digestion on (Un)Bioprocessed Juice Antioxidant Activity
The results of antioxidant activity assessment after in vitro digestion provide valuable insights into the potential health benefits of the bioprocessed juice under simulated physiological conditions. The percentage of DPPH scavenging activity at various time points reflects the antioxidant capacity of the juice before and after undergoing different stages of digestion.
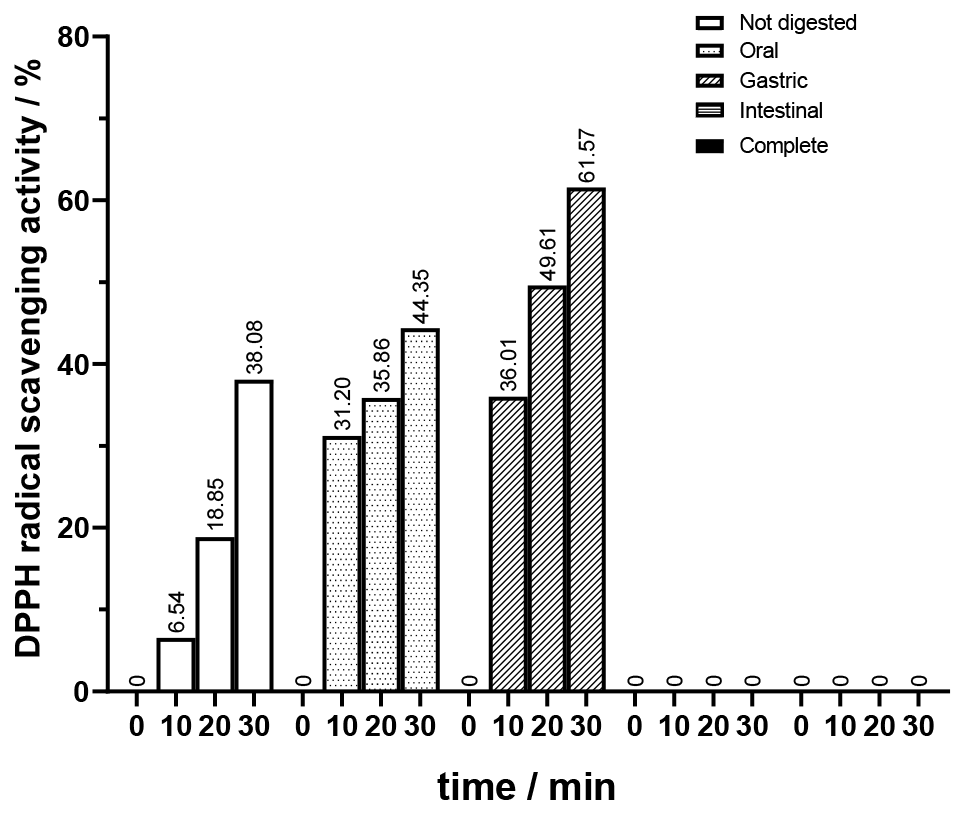
First, it was important to establish a baseline by assessing how digestion impacts the antioxidant activity of the original not bioprocessed juice. Those results are presented in
Figure 9. As previously established, the unbioprocessed juice exhibits antioxidant activity which aligns with the gradual increase in DPPH scavenging activity observed for the not digested sample. Upon oral phase digestion, there was an increase in DPPH scavenging activity compared to the undigested sample. At 10 min, the percentage was roughly five times higher. This suggests that the initial breakdown of juice by amylase in the oral cavity may release antioxidants or enhance their accessibility, leading to a higher antioxidant capacity. During the gastric phase digestion, an even further increase in scavenging activity was observed, reaching the highest registered value for all stages at 30 min with 61.57% of DPPH scavenging activity. This could be attributed to the action of the gastric enzyme, pepsin, and acidic conditions, which may facilitate the release or activation of antioxidant compounds present in the juice. Interestingly, no DPPH scavenging activity was detected in the samples subjected to the intestinal phase and complete digestion. This result may indicate either the degradation or modification of bioactive compounds during the intestinal phase, rendering them inactive or less effective in scavenging free radicals. It is important to note that the absence of DPPH scavenging activity in samples subjected to intestinal and complete digestion does not necessarily imply a lack of bioactive compounds in the juice.
Excluding the intestinal phase, a gradual increase in scavenging activity was observed through digestion, indicating the presence of antioxidants that become more bioavailable as digestion progresses. The results of the intestinal phase align with what was stated earlier: the absorption of phenolics associated with insoluble fiber is ineffective in the small intestine due to gel formation, increased viscosity or binding, and entrapment [
10]. This phase also proves to be, in a way, the limiting phase of complete digestion in terms of losing antioxidant activity. Overall, the results for the digested unbioprocessed juice highlight the dynamic interplay between digestion and antioxidant activity, underscoring the importance of considering oral and gastrointestinal conditions when assessing the bioavailability and efficacy of antioxidants in food and beverage matrices.
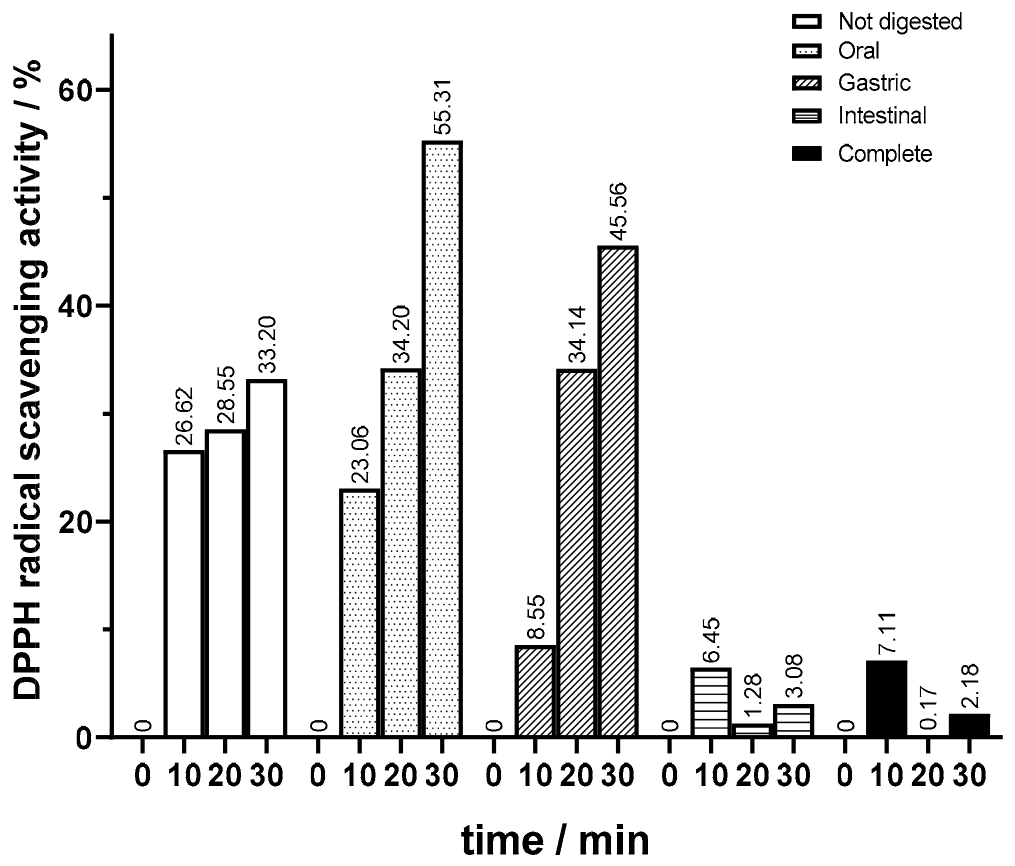
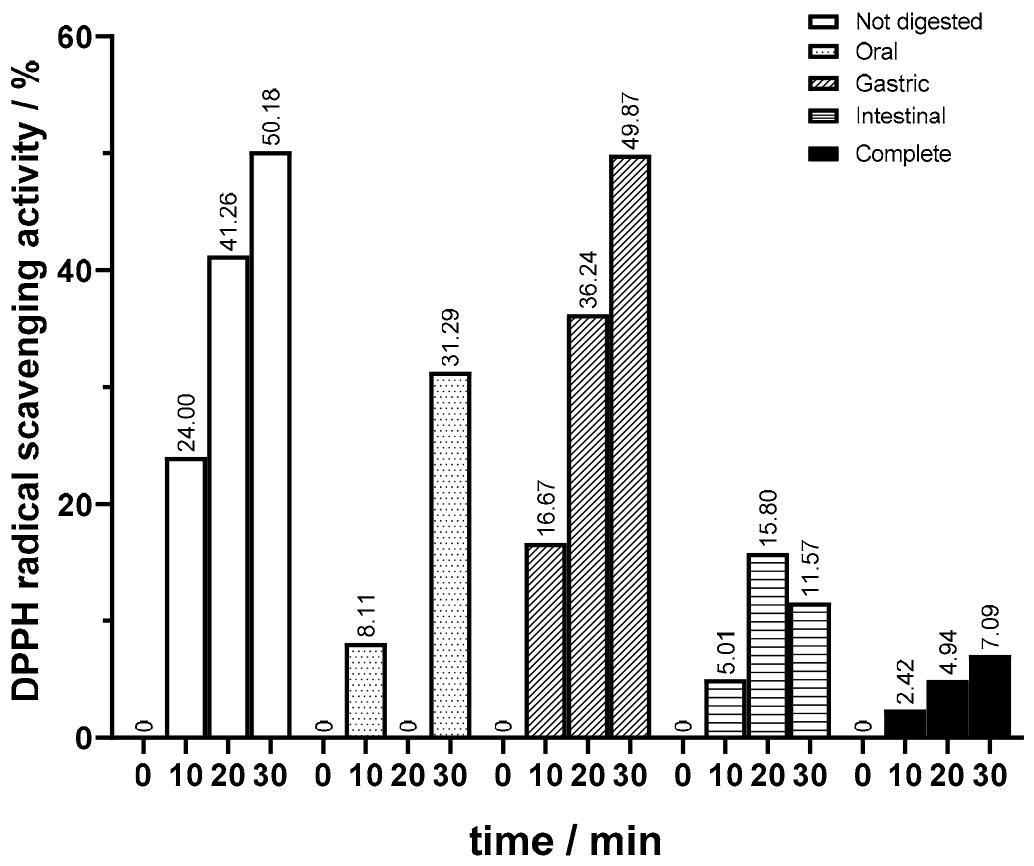
Moreover, in this study, it was a priority to investigate the effect of bioprocessing, employing both regular and magnetized pectinase lenses, on the antioxidant activity of juice throughout different stages of in vitro digestion. Compared to unbioprocessed juice, both bioprocessed samples exhibited modifications in their antioxidant activity profiles throughout in vitro digestion phases, as shown in
Figure 10 and
Figure 11.
In terms of the antioxidant activity of the undigested samples, bioprocessed juice showed a higher antioxidant activity. For juice bioprocessed with magnetized lenses, values of 50% DDPH scavenging activity were attained (
Figure 11), whereas in the juices bioprocessed with regular lenses, there was a more pronounced increase when comparing values at 10 and 20 min (
Figure 11) to those for unbioprocessed juice (
Figure 9). In the oral phase, similar to what happened in the not bioprocessed juice, there was a significant increase in antioxidant activity on samples bioprocessed with regular lenses. This increase was significant, keeping the DPPH scavenging activities consistently higher than those observed in the unbioprocessed juice. On the other hand, the opposite happened in oral phase assays with juice processed with magnetized lenses. The decline in antioxidant activity was quite significant, reaching levels lower than in the unbioprocessed juice. Interestingly, in the gastric phase, juice bioprocessed with magnetized lenses regained antioxidant activity, surpassing samples bioprocessed with regular lenses, which suffered a decrease in DPPH scavenging activity and reached levels below values for unbioprocessed juice. In this phase, the results were in line with those of unbioprocessed juice. The results of antioxidant activity for bioprocessed juice in intestinal and complete digestion showed the most relevant changes. Although there was a decrease in antioxidant activity in both bioprocessed juices, it was not null as in the not bioprocessed juice samples, the intestinal phase scavenging activity being slightly higher than in complete digestion. Samples bioprocessed with magnetized lenses had a higher antioxidant activity after the intestinal phase than after complete digestion and increased DPPH scavenging activity compared to the ones subjected to regular lenses.
In the face of the obtained results, in vitro digestion phases significantly influence the antioxidant activity of juice. Modifications caused by digestion enzymes and pH changes, such as enzymatic hydrolysis and transformations of polyphenols, can augment or decrease the bioavailability and reactivity of antioxidants, influencing their overall antioxidant activity throughout digestion. In the initial phases, the enzymatic breakdown of the cloudy juice matrix seems to facilitate the release of bioactive compounds, including antioxidants, from their natural structures. Through the intestinal phase, antioxidant activity suffers a decrease, which could mean that pancreatin could cause polyphenols and other antioxidant compounds to be broken down into smaller molecules or rendered inactive by enzymatic hydrolysis. The pH levels should not be responsible as the intestinal phase was set at 6, and oral and gastric at 7 and 2, respectively, both displaying increased antioxidant activity.
3.5.2. Effects of In Vitro Digestion on (Un)Bioprocessed Juice Anti-Inflammatory Activity
The anti-inflammatory activity directly correlates to the inhibition percentage of protein denaturation assessed through the albumin method. The anti-inflammatory activity of unbioprocessed and bioprocessed juice underwent dynamic changes throughout different digestion phases presented in
Figure 12, providing insights into its potential health benefits under physiological conditions.
For all juice samples, the initial anti-inflammatory activity before undergoing any digestion was taken as a reference point to obtain percentual values for the different stages of digestion.
As previously mentioned, when assessing the impact of bioprocessing on anti-inflammatory activity on undigested samples, both bioprocessed samples have higher anti-inflammatory activity than the original juice, and the sample bioprocessed with regular lenses outperforms juice treated with pectinase sol–gel magnetized lenses.
First, it was important to establish a baseline by assessing how digestion impacts the anti-inflammatory activity of the original not bioprocessed juice. As the juice progressed into the oral phase, a roughly 75% decrease in anti-inflammatory activity was observed compared to its undigested state. As digestion continued into the gastric phase, there was a substantial increase in anti-inflammatory activity compared to the oral phase. This augmentation to 50% of the undigested sample activity may signify the reactivation of bioactive compounds or the release of anti-inflammatory agents facilitated by the mimicked gastric environment. This suggests that acidic conditions and the enzymatic activity of pepsin could contribute to the enhanced availability or reactivity of anti-inflammatory components within the juice. In the last stage, the intestinal phase, while there was a minor decrease of 10% in activity relative to the gastric phase, the anti-inflammatory activity remained notably higher than that observed in the oral phase. Upon complete digestion, anti-inflammatory activity approaches levels similar to those obtained in the oral phase, indicating that this stage is the most detrimental to anti-inflammatory activity. This decline suggests a potential partial degradation or change in anti-inflammatory compounds present in the juice due to initial digestive processes carried out by α-amylase. The results also demonstrated that the decrease in anti-inflammatory activity upon complete digestion is not equivalent to the sum of the decrease in each digestion phase, implying that there is not a cumulative effect.
The effect of bioprocessing, employing both regular and magnetized pectinase lenses, on the anti-inflammatory activity of juice throughout different stages of in vitro digestion can be observed in
Figure 12. Bioprocessed samples both have a similar profile of percentual inhibition of protein denaturation. In the oral phase, there was an equivalent decrease in the anti-inflammatory activity of juice processed with regular lenses, maintaining its absolute value above the original juice. The sample subjected to magnetized lenses had a slightly more pronounced decline of more than 85%, leading to levels below the original juice. Next, in the gastric phase, as had occurred in the original juice, there was a general regain of anti-inflammatory activity. Bioprocessed juice with regular lenses maintained its values above the original juice. Juice bioprocessed with magnetized lenses had a more pronounced increase of 50% compared with both the other samples and reaching nearly 70% of its activity before undergoing any digestion. Finally, in the intestinal phase, bioprocessed juice stood out for having a higher decrease in the percentual inhibition of protein denaturation being reduced to half. Upon complete digestion, the bioprocessed juices had a similar outcome. Although less highlighted, it also suffered a decrease in activity. Samples bioprocessed with magnetized lenses prevailed for slightly surpassing the unbioprocessed juice after complete digestion.
3.6. Conclusions
This work presents the effective development of sol–gel encapsulated pectinase for bioprocessing. Significant optimization studies involved adjusting the capsule morphology, enzyme concentration, and microreactor configuration with a shift to U-well microplates yielding perfect lens-shaped capsules. Magnetization via iron oxide improved the enzymatic activity and stability, supporting prolonged use in bioconversion. Subsequently, the bioprocessing of peach nectar juice with encapsulated pectinase in sol–gel significantly reduced cloudiness, promoted pectin breakdown, and released bioactive compounds, which improved the antioxidant and anti-inflammatory properties of the juice. In vitro digestion studies in bioprocessed juices showed enhanced bioavailability compared to the controls specifically during gastric and intestinal phases. Overall, the development and application of immobilized biocatalysts represent significant progress in bioprocessing, advancing the benefits of enzyme functionality and bioavailability.