AlH3 as High-Energy Fuels for Solid Propellants: Synthesis, Thermodynamics, Kinetics, and Stabilization
Abstract
1. Introduction
2. Synthesis of AlH3
2.1. Wet Chemical Methods
2.2. Dry Synthesis Methods
2.2.1. Mechano-Chemical Method
2.2.2. Organoaluminum Decomposition Method
2.3. Other Emerging Methods
2.3.1. Supercritical Synthesis Method
2.3.2. Organoaluminum Decomposition Method
2.3.3. High Pressure Hydrogenation Method
2.4. Comparison of Synthetic Methods and Its Development Suggestion
3. Thermodynamics and Kinetics of AlH3
3.1. Thermodynamics
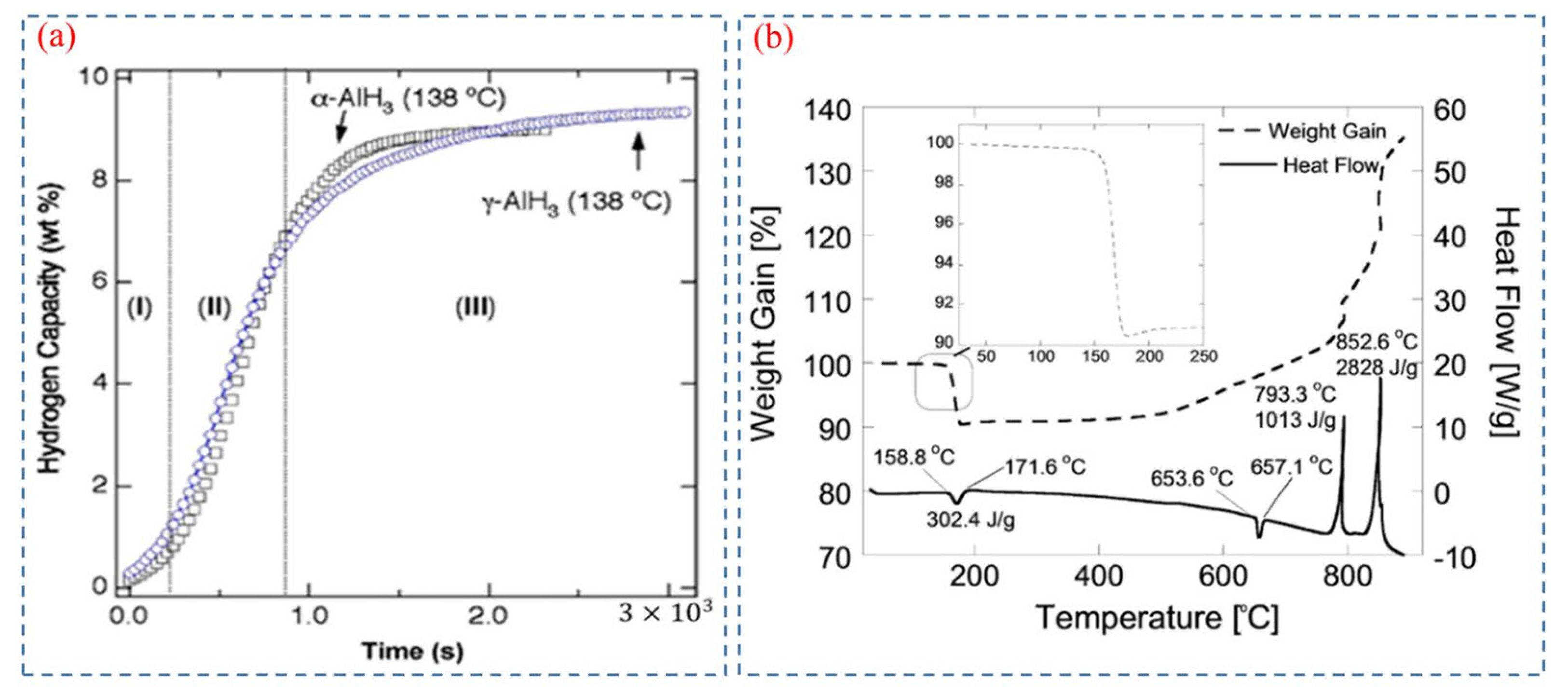
3.2. Kinetics
4. Physical and Chemical Modification of AlH3
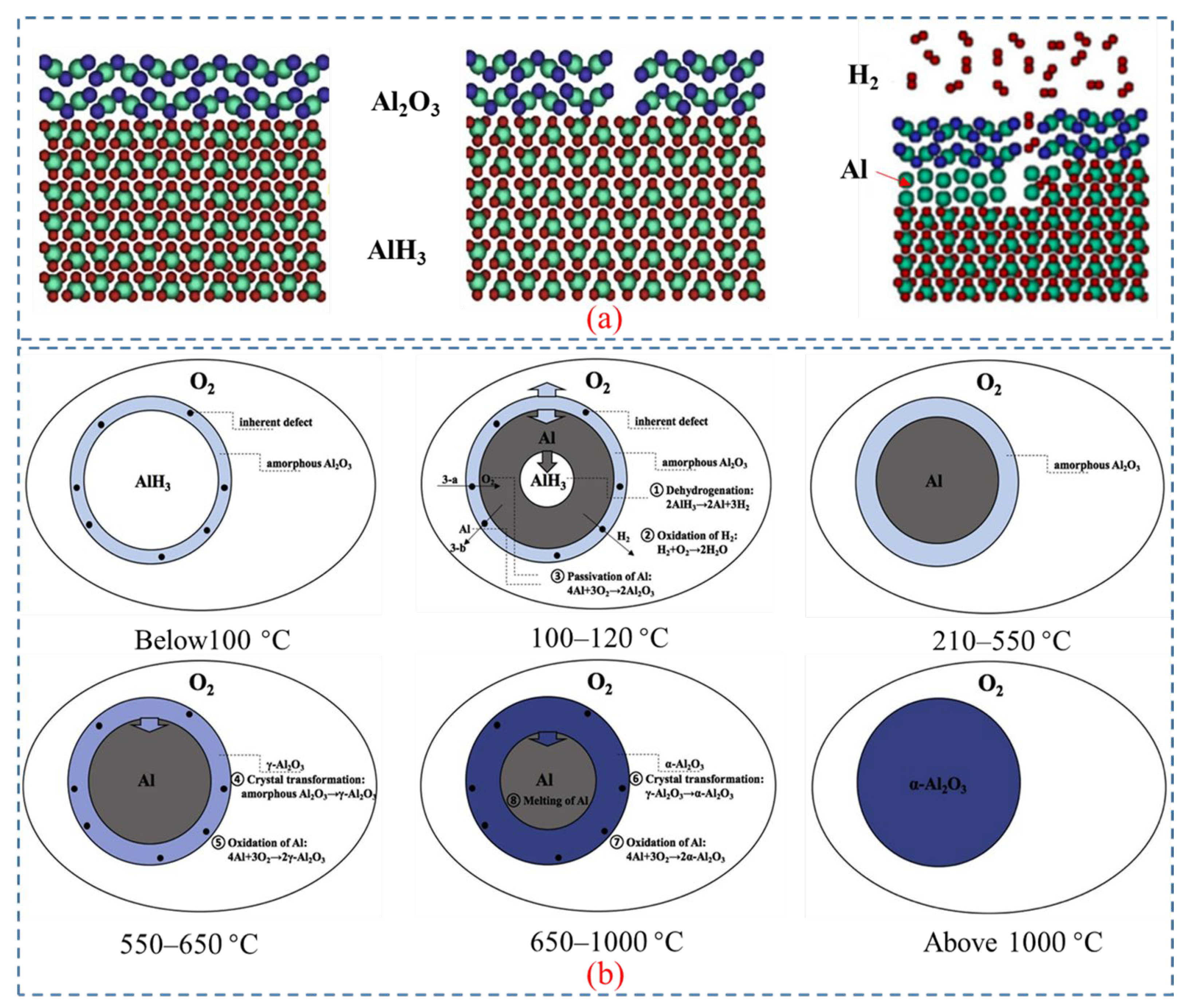
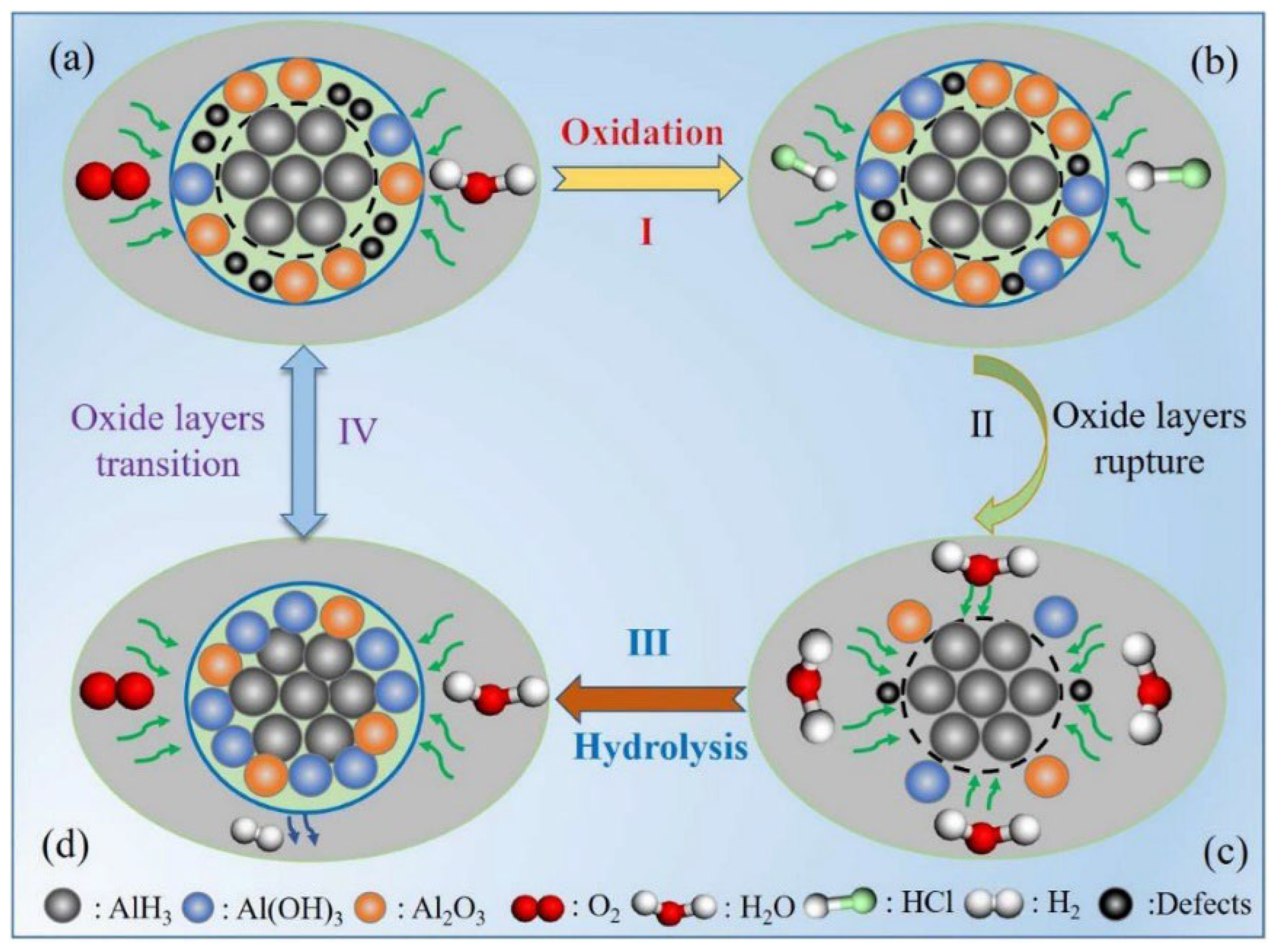
4.1. Surface Passivation Methods
4.2. Doping Methods
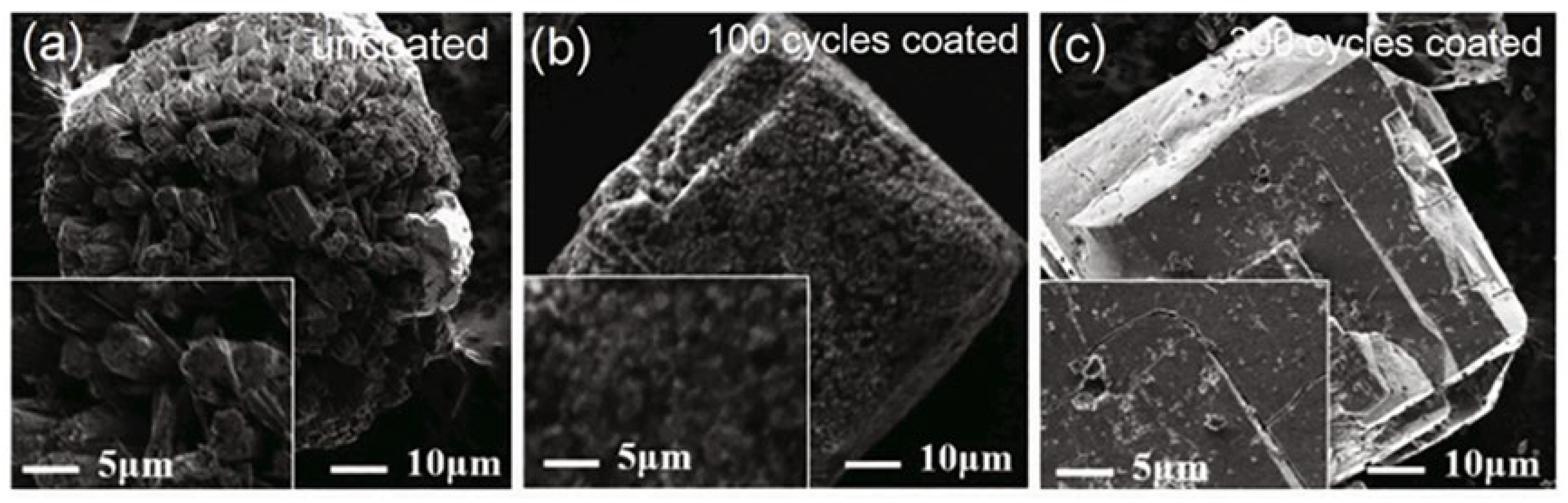
4.3. Surface Coating Methods
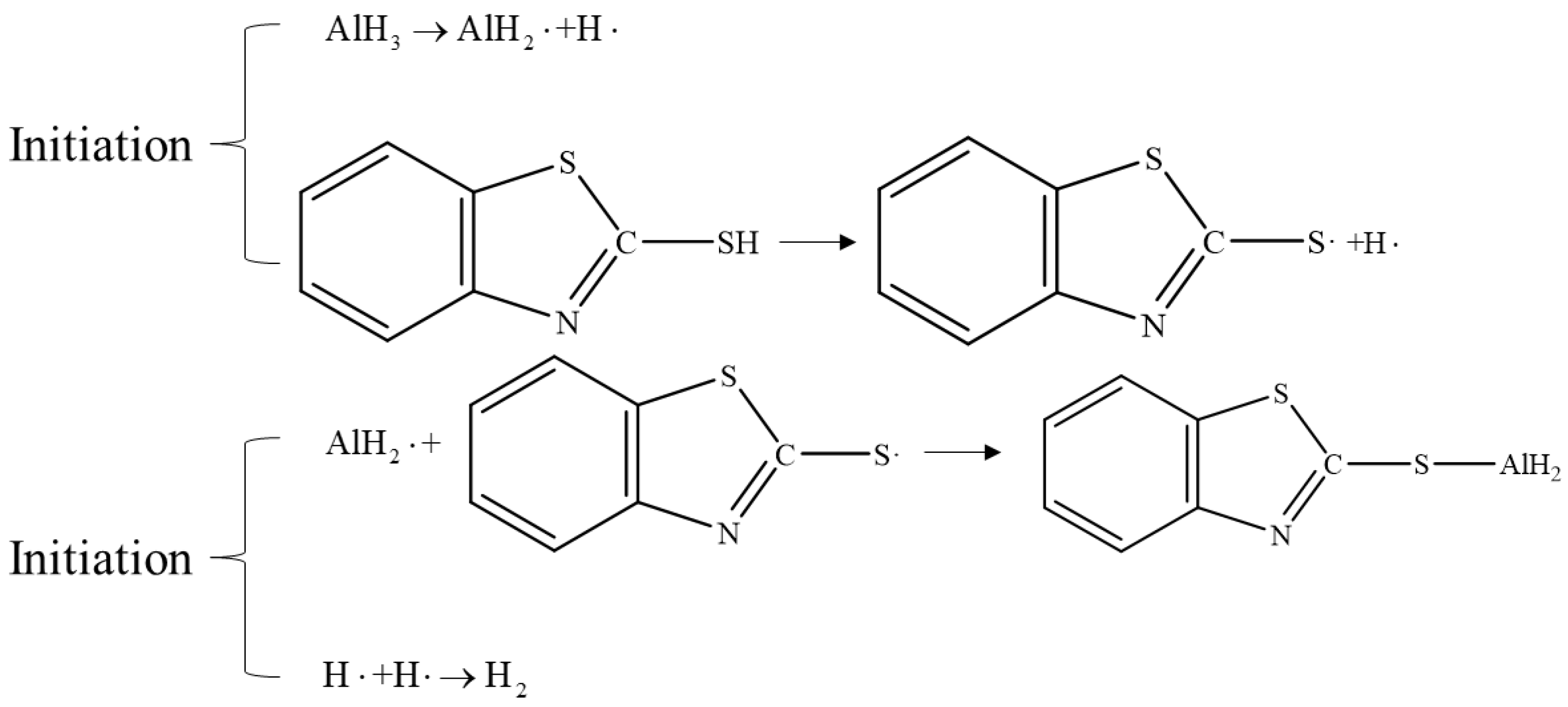
4.3.1. Inorganic and Organic Molecules
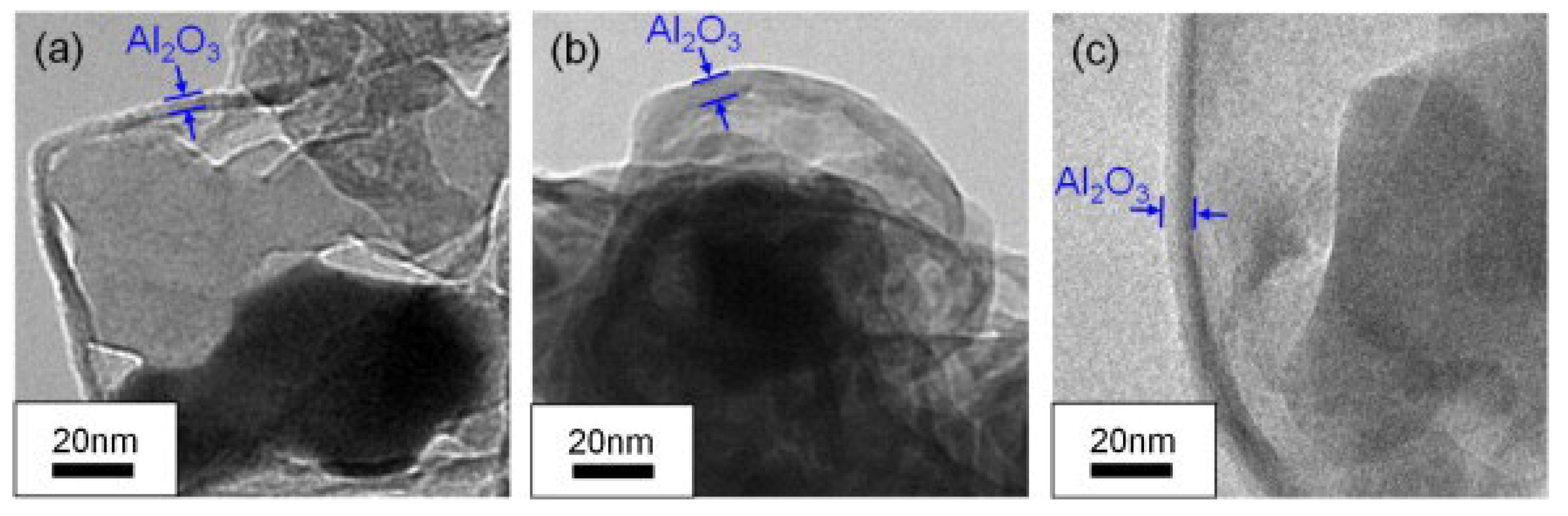
4.3.2. Metal Oxides
4.3.3. Organic Polymers
4.3.4. Carbon Materials
4.3.5. Energetic Components
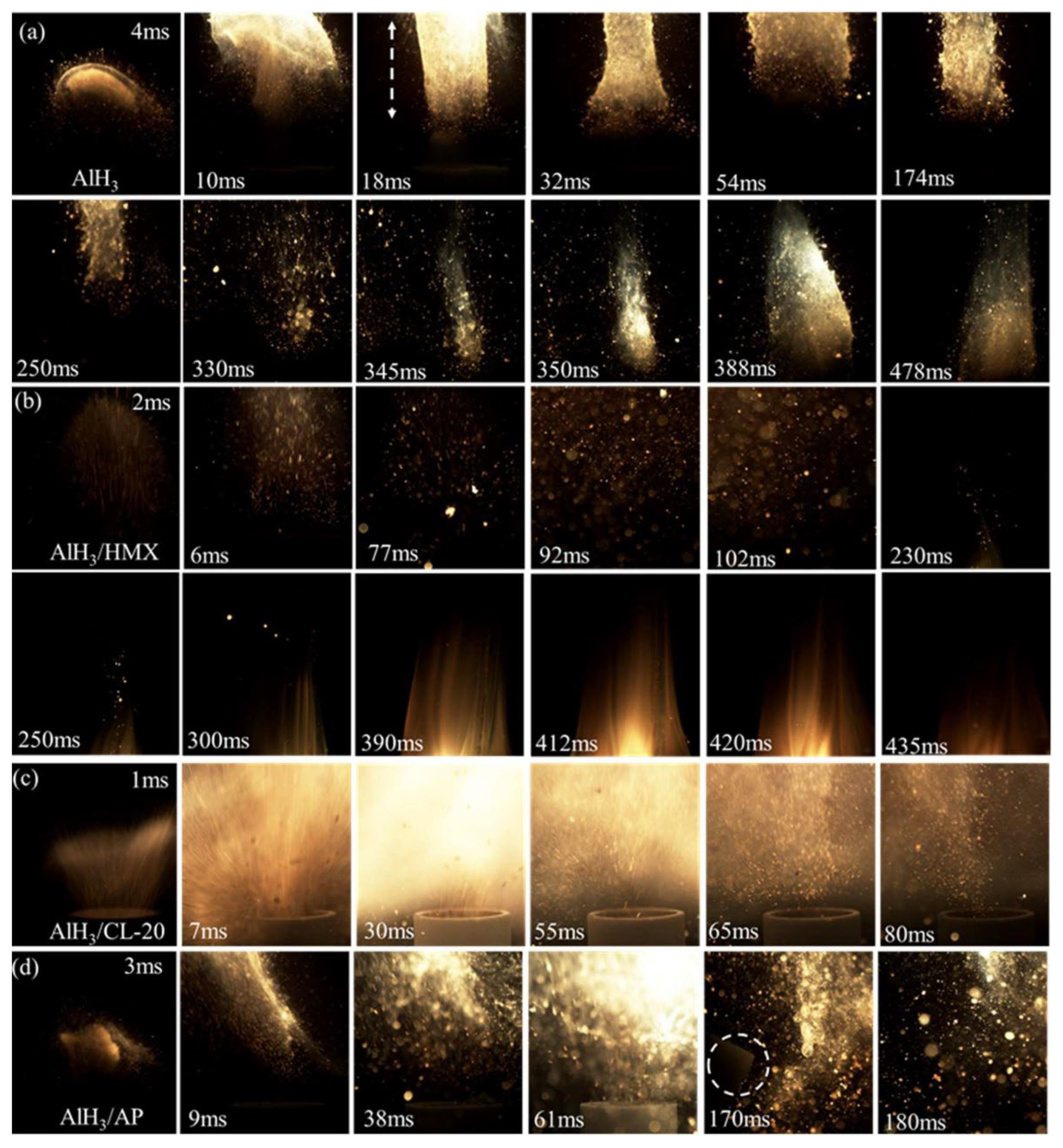
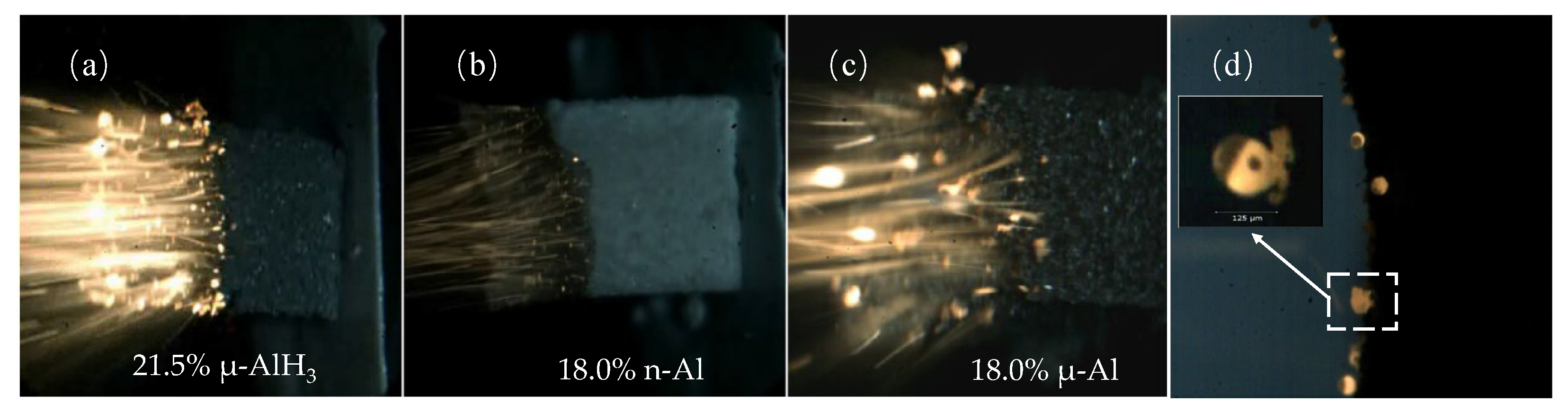
5. High Energy Fuel for Solid Propellant
6. Conclusions and Suggestions
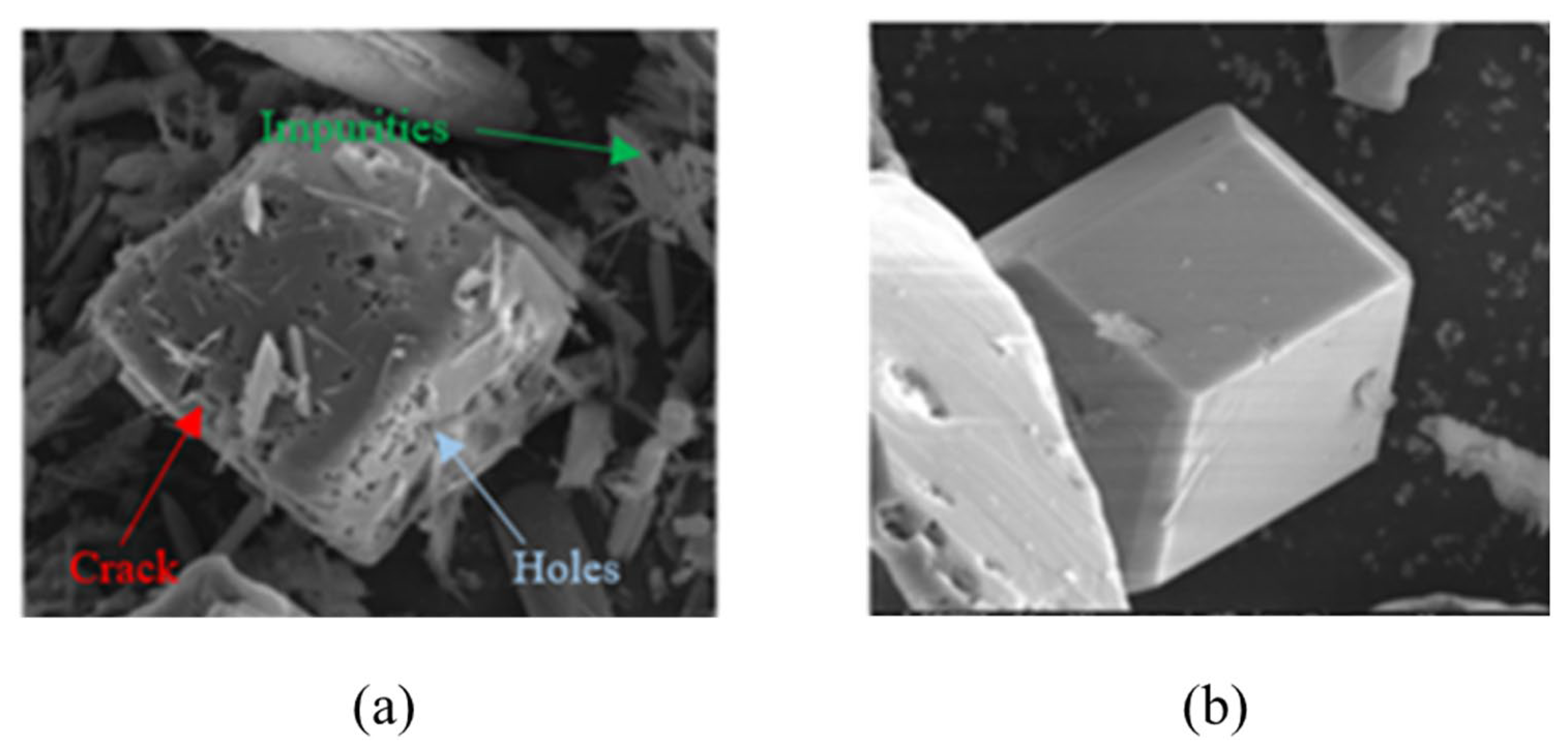
- The commonly used methods of synthesizing AlH3 are wet chemical synthesis and mechano-chemical method, both of which are faced with the difficulty in achieving high crystal purity of the product. Adding crystalline inducer helps to control the proportion of crystal types, but the presence of an inducer in the product will lead to a decrease in the thermal stability of AlH3. Therefore, highly effective inducers are easy to separate, and need to be developed.
- From a kinetics perspective, the acceleratory period is a critical stage that governs the decomposition rate of aluminum hydride, primarily due to the multi-dimensional growth of the aluminum phase. However, the acceleration period is difficult to control. Thus, how to decelerate the decomposition rate by prolonging the induction period through modifying the surface components of AlH3 is the next research direction.
- The stabilization of AlH3 involves enhancing crystal purity, isolating from external stimuli, and eliminating factors contributing to instability. Among the stabilization methods, surface passivation and the doping method will cause energy loss and negatively affect the combustion performance. As for surface coating, the ratio and thickness of the coating layer proves difficult to control, resulting in failure to accurately regulate its impact on the energy performance of α-AlH3. Efficient coating materials are crucial for advancing the application of AlH3 in solid propellants.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Young, G.; Risha, G.A.; Connell, T.L.; Yetter, R.A. Combustion of HTPB Based Solid Fuels Containing Metals and Metal Hydrides with Nitrous Oxide. Propellants Explos. Pyrotech. 2019, 44, 744–750. [Google Scholar] [CrossRef]
- Chen, S.; Tang, Y.; Yu, H.; Bao, L.; Zhang, W.; DeLuca, L.T.; Shen, R.; Ye, Y. The Rapid H2 Release from AlH3 Dehydrogenation Forming Porous Layer in AlH3/Hydroxyl-Terminated Polybutadiene (HTPB) Fuels during Combustion. J. Hazard. Mater. 2019, 371, 53–61. [Google Scholar] [CrossRef]
- DeLuca, L.T.; Galfetti, L.; Severini, F.; Rossettini, L.; Meda, L.; Marra, G.; D’Andrea, B.; Weiser, V.; Calabro, M.; Vorozhtsov, A.B.; et al. Physical and Ballistic Characterization of AlH3-Based Space Propellants. Aerosp. Sci. Technol. 2007, 11, 18–25. [Google Scholar] [CrossRef]
- Ding, X.; Shu, Y.; Liu, N.; Wu, M.; Zhang, J.; Gou, B.; Wang, H.; Wang, C.; Dong, S.; Wang, W. Energetic Characteristics of HMX-Based Explosives Containing LiH. Propellants Explos. Pyrotech. 2016, 41, 1079–1084. [Google Scholar] [CrossRef]
- Liu, L.; Li, J.; Zhang, L.; Tian, S. Effects of Magnesium-Based Hydrogen Storage Materials on the Thermal Decomposition, Burning Rate, and Explosive Heat of Ammonium Perchlorate-Based Composite Solid Propellant. J. Hazard. Mater. 2018, 342, 477–481. [Google Scholar] [CrossRef]
- Yang, Z.; Zhao, F.; Xu, S.; Yang, F.; Yao, E.; Ren, X.; Wu, Z.; Zhang, Z. Investigation on Adsorption and Decomposition Properties of CL-20/FOX-7 Molecules on MgH2(110) Surface by First-Principles. Molecules 2020, 25, 2726. [Google Scholar] [CrossRef]
- Pang, W.; Fan, X.; Zhao, F.; Xu, H.; Zhang, W.; Yu, H.; Li, Y.; Liu, F.; Xie, W.; Yan, N. Effects of Different Metal Fuels on the Characteristics for HTPB-based Fuel Rich Solid Propellants. Propellants Explos. Pyrotech. 2013, 38, 852–859. [Google Scholar] [CrossRef]
- Cooper, M.A.; Oliver, M.S. The Burning Regimes and Conductive Burn Rates of Titanium Subhydride Potassium Perchlorate (TiH1.65/KClO4) in Hybrid Closed Bomb-Strand Burner Experiments. Combust. Flame 2013, 160, 2619–2630. [Google Scholar] [CrossRef]
- Bi, X.; Liu, J. Detonation Properties of High Explosives Containing Ammonia Borane. Z. Anorg. Allg. Chem. 2016, 642, 773–777. [Google Scholar] [CrossRef]
- Li, H.; Liang, D.; Yu, M.; Liu, J.; Wang, Y.; Pang, A.; Tang, G.; Huang, X. Study on Dehydrogenation and Oxidation Kinetics Mechanisms of Micron α-AlH3 in an Oxidative Atmosphere. Int. J. Hydrogen Energy 2020, 45, 24958–24967. [Google Scholar] [CrossRef]
- Graetz, J.; Reilly, J.J.; Yartys, V.A.; Maehlen, J.P.; Bulychev, B.M.; Antonov, V.E.; Tarasov, B.P.; Gabis, I.E. Aluminum Hydride as a Hydrogen and Energy Storage Material: Past, Present and Future. J. Alloys Compd. 2011, 509, S517–S528. [Google Scholar] [CrossRef]
- Chen, R.; Duan, C.L.; Liu, X.; Qu, K.; Tang, G.; Xu, X.-X.; Shan, B. Surface Passivation of Aluminum Hydride Particles via Atomic Layer Deposition. J. Vac. Sci. Technol. A Vac. Surf. Film. 2017, 35, 03E111. [Google Scholar] [CrossRef]
- Jiang, Z.F.; Zhao, F.Q.; Zhang, M.; Li, H.; Zhang, J.-K.; Yang, Y.-J.; Li, N. Research Progress in the Stabilization of Aluminum Hydride. Huozhayao Xuebao/Chin. J. Explos. Propellants 2020, 43, 107–115. [Google Scholar] [CrossRef]
- Qin, M.-N.; Zhang, Y.; Tang, W.; Shi, Q.; Wang, W.; Qiu, S.-J. α-AlH3 Coated with Stearic Acid: Preparation and Its Electrostatic Sensitivity. Hanneng Cailiao/Chin. J. Energ. Mater. 2017, 25, 59–62. [Google Scholar] [CrossRef]
- Xing, J.H.; Xia, Y.; Wang, J.W. A Method for Improving Thermal Stability of Aluminum Hydride. CN Patent 109019507A, 5 November 2021. pp. 11–15. [Google Scholar]
- Cai, X.W.; Yang, J.H.; Zhang, J.L.; Ma, D.X.; Wang, Y.P. Liquid Carbon Dioxide as Anti-Solvent Coating Aluminum Hydride. Propellants Explos. Pyrotech. 2016, 40, 914–919. [Google Scholar] [CrossRef]
- Petrie, M.A.; Bottaro, J.C.; Schmitt, R.J.; Penwell, P.E.; Bomberger, D.C. Preparation of Aluminum Hydride Polymorphs, Particularly Stabilized α-AlH3. U.S. Patent 6228338, 8 May 2001. pp. 5–8. [Google Scholar]
- Schmidt, D. Non-Solvated Particulate Aluminum Hydride Coated with a Cyano-Coating Compound Useful in Solid Propellants. U.S. Patent 3850709, 26 November 1974. pp. 11–26. [Google Scholar]
- Roberts, C.B.; Toner, D.D. Stabilization of Light Metal Hydride (U). U.S. Patent 3850709, 26 November 1974. pp. 11–26. [Google Scholar]
- Flynn, J. Particulate Aluminum Hydride with Nitrocellulose Coating Suitable for Use in Solid Propellants. U.S. Patent 3855022, 17 December 1974. pp. 12–17. [Google Scholar]
- Finholt, A.E.; Bond, A.C.; Schlesinger, H.I. Lithium Aluminum Hydride, Aluminum Hydride and Lithium Gallium Hydride, and Some of Their Applications in Organic and Inorganic Chemistry 1. J. Am. Chem. Soc. 1947, 69, 1199–1203. [Google Scholar] [CrossRef]
- Brower, F.M.; Matzek, N.E.; Reigler, P.F.; Rinn, H.W.; Roberts, C.B.; Schmidt, D.L.; Snover, J.A.; Terada, K. Preparation and Properties of Aluminum Hydride. J. Am. Chem. Soc. 1976, 98, 2450–2453. [Google Scholar] [CrossRef]
- Yu, M.; Zhu, Z.; Li, H.-P.; Yan, Q.-L. Advanced Preparation and Processing Techniques for High Energy Fuel AlH3. Chem. Eng. J. 2021, 421, 129753. [Google Scholar] [CrossRef]
- Park, M.; Kim, W.; Kwon, Y.; Kim, J.; Jo, Y. Wet Synthesis of Energetic Aluminum Hydride. Propellants Explos. Pyrotech. 2019, 44, 1233–1241. [Google Scholar] [CrossRef]
- Xu, B.; Liu, J.; Wang, X. Preparation and Thermal Properties of Aluminum Hydride Polymorphs. Vacuum 2014, 99, 127–134. [Google Scholar] [CrossRef]
- Xu, B.; Liu, J.P. Aluminum Hydride Polymorphs: Preparation, Characterization and Thermal Properties. Adv. Mater. Res. 2013, 712–715, 333–336. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, L.; Ma, H.; Lu, C.; Luo, H.; Wang, X.; Huang, X.; Lan, Z.; Guo, J. Aluminum Hydride for Solid-State Hydrogen Storage: Structure, Synthesis, Thermodynamics, Kinetics, and Regeneration. J. Energy Chem. 2021, 52, 428–440. [Google Scholar] [CrossRef]
- Paskevicius, M.; Sheppard, D.A.; Buckley, C.E. Characterisation of Mechanochemically Synthesised Alane (AlH3) Nanoparticles. J. Alloys Compd. 2009, 487, 370–376. [Google Scholar] [CrossRef]
- Duan, C.W.; Hu, L.X.; Xue, D. Solid State Synthesis of Nano-Sized AlH3 and Its Dehydriding Behaviour. Green Chem. 2015, 17, 3466–3474. [Google Scholar] [CrossRef]
- Duan, C.; Cao, Y.; Hu, L.; Fu, D.; Ma, J.; Youngblood, J. An Efficient Mechanochemical Synthesis of Alpha-Aluminum Hydride: Synergistic Effect of TiF3 on the Crystallization Rate and Selective Formation of Alpha-Aluminum Hydride Polymorph. J. Hazard. Mater. 2019, 373, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, E.-K.; Shim, J.-H.; Cho, Y.W.; Yoon, K.B. Mechanochemical Synthesis and Thermal Decomposition of Mg(AlH4)2. J. Alloys Compd. 2006, 422, 283–287. [Google Scholar] [CrossRef]
- Baranowski, B.; Tkacz, M. The Equilibrium Between Solid Aluminium Hydride and Gaseous Hydrogen. Z. Phys. Chem. 1983, 135, 27–38. [Google Scholar] [CrossRef]
- Appel, M.; Frankel, J.P. Production of Aluminum Hydride by Hydrogen-Ion Bombardment. J. Chem. Phys. 1965, 42, 3984–3988. [Google Scholar] [CrossRef]
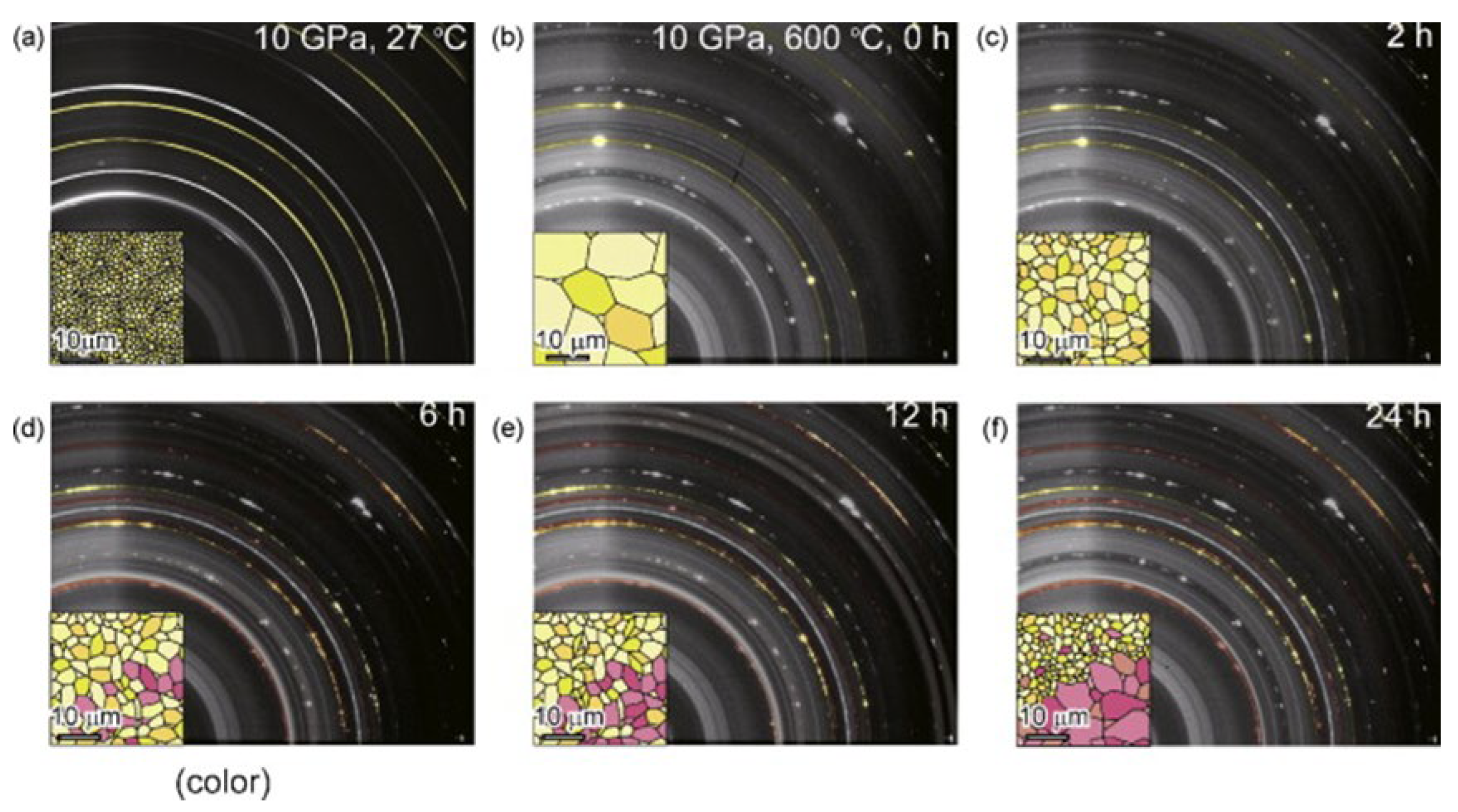
- Saitoh, H.; Machida, A.; Katayama, Y.; Aoki, K. Formation and Decomposition of AlH3 in the Aluminum-Hydrogen System. Appl. Phys. Lett. 2008, 93, 151918. [Google Scholar] [CrossRef]
- Dymova, T.N.; Mal’tseva, N.N.; Konoplev, V.N.; Golovanova, A.I.; Aleksandrov, D.P.; Sizareva, A.S. Solid-Phase Solvate-Free Formation of Magnesium Hydroaluminates Mg(AlH4)2 and MgAlH5 upon Mechanochemical Activation or Heating of Magnesium Hydride and Aluminum Chloride Mixtures. Russ. J. Coord. Chem. Khim. 2003, 29, 385–389. [Google Scholar] [CrossRef]
- Dymova, T.N.; Konoplev, V.N.; Sizareva, A.S.; Aleksandrov, D.P. Magnesium Tetrahydroaluminate: Solid-Phase Formation with Mechanochemical Activation of a Mixture of Aluminum and Magnesium Hydrides. Russ. J. Coord. Chem. Khim. 1999, 25, 312–315. [Google Scholar]
- Fichtner, M.; Frommen, C.; Fuhr, O. Synthesis and Properties of Calcium Alanate and Two Solvent Adducts. Inorg. Chem. 2005, 44, 3479–3484. [Google Scholar] [CrossRef] [PubMed]
- Mal’tseva, N.N.; Golovanova, A.I.; Dymova, T.N.; Aleksandrov, D.P. Solid-Phase Formation of Calcium Hydridoaluminates Ca(AlH4)2 and CaHAlH4 upon Mechanochemical Activation or Heating of Mixtures of Calcium Hydride with Aluminum Chloride. Russ. J. Inorg. Chem. 2001, 46, 1793–1797. [Google Scholar]
- Mamatha, M.; Bogdanović, B.; Felderhoff, M.; Pommerin, A.; Schmidt, W.; Schüth, F.; Weidenthaler, C. Mechanochemical Preparation and Investigation of Properties of Magnesium, Calcium and Lithium–Magnesium Alanates. J. Alloys Compd. 2006, 407, 78–86. [Google Scholar] [CrossRef]
- Duan, C.; Hu, L.; Sun, Y.; Zhou, H.; Yu, H. An Insight into the Process and Mechanism of a Mechanically Activated Reaction for Synthesizing AlH3 Nano-Composites. Dalt. Trans. 2015, 44, 16251–16255. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Cao, Y.; Hu, L.; Fu, D.; Ma, J. Synergistic Effect of TiF3 on the Dehydriding Property of α-AlH3 Nano-Composite. Mater. Lett. 2019, 238, 254–257. [Google Scholar] [CrossRef]
- Wang, L.; Rawal, A.; Quadir, M.Z.; Aguey-Zinsou, K.-F. Formation of Aluminium Hydride (AlH3) via the Decomposition of Organoaluminium and Hydrogen Storage Properties. Int. J. Hydrogen Energy 2018, 43, 16749–16757. [Google Scholar] [CrossRef]
- Liu, H.; Bouchard, M.; Zhang, L. An Experimental Study of Hydrogen Solubility in Liquid Aluminium. J. Mater. Sci. 1995, 30, 4309–4315. [Google Scholar] [CrossRef]
- Mcgrady, G.S. Hydrogenation of Aluminum Using a Supercritical Fluid Medium. U.S. Patent 0241056, 6 December 2008. pp. 10–12. [Google Scholar]
- Liu, F.; Huang, K.; Wu, Q.; Dai, S. Solvent-Free Self-Assembly to the Synthesis of Nitrogen-Doped Ordered Mesoporous Polymers for Highly Selective Capture and Conversion of CO2. Adv. Mater. 2017, 29, 1700445. [Google Scholar] [CrossRef]
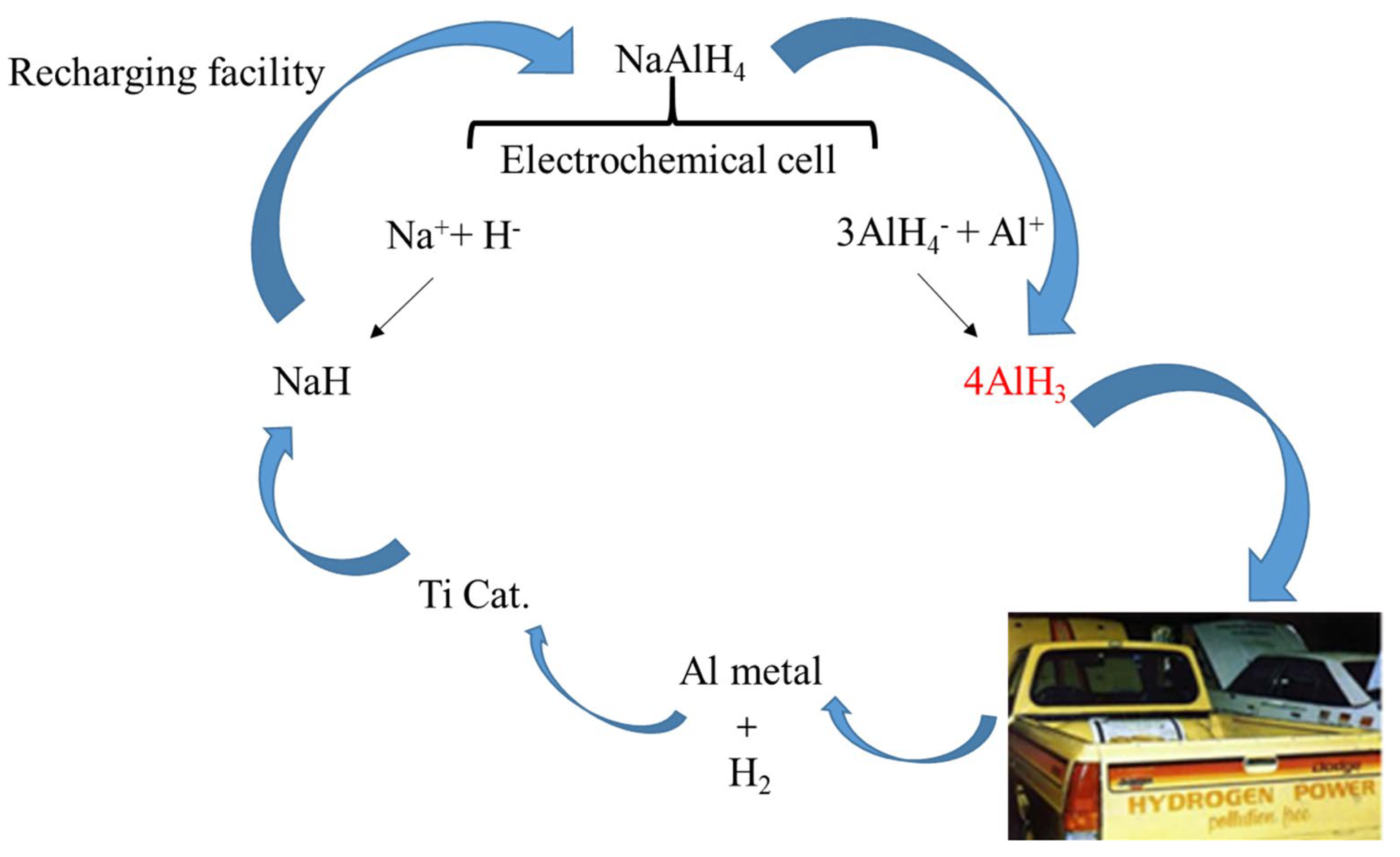
- Zidan, R.; Garcia-Diaz, B.L.; Fewox, C.S.; Stowe, A.C.; Gray, J.R.; Harter, A.G. Aluminium Hydride: A Reversible Material for Hydrogen Storage. Chem. Commun. 2009, 35, 3717. [Google Scholar] [CrossRef]
- Saitoh, H.; Okajima, Y.; Yoneda, Y.; Machida, A.; Kawana, D.; Watanuki, T.; Katayama, Y.; Aoki, K. Formation and Crystal Growth Process of AlH3 in Al–H System. J. Alloys Compd. 2010, 496, L25–L28. [Google Scholar] [CrossRef]
- Sinke, G.C.; Walker, L.C.; Oetting, F.L.; Stull, D.R. Thermodynamic Properties of Aluminum Hydride. J. Chem. Phys. 1967, 47, 2759–2761. [Google Scholar] [CrossRef]
- Chizinsky, G.; Evans, G.G.; Gibb, T.R.P.; Rice, M.J. Non-Solvated Aluminum Hydride 1. J. Am. Chem. Soc. 1955, 77, 3164–3165. [Google Scholar] [CrossRef]
- Sartori, S.; Opalka, S.M.; Løvvik, O.M.; Guzik, M.N.; Tang, X.; Hauback, B.C. Experimental Studies of α-AlD3 and α′-AlD3 versus First-Principles Modelling of the Alane Isomorphs. J. Mater. Chem. 2008, 18, 2361. [Google Scholar] [CrossRef]
- Graetz, J.; Reilly, J.J.; Kulleck, J.G.; Bowman, R.C. Kinetics and Thermodynamics of the Aluminum Hydride Polymorphs. J. Alloys Compd. 2007, 446–447, 271–275. [Google Scholar] [CrossRef]
- Maehlen, J.P.; Yartys, V.A.; Denys, R.V.; Fichtner, M.; Frommen, C.; Bulychev, B.M.; Pattison, P.; Emerich, H.; Filinchuk, Y.E.; Chernyshov, D. Thermal Decomposition of AlH3 Studied by in Situ Synchrotron X-Ray Diffraction and Thermal Desorption Spectroscopy. J. Alloys Compd. 2007, 446–447, 280–289. [Google Scholar] [CrossRef]
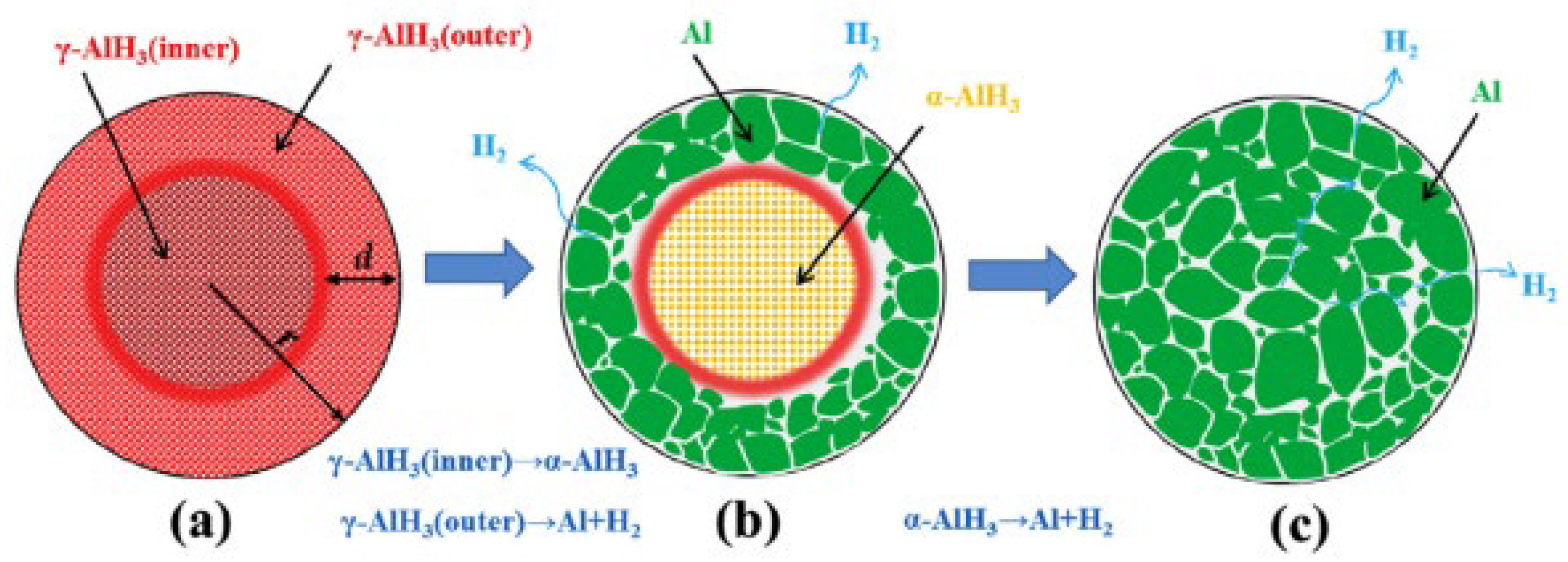
- Liu, H.; Wang, X.; Dong, Z.; Cao, G.; Liu, Y.; Chen, L.; Yan, M. Dehydriding Properties of γ-AlH3. Int. J. Hydrogen Energy 2013, 38, 10851–10856. [Google Scholar] [CrossRef]
- Gao, S.; Liu, H.; Wang, X.; Xu, L.; Liu, S.; Sheng, P.; Zhao, G.; Wang, B.; Li, H.; Yan, M. Hydrogen Desorption Behaviors of γ-AlH3: Diverse Decomposition Mechanisms for the Outer Layer and the Inner Part of γ-AlH3 Particle. Int. J. Hydrogen Energy 2017, 42, 25310–25315. [Google Scholar] [CrossRef]
- Graetz, J.; Reilly, J.J. Thermodynamics of the α, β and γ Polymorphs of AlH3. J. Alloys Compd. 2006, 424, 262–265. [Google Scholar] [CrossRef]
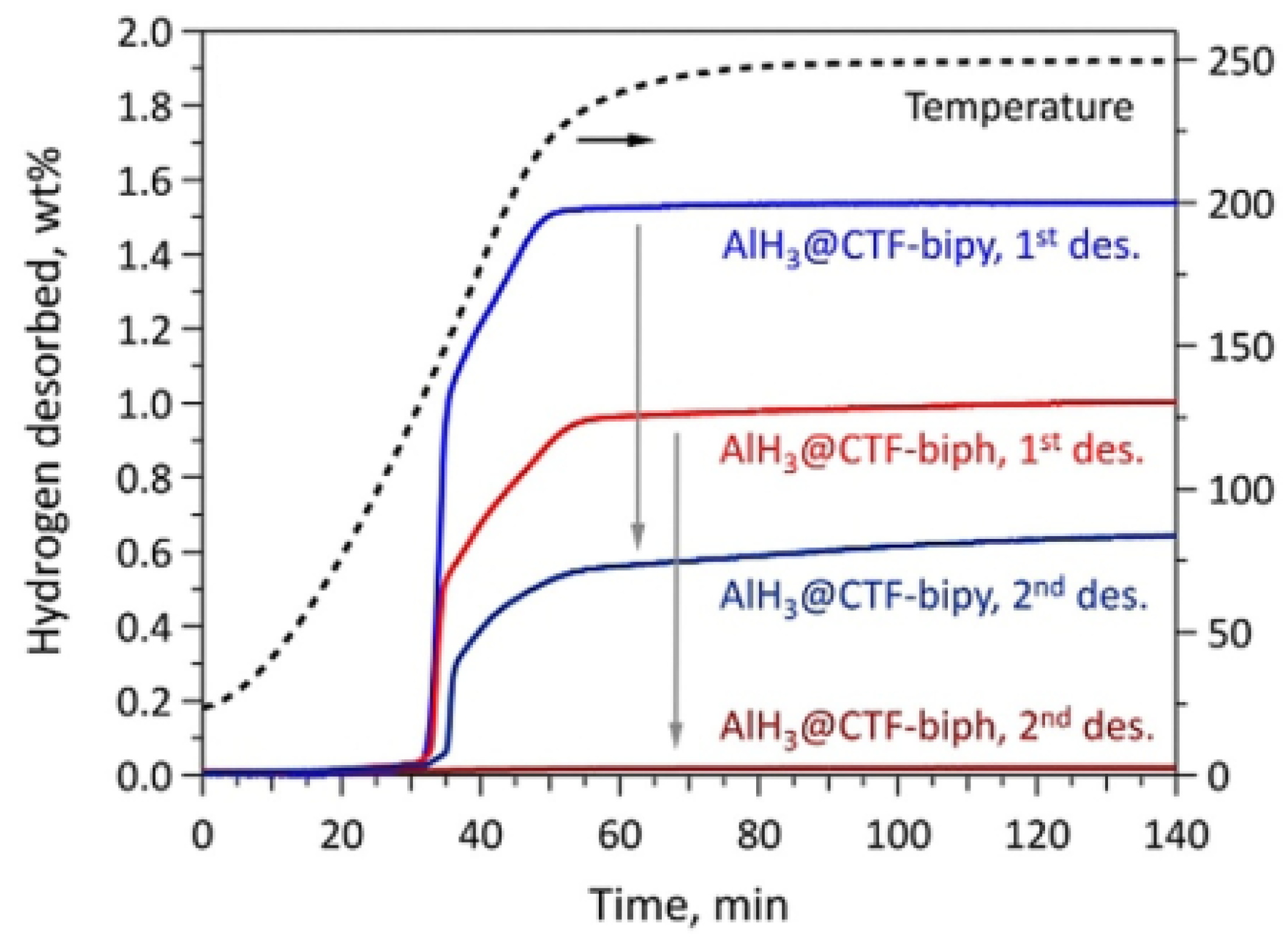
- Stavila, V.; Li, S.; Dun, C.; Marple, M.A.T.; Mason, H.E.; Snider, J.L.; Reynolds, J.E.; El Gabaly, F.; Sugar, J.D.; Spataru, C.D.; et al. Defying Thermodynamics: Stabilization of Alane Within Covalent Triazine Frameworks for Reversible Hydrogen Storage. Angew. Chem. 2021, 133, 26019–26028. [Google Scholar] [CrossRef]
- Herley, P.J.; Christofferson, O.; Irwin, R. Decomposition of Alpha-Aluminum Hydride Powder. 1. Thermal Decomposition. J. Phys. Chem. 1981, 85, 1874–1881. [Google Scholar] [CrossRef]
- Herley, P.J.; Christofferson, O. Decomposition of Alpha.-Aluminum Hydride Powder. 2. Photolytic Decomposition. J. Phys. Chem. 1981, 85, 1882–1886. [Google Scholar] [CrossRef]
- Herley, P.J.; Christofferson, O. Decomposition of Alpha.-Aluminum Hydride Powder. 3. Simultaneous Photolytic-Thermal Decomposition. J. Phys. Chem. 1981, 85, 1887–1892. [Google Scholar] [CrossRef]
- Tarasov, V.P.; Muravlev, Y.B.; Bakum, S.I.; Novikov, A.V. Kinetics of Formation of Metallic Aluminum upon Thermal and Photolytic Decomposition of Aluminum Trihydride and Trideuteride as Probed by NMR. Dokl. Phys. Chem. 2003, 393, 353–356. [Google Scholar] [CrossRef]
- Milekhin, Y.M.; Koptelov, A.A.; Matveev, A.A.; Baranets, Y.N.; Bakulin, D.A. Studying Aluminum Hydride by Means of Thermal Analysis. Russ. J. Phys. Chem. A 2015, 89, 1141–1145. [Google Scholar] [CrossRef]
- Kato, S.; Bielmann, M.; Ikeda, K.; Orimo, S.; Borgschulte, A.; Züttel, A. Surface Changes on AlH3 during the Hydrogen Desorption. Appl. Phys. Lett. 2010, 96, 051912. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, J.; Li, H.; Pang, A.; Xu, P.; Tang, G.; Xu, X. Thermal Oxidation and Heterogeneous Combustion of AlH3 and Al: A Comparative Study. Acta Astronaut. 2021, 179, 636–645. [Google Scholar] [CrossRef]
- Duan, C.W.; Hu, L.X.; Sun, Y.; Zhou, H.P.; Yu, H. Reaction Kinetics for the Solid State Synthesis of the AlH3/MgCl2 Nano-Composite by Mechanical Milling. Phys. Chem. Chem. Phys. 2015, 17, 22152–22159. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Isobe, S.; Wang, Y.; Hashimoto, N.; Ohnuki, S.; Zeng, L.; Liu, S.; Ichikawa, T.; Kojima, Y. Dehydrogenation Process of AlH3 Observed by TEM. J. Alloys Compd. 2013, 580, S163–S166. [Google Scholar] [CrossRef]
- Yu, M.H.; Xie, W.X.; Zhu, Z.Y.; Yan, Q.L. Stability, Reactivity and Decomposition Kinetics of Surface Passivated α-AlH3 Crystals. Int. J. Hydrogen Energy 2022, 47, 8916–8928. [Google Scholar] [CrossRef]
- Evard, E.A.; Voyt, A.P. Hydride Decomposition Characterization by Means of “Morphological Trajectory” Method—Applied to AlH3. J. Alloys Compd. 2011, 509, S667–S670. [Google Scholar] [CrossRef]
- Gabis, I.E.; Voyt, A.P.; Chernov, I.A.; Kuznetsov, V.G.; Baraban, A.P.; Elets, D.I.; Dobrotvorsky, M.A. Ultraviolet Activation of Thermal Decomposition of α-Alane. Int. J. Hydrogen Energy 2012, 37, 14405–14412. [Google Scholar] [CrossRef]
- Pang, A.; Zhu, Z.; Xu, X. Recent Progresses on Synthesis and Evaluation of AlH3. Chin. J. Energ. Mater. 2019, 27, 317–325. [Google Scholar]
- Niles, E.T.; Seaman, B.A.; Wilson, E.J. Stabilization of Aluminum Hydride. U.S. Patent 3869544, 28 June 1975. pp. 3–4. [Google Scholar]
- Roberts, C.B. Stabilization of Aluminum Hydride. U.S. Patent 3821044, 28 June 1974. [Google Scholar]
- Nylack, G.A. Method for Increasing the Thermal Stability of Aluminum Hydride Storage. RU Patent 2175637, 8 May 2001. pp. 5–8. [Google Scholar]
- Norman, M.E.; Herbert, R.C. Stabilization of Light Metal Hydride. U.S. Patent 3857922, 9 April 1974. pp. 12–31. [Google Scholar]
- Cianciolo, A.; Sabatine, S.J. Process for the Preparation of Mercury-Coating Aluminum Hydride Compositions. U.S. Patent 3785890, 15 January 1974. pp. 1–15. [Google Scholar]
- Xing, X.H.; Xia, Y.; Zhang, X.Q.; Chang, W.L.; Wang, J.W. Research Progress in Improving Thermal Stability of Aluminum Trihydride. Chem. Propellants Polym. Mater. 2018, 16, 21–25. [Google Scholar]
- Ardis, A.; Natoli, F. Thermal Stability of Aluminum Hydride through Use of Stabilizers. U.S. Patent 3801707, 2 April 1974. pp. 4–12. [Google Scholar]
- Roberts, C.B.; Toner, D.D. Stabilization of Light Metalhydride(U). U.S. Patent 3803082, 9 April 1974. pp. 4–9. [Google Scholar]
- Maycock, J.N.; Paiverneker, V.R. Crystal Lattice Doping Studies of High Energy Propellant Ingredients AD-507040; Defense Technical Information Center: Fort Belvoir, VA, USA, 1969. [Google Scholar]
- Wang, K.; Liu, Z.; Wang, X.; Cui, X. Enhancement of Hydrogen Binding Affinity with Low Ionization Energy Li2F Coating on C60 to Improve Hydrogen Storage Capacity. Int. J. Hydrogen Energy 2014, 39, 15639–15645. [Google Scholar] [CrossRef]
- Norman, M.E. Non-Solvated Aluminum Hydride Coated with Stabilizer. U.S. Patent 3844853, 29 October 1974. [Google Scholar]
- Shi, Z.; Lu, T.; Zhang, J.; Zhu, Z.; Xu, Y.; Xia, D.; Hu, Y.; Lin, K.; Yang, Y. The Enhanced Thermal Stability and Reduced Hygroscopicity of Aluminum Hydride Coated with Vinyltrimethoxysilane. New J. Chem. 2022, 46, 1643–1649. [Google Scholar] [CrossRef]
- Kempa, P.B.; Thome, V.; Herrmann, M. Structure, Chemical and Physical Behavior of Aluminum Hydride. Part. Part. Syst. Charact. 2009, 26, 132–137. [Google Scholar] [CrossRef]
- Qin, J.; Gong, T.; Yan, N.; Li, J.G.; Hui, L.F.; Hao, H.X. Research Progress on Application of Atomic Layer Deposition in Surface Fabrication of Energetic Material. Chin. J. Explos. Propellant 2019, 42, 425–431. [Google Scholar]
- Su, J.; Li, J.; Guan, H.B. A Method for Coating Aluminum Hydride with Carbon Nanotubes. CN 108163839A, 15 June 2018. [Google Scholar]
- Li, L. Desensitization of Aluminum Hydride (AlH3) by Graphene Oxide. Chin. J. Solid Rocket Technol. 2019, 42, 66–71. [Google Scholar]
- Yu, M.H.; Yang, S.L.; Xie, W.X.; Zhu, Z.Y.; Li, H.P.; Yan, Q.L. Enhanced Stability and Combustion Performance of AlH3 in Combination with Commonly Used Oxidizers. Fuel 2023, 331, 125741. [Google Scholar] [CrossRef]
- Yu, M.H.; Yang, S.L.; Xie, W.X.; Li, Y.J.; Li, H.P.; Yan, Q.L. Stabilization of AlH3 Crystals by the Coating of Hydrophobic PFPE and Resulted Reactivity with Ammonium Perchlorate. Langmuir 2023, 39, 7863–7875. [Google Scholar] [CrossRef] [PubMed]
- Pang, W.; Luigi, D.T.; Fan, X.; Wang, K.; Li, J.; Zhao, F. Progress on Modification of High Active Aluminum Powder and Combustion Agglomeration in Chemical Propellants. J. Solid Rocket Technol. 2019, 42, 42–53. [Google Scholar]
- Cheng, Z.P.; He, X.X. Research Progress of Nano Aluminum Fuel. J. Solid Rocket Technol. 2017, 40, 437–447. [Google Scholar]
- Bazyn, T.; Eyer, R.; Krier, H.; Glumac, N. Combustion Characteristics of Aluminum Hydride at Elevated Pressure and Temperature. J. Propuls. Power 2004, 20, 427–431. [Google Scholar] [CrossRef]
- Maggi, F.; Gariani, G.; Galfetti, L.; DeLuca, L.T. Theoretical Analysis of Hydrides in Solid and Hybrid Rocket Propulsion. Int. J. Hydrogen Energy 2012, 37, 1760–1769. [Google Scholar] [CrossRef]
- Shark, S.; Sippel, T.; Son, S.; Heister, S.; Pourpoint, T. Theoretical Performance Analysis of Metal Hydride Fuel Additives for Rocket Propellant Applications. In Proceedings of the 47th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, San Diego, CA, USA, 31 July–3 August 2011. [Google Scholar]
- Bazyn, T.; Krier, H.; Glumac, N.; Shankar, N.; Wang, X.; Jackson, T.L. Decomposition of Aluminum Hydride Under Solid Rocket Motor Conditions. J. Propuls. Power 2007, 23, 457–464. [Google Scholar] [CrossRef]
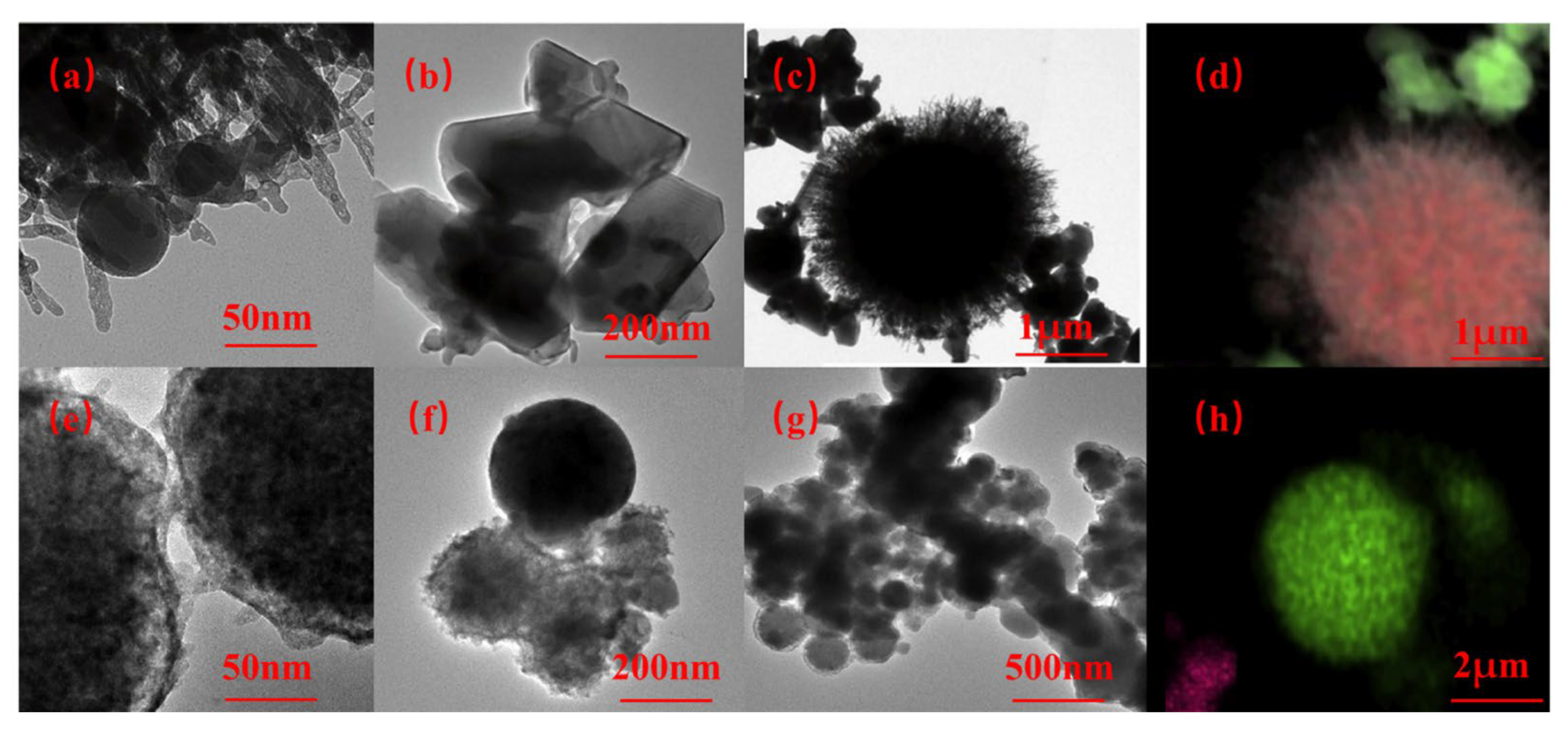


| Hydrides | Molar Mass (g/mol) | Density (g/cm) | Gravimetric Hydrogen Density (wt%) | Volumetric Hydrogen Density (kg/m3) | Tdec (K) |
|---|---|---|---|---|---|
| LiH | 7.95 | 0.82 | 11.5 | 98.3 | 474.15 |
| MgH2 | 26.31 | 1.45 | 7.6 | 110 | 674.15 |
| TiH2 | 49.89 | 3.91 | 4.0 | 91.0 | 624.15 |
| NH3BH3 | 30.81 | 0.78 | 19.6 | 145 | 399.15 |
| BeH2 | 11.03 | 0.65 | 18.28 | 71.2 | 524.15 |
| AlH3 | 30.0 | 1.477 | 10.1 | 148 | 434.15 |
| Polymorphs | Experiment Conditions | Size of Product | Structure | Space Group | Morphology | ||
|---|---|---|---|---|---|---|---|
| LiAlH4:AlCl3:LiBH4 | T (°C) | Time | |||||
| α-AlH3 | 1:4:1 | 65 | 6.5 h | 60 nm | a = 4.449 Å c = 11.804 Å | R¯3c |  |
| 1:3:0 (PDMS, HCl) | 85–93 | 2–8 h | 6–13 μm | ||||
| 1:4:0 (γ → α) | 62 | 11 h | 50–100 μm | ||||
| 1:4:1(β→α) | 65 | 6 h | |||||
| α’-AlH3 | 1:4:0 | 60 | 4 h | 1 μm | a = 6.470 Å b = 11.117 Å c = 6.562 Å | Cmcm |  |
| β-AlH3 | 1:4:1 | 75 | 6 h | <50 μm | a = 9.004 Å | Fd¯3m |  |
| γ-AlH3 | 1:4:0 | 65 | 45 min | <50 μm | a = 7.336 Å b = 5.367 Å c = 5.765 Å | Pnnm | 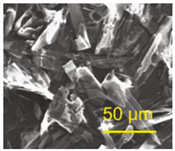 |
| Methods | Advantages | Disadvantages | Development Suggestion |
|---|---|---|---|
| Wet chemical methods | 1. High product quality and yield. 2. Simple process. | 1. Large amount of organic solvents used. 2. Flammable raw materials, intermediates and solvents. | Improving the safety control level and synthesis process. |
| Dry synthesis methods | No or small amount of solvent used. | 1. Serious aggregation of AlH3 crystals. 2. Difficult to separate AlH3 from by-products. | Developing suitable reaction conditions, synthesis devices, and purification technology. |
| Supercritical synthesis method | Direct reaction of activated Al with H2. | The reaction activity is limited by the relatively low temperature | Break the limits of the reaction temperature by suitable medium. |
| Organoaluminum decomposition method | Cheap raw materials. | 1. Low yield. 2. De-etherification and crystal transformation process of intermediate involved. | Developing low-cost exploratory research. |
| High pressure hydrogenation method | Direct reaction of Al with H2. | 1. Harch reaction conditions. 2. Impurities. 3. Low yield. | Developing mild reaction conditions. |
| Methods | Materials Used for Stabilization | α-AlH3 before Stabilization | α-AlH3 after Stabilization |
|---|---|---|---|
| Surface passivation | Mg and N-butylamine (H2O) [15,70] | 1% decomposition for 13.5 d at 60 °C | 1% decomposition for 60 d at 60 °C |
| C2H5OH (98%) [70] | ̅ | 0.1% decomposition for 35~40 d at 60 °C | |
| Mg and KH2PO4/NaOH [77] | ̅ | 0.25% decomposition for 49 d at 60 °C | |
| Mg, N-butylamine (H2O) and KH2PO4/NaOH [77] | 1% decomposition for 33 d at 60 °C | 1% decomposition for 43 d at 60 °C | |
| HCl solution [17] | 1% decomposition for 9.3 d at 60 °C | 1% decomposition for 12.7 d at 60 °C | |
| air (60 °C for 250 h) [72] | 5% decomposition for 95 min at 115 °C | 5% decomposition for 237 min at 115 °C | |
| Doping | MBT/PTA [76] | 7.5% decomposition for 14 d at 60 °C | PTA: 0.97% decomposition 60 °C for 27 d; MBT:0.6% decomposition 60 °C for 17 d |
| Hg [74] | 9.4% decomposition for 24 h at 100 °C | 0.4% decomposition for 24 h at 100 °C | |
| Mg [73] | 1% decomposition for 5 d at 60 °C | 1% decomposition for 26 d at 60 °C | |
| Si [74] | 1% decomposition for 4 d at 60 °C | 1% decomposition for 8 d at 60 °C | |
| Surface coating | NO, N2F4 [80] | 5.63% decomposition for 7 h at 100 °C | 100 °C for 7 h, 0.21% decompose- tion |
| Al2S3 [80] | Rapid decomposition for 2 h at 60 °C after store at room temperature for 114 d | 0.13% decomposition for 22 h at 60 °C after store at room temperature for 114 d | |
| SA [18] | E50: 367 mJ | E50: 5390 mJ | |
| Diphenylacetylene [18] | 1% decomposition for 13 d | 0.84% decomposition for 48 d | |
| Nitrocellulose [20] | Rapid decomposition for 30 d at 50 °C, or for 10 d at 60 °C | 0.3% decomposition for 90 d at 50 °C; 0.63% decomposition for 90 d at 60 °C | |
| FE26 [16] | E50: 63.7 mJ | E50: 85.24 mJ | |
| Al2O3 [12] | 7.8% decomposition for 12 h at 70 °C | 0.49% decomposition for 12 h at 70 °C | |
| GO [85] | I50: 7.3 J | I50: 12.1 J | |
| C60 [15] | ̅ | <1% decomposition for 90 d at 60 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Yang, F.; Zhang, Y.; Wu, Z.; Zhang, Z. AlH3 as High-Energy Fuels for Solid Propellants: Synthesis, Thermodynamics, Kinetics, and Stabilization. Compounds 2024, 4, 230-251. https://doi.org/10.3390/compounds4020012
Liu Y, Yang F, Zhang Y, Wu Z, Zhang Z. AlH3 as High-Energy Fuels for Solid Propellants: Synthesis, Thermodynamics, Kinetics, and Stabilization. Compounds. 2024; 4(2):230-251. https://doi.org/10.3390/compounds4020012
Chicago/Turabian StyleLiu, Youhai, Fusheng Yang, Yang Zhang, Zhen Wu, and Zaoxiao Zhang. 2024. "AlH3 as High-Energy Fuels for Solid Propellants: Synthesis, Thermodynamics, Kinetics, and Stabilization" Compounds 4, no. 2: 230-251. https://doi.org/10.3390/compounds4020012
APA StyleLiu, Y., Yang, F., Zhang, Y., Wu, Z., & Zhang, Z. (2024). AlH3 as High-Energy Fuels for Solid Propellants: Synthesis, Thermodynamics, Kinetics, and Stabilization. Compounds, 4(2), 230-251. https://doi.org/10.3390/compounds4020012







