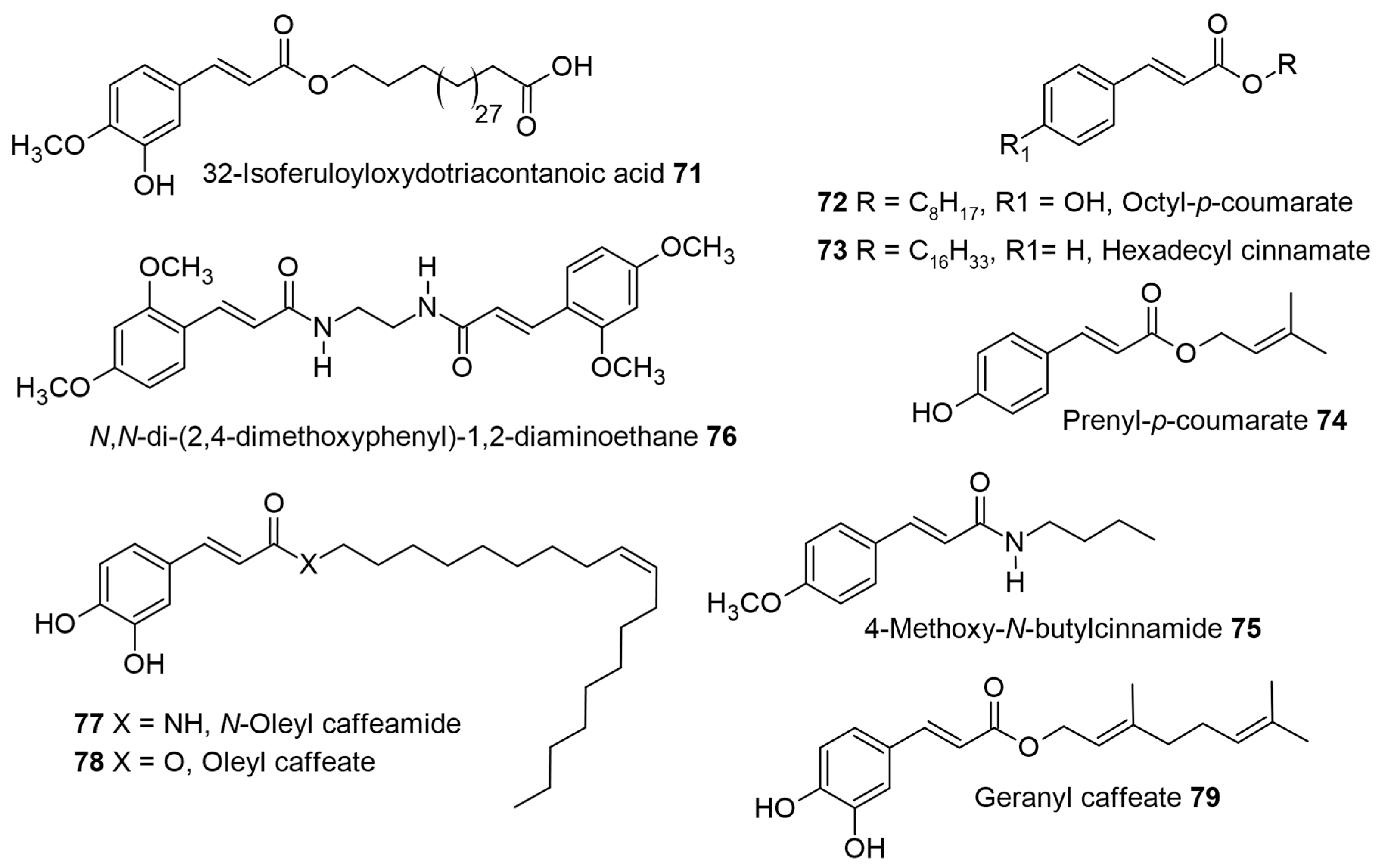
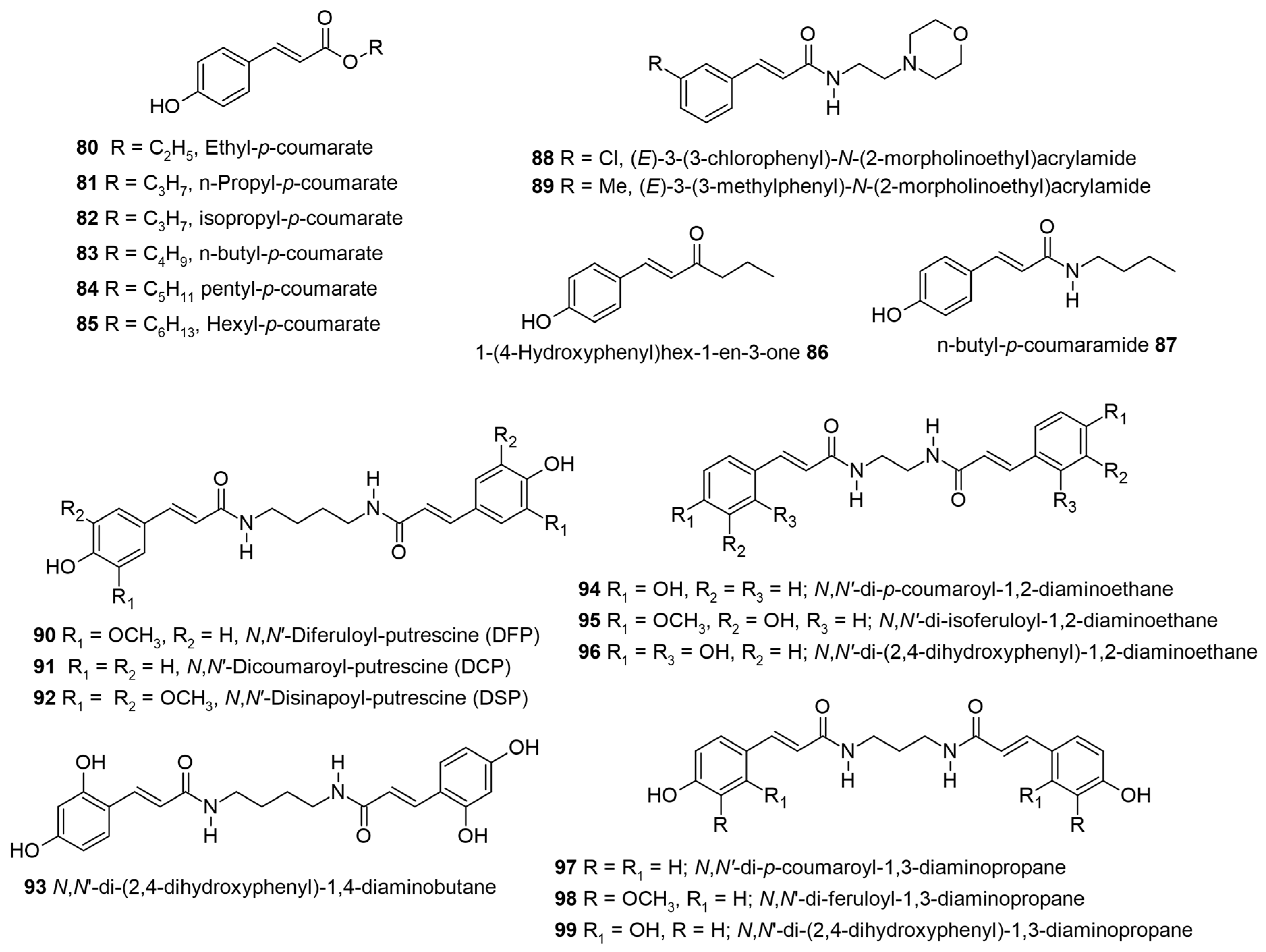
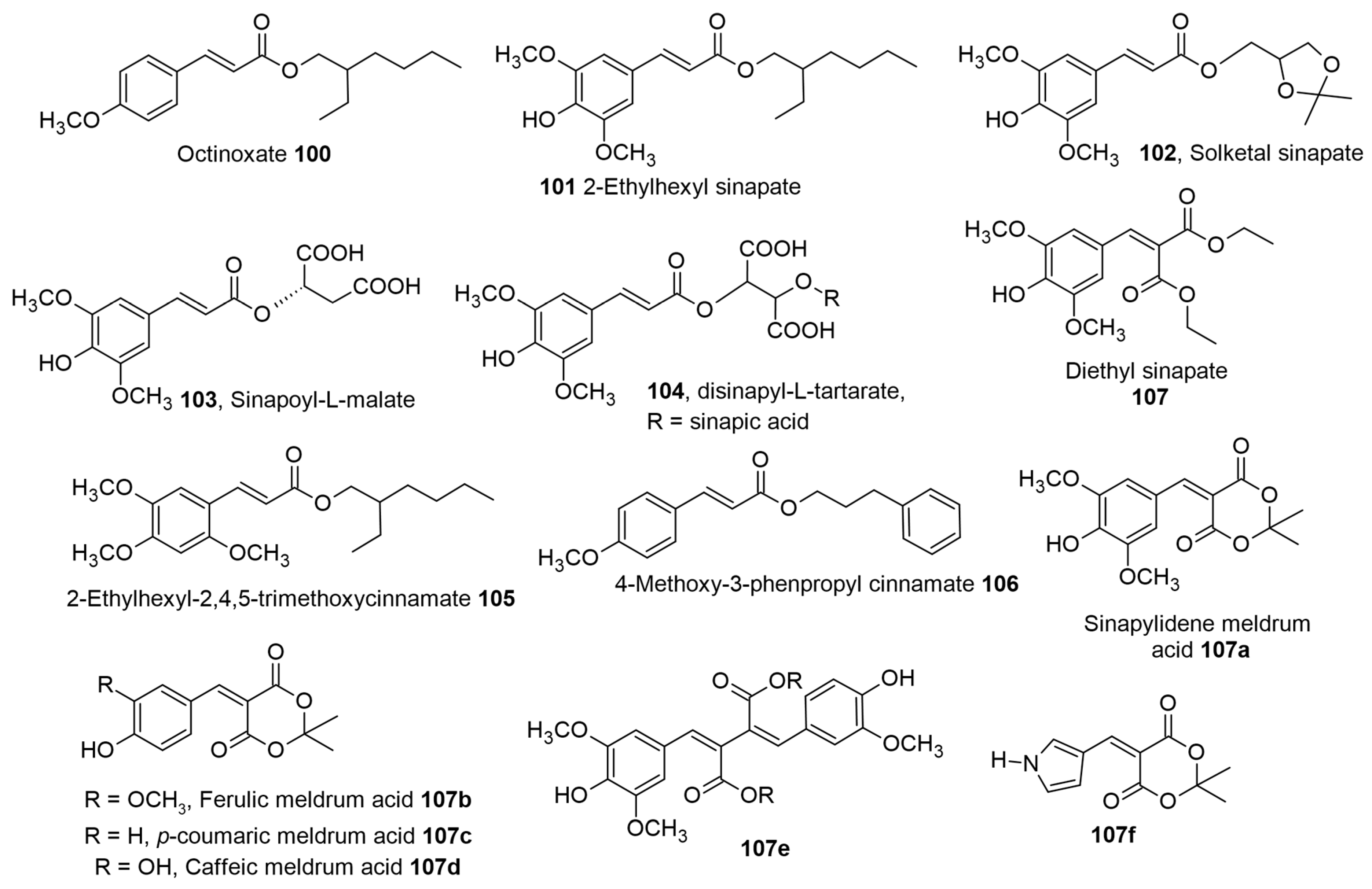
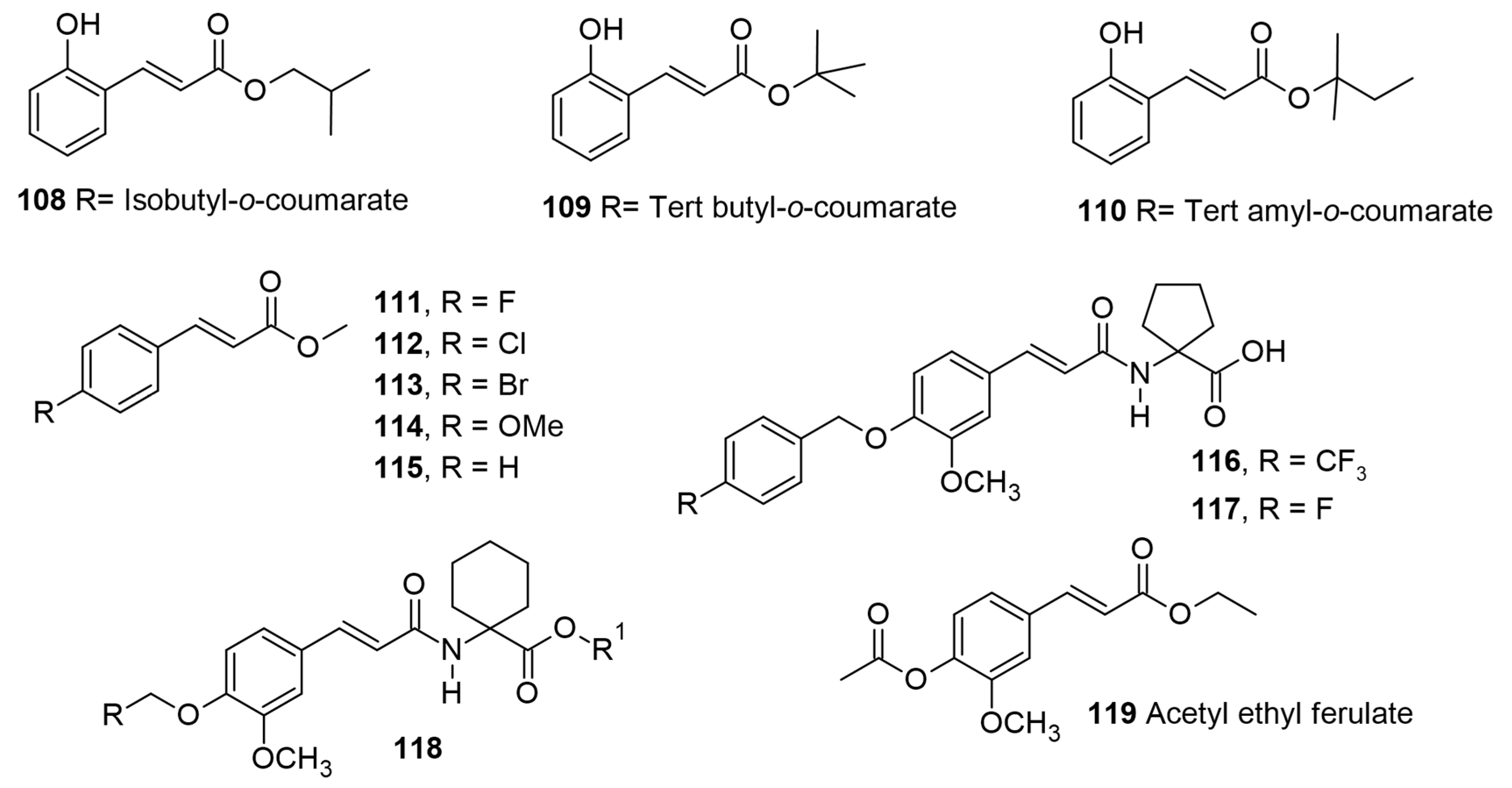
1. Introduction
Alkyl hydroxy cinnamates (AHCs) are bioderived from caffeic, ferulic,
p-coumaric, and sinapic acids arising from the shikimic acid pathway [
1] (
Figure 1). The basic structure of these AHCs comprises a phenyl (C6) decorated with mono- or poly-hydroxy and/or methoxy groups in positions 2-, 3-, 4-, 5-, and 6-. The phenyl is connected to a C3 propenoic acid side chain esterified with saturated aliphatic alcohol of variable length, as reported for various plants [
2]. The ester side chain can be aliphatic (C1-C30) or modified with alkane diols, alkylamines, or diamines. These cinnamates are generally found esterified with fatty alcohols in suberized root tissues and have defense roles in plant physiology [
3]. Alkyl hydroxy cinnamates (AHCs) isolated from medicinal plant species are used in TCM, Indian Ayurveda, and traditional medicines (
Table 1). The occurrence of these long-chain aliphatic alkyl hydroxy cinnamates is varied in the plants, from leaves to components of bark, cuticular waxes, and suberin [
2] (see
Table 1). Recently, Domergue et al. have shown that alkyl hydroxy cinnamates exist in the root tissue of Arabidopsis (
Arabidopsis thaliana) [
3], while salt-induced abiotic stress leads to the production of alkyl-
p-coumarates rather than alkyl caffeates [
4].
Similarly, natural hydroxy cinnamic acid amide (HCAA) metabolites play pivotal functions during plant–pathogen interactions, and this has been recently elaborated [
5]. The biogenesis of HCAA is generally derived from the hydroxy cinnamic acid CoA ligase part and tyramine (4-(2-aminoethyl)phenol), putrescine (1,4-diamino butane), or agmatine (4-aminobutyl-guanidine) via their corresponding transferases (THT, tyramine hydroxy cinnamoyl transferase; PHT, putrescine hydroxy cinnamoyl transferase; or ACT, agmatine coumaryl transferase) [
5]. Several examples of pathogenic infections like late blight caused by the oomycete
Phytophthora infestans lead to HCAA biosynthesis and protection in plants. Apart from defense, HCAA also prevents insect attack on grains during growing or storage and imparts drought tolerance [
5]. Rice plants rapidly biosynthesized 11 of the 12 tested phenol amides on infestation by herbivores, like white-backed planthopper (WBPH)
Sogatella furcifera in a defense response. The level of
p-coumaroylagmatine was induced at a 249-fold higher level in female-gravid-infested plants than its level in non-infested plants [
6].
Several reviews on hydroxy cinnamic esters describing their antioxidant potential and amides describing their pharmacology have been published [
7,
8,
9,
10]. Consequently, this structured review significantly collates the different applications of alkyl hydroxy cinnamates and cinnamides in a comprehensive approach in one place. The review outlines and discusses the applications of alkyl hydroxyl cinnamates and cinnamides in nutraceuticals (as an antioxidant for the preservation of oils and fats), pharmaceuticals/therapies (antioxidant, anticancer therapy, Alzheimer’s and Parkinson’s disease therapy, antimicrobial, antiplasmodial, and metabolic disease), cosmetics (anti-tyrosinase and sunscreens), and biopesticides, presented in that order (see
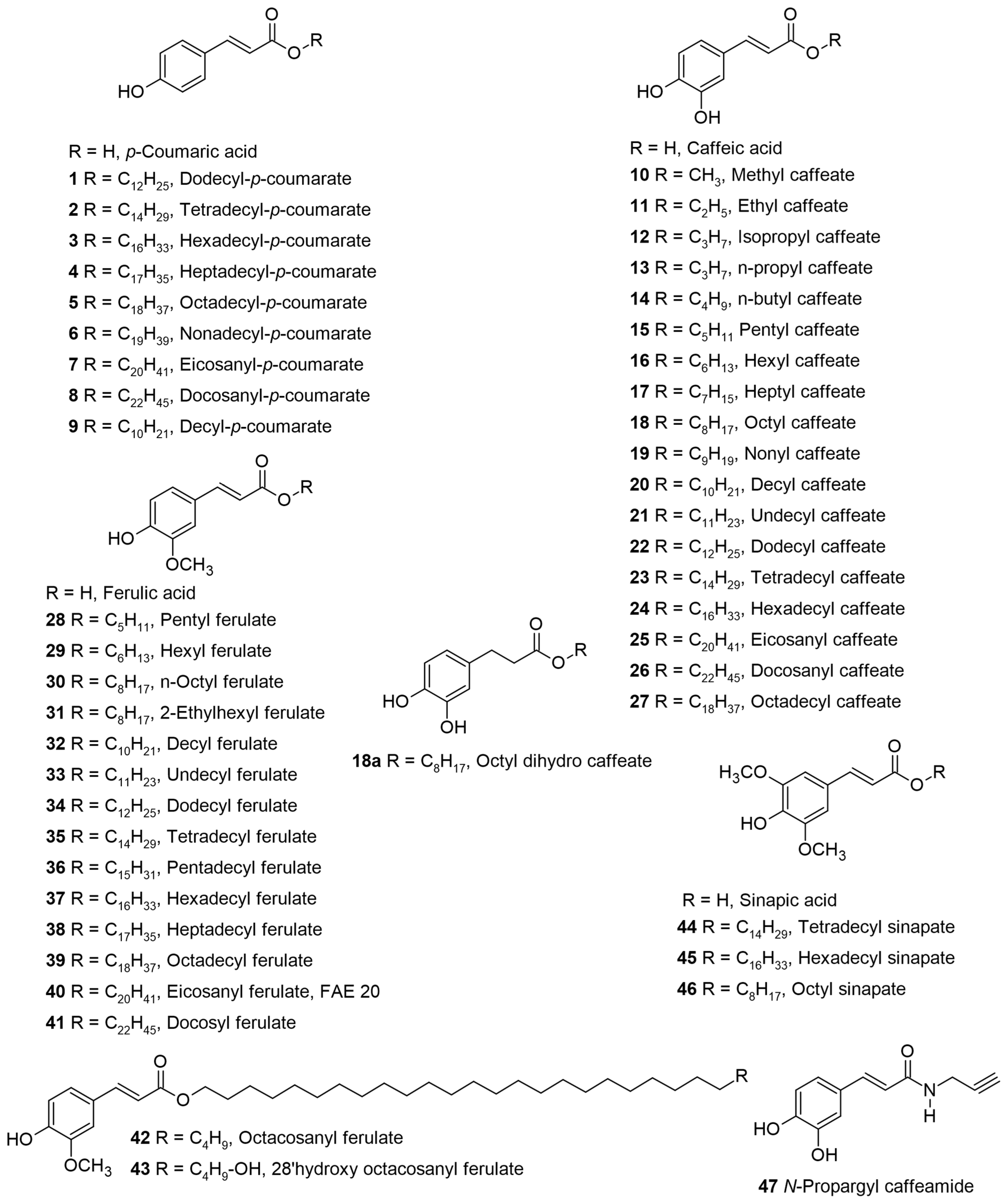
Table 1 for biological activity), along with a relevant discussion of their structure–activity relationships. For ease of understanding and simplicity, the structure of AHCs with common names is depicted in
Figure 2,
Figure 3,
Figure 4,
Figure 5,
Figure 6,
Figure 7,
Figure 8,
Figure 9 and
Figure 10 and denoted by bold numbers or names in the text wherever applicable.
Figure 2 depicts the structures of molecules from 1 to 47 followed by the remaining structures in the following figures as per their appearance in the discussion.
The information collated in
Table 1 shows the various alkyl hydroxyl cinnamates from different plant sources along with their geographical location and reported pharmacological activity.
Table 1.
The selected alkyl hydroxy cinnamates, along with their natural sources, geographical location, pharmacological activity, and references.
Table 1.
The selected alkyl hydroxy cinnamates, along with their natural sources, geographical location, pharmacological activity, and references.
Alkyl Hydroxy Cinnamate
(Alkyl Chain Number) | Plant Sources, Parts, and Geographical Location | Pharmacological Activity and References |
|---|
| Dodecyl-p-coumarate (C12) 1 | Ipomoea sepiaria, whole parts, India | Antioxidant activity [11] |
| Decyl caffeate (C10) 20 | seeds of Phleum pratense | Antioxidant activity comparable with Trolox [12] |
| Eicosanyl caffeate (C20) 25 and Docosanyl caffeate (C22) 26 | Glycyrrhiza glabra, roots, India | Moderate antioxidant activity; Elastase activity [13] |
| Docosanyl caffeate (C22) 26 and octadecyl caffeate (C18) 27 | Artemisia argyi, leaves, China | Antioxidant activity [14] |
| Undecyl ferulate (C11) 33 | Pouzolzia zeylanica, China | Isolated but not tested for Peroxisome proliferator-activated receptors (PPAR) activity [15] |
Tetradecyl ferulate
(C14) 35 | Erythrina sigmoidea and Erythrina eriotricha, roots and bark, Cameroon | Antibacterial activity [16] |
Tetradecyl ferulate
(C14) 35 | Ocimum sanctum, leaves and
Jatropha gossypifolia, roots, India | Reverse Transcriptase (RT)-associated Ribonuclease H inhibition [17]
Isolation [18] |
Hexadecyl ferulate
(C16) 37 | Sophora flavescens, roots, Republic of Korea | 1,1-diphenyl-2-picrylhydrazyl (DPPH) and Peroxynitrite (ONOO) free radical scavengers [19] |
| Pentadecyl ferulate (C15) 36 | Salicornia herbacea, aerial parts, China | Antioxidant and antiproliferative agent
[20] |
| Octacosanyl ferulate (C28) 42 | Potato pulp and Jatropha dioica, stem and roots, Mexico | Antioxidant [21,22] |
| 28-hydroxyoctacosylferulate 43 | Gallesia gorazema, roots,
Brazil | Anti-Herpes simplex 1 (HSV-1) activity
[23] |
Hexadecyl sinapate
(C16) 45 | Sophora flavescens, roots, Republic of Korea | DPPH and ONOO free radical scavengers [19] |
2. The Antioxidant Activity of AHCs: General Considerations
The antioxidant activity or potential of molecules is the result of the ability of the phenolic group(s) on the aromatic ring to react with free radicals and reactive oxygen species (ROS) forming a resonance-stabilized phenoxyl radical. By this mechanism, these antioxidant molecules can inhibit or delay redox reactions via the scavenging of reactive oxygen species. The inherent antioxidant activity of HCAs and their derivatives is the most commonly described health-beneficial property. As a general approximation, caffeic acid and its esters have the highest antioxidant activity, followed by sinapic, ferulic, and lastly by
p-coumaric acid and their esters [
24,
25,
26,
27]. The position of methoxy groups in ferulic and sinapic acid mirrors the hindered phenolic structure similar to BHT (butylated hydroxy toluene) or BHA (butylated hydroxy anisole), which allows them to quench free radicals and form stabilized radicals by the same mechanism [
28,
29]. Teixeira et al. reviewed the electrochemical methods used for redox reactions and the antioxidant capacity of cinnamic acid derivatives. They determined that the redox reaction operates via a quinone intermediate in caffeic acid and its derivatives, while in ferulic and sinapic derivatives, the semiquinone intermediate is formed (see
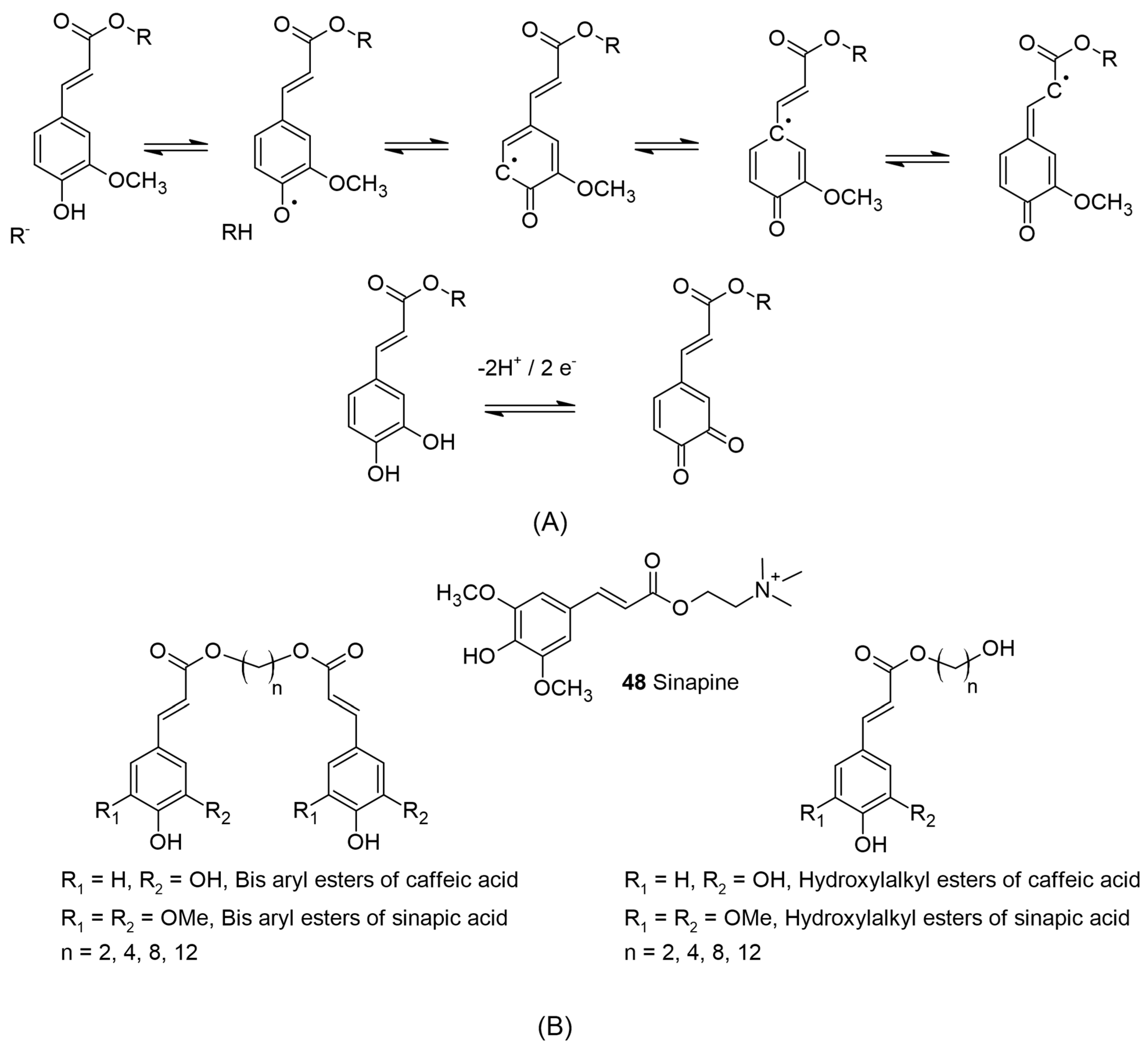
Figure 3A for the representative examples) [
30]. Contributions from the inductive and mesomeric effects of methoxy functions on these acids also provide additional stabilization. The olefinic bond of cinnamic acid derivatives may help to further stabilize the phenoxy radicals formed during the antioxidant activity by delocalization [
31,
32]. Several authors have also pointed out that the olefinic bond of cinnamyl esters may not be required for the antioxidant activity, but it may be more dependent on the hydroxyl or methoxy groups on the aromatic ring [
33,
34,
35].
Figure 3.
(
A) The representative formation of semiquinone in ferulic acid by reaction with reactive species adapted from [
36] and quinone from caffeic esters via the loss of 2 protons and 2 electrons. (
B) Sinapine, a constituent of rapeseed meal by-product and the mono and bis esters of caffeic and sinapic acid from aliphatic α,ω-diols, n = 2, 4, 8, and 10.
Figure 3.
(
A) The representative formation of semiquinone in ferulic acid by reaction with reactive species adapted from [
36] and quinone from caffeic esters via the loss of 2 protons and 2 electrons. (
B) Sinapine, a constituent of rapeseed meal by-product and the mono and bis esters of caffeic and sinapic acid from aliphatic α,ω-diols, n = 2, 4, 8, and 10.
Further, as pointed out by Teixeira et al., it is assumed the third hydroxyl group in the case of 3,4,5-tri hydroxy cinnamic acid can reduce the formation and stabilization of the intermediate, causing a decrease in the oxidation potential. It is assumed that the catechol moiety forms the quinone structure during the antioxidant mechanism via semiquinone formation. This can be applied in the case of commercial trihydroxy benzoates (gallic acid derivatives) to explain the high antioxidant activity observed for such molecules [
24,
37,
38].
Antioxidant assays like DPPH, ABTS (2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic) acid), or fluorescein used for the ORAC (oxygen radical absorbance capacity) assay use organic solvents or aqueous mixtures to allow a better approximation of hydrophilic and lipophilic antioxidant systems [
39]. But these different types of assay methods do not allow a proper correlation due to the non-equivalent substrate and the concentration of antioxidants used [
40], although the available data reported in several studies point out that hydroxy cinnamic acids have good solubility in an aqueous medium and therefore show better antioxidant activity compared to the lipophilic esters [
24,
26,
41]. The lipophilic esters may be better suited in systems like oils, fats, and other applications based on their structure and placement at the oil–aqueous interface in emulsions and therefore merit the following discussion.
A study using higher lipophilic alcohols like tetradecanol, hexadecanol, and octadecanol showed that all caffeic esters (23, 24, and 27) had higher antioxidant activity, followed by the sinapic ester (44) and ferulic esters (35 and 39), as assessed using DPPH and ABTS assays (
Table 2) [
25]. The length of the alkyl chain on the esters did not influence the antioxidant activity, but the aromatic substitution pattern showed a major difference within these series. Similarly, the lipophilic caffeic esters (10, 11, 13, and 14) showed lower DPPH values and higher Ferric-reducing antioxidant power (FRAP) compared to the parent caffeic acid in the antioxidant assay. Overall, the antioxidant activity of caffeates and caffeic acid was superior to ferulic acid and was independent of the lipophilic substituent in the ester side chain [
42].
The antioxidant index for alkyl groups such as pentyl, hexyl, and heptyl ferulates (28, 29) in an ethanol-buffer system was more active than that of the other ferulates and ferulic acid, while hexyl, octyl, and 2-ethyl-1-hexyl ferulates (29, 30, and 31) induced higher inhibitory effects against the oxidation of phosphatidylcholine (PC) liposomes induced by azobis(2-Methylpropionamidine) dihydrochloride (AAPH) [
24]. It was suggested that these ferulates may be located near the surface of the phospholipid membrane to prevent the attack and react with the peroxyl radicals produced by AAPH in the aqueous phase. Similarly, glycerol-derived molecules, such as feruloyl glycerol or feruloyl dioleyl glycerol, and their incorporation in model phospholipid vesicles support this orientation/activity [
43].
The antioxidant activity of linear fatty alcohols, including C7, C8 (branched and linear), C9, C11–13, C15, C16, and C18 esters of ferulic acid in homogeneous phase, was found to be very similar (see
Table 2 for representative examples of C8 and C12 ferulates) [
44], while in the rat liver microsomes (the heterogeneous phase), dodecyl ferulate 34 was the most effective (IC
50 = 11.03 μM), followed by the linear octyl ferulate 30 (IC
50 = 12.40 μM). This clear effect of lipophilization was observed as the lowest activity was obtained for ferulic acid (IC
50 = 243.84 μM). Based on the three-dimensional nuclear magnetic resonance (3D NMR) studies using the Nuclear Overhauser effect (NOE), they concluded that in the case of octyl and dodecyl ferulate (30 and 34), the chain-folding behavior may provide a high degree of conformational flexibility. This ester chain flexibility may have resulted in the penetration/interaction with the microsomal phospholipid bilayer and the subsequent orientation of the phenoxy group for scavenging radicals [
44]. Further, using pulse field gradient NMR diffusion and NMR relaxation studies, Anselmi et al. concluded that the three-dimensional arrangement of alkyl side chains of the most active dodecyl ferulates (34) with the membrane bilayer of human erythrocytes plays a key role in dictating the observed antioxidant ability [
45].
Table 2.
The antioxidant activity of selected alkyl hydroxy cinnamates, and their parent hydroxy cinnamic acids compared with reference standards.
Table 2.
The antioxidant activity of selected alkyl hydroxy cinnamates, and their parent hydroxy cinnamic acids compared with reference standards.
| Molecule (Alkyl Fraction) | DPPH IC50 | ABTS IC50 | Standard/Reference Substance | References |
|---|
| Tetradecyl, hexadecyl and octadecyl caffeate 23, 24 and 27 | 31.9, 33.0, 38.0 µM | 0.98, 0.96 and 1.02 µM | Caffeic acid IC50—34.1 and 0.95 µM | [25] |
| Tetradecyl sinapate 44 | 77.2 µM | 1.18 µM | Sinapic acid IC50—66.0 and 1.14 µM | [25] |
| Tetradecyl and octadecyl ferulate (35 and 39) | 110.2 and 117 µM | 1.66 and 1.80 µM | Ferulic acid IC50—111.6 and 1.76 µM | [25] |
| Heptatriacontanyl caffeate (C37) | 54.8 µg/mL | 63.6 µg/mL | Gallic acid IC50—2.13 and 2.80 µg/mL | [14] |
| Docosyl caffeate (C22) 26 | 35.3 µg/mL | 46.1 µg/mL | Gallic acid IC50—2.13 and 2.80 µg/mL | [14] |
| Octadecyl caffeate (C18) 27 | 27.2 µg/mL | 37.4 µg/mL | Gallic acid IC50—2.13 and 2.80 µg/mL | [14] |
| Eicosanyl caffeate (C20) 25 | 8.8 µg/mL | 20.3 µg/mL | Gallic acid IC50—0.8 and 1.4 µg/mL | [13] |
| Docosyl caffeate (C22) 26 | 13.2 µg/mL | 23.1 µg/mL | Gallic acid IC50—0.8 and 1.4 µg/mL | [13] |
| Propyl caffeate (C3) 13 | 14.1 µM | - | BHT and Vit C IC50—51.2 and 33.3 µM | [46] |
| Ethyl caffeate (C2) 11 | 15.6 µM | - | BHT and Vit C IC50—51.2 and 33.3 µM | [46] |
| Hexadecyl ferulate (C16) 37 | 0.083 nmol/mL | 0.027 nmol/mL | Quercetin IC50—0.030 and 0.002 nmol/mL | [47] |
| Ferulic acid | 0.160 nmol/mL | 0.005 nmol/mL | Quercetin IC50—0.030 and 0.002 nmol/mL | [47] |
| Pentadecyl ferulate 36 | 27.6 µM | - | Ascorbic acid IC50—52.5 µM | [20] |
| Octyl caffeate (C8) 18 | 101.60 µmol/L | 133.54 µmol/L | BHA IC50—127.68 and112.29 µmol/L | [41] |
| Hexadecyl caffeate (C16) 24 | 68.27 µmol/L | 143.74 µmol/L | BHA IC50—127.68 and 112.29 µmol/L | [41] |
| Caffeic acid | 72.94 µmol/L | 82.29 µmol/L | BHA IC50—127.68 and 112.29 µmol/L | [41] |
| Octyl caffeate (C8) 18 | 35.87 µg/mL | 39.45 µg/mL | Trolox IC50—24.55 and 27.93 µg/mL | [26] |
| Rapeseed –linseed Oil +Octyl caffeate 18 | 570 µg/mL | 367 µg/mL | Rapeseed-Linseed Oil
DPPH IC50—20,923 µg/mL
ABTS IC50—11,799 µg/mL | [26] |
| Octyl ferulate (C8) 30 and Dodecyl ferulate (C12) 34 | 22.94 µM and 26.62 µM | - | Ferulic acid IC50—21.98 µM | [44] |
The Antioxidant Activity of AHC in Oils, Emulsions, and Biological Systems—Nutraceutical Applications
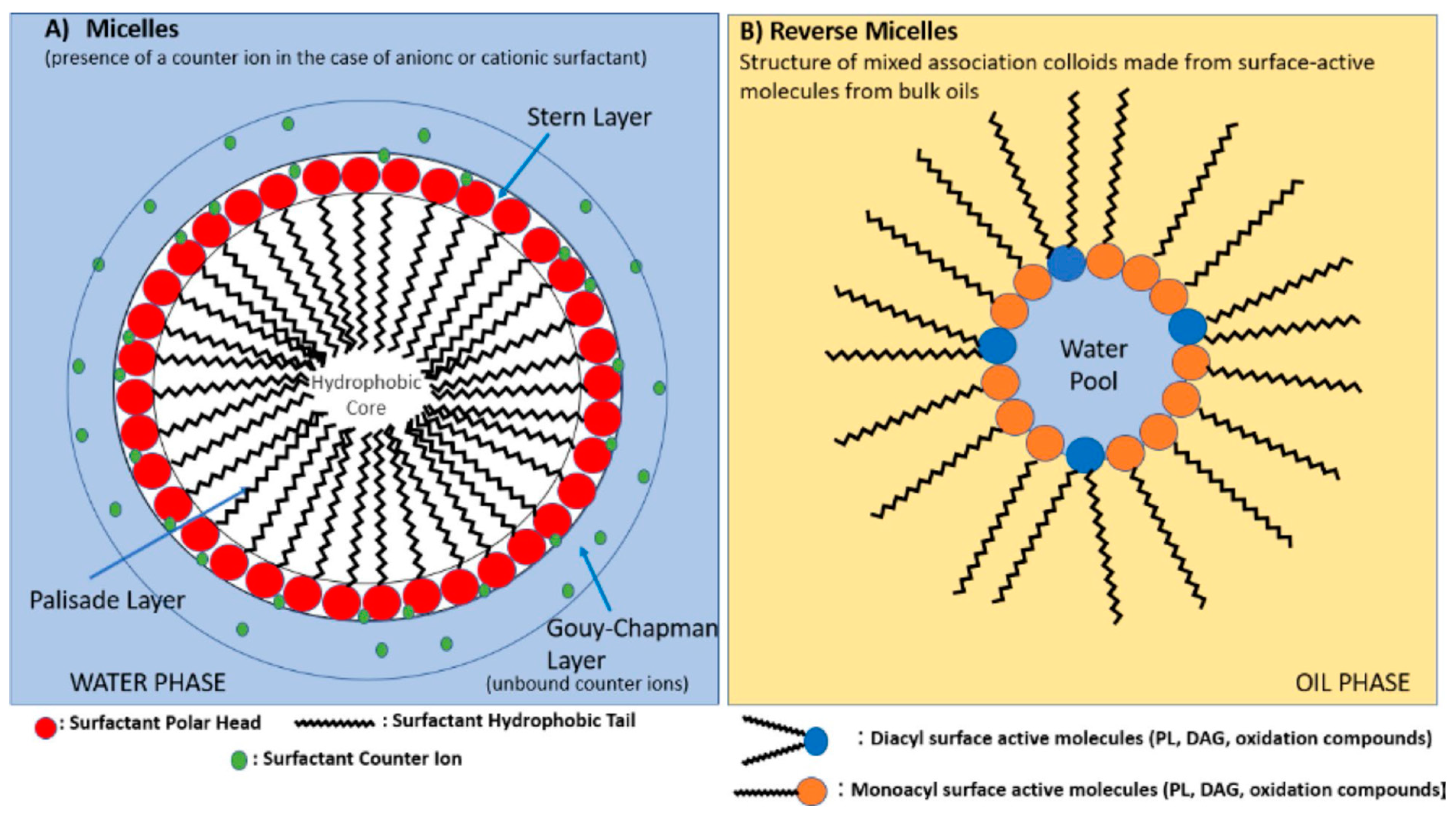
With reference to the emulsion-based antioxidant discussion, it is important to mention that lipid emulsions can exist as oil-in-water (O/W) emulsions or water-in-oil (W/O) emulsions. In O/W emulsions, the micelles form as their hydrophobic parts aggregate around the oil droplets and the hydrophilic parts towards water or the continuous phase, while in W/O emulsions, reverse micelles (association colloids) form, wherein these microstructures are reversed. A recent review discussing the influence of these micellar structures on lipid oxidation should be consulted for detailed understanding [
48]. In brief, Villeneuve et al. discussed the several factors influencing lipid oxidation, like the size of lipid droplets in micelles, the mobility of free radicals between these micelles, the location or orientation of antioxidants based on their hydrophobic and hydrophilic cores, and the interfacial area where oxidation can occur at the air–lipid interface in bulk oils or the O/W interface in emulsions. Further, the presence of additional emulsifiers or surfactants and their loading percentages makes the emulsion system more complex [
48]. These emulsifiers, based on their hydrophilic or lipophilic balance (HLB), can solubilize the antioxidant and change the overall positioning of the antioxidant either at the stern layer or the palisade layer for O/W emulsions (
Figure 4).
In this context of the research on alkyl chlorogenates and rosmarinates, Villeneuve et al. started to see a trend in antioxidant activity, which was dependent on the length of the hydrophobic side chain [
8]. A nonlinear concept called the cut-off effect was applied based on the degree of lipophilization with saturated unbranched fatty alcohols. The antioxidant activity followed a “cut-off effect”, meaning that it increased progressively with hydrophobicity until a critical point (critical chain length-CCL), beyond which the antioxidant activity collapsed [
8].
Figure 4.
Structure of micelles in emulsions and reverse micelles (association colloids) in bulk oils (reproduced with permission from [
48]).
Figure 4.
Structure of micelles in emulsions and reverse micelles (association colloids) in bulk oils (reproduced with permission from [
48]).
Caffeic acid (CA) possesses strong antioxidant activity and is distributed widely in nature. However, its limited solubility in non-polar media limits its application in fat-soluble food. To this end, several derivatives from methyl to octyl caffeates (10 esters) were designed, synthesized, and tested for their antioxidant activities. Concerning their antioxidant behavior, the alkyl caffeates with short chain lengths (n < 5) had better oxidative stability than those with long chain lengths (see
Table 2), while ethyl caffeate 11 had the best protection for unsaturated fatty acids in edible oil at 68.53%, slightly better than BHT (67%) [
46].
Volatile oxidation products like 1-penten-3-one, 1-penten-3-ol, and 2,6-nonadienal represent the decomposition of n-3 fatty acids and indicate the general trend in the development of the resulting fishy off-flavor in storage. The antioxidative effect of octyl dihydro caffeate 18a (
Figure 2) was slightly more efficient than that of oleyl dihydro caffeate in fish-oil-enriched milk emulsions, functioning as a model for a complex food system and reducing the above-described oxidation product levels [
33]. This study also showed that the olefinic bond of cinnamic acid derivatives is not a prerequisite for stabilizing the phenoxy radicals formed during antioxidant activity. As pointed out by Teixeira et al., the ortho-quinone structure formed by the catechol moiety may be the contributing factor in the observed antioxidant activity [
30].
Another interesting study concerning the critical chain length for alkyl hydroxy cinnamates observed that the optimum alkyl chain length of antioxidant phenolipids used may depend on the type of emulsion systems. This was shown by testing the antioxidative effect of lipophilized caffeic acid in mayonnaise (an O/W emulsion with egg components as the emulsifier) and milk enriched with fish oil. The caffeic esters with different chain lengths (C1–C20) performed as better antioxidants in comparison with caffeic acid in both tested emulsion systems. When the medium-alkyl-chain-length butyl caffeate 14, octyl caffeate 18, and dodecyl caffeate 22 were added to the fish-oil-enriched mayonnaise, they resulted in improved oxidative stability than with shorter (methyl caffeate 10) or longer (octadecyl caffeate 27) alkyl chains, whereas the shorter alkyl chains (methyl and butyl caffeates 10 and 14) compared to the medium and long chains (octyl, dodecyl, hexadecyl, and eicosyl) were more effective in fish-oil-enriched milk emulsions. The differences between the milk and mayonnaise systems such as the viscosity of the medium, tocopherol content, protein emulsifiers, pH, and oil droplet characteristics (charge, size, and composition) may have affected the antioxidant properties of these caffeates [
49].
In the O/W emulsion, using the conjugated autoxidizable triene (CAT) assay, antioxidant activity was seen for the critical chain length of the alkyl group in caffeates, up to octyl caffeate (18) and dodecyl caffeate (22), and a further increase in the alkyl chain length resulted in decreased antioxidant activity. Similar results were noted for C8 and C12 ferulates (30 and 34), while dodecyl-
p-coumarate 1 was more active compared to C8 coumarate [
27]. The catechol group (o-dihydroxy benzene) in caffeate-based antioxidants can delay lipid oxidation via the removal of metals from the interface via chelation. In this work, Sørensen observed that higher concentrations of methyl caffeate 10 (200 and 400 µM) were able to chelate iron up to 59% and 75% in comparison to EDTA~99–100% at the same concentrations [
27].
Based on the work of Laguerre et al., the highest concentration of antioxidants with critical chain length (CCL) was assumed to be at the oil–aqueous surface, where lipid oxidation can initiate, while the lower- and higher-alkyl-chain antioxidants move away from the interface [
50]. Similar results were observed by the groups of Paiva-Martins and Bravo-Díaz, who found a cut-off effect for the antioxidant efficiency of octyl caffeate
18 at the interfacial region of olive oil–water emulsions based on their pseudo phase kinetic model [
51].
The presence of natural endogenous tocopherols in oil or food emulsions and added emulsifiers like Citrem (citric acid ester of mono- and diglycerides) and Tween 80 or polysorbate 80 (polyoxyethylene sorbitan monooleate) can have a synergistic or antagonistic impact on the overall antioxidant efficacy. The localization of applied antioxidants in emulsions either at the oil–water interface or away from it will decide their efficacy. Sørensen et al. found that caffeic acid and caffeates (C1, C4, C8, C12, C16, and C20; 10, 14, 18, 22, 24, and 25) were efficient antioxidants in emulsions without the endogenous tocopherols, while caffeic acid, being hydrophilic, was efficient in the presence of the added emulsifiers citrem (HLB~6–9) and tween 80 (HLB~15), along with endogenous tocopherols. The antioxidant activity of alkyl caffeates was superior in tween 80 due to their lipophilic nature and higher partitioning concentration at the O/W interfaces with the absence of tocopherols. The observed interactions between the antioxidants used or between the emulsifier and the antioxidant in the emulsions may be the result of the differences. Therefore, the evaluation of antioxidants in different emulsions for lipid protection should be based on the presence of emulsifiers and natural antioxidants within the emulsified oils [
52]. A similar result was demonstrated for sinapate esters with C4, C8, and C12 alkyl chains in Tween 20 (polyoxyethylene sorbitan monolaurate), dodecyltrimethylammonium bromide (DTAB), and sodium dodecyl sulfate (SDS), which had different molecular structures and electrical charge [
53]. They found that a combination of DTAB, a positively charged emulsifier with a neutral pH, provided the best oxidative stability for echium oil emulsions using sinapic esters [
53].
Higher percentages of unsaturated fatty acids in rapeseed and linseed oils can increase their susceptibility to oxidation. The increase in the lipophilic nature of sinapic acid predominantly present in rapeseed (
Brassica napus L.) and its products could be an alternate strategy to provide antioxidant protection. Therefore, the antioxidant capacity of octyl sinapate 46, octyl caffeate 18, and octyl ferulate 30 was determined by DPPH and ABTS methods and compared with well-known antioxidants (e.g., Trolox). The general trend observed was that higher concentrations of these octyl esters were required to scavenge 50% of the DPPH or ABTS radicals in comparison with the parent acids. The overall antioxidant capacity of the octyl esters was lower by 40–60% in DPPH and over 60% lower in the ABTS assay, compared with their parent hydroxy cinnamic acids [
26]. Further, the antioxidant capacity in rapeseed–linseed oil enriched with 0.5% lipophilic octyl caffeate 18 was about 30 times higher in comparison with the non-supplemented oil based on both methods. The novel octyl ferulate 30 and sinapate 46, due to their lipophilicity, also showed antioxidant properties but were lower compared to 18. Overall, these octyl esters may be promising lipophilic alternatives for the protection of rapeseed–linseed oil mixtures from oxidative processes [
26].
Rabiej-Kozioł et al. evaluated 12 phenolipids based on hydroxy cinnamate derivatives (ethyl, octyl, cetyl, and steryl esters of sinapic acid, caffeic acid (CA), and ferulic acid [FA]) for their antioxidant capacity. The CA esters (11, 18, & 24) had the highest radical scavenging activity (RSA) compared to esters of ferulic acid (FA) and sinapic acid (
Table 2). The antioxidant potential effect of hydroxy cinnamyl esters on real samples like refined rapeseed oil, margarine (water-in-oil emulsion system), and mayonnaise (oil in water) was estimated using a quantity similar to that of commercially allowed antioxidants in food (i.e., 200 mg/kg). The ethyl caffeate 11 provided the best protection, followed by octyl caffeate 18 and 24 in all tested emulsions, similar to the in vitro scavenging experiments [
41].
Nutraceutical supplements like eicosapentaenoic acid (EPA, 20:5) and docosahexanoic acid (DHA, 22:6) are long-chain n-3 polyunsaturated fatty acids (PUFAs) derived from fish sources and are added to various food products to increase their nutritional value. The presence of natural emulsifiers in food emulsions can affect the location of lipophilic antioxidants [
54]. Sorensen et al. observed that methyl ferulate was the most efficient alkyl ferulate for preventing the lipid oxidation of EPA and DHA in milk (O/W emulsion). In comparison with the control having no antioxidants, methyl and butyl ferulates prevented the presence of primary oxidation products (hydroperoxides) and decreased their concentration in fish-oil-enriched milk. Although volatile secondary decomposition products (1-penten-3-one, 1-penten-3-ol, and (E, E)-2,4-heptadienal) were detected, methyl-ferulate-treated milk emulsions had no significant concentrations of the above products. Octyl and dodecyl ferulate (30 and 34) showed a pro-oxidant nature in the test emulsions, while the further elongation of the C16 and C20 alkyl chain resulted in weak pro-oxidative effects [
54]. In the present case of fish-oil-enriched milk, the C1 was the critical alkyl-chain-length observed cut-off effect for the ferulate [
54].
Extra virgin olive oil (EVO) contains oleic acid (65–80% mono unsaturated fatty acid, MUFA), while Nigella sativa oil has higher amounts of linoleic acid (39–43%) followed by oleic acid (33–37%). Nigella sativa oil also has phenolic constituents along with endogenous tocopherols and thymoquinone. Both oils have economical value and various uses in skin and hair care. Therefore, Perinelli et al. tested linear decyl ferulate 32 and decyl-
p-coumarate 9, obtained by chemical esterification, for their antioxidant protection of EVO and Nigella sativa oils. The antioxidant capacity of decyl-
p-coumarate 9 in comparison to 32 for scavenging the superoxide anion and peroxide under physiological conditions was lower than their respective free acids. Overall, the decyl-
p-coumarate 9 displayed a higher ability to scavenge the superoxide anion and hydrogen peroxide; therefore, it was applied for oil-emulsion studies. At the applied test concentration, the decyl-
p-coumarate
9 and
p-coumaric acid did not show any difference in the decrease in hydroperoxide accumulation for EVO or Nigella sativa oils. The emulsions of these oils using 1% polysorbate 80 were prepared, and the C10 coumarate 9 was detected (>99.9%) in the oil phase, while the free
p-coumaric acid was mostly water-based (>42% in Nigella oil and >60% in EVO oil). The
p-coumaric acid and its decyl ester emulsion of Nigella sativa oil showed a higher capacity to scavenge superoxide anion and peroxide in comparison to their EVO-based emulsions, which can therefore help to develop skin care formulations of Nigella sativa oil [
55].
The pentyl and hexyl ferulates (28 and 29) did not significantly differ in their antioxidant activity for encapsulated linoleic acid in gum arabic or maltodextrin but were superior to ferulic acid. Gum arabic (E414) is primarily used in the food and soft drink industry as a stabilizer, while maltodextrin is processed from corn, rice, potato starch, or wheat and added as a food ingredient. The gum arabic suppressed the oxidation without the addition of ferulic acid or its esters. A higher level of suppression in the oxidation of linoleic acid was seen when higher amounts of hexyl ferulate 28 or ferulic acid were added. Based on the partition coefficients, the oxidation of linoleic acid was effectively suppressed due to the location of alkyl ferulates in the linoleic acid phase of the microcapsules [
56]. A similar trend was seen for these alkyl ferulates when added directly to linoleic acid due to higher solubility in the lipophilic medium [
57].
Rapeseed meal, the by-product of the oil industry, contains around 80% total phenolic content, like sinapine 48 (choline derivative,
Figure 3B), while in sunflower meal caffeic acid esters like chlorogenates make up 70% of the total phenolic content. Therefore, Laguna et al. prepared novel hydroxyalkyl sinapoyl and caffeoyl esters using aliphatic α,ω-diols with increasing chain lengths from 2 to 12 [
58]. Bis-cinnamyl esters derived from these aliphatic α,ω-diols (see
Figure 3B) were studied considering their potential for use in polymers derived from bio-sourced materials with antioxidant properties in food packaging. All the mono-hydroxyalkyl esters showed lower DPPH antiradical reactivity compared to their corresponding phenolic acids based on the steric hindrance in them. The oil-in-water emulsion CAT assay was used for antiradical activities to highlight the chemical reactivity/antioxidant activity in combination with the partition of the molecule between the three phases of the system and its interface. The bis-aryl esters gave improved antioxidant capacity in emulsion with an alkyl chain of 4 carbons and 2/8 carbons, after which it decreased for a longer chain (C12) due to intramolecular interactions leading to a negative effect on regeneration phenomena [
58].
Red blood cell (RBC) membranes are composed of 20% phospholipids in the outer layer and are composed of phosphatidylcholine and sphingomyelin and phosphatidylethanolamine and phosphatidylserine in the inner monolayer. Lopes et al. prepared lipophilic hexyl—C6, octyl—C8, and hexadecyl—C16 caffeates (16, 18, and 24), which showed better protection than caffeic acid against red blood cells’ (RBCs’) AAPH-induced oxidative stress. It was observed that such protection was directly dependent on the concentration used and the length of the alkyl chain of these esters. At lower concentrations of 2.5 and 5 μM, the C8 and C16 lipophilic caffeates 18 and 24 showed higher inhibition of hemolysis (or higher antioxidant activity) assuming the possible location within the RBC membrane, while at higher concentrations like 50 μM, the more hydrophilic C1–C4 caffeates showed the same trend. Using fluorescence anisotropy value changes of both the probes 1,6 diphenyl 1,3,5 hexatriene (DPH) and 1,6 diphenyl 1,3,5-hexatriene 4′ trimethylammonium tosylate (DPH-TMA) located at different depths in the liposome membrane, it was observed that at a higher applied concentration, these more lipophilic compounds had better interaction with the RBC membrane, causing changes to the membrane fluidity and disturbing its integrity, but such an effect was not seen with the parent caffeic acid [
59]. Considering the structural similarity of the sinapic acid choline product (sinapine 48,
Figure 3B), with the phosphatidylcholine, it may be possible to have interaction with the phospholipid layer, which may also have additional interaction with the RBC. The high antioxidant activity of sinapine compared with the parent sinapic acid makes it a suitable candidate as an antioxidant [
60,
61]. Moreover, sinapine is water-soluble and isolated in higher amounts from Brassica plants and can be designed and synthesized in higher quantities, making it an attractive option [
60,
61].
Red blood cells contain oxygen, ferrous ions, and PUFAs, which are prone to oxidation by free radicals. Therefore, the system was used for the assessment of isopropyl caffeate 12, which was designed and synthesized using an excess of isopropanol and sulphuric acid in >90% yields. The hemolysis of blood types A, B, and O was induced by isopropyl caffeate 12 at 500 and 1000 μg/mL concentrations. Further, isopropyl caffeate 12 provided protection from osmotic stress to A- and O-type erythrocytes. The erythrocytes were not protected from the oxidation of hemoglobin in the presence of phenylhydrazine but sequestered ROS produced by hydrogen peroxide, providing antioxidant behavior. However, in acute toxicity tests using 300 mg/kg body weight dose, moderate toxicity was seen via depressant effects on the central nervous system, while hepatoprotection was observed [
62].
The dynamic role of macrophages during myocardial infarction injury is via M1/M2 polarization, which can either produce proinflammatory mediators like interleukin 6 (IL-6) and tumor necrosis factor-α (TNF-α) or repair cardiac tissues by the uncontrolled deposition of extracellular matrix components. Biomarkers for the M1 phenotype are the increased level of inducible nitric oxide synthase (iNOS) resulting in the production of toxic NO, while M2 pro-inflammatory activity provides higher levels of arginase-1. This ratio of iNOS/Arg1 defines the macrophage M1 or M2 polarization. Synthetic
N-propargyl caffeamide 47 (
Figure 2) was able to selectively suppress the up-regulation of iNOS compared to cyclooxygenase-2 (COX-2) in lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophage cells. When cells were treated with 47, the up-regulation of M2 biomarkers (e.g., Ym-1 and arginase-1) and the down-regulation of M1 biomarkers (like iNOS, tumor necrosis factor-α (TNF-α), C-X-C motif chemokine 10 (CXCL10) and CD80) were observed.
N-propargyl caffeamide 47 promoted macrophage M2 polarization and reduced the myocardial infarct size in vivo. Mechanistically, 47 was able to decrease LPS-induced NF-ĸB activation and activate the nuclear factor erythroid 2 (NFE2)-related factor 2 (Nrf2)—Heme oxygenase-1(HO-1) pathway [
63].
3. The Anticancer Activity of AHCs—Structure Versus Activity
The herbaceous plant
Ipomoea asarifolia (ginger-leaf morning glory) belongs to the family Convolvulaceae and is common in tropical regions of Asia, Africa, and America. The dichloromethane root extract showed antiproliferative activity against multiple myeloma cells (RPMI8226). During the phytochemical investigation, 15 compounds were isolated, among which hexadecyl isoferulate 49 (
Figure 5) and hexadecyl caffeate 24 were reported for the first time. Alkyl cinnamates like hexadecyl-
p-coumarate 3, octadecyl-
p-coumarate 5, eicosyl-
p-coumarate 7, and octadecyl caffeate 27 were also isolated from the same source, along with other compounds. All isolated compounds were screened against a multiple myeloma cell line (RPMI 8226). Hexadecyl caffeate 24 was found to be the most potent, with an IC
50 value of 3.0 μM followed by a octadecyl caffeate 27 with 9.4 μM against RPMI 8226 cells (
Table 3). Since these IC
50 values were lower than 10 μM, these compounds were also tested against two other multiple-myeloma cell lines, MM.1S and MM.1R (IC
50 values > 10 μM in both cell types). The non-toxicity of 24 and 27 at 50 μM in normal B lymphocytes reveals the tumor cell’s specific activity. Although hexadecyl isoferulate 49, an already synthetic compound, was known to have an affinity for protein kinase C, implicated in cancer, the low isolation yields in this study may have hampered its further assessment. The SAR revealed that the presence of the dihydroxy group on the aromatic ring and the length of the side chain may be the reason for the inactivity of 3, 5, and 7 in the assay [
64].
Conversely, the tetradecyl and hexadecyl-
p-coumarate 2 and 3 and tetradecyl caffeate 23 were selectively active against MOLT-4 (human lymphoblastic leukemia) cells with IC
50 values of 0.123, 0.301, and 1.0 µM, respectively, by 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assay compared with the standard drugs doxorubicin and cisplatin (
Table 3) [
65]. This study evaluated 19 compounds based on C4, C14, C16, and C18 alkyl esters of
p-coumaric, ferulic, sinapic, and caffeic acids for their cytotoxic activity against four human cancer cells (HL-60, MCF-7, MOLT-4, and MES-SA/DX5). The lipophilization of these HCAs was beneficial as an improvement in the cytotoxicity was seen compared to the parent acids. The mechanism of action was derived by observing their effect on cell cycle alteration and apoptosis induction. The tetradecyl chain in the case of 2 and 23 may have provided the optimal membrane anchoring, thereby orienting the HCA and CA moiety toward its intracellular targets for maximum activity [
44].
Figure 5.
Anticancer alkyl cinnamates and cinnamides.
Figure 5.
Anticancer alkyl cinnamates and cinnamides.
Based on the cytotoxic results of the ethyl acetate fraction obtained from
Solanum torvum Swartz. fruit on the MCF-7 cells, methyl caffeate 10 was tested further to explore the molecular mechanism of action. The cytotoxic properties of 10 were investigated against five cell lines (MCF-7, A549, COLO320, HepG-2, and Vero cells). The methyl caffeate 10 showed potent cytotoxic properties against MCF-7 cells compared to the other tested cells (>50% cytotoxicity at a 2.46 µM concentration with an IC50 value of 0.62 µM). Based on Western blotting data, the Bcl-2 protein was downregulated, while Bax and Bid were upregulated, indicating the activation of the intrinsic mitochondrial pathway. Further increases in caspase-3, PARP, and cytochrome c release from mitochondria were detected, indicating that methyl caffeate significantly reduced the cell proliferation and the increased formation of fragmented deoxyribonucleic acid (DNA) and apoptotic bodies in MCF-7 cells. The docking studies displayed a stable bond of methyl caffeate 10 within the active sites of PARP1, B cell CLL/lymphoma-2 (BCL-2), E3 ubiquitin–protein ligase (MDM2), and tubulin, which further strengthened the observed biochemical results [
66], while the isopropyl caffeate 12 had the best antitumor activity in SW620 and HepG2 cells (with inhibition ratios of 78.0% observed in both cases) [
46].
Alkyl caffeates like octyl 18, phenpropyl 50 and decyl caffeate 20 (see
Figure 2 and
Figure 5), were tested in human cancer cells like colorectal carcinoma cells (COLO 205), hepatoblastoma cells (HepG2), and hepatocellular carcinoma cells (Huh7, PLC5, SK-Hep-1). The in vitro cell viability analysis revealed that COLO 205 cells were more affected compared to the other cell types. The highest IC
50 of 48 µM was seen for decyl caffeate 20, while octyl 18 and phenyl-propyl 50 both had IC
50~70 µM. The flow cytometric analysis of COLO 205 cells exposed to 20 showed that the number of apoptotic cells (sub-G1 phase) increased in a time- and dose-dependent manner with altered cell morphology. The intrinsic mitochondrial pathway was activated by the treatment of decyl caffeate, as seen by Western blot analysis. Decyl caffeate 20 stimulated an increase in protein expression levels of p53, Fas, FasL, AIF, and Apaf-1. Additionally, treatment with decyl caffeate 20 changed the expression levels of Bcl-2 family members and subsequently induced the activation of caspase-12, caspase-9, and caspase-3, which was followed by the cleavage of PARP [
67].
Motivated by the activity of octyl and decyl caffeate 18 and 20 on various cancer cells, Chen et al. were interested in further testing the lipophilic octyl, phenyl-propyl, and decyl caffeates on A549 cells. They observed that all three of these caffeates could significantly inhibit the A549 cell viability, with IC
50 ranging from 54.2 to 80.2 µM. The formation of apoptotic bodies was visualized by propidium iodide staining when A549 cells were treated with octyl and decyl caffeates (18 and 20). The apoptosis cascade was identified by the loss of mitochondria membrane potential and an increase in the protein levels of Fas, FasL, and Apaf-1. The activation of caspases-12, -9, and -3, followed by the cleavage of poly (ADP-ribose) polymerase demonstrated that these octyl and decyl caffeates (18 and 20) could induce cell apoptosis in A549 human lung carcinoma cells [
75]. Similarly, the synthetic octyl esters of FA and SA (30 and 46) were toxic to model intestinal cells Caco2, HeLa, and A549 at concentrations below 100 µM [
41].
Octyl caffeate 18 was cytotoxic toward the growth of several human cancer cells, the most prominent being myelogenous leukemia (erythroleukemia type) K562 and histiolytic lymphoma U937 cells. The cytotoxic mechanism of octyl caffeate via apoptosis in U937 cells followed in a dose- and time-dependent manner. Octyl caffeate was more effective in comparison to CAPE, the positive control, while caffeic acid was inactive. The mechanism of apoptosis was seen by DNA fragments, chromatin condensation, and the increased appearance of sub-diploid DNA (sub-G0/G1) detected by flow cytometry. For the caspase 3 inhibitors Z-DEVD-FMK and Z-Asp-CH2D-CB, pretreatment showed that apoptosis was caspase 3-dependent, as seen by the inhibition of DNA fragmentation [
68].
Nine synthetic caffeic acid derivatives, among which N-heptyl caffeamide 54, N-octyl caffeamide 55, (
Figure 5), and octyl caffeate 18 (
Figure 2) are of direct interest to our discussion, are considered. These were studied for their cytoprotective and antioxidant effects on tert-butyl hydroperoxide (t-BHP)-induced oxidative stress in HepG2 cells. The results indicated that all nine CA derivatives significantly inhibited t-BHP-induced cell death of HepG2 cells, and the rank in the order of cytoprotection potency for the three ester/amides was 18 > 54 > 55 >> CA. Their cytoprotective activity was associated with increased lipophilicity [
70].
Jayaprakasam et al. studied the effect of different alkyl esters of CA and FA (27 examples) ranging from C3 to C22 against different cancer cell lines. They identified C12 and C16 caffeates (22 and 24) and also C8 and C12 ferulates (30 and 34) as the optimum alkyl chain length against MCF-7, HCT-116 (colon adenocarcinoma), CCI-H460 (lung adenocarcinoma), SF-26 (CNS tumor), and AGS (gastric adenocarcinoma) cells with an IC
50 range of 2.5 to 18.7 µg/mL (
Table 3). Both the ferulates (30 and 34) and the caffeates (22 and 24) were found to be more active toward the HCT-116 (colon adenocarcinoma) cells, as indicated by their low inhibitory concentrations (
Table 3). The COX-1 and -2 inhibitory activity was dependent on the conformation of these compounds, which determines their interaction with the enzymes. It was observed that the C4 to C12 caffeates showed both COX-1 and -2 enzyme inhibition but were more specific toward COX-2 at a chain length >C12 [
69]. Pentadecyl ferulate (36) isolated from halophyte
Salicornia herbacea also possessed antiproliferative activity against HepG2 and A549 cells (IC
50 = 56 and 48 µM, respectively) by MTT assay [
20].
A comparative study of lipophilic hexyl caffeate 16 and hexyl ferulate 29 to the bioisosteric caffeoylhexylamide 53 and feruloyl hexyl amide 58 (
Figure 5) was carried out in three human breast cancer cell lines, which included estrogen-sensitive (MCF-7) and -insensitive (MDA-MB-231 and HS578T) cells. The caffeic and ferulic acids were non-cytotoxic to the tested cancer cells, but the lipophilic esters predominantly inhibited the MCF-7 cell proliferation and induced alterations of the cell cycle, as seen by a marked increase in the level of sub-G1 phase (apoptotic cells) in a time-dependent manner causing cell death. Further mitochondrial damage was tested using isolated liver mitochondria, which exhibit sufficient similarity with cancer cells and provide a toxicity profile for non-target organ cells. Overall, it was found that these tested lipophilic esters are non-toxic to the liver cells/fractions and can be developed as safe anticancer agents [
76].
Colon cancer development is a multistage process initiated by chronic inflammation associated with excess production of ROS or RNS by neutrophils. Based on their previous works, Tavares-da-Silva et al. tested three amides of caffeic, ferulic, and 3,4,5-trihydroxy cinnamic with n-hexylamine and compared them with the parent acids for the dual modulation of the oxidative burst in human neutrophils, preventing the inflammatory process, and also inhibiting the growth of colon cancer cells [
71]. The induction of the oxidative burst in human neutrophils by phorbol-myristate-acetate (PMA) was inhibited by hexyl caffeamide 53 followed by n-hexyl feruloyl amide 58 (IC
50 = 6.6 and 14 µM, respectively), which was better than their parent caffeic and ferulic acid (IC
50 ≥ 200 µM). The inhibition value of tri-hydroxy cinnamic acid hexyl amide was found to be higher than its parent acid (IC
50 = 36 v/s 19 µM, respectively). The inhibiting growth of two colon cancer cells with different genetic backgrounds and origin localization, WiDr (rectosigmoid) and C2BBe1 (descending colon), was evaluated for these molecules. The dose- and time-dependent effect for inhibition of both cell types was seen for 53 (IC
50 = 30.8 and 22.6 µM at 96 h in WiDr and C2BBe1 cells). The tri hydroxy cinnamic acid hexyl amide showed a similar dose-dependent effect only in WiDr cells (IC
50 = 31.7 µM at 96 h) [
71].
Based on the pharmacological activity of caffeic acid phenethyl ester (CAPE) isolated from propolis, Uwai and colleagues prepared caffeate esters (22 examples) with an alkyl chain from C1 to C22, ranging from straight-chain alcohols and branched alcohols, including geranyl and farnesyl alcohols, and tested their cytotoxic activity against RAW 264.7 macrophage cells. The EC
50 values gradually decreased as the lipophilic chain length increased. The C6–C18 esters’ cytotoxicity was found to be fairly constant between 1.0 and 2.6 µM. More pronounced cytotoxicity effects were seen in the case of C11 and C12 caffeate ester (21 and 22, IC
50 = 1.188 and 1.0 µM). A similar trend was seen in the inhibition of NO for the C6-C18 caffeate esters, with undecyl caffeate 21 being the most potent (EC
50 = 0.018 µM) [
72]. Uwai et al. further observed that previous reports had suggested that esters are labile in biological fluids. Hence, they separately pretested caffeic acid, undecanol, undecyl caffeate 21, and the mixture of caffeic acid + undecanol for 1 h before LPS treatment. Only undecyl caffeate 21 gave a significant difference compared to other tested compounds. Further, the bioisosteric C11 caffeamide 56 and 1-(3′,4′-dihydroxyphenyl)pentadec-1-en-3-one 59 (
Figure 5) also gave a similar inhibition of NO (EC
50 = 0.01–0.08 µM). Overall, they concluded that the ester linkage and length of the alkyl chain were important for the inhibition of NO [
72].
Further, the mechanisms of cytotoxicity and induced apoptosis using undecyl caffeate 21 and its parent compound, caffeic acid phenethyl ester (CAPE) in the human B cell leukemia cell line NALM-6, were evaluated. No significant effect after treatment with undecyl caffeate (concentrations > 0.3 μM up to 6 μM) was seen on the survival of normal lymphocytes, but the cell survival of NALM-6 cells was reduced. The IC
50 values for undecyl caffeate 21 were 10-fold higher in comparison to CAPE in 24 and 72 h (0.33 and 0.16 μM, v/s 5.39 and 1.74 μM, respectively). The mechanism of apoptosis was concluded based on the appearance of cleaved poly (adenosine diphosphate (ADP)-ribose) polymerase and activated caspase-3 after undecyl caffeate 21 treatment (0.1 to 1.0 µM). A broad-spectrum caspase inhibitor (Z-VAD-FMK) completely blocked the induced apoptosis by undecyl caffeate. The applied concentration- and time-dependent decrease in both the mitochondrial membrane potential (judged by the uptake of R123, a substrate of mitochondrial membrane permeability) and the downregulated Bcl-2 expression indicated the intrinsic pathway. The knockdown of the Bcl-2 protein by siRNA led to an enhanced level of induced apoptosis. All these mechanistic data pointed out the co-treatment and selective action option using undecyl caffeate for the therapy of leukemia via Bcl-2 protein inhibition [
73].
Several hydroxy cinnamic esters based on the phenethyl caffeate (CAPE) were designed, synthesized, and tested for the inhibition of cancer cell growth in breast and prostate cancer cells (eight examples). Among them, the 3,4,5-trihydroxy cinnamic acid decyl ester 61 (
Figure 5) at a 4 µM concentration induced apoptosis and was the most potent for inhibiting 98% of MCF-7 and 94% of PC-3 cancer cell growth. It was interesting to note that an increase in the alkyl chain linking the phenyl ring to the ester group from phenethyl to phenhexyl [C
6H
5-CH
2 to C
6H
5-(CH
2)
6] drastically improved the cytotoxicity from 14% to 41% in MCF-7 cells, while in PC-3 cells, this effect was less pronounced (62% to 80%) [
77].
Natural curcumin is known to interact with the protein kinase C (PKC) family of serine/threonine kinase isoforms through the C1 domain and modulate the PKC activity. The 4-hydroxy-3-methoxy phenyl moiety in curcumin is similar to that in ferulic acid. This structural feature was used by Mamidi et al. to design AHC (eight examples) with hexadecyl and octyl esters for the binding and molecular docking study [
74]. The binding study by the in vitro fluorescence quenching method indicated that octyl 3, 4-dihydroxy-5-methoxycinnamate 62 had a good binding affinity for the C1b subdomain of PKCϴ (EC
50 = 3.51 µM) and was comparable with octyl ferulate and caffeate 30 and 18 (EC
50 = 5.3 and 4.78 µM) and the corresponding hexadecyl-3,4-dihydroxy-5-methoxycinnamate 63 (EC
50 = 2.98 µM). The binding study with hexadecyl ester 63, 37, and 24 containing liposomes and proteins also showed comparable binding affinity (EC
50 = 0.54, 0.91, and 0.85 nM, respectively). The membrane localization of these hexadecyl esters in the liposomes was detected to be at the liposome surface and hence accessible for protein binding under the liposomal environment. The molecular docking studies supported the presence of both hydroxyl and methoxyl groups in these molecules, which were crucial for their interaction with PKCs [
74]. Building on this study, the authors further used these compounds to study in vitro effects in MDA-MB-231 and MCF-7 breast cancer cells, which show higher levels of PKCs [
78]. The hexadecyl 3, 4-dihydroxy-5-methoxycinnamate 63 translocated ~16% PKCα from the cytosol to the plasma membrane (comparable to that of curcumin at 17%) and presented an IC
50 of 4.13 μg/mL against MDAMB-231 cancer cells. Once over-activated, the PKC isoforms undergo proapoptotic mechanisms, and these effects were seen by the downstream activation of caspase-9 and caspase-3. The classical mitochondrial pathway of apoptosis with cytochrome c release from mitochondria to cytosol was noted [
78]. The results of these AHCs in the highly aggressive and metastatic triple-negative breast cancer cell line MDAMB-231 compared to the ER-positive and non-metastatic MCF-7 breast cancer cell line could provide promising anticancer therapy.
4. Therapeutic Role of AHCs in Alzheimer’s, Parkinson’s, and Neurodegenerative Diseases
Neurodegenerative diseases like Parkinson’s and Alzheimer’s can be caused by the combined effects from environmental factors like diet, exposure to occupational toxic metals, solvents, viral infections, genetics, and pesticides. Natural hydroxy cinnamic acid (HCA) derivatives like ferulic, caffeic, and coumaric acids are a fundamental part of the biosphere of the environment and have been tested as agents for the prevention and treatment of neurodegenerative diseases [
79]. 3,4-Dimethoxycinnamic acid isolated from coffee beans is a bioavailable component and has shown the inhibition (~90%) of α-syn fibrillation, and it also has anti-prion activity. Both of these are implicated in neurodegenerative diseases like Parkinson’s and Alzheimer’s [
79]. The design of multifunctional drugs combining the anti-oxidant molecular entities like caffeic acid, rosmarinic acid, and Trolox with choline (part structure of acetylcholine) was synthesized and evaluated for acetylcholinesterase (AChE) enzyme inhibition. Among these, the 3,4-dimethoxy cinnamoyl choline ester 64 (see
Figure 6) was the strongest inhibitor, with IC
50 values (7.3 μM) comparable to standard AChE inhibitors (galantamine-1.07 μM and rivastigmine-1.5 μM,
Table 4) [
80], while the caffeoyl choline trifluoroacetate 65 (see
Figure 6) showed lower activity (IC
50 = 91 μM) in comparison to the dimethoxy derivative. A possible reason for the higher activity of the 3,4-dimethoxy cinnamic choline ester may be favorable hydrophobic interactions within the active site of the AChE enzyme. The 3,4-dimethoxy group is also found in donepezil, the most prescribed drug for the treatment of all stages of AD [
81]. Further, the rosmarinic and Trolox esters also had low activity and were ascribed to bulky groups and unfavorable interactions within the active site. Rosmarinic acid was the best antioxidant, with an EC
50 value of 6.4 μM, while the choline ester improved the inhibition slightly (EC
50 of 4.3 μM, Trolox EC
50 = 13.2 µM). The additional catechol group in the rosmarinic structure may have contributed to the observed antioxidant effect in comparison with other tested molecules [
80].
The by-product of the
Brassica napus (rapeseed) oil extraction industry provides rapeseed pomace (RSP), which has the highest content of sinapine (109.1 mg/g RSP extract) and also contains sinapic, caffeic, ferulic, and syringic acids (0.159–3.91 mg/g RSP extract). The concentration of sinapine (see 48 in
Figure 3B) as a secondary metabolite present in RSP is dependent on the growing conditions, the country of origin, the rapeseed processing, and the extraction parameters. The defatted RSP extract harvested in the northeast of Scotland revealed more than 50% activity relative to the extract in the antioxidant assays. Furthermore, sinapine, with similarity to acetylcholine, inhibited acetylcholinesterase activity by 85%, while the phenolic acid mixture showed only 25% inhibition. The structural resemblance of sinapine and neostigmine concerning the trimethylammonium group led to their interaction with several important amino acid residues in the binding cavity of the AChE enzyme, giving comparable binding scores (BS) of −7.4 and −7.1 in the molecular docking [
82].
The neurodegenerative disorder called Alzheimer’s disease (AD) has been identified as one of the causes of memory loss (dementia) affecting elderly adults. The secretases (α-, β-, and γ-secretase) can cleave the amyloid protein precursor (APP), leading to extracellular deposits of amyloid beta peptide (Aβ) called amyloidal plaques. This aspect is the key feature of the neuropathology of AD. The various species of Aβ oligomers formed after cleavage can aggregate due to their hydrophobic nature and form senile plaques. These senile plaque deposits can induce neuronal death and cognitive dysfunction. Therefore, agents preventing the formation of Aβ aggregates can be used for the therapy of AD. Taguchi et al. designed and synthesized pentyl caffeate 15 (
Figure 2) and the bioisosteric ketone (1-(3′,4′-dihydroxyphenyl)non-1-en-3-one) 66 (see
Figure 6), which had the best activity (EC
50 = 2.58 and 2.82 µM, respectively;
Table 4) compared to other derivatives studied. Hexyl caffeate 16 was the next best alkyl-substituted molecule with EC
50 = 5.13 µM. Further, the bioisosteric change from pentyl ester to pentyl caffeamide 52 gave EC
50 = 11.4 µM, which was comparable to heptyl and nonyl caffeates 17 and 19 (EC
50 = 10.4 µM and 9.52 µM) [
83].
Nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF), or neurotrophins, maintain and develop the nervous system by promoting neuronal survival, proliferation, and differentiation. The therapeutic application of neurotrophins’ role can be focused on neurodegenerative diseases such as Alzheimer’s and Parkinson’s. Small molecules capable of mimicking the action of neurotrophins can effectively provide improved pharmacokinetic profiles. The increased lipophilicity and antioxidant capacity of caffeic acid esters (C1–C4) inhibited peroxide-induced neuronal PC12 cell death (40–60%) assessed at concentrations of 5 and 25 µM [
42]. To this end, Hosseini et al. prepared 30 ester derivatives of hydroxy cinnamic acids (HCAs) with different alkyl groups (C1–C12). Their neuroprotective effects in serum-deprived conditions and neurite outgrowth promotion in PC12 cells were studied. Only caffeic acid and all its alkyl esters, especially C10 and C12 caffeate (20 and 22), significantly promoted neuronal survival at 25 μm, while the other HCAs, like
p-coumaric, ferulic, and sinapic acids and their esters, did not alter cell survival. The lower alkyl esters, like propyl and butyl caffeates, also significantly increased the number of neurites by 25- and 22-fold at 5 μm [
84].
The biological effects of neurotrophins (NGF and BDNF) are mediated by receptor tyrosine kinases like tropomyosin-receptor kinases (Trk) with three Trk receptors, NTRK1, NTRK2, and NTRK3 (TrkA, TrkB, and TrkC). These Trks affect neuronal survival and differentiation through several signaling cascades. Hosseini et al. found that the neurotrophic mechanisms regulating the HCAs’ protective effects were via the phosphorylation of the tropomyosin-receptor kinases A (TrkA) receptor and its downstream signaling pathways (ERK1/2 and Akt). Among the eight caffeate esters studied, decyl caffeate 20 and dodecyl caffeate 22 caused the phosphorylation of extracellular-signal-regulated kinase (ERK1/2) and Akt serine-threonine kinase in PC12 cells [
85]. Dodecyl caffeamide 57 (
Figure 5), when applied at 25 µM, significantly increased survival in PC12 neuronal cells against serum-deprivation-induced cell death, along with the butyl, hexyl, and octyl caffeic amides. The butyl, hexyl, and octyl caffeamides (51, 53, and 55, see
Figure 5) also improved neuronal differentiation (at 5 µM), seen as neurite outgrowth, at a low dosage of nerve growth factor (NGF-5 ng/mL). The dodecyl caffeamide 57 induced ERK1/2 and increased AKT phosphorylation in PC12 cells at 5 and 25 µM (with or without NGF—5 ng/mL), while butyl caffeamide 51 only induced ERK1/2 at these concentrations. Finally, the docking analysis revealed that dodecyl caffeamide
57 displayed higher binding energy with phosphoinositide 3-kinase (PI3K) compared to caffeic acid [
86].
Figure 6.
The neuroprotective pharmacological activity of 3,4-dimethoxy cinnamoyl choline, caffeoyl choline esters, and other cinnamyl ester or amide derivatives.
Figure 6.
The neuroprotective pharmacological activity of 3,4-dimethoxy cinnamoyl choline, caffeoyl choline esters, and other cinnamyl ester or amide derivatives.
Parkinson’s disease affects the population aged > 60 years with the progressive loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) and can lead to the insufficient production of nerve growth factor. These NGFs are essential for neuronal system homeostasis by regulating the growth, maintenance, proliferation, and survival of neurons. In PD patients, the levels of neurotrophins (NGF and BDNF) are decreased, which can be achieved in mice study models on exposure to MPTP. This approach was used to study the effect of
N-propargyl caffeamide 47 (
Figure 2) in MPTP-challenged mice. The natural-product-derived 47 could potentiate or mimic the activities of neurotrophins and reduce motor impairments in MPTP-challenged mice. Further Western blot showed that 47 conserved the level of tyrosine hydroxylase (TH) in the midbrain substantia nigra pars compacta, increased NGF expression, and decreased proNGF accumulation. The production of NGF, BDNF, and neurotrophic factor tyrosine kinase receptors (Trks) is tightly regulated. The application of 47 at a dose of 15 mg/kg/day led to increased levels of NGF in the midbrains, including astrocytes, and also induced TrkA activation in MPTP-treated mice. NGF provides neuroprotective effects when bound to receptor TrkA and the activation of the extracellular signal-regulated kinase (ERK) and phosphatidylinositol 3-kinase (PI3K)/Akt pathways. At the same dosage, 47 phosphorylated PI3K/Akt, ERK1/2, CREB, and TrkA in the midbrain of MPTP-induced mice [
87].
During normal cell homeostasis/functioning, the Nrf2 forms a protein complex with Keap-1 in the cytosol. Several studies have shown the role of Nrf2 as a mechanism of resistance to disease-mediated oxidative stress [
88]. Therefore, under induced conditions or basal expression, several antioxidant response element (ARE)-dependent genes are controlled by Nrf2 to regulate the physiological outcomes of exposure to oxidants. The oxidative stress leads Keap1 toward oxidation, with the subsequent release of Nrf2. This free Nrf2 gets translocated into the cell nucleus and leads to the activation and expression of various antioxidant enzymes, phase II defense enzymes like HO-1, or transporters [
89]. The Nrf2/HO-1 pathway has gained therapeutic potential in the treatment of various neurodegenerative disorders including PD. Yang et al. reported that 47 treatment significantly increased the levels of nuclear Nrf2 in a concentration-dependent manner. To identify the target proteins of 47, a biotin tag was introduced within the 47 molecule via azide click chemistry. The binding proteins isolated from treated PC12 cells with this probe were predominately identified to be kelch-like ECH-associated protein 1 (Keap-1). Once the 47–Keap1 conjugates are formed, they induce the nuclear translocation of the Nrf2 transcription factor and the expression of antioxidant heme oxygenase-1 (HO-1). Further, 47 potentiates nerve growth factor (NGF) induced neurite outgrowth and diminished the 6-hydroxydopamine-induced production of neurotoxic reactive oxygen and nitrogen species exhibiting neuroprotective activities [
90]. This work also reinforces the work by Hosseini et al., with alkyl caffeates capable of mimicking the action of neurotrophins (NGFs) to provide improved pharmacokinetic profiles [
84].
Yet another interesting SAR development of the 47 derivative was achieved by combining the propargyl amine and piperidinyl fragments of an anti-AD ligand (Contilisant) with ferulic acid to give multifunctional
N-hydroxy-
N-propargylamide ferulamide hybrid, which was further modified with
N-ethyl piperidinyl fragment 67 (
Figure 6). The inhibition of hAChE (IC
50 = 2.63 µM) by 67 was found in the micromolar range and also showed lower DPPH radical-scavenging capacity than resveratrol, which can combat the oxidative stress seen in AD. Further, ferulamide 67 effectively chelated copper(II) ions, which are associated with amyloid β pathology [
91].
The novel molecule 68 (
E)-5-(1,2-dithiolan-3-yl)-
N-(3-(3-(4-hydroxy-3-methoxyphenyl)acrylamido)propyl)pentanamide (
Figure 6), designed by coupling ferulic acid, 1,3-diaminopropane, and lipoic acid (an organosulfur compound derived from octanoic acid), showed the best activity as a neuroprotective agent with moderate antioxidant activity [
92]. The observed neuroprotection against Aβ
1–42, high stability in simulated gastric fluid at pH 1.2, simulated intestinal fluid at pH 6.8, and human plasma (with half-life > 72 h), displayed by 68 reflect the potential for development of a new anti-Alzheimer agent.
Cognitive impairments can be devastating to quality of life, and thus, preventing or counteracting them is of great value.
Rhodiola rosea L., a perennial plant from the Northern Hemisphere (Europe and Asia), belongs to the family Crassulaceae, which grows naturally in higher-altitude regions. The roots, when consumed as a decoction, help the body adapt to stress and hence are considered adaptogens. Michels et al. studied the potential of the plant
Rhodiola rosea and identified one of the root constituents, eicosyl ferulate 40, as a memory enhancer across different species. Michels et al. found that 40 in dried root material from
R. rosea food supplements could improve the odor–taste reward-associative memory scores in larval Drosophila in a dose-dependent manner. They further found that 40 can compensate for the age-related decline in this appetitive memory in adult Drosophila flies. Acute feeding on food supplemented with
Rhodiola root material before training improved memory acquisition, while after training, feeding improved memory consolidation in honeybees. Since natural 40 extraction would need a lot of dried root material, a synthetic sample was generated and used for further studies. The synthetic 40 could partially compensate for both age-related memory decline in adult flies and genetically induced early onset loss of memory function in young flies. Further, in mice, 40 could increase the excitability in mouse hippocampal CA1 neurons. This led to more stable contextual fear memory in 3-month-old adult mice and increased memory scores in the tested 2-year-old mice. These memory-enhancer effects and the utility of the discovered 40 from
R. rosea have translational potential for clinical studies [
93]. In the same study, structure–activity relationships were derived from the structural changes to 40 using 4-hydroxy coumaric ester 7 and dihydro isomer (7,8-dihydro-ferulic acid eicosyl ester). Neither of these molecules showed memory enhancement scores, as seen for ferulate 40 supplementations [
93]. Recently, Michels et al. have identified the memory-enhancing compounds from
R. rosea using detail chromatography and spectral data and discussed their habit-forming or addictive potential [
94]. The physiological hydrolysis of 40 may produce ferulic acid and 1-eicosanol, which were also tested as effective rewards. The highest test concentration (7.06 μmol L
−1) of ferulic acid was found to be rewarding compared with the solvent control, while no such effect could be observed for 1-eicosanol.
In traditional Indian Ayurveda,
Withania somnifera (L.) Dunal, also known as “Ashwagandha” or Indian ginseng, is an adaptogenic plant capable of providing relief for neurological disorders. The methanolic root extract possesses GABA-mimetic activity. Therefore, extraction and isolation provided the most active compound, docosanyl ferulate 41, from
Withania somnifera and was able to enhance the GABAA receptor’s inhibitory postsynaptic currents with an IC
50 value of 7.9 μM [
95]. Docosanyl ferulate 41 was previously isolated and identified from roots of
Lupinus luteus, as well as
Pavetta owariensis stem bark [
94,
95]. As the docosanyl ferulate 41, the octadecyl (C18) ferulate 39, and eicosanyl (C20) ferulate 40 were originally isolated as an inseparable mixture; they were individually synthesized using a mixed anhydride obtained via a reaction of ferulic acid and ethyl chloroformate followed by a reaction with the fatty alcohols and DMAP and final deprotection for further studies [
95]. In a separate study, the aqueous extract of
W. somnifera containing withaferin A and with anolide A, the main constituents, did not activate the main inhibitory receptors GABA type A receptors (GABAAR) in the central nervous system (see reference 16 in [
95]). Anxiolytic drugs like benzodiazepine often lead to side effects like drowsiness, dependence after long use, impaired coordination, and so on. The docosanyl ferulate, 41, isolated from
W. somnifera, was further able to manifest potent anxiolytic properties without the side effects observed with typical benzodiazepine (BDZ)-like molecules [
96]. The dose-dependent anxiolytic effects of DF (at 0.25 and 2 mg/kg) were mediated via the modulation of the GABAAR complex activity by probable interaction with the BDZ binding site. This was ascertained by using flumazenil (FMZ), a BDZ-competitive antagonist (at 10 mg/kg), which reverses the DF’s effects, as seen in the elevated plus maze test. The behavioral and pharmacological characterization in mice showed that DF does not impair motor coordination, and anterograde memory (observed by the time spent on new object exploration) in comparison to BDZ [
96]. Drug addiction effects concerning the repeated intake of alcohol and morphine can lead to neuroadaptations of several neural circuits, leading to complications like substance abuse, multi-organ failure, and drug dependence. To study the effect of docosanyl ferulate on these neuronal processes, Maccioni et al. used the conditioned place preference (CPP) method, which allowed them to understand the rewarding properties of unconditioned stimuli. DF affected only the acquisition of ethanol- and morphine-induced conditioned place preference (CPP) but not the expression. The mitogen-activated protein kinase (MAPK) family consists of extracellular signal-regulated kinase 1/2 (ERK), which plays effective roles in signaling cascades and transmits signals to intracellular targets. They also play roles in maintaining neuroplasticity and gene expression. The expression of ERKs is related to rewarding areas of the human brain; hence, it was of interest to evaluate these effects. DF prevented the alcohol/morphine-induced ERK phosphorylation in the nucleus accumbens shell (AcbSh) of mice [
97].
Although the authors reporting the GABA receptor’s inhibitory activity for docosanyl ferulate had reservations about the high logP and solubility issues that may crop up later, the long-chain hydroxyl cinnamates, namely octyl ferulate or hexadecyl ferulate or eicosanyl-
p-coumarate or dodecyl ferulate, in pharmaceutical formulations have shown memory-enhancing activity in vivo, but a pronounced effect was seen with the administration of eicosanyl ferulate [
98]. The key to understanding the beneficial effects of long-chain alkyl groups for improving brain functions depends on their ability to cross the blood–brain barrier (BBB). Such long-chain alkyl lipophilic esters (MW of 40 is below 500) stand a better chance of overcoming this barrier by membrane permeation [
93]. It is also known that small molecules, proteins, or peptides with an MW greater than 500 Da are generally not able to pass the BBB [
99]. Therefore, to reach the brain, most molecules must cross the BBB through interactions with specific transporters and/or receptors expressed at the luminal (blood) side of the endothelial cells. Considering the potential of ferulates discussed above, there is a high probability that these may interact with transient receptor potential vanilloid 2 (TRPV2) and TRPV4 expressed in the human cerebral microvessel endothelial cells D3 cells [
100]. Similarly, the monounsaturated and polyunsaturated fatty acids (MUFA and PUFA) available after daily intake of brain superfoods like almonds, hazelnuts, and walnuts show cognitive improvement and the inhibition of β-secretase 1 (BACE1) [
101].
Natural isopentyl ferulate (69,
Figure 6) showed an anticonvulsant effect in mice (Mus musculus) when subjected to pilocarpine (400 mg/kg, intraperitoneal) and pentylenetetrazole (70 mg/kg, intraperitoneal) models of induced seizures. The protective effect against these induced seizures was obtained using isopentyl ferulate 69 at doses of 25, 50, and 75 mg/kg (intraperitoneal). Further, using the GABAergic antagonist, flumazenil, (at 5 mg/kg) pre-treatment significantly reversed the anticonvulsant action of diazepam (2 mg/kg) and 69 (75 mg/kg) in mice. This suggests that the isopentyl ferulate 69 could act on the modulatory site of benzodiazepine in the GABAA receptor in the mouse brain. The safety (or neurotoxicity) of 69 was assessed for behavioral changes, and the results obtained showed that the doses tested above did not induce significant changes in locomotor activity or motor coordination when compared with the control and diazepam groups. Therefore, isopentyl ferulate 69 can have a potential application against epileptic seizure therapy [
102]. Further, the same authors found no toxicity of 69 in
Artemisa salina. The evaluation of the anxiolytic-like effect and neuronal mechanism of action of isopentyl ferulate 69 was determined by the marble burying test in Swiss albino mice. It was seen that mice treated with 69 at 25, 50, 75, and 150 mg/kg body weight had a reduced number of marbles buried in 82%, 85%, 88%, and 94% of cases, respectively, in comparison with the groups treated with ascorbic acid (250 mg/kg) and diazepam (2 mg/kg), which decreased the number of marbles buried in 19.8% and 50.6% of cases. At the higher concentration of 69 (150 mg/kg), pre-treatment with flumazenil significantly reversed the behavioral activity of mice, as seen previously with the 75 mg/kg. However, the exact mechanism of binding between 69 and benzodiazepine receptor is yet to be deduced. The study also evaluated the antioxidant enzyme levels in the mouse hippocampus and found that 69 had a neuroprotective role, as seen by their increased levels [
103].
The leaves of
Pycnanthus angolensis (Welw.) Warb., Myristicaceae, are traditionally used as a memory enhancer and anti-aging agent in Nigerian ethnomedicine. The dimethyl-substituted cinnamic acid derivative named omifoate A [12-(4-hydroxy-3-methyl-oxo-cyclopenta-1,3-dien-1yl)-11-methyl-dodecyl]-(
E)-3-(3,4-dimethylphenyl)prop-2-enoate 70 was isolated from this plant with IC
50 of 6.51 µg/mL (AChE), and 9.07 µg/mL (BuChE), respectively. These cholinesterase inhibitory activity results showed that the traditional preparations of this plant may be responsible for its memory-enhancing action [
104].
5. Antimicrobial and Antifungal Activity of AHCs
Cinnamic acids and related molecules are commonly found in preparations involving cinnamon, storax, and propolis and are partly responsible for the observed antimicrobial effects observed in these preparations. Guzman has comprehensively reviewed the effects of natural cinnamic acids, their esters, amides, aldehydes, and alcohols in several bacterial and fungal species, collating their minimum inhibitory concentration (MIC) against the reported microorganisms [
105]. For an effective understanding of the bio-application of alkyl cinnamyl ester for antimicrobial activity, it seems important to understand the outer cell wall structure of Gram-positive and negative microorganisms. Most Gram-negative bacteria’s cell structures are made up of a thin peptidoglycan cell wall, which is surrounded by a lipopolysaccharide (LPS) outer membrane. This LPS molecule causes contact with the human innate immune system to trigger an endotoxic shock, while Gram-positive bacteria have several layers of peptidoglycan and lack an outer membrane [
106]. The outer membrane proteins are generally composed of lipoproteins and β-barrel proteins, which form hollow cylindrical structures called porins, allowing the diffusion of molecules up to 700 daltons [
106].
The fruits of
Solanum torvum Swartz. (Solanaceae) are traditionally used for the treatment of bacterial and fungal infections. Methyl caffeate (MC) 10 isolated from the
Solanum torvum Swartz fruit has previously shown antiproliferative activity against cervix adenocarcinoma and anticancer activity in lung and leukemia cell lines. Therefore, Balachandran et al. tested MC for antimicrobial activity using five Gram-positive bacteria, six Gram-negative bacteria, seven clinical isolates, and four fungi. The anti-mycobacterial activity was screened against two Mycobacterium strains (
Table 5). A moderate activity of methyl caffeate in comparison to the control drugs used was seen (inhibition zone—0 to 22 mm). The lowest minimum inhibitory concentration (MIC) values of methyl caffeate were seen against Gram-negative bacteria
Proteus vulgaris (50 µg/mL) and
Klebsiella pneumoniae clinical isolate (25 µg/mL). Methyl caffeate 10 showed prominent antimycobacterial activities against two strains of
Mycobacterium tuberculosis (H37Rv and RifR) with 8 µg/mL compared with control drug rifampicin (MIC—0.12 and 32 µg/mL) [
107]. Mycobacterium is classified as Gram-positive based on the cell wall structure, which can be composed of both Gram-negative and -positive types of lipid structures [
106].
Dioscorea, commonly known as the “yam” taxon, is well known for its phytochemical diversity, and rhizomes of some species are used as food and medicine. (
E)-32-((3-(3-hydroxy-4-methoxyphenyl) isoferuloyl) oxy) dotriacontanoic acid 71 (see
Figure 7) was isolated from the chloroform extracts of
Dioscorea cotinifolia and showed antibiotic-potentiating activities determined using two MDR
S. aureus strains. The MDR
S. aureus strain SA-1199B possessed the well-characterized and overexpressed efflux protein, namely the NorA MDR pump, which is resistant to fluoroquinolones, while the XU212 strain expresses the TetK efflux protein, which is tetracycline-resistant. The ω-hydroxy fatty acid isoferulic ester 71 was very active at 100 mg/L and resulted in an 8-and 16-fold reduction in the MIC of norfloxacin and tetracycline, respectively [
108].
Figure 7.
Antimicrobial, antifungal, antiplasmodial, and antiviral activity of alkyl hydroxy cinnamates and cinnamides.
Figure 7.
Antimicrobial, antifungal, antiplasmodial, and antiviral activity of alkyl hydroxy cinnamates and cinnamides.
A recent European Medicines Agency (EMA) monograph advised the traditional use of rhizomes from
Agropyron repens (commonly called couch grass, quack grass, or graminis rhizoma) for the treatment of urinary tract infections. The acetone extract indicated significant antiadhesive activity against the bacterial attachment to human T24 bladder cells at concentrations > 250 μg/mL. The identification of hexadecyl-
p-coumarate 3 from the bioassay-guided isolation was responsible for inhibiting the
Escherichia coli strains 2980 and uropathogenic
E. coli (UPEC) NU14 to T24 bladder cells (IC
50 of about 1 mmol;
Table 5). A specific invasion assay revealed that 3 reduced the bacterial UPEC invasion into the bladder T24 cells. Beydokthi et al. reasoned that the presence of 3 in the rhizomes may be very low (0.1%) as detected by mass spectrometry, and extraction of such lipophilic compounds by a hydroalcoholic solvent mixture or water is limited and difficult. Therefore, they chemically synthesized 3 along with synthetic structural analogs octyl-
p-coumarate 72 and hexadecyl cinnamate 73 (
Figure 7). Additionally, the structure–activity relationships were derived using these synthetic structural analogs. Their antiadhesive activity indicated that a shorter alkyl chain (octyl) at the ester function reduced activity, and the lack of hydroxylation of the phenyl moiety (cinnamate vs.
p-hydroxy) eliminated the antiadhesive activity [
109].
The onychomycosis infection of the nails is caused by a complex of species including
Fusarium oxysporum, Fusarium solani, and
Fusarium fujikuroi. Prenyl-
p-coumarate (
p-Coumaric acid 3,3′-dimethyl allyl ester), 74, a component of propolis, showed the best activity among 13 tested cinnamates (esters and ethers), while the parent
p-coumaric, caffeic, and ferulic acid did not show any effect on the growth of clinical isolates of
Fusarium spp. [
110]. The values obtained for the tested pathogenic strains, MIC and LD50, for 74 were comparable to those obtained for fungicides terbinafine (TRB) and amphotericin B (AmB) by fungal growth quantification.
Similarly, octyl caffeamide (55) had specific inhibition on the growth of oral microbes like
S. aureus and Methicillin-resistant
S. aureus (MRSA), as seen from the MIC and MBC values (
Table 5). The growth inhibition of these organisms was delayed in the presence of 55 at an increasing concentration by at least 24 h. The expression of drug resistance genes of MRSA (mRNA expression of mecR1, mecI, and mecA) was inhibited in the presence of 25 and 50 µM of 55, along with the decreased ability for MRSA biofilm formation. The improved stability over a wide pH range and temperature was observed for 55, as judged by the inhibition zones of
Streptococcus mutans [
111]. 4-methoxy-
N-butyl cinnamide 75 was the most potent antifungal (MIC 0.08–0.16 mg/mL) against all
Sporothrix brasilensis isolates (
Figure 7) [
112].
Microbial lipopolysaccharide (LPS) stimulates acute inflammation that can lead to sepsis and acute lung injury, a common cause of death in hospitals’ intensive care units. Based on the dimeric cinnamides discussed earlier, Chen et al. designed and synthesized several mono- and dimeric cinnamides, among which
N,
N′-di-(2,4-dimethoxyphenyl)-1,2-diaminoethane 76 (
Figure 7) could inhibit the TNF-α and IL-6 production induced by LPS in macrophages.
N,
N′-di-(2,4-dimethoxyphenyl)-1,2-diaminoethane 76 could block LPS-induced MD2/TLR4 pro-inflammatory signaling activation in vitro. Further, it directly interacts dose-dependently with MD2 proteins. This was confirmed by a competitive co-binding experiment with the fluorescent probe bis-ANS, which was displaced and showed a lower intensity of fluorescence. In vivo sepsis experiments with mice showed that using 76 at a dose of 10 mg/kg before LPS injection prevented death compared to the LPS-only group. The docking experiments revealed the binding of 76 with Arg90 and Tyr102 residues in MD2 and inhibited the MD2/TLR4 complex formation [
113].
Lauryl ferulate (LF) or dodecyl ferulate 34 was assessed for the antibacterial activities in vitro against three food-related bacteria (Gram-positive
Listeria monocytogene and
S. aureus and Gram-negative
E. coli). Probably due to the higher lipophilicity of the lauryl alkyl part, LF exhibited a stronger antibacterial activity against
E. coli (zone of inhibition—25 mm) than the Gram-positive (21.5–23.2 mm) bacteria. This was accompanied by the lowest MIC value (1.25 mM) for
E. coli followed by
L. monocytogenes (2.5 mM) and
S. aureus (5 mM). The corresponding MBCs for
L. monocytogenes,
S. aureus, and
E. coli were 10, 20, and 5 mM [
114].
Hexyl ferulate 29 was the most active in a series of ferulates (ethyl, butyl, octyl, decyl, and dodecyl) against
E. coli ATCC 25922 and
L. monocytogenes ATCC 19115 with MICs of 1.6 and 0.1 mM and MBCs of 25.6 and 0.2 mM, respectively. With food-related applications in mind, Shi et al. studied the detailed mechanism of 29’s antibacterial actions against
E. coli. It had a higher affinity for cell membranes compared to ferulic acid, and the partial cell lysis and inhibition of the growth of
E. coli caused by 29 was indicated by the growth and time–kill curves. This was confirmed a by higher uptake of propidium iodide, the leakage of cell constituents, the visually observed cell elongation and cardiolipin microdomains, and the membrane hyperpolarization by zeta potential. A binding to minor grooves of
E. coli DNA to form complexes and anti-biofilm formation activity was also observed [
115].
Further, Shi et al. used hexyl ferulate 29 as a multi-functional food additive to effectively maintain the sensory quality of sturgeon caviar. As seen earlier and confirmed in this study, the cell membrane morphology and hyperpolarization of
L. monocytogenes cells treated with 29 were altered. The occurrence of cell lysis and a significant inhibition of the growth of
L. monocytogenes caused by 29 was seen by growth curves and time-kill assay (lowest MIC of 0.1 mM and lowest MBC of 0.2 mM for
L. monocytogenes) values. The membrane integrity and permeability were compromised on treatment with 29, judged by the PI uptake analysis and massive leakage of cell constituents (K+, proteins, and nucleotide). The cell membranes of
L. monocytogenes cells treated with 29 showed alterations in morphology and membrane hyperpolarization, confirming the cell membrane disruption. SDS page analysis of protein bands showed strong intensities in untreated bacteria while the 29-treated bacterial proteins faded or disappeared. The biofilm formation was reduced by 2.2 times in
L. monocytogenes using 1/2 × MIC of 29. The artificially inoculated Amer sturgeon caviar with
L. monocytogenes (10
2 CFU g
−1) grew to 8.4 Log CFU g
−1 in 7 days of incubation at 10 °C, while the 1 × MIC 29-treated Amer sturgeon caviar growth was recorded at 1.2 Log CFU g
−1 at 10 °C for 7 days. A sensory evaluation by 15 trained judges for the general appearance, color, texture, and flavor of the treated samples was awarded scores ranging from 7.2 to 8.8, while lower scores were attributed to the presence of off-flavors. Therefore, the dual-mode antioxidant and antibacterial 29 was effective against
L. monocytogenes as an effective and safe multifunctional food additive [
116].
Song et al. recently designed and synthesized ferulic acid esters with different fatty alcohols (including 1-propanol, 1-hexanol, nonanol, and lauryl alcohol) by the enzyme catalytic use of Novozym 435. The increase and decrease in the cut-off factor for antibacterial activity were observed based on the fatty alkyl chain of the alcohol used. Among the four ferulates, hexyl ferulate 29 was judged as the best antibacterial molecule with activities against
E. coli and
P. aeruginosa (MIC was 0.5 mg/mL, and 0.4 mg/mL, respectively). The hexyl ferulate 29 also showed similar antibacterial activities against
S. aureus (MIC = 0.4 mg/mL). The MIC of
Bacillus subtilis (1.1 mg/mL) with hexyl ferulate 29 was comparatively found to be lower than that observed for the other three studied microorganisms. A further mechanism of action for hexyl ferulate was studied in
P. aeruginosa considering the strong effect on its cell wall permeability with the leakage of the cell contents and biofilm inhibition [
117].
The alkyl ester side-chain length can influence the activity of caffeic acid alkyl esters (ethyl, butyl, hexyl, octyl, and decyl) against
E. coli and
S. aureus. Therefore, their mode of action was judged by indices like changes in physicochemical surface properties (an increase in bacterial surface hydrophobicity for hexyl and octyl esters, zeta potential) and intracellular potassium leakage, along with MIC and MBC. The trend of a steady decrease in MIC and MBC values as the alkyl chain length increased (C2 to C10) was noted. Octyl caffeate 18 and decyl caffeate 20 had the best activity (MIC—0.1 mM against both
E. coli and
S. aureus). The lowest MBC of butyl caffeate (3.25 mM) against
E. coli indicated that a medium chain length was more effective against the Gram-negative bacterium. The action of these esters was dependent on the outer cell wall structures; hence, Gram-negative
E. coli was less susceptible than Gram-positive
S. aureus. The potassium ion release was significantly increased in
S. aureus exposed to hexyl, octyl, and decyl caffeates compared to the control, while it was not significant in the case of
E. coli [
118].
Caffeic acid and eight of its esters were tested for antifungal potential against
Candida albicans ATCC 90028 and 9 clinical isolates. Propyl, butyl, and hexyl caffeate (13, 14, and 16) showed the best antifungal activity against the
C. albicans ATCC 90028 (MIC for 13 and 14 was 15.62 µg/mL and for 16 was 7.81 µg/mL). These three were taken for tests against nine clinical isolates, wherein propyl caffeate (13) showed the overall better activity with an MIC of 7.81 to 62.5 µg/mL compared with the butyl 14 (31.25–125 µg/mL) and hexyl caffeate 16 (HC-7.81 ≥ 250 µg/mL). Interestingly among the nine clinical isolates tested, 13 and 16 had similar MIC and MFC—7.81 µg/mL—against the same two clinical isolates. Since many antifungals are routinely used for the clinical treatment of oral candidiasis, a synergism assay with fluconazole and nystatin was also performed. The combination of propyl caffeate 13 and fluconazole reduced the MIC of fluconazole with a synergistic effect of up to 64-fold. Finally, 13 was non-cytotoxic against oral keratinocytes (NOK cells—IC
50 value = 420 µg/mL), and the combination with fluconazole and nystatin also had high selectivity for yeast cells and low toxicity to NOK cells [
119].
Melanin pigment synthesis and its protective nature help several fungal organisms like
Cryptococcus neoformans with pathogenesis and colonization. Melanized cells of such fungal colonies offer more resistance to treatment with fluconazole (FCZ) and amphotericin B (AmB). Infection with
C. neoformans can give rise to pneumonia and meningoencephalitis in immunocompromised people. To have a structure–activity assessment, natural-product-derived compounds like
p-coumaric esters, phenylpropanones, and allylic phenols were tested for antimelanogenic and antifungal activities. Among them, ethyl-
p-coumarate 80 and the bioisosteric 1-(4-hydroxyphenyl)hex-1-en-3-one 86 (see
Figure 8) gave effective tyrosinase inhibition activity and also had a higher disk diffusion index level of 2.58 and 2.92, compared to the positive control (Timol-1.75). The structural similarity between the
p-coumaric acid ester and phenyl propanone and the amino acid L-tyrosine (substrate for melanin biosynthesis) may have influenced the inhibitory activity. The administration of antifungals like AmB can generate ROS, which is neutralized by melanin. Therefore, when combined with AmB, ethyl-
p-coumarate 80 showed a 16-fold concentration reduction of AmB and no appreciable cytotoxicity to murine fibroblasts’ NCTC cells. The inhibitory action of 80 on melanin biosynthesis may have increased the susceptibility of the cells to the oxidative stress generated by AmB [
120].
Figure 8.
Natural and synthetic alkyl hydroxy cinnamates, cinnamides, and polyamine derivatives as anti-tyrosinase agents.
Figure 8.
Natural and synthetic alkyl hydroxy cinnamates, cinnamides, and polyamine derivatives as anti-tyrosinase agents.
6. Antiplasmodial and Antiviral Activity of AHCs
Leishmaniasis is a chronic parasitic disease classified as a neglected tropical disease (NTD) by the World Health Organization (WHO) and forms part of the eradication strategy to achieve Goal 3 of the Sustainable Development Goals of the United Nations. Leishmaniasis is caused by the protozoa of the Leishmania genus transmitted by the bite of phlebotomine sandflies. This neglected tropical disease (NTD) is endemic to East Africa; South America, especially Brazil; the Eastern Mediterranean region; Europe; and South East Asian countries. The Leishmania parasites consist of two life forms, amastigotes and promastigotes, depending on the stage in their life cycle [
121]. Lopes et al. designed and synthesized 12
p-coumaric esters (methyl, ethyl, propyl, isopropyl, methoxyethyl, butyl, n-pentyl, isopentyl, hexyl, dodecyl, 4-methyl benzyl, and 4-isopropyl benzyl) for testing against antiparasitic activities. Among these tested derivatives, eight cinnamyl esters presented antiparasitic activities, with the highest potency against the
Leishmania braziliensis amastigote form observed for hexyl
p-coumarate 85 (EC
50 = 4.14 μg/mL, Amphotericin B EC
50 = 0.3 μg/mL,
Table 5). In the case of
Plasmodium falciparum activity, only the methyl
p-coumarate demonstrated the highest bioactivity (EC
50 = 64.59 μg/mL; Chloroquine—EC
50 = 3.35 μg/mL) among the 12 tested compounds. The molecular docking analysis revealed key enzymes in the development of
L. braziliensis like aldehyde dehydrogenase (ALDH), mitogen-activated kinase protein (MPK4), and DNA topoisomerase 2 (TOP2), which may be inhibited by 85 via van der Waals and hydrogen bonds or hydrophobic interactions [
122].
Octyl caffeate 18 was the most active in the leishmanial activity against axenic amastigotes of
Leishmania panamensis with an EC
50 of 2.3 µg/mL (
Table 5) but was equally cytotoxic to U937 cells (LC
50 = 9.9 µg/mL). Further, it was tested for leishmanial activity against intracellular amastigotes of
L. panamensis and gave comparable EC
50 values (3.2 µg/mL). The positive control Amphotericin B in both assays gave EC
50 values of 0.05 and 0.06 µg/mL, respectively [
123]. The high leishmanial activity and cytotoxicity (in L-6 cells) for octyl caffeate 18 were independently verified by Bernal et al. in
Leishmania donovani [
124]. The caffeate esters with n-propyl, isopropyl, n-butyl, and tert-butyl groups were active against both tested parasites in vitro (
L. donovani and
Trypanosoma brucei rhodesiense) [
124].
Lopes et al. further tested their set of 12
p-coumaric esters against another neglected tropical disease (NTD) caused by a Trypanosoma species. Infection with the
Trypanosoma cruzi protozoan is generally transmitted by triatomine bugs (vector), causing Chagas disease or American trypanosomiasis, responsible for chronic and systemic infections in millions of people. The parasitic life forms are called amastigotes or epimastigotes, which develop intracellularly and then differentiate into the blood trypomastigotes. The best activity was observed for pentyl
p-coumarate 84 (IC
50 = 5.16 µM; and 61.63 µM) against epimastigote and trypomastigote forms, respectively (
Table 5). The cell viability of host LLC-MK2 cells in the presence of an increasing concentration of pentyl-
p-coumarate 84 gave an IC
50 of 123 µM. Analysis by scanning electron microscopy revealed epimastigote cell death by necrosis as a result of the increase in the % of 7-AAD labeled cells when treated with pentyl-
p-coumarate 84 (IC
50 and 2 X IC
50 concentrations of 5.164 and 10.329 µM). The 7-AAD molecule does not penetrate cells with intact membranes, and this was confirmed by noting the loss of cellular integrity by scanning electron microscopy. An increased production of ROS and the loss of mitochondrial membrane potential were noted after treatment with 84. The docking analysis revealed that the observed activity may operate by utilizing two mechanisms, one by linking with aldo-keto reductases (AKR) or the second with key
T. cruzi development enzyme cruzain (CZ). The binding was favored by van der Waals and hydrophobic interactions between ligands and receptors [
125].
Ocimum sanctum or holy basil (Tulsi) is an adaptogenic plant that traditionally has several health benefits in the Indian Ayurveda system. The HIV-1 Reverse Transcriptase (RT) enzyme is multifunctional with DNA polymerase (DP) and ribonuclease H (RNase H) activities. A bio-guided fractionation using an HIV-1 Reverse Transcriptase (RT)-associated RNase H inhibition assay led to the isolation of tetradecyl ferulate 35 from the dichloromethane extract of the
Ocimum sanctum leaves along with triterpenes and showed an RNase H IC
50 value of 12.1 µM but had >100µM in the RDDP assay (the dichloromethane extract had an RNase H activity of 4.2 µg/mL). Considering the dual RT activity, it was pertinent to develop natural-based compounds having activity against both. Therefore, Sonar et al. designed and synthesized 18 hydroxy cinnamic acid derivatives based on tetradecyl ferulate 35, including the caffeate moiety, and tested them all against both RDDP and RNase H. The most active molecules were oleyl (C18:1) caffeate 78, geranylgeranyl (C20) caffeate, and oleyl (C18:1) caffeamide 77 (RNase H IC
50 = 1.06, 1.04, and 0.68 µM; RDDP IC
50 = 1.6, 1.5 and 2.3 µM, respectively,
Table 5) (
Figure 7). The bioisosteric replacement of ester to amide was noticeable with a further improvement in the RNase activity for oleyl (C18:1) caffeamide 77. They initially concluded that the ferulic ester structure with an aliphatic chain is important for activity in comparison with vanillin or ferulic acid. Secondly, the SAR based on the 18 compounds revealed that the dihydroxy group and the introduction of double bonds in a long alkyl chain aliphatic chain like oleyl or geranylgeraniol is required for improving the dual RT activity [
17]. The molecular modeling studies indicated the strong inhibitory activity toward both RNase H and DNA polymerase possibly occurred by binding at the two allosteric sites, one located close to the NNRTI binding pocket and the other near the RNase H catalytic site [
17].
The development of anti-HCV compounds based on the multifunctional caffeic acid phenethyl ester (CAPE) resulted in the testing of five alkyl-substituted caffeic ester derivatives on hepatitis C virus (HCV) replication. Based on the EC
50 values, the SAR for anti-HCV activity indicated that the length of the n-alkyl side chain and the catechol moiety were important. The most potent was octyl caffeate 18 for the inhibition of viral replication in all tested replicon cell lines at similar levels. However, it exhibited the lowest EC
50 value of 1.0 mM and an SI value of 63.1 for strain Con1 replicon cells of genotype 1b (
Table 5). Noted synergistic combinations with known anti-HCV drugs like interferon-alpha 2b, daclatasvir, and VX-222, but not with telaprevir or danoprevir, can formulate its use in the anti-HCV chemotherapy [
126].
In traditional Brazilian medicine, teas made from the leaves and bark of
Gallesia gorazema (a garlic plant in Brazil) are used for relief from various ailments (antispasmodic, anthelmintic, antihemorrhagic, and febrifuge agents). The crude leaves of this plant provide a remedy in the treatment of gonorrhea, rheumatic pain relief, abscesses, and orchitis. The dichloromethane root extract (DRE) and ethanolic leaf (ELE) extracts displayed significant anti-nociceptive and anti-inflammatory activities in in vivo experiments with mice. The DRE was most active against the herpes simplex viruses HSV-1 and HSV-2, with a selective antiviral effect against HSV-1. Further, DRE fractionation led to the isolation of 28-hydroxyoctacosyl ferulate, with 43 having strong antiviral activity against HSV-1 (EC
50 = 21.6 μg/mL,
Table 5), with a selective index above 9 [
23]. Docosanol (C22 fatty alcohol) is traditionally used as an emollient (CosIng ref No. 74589), emulsifier, and thickener in cosmetics. A 10% docosanol cream finds applications to treat cold sores (infection with Herpes labialis); therefore, such natural products and ester with ferulic acid can have further potential for effective anti-herpes therapy.
8. Natural and Synthetic AHC as Anti-Tyrosinase Agents and Their Applications
p-Coumaric acid is a known inhibitor of tyrosinase, an enzyme involved in the initial steps of melanin synthesis in humans and other species [
132,
133]. The inherent low lipophilicity reduces the penetration through the skin and its potential efficacy as an anti-melanogenic agent [
133]. Therefore, Varela et al. showed that the ethyl, n-propyl, isopropyl, and n-butyl-
p-coumarates (80–83) have tyrosinase inhibition and can function as anti-melanogenic agents in in vitro, in silico and ex vivo assays. The compounds showed higher tyrosinase inhibitory activity (IC
50 = 95, 124, 221, and 69 µM) than the parent acid (IC
50 = 296 µM) (
Table 6). Further, bioisosteric n-butyl coumaramide 87 (
Figure 8) also had better activity (IC
50 = 209 µM) compared to coumaric acid. Docking analysis revealed that these
p-coumaric esters are competitive inhibitors per se and may act as prodrugs for efficient transport and subsequent hydrolysis. Moreover, the isopropyl coumarate 82 showed efficacy in reducing melanin deposition in human skin tissue at a 0.1% concentration [
134]. Another report showed that ethyl-
p-coumarate 80 isolated from
Camellia pollen had non-competitive inhibitory activity (IC
50 = 4.89 μg/mL) and was about 10-fold stronger than the standard arbutin used (IC
50 = 51.54 μg/mL). The ethyl-
p-coumarate 80 did not interact with the copper ions but induced a conformational change in the catalytic region of the tyrosinase enzyme [
135]. The structural similarity between
p-coumaric acid and its derivatives and two tyrosinase enzyme substrates, namely tyrosine and 3,4-dihydroxyphenylalanine, can provide competitive inhibition of tyrosinase activity at the sites of the enzyme.
Khoshneviszadeh et al. designed cinnamic acid derivatives based on hydroxy cinnamides with 4-(2-Aminoethyl) morpholine to generate 19 cinnamides with antityrosinase activity. The authors designed the cinnamide fragment with morpholino ethyl moiety, assuming additional hydrogen bonding and hydrophobic interactions with the enzyme. This was indeed seen by the H-bonding of the NH group with Glu256 at a distance of 1.75 Å in the binding site of mushroom tyrosinase. Further, the morpholine hydrophobic interaction was seen with Val248 and His244 at 4.42 and 5.32 Å. The 3-chloro substituted cinnamyl amide
88 (
Figure 8) gave the best activity against mushroom tyrosinase with IC
50 = 15.2 µM, comparable to kojic acid (IC
50 = 14.4 µM), while the second best was 3-methyl 89 with IC
50 = 21.3 µM [
136]. Previously, this type of morpholine-appended approach was used to target the melanosome. The morpholine group can be protonated and accumulated in both melanosomes and lysosomes because they have an acidic pH (about pH 4.2–4.6), and the tyrosinase enzyme is normally present in melanosomes [
137].
Putrescine (1,4-diaminobutane) is a polyamine conjugated with ferulic acid or coumaric acids to form di feruloyl putrescine (DFP) 90 and dicoumaroylputrescine (DCP) 91 constituents of corn bran derived as a by-product of the corn milling process. Recently, DFP has been identified in broa, an ethnic maize bread made and consumed in Portugal [
138]. The functional benefits of these DFP and DCP as corn bran isolates were investigated by Choi et al. as useful anti-melanogenic agents. DFP 90 inhibited tyrosinase (IC
50 = 291 µM) while DCP 91 (IC
50 = 181 µM) was the most potent toward tyrosinase enzyme activity with L-tyrosine as the substrate compared to L-ascorbic acid (IC
50 = 331 µM;
Table 6). The most potent inhibitor for melanin synthesis in B16 melanoma cells was
90 (IC
50 = 733.64 µM) compared with arbutin (IC
50 = 1110.4 µM) [
139].
p-coumaric acid and its derivatives have been shown to have anti-melanogenic activity [
133]. Therefore, it was interesting to note that the dimeric
N,
N′-di-
p-coumaroyl-1,2-diaminoethane 94 was the most potent molecule to inhibit mushroom tyrosinase activity (IC
50 = 5.7 µM) compared with
p-coumaric acid and the well-known natural tyrosinase inhibitor kojic acid (IC
50 = 200 µM and 75 µM). Similarly, the dimeric
N,
N′-di-(4-methoxy-3-hydroxyphenyl)-1,2-diaminoethane 95 was equally potent (IC
50 = 8.5 µM) compared to kojic acid and isoferulic acid. Further, the dimeric
N,
N′-di-
p-coumaroyl-1,2-diaminoethane 94 inhibited 14% of melanin synthesis in normal human melanocytes more efficiently at 10 µM than kojic acid (17.7% inhibition at 300 µM) [
140]. Dimeric cinnamoyl amide analogs (DCAs), based on
N,
N′-diferuloyl-putrescine (DFP) like
N,
N′-di-(2,4-dihydroxyphenyl)-1,4-diaminobutane 93,
N,
N′-di-(2,4-dihydroxyphenyl)-1,3-diaminopropane 99, and
N,
N′-di-(2,4-dihydroxyphenyl)-1,2-diaminoethane 96, showed the best tyrosinase inhibition (IC
50 = 0.82, 0.078 and 0.034 µM) in comparison to kojic acid (IC
50 = 52.2 µM) among the 12 tested dimeric amides. Similarly, the
N,
N′-di-
p-coumaroyl-1,3-diaminopropane 97 showed effective tyrosinase inhibition activity (IC
50 = 6.4 µM) [
141]. Further depigmenting activity in B16/F1 melanoma cells was also seen for 93, 99, and 96 (IC
50 = 2.1, 2.4, and 8.7 µM) compared to kojic acid (IC
50 > 1 mM). [
141]. Another study by Ha and Park observed that the carbon length of the diamide linkage between two cinnamic acid derivatives and the position of hydroxyl groups may play an important role in effective tyrosinase inhibition. The
N,
N′-di-
p-coumaroyl-1,3-diaminopropane 97 showed effective depigmenting activity (IC
50 = 3.4, 4.6, and 45.6 µM) in mushroom tyrosinase inhibition, tyrosinase inhibition, and melanin production in B16F1 cells in comparison to the dimeric coumaryl amides 94 and 91 (n = 2 and 4; IC
50 = 4.6 and 6.5 µM for mushroom tyrosinase; > 150 µM in all tyrosinase inhibition and melanin production in B16F1 cells for both amides). The structural similarity of the
p-hydroxy benzyl group with the tyrosine molecule can provide competitive inhibition at the binding site. The m-coumaryl, isoferulic, or ferulic diamides were less effective for the inhibition of mushroom tyrosinase activity and tyrosinase inhibitory activity and melanin production in B16F1 cells (IC
50 >150 µM) [
142]. Although the
N,
N′-diferuloyl-1,3-diaminopropane 98 was less effective in the in vitro inhibition tests, it had increased UV absorbance based on the addition of copper (II) ions (observed in the region 280–325 nm). The authors concluded that melanin production may be dependent on the inhibition of tyrosinase via copper chelation [
142].
9. Functional Role of AHC in Sunscreen Applications
UV radiation received on Earth is classified as UVA (320–400 nm), UVB (290–320 nm), and UVC (200–290 nm). Octinoxate (2-ethylhexyl-4-methoxycinnamate; tradename Parsol MCX, see 100 in
Figure 9) has a high molar absorption coefficient (approximately ε 22,000–24,000 M
−1 cm
−1 at 309 nm) and is the most widely used cinnamyl class UV filter in several sunscreen creams [
143]. Octinoxate undergoes reversible trans–cis photoisomerization, which can lead to significant changes in the absorption spectrum at ∼310 nm and considerable loss of absorbance over 10 min of irradiation. The serious environmental impacts of octinoxate and other UV filters on the coral reef led the state of Hawaii in the USA to permanently ban their use and sale since 2021. The longer wavelength of UVA (320–400 nm) allows deeper penetration into the skin through the epidermis and dermis. In terms of absorbance among the
p-hydroxy cinnamic acids, sinapic acid or esters (λ
max~330 nm) are more efficient UV absorbers, while ferulic and caffeic acid or esters (λ
max~319 and 325 nm) and
p-coumaric acid or esters (λ
max~315 nm) are slightly lower; therefore, all these can target the UVA and UVB regions [
60,
144,
145].
There is a need to develop newly modified and safer sunscreen molecules compared with octinoxate that have more coverage of UVA and UVB to prevent skin damage. Sinapic acid is widely described for its UV protection in plants [
60]. This fact prompted Peyrot et al. to prepare sinapate esters with different aliphatic alkyl and aromatic alcohols. In the case of 2-ethyhexyl sinapate 101 (
Figure 9), the high absorbance (λ
max—332 nm), which can effectively target both UVB (280–315 nm) and UVA (315–400 nm) wavelengths, with a hypsochromic shift compared to octinoxate (λ
max~310 nm), was seen. The free phenolic group gave a higher absorbance compared to its protection with methyl or acetyl groups. Further, among the sinapic esters with ethyl, tert-butyl, oleyl, isopropyl, solketal (ketal of glycerol), and heptyl groups, only the bulky secondary or tertiary alcohols resulted in a comparative gain of absorbance. This may be due to the steric hindrance of the esterified group, which increases the cis/trans isomerization upon UVA/B exposure. They also concluded that the phenolic ester moiety gave the best absorbance, but the steric hindrance must be carefully chosen to avoid a negative impact on the absorption [
146]. The loss of absorbance is directly related to photostability, as mentioned earlier for octinoxate, and was also studied for all the sinapate esters prepared by Peyrot. Among all the esters, the solketal sinapate 102 (
Figure 9) had the best photostability compared to octinoxate 100 (13% vs. 25%), along with water solubility, non-toxicity, and renewable sources [
146].
Plants produce sinapoyl-L-malate to protect themselves from the negative effects of UV. Therefore, Peyrot et al. designed sinapoyl-L-malate (SM) 103 (see
Figure 9), a bio-based, safe, and natural skin product, as an excellent alternative to substitute chemical filters in sunscreens. They used a greener synthetic pathway involving Meldrum’s acid and hydroxy acids like lactic, malic, and tartaric acids and their subsequent Knoevenagel–Doebner condensation with biomass-derived
p-hydroxy benzaldehydes [
147]. All these compounds showed similar wavelength coverage and intensity to octinoxate throughout the series, but the tartarate dimers (104,
Figure 9) had significantly higher absorbance and high molar absorption coefficients (λ
max—333 nm and ε 36,493 vs. 22,000–24,000 M
−1 cm
−1) than octinoxate (22,000–24,000 M
−1 cm
−1 at 309 nm). The photostability against UV radiation revealed that tartaric acid dimers were more stable with ~15% LoA (loss of activity) as compared to octinoxate (LoA—25%). These molecules also showed antimicrobial activities and did not have endocrine-disrupting activities, making them safe and water-soluble sunscreens [
147].
Figure 9.
Cinnamic acid derivatives for sunscreen applications.
Figure 9.
Cinnamic acid derivatives for sunscreen applications.
With a probable role in cosmetic and other applications, the hydrophilic–lipophilic balance of sinapoyl malate was reduced or controlled by introducing fatty aliphatic chains of variable length on the malate acid groups modulating the lipophilicity [
148]. The lipophilic SM molecules exhibit similar UV properties to SM (λ
max~335 nm), indicating that the additional fatty chains do not disrupt the UV absorption range. The photostability of dioctyl SM was similar to that of octinoxate (27%), while the remaining SM diesters with unsaturated fatty alcohols like citronellol, geraniol, farnesol, and oleyl exhibited better photostability (i.e., loss of absorbance) than Octinoxate™ (15–18% vs. 27%) [
148].
Lipophilic dodecyl and 2-ethylhexyl ferulate (34 and 31) were prepared using catalysts like
p-TSA and solvents like benzene and toluene, with >70% yields for use as antioxidants in sunscreen and cosmetic formulations. The UV absorption (λmax—332 nm) and high molar absorptivity (ε 19,800 M
−1 cm
−1) resulted in topical formulations of sun oil, sunscreen cream, lotion, hair spray, cologne, liquid foundation, and anti-wrinkle cream, which were prepared using up to 10%
w/
w of 31. The antioxidant activity was measured in lard oil against the peroxide value and showed excellent protection compared with octinoxate. The thermal stability was improved in comparison to ferulic acid and octinoxate 100 [
149].
Another aspect of the development of safer sunscreens based on the cinnamic acid scaffold is based on the modification of the groups on the aromatic ring or the alkyl spacer. Previously, 2-ethylhexyl-2,4,5-trimethoxycinnamate (see 105 in
Figure 9) has shown effective coverage of UVA and UVB regions up to 350 nm [
150], while the 2,4-dihydroxy-5-methoxy cinnamic acid isolated from the whole plant of
Viola Betonicifolia had UV λmax at 345 nm [
151]. Similarly, the new development of 4-methoxy-3-phenpropyl cinnamate (106,
Figure 9) had comparative molar absorptivity (ε 22,400 M
−1 cm
−1) than that reported for octinoxate 100 and was less toxic [
152]. Another important consideration was to introduce an identical ester moiety on the double bond of ethyl sinapate to prevent the cis/trans isomerization from leading to indistinguishable isomers with a blue shift of UV absorbance. Diethyl 2-(4-hydroxy-3,5-dimethoxybenzylidene)malonate (diethyl sinapate, 107
Figure 9) had a λ
max 364 nm compared to ethyl sinapate at λ
max of 352 nm and was photostable for more than 2 h with a minor loss of 3.3% [
153]. The symmetrical substitution pattern of 107 provides non-radiative relaxation and also compensates for the loss of absorbance due to the locked double-bond geometry [
154]. Similarly, Peyrot et al. also prepared new benzylidene Meldrum acid derivatives, among which the derivatives of sinapic, ferulic,
p-coumaric, and caffeic (107a-d) with auxochromes (-OH, OMe) resulted in a bathochromic absorbance shift with the proportionate high molar absorption coefficient (approximately ε 24,600–29,400 M
−1 cm
−1 at their individual λ
max). This increase in wavelength coverage was able to include part of the UVA and the blue light regions (all absorbing > 351 nm of avobenzene up to 405 nm for 107a). The loss of absorbance was below 5% for only the
p-coumaric derivative 107c in line with the standard cosmetic industry requirement for commercial UV filters [
155]. The dimerization of sinapic esters using copper(I)-catalyzed process using the REACH-compliant bio-based solvent Cyrene resulted in sinapic dimers 107e with extended conjugation with higher UV absorbance covering both UVA and UVB regions [
156].
Generally, ferulic, coumaric, and sinapic acids and their esters have UV λ
max ~300–330 nm; therefore, they can target the UVA and UVB regions. Improvement in the structure can further their range and also increase the molar absorption coefficient and loss of absorption values. Another important way to improve the range of UV absorption in the UVA region would be the incorporation of the amino substituent in the aromatic ring at the para position. Although these types of molecules are synthetic, they are based on the natural cinnamic acid core which is conjugated. The aminoalkyl groups are electron donating and hence can increase the conjugation by the delocalization of a lone pair on the nitrogen atom into the aromatic ring toward the double bond. Such compounds have a λ
max > 360 nm up to 410 nm and high molar absorption coefficients (30,000–40,000 M
−1 cm
−1) [
157]. Peyrot et al. experimented by replacing the hydroxyphenyl group with the pyrrole group 107f, obtaining λ
max of 393 nm and a high molar absorption coefficient (40,225 M
−1 cm
−1). The loss of absorbance in this case was 0.1%, while 0.6% was seen for avobenzene, and both are well below the 5% requirement for standard commercial cosmetic industry UV filters [
155].
Changes in the modern environment lead to an increase in UV exposure and contribute to skin aging. UVA rays (315–400 nm) can penetrate skin layers and cause photosensitized oxidative damage via ROS. Singlet oxygen (
1O
2) is a highly reactive species and causes serious photodamage to skin cells. The natural
N,
N′-diferuloyl-putrescine (DFP) 90, with UV absorption at λ
max 294 and 320 nm, could reduce the melanin content and provide skin-whitening effects, along with antioxidative activities. To modulate these effects, two additional isomers with two- and three-carbon linkage chain lengths connecting these dimeric ferulamide moieties were prepared. The
N,
N′-diferuloyl-1,3-diaminopropane 98 (having λ
max at 294, 321 nm) showed 85% cellular viability against UVA at an applied concentration of 100 µM in human keratinocyte (HaCaT) cells, indicating protective effects.
N,
N′-diferuloyl-1,3-diaminopropane 98 at 25 µM protected cell hemolysis induced by singlet oxygen by τ
50 (half the time of hemolysis) = 79 min compared to other samples and the control α-tocopherol (τ
50 = 40 min). The mechanism of action was deduced as the combined effect of high cellular uptake into the cell membrane, along with scavenging and antioxidant effects [
158]. Previously Hwang et al., have reported that
N,
N′-disinapoyl-1,4-diaminobutane (or
N,
N′-disinapoyl putrescine) 92 had better DPPH-scavenging activity FSC
50 = 12.92 ± 0.72 μM compared with DFP 90 at 61.25 μM (compared to ~20 μM for L-ascorbic acid) and protected human erythrocytes (τ
50 = 80.2 min) from oxidative damage more than that observed with α-tocopherol (τ
50 = 43.6 min) [
158]. A corn bran extract containing DFP, DCP, or
N-
p-coumaroyl-
N′-feruloylputrescine (CFP) showed comparable activity to the active ingredient in cosmetic creams and formulations with the UV absorption and the sun-protection factor (SPF) of octinoxate (7.71), and it reduced erythema and skin safety using human subjects for testing [
159]. The UV-Vis spectrum of DFP was compared with octinoxate in the Korean patent KR20110028012A, and a visual comparison showed similar peak heights, which indicates they may have similar molar extinction coefficients [
159]. These molecules, the
N,
N′-di-(2,4-dimethoxyphenyl)-1,2-diaminoethane (sepsis inhibitor) 76 and
N,
N′-di-(2,4-dihydroxyphenyl)-1,2-diaminoethane 96 (tyrosinase inhibitor), are interesting from the viewpoint of UV-based application. Firstly, diamide linkage may be more stable to hydrolysis and provide more stability concerning the loss of absorbance (LoA) on irradiation; secondly, the presence of 2,4-dimethoxy/dihydroxy cinnamic acid can improve the UV λ
max to ~340 nm or greater wavelengths (in UVA regions), and the dual cinnamyl chromophores may increase the molar absorption values and provide naturally safe sunscreens [
152,
160,
161]. Peyrot prepared the aniline-derived amide with sinapic acid, which showed comparable LoA and molar absorption coefficient with octinoxate [
146]. Therefore, it may validate the use of 2,4-dimethoxy/dihydroxy cinnamic-acid-based putrescine or 1,2-diaminoethane-type molecules with comparable molar extinction coefficients to known sunscreens and can be applied for UV filter applications [
159].
10. AHCs as Potential Biopesticides for Agricultural Applications
As mentioned in the preamble, Goal 2—Zero Hunger of the United Nations Sustainable Development Goals (UN-SDG) states, “The food and agriculture sector offers key development solutions, and is central for hunger and poverty eradication”. In line with the goal, this section highlights the various AHCs that have shown potential for their application as biopesticides to contribute to improving agricultural practices.
The alkyl hydroxy cinnamates like hexadecyl, heptadecyl, and octadecyl ferulates (37, 38, and 39, in
Figure 2) were isolated from the roots of three different varieties of
Ipomoea batatas L. (Lam.) [
2]. The C16, C18, and C20-
p-coumarates (3, 5, and 7,
Figure 2) could repel the herbivore insects and provide resistance to sweet potato latex (
Ipomoea batatas) [
8].
The fungus
Alternaria alternata can cause the food contamination of fresh fruits and vegetables and lead to postharvest decay during processing and storage. The appearance of black spot rot and the production of mycotoxins can raise food safety issues. In attempts to provide alternate methods of postharvest disease management, plant-derived products are preferred instead of traditional chemical fungicides. Based on the isolation of
p-coumaric acid from fruits (jujubes, pears, and sweet cherries) and its known antimicrobial potential, Li et al. assessed the underlying antifungal activity mechanism of commercially available lipophilic ethyl-
p-coumarate 80 (
Figure 8) against
A. alternate in vitro and in vivo. Ethyl-
p-coumarate 80 was tested against common postharvest fungi like
A. alternata,
Botrytis cinerea,
Rhizopus stolonifer, and
Penicillium expansum, and their MIC values were 250, 125, 62.5, and 125 μg/mL, respectively. A pronounced antifungal activity against in vitro mycelial growth of
A. alternata, with a half-inhibition concentration of 176.8 μg/mL, was seen for 80 after 9 days of incubation. Treatment with 80 in a dose-dependent manner inhibited spore germination of the
A. alternata. Mechanistically, the treatment of
A. alternata with 100 and 800 μg/mL 80 as a function of time increased the plasma membrane permeability and led to the leakage of intercellular electrolytes, soluble proteins, and sugars. The membrane integrity of
A. alternata was checked by propidium iodide staining (25.92% at 800 μg/mL dose of 80), indicating a loss of membrane integrity and damage to the cell membrane. Further, the treatment with 800 μg/mL of 80 also affected the cell morphology of the pathogen, seen as distorted, sunken, and shriveled spores and mycelia. The production of high levels of endogenous ROS at a higher concentration of 80 in the spores of
A. alternata led to oxidative damage to cellular membranes and organelles, as demonstrated by the severe occurrence of lipid peroxidation. Moreover, the in vivo test for the disease development of black spot rot in jujube fruit caused by
A. alternata was significantly reduced at 100 and 800 μg/mL doses of 80 after 5 and 9 days of incubation [
162].
The biotic-stress-related expression of AHC was shown in a bioassay of the chief antifungal fraction of the leaves of
Ipomoea carnea subsp. fistulosa (Convulvulaceae, bush morning glory), which was achieved using
Colletotrichum gloeosporioides and
Cladosporium cucumerinum as test organisms. Geographically,
Ipomoea carnea subsp. fistulosa is native to South America and is sparsely distributed in India and Bangladesh. The activity of the purified fraction was further confirmed by the dose-dependent inhibition of the spore germination of
A. alternata and
Alternaria porri. The active fraction was identified as a mixture of (
E)-octadecyl-
p-coumarate 5 and (
Z)-octadecyl-
p-coumarate [
163]. The hydroxy cinnamic acid esters in the latex of sweet potato
Ipomoea batatas reduced the damage caused by weevil larvae (insects), feeding, and oviposition [
164]. The control of adult insects of two species
Cylas puncticollis and
Cylas brunneus was of economic and agricultural importance in Africa. Therefore, when octadecyl-
p-coumarate 5 and octadecyl caffeate 27 (
Figure 2) were applied to the surface of insect-susceptible varieties of
Ipomoea in laboratory bioassays, they reduced the feeding and oviposition, as observed on roots of resistant varieties, and were therefore implicated in weevil insect resistance [
164]. Methyl cinnamate isolated from
Ocimum gratissimum (African basil) and sitosterol cinnamate from
Vitellaria paradoxa (indigenous to Africa) had insecticidal activity against adults and larvae of
Tribolium castaneum, supporting the use of plant isolates for storage pest management in sub-Saharan Africa [
165].
A non-conventional molecule, isobutyl-
o-coumarate 108 (
Figure 10), was able to penetrate more effectively in vivo against
Venturia inaequalis on immature apple leaves compared to the acid. The formulation mixture with the isobutyl-
o-coumarate 108 in acetone as a solvent, Tween 80, and Triton X-100 was applied to the shoots of the apple variety Cox’s Orange Pippin, and its presence in the upper epidermis of the first five leaves above those to which it had been applied was shown by the thin-layer chromatography of the extracted material [
166].
Figure 10.
Alkyl o-hydroxy cinnamates, halogen, and methoxy substituted alkyl cinnamides and substituted ferulic acid amides with 1-aminocyclopentane carboxylic acid (Ac5c) and 1-aminocyclohexane carboxylic acid (Ac6c) as potential biopesticides or additive for agricultural and aquaculture applications.
Figure 10.
Alkyl o-hydroxy cinnamates, halogen, and methoxy substituted alkyl cinnamides and substituted ferulic acid amides with 1-aminocyclopentane carboxylic acid (Ac5c) and 1-aminocyclohexane carboxylic acid (Ac6c) as potential biopesticides or additive for agricultural and aquaculture applications.
Plant fungal pathogens or mycosis can lead to yield and quality loss in agricultural production worldwide. Therefore, Zhou et al. designed t-butyl-
o-coumarate 109 and t-amyl-
o-coumarate 110 (
Figure 10, screened from 60 examples), which showed higher activity, with average EC
50 values of 17.4 and 18.5 μg/mL (compared with the mean value of standard drug Kresoxim-methyl EC
50, 43.9 μg/mL) against four plant fungal strains, namely
Fusarium solani,
Pyricularia grisea,
Valsa mali, and
Botryosphaeria dothidea. These naturally derived ortho-hydroxy cinnamic acids are safe and have the potential for the development of environmentally friendly plant antifungal agents [
167].
The plant parasite of the genus
Meloidogyne (also called Root-knot nematode because of the appearance of knots in the infected roots) can infect ~2000 plant species worldwide and cause a loss of agricultural production. The toxicity of commercial nematicides has led to their withdrawal from the markets. Therefore, the search for effective biopesticides that are safe for the environment and can control the infections is required. Although cinnamaldehyde was found to be very active against the plant parasite
Meloidogyne incognita, killing 97% of second-stage J2 juveniles at a 52 μg/mL dosage compared to 63% with carbofuran (commercial nematicide) at a 173 μg/mL dosage, its low persistence or high oxidation rate in the soil was the cause of further development. Therefore, Vanegas et al. evaluated 15 cinnamic ester derivatives on the motility and mortality of
M. incognita and found the three most potent compounds (
Figure 10) against
M. incognita second-stage juveniles (J2);
p-fluoro methylcinnamate 111,
p-chloro methylcinnamate 112, and
p-bromo methylcinnamate 113 (lethal concentrations with LC
50 of 168, 95, and 216 μg/mL, respectively) equivalent to the nematicidal activity of carbofuran and fluensulfone (LC
50 values of 160 and 34 μg/mL). The 4-methoxy methyl cinnamate 114 and methyl cinnamate 115 were also active but comparatively less potent. These last two molecules (114 and 115) are already used as biopesticides or nematocides against other species. The
p-chloro 112 was found to be the most active against
M. incognita nematode eggs, which were the most relevant, considering the field application followed by
p-fluoro and
p-bromo cinnamyl esters (111 and 113). From the pharmacophoric search for these three esters in the ligand expo database, BRENDA and ChEMBL provided the conclusive information that they probably act via the inhibition of histone deacetylase, and docking analysis was performed for confirmation [
168].
Rice crops are attacked by
Xanthomonas oryzae pv oryzae (Xoo) and
X. oryzae pv. oryzicola (Xoc) pathogens, which can reduce agricultural yields by 50%. Similarly,
X. axonopodis pv. citri (Xac) causes citrus canker in citrus-bearing fruit trees, which affects the quality and supply of citrus fresh fruits. The effectiveness of traditional pesticides like thiazolyl zinc and others gets compromised by resistance and cross-resistance. Therefore, Zhang et al. modified ferulic acid using 1-aminocyclopentane carboxylic acid (Ac5c), or cycloleucine, which is a non-metabolic amino acid and a ATP competitor widely used in drug discovery. The incorporation of this moiety into ferulic acid and also changes in the 4-hydroxyl substituent with bioisosteric type 4-trifluoromethyl benzyl ether provided a new cinnamic acid hybrid molecule 116 with antibacterial activity (EC
50 = 1.9 μg/mL) against Xoo. Moderate antifungal and insecticidal activity was also noted for these derivatives. The trifluoromethyl group was introduced to modulate the physicochemical properties and to increase the binding affinity of the drug molecule [
169]. The 4-fluoro substituted analog 117 also gave potent activity against Xoo, with EC
50 values of 4.0 μg/mL. Previously, the same authors have studied 38 examples of the ferulic amide derivatives of 1-amino cyclohexane carboxylic acid (Ac6c) 118 against the
Xanthomonas oryzae pv oryzae (
Xoo) organism and found that EC
50 values (11.6 to 83.1 μg/mL) were better than commercial bismerthiazol (BMT, EC
50 = 84.3 μg/mL) and thiediazole copper (TDC, EC
50 = 137.8 μg/mL) [
170].
O-acetyl ethyl ferulate 119 (
Figure 10) was shown to be an efficient and safe natural feed additive for the aquaculture farming of genetically improved farmed Tilapia (
Oreochromis niloticus). Several animal feed studies have reported the use of ferulic acid for improved growth. This aquaculture-related study observed that Tilapia fish fed the diets with increasing amounts of acetylated ethyl ferulate showed an increase in weight, along with improved serum biochemical parameters and an increase in antioxidant enzymes compared with control diets. The increased lipophilic nature of acetylated ethyl derivative compared to ferulic acid may have influenced the migration into cells to provide the observed effects [
171]. This method of converting free hydroxyl groups into their acetate derivatives can be used for delayed metabolism and higher bioavailability, leading to the observed effects.
11. Discussion
The lipophilic nature of hydroxy cinnamic esters can provide a clear advantage over the parent acid molecules in terms of antioxidant activity (
Table 2). We saw that there exists a critical chain length the surpassing of which may affect the way these molecules orient in the lipophilic medium employed for the assessment. Accordingly, in lipophilic assay media, the activity of esterified molecules stood out or leveled off in comparison to the free phenolic acids [
24]. Most active molecules were caffeates followed by sinapates, ferulates, and
p-coumarates [
25,
26]. We saw that overall, one antioxidant molecule cannot play a specific role for all types of emulsions or lipid systems, and each system/antioxidant pair needs evaluation before its application. Ferulates and caffeates with C8 and C12 alkyl groups were more active in comparison with other higher alkyl groups evaluated [
27,
44,
49,
54]. The long-term and accelerated stability data of oils and emulsions with promising natural cinnamic acid derivatives like octyl or dodecyl caffeate [
27,
49,
51] or ferulate [
54], which may occupy the oil–aqueous interface where lipid oxidation can initiate, may be required considering their commercial application as antioxidants to replace the use of current petroleum-based active molecules such as BHT or BHA. Secondly, the food system usually presents trace metals, which can use these lipophilic caffeates for their efficient metal-chelating activity [
27,
31].
In the anticancer section, several caffeate molecules with short, medium, and long alkyl chains such as C1, C6, C8, C10, C11, C12, C14, C16, and C18 were mostly active in different cancer cell assays (
Table 3). Among these, the medium chain octyl caffeate was active in different cancer cell lines [
68,
70,
75], while decyl, undecyl and tetradecyl caffeates also had their share of activity [
65,
67,
72,
73]. Similarly, the C6, C8, C12, and C15 ferulates were also active against cancer cell growth [
20,
69,
74,
76]. Tetradecyl and hexadecyl-
p-coumarates had anticancer activity against leukemia cell lines [
65]. Trisubstituted decyl-3,4,5-trihydroxy cinnamate, octyl-3,4-dihydroxy-5-methoxycinnamate, and hexadecyl-3,4-dihydroxy-5-methoxycinnamate were active in cancer cells (
Table 3). The variable lipophilicity of these molecules compared to their parent acid may be the reason for anticancer activity [
65,
67,
69,
70]. Most of the reported studies were carried out in vitro, and there is a need to test the potential substances in vivo studies.
In the neurotherapy section, pentyl, C10 and C12 caffeate, dodecyl caffeamide, and isopentyl ferulate had a prominent neuroprotective effect (
Table 4). Similarly, the long-chain alcohol ester, eicosanyl ferulate (C20), has shown memory enhancement activity, and the docosanyl ferulate (C22) has shown GABA mimetic activity; therefore, such lipophilic molecules can provide new areas for the development of PD and AD therapy [
83,
85,
93,
95]. Owing to the antioxidant activity, natural sinapic acid derivatives like sinapine have shown potential application for AD therapy [
60,
82].
The C1-C6 caffeates, the C6 and C12 ferulates, and the C16-
p-coumarate had pronounced antimicrobial activity against Gram-positive and Gram-negative organisms, which may be dependent on their lipophilicity and interaction with outer cell wall structures (
Table 5). The most active molecules for antiplasmodial activity were hexyl
p-coumarate, pentyl-
p-coumarates, and octyl caffeate, while tetradecyl ferulate, oleyl caffeate, oleyl caffeamide, and 28-hydroxyoctacosyl ferulate showed antiviral activity (
Table 5).
The lipophilic long-chain alkyl caffeates like octadecyl caffeate, eicosanyl caffeate, docosanyl caffeate, and 3,4,5-trihydroxy cinnamic acid decyl ester and decyl caffeate improved the glucose uptake and elastase and lipase inhibition in vivo, respectively. Among the esters of
p-coumaric acid, a known inhibitor of tyrosinase, the n-butyl-
p-coumarate showed anti-tyrosinase activity, while the di feruloyl putrescine (DFP) and the other polyamine-based hydroxy cinnamic acid amides were the most active (
Table 6).
Among the sunscreen active molecules, the sinapic acid derivatives such as 2-ethylhexyl sinapate, solketal sinapate, diethyl 2-(4-hydroxy-3,5-dimethoxybenzylidene)malonate, and 2-ethylhexyl-2,4,5-trimethoxycinnamate could target the UVA and UVB regions. The sinapic acid or esters having λ
max~330 nm are more efficient UV absorbers, as exemplified by the sinapic acid solketal ester, which had the best photostability compared to the commercial octinoxate. The natural polyamine-based antioxidant
N,
N′-disinapoyl putrescine and di-feruloyl putrescine can provide dual cinnamyl-based chromophores and improve their molar absorption values, while the inclusion of alkylated amino groups on the aromatic ring can move the UV-λ
max into the UVA regions. Stability studies on these hydroxy cinnamyl di-amides concerning the loss of absorbance (LoA) on irradiation can provide naturally safe sunscreens for skincare and UV filter/sunscreen applications. Since fatty alcohols can function naturally as an emollient, it is also proposed that several of the long-chain cinnamyl esters or vegetable-oil-based phenolipids can be efficient molecules for commercial topical applications such as sunscreens or in skin cream formulations, and hence, such conjugated molecules can have dual functionality [
172]. For effective cosmetic formulation and development, one molecule with several functionalities like UV absorption, antimicrobial, and endocrine safety is the need of the hour [
146,
147,
155].
The application of natural lipophilic octadecyl caffeate against potato insects as a biopesticide was noteworthy [
164]. The agricultural applications saw the most use of natural
o/
p-coumaric acids derivatives such as isobutyl-
o-coumarate, t-butyl-
o-coumarate, t-amyl-
o-coumarate, ethyl-
p-coumarate, octadecyl-
p-coumarate, and halogenated derivatives such as
p-fluoro,
p-chloro and
p-bromo methyl cinnamate as biodegradable pesticide molecules.