Antimicrobial Activity of Dimeric Flavonoids
Abstract
1. Introduction
2. Dimeric Flavonoids
2.1. Antiviral Activity of Dimeric Flavonoids
2.2. Antifungal Activity of Dimeric Flavonoids
2.3. Antiparasitic Activity of Dimeric Flavonoids
2.4. Antibacterial Activity of Dimeric Flavonoids
2.5. Potential of Dimeric Flavonoids as Antimicrobials: Form Lab to Clinics
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tanwar, J.; Das, S.; Fatima, Z.; Hameed, S. Multidrug resistance: An emerging crisis. Interdiscip. Perspect. Infect. Dis. 2014, 2014, 541340. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, S.R.; Guarner, J. Emerging and reemerging fungal infections. Semin. Diagn. Pathol. 2019, 36, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Benedict, K.; Richardson, M.; Vallabhaneni, S.; Jackson, B.R.; Chiller, T. Emerging issues, challenges, and changing epidemiology of fungal disease outbreaks. Lancet Infect. Dis. 2017, 17, e403–e411. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Denning, D.W.; Gow, N.A.R.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef] [PubMed]
- Ferri, M.; Ranucci, E.; Romagnoli, P.; Giaccone, V. Antimicrobial resistance: A global emerging threat to public health systems. Crit. Rev. Food Sci. Nutr. 2015, 57, 2857–2876. [Google Scholar] [CrossRef] [PubMed]
- Huemer, M.; Shambat, S.M.; Brugger, S.D.; Zinkernagel, A.S. Antibiotic resistance and persistence—Implications for human health and treatment perspectives. Embo Rep. 2020, 21, e51034. [Google Scholar] [CrossRef] [PubMed]
- Larsson, D.G.J.; Flach, C.F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef]
- Muhaj, F.F.; George, S.J.; Nguyen, C.D.; Tyring, S.K. Antimicrobials and resistance part II: Antifungals, antivirals, and antiparasitics. J. Am. Acad. Dermatol. 2022, 86, 1207–1226. [Google Scholar] [CrossRef]
- Pramanik, P.K.; Alam, N.; Chowdhury, D.R.; Chakraborti, T. Drug Resistance in Protozoan Parasites: An Incessant Wrestle for Survival. J. Glob. Antimicrob. Resist. 2019, 18, 1–11. [Google Scholar] [CrossRef]
- Strasfeld, L.; Chou, S. Antiviral drug resistance: Mechanisms and clinical implications. Infect. Dis. Clin. N. Am. 2010, 24, 809–833. [Google Scholar] [CrossRef]
- Rocha, M.F.G.; Sales, J.A.; da Rocha, M.G.; Galdino, L.M.; de Aguiar, L.; Pereira-Neto, W.d.A.; Cordeiro, R.d.A.; Castelo-Branco, D.d.S.C.M.; Sidrim, J.J.C.; Brilhante, R.S.N. Antifungal effects of the flavonoids kaempferol and quercetin: A possible alternative for the control of fungal biofilms. Biofouling 2019, 35, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, F.; Maia, M.; Gabriel, C.; Medeiros, R.; Cravo, S.; Ribeiro, A.I.; Dantas, D.; Dias, A.M.; Saraiva, L.; Raimundo, L.; et al. Mechanism of Antifungal Activity by 5-Aminoimidazole-4-Carbohydrazonamide Derivatives against Candida albicans and Candida krusei. Antibiotics 2021, 10, 183. [Google Scholar] [CrossRef]
- Jin, Y.-S. Recent advances in natural antifungal flavonoids and their derivatives. Bioorganic Med. Chem. Lett. 2019, 29, 126589. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, W.; Tang, F.; Chen, X.; Ren, L. Antibacterial Activities of Flavonoids: Structure-Activity Relationship and Mechanism. Curr. Med. Chem. 2014, 22, 132–149. [Google Scholar] [CrossRef] [PubMed]
- Badshah, S.L.; Faisal, S.; Muhammad, A.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Antiviral activities of flavonoids. Biomed. Pharmacother. 2021, 140, 111596. [Google Scholar] [CrossRef] [PubMed]
- Penna-Coutinho, J.; Aguiar, A.C.; Krettli, A.U. Commercial drugs containing flavonoids are active in mice with malaria and in vitro against chloroquine-resistant Plasmodium falciparum. Mem. Inst. Oswaldo Cruz. 2018, 113, e180279. [Google Scholar] [CrossRef]
- Janabi, A.H.W.; Kamboh, A.A.; Saeed, M.; Xiaoyu, L.; BiBi, J.; Majeed, F.; Naveed, M.; Mughal, M.; Korejo, N.A.; Kamboh, R.; et al. Flavonoid-rich foods (FRF): A promising nutraceutical approach against lifespan-shortening diseases. Iran. J. Basic Med. Sci. 2020, 23, 140–153. [Google Scholar]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Sun, Z.-G.; Li, Z.-N.; Zhang, J.-M.; Hou, X.-Y.; Yeh, S.M.; Ming, X. Recent Developments of Flavonoids with Various Activities. Curr. Top. Med. Chem. 2022, 22, 305–329. [Google Scholar] [CrossRef]
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012, 3, 34352. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Chen, H.; Zhou, X.; Deng, Q.; Hu, E.; Zhao, C.; Gong, X. Optimized microwave-assistant extraction combined ultrasonic pretreatment of flavonoids from Periploca forrestii Schltr. and evaluation of its anti-allergic activity. Electrophoresis 2017, 38, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Kawai, M.; Hirano, T.; Higa, S.; Arimitsu, J.; Maruta, M.; Kuwahara, Y.; Ohkawara, T.; Hagihara, K.; Yamadori, T.; Yoshihito, S.; et al. Fla-vonoids and Related Compounds as Anti-Allergic Substances. Allergol. Int. 2007, 56, 113. [Google Scholar] [CrossRef] [PubMed]
- Brasiel, P.G.d.A.; Guimarães, F.V.; Rodrigues, P.M.; Bou-Habib, D.C.; Carvalho, V.d.F. Therapeutic Efficacy of Flavonoids in Allergies: A Systematic Review of Randomized Controlled Trials. J. Immunol. Res. 2022, 2022, 8191253. [Google Scholar] [CrossRef]
- Rakha, A.; Umar, N.; Rabail, R.; Butt, M.S.; Kieliszek, M.; Hassoun, A.; Aadil, R.M. Anti-inflammatory and anti-allergic potential of dietary flavonoids: A review. Biomed. Pharmacother. 2022, 156, 113945. [Google Scholar] [CrossRef] [PubMed]
- Shukitt-Hale, B.; Galli, R.L.; Meterko, V.; Carey, A.; Bielinski, D.F.; McGhie, T.; Joseph, J.A. Dietary supplementation with fruit poly-phenolics ameliorates age-related deficits in behavior and neuronal markers of inflammation and oxidative stress. Age 2005, 27, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Serafini, M.; Peluso, I.; Raguzzini, A. Flavonoids as anti-inflammatory agents. Proc. Nutr. Soc. 2010, 69, 273–278. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef]
- Ginwala, R.; Bhavsar, R.; Chigbu, D.G.I.; Jain, P.; Khan, Z.K. Potential role of flavonoids in treating chronic inflammatory diseases with a special focus on the anti-inflammatory activity of apigenin. Antioxidants 2019, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, F.; Cordeiro-Da-Silva, A.; Araújo, N.; Cidade, H.; Kijjoa, A.; Nascimento, M.S.J. Inhibition of lymphocyte proliferation by prenylated flavones: Artelastin as a potent inhibitor. Life Sci. 2003, 73, 2321–2334. [Google Scholar] [CrossRef]
- Cerqueira, F.; Cidade, H.; van Ufford, L.; Beukelman, C.; Kijjoa, A.; Nascimento, M.S.J. The natural prenylated flavone artelastin is an inhibitor of ROS and NO production. Int. Immunopharmacol. 2008, 8, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Lncap, P.; Hela, C.H.; Horta, B.; Freitas-Silva, J.; Silva, J.; Dias, F.; Lu, A.; Medeiros, R.; Cidade, H.; Pinto, M.; et al. Antitumor Effect of Chalcone Derivatives against Human Macrophage Functions. Molecules 2023, 28, 2159. [Google Scholar]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as anticancer agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef] [PubMed]
- Tuli, H.S.; Garg, V.K.; Bhushan, S.; Uttam, V.; Sharma, U.; Jain, A.; Sak, K.; Yadav, V.; Lorenzo, J.M.; Dhama, K.; et al. Natural flavonoids exhibit potent anticancer activity by targeting microRNAs in cancer: A signature step hinting towards clinical perfection. Transl. Oncol. 2023, 27, 101596. [Google Scholar] [CrossRef] [PubMed]
- de Luna, F.C.F.; Ferreira, W.A.S.; Casseb, S.M.M.; de Oliveira, E.H.C. Anticancer Potential of Flavonoids: An Overview with an Emphasis on Tangeretin. Pharmaceuticals 2023, 16, 1229. [Google Scholar] [CrossRef] [PubMed]
- Lúcio, M.; Giannino, N.; Barreira, S.; Catita, J.; Gonçalves, H.; Ribeiro, A.; Fernandes, E.; Carvalho, I.; Pinho, H.; Cerqueira, F.; et al. Nanostructured Lipid Carriers Enriched Hydrogels for Skin Topical Administration of Quercetin and Omega-3 Fatty Acid. Pharmaceutics 2023, 15, 2078. [Google Scholar] [CrossRef] [PubMed]
- Al Aboody, M.S.; Mickymaray, S. Anti-fungal efficacy and mechanisms of flavonoids. Antibiotics 2020, 9, 45. [Google Scholar] [CrossRef]
- Shamsudin, N.F.; Ahmed, Q.U.; Mahmood, S.; Shah, S.A.A.; Khatib, A.; Mukhtar, S.; Alsharif, M.; Parveenn, H.; Zakaria, Z.A. Anti-bacterial Effects of Flavonoids and Their Structure-Activity Relationship Study: A Comparative Interpretation. Molecules 2022, 27, 1149. [Google Scholar] [CrossRef]
- Mead, J.R.; McNair, N. Antiparasitic activity of flavonoids and isoflavones against Cryptosporidium parvum and Encephalitozoon intestinalis. FEMS Microbiol. Lett. 2006, 259, 153–157. [Google Scholar] [CrossRef]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2018, 18, 241–272. [Google Scholar] [CrossRef]
- Wang, J.-F.; Liu, S.-S.; Song, Z.-Q.; Xu, T.-C.; Liu, C.-S.; Hou, Y.-G.; Huang, R.; Wu, S.-H. Naturally Occurring Flavonoids and Isoflavonoids and Their Microbial Transformation: A Review. Molecules 2020, 25, 5112. [Google Scholar] [CrossRef] [PubMed]
- Parshikov, I.A.; Sutherland, J.B. Biotransformation of Steroids and Flavonoids by Cultures of Aspergillus niger. Appl. Biochem. Biotechnol. 2015, 176, 903–923. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, W.; Grigori, L.; Just, E.; Santos, C.; Seleem, D. The in vivo anti-Candida albicans activity of flavonoids. J. Oral Biosci. 2021, 63, 120–128. [Google Scholar] [CrossRef]
- Gontijo, V.S.; dos Santos, M.H.; Viegas, C., Jr. Biological and Chemical Aspects of Natural Biflavonoids from Plants: A Brief Review. Mini-Rev. Med. Chem. 2017, 17, 834–862. [Google Scholar] [CrossRef] [PubMed]
- Menezes, J.C.; Campos, V.R. Natural biflavonoids as potential therapeutic agents against microbial diseases. Sci. Total Environ. 2021, 769, 145168. [Google Scholar] [CrossRef]
- Oliveira, T.T.D.; Silva, R.R.D.; Dornas, W.C.A.; Nagem, T.J. Flavonóides e Aterosclerose. Biologia 2010, 42, 49–54. [Google Scholar]
- Canard, B.; Figadère, B.; Guillemot, J.; Claude Nour, M. Biflavonoids of Dacrydium balansae with potent inhibitory activity on dengue 2 NS5 polymerase. Planta Medica 2012, 78, 672–677. [Google Scholar]
- Konziase, B. Protective activity of biflavanones from Garcinia kola against Plasmodium infection. J. Ethnopharmacol. 2015, 172, 214–218. [Google Scholar] [CrossRef]
- Lopes Andrade, A.W.; Dias Ribeiro Figueiredo, D.; TorequlIslam, M.; Viana Nunes, A.M.; da Conceição Machado, K.; da Conceição Machado, K.; Uddin, S.J.; Shilpi, J.A.; Rouf, R.; Carvalho Melo-Cavalcante, A.A.; et al. Toxicological evaluation of the biflavonoid, agathisflavone in albino Swiss mice. Biomed. Pharmacother. 2019, 110, 68–73. [Google Scholar] [CrossRef]
- Antia, B.S.; Pansanit, A.; Ekpa, O.D.; Ekpe, U.J.; Mahidol, C.; Kittakoop, P. α-Glucosidase inhibitory, aromatase inhibitory, and antiplasmodial activities of a biflavonoid gb1 from garcinia kola stem bark. Planta Medica 2010, 76, 276–277. [Google Scholar] [CrossRef]
- Wang, T.Y.; Li, Q.; Bi, K.S. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kerr, R.A.; Korshavn, K.J.; Lee, J.; Kang, J.; Ramamoorthy, A.; Ruotolo, B.T.; Lim, M.H. Effects of hydroxyl group variations on a flavonoid backbone toward modulation of metal-free and metal-induced amyloid-β aggregation. Inorg. Chem. Front. 2016, 3, 381–392. [Google Scholar] [CrossRef]
- Sagrera, G.; Bertucci, A.; Vazquez, A.; Seoane, G. Synthesis and antifungal activities of natural and synthetic biflavonoids. Bioorganic Med. Chem. 2011, 19, 3060–3073. [Google Scholar] [CrossRef] [PubMed]
- Kpabi, I.; Munsch, T.; Agban, A.; Théry-Koné, I.; Dorat, J.; Boudesocque-Delaye, L.; Delaye, P.-O.; Neveu, C.; Lanoue, A.; Enguehard-Gueiffier, C. Cassia sieberiana root bark used in traditional medicine in Togo: Anthelmintic property against Haemonchus contortus and tannins composition. S. Afr. J. Bot. 2022, 151, 549–558. [Google Scholar] [CrossRef]
- Chaves, O.A.; Lima, C.R.; Fintelman-Rodrigues, N.; Sacramento, C.Q.; de Freitas, C.S.; Vazquez, L.; Temerozo, J.R.; Rocha, M.E.N.; Dias, S.S.G.; Carels, N.; et al. Agathisflavone, a natural biflavonoid that inhibits SARS-CoV-2 replication by targeting its proteases. Int. J. Biol. Macromol. 2022, 222, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Šamec, D.; Karalija, E.; Dahija, S.; Hassan, S.T.S. Biflavonoids: Important Contributions to the Health Benefits of Ginkgo (Ginkgo biloba L.). Plants 2022, 11, 1381. [Google Scholar] [CrossRef]
- Nazerian, Y.; Ghasemi, M.; Yassaghi, Y.; Nazerian, A. Role of SARS-CoV-2-induced cytokine storm in multi-organ failure: Molecular pathways and potential therapeutic options. Int. Immunopharmacol. 2022, 113, 109428. [Google Scholar] [CrossRef] [PubMed]
- Bhalerao, A.; Raut, S.; Noorani, B.; Mancuso, S.; Cucullo, L. Molecular mechanisms of multi-organ failure in COVID-19 and potential of stem cell therapy. Cells 2021, 10, 2878. [Google Scholar] [CrossRef]
- Hartini, Y.; Saputra, B.; Wahono, B.; Auw, Z.; Indayani, F.; Adelya, L.; Namba, G.; Hariono, M. Biflavonoid as potential 3-chymotrypsin-like protease (3CLpro) inhibitor of SARS-Coronavirus. Results Chem. 2021, 3, 100087. [Google Scholar] [CrossRef]
- Miki, K.; Nagai, T.; Suzuki, K.; Tsujimura, R.; Koyama, K.; Kinoshita, K.; Furuhata, K.; Yamada, H.; Takahashi, K. Anti-influenza virus activity of biflavonoids. Bioorganic Med. Chem. Lett. 2007, 17, 772–775. [Google Scholar] [CrossRef]
- Miki, K.; Nagai, T.; Nakamura, T.; Tuji, M.; Koyama, K.; Kinoshita, K.; Furuhata, K.; Yamada, H.; Takahashi, K. Synthesis and Evaluation of Influenza Virus Sialidase Inhibitory Activity of Hinokiflavone-Sialic Acid Conjugates. Heterocycles 2008, 75, 879. [Google Scholar] [CrossRef]
- De Freitas, C.S.; Rocha, M.E.N.; Sacramento, C.Q.; Marttorelli, A.; Ferreira, A.C.; Rocha, N.; de Oliveira, A.C.; de Gomes, A.M.O.; dos Santos, P.S.; de Silva, E.D.O.; et al. Agathisflavone, a Biflavonoid from Anacardium occidentale L., Inhibits Influenza Virus Neuraminidase. Curr. Top. Med. Chem. 2020, 20, 111–120. [Google Scholar] [CrossRef]
- Lin, Y.-M.; Anderson, H.; Flavin, M.T.; Pai, Y.-H.S.; Mata-Greenwood, E.; Pengsuparp, T.; Pezzuto, J.M.; Schinazi, R.F.; Hughes, S.H.; Chen, F.-C. In Vitro Anti-HIV Activity of Biflavonoids Isolated from Rhus succedanea and Garcinia multiflora. J. Nat. Prod. 1997, 60, 884–888. [Google Scholar] [CrossRef] [PubMed]
- Ito, C.; Itoigawa, M.; Miyamoto, Y.; Rao, K.S.; Takayasu, J.; Okuda, Y.; Mukainaka, T.; Tokuda, H.; Nishino, H.; Furukawa, H. A new biflavonoid from Calophyllum panciflorum with antitumor-promoting activity. J. Nat. Prod. 1999, 62, 1668–1671. [Google Scholar] [CrossRef] [PubMed]
- Zakaryan, H.; Arabyan, E.; Oo, A.; Zandi, K. Flavonoids: Promising natural compounds against viral infections. Arch. Virol. 2017, 162, 2539–2551. [Google Scholar] [CrossRef] [PubMed]
- Coulerie, P.; Nour, M.; Maciuk, A.; Eydoux, C.; Guillemot, J.C.; Lebouvier, N.; Hnawia, E.; Leblanc, K.; Lewin, G.; Canard, B.; et al. Structure-Activity Relationship Study of Biflavonoids on the Dengue Virus Polymerase DENV-NS5 RdRp. Planta Medica 2013, 79, 1313–1318. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, L.R.F.; Wu, H.; Nebo, L.; Fernandes, J.B.; das Graças Fernandes da Silva, M.F.; Kiefer, W.; Kanitz, M.; Bodem, J.; Diederich, W.E.; Schirmeister, T.; et al. Flavonoids as noncompetitive inhibitors of Dengue virus NS2B-NS3 protease: Inhibition kinetics and docking studies. Bioorg. Med. Chem. 2015, 23, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-M.; Zembower, D.E.; Flavin, M.T.; Schure, R.M.; Anderson, H.M.; Korba, B.E.; Chen, F.-C. Robustaflavone, a naturally occurring biflavanoid, is a potent non-nucleoside inhibitor of hepatitis B virus replication in vitro. Bioorganic Med. Chem. Lett. 1997, 7, 2325–2328. [Google Scholar] [CrossRef]
- Yang, G.; Chen, D. Biflavanones, flavonoids, and coumarins from the roots of Stellera chamaejasme and their antiviral effect on hepatits B virus. Chem. Biodivers. 2008, 5, 1419–1424. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-P.; Liao, S.-X.; Huang, Y.-H.; Hou, M.-C.; Lan, K.-H. Inhibitory Effects of Amentoflavone and Orobol on Daclatasvir-Induced Resistance-Associated Variants of Hepatitis C Virus. Am. J. Chin. Med. 2018, 46, 835–852. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Song, X.; Su, G.; Wang, Y.; Wang, Z.; Jia, J.; Qing, S.; Huang, L.; Wang, Y.; Zheng, K.; et al. Amentoflavone Inhibits HSV-1 and ACV-Resistant Strain Infection by Suppressing Viral Early Infection. Viruses 2019, 11, 466. [Google Scholar] [CrossRef]
- Fidelis, Q.C.; Ribeiro, T.A.; Araújo, M.F.; de Carvalho, M.G. Ouratea genus: Chemical and pharmacological aspects. Rev. Bras. Farm. 2014, 24, 1–19. [Google Scholar] [CrossRef]
- Boff, L.; Silva, I.T.; Argenta, D.F.; Farias, L.M.; Alvarenga, L.F.; Pádua, R.M.; Braga, F.C.; Leite, J.P.V.; Kratz, J.M.; Simões, C.M.O. Strychnos pseudoquina A. St. Hil.: A Brazilian medicinal plant with promising in vitro antiherpes activity. J. Appl. Microbiol. 2016, 121, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Hayashi, T.; Morita, N. Mechanism of action of the antiherpesvirus biflavone ginkgetin. Antimicrob. Agents Chemother. 1992, 36, 1890–1893. [Google Scholar] [CrossRef]
- Wilsky, S.; Sobotta, K.; Wiesener, N.; Pilas, J.; Althof, N.; Munder, T.; Wutzler, P.; Henke, A. Inhibition of fatty acid synthase by amentoflavone reduces coxsackievirus B3 replication. Arch. Virol. 2012, 157, 259–269. [Google Scholar] [CrossRef]
- Salas, M.P.; Céliz, G.; Geronazzo, H.; Daz, M.; Resnik, S.L. Antifungal activity of natural and enzymatically-modified flavonoids isolated from citrus species. Food Chem. 2011, 124, 1411. [Google Scholar] [CrossRef]
- Arif, T.; Bhosale, J.; Kumar, N.; Mandal, T.; Bendre, R.; Lavekar, G.; Dabur, R. Natural products antifungal agents derived from plants. J. Asian Nat. Prod. Res. 2009, 11, 621–638. [Google Scholar] [CrossRef]
- Bagla, V.P.; McGaw, L.J.; Elgorashi, E.E.; Eloff, J.N. Antimicrobial activity, toxicity and selectivity index of two biflavonoids and a flavone isolated from Podocarpus henkelii (Podocarpaceae) leaves. BMC Complement. Altern. Med. 2014, 14, 383. [Google Scholar] [CrossRef]
- Jung, H.J.; Park, K.; Lee, I.-S.; Kim, H.S.; Yeo, S.H.; Woo, E.R.; Lee, D.G. S-Phase Accumulation of Candida albicans by anticandidal effect of Amentoflavone Isolated from Selaginella tamariscina. Biol. Pharm. Bull. 2007, 30, 1969–1971. [Google Scholar] [CrossRef]
- Krauze-Baranowska, M.; Wiwart, M. Antifungal Activity of Biflavones from Taxus baccata and Ginkgo biloba. Z. Naturforschung Sect. C J. Biosci. 2003, 58, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Wang, H.; Zhu, L. Quercetin Assists Fluconazole to Inhibit Biofilm Formations of Fluconazole-Resistant Candida Albicans in In Vitro and In Vivo Antifungal Managements of Vulvovaginal Candidiasis. Cell. Physiol. Biochem. 2016, 40, 727–742. [Google Scholar] [CrossRef] [PubMed]
- Gonçalez, E.; Felicio, J.; Pinto, M. Biflavonoids inhibit the production of aflatoxin by Aspergillus flavus. Braz. J. Med. Biol. Res. 2001, 34, 1453–1456. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, A.L.D.; Kaplum, V.; Rossi, D.C.P.; da Silva, L.B.R.; Melhem, M.d.S.C.; Taborda, C.P.; de Mello, J.C.P.; Nakamura, C.V.; Ishida, K. Proanthocyanidin polymeric tannins from Stryphnodendron adstringens are effective against Candida spp. isolates and for vaginal candidiasis treatment. J. Ethnopharmacol. 2018, 216, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.W.L.; Pang, L.M.; Wang, Y. Impact of the host microbiota on fungal infections: New possibilities for intervention? Adv. Drug Deliv. Rev. 2023, 198, 114896. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.J.; Woo, S.S.; Yeo, S.H.; Hyun, S.K.; Lee, I.S.; Woo, E.R.; Dong, G.L. Antifungal effect of amentoflavone derived from Selaginella tamariscina. Arch. Pharm. Res. 2006, 29, 746–751. [Google Scholar]
- Martino, R.; Canale, F.; Sülsen, V.; Alonso, R.; Davicino, R.; Mattar, A.; Anesini, C.; Micalizzi, B. A Fraction containing kaempferol-3,4-dimethylether from Larrea divaricata Cav. Induces macrophage activation on mice infected with Candida albicans. Phytother. Res. 2014, 28, 917–924. [Google Scholar] [CrossRef]
- Gazoni, V.F.; Balogun, S.O.; Arunacham, K.; Oliveira, D.M.; Filho, V.C.; Lima, S.R.; Colodel, E.M.; Soares, I.M.; Ascêncio, S.D.; de Martins, D.T.O. Assessment of toxicity and differential antimicrobial activity of methanol extract of rhizome of Simaba ferruginea A. St.-Hil. and its isolate canthin-6-one. J. Ethnopharmacol. 2018, 223, 122–134. [Google Scholar] [CrossRef]
- Prevention C for DC and Parasites. About Parasites. 2023. Available online: https://www.cdc.gov/parasites/ (accessed on 13 June 2023).
- Gontijo, V.S.; Judice, W.A.; Codonho, B.; Pereira, I.O.; Assis, D.M.; Januário, J.P.; Caroselli, E.E.; Juliano, M.A.; Dosatti, A.d.C.; Marques, M.J.; et al. Leishmanicidal, antiproteolytic and antioxidant evaluation of natural biflavonoids isolated from Garcinia brasiliensis and their semisynthetic derivatives. Eur. J. Med. Chem. 2012, 58, 613–623. [Google Scholar] [CrossRef]
- Mercado-Camargo, J.; Cervantes-Ceballos, L.; Vivas-Reyes, R.; Pedretti, A.; Serrano-García, M.L.; Gómez-Estrada, H. Homology Modeling of Leishmanolysin (gp63) from Leishmania panamensis and Molecular Docking of Flavonoids. ACS Omega 2020, 5, 14741–14749. [Google Scholar] [CrossRef]
- Muhammad, A.; Anis, I.; Ali, Z.; Awadelkarim, S.; Khan, A.; Khalid, A.; Shah, M.R.; Galal, M.; Khan, I.A.; Iqbal Choudhary, M. Methylenebissantin: A rare methylene-bridged bisflavonoid from Dodonaea viscosa which inhibits Plasmodium falciparum enoyl-ACP reductase. Bioorg. Med. Chem. Lett. 2012, 22, 610–612. [Google Scholar] [CrossRef]
- Weniger, B.; Vonthron-Sénécheau, C.; Kaiser, M.; Brun, R.; Anton, R. Comparative antiplasmodial, leishmanicidal and antitrypa-nosomal activities of several biflavonoids. Phytomedicine 2006, 13, 176–180. [Google Scholar] [CrossRef]
- Kunert, O.; Swamy, R.C.; Kaiser, M.; Presser, A.; Buzzi, S.; Rao, A.A.; Schühly, W. Antiplasmodial and leishmanicidal activity of biflavonoids from Indian Selaginella bryopteris. Phytochem. Lett. 2008, 1, 171–174. [Google Scholar] [CrossRef]
- Florencio, M.; Tomás Nery, E.; Rosa, D.; Auxiliadora Nascimento Ribeiro, T.; de Brito Braz Moraes, J.; Araujo Zuma, A.; da Silva Trindade, J.D.A.; Dutra Barbosa da Rocha, R.F.; Decote-Ricardo, D.; Pinto-da-Silva, L.H.; et al. The effect of the biflavonoid 2″,3″-dihydroochnaflavone on Trypanosoma cruzi Y strain. Parasitol. Int. 2020, 79, 102180. [Google Scholar] [CrossRef] [PubMed]
- da Rocha, C.Q.; Queiroz, E.F.; Meira, C.S.; Moreira, D.R.M.; Soares, M.B.P.; Marcourt, L.; Vilegas, W.; Wolfender, J.-L. Dimeric Flavonoids from Arrabidaea brachypoda and Assessment of Their Anti-Trypanosoma cruzi Activity. J. Nat. Prod. 2014, 77, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Boniface, P.K.; Ferreira, E.I. Flavonoids as efficient scaffolds: Recent trends for malaria, leishmaniasis, Chagas disease, and dengue. Phytother. Res. 2019, 33, 2473–2517. [Google Scholar] [CrossRef] [PubMed]
- Ichino, C.; Kiyohara, H.; Soonthornchareonnon, N.; Chuakul, W.; Ishiyama, A.; Sekiguchi, H.; Namatame, M.; Otoguro, K.; Omura, S.; Yamada, H. Antimalarial Activity of Biflavonoids from Ochna integerrima. Planta Medica 2006, 72, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Lage, P.S.; Chávez-Fumagalli, M.A.; Mesquita, J.T.; Mata, L.M.; Fernandes, S.O.A.; Cardoso, V.N.; Soto, M.; Tavares, C.A.P.; Leite, J.P.V.; Tempone, A.G.; et al. Antileishmanial activity and evaluation of the mechanism of action of strychnobiflavone flavonoid isolated from Strychnos pseudoquina against Leishmania infantum. Parasitol. Res. 2015, 114, 4625–4635. [Google Scholar] [CrossRef] [PubMed]
- Rizk, Y.S.; Fischer, A.; Cunha, M.d.C.; Rodrigues, P.O.; Marques, M.C.S.; Matos, M.d.F.C.; Kadri, M.C.T.; Carollo, C.A.; de Arruda, C.C.P. In vitro activity of the hydroethanolic extract and biflavonoids isolated from Selaginella sellowii on Leishmania (Leishmania) amazonensis. Mem. Inst. Oswaldo Cruz. 2014, 109, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Rocha, V.P.C.; da Rocha, C.Q.; Queiroz, E.F.; Marcourt, L.; Vilegas, W.; Grimaldi, G.B.; Furrer, P.; Allémann, E.; Wolfender, J.-L.; Soares, M.B.P. Antileishmanial activity of dimeric flavonoids isolated from Arrabidaea brachypoda. Molecules 2018, 24, 1. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Sun, W.; Simeonov, A. Drug repurposing screens and synergistic drug-combinations for infectious diseases. Br. J. Pharmacol. 2017, 175, 181–191. [Google Scholar] [CrossRef]
- Thévenin, M.; Mouray, E.; Grellier, P.; Dubois, J. Facile formation of methylenebis(chalcone)s through unprecedented methylenation reaction. Application to antiparasitic and natural product synthesis. Eur. J. Org. Chem. 2014, 2014, 2986–2992. [Google Scholar] [CrossRef]
- Cane, H.P.C.A.; Saidi, N.; Yahya, M.; Darusman, D.; Erlidawati, E.; Safrida, S.; Musman, M. Macrophylloflavone: A New Biflavonoid from Garcinia macrophylla Mart. (Clusiaceae) for Antibacterial, Antioxidant, and Anti-Type 2 Diabetes Mellitus Activities. Sci. World J. 2020, 2020, 2983129. [Google Scholar] [CrossRef]
- Lee, J.; Kim, M.; Jeong, S.E.; Park, H.Y.; Jeon, C.O.; Park, W. Amentoflavone, a novel cyanobacterial killing agent from Selaginella tamariscina. J. Hazard. Mater. 2020, 384, 121312. [Google Scholar] [CrossRef]
- Xu, H.-X.; Mughal, S.; Taiwo, O.; Lee, S.F. Isolation and characterization of an antibacterial biflavonoid from an African chewing stick Garcinia kola Heckel (Clusiaceae). J. Ethnopharmacol. 2013, 147, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Linden, M.; Brinckmann, C.; Feuereisen, M.M.; Schieber, A. Effects of structural differences on the antibacterial activity of biflavonoids from fruits of the Brazilian peppertree (Schinus terebinthifolius Raddi). Food Res. Int. 2020, 133, 109134. [Google Scholar] [CrossRef] [PubMed]
- Nandu, T.G.; Subramenium, G.A.; Shiburaj, S.; Viszwapriya, D.; Iyer, P.M.; Balamurugan, K.; Rameshkumar, K.B.; Pandian, S.K. Fukugiside, a biflavonoid from Garcinia travancorica inhibits biofilm formation of Streptococcus pyogenes and its associated virulence factors. J. Med. Microbiol. 2018, 67, 1391–1401. [Google Scholar] [CrossRef]
- Mkounga, P.; Fomum, Z.T.; Meyer, M.; Bodo, B.; Nkengfack, A.E. Globulixanthone F. a new polyoxygenated xanthone with an isoprenoid group and two antimicrobial biflavonoids from the stem bark of Symphonia globulifera. Nat. Prod. Commun. 2009, 4, 803–808. [Google Scholar] [CrossRef]
- Bitchagno, G.T.M.; Tankeo, S.B.; Tsopmo, A.; Mpetga, J.D.S.; Tchinda, A.T.; Fobofou, S.A.T.; Nkuete, A.H.L.; Wessjohann, L.A.; Kuete, V.; Tane, P. Ericoside, a new antibacterial biflavonoid from Erica mannii (Ericaceae). Fitoterapia 2016, 109, 206–211. [Google Scholar] [CrossRef]
- Makhafola, T.J.; Samuel, B.B.; Elgorashi, E.E.; Eloff, J.N. Ochnaflavone and Ochnaflavone 7-O-Methyl Ether two Antibacterial Biflavonoids from Ochna pretoriensis (Ochnaceae). Nat. Prod. Commun. 2012, 7, 1601–1604. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.G.d.F.; Pacífico, M.; Vilegas, W.; Dos Santos, L.C.; Icely, P.A.; Miró, M.S.; Scarpa, M.V.C.; Bauab, T.M.; Sotomayor, C.E. Evaluation of Syngonanthus nitens (Bong.) Ruhl. extract as antifungal and in treatment of vulvovaginal candidiasis. Med. Mycol. 2013, 51, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, G.; Santamaria, A.R.; D’Auria, F.D.; Mulinacci, N.; Innocenti, M.; Cecchini, F.; Pericolini, E.; Gabrielli, E.; Panella, S.; Antonacci, D.; et al. Evaluation of anti-Candida activity of Vitis vinifera L. seed extracts obtained from wine and table cultivars. BioMed Res. Int. 2014, 2014, 127021. [Google Scholar] [CrossRef] [PubMed]
- Seleem, D.; Benso, B.; Noguti, J.; Pardi, V.; Murata, R.M. In vitro and in vivo antifungal activity of lichochalcone-A against candida albicans biofilms. PLoS ONE 2016, 11, e0157188. [Google Scholar] [CrossRef]
- Marín, C.; Ramírez-Macías, I.; López-Céspedes, A.; Olmo, F.; Villegas, N.; Díaz, J.G.; Rosales, M.J.; Gutiérrez-Sánchez, R.; Sánchez-Moreno, M. In Vitro and in Vivo Trypanocidal Activity of Flavonoids from Delphinium staphisagria against Chagas Disease. J. Nat. Prod. 2011, 74, 744–750. [Google Scholar] [CrossRef]
- Gervazoni, L.F.O.; Gonçalves-Ozório, G.; Almeida-Amaral, E.E. 2′-Hydroxyflavanone activity in vitro and in vivo against wild-type and antimony-resistant Leishmania amazonensis. PLoS Neglected Trop. Dis. 2018, 12, e0006930. [Google Scholar] [CrossRef]
- Pereira, V.R.D.; Junior, I.J.A.; da Silveira, L.S.; Geraldo, R.B.; Pinto, P.d.F.; Teixeira, F.S.; Salvadori, M.C.; Silva, M.P.; Alves, L.A.; Capriles, P.V.S.Z. In Vitro and in Vivo Antischistosomal Activities of Chalcones. Chem. Biodivers. 2018, 15, e1800398. [Google Scholar] [CrossRef]
- Zhi, H.-J.; Zhu, H.-Y.; Zhang, Y.-Y.; Lu, Y.; Li, H.; Chen, D.-F. In vivo effect of quantified flavonoids-enriched extract of Scutellaria baicalensis root on acute lung injury induced by influenza A virus. Phytomedicine 2019, 57, 105–116. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, L.; Lu, A.; Xue, W. Synthesis and Biological Activity of Novel Oxazinyl Flavonoids as Antiviral and Anti-Phytopathogenic Fungus Agents. Molecules 2022, 27, 6875. [Google Scholar] [CrossRef]
- Ferrazzano, G.F.; Cantile, T.; Roberto, L.; Ingenito, A.; Catania, M.R.; Roscetto, E.; Palumbo, G.; Zarrelli, A.; Pollio, A. Determination of the in vitro and in vivo antimicrobial activity on salivary streptococci and lactobacilli and chemical characterisation of the phenolic content of a plantago lanceolata infusion. BioMed Res. Int. 2015, 2015, 286817. [Google Scholar] [CrossRef]
- Machado, G.H.A.; Marques, T.R.; de Carvalho, T.C.L.; Duarte, A.C.; de Oliveira, F.C.; Gonçalves, M.C.; Piccoli, R.H.; Corrêa, A.D. Antibacterial activity and in vivo wound healing potential of phenolic extracts from jaboticaba skin. Chem. Biol. Drug Des. 2018, 92, 1333–1343. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Plant Secondary Metabolism: Diversity, Function and its Evolution. Nat. Prod. Commun. 2010, 1, 9–12. [Google Scholar] [CrossRef]
| Virus | Compound | Conclusions | Literature Reference |
|---|---|---|---|
| SARS-CoV-2 | Agathisflavone | Replication block by Mpro protease inhibition. Reduces TNF-α (tumor necrosis factor-alpha) levels in infected cells. | [57] |
| 5,6,7-trihydroxy-2-phenyl-4H-chromen-4-one | Interruption of viral RNA replication by blocking the Chymotrypsinlike protease | [61] | |
| Influenza | Ginkgetin | Inhibits sialidase activity. | [62] |
| Hinokiflavone | [63] | ||
| Agathisflavone | Inhibits neuraminidase activity. | [64] | |
| Human immunodeficiency virus (HIV) | Robustaflavone and hinokiflavone | Blocks reverse transcriptase activity. | [65] |
| Morelloflavone | Activity against HIV-1. | ||
| Epstein−Barr virus (EBV) | Garcinianin and talbotaflavone | Inhibit the 12-O-tetradecanoylphorbol-13-acetate-(TPA)-induced Epstein−Barr virus early antigen (EBV-EA) activation in Raji cells. | [66] |
| 3′, 4′, 5, 7-tetrahydroxyflavone | Inhibit the reactivation of EBV through early genes (Zta and Rta) block and interfering with binding of transcription factor Sp1. | [67] | |
| Dengue virus | Hinokiflavone and Amentoflavone | Inhibits RNA-dependant RNA polymerase (DV-NS5 RdRp). | [48,68] |
| Sotetsuflavone and robustaflavone | Inhibits dengue virus NS5 RNA dependent RNA polymerase. | [68] | |
| Agathisflavone | Inhibits NS2B-NS3 protease. | [69] | |
| Podocarpusflavone A | Inhibits the DV-NS5. | [48] | |
| Hepatitis B virus (HBV) | Robustaflavone | Inhibits the DNA polymerase. | [70] |
| Sikokianin A | Reduces HBsAg secretion. | [71] | |
| Hepatitis C virus (HBC) | Amentoflavone | Deregulates all the virus life cycle, including viral entry, replication, and translation. Inhibitor of NS5A. | [72] |
| Herpes simplex virus (HSV) | Amentoflavone | Affects the expression of UL52 (early gene), UL54 (immediate-early gene) and UL27 (late gene). More active against HSV-1. | [73] |
| Agathisflavone | Active against HSV-1 and HSV-2. | [74] | |
| Strychnobiflavone | Interferes with the initial stages of viral infection and reduces HSV-1 protein expression. | [75] | |
| Ginkgetin | Activity against HSV-1 and HSV-2. Inhibits the transcription step in the protein synthesis of HSV-infected cells. | [76] | |
| Coxsackievirus B3 | Amentoflavone | Inhibits fatty acid synthase. | [77] |
| Fungus | Compound | Conclusions | Literature Reference |
|---|---|---|---|
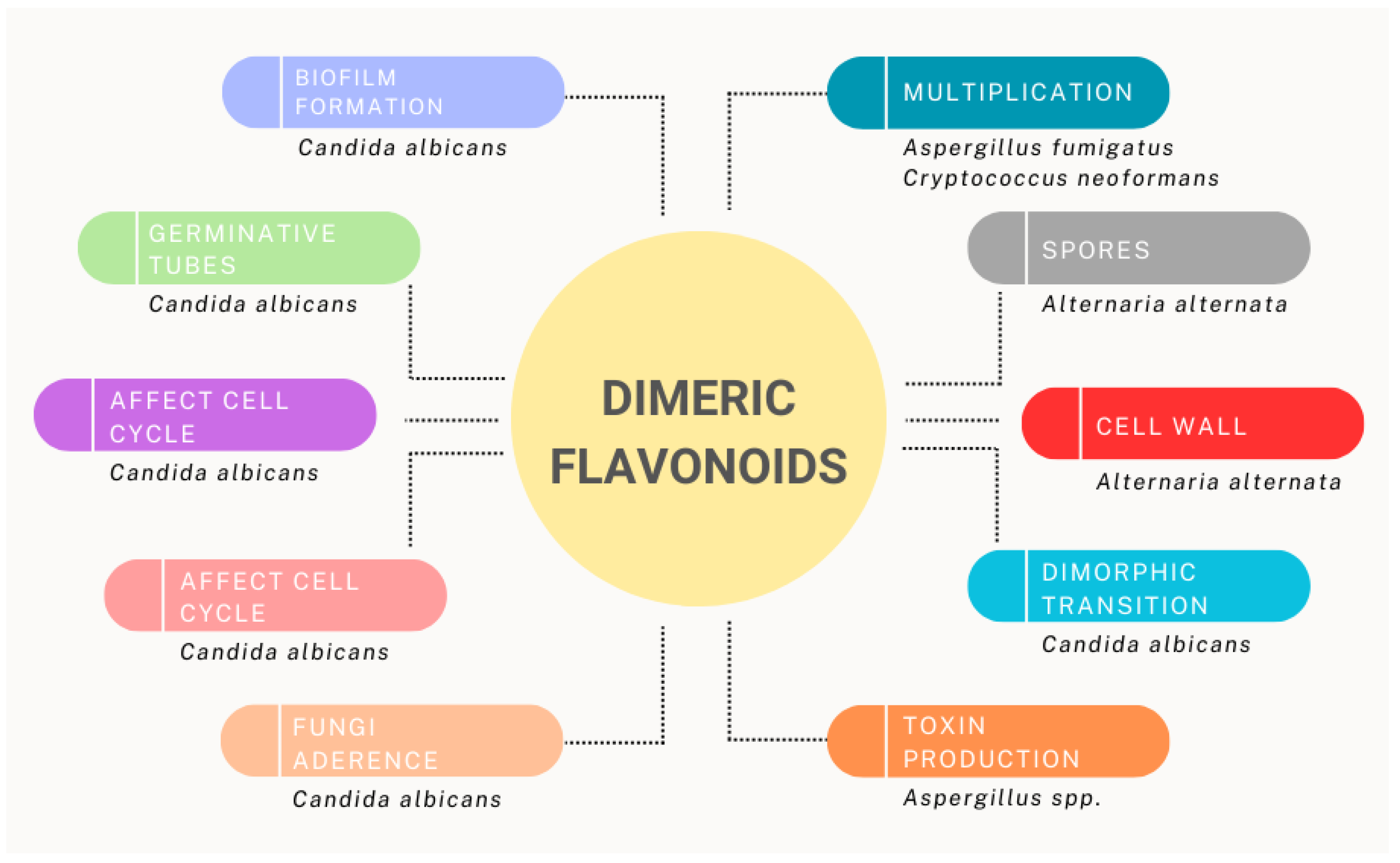
| Candida albicans | Amentoflavone | Fungistatic. Affects cell cycle progress during S-phase. | [81] |
| Interrupts dimorphic transition. Enhances the intracellular trehalose level, which induces a stress response in fungal cells. | [87] | ||
| Quercetin | Inhibits fungal adherence and biofilm formation. | [83] | |
| Kaempferol-3,40-dimethylether | Activates macrophages and increases lysosomal activity. | [88] | |
| Kaempferol, canthin- 6-one, and morin) | Cell membrane damage. | [89] | |
| Proanthocyanidin | Inhibits proliferation and dispersion cells from pre-formed biofilms. | [85] | |
| Aspergillus flavus | Amentoflavone, 7,7″- Dimethoxyagastisflavone, 6,6″-bigenkwanin, and tetramethoxy-6,6″-bigenkwanin | Reduces the production of aflatoxin B1 (AFB1) and B2 (AFB2). | [84] |
| Aspergillus fumigatus | Isoginkgetin | Growth inhibition. | [80] |
| Cryptococcus neoformas | Isoginkgetin | Growth inhibition. | [80] |
| Podocarpusflavone | |||
| Fusarium culmorum | Bilobetin | Inhibits the growth of germinating tubes. | [82] |
| Cladosporium oxysporum | Bilobetin | Inhibits the growth of germinating tubes. | [82] |
| Alternaria alternata | Ginkgetin and 7-O-methylamentoflavone | Inhibits the growth of fungal spores. Small changes in the cell wall. | [82] |
| Protozoa | Compound | Conclusions | Literature Reference |
|---|---|---|---|
| Plasmodium falciparum | 3″,4′,4‴,5,5″,7,7″-heptahydroxy-3,8-biflavanone | Inhibition of α-glucosidase and aromatase. | [51] |
| Lanaroflavone | Mechanism of action unknown. | [94] | |
| 7,4′,7″-tri-O-methylamentoflavone | Mechanism of action unknown. | [95] | |
| Methylenebissantin | Inhibits enoyl-ACP reductase. | [93] | |
| 3,3″-di(7,4″-dihydroxyflavanone-3-yl) | Mechanism of action unknown. | [98,99] | |
| Leishmania panamensis | Lanaroflavone Podocarpusflavone A Podocarpusflavone B Amentoflavona | Interact with Glycoprotein 63. | [92] |
| Leishmania infantum | Strychnobiflavone | Causes depolarization of parasitic mitochondria. | [100] |
| Amentoflavone | Activity against intracellular amastigotes. | [95] | |
| Leishmania donovani | 2,3-Dihydrohinokiflavone | Tested on axenic amastigotes. | [91] |
| Leishmania mexicana | Morelloflavone and Acetate | Interact with recombinant cysteine protease type 2.8 | [91] |
| Leishmania amazonensis | Amentoflavone and robustaflavone | Effective antioxidant activity by increasing nitric oxide (NO) production in macrophages. Strong activity against promastigote and amastigote forms. | [91] |
| 7-O-methyl ochnaflavone | Activity against promastigote forms. | [101] | |
| Brachydin | Reduces the number of amastigotes and infected macrophages. Presents a synergic effect with amphotericin B. Also showed ability to induce damage in Golgi apparatus by accumulation of vesicles. | [102] | |
| Trypanosoma cruzi | 2″,3″-Dihydroochnaflavone | Kills approximately 62% of amastigote forms and 100% of trypomastigotes in infected murine macrophages. The mechanism is unknown. It is also able to inhibit topoisomerase I and topoisomerase II-α, which may be the cause of mitochondrial alterations in the parasitic form. | [96] |
| Brachydin B and C | Inhibits the parasite invasion and its intracellular multiplication in host cells, reducing parasitemia. | [97] |
| Bacteria | Compound | Conclusions | Literature Reference |
|---|---|---|---|
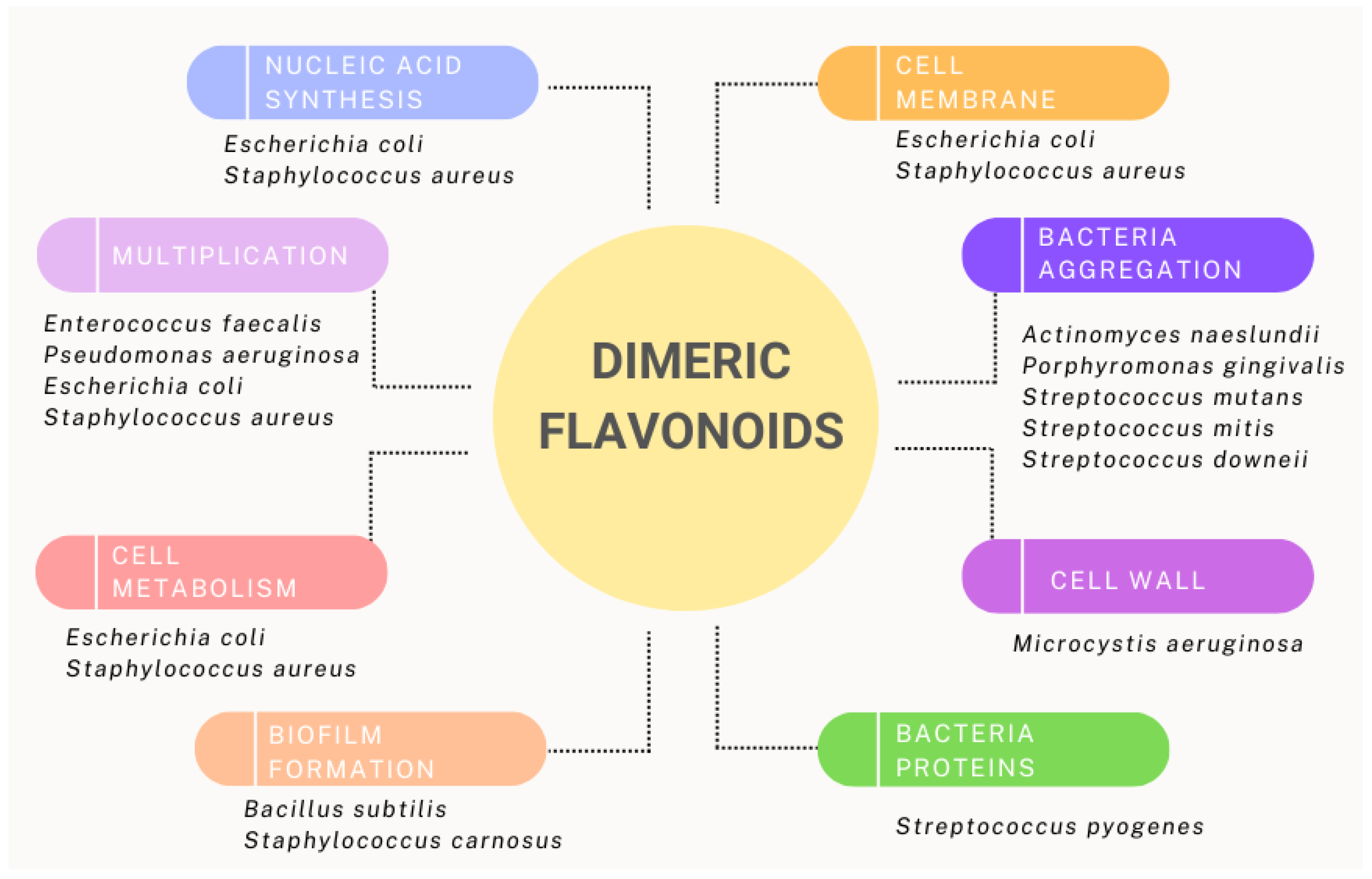
| Staphylococcus aureus | 7, 4′, 7″, 4‴-Tetramethoxy amenthoflavone | The lipophilic nature of the molecules and the external porous peptide cell wall structure of Gram-positive bacteria determined their effect. In Gram-negative bacteria, growth inhibition is lower. | [80] |
| Macrophylloflavone 18 | Inhibits nucleic acid synthesis, cytoplasmic membrane function, energy metabolism, and porins in cell membranes. | [105] | |
| Isoginkgetin | Growth inhibition. | [80] | |
| Podocarpusflavone—A | Mechanism of action unknown. | [80] | |
| Manniflavanone | Mechanism of action unknown. | [110] | |
| Escherichia coli | Macrophylloflavone 18 | Inhibits nucleic acid synthesis, cytoplasmic membrane function, energy metabolism, and porins in cell membranes. | [105] |
| Isoginkgetin | Growth inhibition. | [80] | |
| Ericoside | Mechanism of action unknown. | [111] | |
| Bacillus subtilis and Staphylococcus carnosus | Agatisflavone 2, amentoflavone 1, and Tetrahydroamentoflavone (THAF) | Inhibition of biofilm formation. Dimerization and a reduced C ring contribute to greater activity of the compounds. | [108] |
| Streptococcus pyogenes | Fukugiside | Exhibited concentration-dependent biofilm inhibition by destabilizing the biofilm matrix and by inhibiting M proteins. | [109] |
| Pseudomonas aeruginosa | Ochnaflavone and ochnaflavone 7-O-methylether 15c | Mechanism of action unknown. | [112] |
| Microcystis aeruginosa | Amentoflavone | Bacteria lose their round shape and eventually succumb completely. Affects the peptidoglycan layer and reduces pressure, which ends with the leaking of cell contents. Effects are dose-dependent. | [106] |
| Enterococcus faecalis | Podocarpusflavone—A | Mechanism of action unknown. | [80] |
| Manniflavanone | Mechanism of action unknown. | [88] | |
| Isoginkgetin | Growth inhibition. | [80] | |
| Ochnaflavone and ochnaflavone 7-O-methylether 15c | Mechanism of action unknown. | [112] | |
| Actinomyces naeslundii, Porphyromonas gingivalis, Streptococcus mutans, Streptococcus mitis and Streptococcus downeii | 3″,4′,4‴,5,5″,7,7″-heptahydoxy-3-8″-biflavone | Inhibition of glucan synthesis, glucose uptake and metabolism. Induces bacterial aggregation. | [107] |
| Klebsiella pneumoniae | Ericoside | Mechanism of action unknown. | [111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, I.; Campos, C.; Medeiros, R.; Cerqueira, F. Antimicrobial Activity of Dimeric Flavonoids. Compounds 2024, 4, 214-229. https://doi.org/10.3390/compounds4020011
Lopes I, Campos C, Medeiros R, Cerqueira F. Antimicrobial Activity of Dimeric Flavonoids. Compounds. 2024; 4(2):214-229. https://doi.org/10.3390/compounds4020011
Chicago/Turabian StyleLopes, Inês, Carla Campos, Rui Medeiros, and Fátima Cerqueira. 2024. "Antimicrobial Activity of Dimeric Flavonoids" Compounds 4, no. 2: 214-229. https://doi.org/10.3390/compounds4020011
APA StyleLopes, I., Campos, C., Medeiros, R., & Cerqueira, F. (2024). Antimicrobial Activity of Dimeric Flavonoids. Compounds, 4(2), 214-229. https://doi.org/10.3390/compounds4020011







