Identifying the Structural Components Responsible for the Antiproliferative Properties of Hydroxychavicol
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Chemical Solution Preparation
2.3. Treatment of Cells
2.4. Cell Counting and Viability Determination
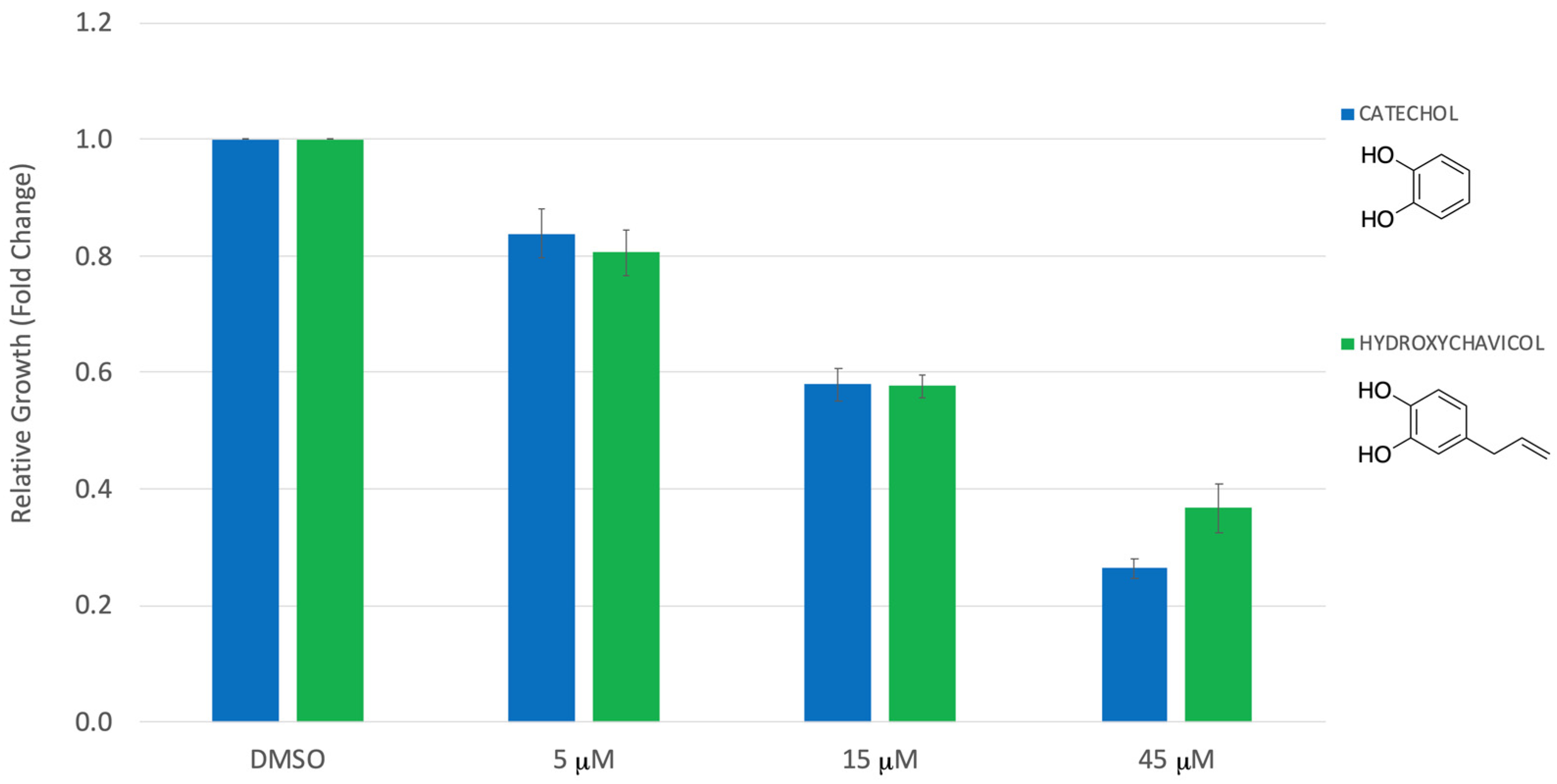
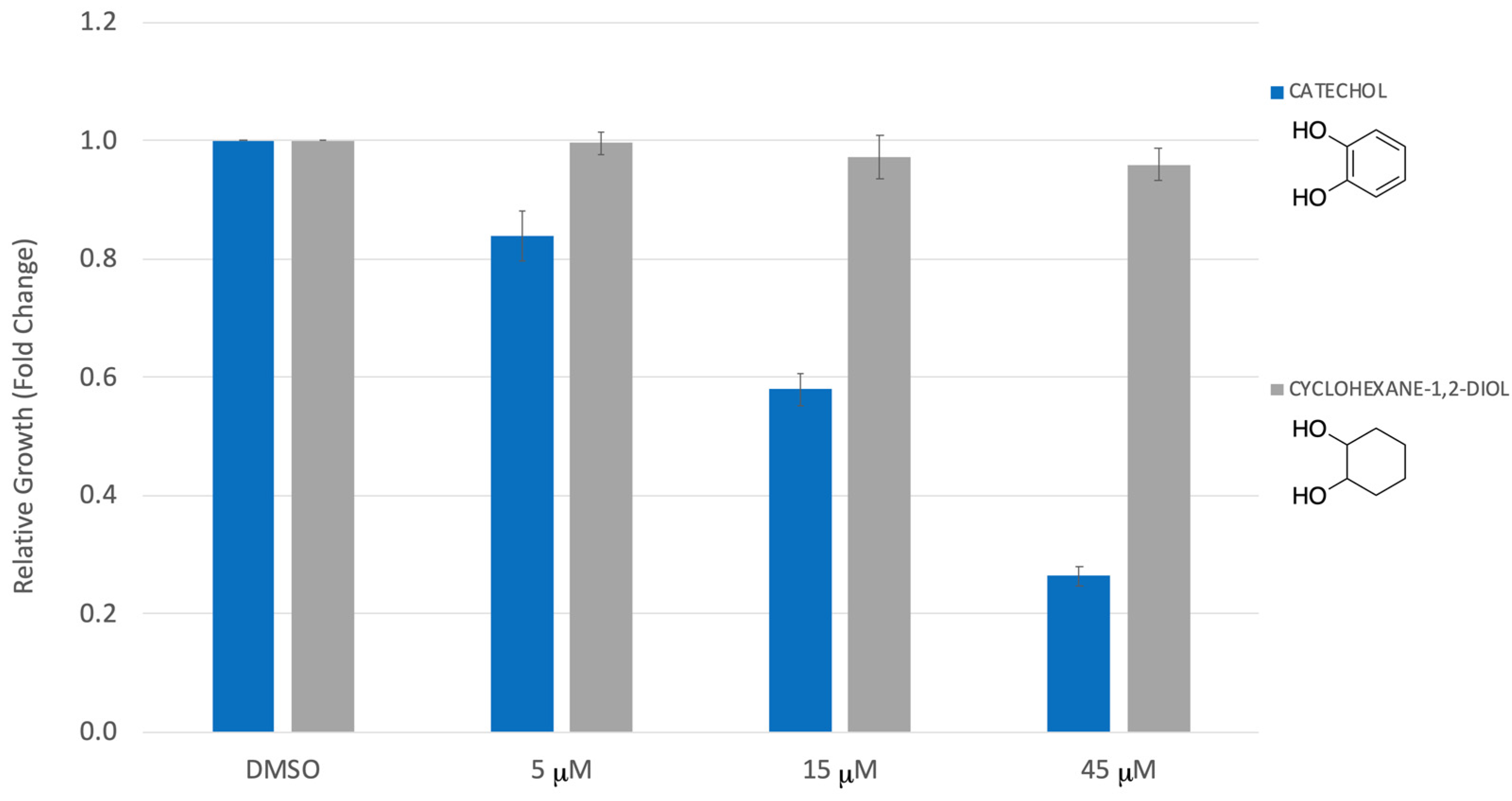
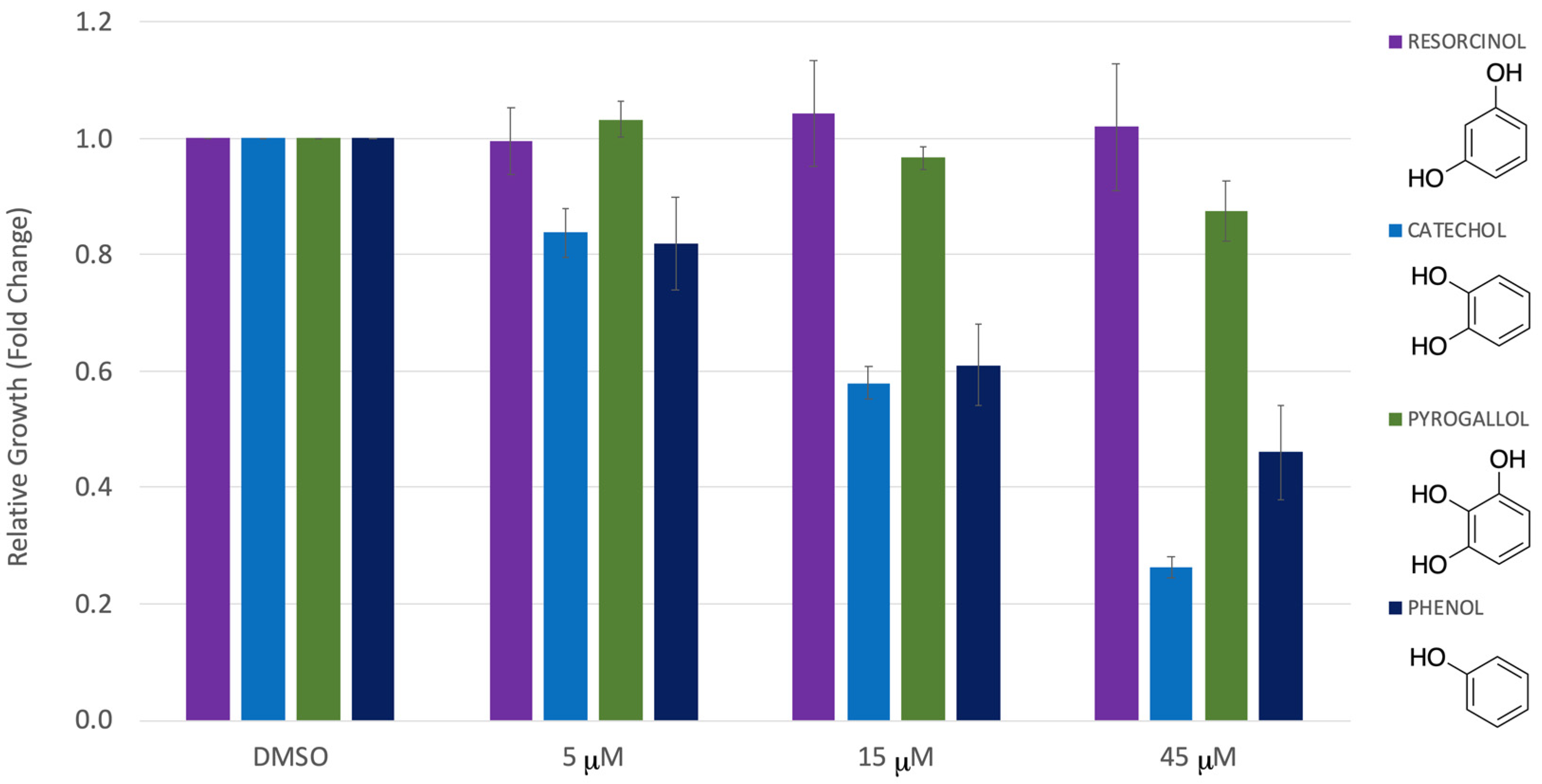
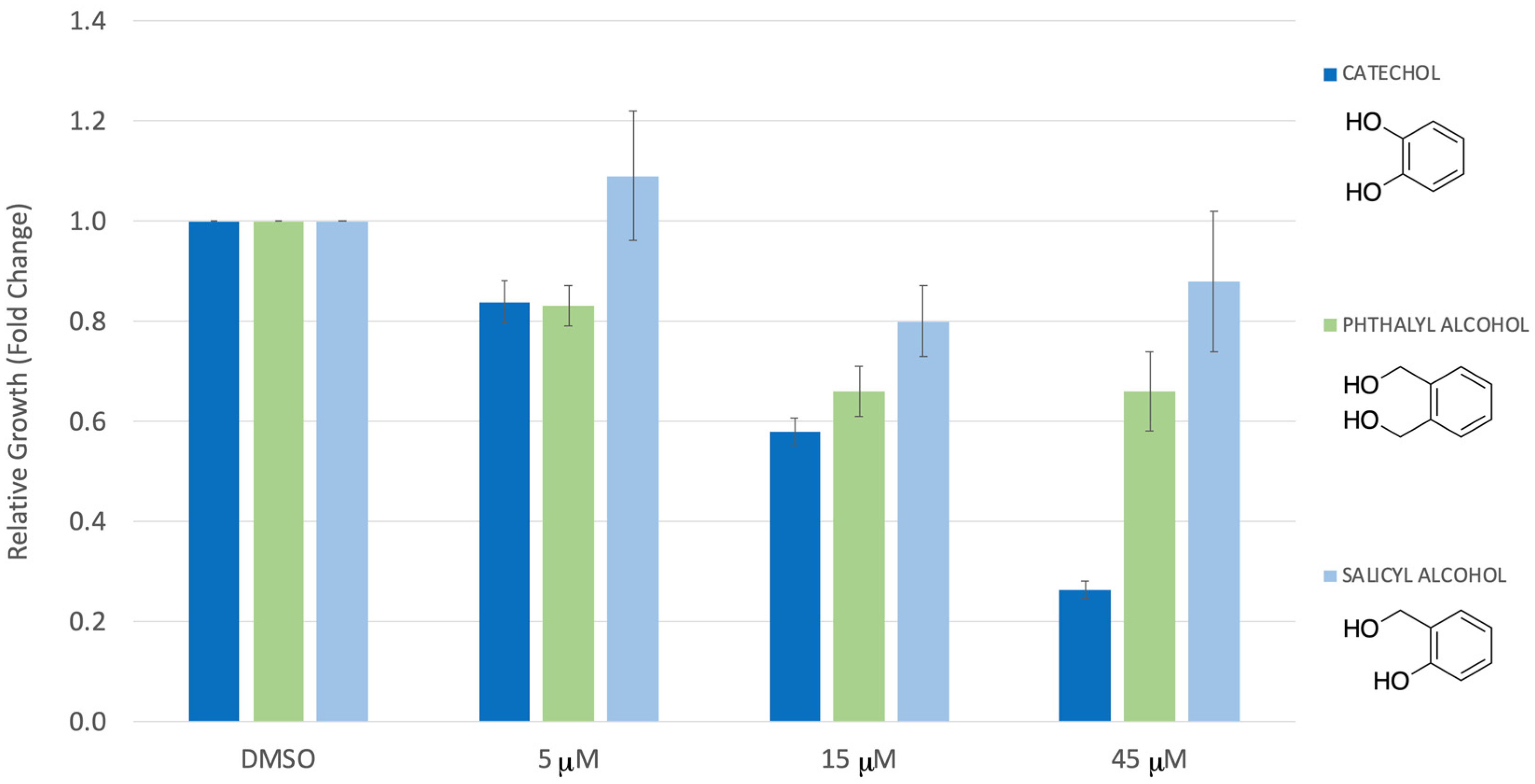
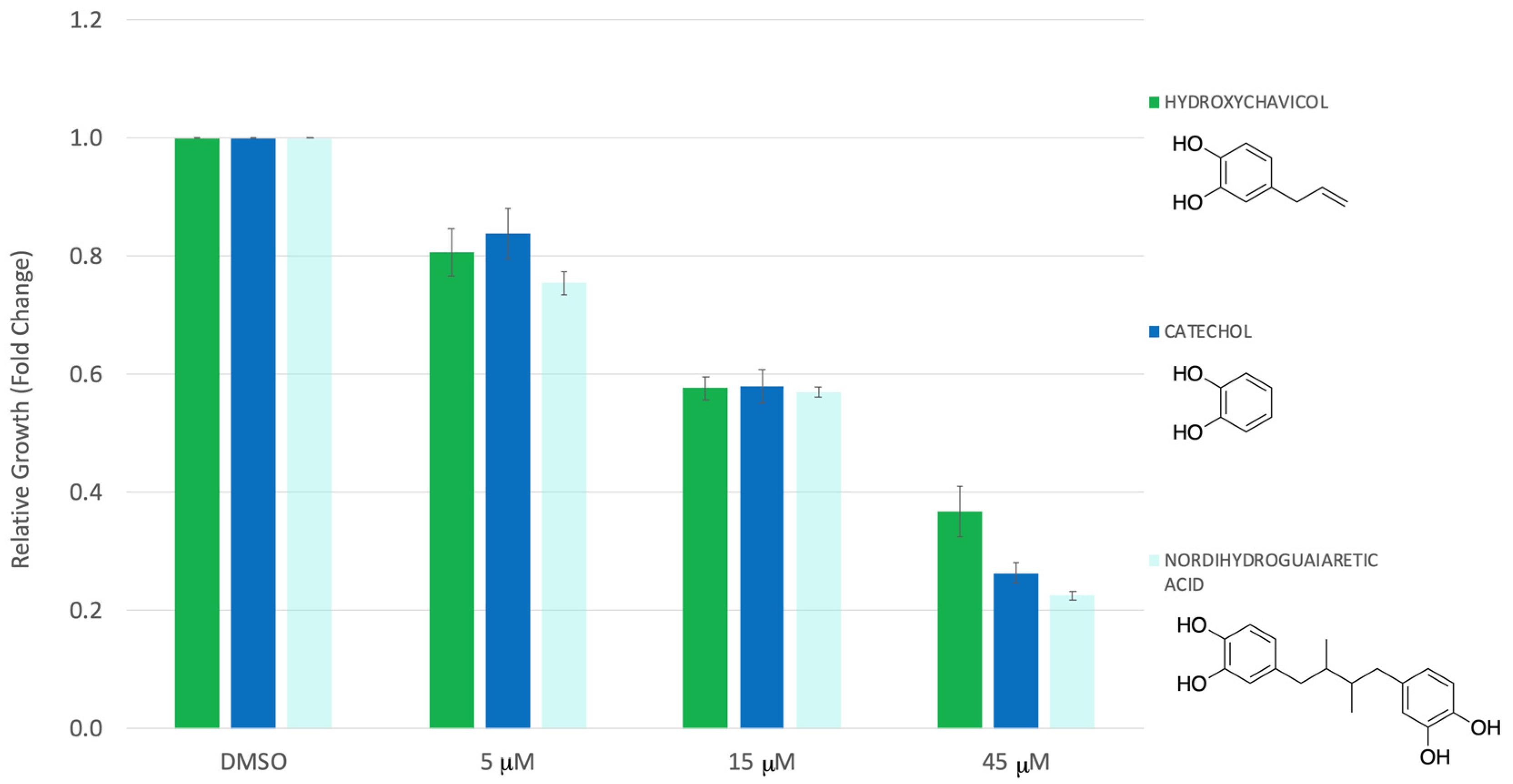
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Madhumita, M.; Guha, P.; Nag, A. Bio-actives of betel leaf (Piper betle L.): A comprehensive review on extraction, isolation, characterization, and biological activity. Phytother. Res. 2020, 34, 2609–2627. [Google Scholar] [CrossRef] [PubMed]
- Abrahim, N.N.; Kanthimathi, M.S.; Abdul-Aziz, A. Piper betle shows antioxidant activities, inhibits MCF-7 cell proliferation and increases activities of catalase and superoxide dismutase. Chin. J. Integr. Med. 2012, 12, 220. [Google Scholar] [CrossRef] [PubMed]
- Nayaka NM DM, W.; Sasadara MM, V.; Sanjaya, D.A.; Yuda PE, S.K.; Dewi NL KA, A.; Cahyaningsih, E.; Hartati, R. Piper betle (L): Recent Review of Antibacterial and Antifungal Properties, Safety Profiles, and Commercial Applications. Molecules 2021, 26, 2321. [Google Scholar] [CrossRef] [PubMed]
- Fazal, F.; Mane, P.P.; Rai, M.P.; Thilakchand, K.R.; Bhat, H.P.; Kamble, P.S.; Palatty, P.L.; Baliga, M.S. The phytochemistry, traditional uses and pharmacology of Piper Betel. linn (Betel Leaf): A pan-asiatic medicinal plant. Chin. J. Integr. Med. 2014. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.T.; Nguyen, T.B.; Nguyen, H.C.; Do, N.H.N.; Le, P.K. Recent applications of natural bioactive compounds from Piper betle (L.) leaves in food preservation. Food Control 2023, 152, 110026. [Google Scholar] [CrossRef]
- Mohamad, N.A.; Rahman, A.A.; Kadir, S.H.S.A. Hydroxychavicol as a potential anticancer agent (Review). Oncol. Lett. 2023, 25, 34. [Google Scholar] [CrossRef] [PubMed]
- Vinusri, S.; Gnanam, R.; Caroline, R.; Santhanakrishnan, V.P.; Kandavelmani, A. Anticancer Potential of Hydroxychavicol Derived from Piper betle L: An in Silico and Cytotoxicity Study. Nutr. Cancer 2022, 74, 3701–3713. [Google Scholar] [CrossRef]
- Yadav, Y.; Owens, E.A.; Sharma, V.; Aneja, R.; Henary, M. Synthesis and evaluation of antiproliferative activity of a novel series of hydroxychavicol analogs. Eur. J. Med. Chem. 2014, 75, 1–10. [Google Scholar] [CrossRef]
- Gundala, S.R.; Aneja, R. Piper betel leaf: A reservoir of potential xenohormetic nutraceuticals with cancer-fighting properties. Cancer Prev. Res. 2014, 7, 477–486. [Google Scholar] [CrossRef]
- Gupta, R.K.; Guha, P.; Srivastav, P.P. Phytochemical and biological studies of betel leaf (Piper betle L.): Review on paradigm and its potential benefits in human health. Acta Ecol. Sin. 2023, 43, 721–732. [Google Scholar] [CrossRef]
- Vazhappilly, C.G.; Hodeify, R.; Siddiqui, S.S.; Laham, A.J.; Menon, V.; El-Awady, R.; Matar, R.; Merheb, M.; Marton, J.; Al Zouabi, H.A.K.; et al. Natural compound catechol induces DNA damage, apoptosis, and G1 cell cycle arrest in breast cancer cells. Phytother. Res. 2021, 35, 2185–2199. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.A.; Rahman, U.; Khan, M.R.; Sahar, A.; Mehmood, T.; Khan, M. Essential oil eugenol: Sources, extraction techniques and nutraceutical perspectives. RSC Adv. 2017, 7, 32669–32681. [Google Scholar] [CrossRef]
- Sharma, S.; Khan, I.A.; Ali, I.; Ali, F.; Kumar, M.; Kumar, A.; Johri, R.K.; Abdullah, S.T.; Bani, S.; Pandey, A.; et al. Evaluation of the antimicrobial, antioxidant, and anti-inflammatory activities of hydroxychavicol for its potential use as an oral care agent. Antimicrob. Agents Chemother. 2009, 53, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.K.N.; Hadisurya, M.; Cornwall, R.G. Antimicrobial Investigation and Structure- Activity Analysis of Natural Eugenol Derivatives Against Several Oral Bacteria. J. Pharm. Microbiol. 2019, 5, 1. [Google Scholar]
- Syahidah, A.; Saad, C.R.; Hassan, M.D.; Rukayadi, Y.; Norazian, M.H.; Kamarudin, M.S. Phytochemical Analysis, Identification and Quantification of Antibacterial Active Compounds in Betel Leaves, Piper betle Methanolic Extract. Pak. J. Biol. Sci. 2017, 20, 70–81. [Google Scholar] [CrossRef][Green Version]
- Al-Sharif, I.; Remmal, A.; Aboussekhra, A. Eugenol triggers apoptosis in breast cancer cells through E2F1/survivin down-regulation. BMC Cancer 2013, 13, 600. [Google Scholar] [CrossRef]
- Abdullah, M.L.; Hafez, M.M.; Al-Hoshani, A.; Al-Shabanah, O. Anti-metastatic and anti-proliferative activity of eugenol against triple negative and HER2 positive breast cancer cells. BMC Complement. Altern. Med. 2018, 18, 321. [Google Scholar] [CrossRef]
- Manikandan, P.; Murugan, R.S.; Priyadarsini, R.V.; Vinothini, G.; Nagini, S. Eugenol induces apoptosis and inhibits invasion and angiogenesis in a rat model of gastric carcinogenesis induced by MNNG. Life Sci. 2010, 86, 936–941. [Google Scholar] [CrossRef]
- Jaganathan, S.K.; Supriyanto, E. Antiproliferative and Molecular Mechanism of Eugenol-Induced Apoptosis in Cancer Cells. Molecules 2012, 17, 6290–6304. [Google Scholar] [CrossRef]
- Petrocelli, G.; Farabegoli, F.; Valerii, M.C.; Giovannini, C.; Sardo, A.; Spisni, E. Molecules Present in Plant Essential Oils for Prevention and Treatment of Colorectal Cancer (CRC). Molecules 2021, 26, 885. [Google Scholar] [CrossRef]
- Fathy, M.; Fawzy, M.A.; Hintzsche, H.; Nikaido, T.; Dandekar, T.; Othman, E.M. Eugenol Exerts Apoptotic Effect and Modulates the Sensitivity of HeLa Cells to Cisplatin and Radiation. Molecules 2019, 24, 3979. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.L.; Al-Shabanah, O.; Hassan, Z.K.; Hafez, M.M. Eugenol-Induced Autophagy and Apoptosis in Breast Cancer Cells via PI3K/AKT/FOXO3a Pathway Inhibition. Int. J. Mol. Sci. 2021, 22, 9243. [Google Scholar] [CrossRef] [PubMed]
- Rajedadram, A.; Pin, K.Y.; Ling, S.K.; Yan, S.W.; Looi, M.L. Hydroxychavicol, a polyphenol from Piper betle leaf extract, induces cell cycle arrest and apoptosis in TP53-resistant HT-29 colon cancer cells. J. Zhejiang Univ. Sci. B 2021, 22, 112–122. [Google Scholar] [CrossRef]
- Chakraborty, J.B.; Mahato, S.K.; Joshi, K.; Shinde, V.; Rakshit, S.; Biswas, N.; Choudhury, I.; Mandal, L.; Ganguly, D.; Chowdhury, A.A.; et al. Hydroxychavicol, a Piper betle leaf component, induces apoptosis of CML cells through mitochondrial reactive oxygen species-dependent JNK and endothelial nitric oxide synthase activation and overrides imatinib resistance. Cancer Sci. 2012, 103, 88–99. [Google Scholar] [CrossRef]
- Thamaraikani, I.; Kulandhaivel, M. Purification of Hydroxychavicol from Piper Betle Linn and evaluation of antimicrobial activity against some food poison causing bacteria. J. Pure Appl. Microbiol. 2017, 11, 1883–1889. [Google Scholar] [CrossRef]
- Lim, D.Y.; Shin, S.H.; Lee, M.-H.; Malakhova, M.; Kurinov, I.; Wu, Q.; Xu, J.; Jiang, Y.; Dong, Z.; Liu, K.; et al. A natural small molecule, catechol, induces c-Myc degradation by directly targeting ERK2 in lung cancer. Oncotarget 2016, 7, 35001–35014. [Google Scholar] [CrossRef] [PubMed]
- Grigalius, I.; Petrikaite, V. Relationship between Antioxidant and Anticancer Activity of Trihydroxyflavones. Molecules 2017, 22, 2169. [Google Scholar] [CrossRef]
- Park, Y.; Lim, Y.; Woo, Y.; Lee, S. Relationships between structure and anti-oxidative effects of hydroxyflavones. Bull. Korean Chem. Soc. 2009, 30, 1397–1400. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jackson, J.; Romero, G.M.; Hawkins, D.; Cornwall, R.G.; Lukov, G.L. Identifying the Structural Components Responsible for the Antiproliferative Properties of Hydroxychavicol. Compounds 2023, 3, 552-560. https://doi.org/10.3390/compounds3040039
Jackson J, Romero GM, Hawkins D, Cornwall RG, Lukov GL. Identifying the Structural Components Responsible for the Antiproliferative Properties of Hydroxychavicol. Compounds. 2023; 3(4):552-560. https://doi.org/10.3390/compounds3040039
Chicago/Turabian StyleJackson, Joshua, Gerome M. Romero, Diana Hawkins, Richard G. Cornwall, and Georgi L. Lukov. 2023. "Identifying the Structural Components Responsible for the Antiproliferative Properties of Hydroxychavicol" Compounds 3, no. 4: 552-560. https://doi.org/10.3390/compounds3040039
APA StyleJackson, J., Romero, G. M., Hawkins, D., Cornwall, R. G., & Lukov, G. L. (2023). Identifying the Structural Components Responsible for the Antiproliferative Properties of Hydroxychavicol. Compounds, 3(4), 552-560. https://doi.org/10.3390/compounds3040039





