Abstract
The thionamide antithyroid agents were discovered largely through observations carried out by various researchers in the 1940s that found that sulfhydryl-containing substances were goitrogenic in animals. Prof. Edwin B. Astwood started using these drugs to treat hyperthyroidism. In the current paper, we summarize the development background of these agents and the coordination possibility of 2-thiouracil and its derivatives, as well as the biological activities of some of its complexes. Some of them are used as agents for the treatment of tuberculosis, and arthritis, others have bactericidal and fungicidal activity, the third cytotoxic properties, and could be used to treat various types of cancer.
1. Introduction
Uracil is a pyrimidine derivative identified in nucleic acids [1]. It is a pyrimidine base with four distinct binding sites, such as N1, N3, O2, and O4 atoms. It belongs to a group of pyrimidines that have a significant role in the structure and function of certain enzymes and medicines.
2-thiouracil has been found to inhibit thyroid hormone biosynthesis. In 1942, Prof. Edwin W. Astwood started using 2-thiouracil to treat patients with Graves’ disease [2]. The thyroid gland (Lat. Glandula thyr(e)oidea) is an important endocrine gland. It reproduces the hormones T3, T4, and calcitonin, whose main role is to stimulate the metabolism, growth, and development of cells and several organs. It should be noted that possible ways to treat patients with Graves’ disease include conservative treatment, which consists of taking antithyroid drugs—thiamazole (methizol, tyrozole) and propylthiouracil (propicillin), surgical treatment or radioactive iodine therapy. Methylthiouracil has been identified as an antithyroid drug. It enhances cell growth and proliferation, accelerates wound, ulcer, and burn healing, and increases resistance to infection. A characteristic feature of the drug is that it stimulates the effect of hematopoiesis (formation and strengthening of leukocytes and erythrocytes in bone marrow). It shows a similar mechanism of action and side effects as propylthiouracil. The drug acts to reduce the production of stored hormones, such as thyroglobulin in the thyroid gland.
Recently, Mao et al. have assessed the reduction effects associated with intrathyroidal injection of dexamethasone on the relapse rate of hyperthyroidism in individuals newly diagnosed with Graves’ disease [3]. At present, propylthiouracil is regarded as the preferred treatment for hyperthyroidism during pregnancy [4]. The literature contains various reviews on the subject [5,6,7,8].
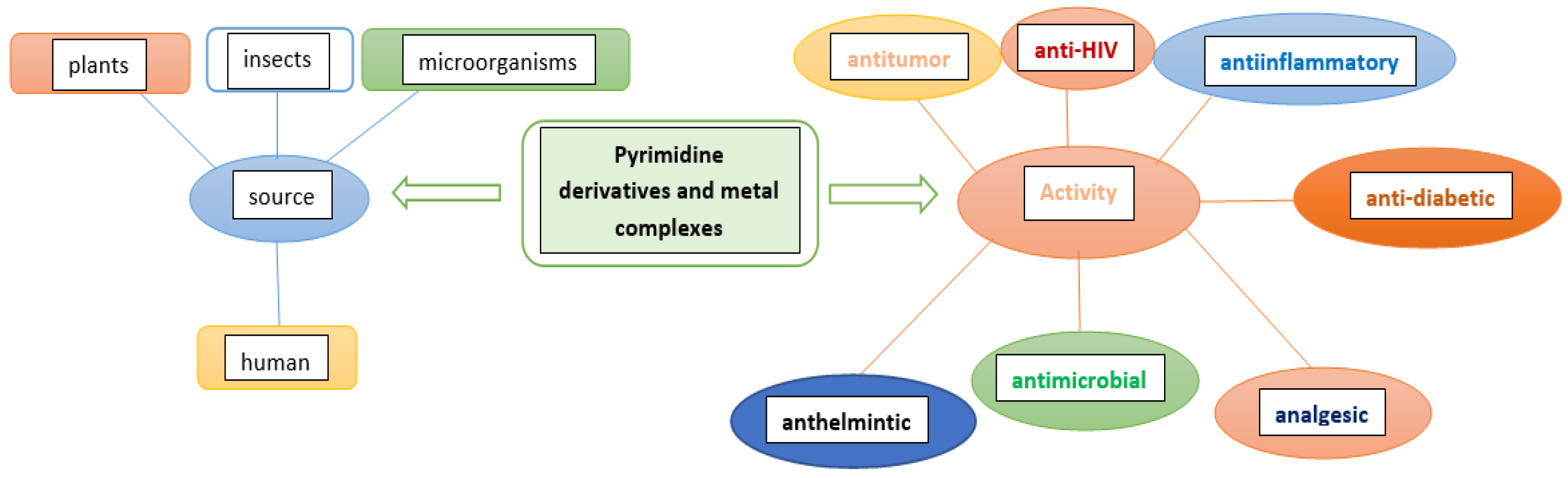
Patil presented a review of the biological potency and the structure–activity relationship of pyrimidines such as antitumor, anti-HIV, anti-diabetic, antimicrobial, anti-inflammatory, analgesic, anthelmintic, CNS depressants, and cardiac agents [9]. Verbitskiy et al. reported a new antituberculosis drug among 1,3- and 1,4-diazines [10] (Scheme 1). Many new pyrimidine derivatives were both recently designed and developed for their antitumor properties. Mahapatra et al. focused on the structure–activity relationship (SAR) of pyrimidine derivatives as an antitumor drug in recent years [11]. Wu et al. synthesized new pyrimidine derivatives containing an amide moiety and assessed against different pathogenic fungi [12]. The new pyrimidine derivatives were obtained, characterized, and designed with regard to their anticancer activities [13].
Scheme 1.
Different applications of bioactive pyrimidines and their metal complexes.
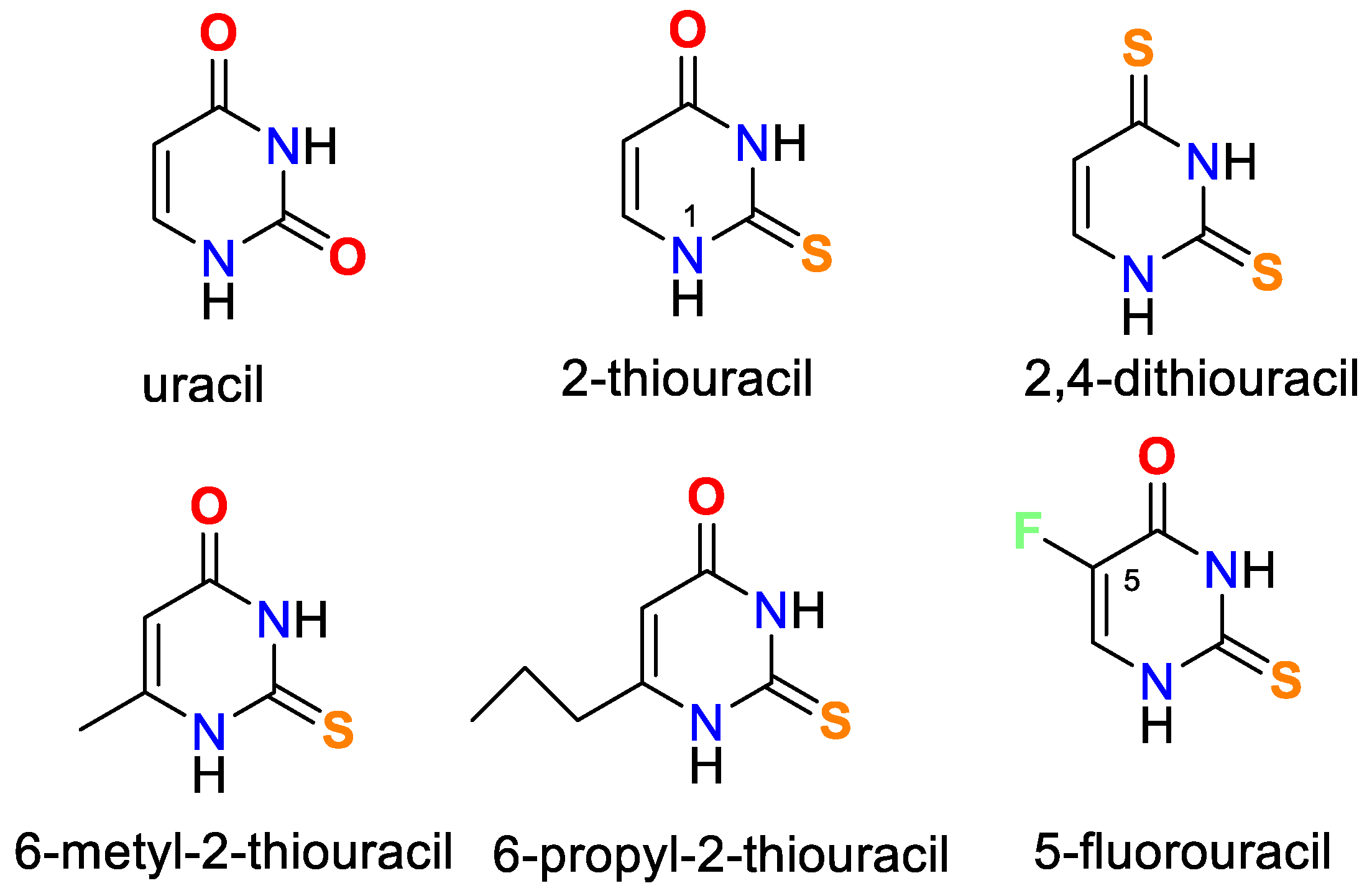
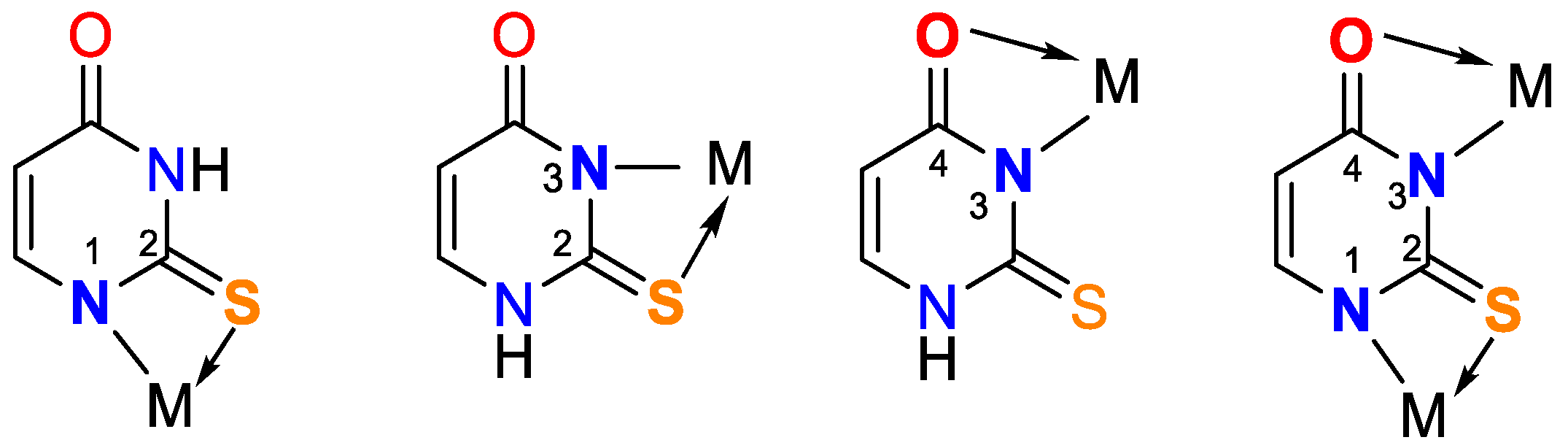
Oladipo and Isola have published a thorough review of uracil’s coordination capabilities and the practical use of some of its complexes [14]. Recently, Masoud et al. have presented a review of complex properties and applications of some biological activities of nucleic acid [15]. In Figure 1 the chemical structure of uracil, 2-thiouracil, and some of their derivatives is given.
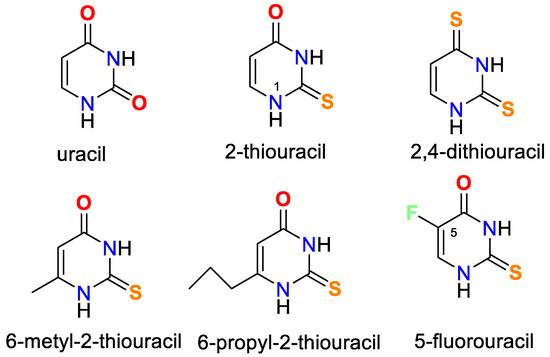
Figure 1.
Structures of uracil, 2-thiouracil, and some of their derivatives.
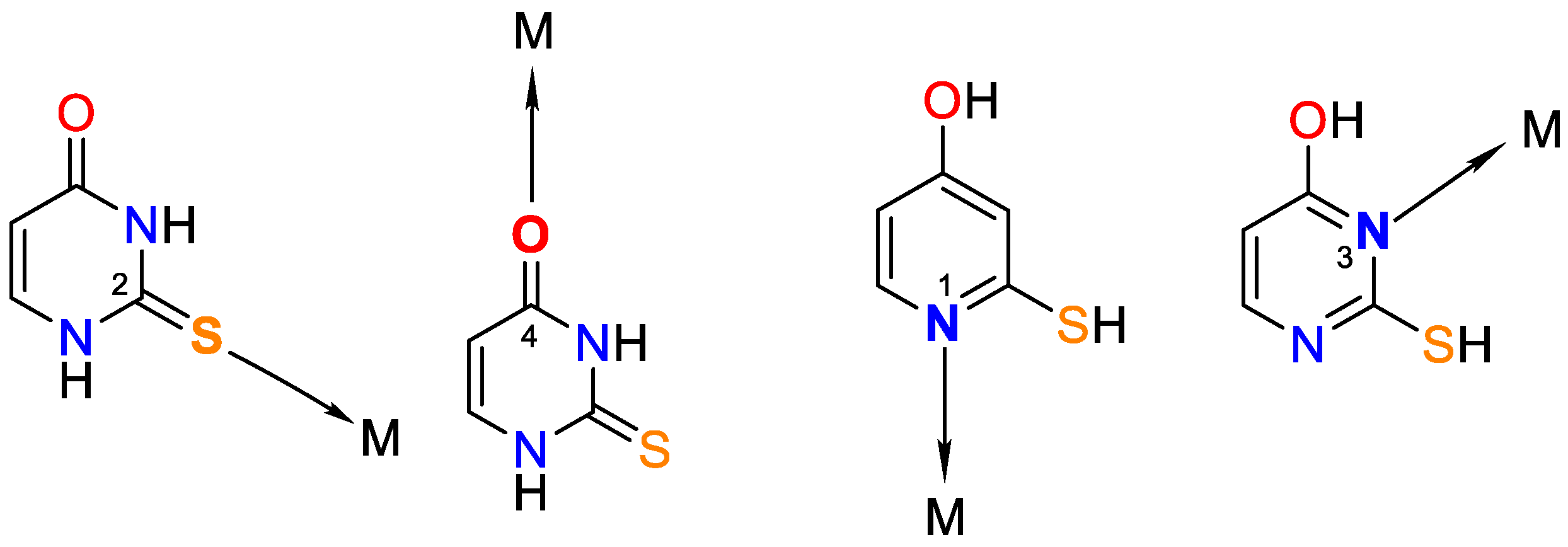
It is worth noting that uracil, 2-thiouracil, 2,4-dithiouracil, and its derivatives are of chemical interest as they have four donor atoms for coordination with a metal ion/two O- and two N- or 1-O, 1-S- and 2-N atoms or 2 S- and 2-N atoms. It is evident that they demonstrate various possible ways to coordinate 2-thiouracil with metal ions, as follows in Figure 2 and Figure 3:
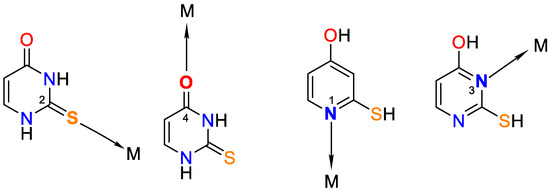
Figure 2.
Monodentate coordination of 2-thiouracil with one of the four donor atoms, without any prior deprotonation (the tautomeric form participates in N1 and N3 coordination).
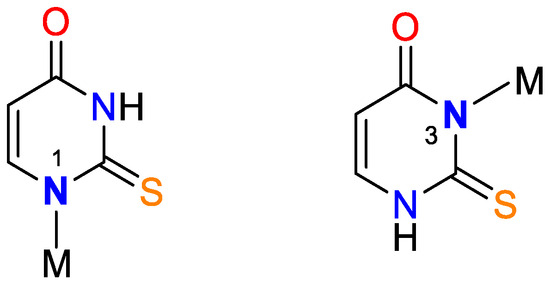
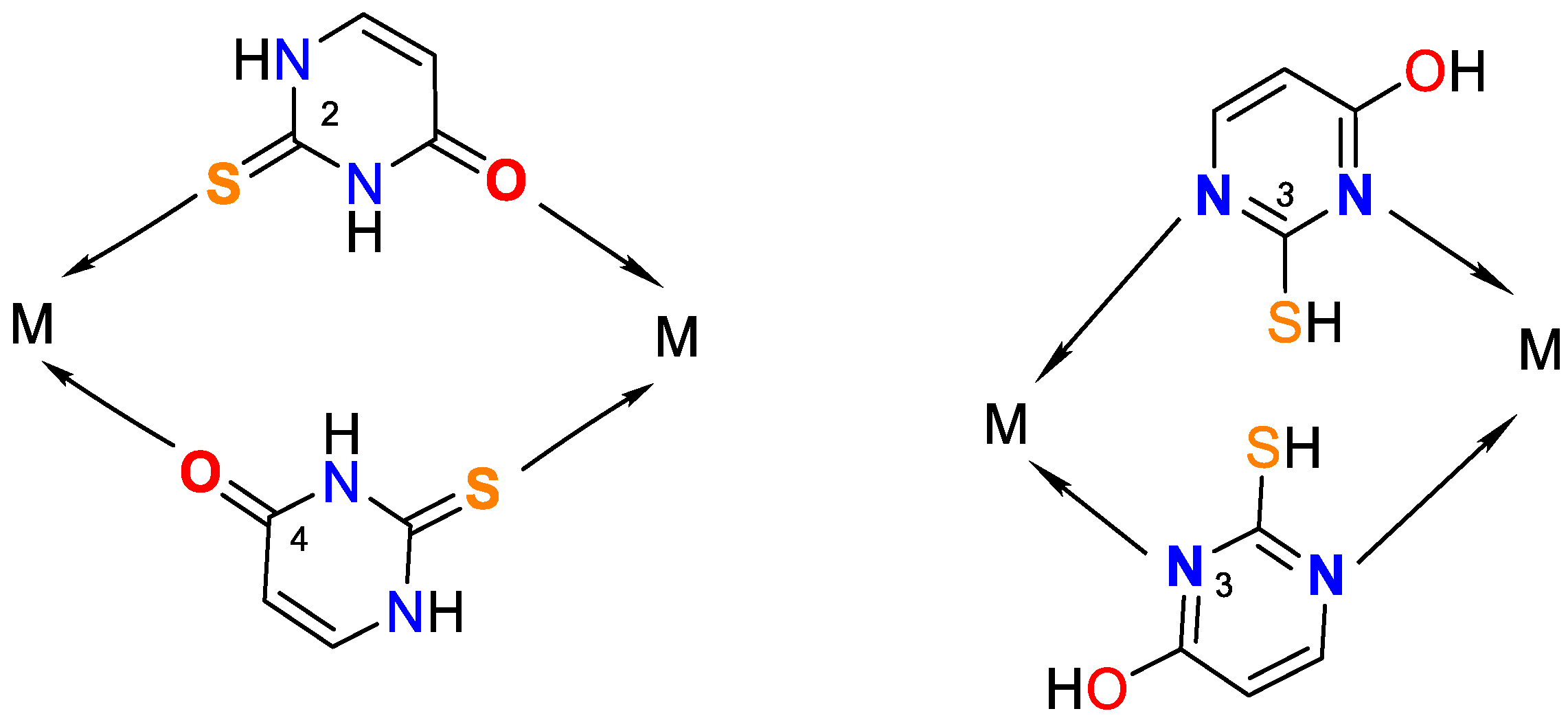
Figure 3.
Monodentate coordination with deprotonated ligands that participate as a monoanionic (through a deprotonated nitrogen atom in the first position N1 or a nitrogen atom in the third position N3).
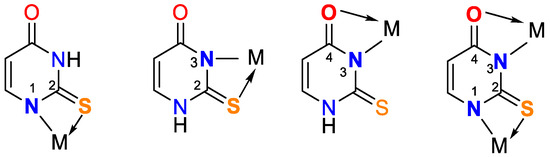
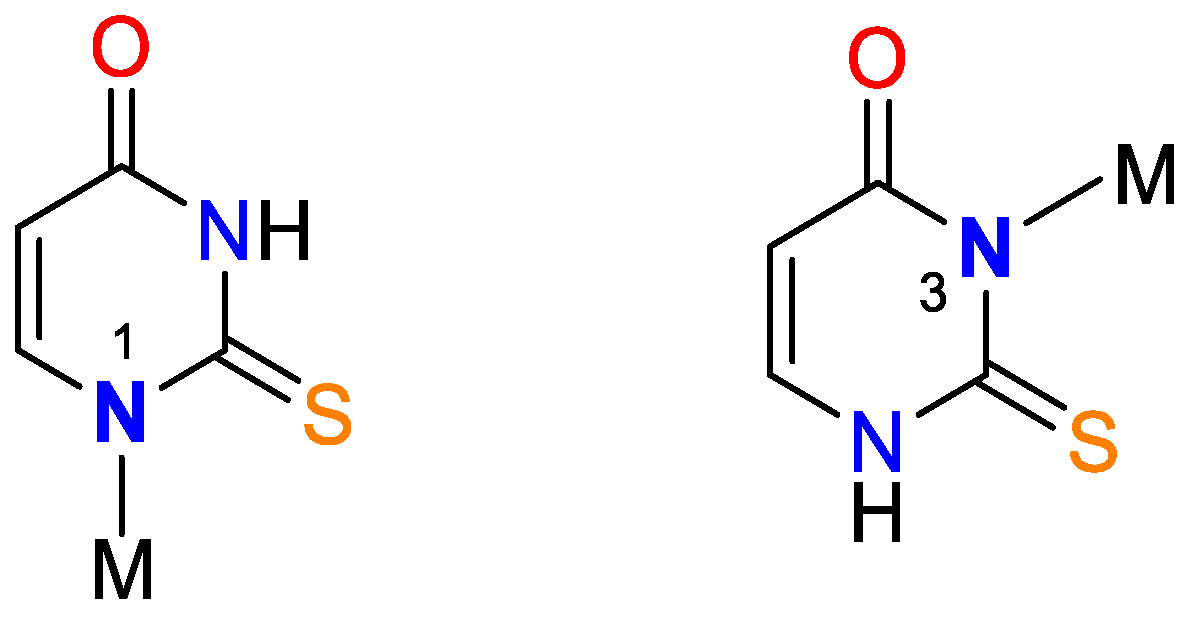
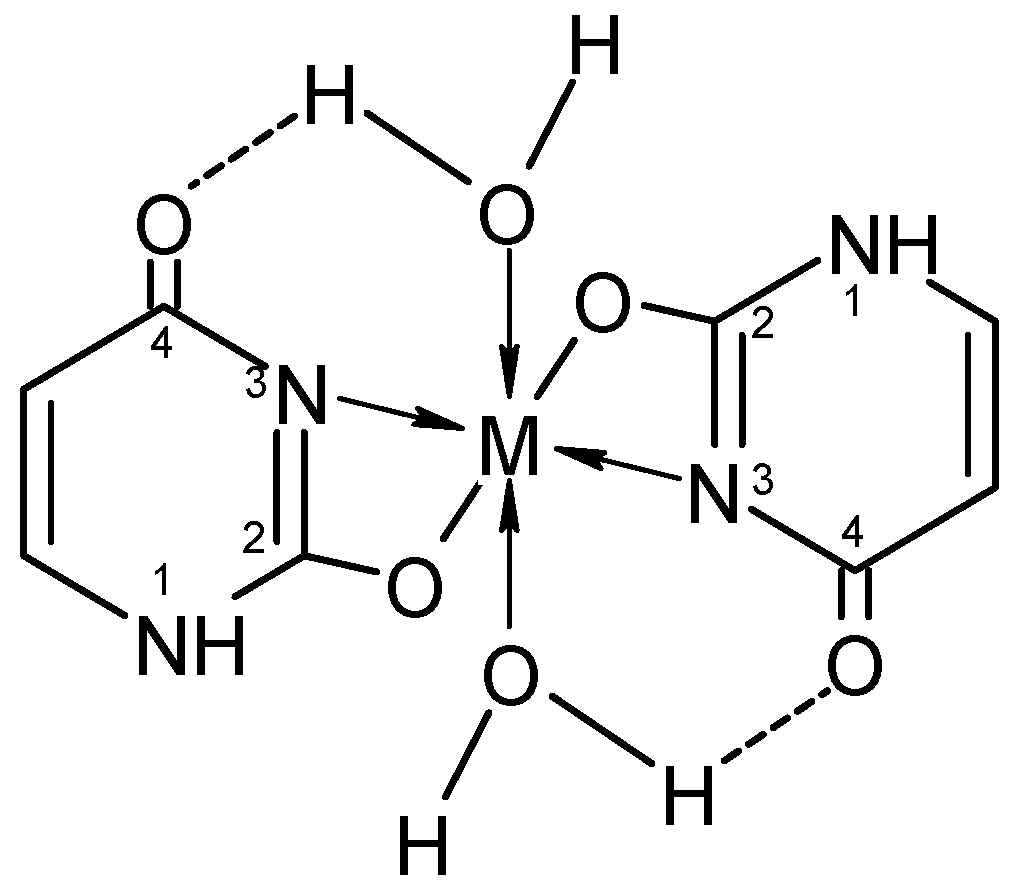
Figure 4.
Bidentate coordination with formed chelate.
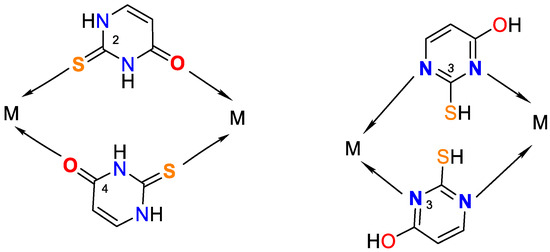
Figure 5.
Possible bridge ways of 2-thiouracil coordination with metal ions.
It presents the biological activity of 2-thiouracil and metal complexes: some of them are used as agents for fighting tuberculosis and arthritis, others have bactericidal and fungicidal action, and the third is cytotoxic activity.
1.1. Synthesis of Metal Complexes with Uracil and Its Derivatives
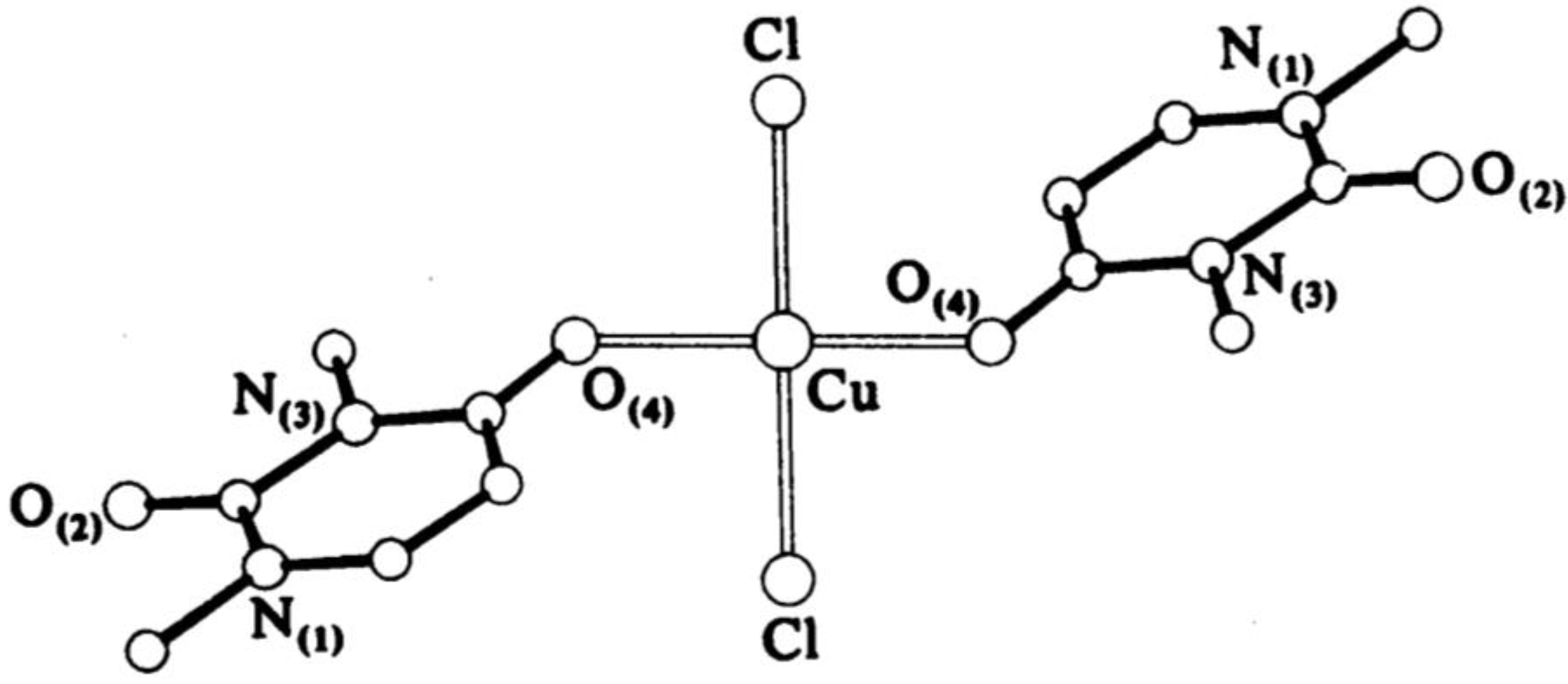
Narang et al. [16] have obtained complexes of Fe(III) and Cr(III) with uracil through the basic formula [M(Uracil)(H2O)2–(OH)Cl], where M = Cr(III) or Fe(III). The complexes have been characterized via elemental analysis, UV-Vis, EPR, and IR spectroscopic methods. Based on the obtained results, the authors suggest a polymer structure with an octahedral geometry of the metal center, with the ligand coordinated through the O(4)- and N(1)-atoms, while histidine coordinates through the O atom of –CO2− and the N atom of the –NH2 groups. Cartwright et al. [17] have obtained a bis-(1,3-dimethyluracil)-dichloridocopper(II) complex, which has been studied via elemental analysis, IR spectroscopy, and X-ray diffraction. The data from the analysis show that the uracil coordinates to the metal center monodentate with the participation of an O-atom in the fourth position of the pyrimidine ring and two chloride ions coordinate to Cu(II), so the complex is neutral with a planar-square geometry. The complexation structure is given in Figure 6.
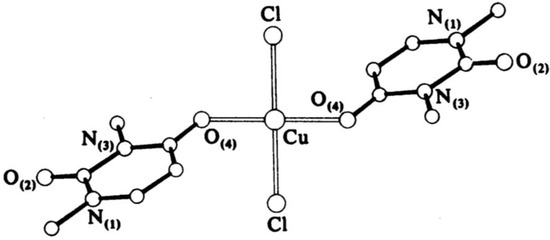
Figure 6.
The structure of bis-(1,3-dimethyluracil)-dichloridocopper(II) complex [17].
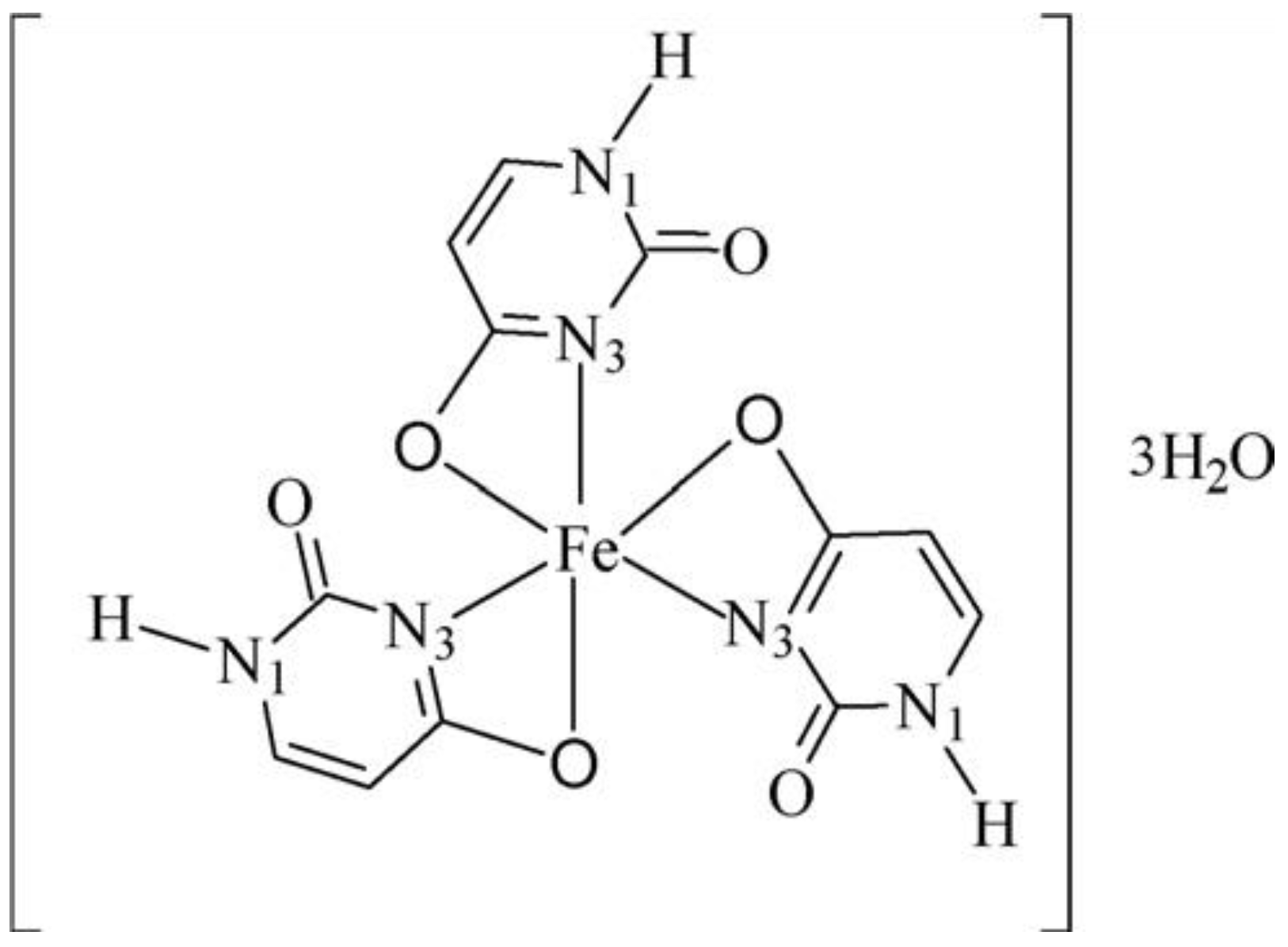
A complex of Fe(III) with uracil and complexes of thiouracil and 5-(phenylazo)-thiouracil with Co(II), Ni(II), and Cu(II) have been obtained [18]. The new metal complexes have been characterized via elemental analysis, DTA, UV-Vis, IR, and Mössbauer spectroscopy. In all complexes, uracil and other free ligands act in a bidentate fashion with coordination occurring through the O(4)- and N(3)-atoms. The coordination bond lengths of the octahedral Co(II) and Ni(II) complexes are smaller compared to the square-planar Cu(II) complex [18]. In Figure 7 the suggested structure of iron(III)-uracil complex is given.
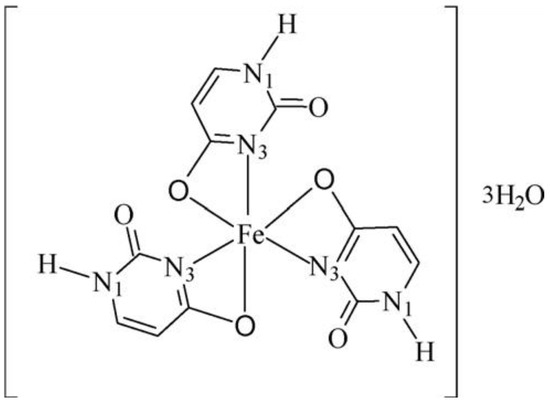
Figure 7.
Suggested structure of the iron(III)–uracil complex [18].
Ghosh et al. [19] have synthesized new complexes of uracil with a general formula: [ML2(H2O)2], where M = Mn, Fe, Co, Ni, or Cu; L = uracil. Electronic spectra indicate octahedral coordination for all complexes and data from comparative IR spectra show chelate structure via the O(2)- and N(3)-atoms of uracil. In Figure 8 the proposed structure of the complexes is given.
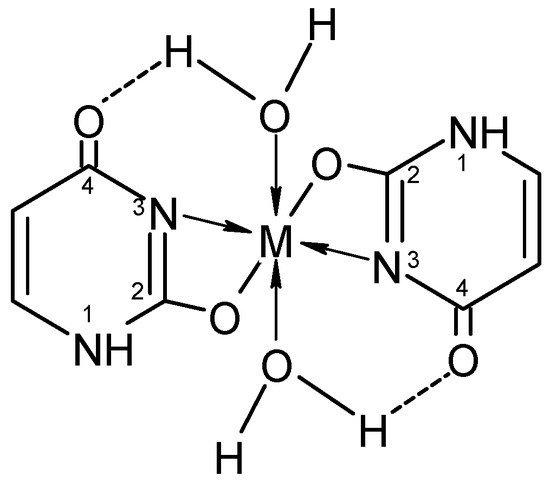
Figure 8.
The proposed structure of the complexes [19].
The synthesis, spectroscopic analysis, and investigation of the biological properties of Ni(II), Cu(II), and Co(II) complexes formed by Schiff base ligands obtained from 5-aminouracil, 2-hydroxy-1-naphthaldehyde, 2,4-dihydroxybenzaldehyde, and salic-ylaldehyde have been reported [20]. In each instance, the complexes seem to exist as monomers. The ligands exhibit bidentate coordination with Ni(II) and Co(II), while with Cu(II), they coordinate in a tridentate manner, involving the carbonyl oxygen atom in the fourth position of the uracil ring. In Figure 9 the proposed structure of new metal complexes is presented.
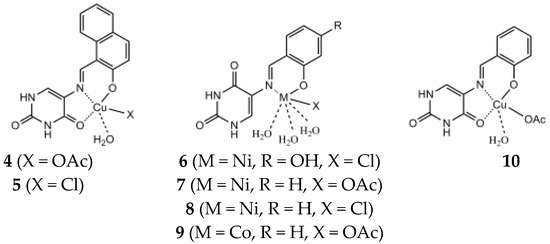
Figure 9.
Proposed structures of the metal complexes [20].
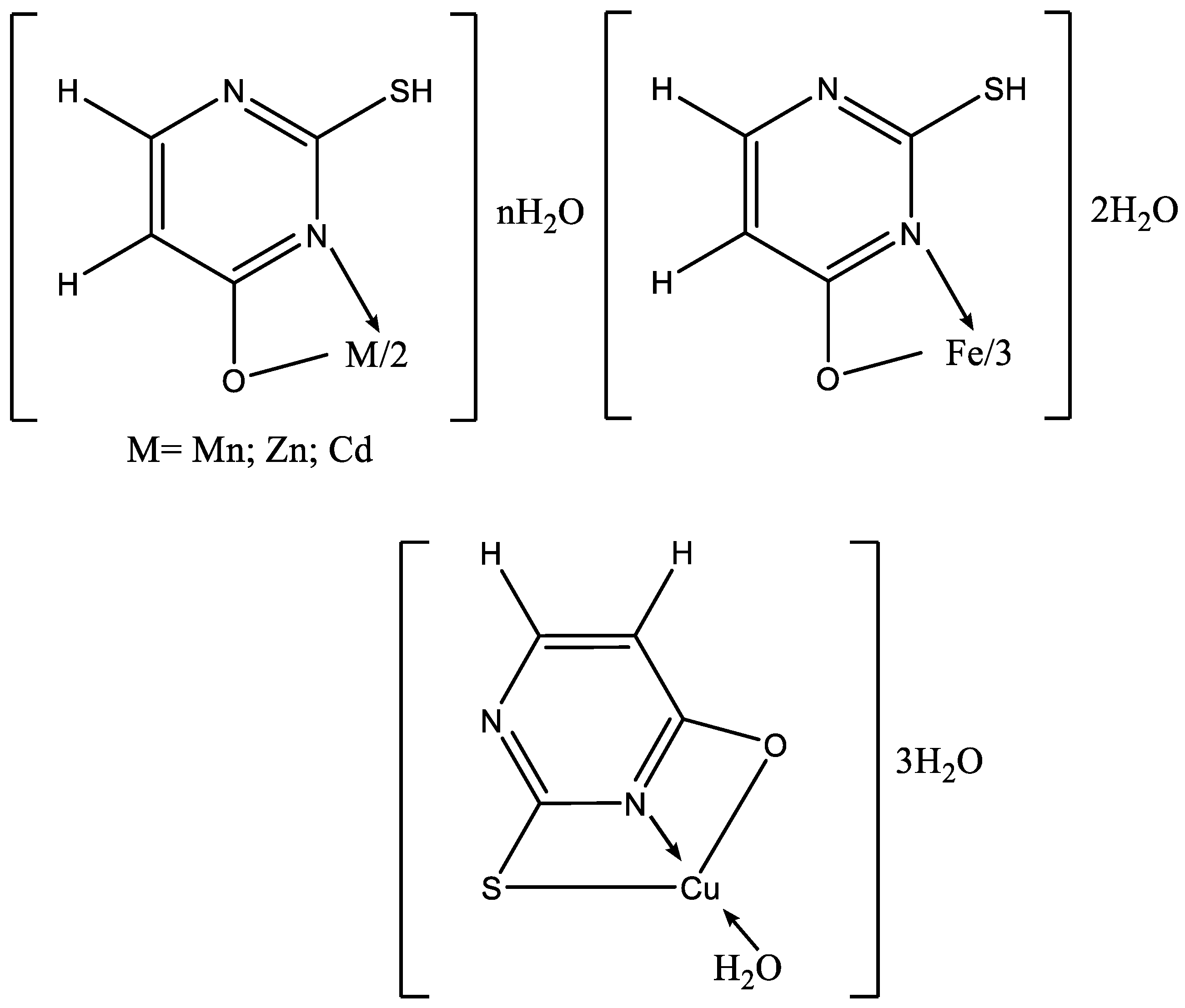
The complexes of M2+, M = Co, Ni, and Zn with 6-chloromethyluracil, 5-hydroxymethyluracil, uracil, 6-methyluracil, and dimethyl-6-uracilmethylphosphonate have been synthesized [21]. Based on the data obtained, we may assume that the ligands are coordinated to the metal ion through the N(3)-atom of Co(II) and Ni(II), as well as a hydroxo complex MLH−1 with deprotonated water of the inner coordination sphere [21]. Mixed ligand complexes play a crucial role in different biological environments. Some mixed ligand complexes of Co(II), Ni(II), Cu(II), Zn(II), and Cd(II) have been obtained by Tyagi et al. by using the corresponding nitrates as starting metal salts and 5-fluorouracil and histamine [22]. New complexes have been characterized by elemental analysis, UV-Vis, and IR spectroscopy, as well as by the X-ray diffraction method. Data from X-ray structural analysis indicates that 5-fluorouracil is coordinated through the N(3)-atom. Mixed ligand complexes of Co(II), Ni(II), Cu(II), Zn(II), and Cd(II) with adenine and uracil have been obtained [23]. The complexes have been characterized via elemental analysis, UV-Vis, and IR spectroscopy, as well as using powder X-ray diffraction studies. The authors suggest the complexes may be polymeric in nature using adenine, as well as –OH group as bridging ligands. During polymerization, one metal atom is bound via the N(3)-atom of one adenine ligand and the N(7)-atom of another adenine ligand. Uracil acted in coordination via O-atom in the second position of the pyrimidine ring [23]. Based on the results obtained, Abdullah suggests a polymer structure with an octahedral geometry of the metal center for all synthesized complexes. Mixed ligand complexes of Th(II), Ce(II), and Gd(II) with uracil and omeprazole have been synthesized as per the general formula: [M(Ome)(ura)·4H2O)]SO4·xH2O [24]. The compounds have been researched via elemental analysis, UV-Vis, IR-, Mass, and 1H NMR spectroscopy. Based on the data obtained, it is suggested that uracil acts as a bidentate ligand, binding through the N(3)- and O(2)-atoms [24]. In Figure 10 the suggested structure of metal complex is given.
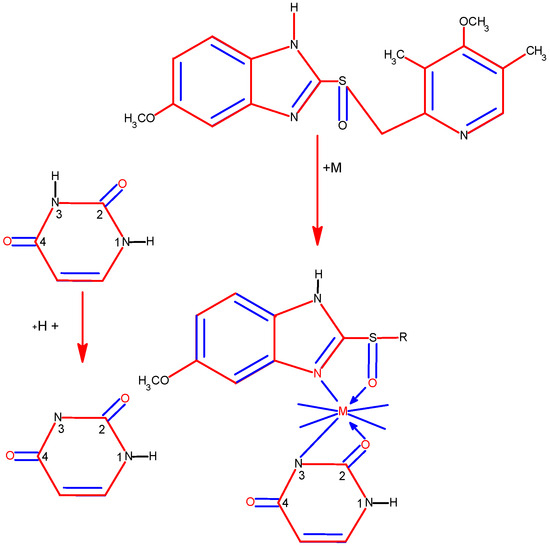
Figure 10.
Proposed scheme of formation of representative ternary complex (M-OME-URA) [24].
Mixed ligand complexes of Ni(II), Cu(II), and Zn(II) with 5-fluorouracil and amino acids have been obtained by Shobana et al. [25]. Magnetic moment values together with the electronic spectral data indicate 5-fluorouracil coordinates with the metal ion in a bidentate manner through the C(4)=O and N(3) atoms. They also prescribe the amino acids behavior as bidentate by nitrogen and carboxylate oxygen with formed 4, 5, and 6-membered chelate rings. All the Cu(II) mixed ligand compounds demonstrate a distorted tetrahedral geometry, further supported by the ESR studies. In Figure 11 the suggested structure of metal complexes is presented.
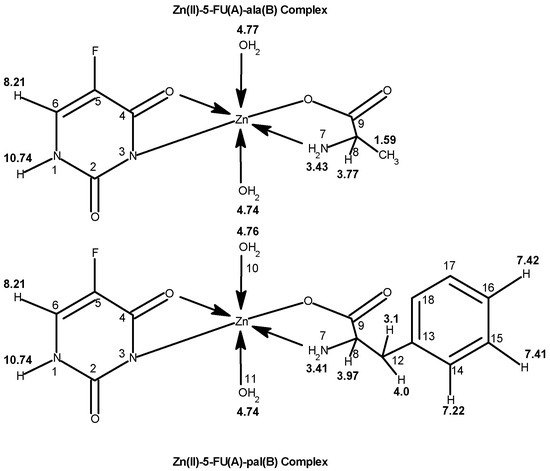
Figure 11.
The structure of metal complexes [25].
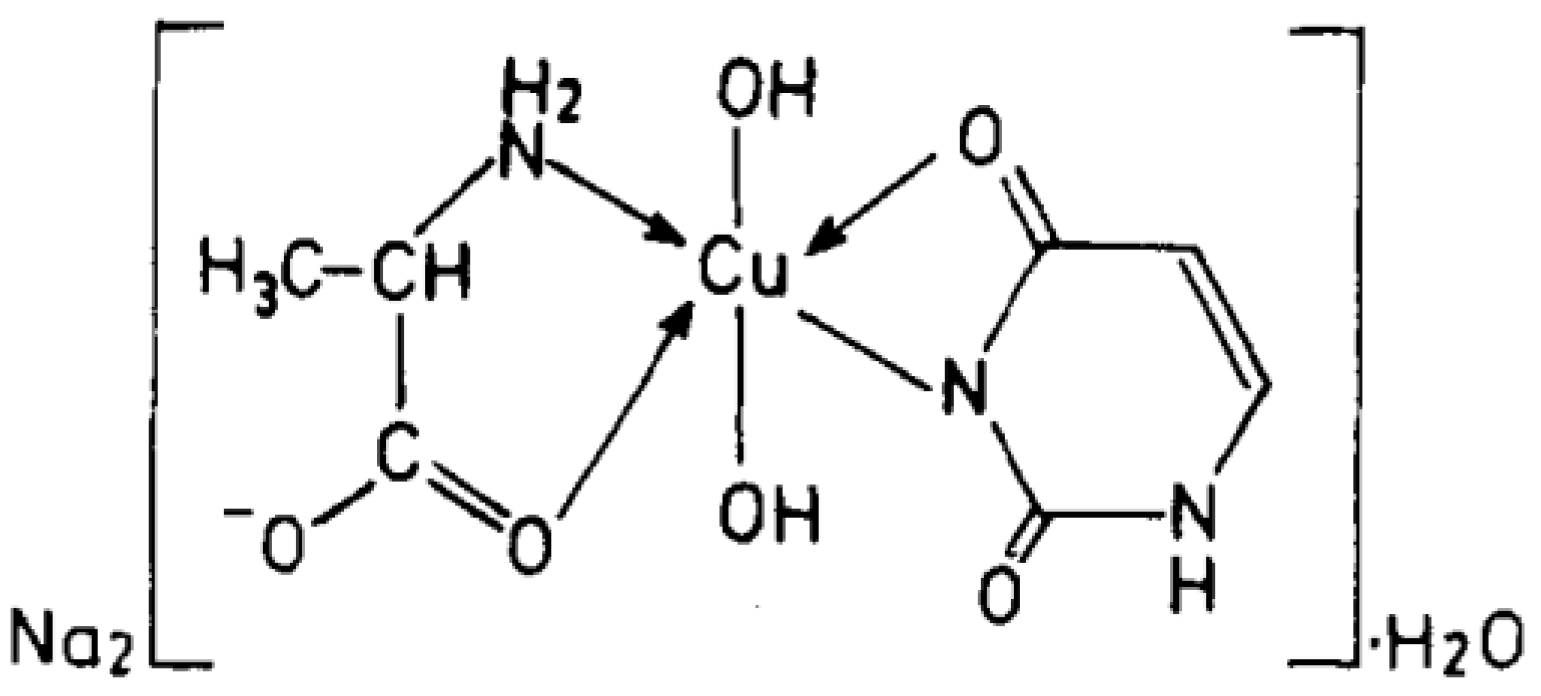
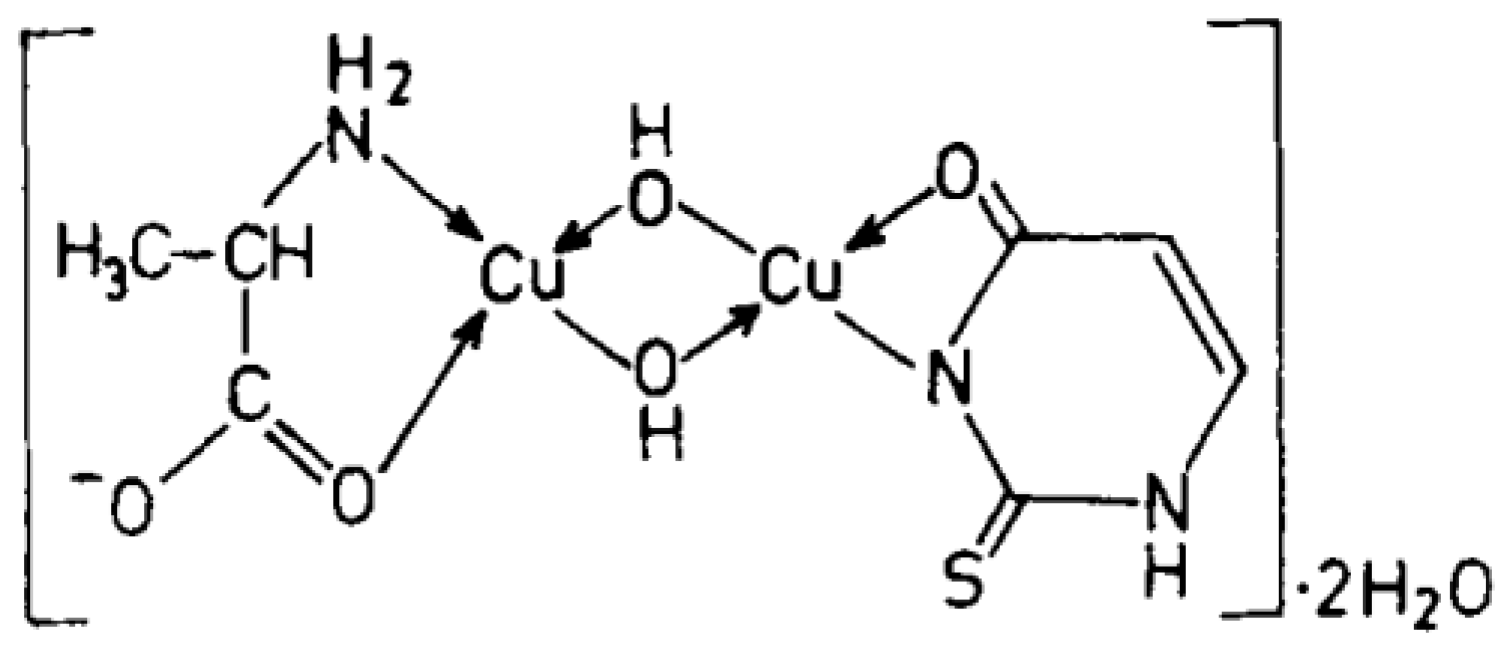
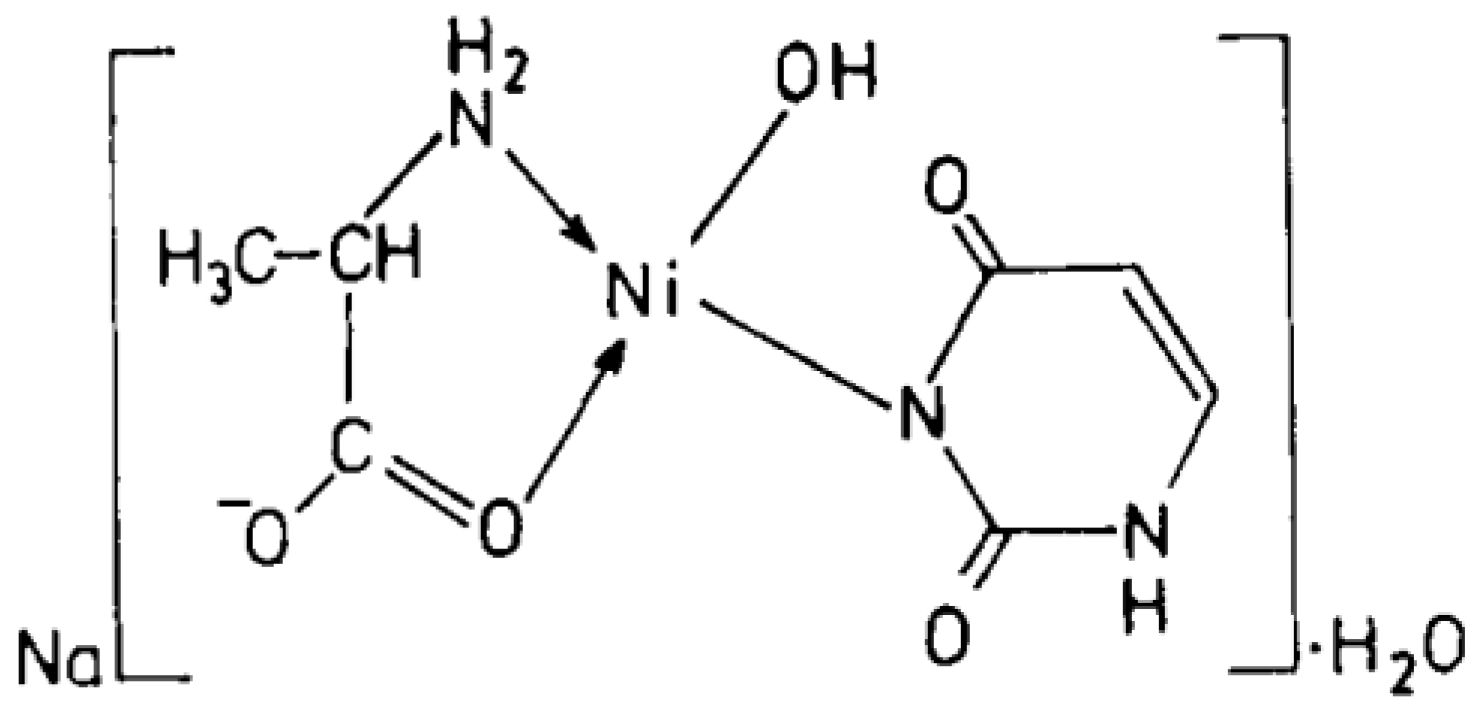
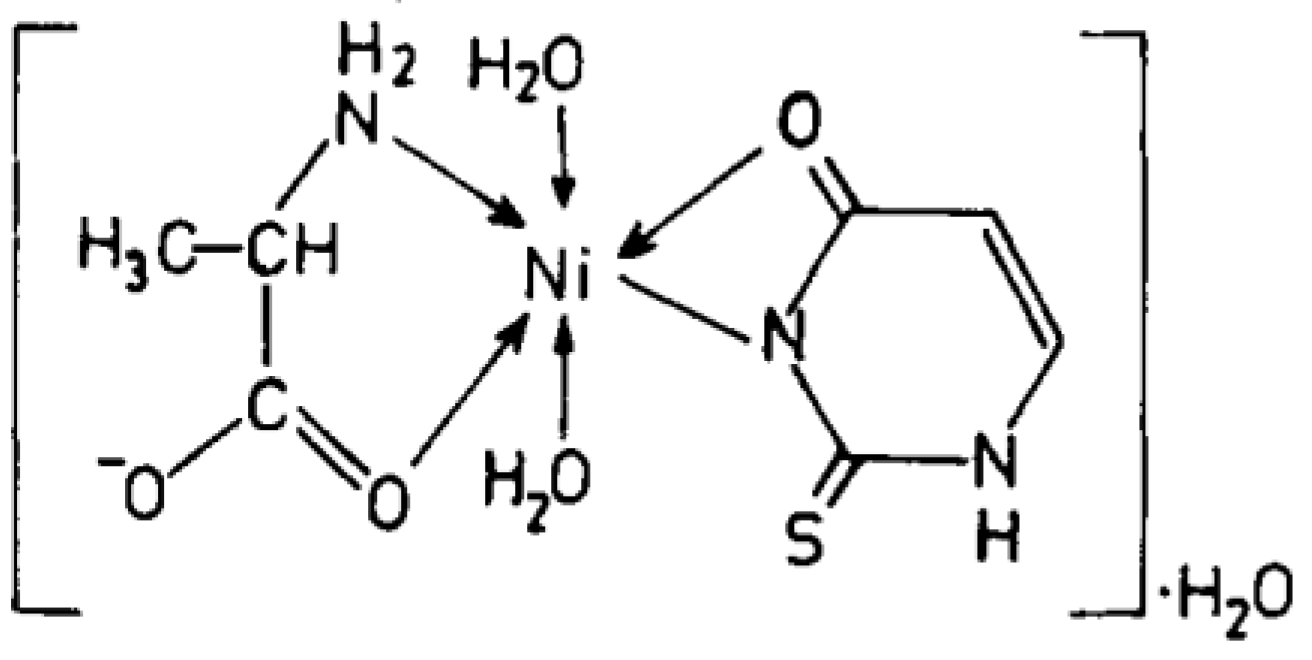
Cu(II), Ni(II), Co(II), and Zn(II) mixed ligand complexes, comprising alanine in conjunction with either uracil or 2-thiouracil, have been synthesized and characterized [26]. The findings indicate alanine consistently exhibits bidentate coordination, utilizing both –NH2 and COO− groups. Uracil acts as a bidentate ligand in the Cu(II) complex, coordinating via one carbonyl O- and N-atoms, while in other instances, it is coordinating solely via N-atom, as monodentate. In the presence of thiouracil, Cu(II) and Ni(II) complexes involve coordination from carbonyl O- and one N-atoms, whereas the Co(II) complex coordination occurs from S- and N-atoms. The Zn(II) complex displays tridentate behavior, coordinating through O-, S-, and N-atoms. The mixed complexes of Cu(II), Co(II), and Zn(II) with uracil, as well as Ni(II) and Zn(II) complexes with thiouracil, exhibit octahedral geometry. The mixed Ni(II) complex with uracil displays distorted tetrahedral geometry, while the mixed Co(II) thiouracil complex adopts a square-planar structure. Notably, the mixed Cu(II) thiouracil complex features a binuclear structure with a square-planar arrangement around each copper atom [26]. The suggested structure of the complexes is given in Figure 12, Figure 13, Figure 14 and Figure 15.
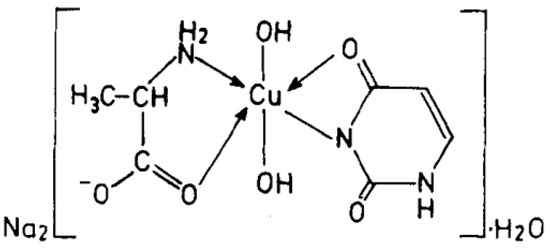
Figure 12.
Cu(II)-Alanine-Uracil Complex [26].
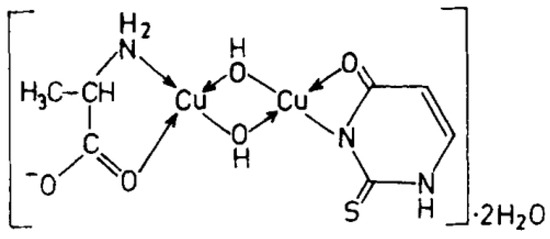
Figure 13.
Cu(II)-Alanine-2-Thiouracil Complex [26].
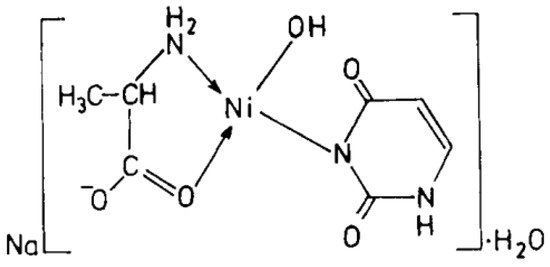
Figure 14.
Ni(II)-Alanine-Uracil Complex [26].
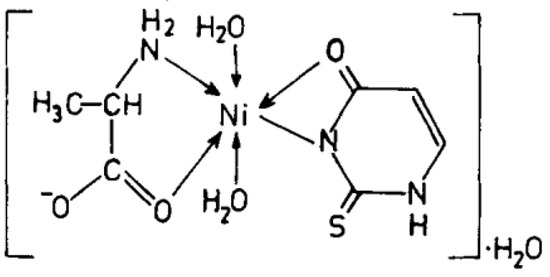
Figure 15.
Ni(II)-Alanine-2-Thiouracil Complex [26].
Srivastava et al. have published papers on the synthesis and characterization of mixed ligand complexes of glycine and uracil or 2-thiouracil, thymine or adenine with histidine and uracil, thymine or 2-thiouracil with glycine, alanine, valine, and leucine with Cu(II), Ni(II), Co(II), and Zn(II) [27,28,29,30].
1.2. Synthesis of Metal Complexes with 2-Thiouracil and Its Derivatives
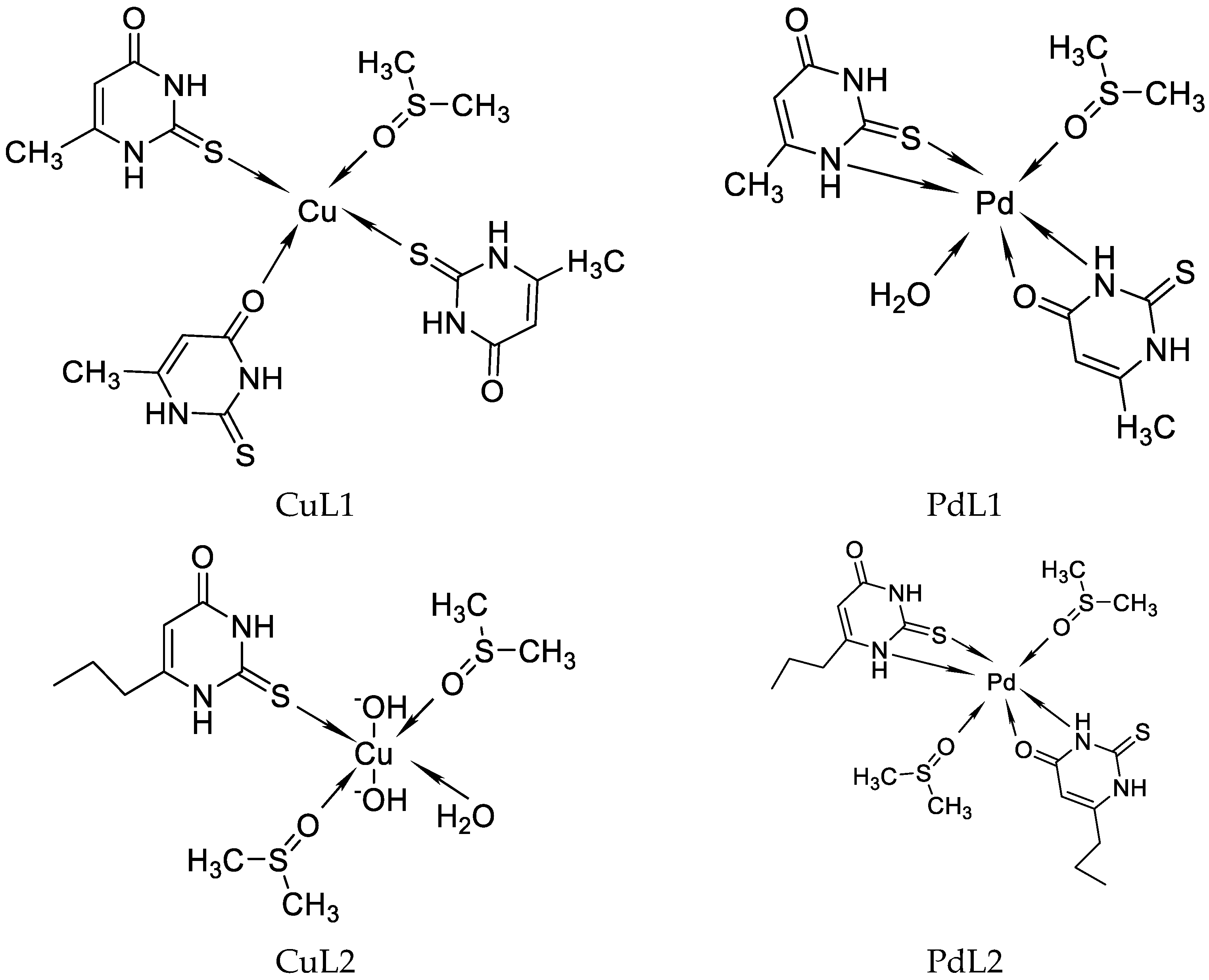
Recently, we have reported on Cu((II), Pd(II), and Au(III) complexes with 2-thiouracil [31]. The compound structure has been analyzed with UV-Vis, IR, 1H, 13C NMR, and Raman spectral data. The interpretation of complex spectra is assisted by the data for 2-thiouracil obtained from 1H–1H COSY, DEPT-135, HMBC, and HMQC spectra. The spectral data reveal the bonding of the ligand through sulfur, oxygen, and nitrogen atoms in Cu(II) and Pd(II) complexes and through S and N atoms in the complex of gold. In addition, the antimicrobial activity against both Gram-positive and Gram-negative bacteria and yeasts has also been studied. New complexes of Cu(II) and Pd(II) with 6-methyl-2-thiouracil and 6-propyl-2-thiouracil have been obtained [32]. All metal complexes have been obtained after mixing water solutions of the corresponding metal salts and the ligand dissolved in DMSO and water solutions of NaOH, in a metal-to-ligand ratio of 1:4:2. The compound structure has been reviewed based on melting point analysis, MP-AES for Cu and Pd, UV-Vis, IR, ATR, 1H NMR, 13C NMR, and Raman spectroscopy. The interpretation of complex spectra is assisted by the data for 6-methyl-2-thiouracil and 6-propyl-2-thiouracil obtained from 1H-1H COSY, DEPT-135, HMBC, and HMQC spectra [32]. We have suggested that in the Cu(II)L1 complex, the ligand could probably coordinate in a monodentate way through S2 and/or O4 atoms. The coordination binding site we would suggest for 6-propyl-2-thiouracil in Cu(II)L2 is a monodentate coordination mode binding through S-atom. For Pd(II)L1 and Pd(II)L2 complexes, we suggested that one of the ligands participates in coordination via N1 and S2 atoms and others with N3 and O4 atoms. In all the complexes, the solvent DMSO acts as a ligand (one molecule coordinate in Cu(II)L1 and Pd(II)L1 and two molecules in Cu(II)L2 and Pd(II)L2). The formation of polymeric complexes in a solid state has also been suggested, whereas dissolution in DMSO decreases. The suggested structure of the new complexes is given in Figure 16.
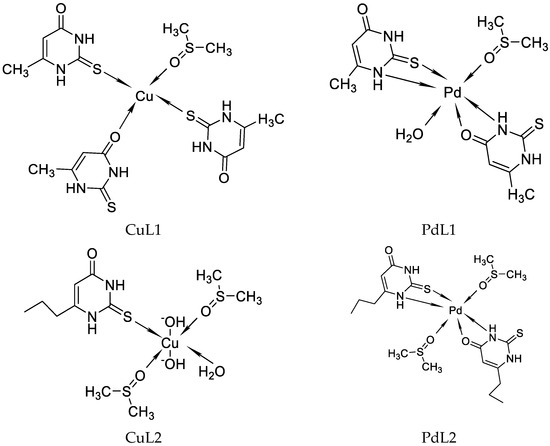
Figure 16.
The representation of suggested coordination binding sites for 6-metyl-2-thiouracil and 6-propyl-2-thiouracil [32].
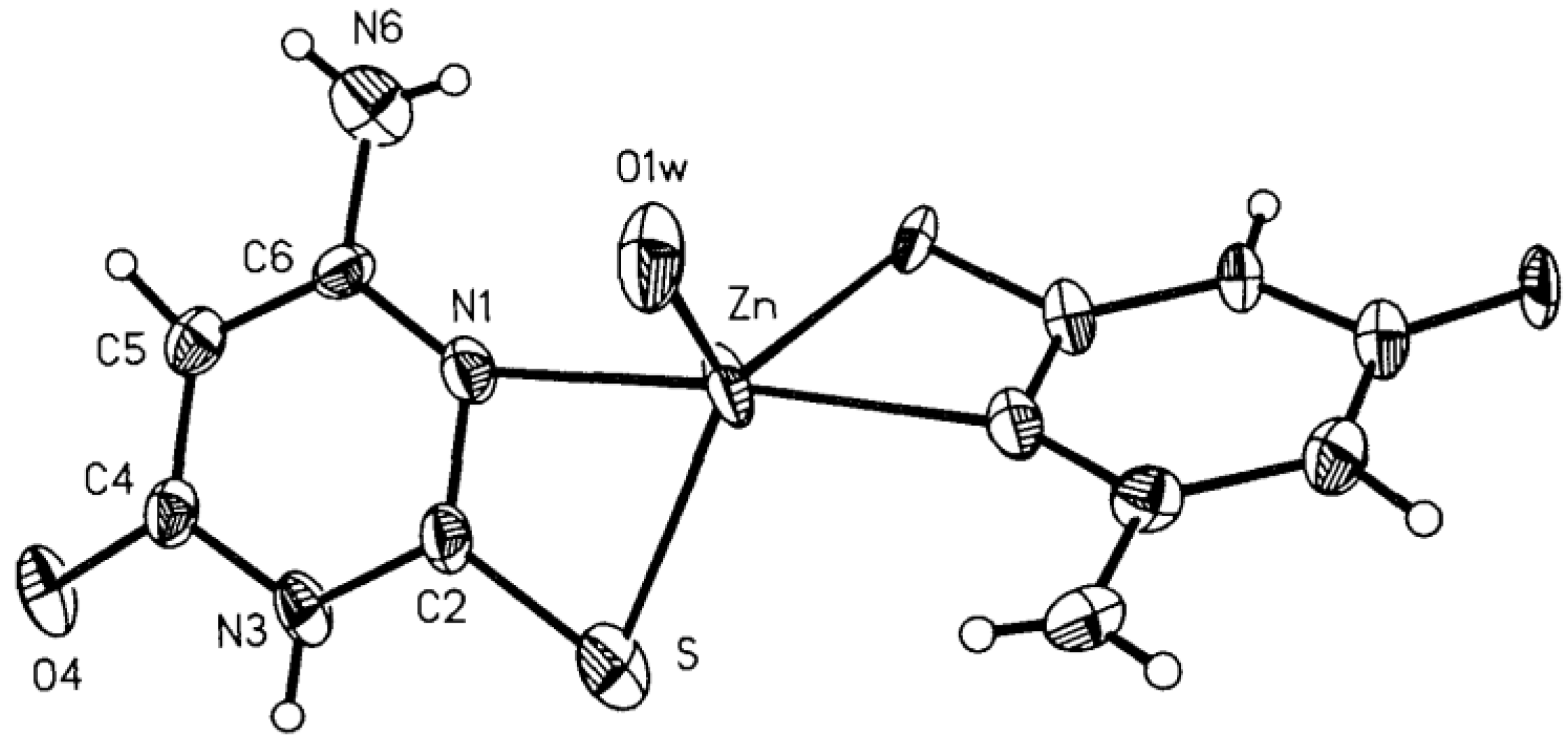
Complexes of Cu(I), Ni(II), Co(II), Zn(II), Ag(I), Cd(II), and Hg(II) with 6-amino-2-thiouracil have been synthesized, isolated, and studied by thermogravimetric analysis, differential scanning calorimetry (DSC) and IR spectroscopy [33]. Based on the results obtained, the authors suggest that the ligand acts as a monoanion and coordinates via the N-atom in the complexes of Cu(I), Ni(II), Co(II), Zn(II), Ag(I), and Cd(II), and via the S-atom in Hg(II) and Ni(II). The Zn(II) and Cd(II) complexes are assumed to be polymeric, and the ligand is bridged and acts as a dianion. It is crucial to mention the water in the first six complexes is both coordination and crystallization, whereas in the last it is only crystallization [33]. Complexes of 6-amino-2-thiouracil with Ni(II), Co(II), Zn(II), Cd(II), Cu(I), Ag(I), and Hg(II) have been obtained by Romero et al. [34]. The complexes have been studied via elemental analysis, IR-, UV-Vis, and NMR spectroscopy, as well as magnetochemical measurements. The structure of one of these has been established by X-ray structure analysis [Zn(6-amino-2-thiouracil)2H2O]·2H2O, in which the ligand is coordinated bidentate chelate through S-atom in second and N-atom in the first position. The structure of the new Zn(II) complex is given in Figure 17.
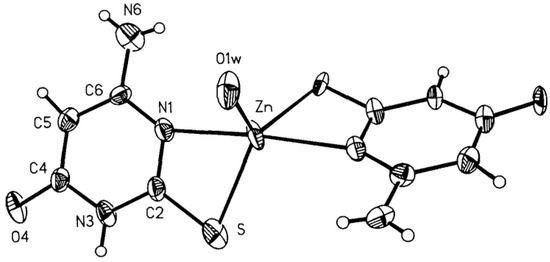
Figure 17.
View of the molecule of [Zn(6-amino-2-thiouracil)2H2O]·2H2O 50% probability thermal ellipsoids are shown [34].
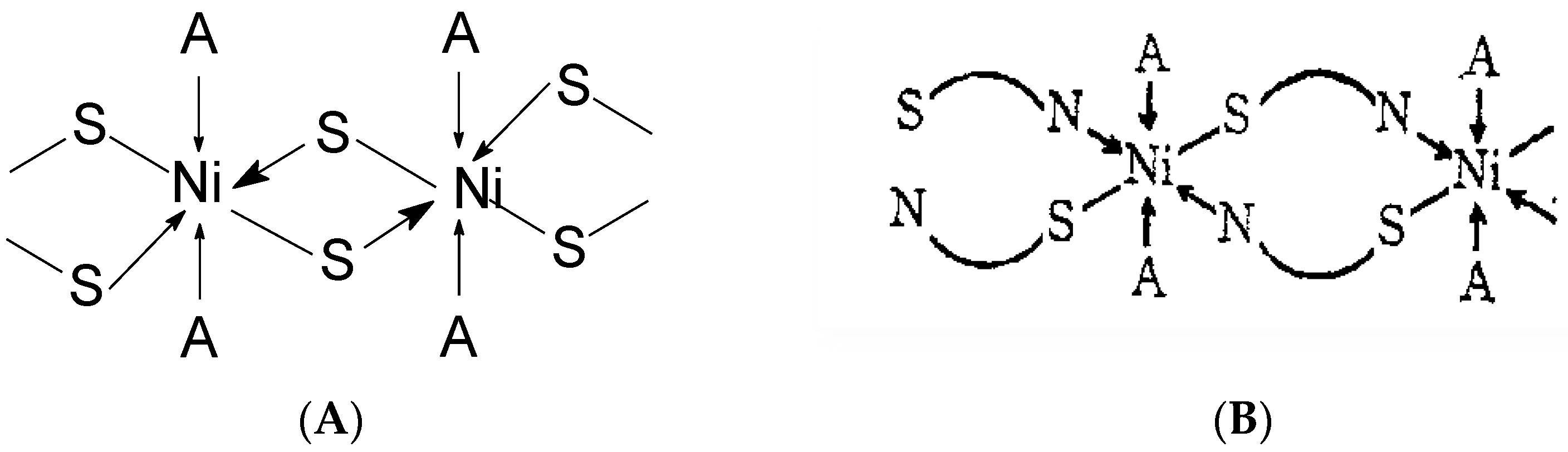
It is important to note that complexes of 2-thiouracil with Cu(II), Ni(II), Co(II), and Fe(III) have been synthesized [35]. The compounds have been analyzed via IR and UV-Vis spectroscopic methods, as well as magnetochemical measurements and DTA (differential thermal analysis). Based on the results obtained, the authors suggest that 2-thiouracil has coordinated to the metal center via a deprotonated N-atom and via an S-atom from the thiocarbonyl group in the Cu(II) and Ni(II) complexes; in addition, there is the presence of two water molecules. In Figure 18 the proposed structure of Ni(II) complexes is given. The authors also suggest, that in the complex of Co(II), one ligand is not deprotonated and binds via an S-atom from the thiocarbonyl group and an O-atom from the carbonyl group. For the Fe(III) complex, coordination is most likely via N- and O-atoms [35]. All of these complexes exhibit high insolubility in typical organic solvents, indicating their likely polymeric nature. As a result, the structure of the nickel(II) complex seems to be octahedral, while the structure of the other complexes remains uncertain. However, tentative structures are proposed as follows:
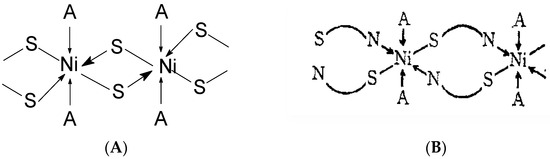
Figure 18.
Polymeric structure with thio bridges as in structure (A); polymeric as shown in structure (B) (A = H2O or py) [35].
- Fe(III) complex—octahedral;
- Co(II) complex—A tetrahedral structure has been observed, where each cobalt atom within the dimer forms bonds with three nitrogen atoms and one water molecule. Four ligands exhibit monodentate bonding in the dimer, connecting through deprotonated nitrogen. The fifth ligand has both nitrogen atoms protonated, with each nitrogen atom bonding to one cobalt atom [35];
- Cu(II) complex—octahedral possibly having Cu–Cu bond [35].
Garrett et al. have reported on complexes of 2-thiouracil, 6-n-propyl-2-thiouracil, 6-methyl-2-thiouracil, 5-methyl-2-thiouracil, 5,6-dimethyl-2-thiouracil, 2-ethylmercapto-4-hydroxypyrimidine, and 6-methyl-N,N′-diethyl-2-thiouracil with Cu(II), Cd(II), Pb(II), Fe(II), and Fe(III) [36]. The structure of some of metal complexes synthesized by Garrett et al. is given in Figure 19.
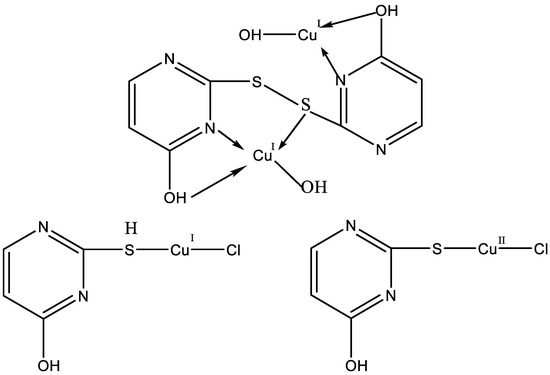
Figure 19.
The structure of some of metal complexes synthesized by Garrett et al. [36].
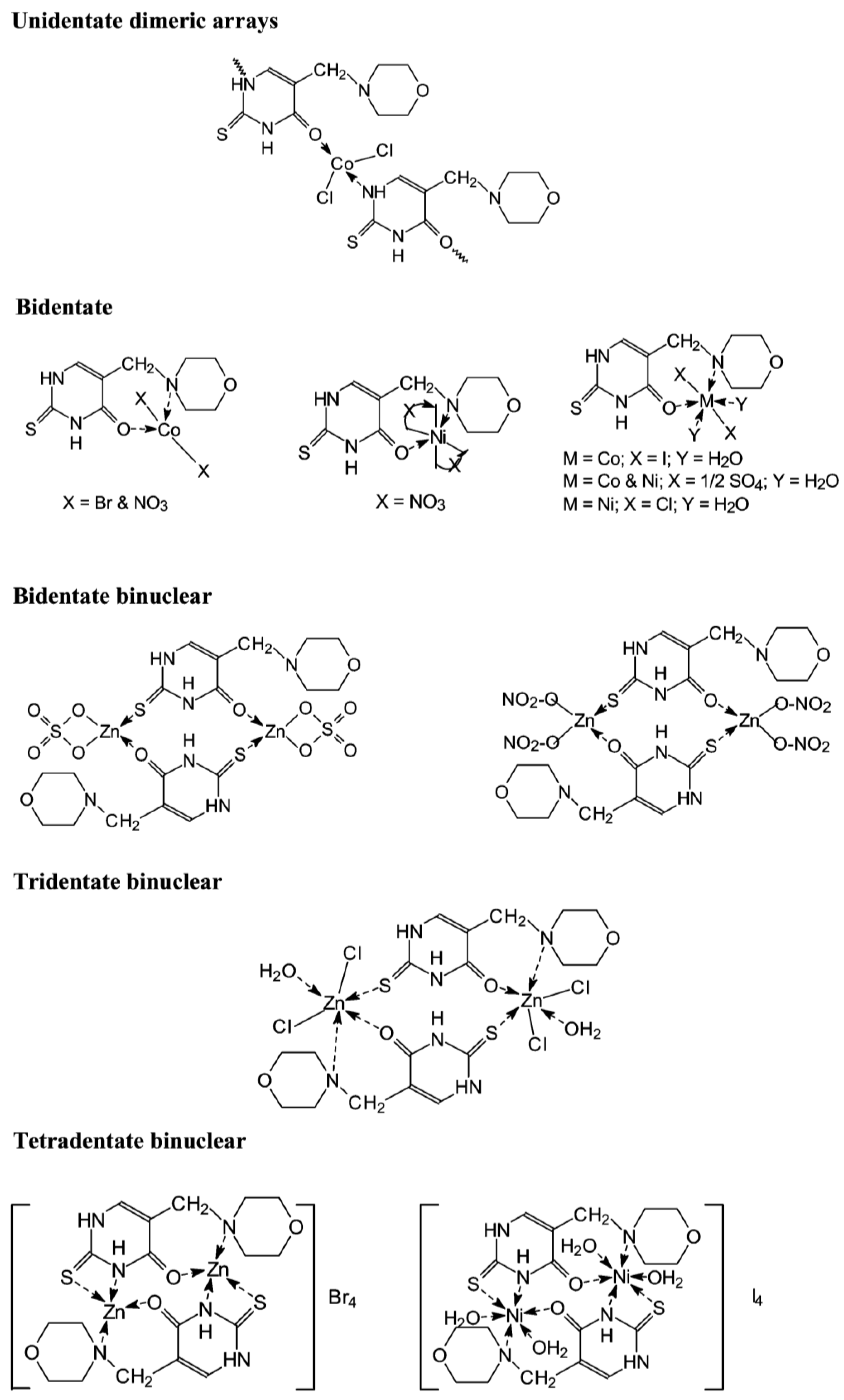
Dozens of complexes have been prepared and isolated using chlorides, bromides, iodides, sulfates, and nitrates of Co(II), Zn(II), and Ni(II) with 5-morpholinomethyl-2-thiouracil [37]. The complexes were formed by mixing a solution of the ligand in 2-propanol and the metal salt in ethanol, the molar ratio was 2:1, and the temperature of the reaction mixture was 70 °C. The suggested structure of the new complexes is given in Figure 20.
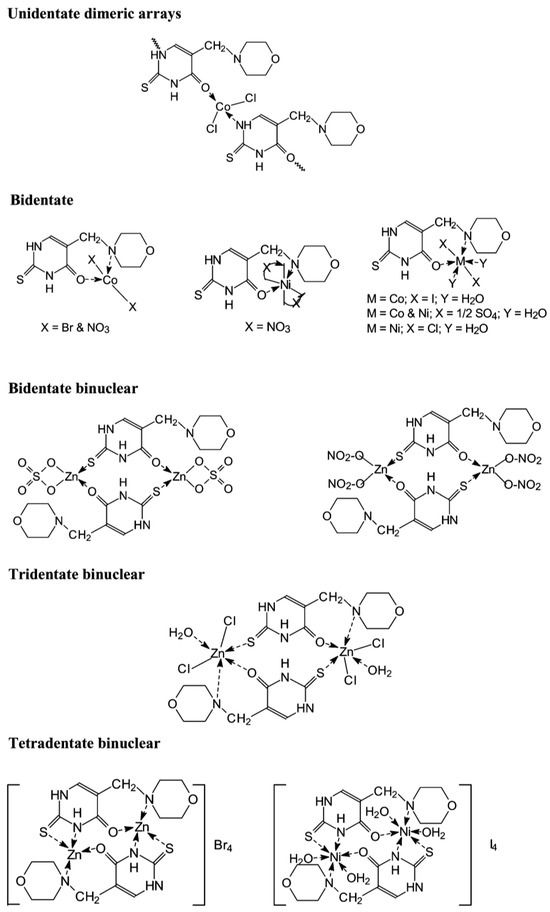
Figure 20.
The structure of the metal complexes reported by Kamalakannan et al. [37].
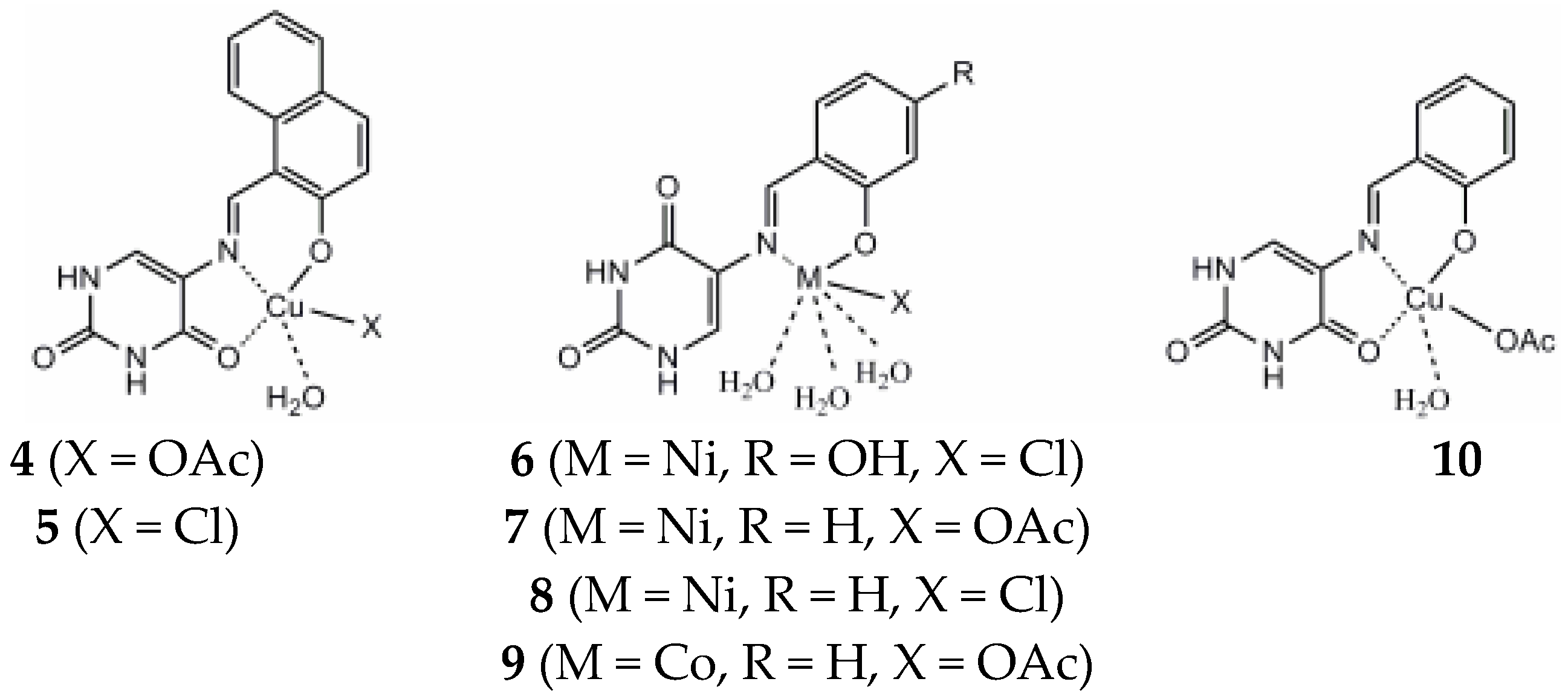
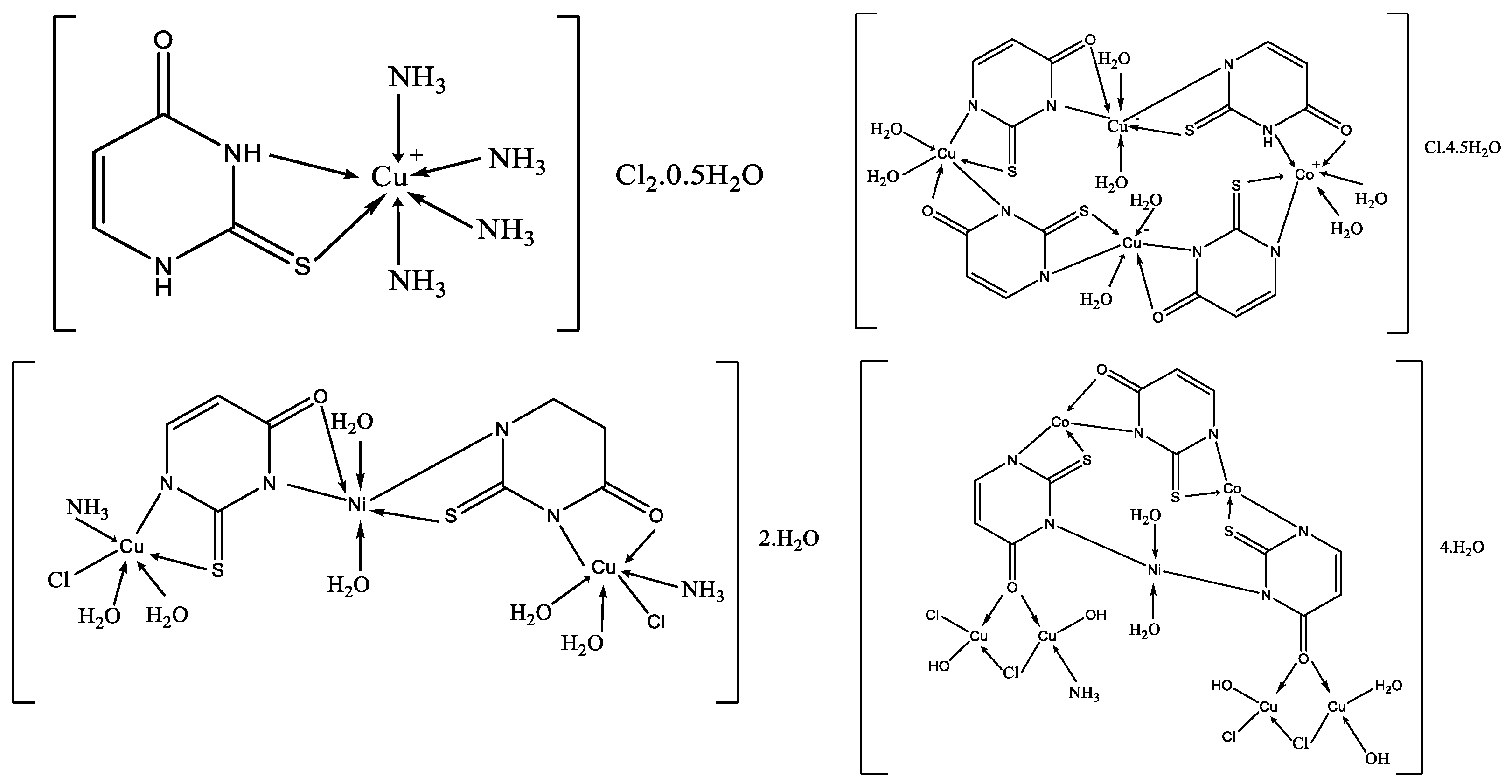
Complexes of 2-thiouracil and 6-methyl-2-thiouracil and their derivatives with W(CO)5 have been obtained [38]. One of the compound structures has been established via X-ray structural analysis. The authors prove the ligand coordinated with the metal ion through the S-atom of the 2-thiouracil. The pentacarbonyl complexes of 2-thiouracilate and 6-methyl-2-thiouracilate have been demonstrated to undergo cis CO dissociation with stereoselectivity, leading to the concurrent formation of tetracarbonyl derivatives chelated by the exocyclic sulfur and the endocyclic N(1) [38]. A mononuclear complex with the general formula [CuL(NH3)4]Cl2·0.5H2O and three heterometallic complexes [Cu2Ni(L)2(NH3)2Cl2.6H2O]·2H2O, [Cu3Co(L)4·8H2O]Cl·4.5H2O, and [Cu4Co2Ni(L)3(OH)4(NH3)Cl4·3H2O]·4H2O, where the ligand is 2-thiouracil has been obtained [39]. The complexes have been analyzed via elemental analysis, magnetochemical studies, and IR, as well as UV-Vis, EPR, TG, DTG, and DTA. The results show the ligand acts bidentate or tetradentate, with the geometry of the metal center on an octahedron, except for [Cu4Co2Ni(L)3(OH)4(NH3)Cl4·3H2O]·4H2O, in which a planar-square structure of Co(II), Ni(II), and Cu(II) is suggested. In Figure 21 the proposed structure of mononuclear and hetero-metallic complexes is given.
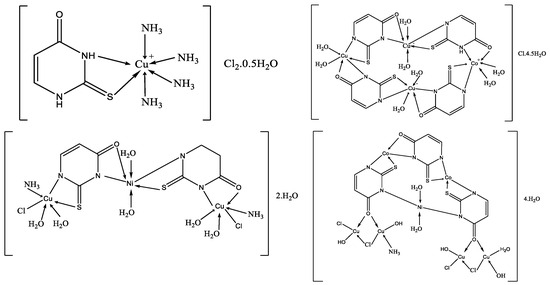
Figure 21.
Proposed structures for mononuclear copper(II) complex and its hetero-metallic complexes [39].
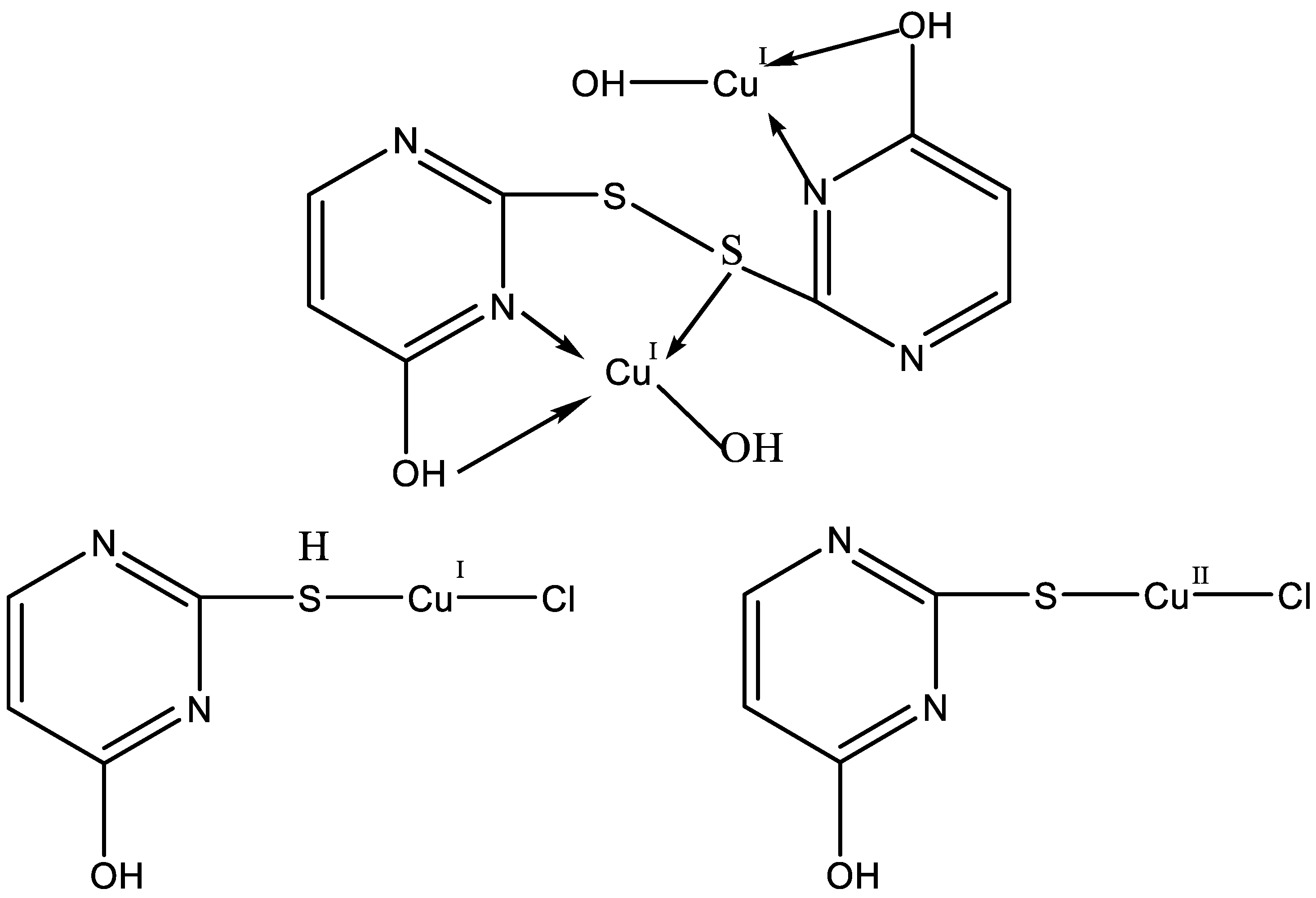
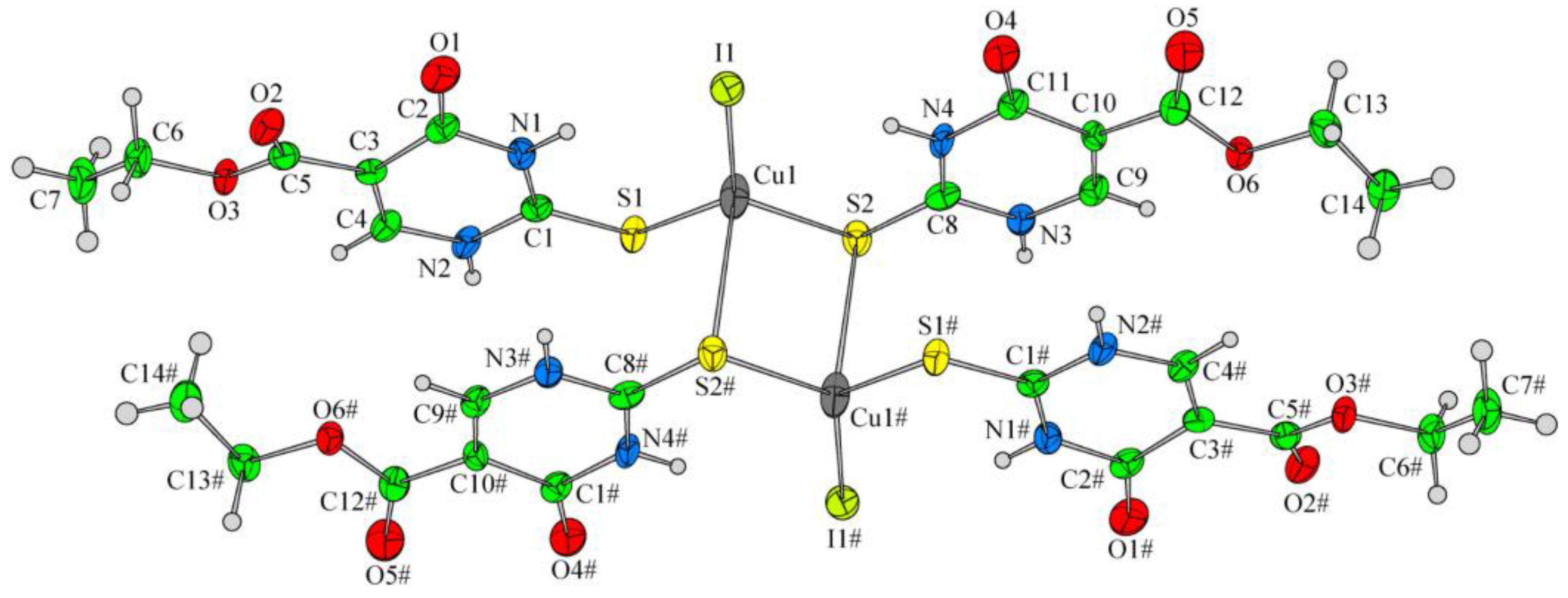
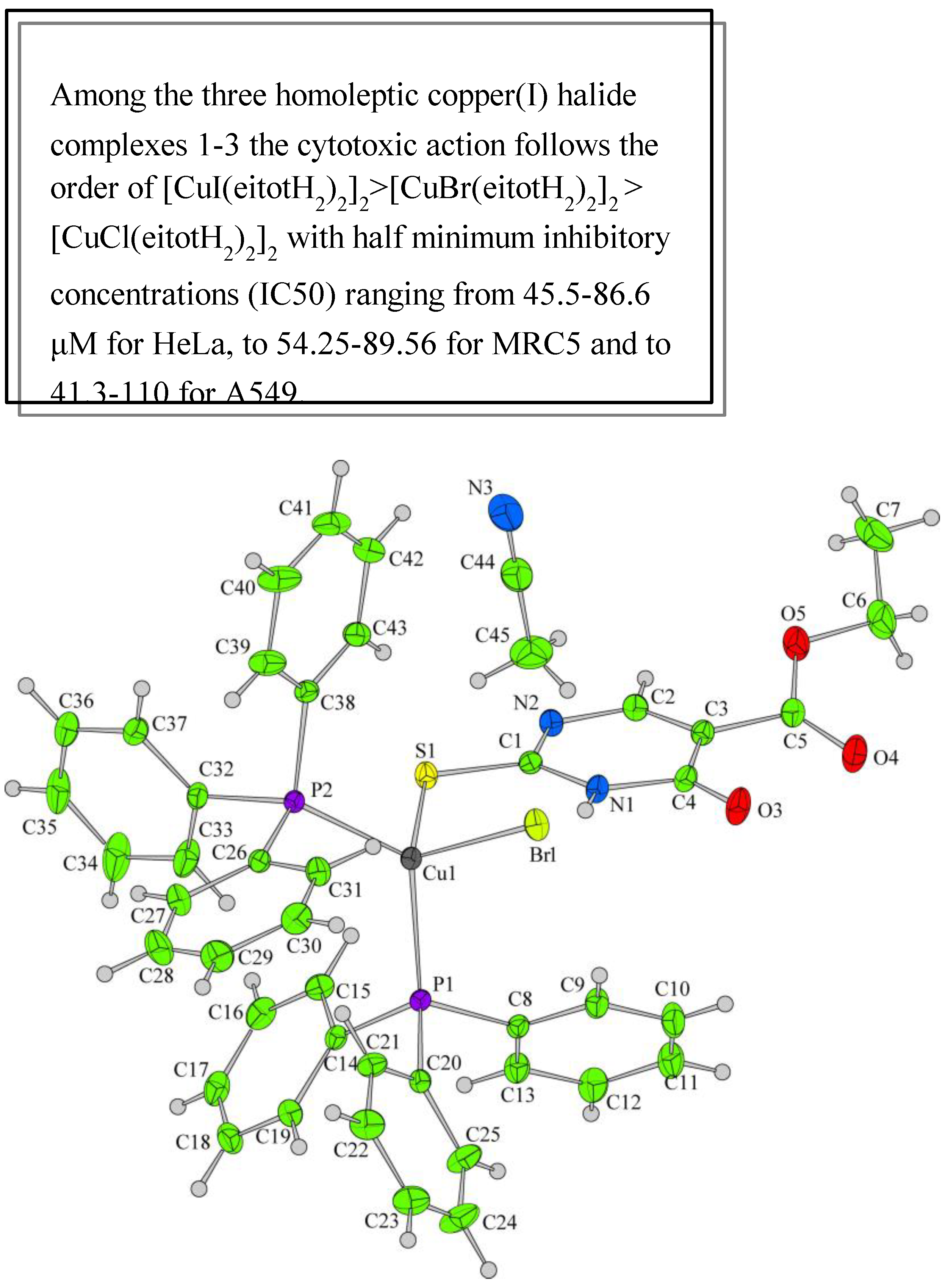
Papazoglou et al. have prepared a dinuclear complex of Cu(I) (CuX, where (X = Cl, Br, I)) involving 5-carbethoxy-2-thiouracil (eitotH2) as a ligand with the general formula [CuX(eitotH2)2]2 [40]. The molecular crystal structure of the complex is presented in Figure 22 and mixed-ligand mononuclear complexes of Cu(I) with the general formula [CuX(PPh3)2(eitotH2)], the crystal structure of which is presented in Figure 23.
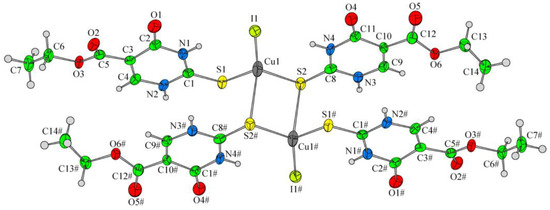
Figure 22.
Molecular crystal structure of Cu(I) dinuclear complex reported by Papazoglou et al. [40].
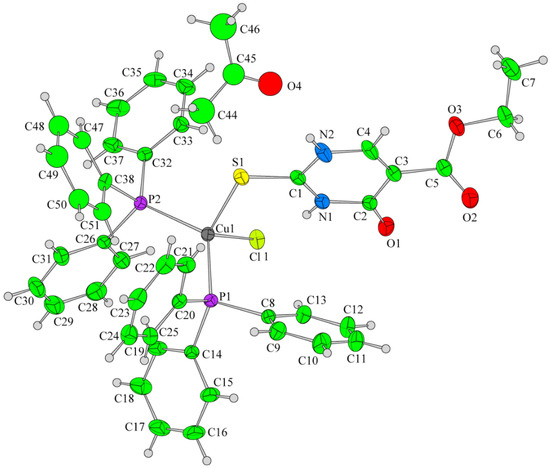
Figure 23.
Molecular crystal structure of mononuclear Cu(I) complex with general formula [CuX(PPh3)2(eitotH2)] reported by Papazoglou et al. [40].
New dinuclear copper(I) complexes with 5-carbethoxy-2-thiouracil have been synthesized recently [41].
It is known that heavy metal compounds such as platinum, gold, rhodium, palladium, ruthenium, and others can be used as biologically active agents in chemotherapy and for the treatment of various types of human tumors. We should take into consideration the coordination compounds of Pd with amino acids, catecholamines, and some heterocyclic nitrogen-containing compounds that have found application as immunomodulators, facilitating the recovery of cells after radiation damage [42]. The treatment with gold preparations, called chrysotherapy, was known as early as 2500 BC in China. In the form of official pharmaceuticals, Au compounds were used in the 1920s [42].
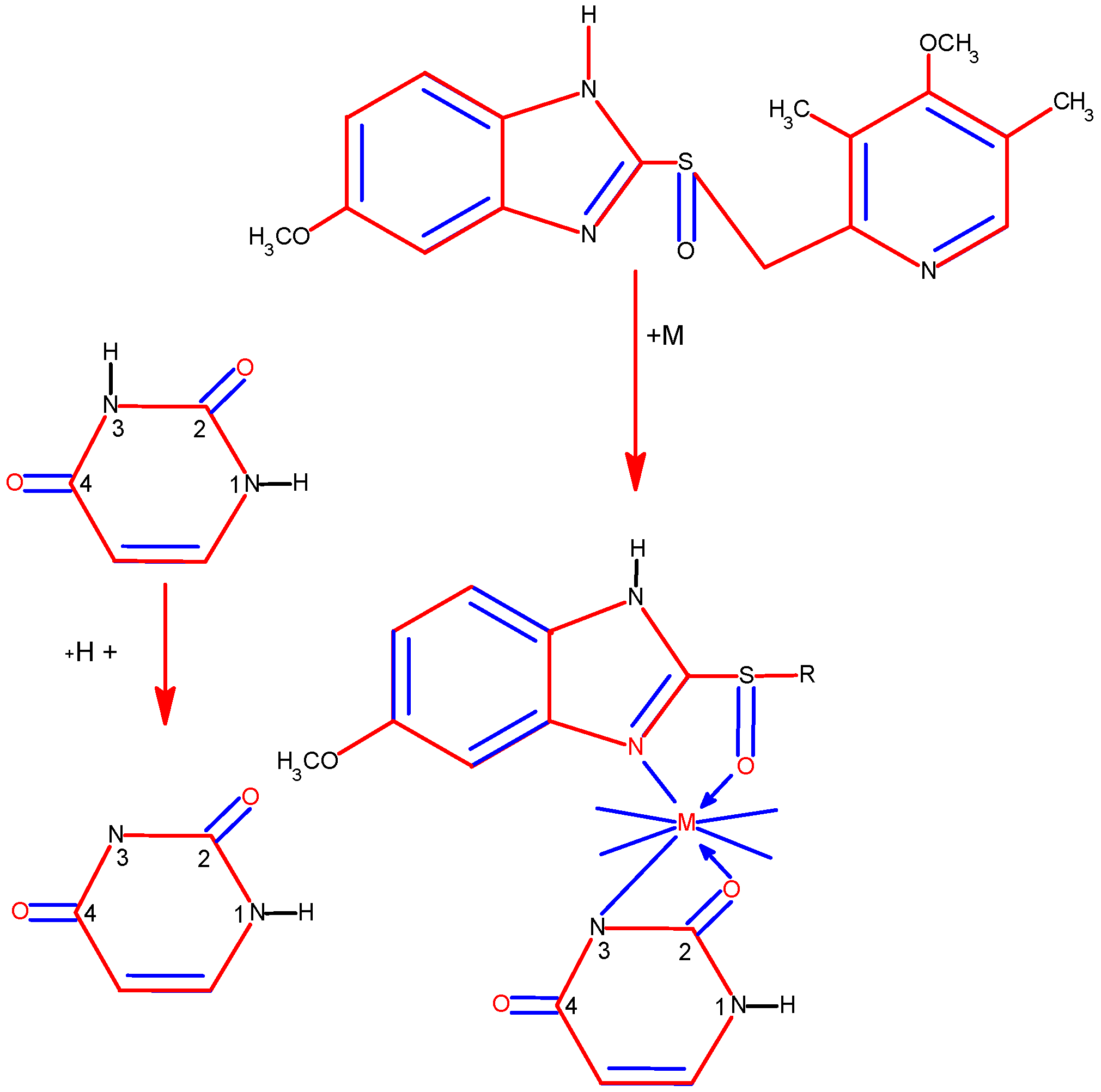
New complexes of rhenium(I) involving certain 5-nitrosopyrimidines, characterized with the general formula [ReCl(CO)3L], have been synthesized and identified through elemental analysis, conductivity measurements, and spectroscopic methods including IR, 1H, 13C, and 15N NMR. [43]. The complexes seem to exist as monomers, where the pyrimidine ligands function in a neutral form. The structure of [ReCl(CO)3(DANU)]·CH3CN has been elucidated through X-ray diffraction. The coordination environment around Re(I) is best described as a distorted octahedron, with the ligand adopting a bidentate configuration through N5 and O4 atoms, forming a five-membered chelate ring.
Abou-Melha has synthesized new complexes of VO(II), Ni(II), Pd(II), Pt(IV), and UO2(II) with N-(4-((Z)-(6-oxo-2-thioxo-1,2,3,4-tetrahydro-6l5-pyrimidin-5-yl)diazenyl)phenyl)-4-((E)-(6-oxo-2-thioxo-1,2,5,6-tetrahydropyrimidin-5-yl)diazenyl)benzamide [44]. The structure of the complexes has been studied by IR, UV-Vis, 1H NMR, EPR, 13C NMR, TGA, TEM, and XRD methods. The structure of new metal complexes is given in Figure 24.
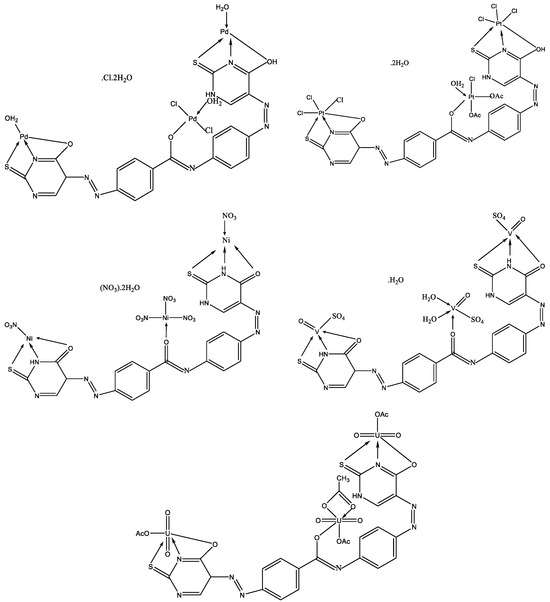
Figure 24.
The geometries of all investigated complexes [44].
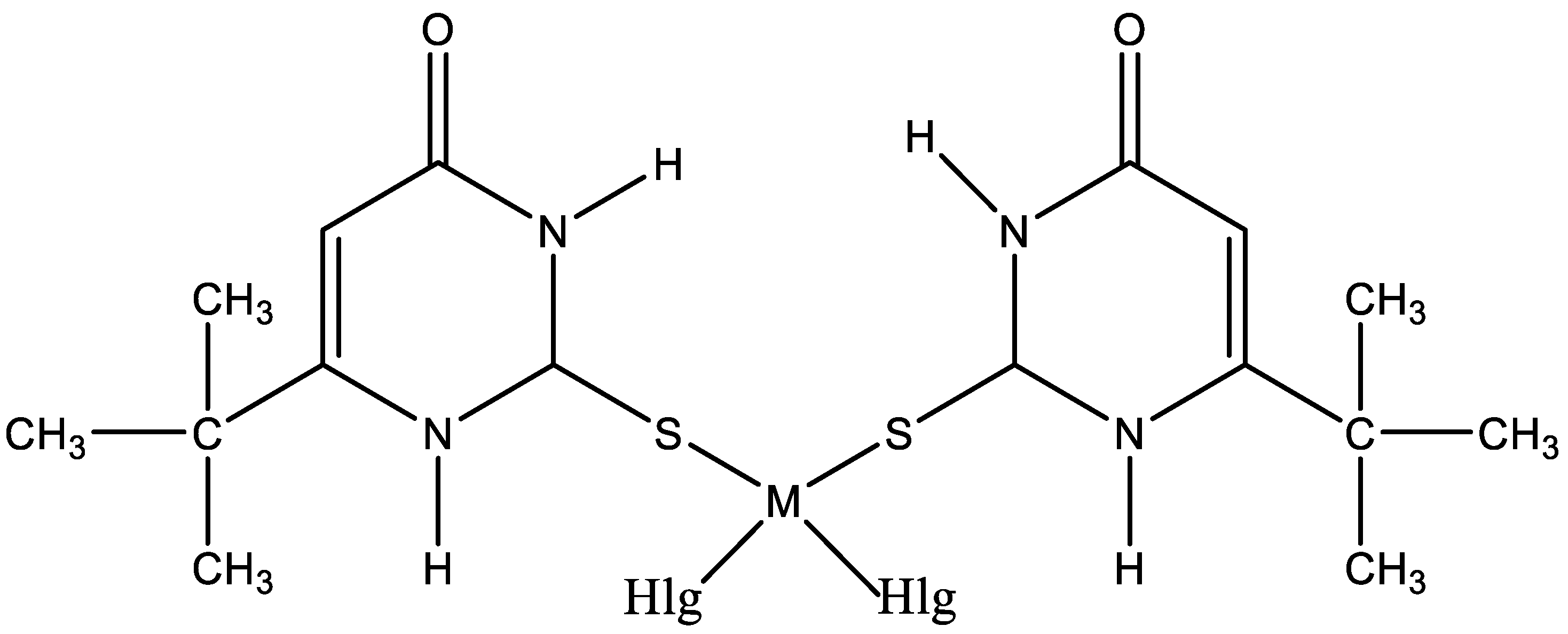
The interest in platinum and palladium complexes originates from their high cytostatic activity. Recently, cis-dihalogeno complexes of platinum(II) and palladium(II) with 6-tert-butyl-2-thiouracil have been synthesized [45]. The structure of platinum and palladium complexes with 6-tert-butyl-2-thiouracil is presented in Figure 25.
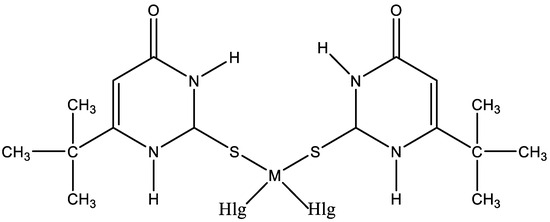
Figure 25.
The structure of platinum and palladium complexes with 6-tert-butyl-2-thiouracil [45].
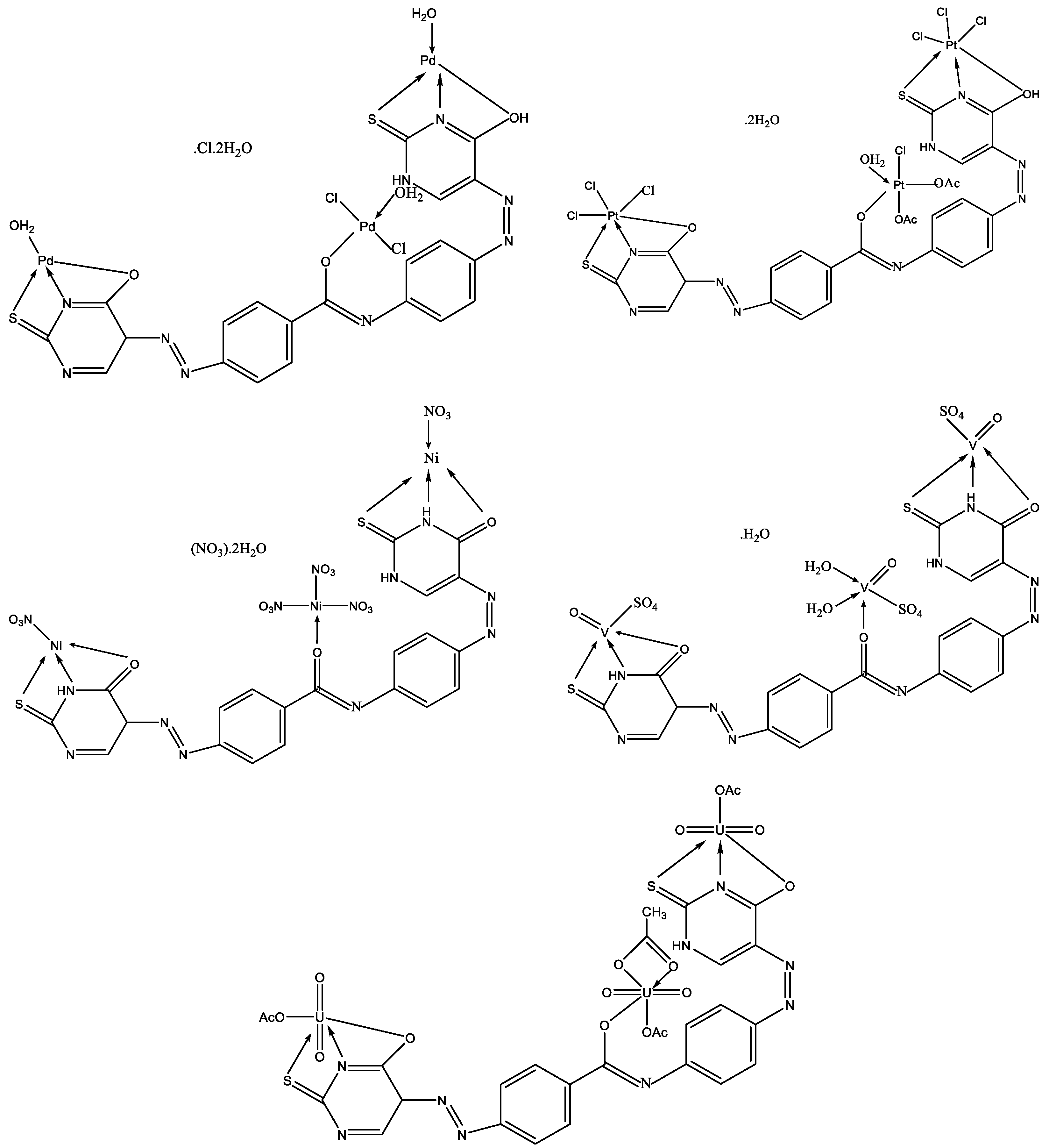
To date, numerous metal complexes of uracil and thiouracil derivatives have been synthesized and their composition and structure with various metals like copper, iron, cobalt, nickel, zinc, manganese, cadmium, and vanadium [18,26,46,47,48], as well as palladium, platinum, and gold have been studied [49]. The suggested structure of metal complexes with 2-thiouracil proposed by Masoud et al. is given in Figure 26. In Figure 27 the structure of some complexes is presented.
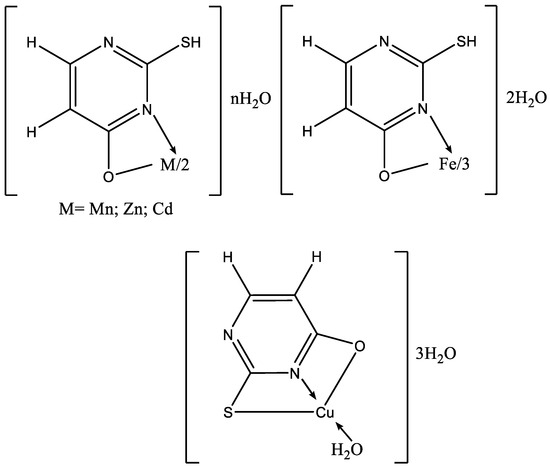
Figure 26.
The suggested structure of metal complexes with 2-thiouracil proposed by Masoud et al. [47].
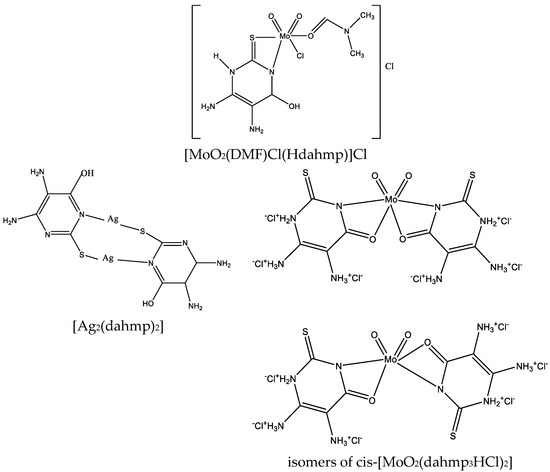
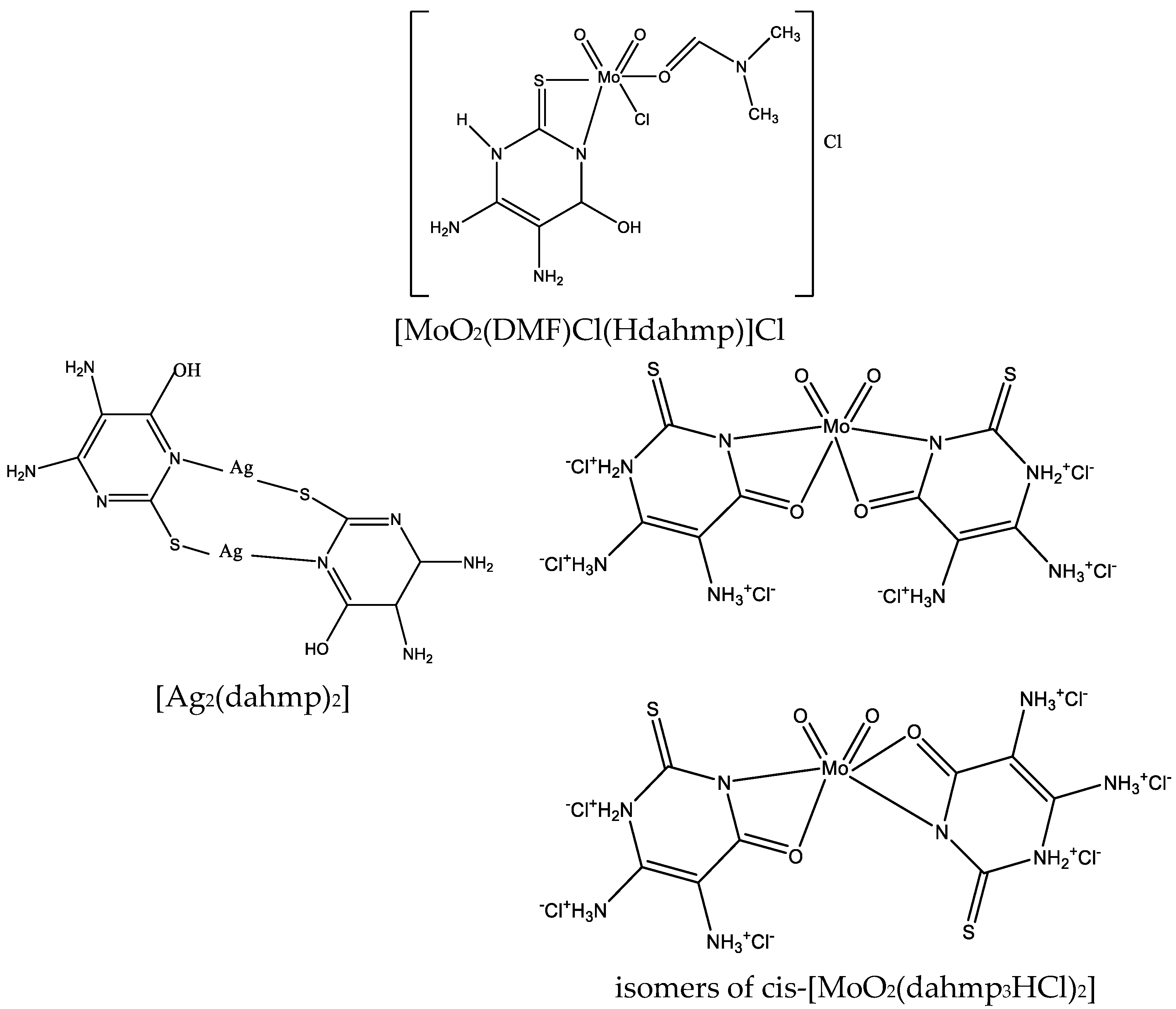
Figure 27.
Structure of [MoO2(DMF)Cl(Hdahmp)]Cl, [Ag2(dahmp)2], and isomers of cis-[MoO2(dahmp3HCl)2] [49].
New thiolate gold(I) complexes with P(NMe2)3 (HMPT) as a phosphane group have been synthesized [Au(SR)(HMPT)] (R = Spy, Spyrim, SMe2pyrim, Sbenzothiazole, Sthiazoline, Sbenzimidazole and 2-thiouracil). Two of the thiolate gold(I) complexes appear suitable effective candidates to be used in chemotherapy [50]. The chemistry of AuIII is much less developed than that of the isoelectronic and often isostructural PtII atom. The first crystallographically characterized AuIII complex with a pyrimidine derivative is the N(3)-bonded compound with 1-methylcytosine [51]. In the same manner, a gold complex has been isolated [52] by reacting HAuCl4 with 6-amino-1,3-dimethyl-5-(2-chlorophenylazo)uracil (DZCH) to give [Au(DZC)Cl2] complex. X-ray diffraction has demonstrated that the crystal of [Au(DZC)Cl2] contains individual complex molecules where the metal has a slightly distorted square-planar coordination. Two cis corners are occupied by Cl ligands. The uracil derivative has formed a six-membered chelate ring via the deprotonated amino group and the phenylsubstituted nitrogen atom of the azo group [52]. Metal complexes with 5-carboxy-2-thiouracil were synthesized by Singh et al. [53]. The structure of complexes with 5-carboxy-2-thiouracil is given in Figure 28.
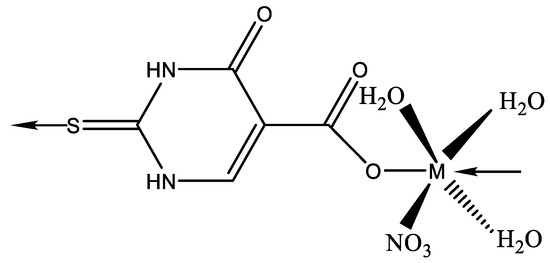
Figure 28.
The structure of complexes with 5-carboxy-2-thiouracil M(II) = Mn(II), Co(II), Ni(II), Cu(II), Zn(II), and Cd(II) [53].
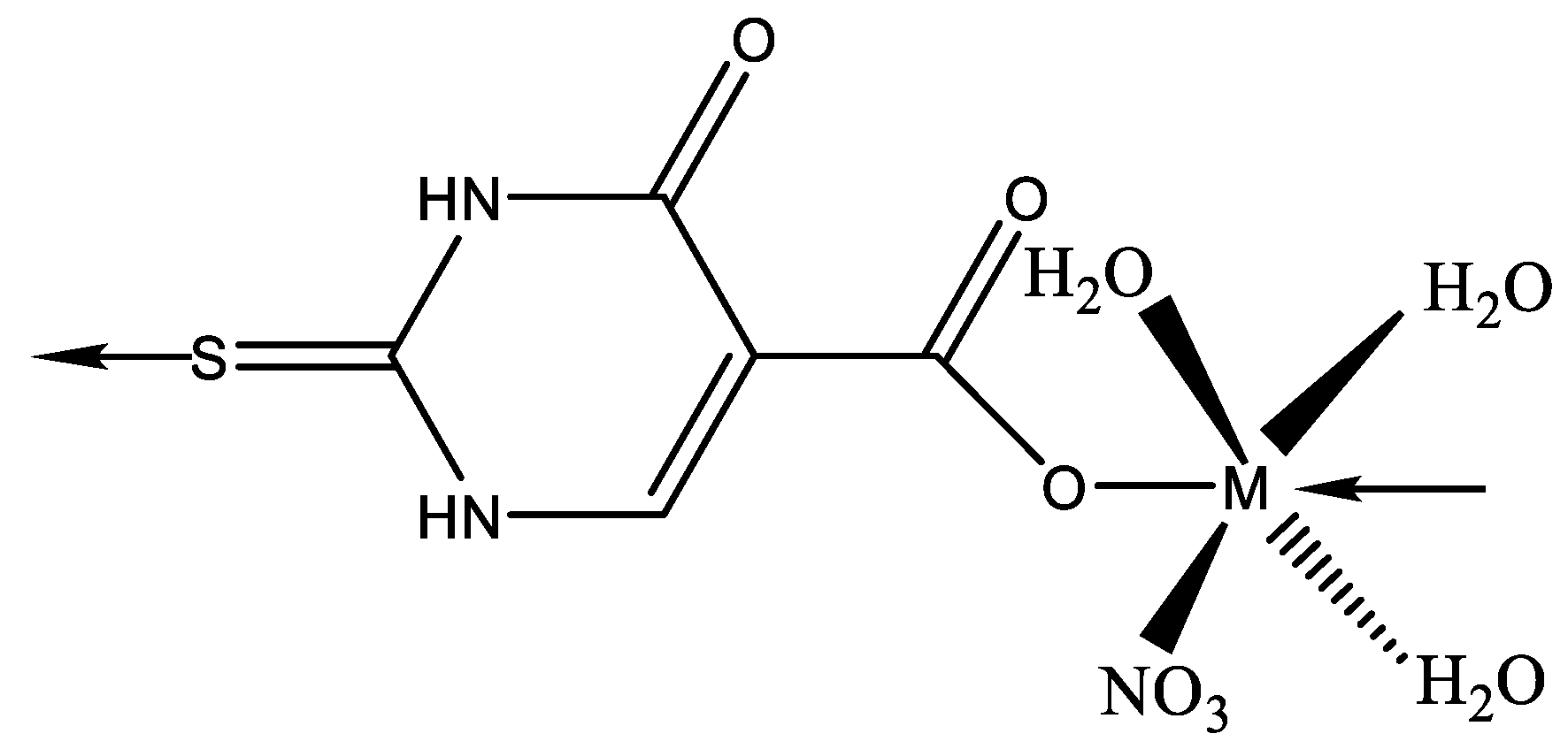
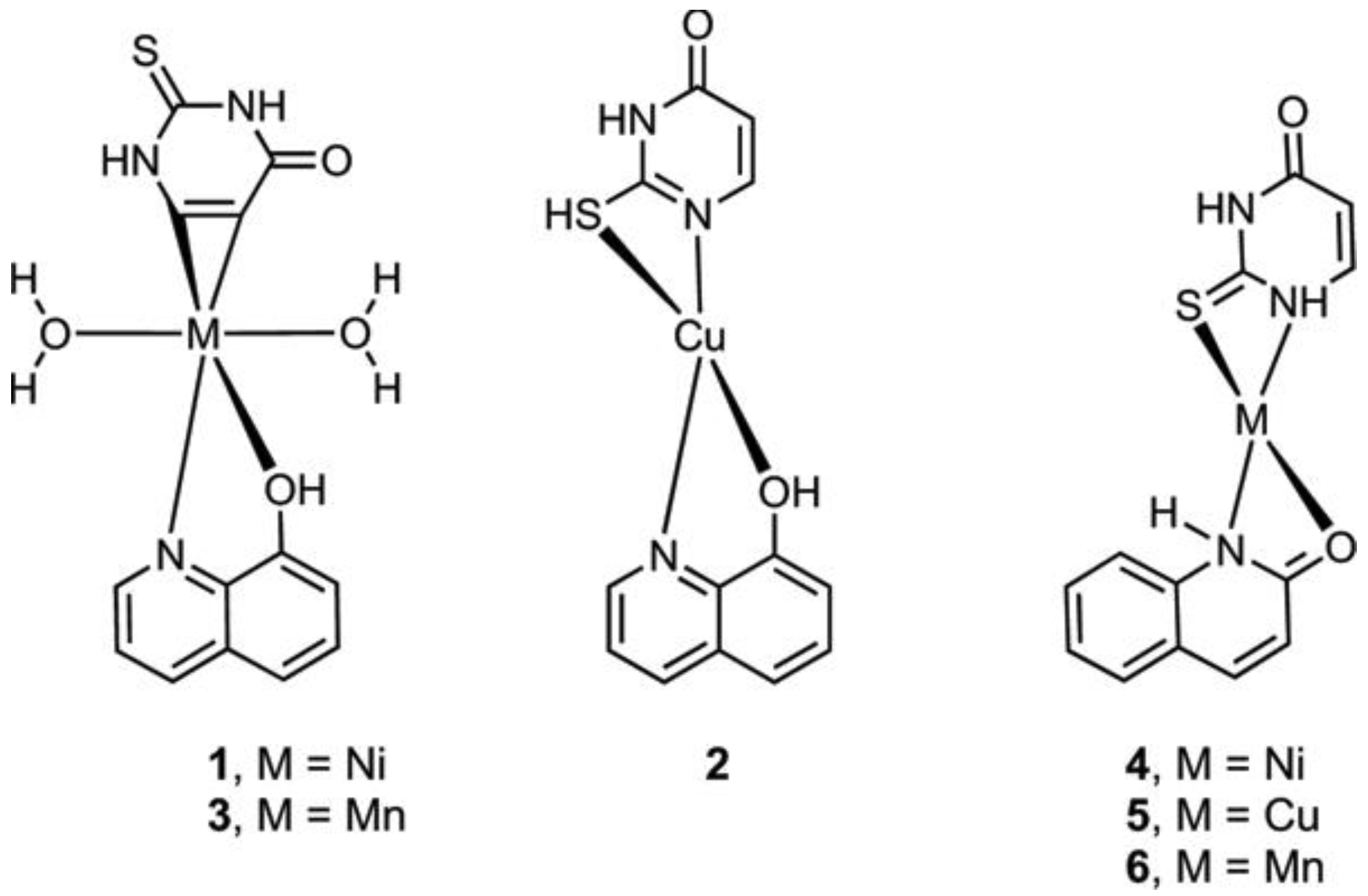
Metal complexes of Ni, Cu, and Mn with mixed ligands involving 2-thiouracil and 8-hydroxyquinoline (1–3), as well as with 2-hydroxyquinoline (4–6), were synthesized [54]. An assessment of their antimicrobial and antioxidant properties was conducted. The structure of Cu, Ni, Mn complexes is given in Figure 29.
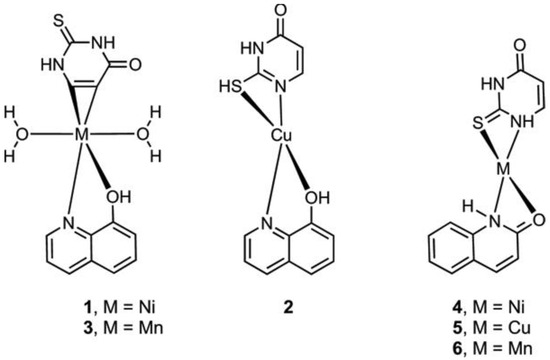
Figure 29.
The structure of Cu, Ni, and Mn complexes [54].
- Summary data on the structure of the complexes and the donor atoms involved in the coordination is given in Table 1.
 Table 1. Summary data on the structure of the complexes and the donor atoms involved in the coordination.
Table 1. Summary data on the structure of the complexes and the donor atoms involved in the coordination.
1.3. Biological Activities
Antibacterial Activity
The antimicrobial properties of the numerous metal complexes of thiouracil derivatives were screened in vitro against Gram-positive and Gram-negative bacteria, filamentous fungi, and yeast. The in vitro testing of the biological activities of both Schiff bases and metal complexes was conducted against several bacteria and a fungus [20]. Nickel(II) complexes, formed from the Schiff base ligand derived from salicylaldehyde, exhibited effective antimicrobial activity. Meanwhile, a cobalt(II) complex derived from the same ligand demonstrated notable anticandidal activity. The in vitro antimicrobial analyses showed more potent properties for both Ni(II) and Cu(II) mixed ligand complexes [25]. It was determined that the free ligands and their mixed ligand complexes display significant activity against pathogenic Gram-positive bacterial strains such as Bacillus subtilis, as well as against Escherichia coli and fungal strains like Aspergillus niger and Enterobacter species. The antimicrobial activities of different uracil and 2-thiouracil derivatives and their complexes are presented in Table 2 and Table 3.

Table 2.
Antimicrobial activities of different uracil derivatives and the complexes.

Table 3.
Antimicrobial activity of 2-thiouracil and derivatives and their complexes (inhibition zone, mm).
The strongest activity against Gram-negative bacteria representatives was noted with the Cu(II) complex when tested against S. enterica [31]. The free ligand 2-thiouracil did not hinder the growth and proliferation of P. vulgaris, but its Cu(II) and Au(III) complexes displayed mild to moderate activity. The introduction of metal ions to 2-thiouracil generally maintained or enhanced antimicrobial activity against Gram-positive bacteria, with two exceptions. Among these bacteria, S. aureus exhibited the greatest susceptibility, particularly in response to the influence of the Cu(II) complex [31]. Both yeast strains were impacted by the unbound ligand and its complexes. C. albicans exhibited higher sensitivity to the complexes, with the addition of Cu(II), Pd(II), and Au(III) to 2-thiouracil showing a more pronounced antimicrobial effect. S. cerevisiae was generally more susceptible among the yeast strains, except in the case of the Pd(II) complex. The most potent antifungal activity was observed with the Cu(II) complex against S. cerevisiae [31]. The highest antimicrobial activity was exhibited by the Cu(II) complex with 6-methyl-2-thiouracil [32]. It was active against all of the test microorganisms. The Pd(II)L1 complex did not hinder the growth of S. aureus, E. faecalis, and S. cerevisiae. 6-methyl-2-thiouracil exhibited the narrowest antimicrobial spectrum, being inactive against S. aureus, E. coli, S. enterica, and S. cerevisiae. The introduction of Cu(II) enhanced the antimicrobial activity of 6-methyl-2-thiouracil against all test microorganisms, except B. cereus. However, isolated cell colonies were observed in the inhibition zones (IZ) against E. coli, P. vulgaris, and K. pneumoniae, indicating varying resistance within the microbial population. Conversely, the addition of Pd(II) resulted in a loss of activity against E. faecalis and reduced activity against L. monocytogenes and C. albicans [32].
Among the compounds tested, the cobalt(II) bromo complex was identified as the most active against S. aureus and E. coli [37]. It should be noted that the most active are the complexes of Co(II). The order of activity of the complexes synthesized from the corresponding salts is CoIIBr2 ˃ CoIICl2 ˃ ZnIISO4 ˃ ZnIICl2 ˃ NiIII2 ˃ standard ˃ ligand. Tetrahedral complexes appeared to be more active compared to octahedral [37]. Studies on the antimicrobial activity of the complexes obtained by Masoud et al. have been performed against various microorganisms [39]. Among the representatives of prokaryotes, Gram(+) and Gram(−) bacteria such as Staphylococcus aureus, Escherichia coli, etc. were used. Yeasts (Candida albicans and Aspergillus flavus) have been used as representatives of eukaryotes. The results of the antimicrobial studies carried out show that the complexes possessed remarkable activity against Escherichia coli, Staphylococcus aureus, and Candida albicans yeasts but were not active against Aspergillus flavus. The complex with the general formula [Cu4Co2Ni(L)3(OH)4(NH3)Cl4·3H2O]·4H2O appears to be having the most significant activity against the tested microorganisms. The antibacterial activity under in vitro conditions of complexes of VO(II), Ni(II), Pd(II), Pt(IV), and UO2(II) with N-(4-((Z)-(6-oxo-2-thioxo-1,2,3,4-tetrahydro-6l5-pyrimidin-5-yl)diazenyl)phenyl)-4-((E)-(6-oxo-2-thioxo-1,2,5,6-tetrahydropyrimidin-5-yl)diazenyl)benzamide against Gram(−) E. coli, Klebsiella sp., and Gram(+) Bacillus subtilis bacteria was reported [44]. The agar diffusion method was used to determine susceptibility to the above substances. It was found that Pt(IV) and Pd(II) complexes showed bacteriostatic effects against Proteus, while the Klebsiella strain was resistant to all tested compounds.
1.4. Antifungal Activity
The complex of uracil and omeprazole with Th(II) showed a smaller inhibitory zone compared to that of the complexes of Gd(II) and Ce(II) against A. niger culture [19]. The research results show the complexes are more active compared to the activity of the free ligands. Mixed ligand complexes of 5-fluorouracil, alanine, and phenylalanine with Ni(II), Cu(II), and Zn(II) were found to show antifungal activity against Aspergillus niger, Enterobacter ssp., and Candida albicans [25]. The results of antifungal activity studies show that complexes of CoIINO3 with 5-morpholinomethyl-2-thiouracil are more active compared to the free ligand [37]. The metal complex 2TU-Mn-8HQ, where 2-TU represents 2-thiouracil and 8HQ stands for 8-hydroxyquinoline, demonstrated significant antimicrobial activity. Its minimal inhibitory concentration (MIC) ranged from 11.07 to 708.64 µM, displaying efficacy against both Gram-negative bacteria and Gram-positive bacteria, as well as diploid fungus. This performance surpassed that of other metal complexes, such as 2TU-Cu-8HQ (MIC of 23.68–757.70 µM) and 2TU-Ni-8HQ (MIC of 350.67–701.35 µM) [54]. Strong antifungal activity was exhibited by all five of the tested compounds with a strong emphasis on compound 2-thiouracil-5-sulphonic acid N-(4-(3-substituted-2-propen-l-oxo)phenyl) amide derivative, a chalcone derivative (α, β—unsaturated ketone). This displayed potent activity, attributed to its ability to easily penetrate the fungal cell membrane due to its high lipophilicity. Additionally, compound 2-thiouracil-5-sulphonic acid N-(4-(1,2,3-thiadiazolo) phenyl) amide derivative exhibits high levels of activity in the antifungal assessment [55]. Nevertheless, the addition of 4-amino-1,5-dimethyl-2-phenyl-1,2-dihydro-3H--pyrazol-3-one (4-aminoantipyrine) or anthranilic acid ring to the thiouracil derivatives 4-(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-ylamino)-6-aryl-2-thioxo-1,2-dihydropyrimidine-5-carbonitrile, and 2-[5-cyano-6-aryl-2-thioxo-1,2-dihydropyrimidine--4-yl-amino]-benzoic acid, resulted in reduced antimicrobial activity [56]. The structure–activity relationship indicated that thiouracil-containing amide or hydrazine hydrate part exhibited superior antibacterial and antifungal activities compared to other derivatives [56]. The antimicrobial studies conducted revealed that compounds 4-[(2E)-2-benzylidene-hydrazino]-6-(4-fluoro-phenyl)-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbonitrile, 4-[(2E)-2-benzylidene-hydrazino]-6-(4-bromo-phenyl)-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbonitrile, 4-[(2E)-2-benzylidene-hydrazino]-6-(3,4,5-trimethoxy-phenyl)-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbonitrile, 4-[(2E)-2-(4-fluorobenzylidene)-hydrazino]-6-(4-fluoro-phenyl)-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbonitrile, 4-[(2E)-2-(4-fluorobenzylidene)-hydrazino]-6-(4-bromo-phenyl)-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbonitrile, 4-[(2E)-2-(4-fluorobenzylidene)-hydrazino]-6-(3,4,5-trimethoxy-phenyl)-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbonitrile, 4-[(2E)-2-(4-methoxybenzylidene)-hydrazino]-6-(4-fluoro-phenyl)-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbonitrile, 4-[(2E)-2-(4-methoxybenzylidene)-hydrazino]-6-(4-bromo-phenyl)-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbonitrile, 4-[(2E)-2-(4-methoxybenzylidene)-hydrazino]-6-(3,4,5-trimethoxy-phenyl)-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbonitrile and 7-(4-Fluoro-phenyl)-5-thioxo-5,6-dihydro[1,2,4] triazolo[4,3-c]-pyrimidine-8-carbonitrile, 7-(4-Bromo-phenyl)-5-thioxo-5,6-dihydro[1,2,4]triazolo[4,3-c]-pyrimidine-8-carbonitrile, and 5-Thioxo-7-(3,4,5-trimethoxy-phenyl)-5,6-dihydr[1,2,4]triazolo[4,3-c]-pyrimidine-8-carbonitrile, exhibit significant antimicrobial activity against Staphylococcus aureus, Bacillus cereus, Escherichia coli, Candida albicans, and Aspergillus flavus when compared to reference drugs [57]. In contrast, compounds 6-(4-Fluoro-phenyl)-4-hydrazino-2-thioxo-1,2-dihydropyrimidine-5-carbonitrile, 6-(4-Bromo-phenyl)-4-hydrazino-2-thioxo-1,2-dihydropyrimidine-5-carbonitrile, 4-hydrazino-2-thioxo-6-(3,4,5-trimethoxy-phenyl)-1,2-dihydropyrimidine-5-carbonitrile, 4-(3,5-dimethyl-1H-pyrazol-1-yl)-6-(4-fluoro-phenyl)-2-thioxo-1,2-dihydropyrimidine-5-carbonitrile, 4-(3, 5-dimethyl-1H-pyrazol-1-yl)-6-(4-bromo-phenyl)-2-thioxo-1,2-dihydro pyrimidine-5-carbonitrile, 4-(3,5-Dimethyl-1H-pyrazol-1-yl)-2-thioxo-6(3,4,5-trimethoxy-phenyl)-1,2-dihydropyrimidine-5-carbonitrile, and 7-(4-Fluoro-phenyl)-5-thioxo-5,6-dihydrotetrazolo[1,5-c]pyrimidine-8-carbonitrile, 7-(4-Bromo-phenyl)-5-thioxo-5,6-dihydrotetrazolo[1,5-c]pyrimidine-8-carbonitrile, and 5-Thioxo-7-(3,4,5-trimethoxy-phenyl)-5,6-dihydrotetrazolo[1,5-c]pyrimidine-8-carbonitrile displayed notable antibacterial effects against Staphylococcus aureus, Bacillus cereus, and Escherichia coli but lacked antifungal activity, except for compound 6-(4-Fluoro-phenyl)-4-hydrazino-2-thioxo-1,2-dihydropyrimidine-5-carbonitrile, which showed moderate activity against Aspergillus flavus. Compounds 7-(4-Fluoro-phenyl)-3,5-dithioxo-2,3,5,6-tetrahydro[1,2,4]triazolo[4,3-c]pyrimidine-8-carbonitrile, 7-(4-Bromo-phenyl)-3,5-dithioxo-2,3,5,6-tetrahydro[1,2,4]triazolo[4,3-c]pyrimidine-8-carbonitrile, and 3,5-Dithioxo-7-(3,4,5-trimethoxy-phenyl)-2,3,5,6-tetrahydro[1,2,4]triazolo[4,3-c]pyrimidine-8-carbonitrile demonstrated high activities against Bacillus cereus, Escherichia coli, and Candida albicans, but were inactive against Staphylococcus aureus and Aspergillus flavus. Compounds 8-(4-Fluoro-phenyl)-3,4-dioxo-6-thioxo-3,4,6,7-tetrahydro-2H-pyrimido[6,1-c]-[1,2,4]triazine-9-carbonitrile, 8-(4-Bromo-phenyl)-3,4-dioxo-6-thioxo-3,4,6,7-tetrahydro-2H-pyrimido [6,1-c]-[1,2,4]triazine-9-carbonitrile, and 3,4-Dioxo-6-thioxo-8(3,4,5-trimethoxy-phenyl)-3,4,6,7-tetrahydro-2H-pyrimido [6,1-c]-[1,2,4]triazine-9-carbonitrile exhibited activity against Staphylococcus aureus and Aspergillus flavus only, while compounds 7-(4-Fluoro-phenyl)-3-oxo-5-thioxo-2,3,5,6-tetrahydro[1,2,4]-triazolo[4,3-c]pyrimidine-8-carbonitrile, 7-(4-Bromo-phenyl)-3-oxo-5-thioxo-2,3,5,6-tetrahydro[1,2,4]-triazolo[4,3-c]pyrimidine-8-carbonitrile, 3-Oxo-5-thioxo-7-(3,4,5-trimethoxy-phenyl)-2,3,5,6-tetrahydro [1,2,4]-triazolo [4,3-c] pyrimidine-8-carbonitrile showed high activity specifically against Staphylococcus aureus. In summary, the study revealed that the conversion of 4-hydrazino pyrimidines to hydrazones 4-[(2E)-2-benzylidene-hydrazino]-6-(4-fluoro-phenyl)-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbonitrile, 4-[(2E)-2-benzylidene-hydrazino]-6-(4-bromo-phenyl)-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbonitrile, 4-[(2E)-2-benzylidene-hydrazino]-6-(3,4,5-trimethoxy-phenyl)-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbonitrile, 4-[(2E)-2-(4-fluorobenzylidene)-hydrazino]-6-(4-fluoro-phenyl)-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbonitrile, 4-[(2E)-2-(4-fluorobenzylidene)-hydrazino]-6-(4-bromo-phenyl)-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbonitrile, 4-[(2E)-2-(4-fluorobenzylidene)-hydrazino]-6-(3,4,5-trimethoxy-phenyl)-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbonitrile, 4-[(2E)-2-(4-methoxybenzylidene)-hydrazino]-6-(4-fluoro-phenyl)-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbonitrile, 4-[(2E)-2-(4-methoxybenzylidene)-hydrazino]-6-(4-bromo-phenyl)-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbonitrile, 4-[(2E)-2-(4-methoxybenzylidene)-hydrazino]-6-(3,4,5-trimethoxy-phenyl)-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbonitrile or triazolo[4,3-c]pyrimidines 7-(4-Fluoro-phenyl)-5-thioxo-5,6-dihydro[1,2,4] triazolo[4,3-c]-pyrimidine-8-carbonitrile, 7-(4-Bromo-phenyl)-5-thioxo-5,6-dihydro[1,2,4]triazolo[4,3-c]-pyrimidine-8-carbonitrile, and 5-Thioxo-7-(3,4,5-trimethoxy-phenyl)-5,6-dihydr[1,2,4]triazolo[4,3-c]-pyrimidine-8-carbonitrile led to a pronounced inhibitory effect against Gram-positive (Staphylococcus aureus, Bacillus cereus), Gram-negative (Escherichia coli) bacteria, and fungi (Candida albicans and Aspergillus flavus) [57]. Cyclization to triazolo[4,3-c]pyrimidines, pyazolopyrimidines, or tetrazolo pyrimidines, as seen in 7-(4-Fluoro-phenyl)-3,5-dithioxo-2,3,5,6-tetrahydro[1,2,4]triazolo[4,3-c]pyrimidine-8-carbonitrile, 7-(4-Bromo-phenyl)-3,5-dithioxo-2,3,5,6-tetrahydro [1,2,4]triazolo[4,3-c]pyrimidine-8-carbonitrile, 3,5-Dithioxo-7-(3,4,5-trimethoxy-phenyl)-2,3,5,6-tetrahydro[1,2,4]triazolo[4,3-c]pyrimidine-8-carbonitrile, 4-(3,5-dimethyl-1H-pyrazol-1-yl)-6-(4-fluoro-phenyl)-2-thioxo-1,2-dihydropyrimidine-5-carbonitrile, 4-(3, 5-dimethyl-1H-pyrazol-1-yl)-6-(4-bromo-phenyl)-2-thioxo-1,2-dihydro pyrimidine-5-carbonitrile, 4-(3,5-Dimethyl-1H-pyrazol-1-yl)-2-thioxo-6(3,4,5-trimethoxy-phenyl)-1,2-dihydropyrimidine-5-carbonitrile, and 7-(4-Fluoro-phenyl)-5-thioxo-5,6-dihydrotetrazolo[1,5-c]pyrimidine-8-carbonitrile, 7-(4-Bromo-phenyl)-5-thioxo-5,6-dihydrotetrazolo[1,5-c]pyrimidine-8-carbonitrile, and 5-Thioxo-7-(3,4,5-trimethoxy-phenyl)-5,6-dihydrotetrazolo[1,5-c]pyrimidine-8-carbonitrile, increased antibacterial activity, while cyclization to triazolo[4,3-c]pyrimidines or pyrimidotriazines, as observed in 7-(4-Fluoro-phenyl)-3-oxo-5-thioxo-2,3,5,6-tetrahydro[1,2,4]-triazolo [4,3-c]pyrimidine-8-carbonitrile, 7-(4-Bromo-phenyl)-3-oxo-5-thioxo-2,3,5,6-tetrahydro[1,2,4]-triazolo[4,3-c]pyrimidine-8-carbonitrile, 3-Oxo-5-thioxo-7-(3,4,5-trimethoxy-phenyl)-2,3,5,6-tetrahydro [1,2,4]-triazolo [4, 3-c] pyrimidine-8-carbonitrile, 8-(4-Fluoro-phenyl)-3,4-dioxo-6-thioxo-3,4,6,7-tetrahydro-2H-pyrimido[6,1-c]-[1,2,4]triazine-9-carbonitrile, 8-(4-Bromo-phenyl)-3,4-dioxo-6-thioxo-3,4,6,7-tetrahydro-2H-pyrimido[6,1-c]-[1,2,4]triazine-9-carbonitrile, and 3,4-Dioxo-6-thioxo-8(3,4,5-trimethoxy-phenyl)-3,4,6,7-tetrahydro-2H-pyrimido [6,1-c]-[1,2,4]triazine-9-carbonitrile, enhanced antibacterial activity only against Staphylococcus aureus or both Staphylococcus aureus and Aspergillus flavus, respectively [57]. In terms of antifungal activity, the compound 5-Oxo-3-phenyl-7-(thiophen-2-yl)-1,5-dihydro-[1,2,4]triazolo[4,3-a] pyrimidin e-6-carbonitrile demonstrated significant efficacy, comparable to the reference drug Colitrimazole, when tested against Candida albicans [58]. Similarly, compounds 2-(4-methoxybenzylidene)-3,5-dioxo-7-(thiophen-2-yl)-2,3-dihydro-5H-thiazolo[3,2-a]pyrimidine-6-carbonitrile, 3-(4-Formylphenyl)-6-oxo-8-(thiophen-2-yl)-3,4-dihydro-2H,6H-pyrimido [2, 1-b][1,3,5] thiadiazine-7-carbonitrile and 6-Oxo-8-(thiophen-2-yl)-3-(p-tolyl)-3,4-dihydro-2h,6h-pyrimido[2,1-b][1,3,5]thiadiazine-7-carbonitrile exhibited noteworthy activity against Aspergillus flavus. Moreover, compounds 6-Oxo-3-(thiazol-2-yl)-8-(thiophen-2-yl)-3,4-dihydro-2h,6hpyrimido[2,1-b] [1,3,5] thiadiazine-7-carbonitrile, 6-Oxo-3-(pyridin-2-yl)-8-(thiophen-2-yl)-3,4-dihydro-2h,6h-pyrimido[2,1-b] [1,3,5] thiadiazine-7-carbonitrile, and Ethyl-(4-(7-cyano-6-oxo-8-(thiophen-2-yl)-2h,6h-pyrimido[2,1-b][1,3,5] thiadiazin-3(4H)-yl))benzoate displayed moderate activity against Candida albicans. Additionally, compounds 6-Oxo-3-(thiazol-2-yl)-8-(thiophen-2-yl)-3,4-dihydro-2h,6hpyrimido[2,1-b] [1,3,5], thiadiazine-7-carbonitrile, and 2-(((Naphthalen-2-ylamino)methyl)thio)-6-oxo-4-(thiophen-2-yl)-1,6-dihydropyrimidine-5-carbonitrile exhibited moderate activity against Aspergillus flavus [58]. Recently, two new copper(II) complexes were successfully synthesized and characterized [59]. The investigation focused on determining the antifungal and mutagenic potential of these complexes. Complex 1, denoted as [CuCl2(Bipy)(L1)], demonstrated notable fungicidal activity against all tested Candida isolates, exhibiting effectiveness against both planktonic and sessile cells. Particularly, Candida krusei displayed heightened sensitivity to Complex 1. However, Complex 2, identified as [CuCl2(Bipy)(L2)], along with the free ligands, exhibited no discernible antifungal activity at the assessed concentrations. Crucially, the study found that Complex 1 showed no mutagenic potential within the tested concentrations. As a result, the researchers propose Complex 1 as a promising candidate for a new drug in the realm of anti-Candida therapy, based on its potent antifungal properties and lack of mutagenicity [59].
1.5. Antitumor and Cytotoxic Activities
The in vivo and in vitro antitumor activity results show that Cr(III) and Fe(III) complexes demonstrate significant activity against P815 murine mastocytoma whereas Al(III) complexes show a poor one [16]. It has been observed that the ligand 5-fluorouracil has significant antitumor activity against Dalton’s lymphoma tumor system [22]. The complexes with the general formula Co(II)-SFU-Hm and Zn(II)-SFU-Hm exhibit significant antitumor activity [22]. Complexes of 5-morpholinomethyl-2-thiouracil with Co(II), Ni(II), and Zn(II) have been found to exhibit antitumor activity against Dalton’s lymphoma and a murine leukemia cell line of P-338 [37]. The results of these studies show that the complexes are more active compared to the activity exhibited by the free ligand. Of all the complexes, the complex synthesized from ZnIISO4 starting salt showed the highest cytotoxic activity. The screening data of complexes for antitumor activity against Dalton’s lymphoma [22,37] and Sarcoma-180 [53] in vivo is given in Table 4.

Table 4.
Screening data of complexes for antitumor activity against Dalton’s lymphoma [22,37] and Sarcoma-180 [53] in vivo.
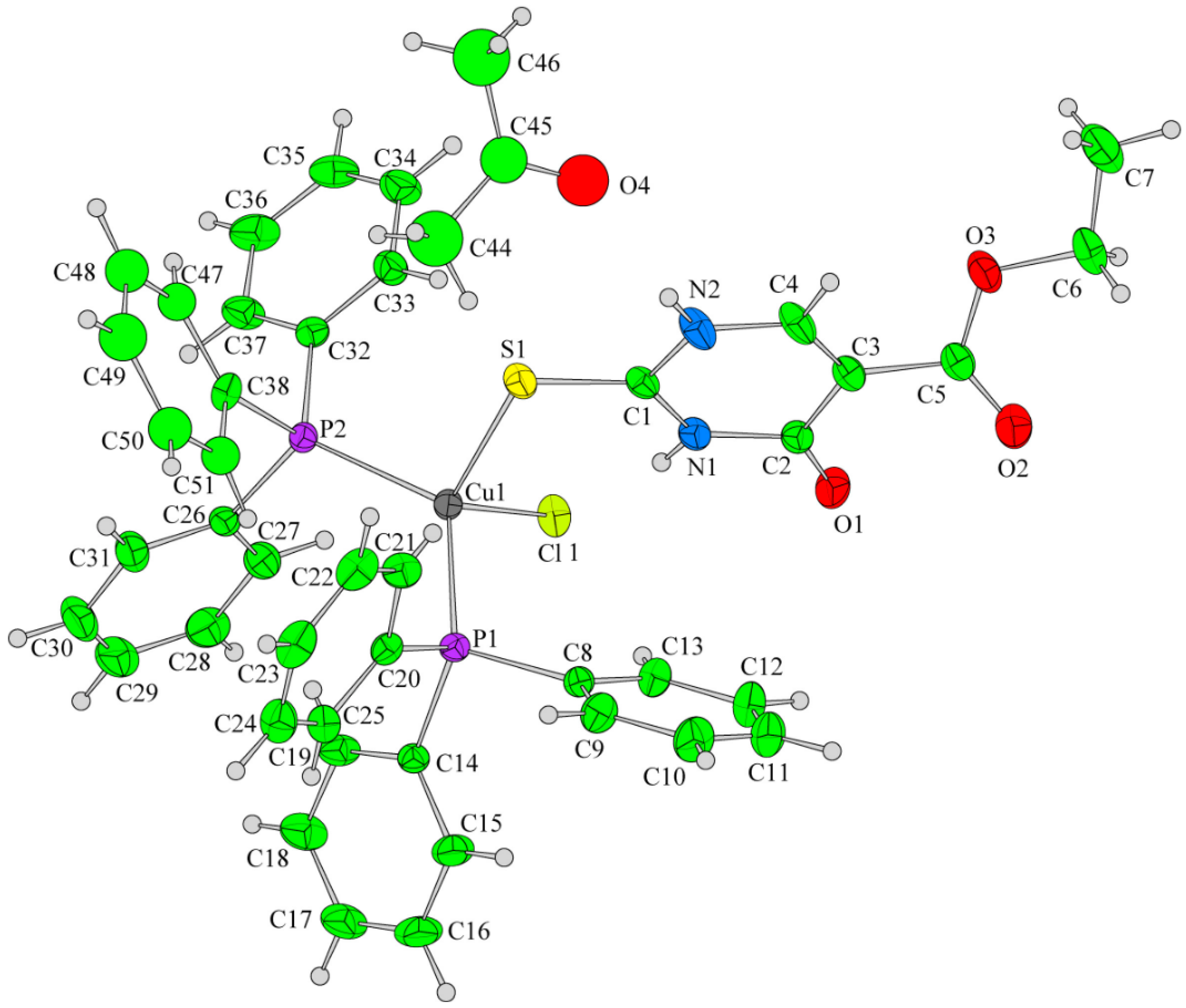
The new copper(I) complexes have been evaluated for in vitro antitumor properties against two tumor cell lines, A549 (human pulmonary carcinoma cell line) and HeLa (human epithelial carcinoma cell line) and one normal immortalized cell line MRC5 (human fetal lung fibroblast) [40]. The mixed-ligand complexes possessing triphenylphosphine appear to be highly cytotoxic in contrast to the phosphine-free ones, which inhibited cell proliferation only in relatively high concentrations. The SAR studies and chemical structures of new copper(I) complexes are presented in Figure 22, Figure 23 and Figure 30.
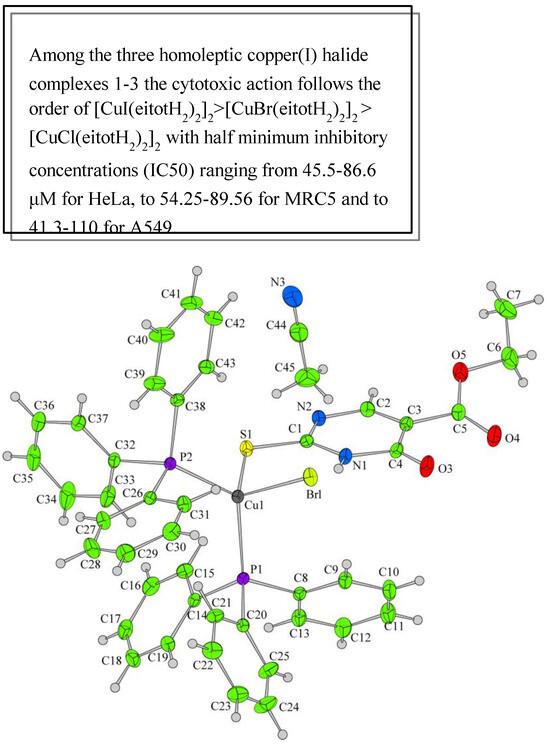
Figure 30.
SAR studies and chemical structure of new copper(I) complexes [40].
The copper(I) complexes have been studied against two different tumor cell lines. The cytotoxicity of all the above complexes is in contrast to that of each of the free ligands, which were higher in all the cell lines that had been tested [41]. The assessment of the antiproliferative effects on five human tumor cell lines (MCF-7 and EVSA-T for breast cancer, NB69 for neuroblastoma, H4 for glioma, and ECV for bladder carcinoma) indicates a modulating influence on cell growth at low concentrations, attributed to their estrogenic-like characteristics [43]. The metal complexes of Cu(II), Ni(II), Co(II), Zn(II), Cd(II), and Mn(II) with 5-carboxy-2-thiouracil have been screened against Sarcoma-180 tumor cells [53]. The results show that some complexes have antitumor activity both in vivo and in vitro against S-180 tumor cells.
1.6. Possible Mechanism of Biological Activities
The results presented above lead to the conclusion that the [Cu4Co2Ni(L)3(OH)4(NH3)Cl4·3H2O]4H2O complex showed the highest antimicrobial activity, particularly in terms of inhibition zone diameter [39]. Notably, the presence of different metal ions within the chelated complex played a crucial role in achieving a high level of biological activity. The increased lipophilic nature of these complexes, resulting from chelation, likely contributed to their enhanced activity. Additionally, it was observed that the toxicity of the metal chelates escalated with higher metal ion concentrations. This may be attributed to the faster diffusion of the chelates through the cell membrane as a whole or the chelation theory. The bound metals could potentially hinder enzymatic activity within the cell or catalyze toxic reactions among cellular constituents. The variation in the activity of different complexes against various organisms is dependent on factors such as the impermeability of microbial cell membranes or discrepancies in ribosome composition in microbial cells.
The cobalt(II) bromo complex exhibited the highest antibacterial activity against S. aureus and E. coli among the tested compounds [37]. This heightened activity is attributed to the potential interaction of the cobalt(II) complex with RNA. Additionally, the essential role of cobalt(II) as a micronutrient in transcription and transformation processes may contribute to its antibacterial efficacy. The order of activity observed was MMTU < standard < nickel(II) iodide complex < zinc(II) chloro complex < zinc(II) sulfato complex < cobalt(II) chloro complex < cobalt(II) bromo complex. Notably, the metal ion complexes exhibited greater biological activity compared to MMTU (5-morpholinomethyl-2-thiouracil) in all cases. Further analysis revealed a correlation between the lability (as determined from thermal studies) of M–O, M–N, M–S, and M–X (X = anionic counterpart of the metal ion) and their biological activities. The observed trend indicated that the activity increased with the rising lability of the metal complexes. Thermally labile complexes were found to be more active, with tetrahedral complexes exhibiting greater activity than octahedral ones, likely due to reduced steric constraints in tetrahedral structures. In antifungal studies, the compound MMTU exhibited lower activity compared to its metal ion complexes with Co(NO3)2, with MMTU itself being the most active. The antifungal mechanism is suggested to be related to chelation theory. Chelation reduces the metal ion’s polarity by partially sharing its positive charge with donor groups and possible π-delocalization over the chelate ring. This enhances the lipophilic character of the neutral chelate, promoting permeation through the lipid layers of fungal membranes [37]. Additionally, the compounds may act by forming hydrogen bonds with uncoordinated heteroatoms O, S, and N, interfering with normal cell processes. Compounds with lipophilic and polar substituents like C=O, C=S, and NH are expected to have increased fungal toxicity, enhancing interaction with nucleotide bases, essential metal ions, coordinatively unsaturated metals, or enzymatic functional groups in the biosystem. Some complexes exhibit low activity due to mismatched geometry and charge distribution around the molecule compared to the pores of the fungal cell wall, preventing penetration and toxic reactions within the pores. Consequently, these complexes may fail to reach the desired site of action on the cell wall to interfere with normal cell activity [37]. The study focused on antitumor screening, evaluating the impact of MMTU (a ligand) and its cobalt(II), nickel(II), and zinc(II) complexes on the growth of P-338 leukemic and Dalton’s lymphoma cells. Metal ion complexes exhibited higher activity compared to the ligand, with the zinc(II) complex showing significant activity, surpassing other complexes and free MMTU. The zinc(II) sulfato complex stood out as the most active among the tested complexes. The study proposed hydrogen bonding as a potential factor in the antitumor mechanism, highlighting the amine groups of adenine, guanine, and cytosine as capable of forming hydrogen bonds with the compounds, inhibiting normal cell metabolism. The presence of an endogenous receptor site, oxygen, further contributed to increased activity. The order of activity observed was MMTU < NiI2·MMTU < CoBr2·MMTU < ZnCl2·MMTU·H2O < ZnSO4·MMTU. The study emphasized that free MMTU was less effective in reaching cancer cells, making it less active. However, the enhanced activity of the zinc(II) chelate was explained by the significant zinc requirement of rapidly dividing tumor cells, facilitating the compound’s uptake by cancerous cells. Upon reaching the receptor site, the labile Zn–N, Zn–O, or Zn–S bonds in the complexes underwent dissociation, producing a free active antimetabolite with inherent activity. Comparing activities against leukemic and lymphoma cells, it was observed that leukemic cells were relatively more resistant. Additionally, the coordinatively unsaturated tetrahedral complex exhibited greater activity than the octahedral complex. The study concluded that there is a correlation between the lability of M–O and M–N linkages and biological activity, with activity being directly proportional to the lability of the metal ion–MMTU bond [37].
The utilization of spherical gold nanoparticles, modified with biologically active molecules, holds significant potential for various medical applications [60]. Specifically, considering the known applications of 2-TU in treating hyperthyroidism and skin cancer, the compound 2-TUAuNPs emerges as a promising drug candidate for these diseases, offering the advantage of substantial side effect reduction. While there are alternative nanoparticle geometries with potential applications in cancer treatment, the use of small-diameter spherical AuNPs is particularly advantageous due to their ease of transport within cells, facilitated by the heightened vascularity characteristic of cancer cells [60].
2. Conclusions
We have summarized in this review the coordination sites and the applications of some synthesized metal complexes of 2-thiouracil and its derivatives. Structural reviews of metal complexes of 2-thiouracil and its derivatives as single ligands and mixed ligands have provided significant information on the nature of metal interactions with 2-thiouracil and its derivatives. Spectrophotometric and X-ray analysis of the metal complexes of uracil, 2- thiouracil, and its derivatives reveal the tendency of uracil and its derivatives to act as bidentate and monodentate agents in both binary and ternary complexes coordinating to a metal via any of O, N, S-atoms of the ring. Some of these complexes of 2-thiouracil and its derivatives have shown a variety of biological applications and should therefore be investigated. We have also researched the cytotoxic effect against a different tumor cell line of the various metal complexes of thiouracil derivatives.
Funding
We acknowledge the financial support of the Fund for Scientific Research of Plovdiv University, project CΠ 23-XΦ-006.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Garrett, R.H.; Grisham, C.M. Principles of Biochemistry with a Human Focus; Brooks/Cole Thomson Learning: Pacific Grove, CA, USA, 2001; p. 939. ISBN 0-03-097369-4. [Google Scholar]
- Astwood, E.B. The chemical nature of compounds which inhibit the function of the thyroid gland. J. Pharmacol. Exp. Ther. 1943, 78, 79–89. [Google Scholar]
- Mao, X.-M.; Li, H.-Q.; Li, Q.; Li, D.-M.; Xie, X.-J.; Yin, G.-P.; Zhang, P.; Xu, X.-H.; Wu, J.-D.; Chen, S.-W.; et al. Prevention of Relapse of Graves’ Disease by Treatment with an Intrathyroid Injection of Dexamethasone. J. Clin. Endocrinol. Metab. 2009, 94, 4984–4991. [Google Scholar] [CrossRef][Green Version]
- Rosenfeld, H.; Ornoy, A.; Shechtman, S.; Diav-Citrin, O. Pregnancy outcome, thyroid dysfunction, and fetal goiter after in utero exposure to propylthiouracil: A controlled cohort study. Br. J. Clin. Pharmacol. 2009, 68, 609–617. [Google Scholar] [CrossRef]
- Cooper, D.S. Antithyroid Drugs. N. Eng. J. Med. 2005, 352, 905–917. [Google Scholar] [CrossRef]
- Volpé, R. The Immunomodulatory Effects of Anti-thyroid Drugs are Mediated via Actions on Thyroid Cells, Affecting Thyrocyte-immunocyte Signalling: A Review. Curr. Pharm. Des. 2001, 7, 451–460. [Google Scholar] [CrossRef]
- Burch, H.B.; Cooper, D.S. Antithyroid drug therapy: 70 years later. Eur. J. Endocrinol. 2018, 179, R261–R274. [Google Scholar] [CrossRef]
- Fernandez, M.G. Hyperthyroidism and pregnancy. Endocrinol. Nutr. 2013, 60, 535–543. [Google Scholar] [CrossRef]
- Patil, S.B. Recent medicinal approaches of novel pyrimidine analogs: A review. Heliyon 2023, 9, e16773. [Google Scholar] [CrossRef] [PubMed]
- Verbitskiy, E.V.; Rusinov, G.L.; Charushin, V.N.; Chupakhin, O.N. Development of new antituberculosis drugs among of 1,3- and 1,4-diazines. Highlights and perspectives. Russ. Chem. Bull. Int. Ed. 2019, 68, 2172–2189. [Google Scholar] [CrossRef]
- Mahapatra, A.; Prasad, T.; Sharma, T. Pyrimidine: A review on anticancer activity with key emphasis on SAR. Futur. J. Pharm. Sci. 2021, 7, 123. [Google Scholar] [CrossRef]
- Wu, W.; Lan, W.; Wu, C.; Fei, Q. Synthesis and Antifungal Activity of Pyrimidine Derivatives Containing an Amide Moiety. Front. Chem. 2021, 9, 695628. [Google Scholar] [CrossRef]
- Tyli´nska, B.; Wiatrak, B.; Czyznikowska, Z.; Cie´sla-Niechwiadowicz, A.; Gebarowska, E.; Janicka-Kłos, A. Novel Pyrimidine Derivatives as Potential Anticancer Agents: Synthesis, Biological Evaluation and Molecular Docking Study. Int. J. Mol. Sci. 2021, 22, 3825. [Google Scholar] [CrossRef] [PubMed]
- Oladipo, M.A.; Isola, K.T. Coordination Possibility of Uracil and Applications of Some of Its Complexes: A Review. Res. J. Pharm. Biol. Chem. Sci. 2013, 4, 386–394. [Google Scholar] [CrossRef]
- Masoud, M.S.; Ramadana, M.S.; Ramadana, A.M.; Al-Saify, M.H. Complexing Properties and Applications of Some Biologically Active Nucleic Acid Constituents. Int. J. Innov. Res. Technol. Sci. Eng. 2020, 6, 23–39. [Google Scholar]
- Narang, K.K.; Singh, V.P.; Bhattacharya, D. Synthesis, characterization and antitumor activity of uracil and uracil–histidine complexes with metal(III) ions. Trans. Metal. Chem. 1997, 22, 333–337. [Google Scholar] [CrossRef]
- Cartwright, B.A.; Goodgame, M.; Johns, K.W.; Skapski, A.C. Strong Metal-Oxygen Interaction in Uracils. X-ray crystal structure of bis-(1,3-dimethyluracil)dichlorocopper(II). Biochem. J. 1978, 175, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Masoud, M.S.; Ibrahim, A.A.; Khalil, E.A.; El-Marghany, A. Spectral properties of some metal complexes derived from uracil–thiouracil and citrazinic acid compounds. Spectrochim. Acta Part A 2007, 67, 662–668. [Google Scholar] [CrossRef]
- Ghosh, P.; Mukhopadhyay, T.K.; Sarkar, A.R. Interaction of Divalent Metal Ions with Uracil III. Complexes of MnII, FeII, CoII, NiII and Cull with Uracil Acting as Bidentate Ligand. Trans. Metal Chem. 1984, 9, 46–48. [Google Scholar] [CrossRef]
- Koz, G.; Kaya, H.; Astley, D.; Yaşa, İ.; Astley, S.T. Synthesis, Characterization and Antimicrobial Screening of Ni(II), Cu(II) and Co(II) Complexes of Some Schiff Base Ligands Derived from 5-Aminouracil. Gazi Univ. J. Sci. 2011, 24, 407–413. [Google Scholar]
- Kufelnicki, A.; Jaszczak, J.; Kalinowska-Lis, U.; Wardak, C.; Ochocki, J. Complexes of Uracil (2,4-Dihydroxypyrimidine) Derivatives Part III. pH-Metric, ISE, and Spectrophotometric Studies on Co(II), Ni(II), and Zn(II) Complexes. J. Solution Chem. 2006, 35, 739–751. [Google Scholar] [CrossRef]
- Tyagi, S.; Singh, S.M.; Gencaslan, S.; Sheldrick, W.S.; Singh, U.P. Metal-5-fluorouracil-histamine complexes: Solution, structural, and antitumor studies. Metal Based Drugs 2002, 8, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, A.A. Synthesis, structural studies of some nucleic acids metal complexes. Basrah J. Sci. 2006, 24, 115–128. [Google Scholar]
- Verma, S.; Shrivastva, S.; Rani, P. Synthesis and spectroscopic studies of mixed ligand complexes of transition and inner transition metals with a substituted benzimidazole derivative and RNA bases. J. Chem. Pharm. Res. 2012, 4, 693–699. [Google Scholar]
- Shobana, S.; Dharmaraja, J.; Kamatchi, P.; Selvaraj, S. Mixed ligand complexes of Cu (II)/Ni (II)/Zn (II) ions with 5-Fluorouracil (5-FU) in the presence of some amino acid moieties: Structural and antimicrobial studies. J. Chem. Pharm. Res. 2012, 4, 4995–5004. [Google Scholar]
- Gupta, M.; Srivastava, M.N. Synthesis and characterization of complexes of copper(II), nickel(II), cobalt(II) and zinc(II) with alanine and uracil or 2-thiouracil. Synth. React. Inorg. Met.-Org. Chem. 1996, 26, 305–320. [Google Scholar] [CrossRef]
- Gupta, M.; Srivastava, M.N. Synthesis and characterization of mixed ligand complexes of copper(II), nickel(II), cobalt(II and zinc(II) with glycine and uracil or 2-thiouracil. Polyhedron 1985, 4, 475–479. [Google Scholar] [CrossRef]
- Gupta, M.; Srivastava, M.N. Synthesis and characterization of complexes of copper (II), nickel (II), cobalt (II) and zinc (II) with histidine and uracil, thymine or 2-thiouracil. Bull Chem. Soc. Fr. 1991, 128, 859. [Google Scholar]
- Gupta, M.; Srivastava, M.N. Synthesis and Characterization of Mixed-Ligand Complexes of Copper (II), Nickel (II), Cobalt (II) and Zinc (II) With Glycine and Thymine or Adenine. Bull. Pol. Acad. Sci. 1992, 40, 277–285. [Google Scholar]
- Saxena, V.K.; Srivastava, M.N. PMR Spectral Studies of ktixed-Ligand Amino Acid Chelates of Cobalt(II), Nickel(II), Copper(II) and Zinc(II) with Nitrilotriacetic Acid and glycine, α-alanine, Valine, or Leucine. J. Inorg. Bio-Chem. 1990, 38, 37. [Google Scholar] [CrossRef]
- Marinova, P.; Tsoneva, S.; Frenkeva, M.; Blazheva, D.; Slavchev, A.; Penchev, P. New Cu(II), Pd(II) and Au(III) complexes with 2-thiouracil: Synthesis, Characteration and Antibacterial Studies. Russ. J. Gen. Chem. 2022, 92, 1578–1584. [Google Scholar] [CrossRef]
- Marinova, P.; Hristov, M.; Tsoneva, S.; Burdzhiev, N.; Blazheva, D.; Slavchev, A.; Varbanova, E.; Penchev, P. Synthesis, Characterization and Antibacterial Studies of new Cu(II) and Pd(II) complexes with 6-methyl-2-thiouracil and 6-propyl-2-thiouracil. Appl. Sci. 2023, 13, 13150. [Google Scholar] [CrossRef]
- Moreno-Carretero, M.N.; Romero-Molina, M.A.; Salas-Peregrin, J.M.; Sanchez-Sanchez, M.P. Thermal analysis applied to the study of metal complexes: Thermal behaviour of 6-amino-2-thiouracil and its complexes with several transition metal ions. Thermochim. Acta 1992, 200, 271–280. [Google Scholar] [CrossRef]
- Romero, M.A.; Sanchez, M.P.; Quiros, M.; Sanchez, F.; Salas, J.M.; Moreno, M.; Faure, R. Transition metal complexes of 6-amino 2-thiouracil; crystal structure of bis(6-amino-2-thiouracilato)aquazinc(II) dihydrate. Can. J. Chem. 1993, 71, 29–33. [Google Scholar] [CrossRef]
- Khullar, I.P.; Agarwala, U. 2-Mercaptopyrimidin-4-ol (2-Thiouracil) Complexes of Copper(II), Nickel(II), Cobalt(II) and Iron(III). Aust. J. Chem. 1974, 27, 1877–1883. [Google Scholar] [CrossRef]
- Garrett, E.R.; Weber, D.J. Metal Complexes of Thiouracils II: Solubility Analyses and Spectrophotometric Investigations. J. Pharm. Sci. 1971, 60, 845–853. [Google Scholar] [CrossRef]
- Kamalakannan, P.; Venkappayya, D.; Balasubramanian, T. A new antimetabolite, 5-morpholinomethyl-2-thiouracil—Spectral properties, thermal profiles, antibacterial, antifungal and antitumour studies of some of its metal chelates. J. Chem. Soc. Dalton Trans. 2002, 17, 3381–3391. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Frost, B.J.; Derecskei-Kovacs, A.; Reibenspies, J.H. Coordination Chemistry, Structure, and Reactivity of Thiouracil Derivatives of Tungsten(0) Hexacarbonyl: A Theoretical and Experimental Investigation into the Chelation/Dechelation of Thiouracil via CO Loss and Addition. Inorg. Chem. 1999, 38, 4715–4723. [Google Scholar] [CrossRef]
- Masoud, M.S.; Soayed, A.A.; El-Husseiny, A.F. Coordination modes, spectral, thermal and biological evaluation of hetero-metal copper containing 2-thiouracil complexes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 99, 365–372. [Google Scholar] [CrossRef]
- Papazoglou, I.; Cox, P.J.; Hatzidimitriou, A.G.; Kokotidou, C.; Choli-Papadopoulou, T.; Aslanidis, P. Copper(I) halide complexes of 5-carbethoxy-2-thiouracil: Synthesis, structure and in vitro cytotoxicity. Eur. J. Med. Chem. 2014, 78, 383–391. [Google Scholar] [CrossRef]
- Kumar, B.; Suman, A. Synthesis, spectroscopic characterization and biological application of copper complex of 5-carbethoxy-2-thiouracil. J. Drug Deliv. Ther. 2020, 10, 145–148. [Google Scholar] [CrossRef]
- Kostova, I. General and inorganic chemistry; Softtrade: Sofia, Bulgaria, 2016; ISBN 978-954-334-185-6. [Google Scholar]
- Illán-Cabeza, N.A.; García-García, A.R.; Moreno-Carretero, M.N.; Martínez-Martos, J.M.; Ramírez-Expósito, M.J. Synthesis, characterization and antiproliferative behavior of tricarbonyl complexes of rhenium(I) with some 6-amino-5-nitrosouracil derivatives: Crystal structure of fac-[ReCl(CO)3(DANU-N5,O4)] (DANU = 6-amino-1,3-dimethyl-5-nitrosouracil). J. Inorg. Biochem. 2005, 99, 1637–1645. [Google Scholar] [CrossRef]
- Abou-Melha, K.S. A Series of Nano-sized Metal ion-thiouracil Complexes, tem, Spectral, γ- irradiation, Molecular Modeling and Biological Studies. Orient. J. Chem. 2015, 31, 1897–1913. [Google Scholar] [CrossRef]
- Golubyatnikova, L.G.; Khisamutdinov, R.A.; Grabovskii, S.A.; Kabal’nova, N.N.; Murinov, Y.I. Complexes of Palladium(II) and Platinum(II) with 6-tert-Butyl-2-thiouracil. Russ. J. Gen.Chem. 2017, 87, 117–121. [Google Scholar] [CrossRef]
- Jayabharathi, J.; Thanikachalam, V.; Jayamoorthy, K.; Perumal, M.V. Computational studies of 1,2-disubstituted benzimidazole derivatives. Spectrochim. Acta A 2012, 97, 6. [Google Scholar] [CrossRef] [PubMed]
- Masoud, M.S.; Amira, M.F.; Ramadan, A.M.; El-Ashry, G.M. Synthesis and characterization of some pyrimidine, purine, amino acid and mixed ligand complexes. Spectrochim. Acta Part A 2008, 69, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Masoud, M.S.; El-Hamid, O.H.A.; Zaki, Z.M. 2-thiouracil-based cobalt(II), nickel(II) and copper(II) complexes. Trans. Met. Chem. 1994, 19, 21–24. [Google Scholar] [CrossRef]
- El-Morsy, F.A.; Jean-Claude, B.J.; Butler, I.S.; El-Sayed, S.A.; Mostafa, S.I. Synthesis, characterization and anticancer activity of new zinc(II), molybdate(II), palladium(II), silver(I), rhodium(III), ruthenium(II) and platinum(II) complexes of 5,6-diamino-4-hydroxy2-mercaptopyrimidine. Inorg. Chim. Acta 2014, 423, 144–155. [Google Scholar] [CrossRef]
- Abás, E.; Pena-Martínez, R.; Aguirre-Ramírez, D.; Rodríguez-Diéguez, A.; Laguna, M.; Grasa, L. New selective thiolate gold(I) complexes inhibit the proliferation of different human cancer cells and induce apoptosis in primary cultures of mouse colon tumors. Dalton Trans. 2020, 49, 1915–1927. [Google Scholar] [CrossRef]
- Holowczak, M.S.; Stancl, M.D.; Wong, G.B. Trichloro( 1-metbylcytosinato)gold(III). Model for DNA interactions. J. Am. Chem. Soc. 1985, 107, 5789–5790. [Google Scholar] [CrossRef]
- Rodriguez, E.C.; Sánchez, J.R.; López-González, J.D.; Salas-Peregrin, J.M.; Olivier, M.J.; Quirós, M.; Beauchamp, A.L. Thermal Behavior and Crystal Structure of Dichloro[ 6-amino-l,3-dimethyl-5-(2-chlorophenylazo)uracilato]gold(III). Inorg. Chim. Acta 1990, 171, 151–156. [Google Scholar] [CrossRef]
- Singh, U.P.; Singh, S.; Singh, S.M. Synthesis, characterization and antitumour activity of metal complexes of 5-carboxy-2-thiouracil. Metal-Based Drugs 1998, 5, 35–39. [Google Scholar] [CrossRef]
- Worachartcheewan, A.; Pingaew, R.; Lekcharoen, D.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Synthesis, Antioxidant and Antimicrobial Activities of Metal Complexes of 2-thiouracil-hydroxyquinoline Derivatives. Lett. Drug Des. Discov. 2018, 15, 602–611. [Google Scholar] [CrossRef]
- Fathalla, O.A.; Awad, S.M.; Mohamed, M.S. Synthesis of new 2-thiouracil-5-sulphonamide derivatives with antibacterial and antifungal activity. Arch. Pharm. Res. 2005, 28, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.S.; Awad, S.M.; Ahmed, N.M. Synthesis and antimicrobial evaluation of some 6-aryl-5-cyano-2-thiouracil derivatives. Acta Pharm. 2011, 61, 171–185. [Google Scholar] [CrossRef]
- Mohamed, M.S.; Youns, M.M.; Ahmed, N.M. Synthesis, antimicrobial, antioxidant activities of novel 6-aryl-5-cyano thiouracil derivatives. Eur. J. Med. Chem. 2013, 69, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Rizk, S.A.; El-Naggar, A.M.; El-Badawy, A.A. Synthesis, spectroscopic characterization and computational chemical study of 5-cyano-2-thiouracil derivatives as potential antimicrobial agents. J. Mol. Struct. 2018, 1155, 720–733. [Google Scholar] [CrossRef]
- da Silva Dantas, F.G.; de Almeida-Apolonio, A.A.; de Araújo, R.P.; Favarin, L.R.V.; de Castilho, P.F.; de Oliveira Galvão, F.; Svidzinski, T.I.E.; Casagrande, G.A.; de Oliveira, K.M.P. A Promising Copper(II) Complex as Antifungal and Antibiofilm Drug against Yeast Infection. Molecules 2018, 23, 1856. [Google Scholar] [CrossRef]
- Lorenzana-Vázquez, G.; Pavel, I.; Meléndez, E. Gold Nanoparticles Functionalized with 2-Thiouracil for Antiproliferative and Photothermal Therapies in Breast Cancer Cells. Molecules 2023, 28, 4453. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).