Abraham Solvation Parameter Model: Revised Predictive Expressions for Solute Transfer into Polydimethylsiloxane Based on Much Larger and Chemically Diverse Datasets
Abstract
1. Introduction
2. Prior Abraham Model Studies Describing Solute Transfer into Polydimethylsiloxane
(N = 32, R2 = 0.969; SE = 0.127; and F = 155)
(N = 32, R2 = 0.995, and F = 1056)
(N = 170, R2 = 0.993, Radj2 = 0.993, SD = 0.171, F = 4475.2)
(N = 142, R2 = 0.995, Radj2 = 0.994, SD = 0.180, F = 4919.0)
(N = 277, Radj2 = 0.764, RMSE = 0.812, F = 175)
(N = 200, Radj2 = 0.867, RMSE = 0.753; F = 644)
3. Construction of Databases and Determination of Updated Abraham Model Correlations
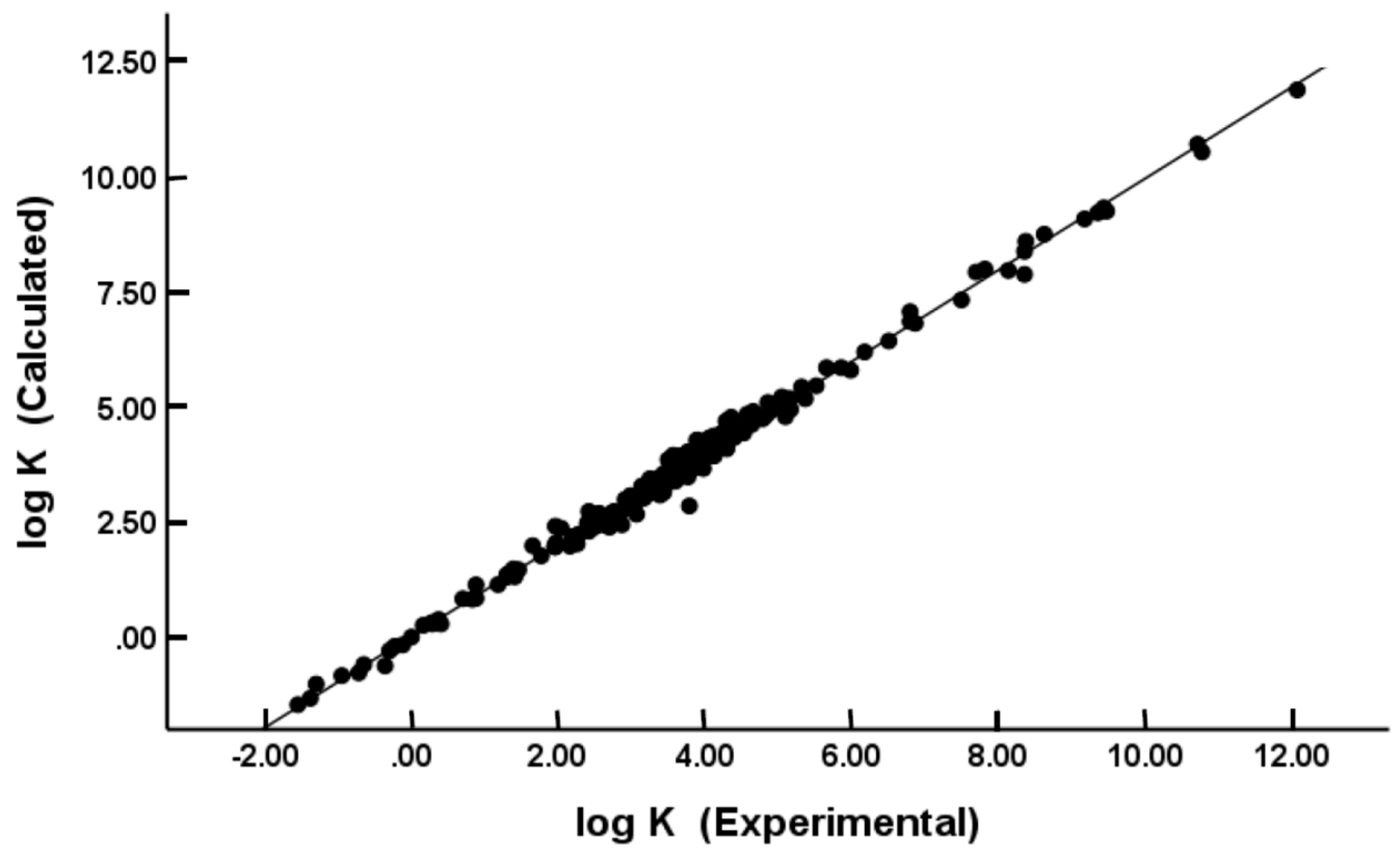
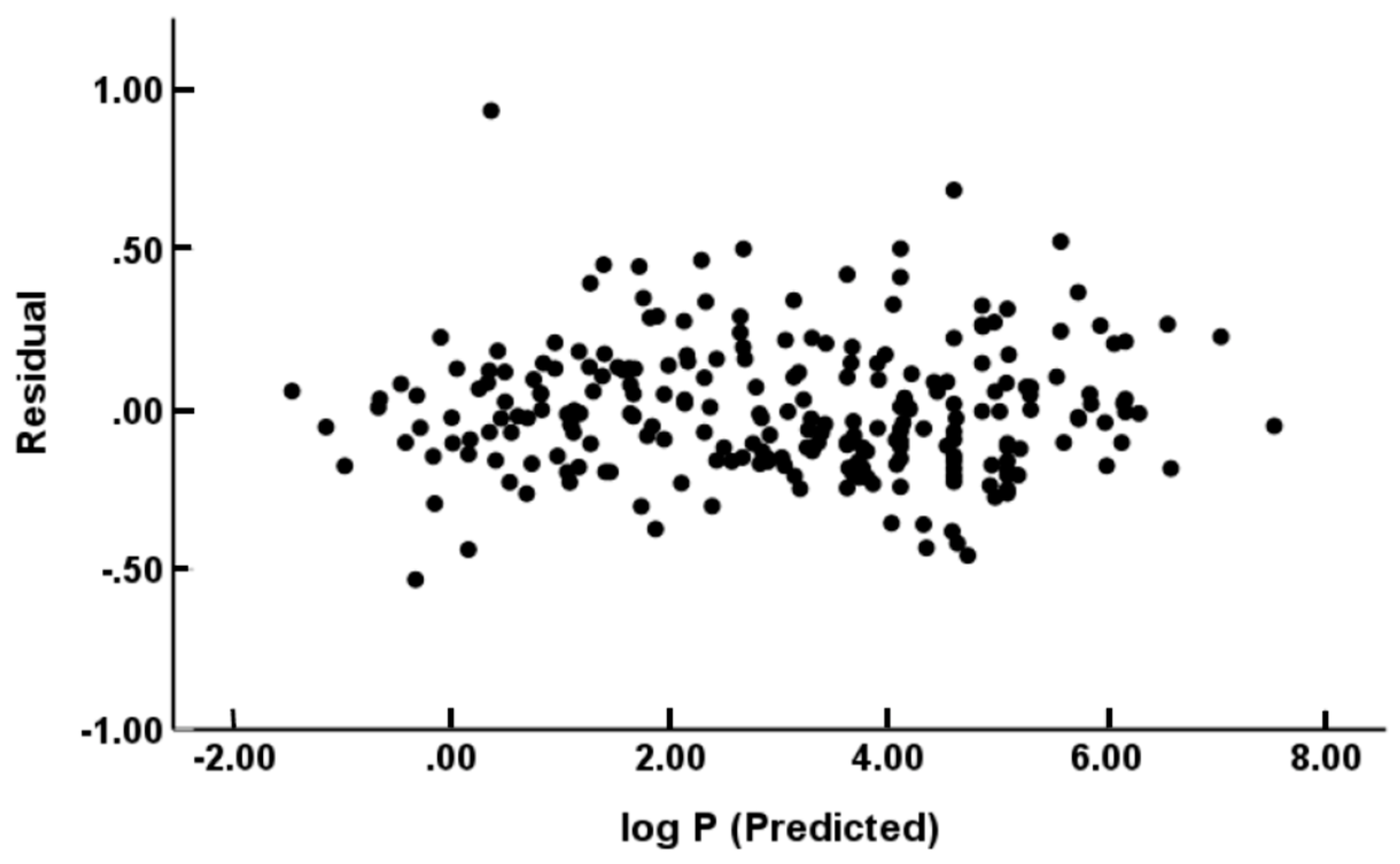
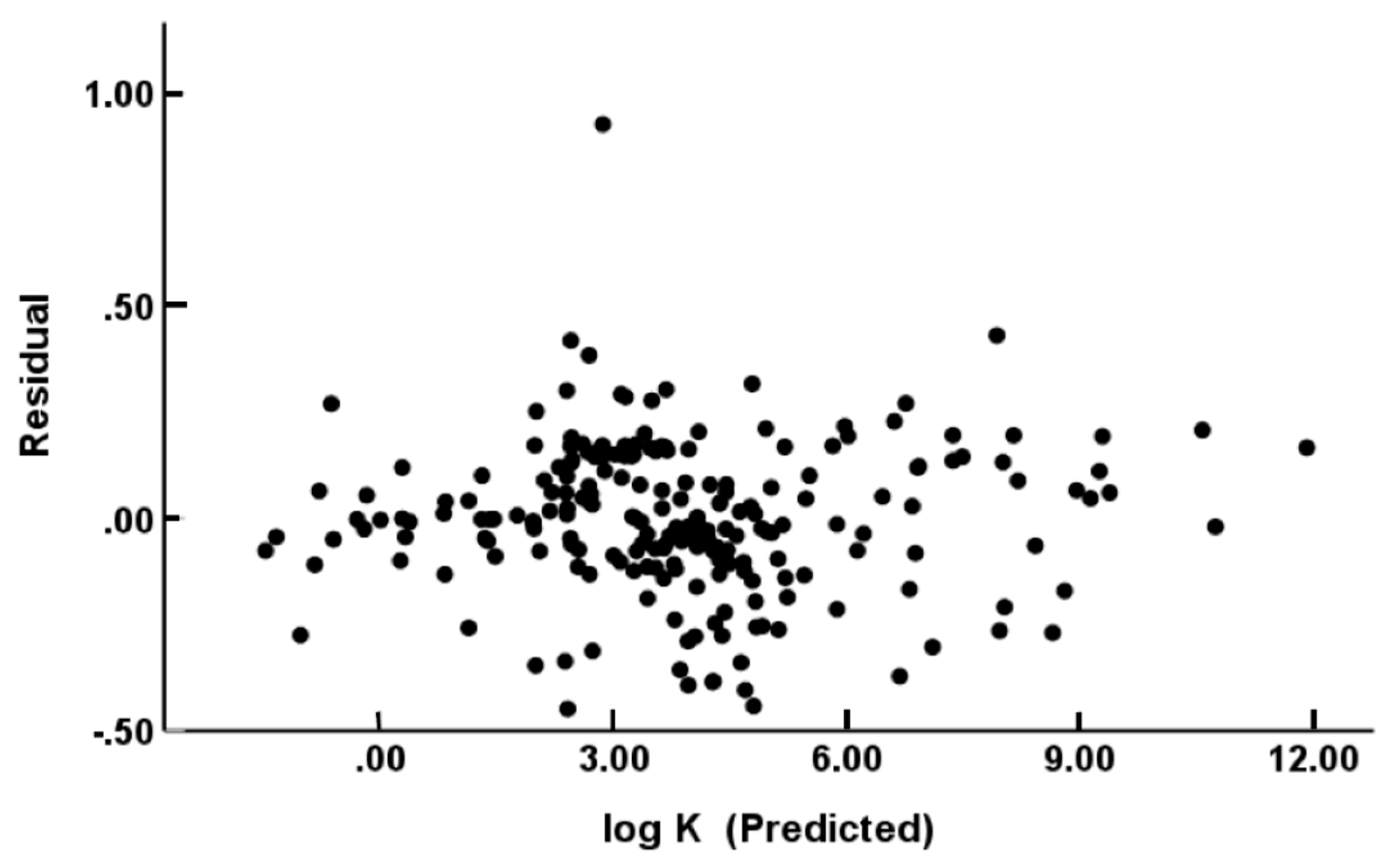
(N = 244, R2 = 0.988, Radj2 = 0.988, SD = 0.206, SEE = 0.208, F = 4088)
(N = 229, R2 = 0.994, Radj2 = 0.994, SD = 0.176, SEE = 0.178, F = 7282)
(N = 122, R2 = 0.989, Radj2 = 0.988, SD = 0.201, SEE = 0.205, F = 1879)
(N = 115, R2 = 0.995, Radj2 = 0.994, SD = 0.155, SEE = 0.159, F = 3799)
4. Summary
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Amiri, A.; Ghaemi, F. Thermally stable carbon nanofibers functionalized with poly(dimethylsiloxane) for solid-phase microextraction of polycyclic aromatic hydrocarbons prior to GC analysis. Microchim. Acta 2016, 183, 1917–1924. [Google Scholar] [CrossRef]
- Orazbayeva, D.; Koziel, J.A.; Trujillo-Rodriguez, M.J.; Anderson, J.L.; Kenessov, B. Polymeric ionic liquid sorbent coatings in headspace solid-phase microextraction: A green sample preparation technique for the determination of pesticides in soil. Microchem. J. 2020, 157, 104996. [Google Scholar] [CrossRef]
- Grant, S.; Schacht, V.J.; Escher, B.I.; Hawker, D.W.; Gaus, C. Experimental solubility approach to determine PDMS-water partition constants and PDMS activity coefficients. Environ. Sci. Technol. 2016, 50, 3047–3054. [Google Scholar] [CrossRef] [PubMed]
- Poole, C.; Mester, Z.; Miro, M.; Pedersen-Bjergaard, S.; Pawliszyn, J. Extraction for analytical scale sample preparation (IUPAC Technical Report). Pure Appl. Chem. 2016, 88, 649–687. [Google Scholar] [CrossRef]
- Rykowska, I.; Ziemblinska, J.; Nowak, I. Modern approaches in dispersive liquid-liquid microextraction (DLLME) based on ionic liquids: A review. TrAC Trends Anal. Chem. 2015, 72, 153–168. [Google Scholar] [CrossRef]
- Sharifi, V.; Abbasi, A.; Nosrati, A. Application of hollow fiber liquid phase microextraction and dispersive liquid-liquid microextraction techniques in analytical toxicology. J. Food Drug Anal. 2016, 24, 264–276. [Google Scholar] [CrossRef]
- Makos, P.; Slupek, E.; Gebicki, J. Hydrophobic deep eutectic solvents in microextraction techniques-A review. Microchem. J. 2020, 152, 104384. [Google Scholar] [CrossRef]
- Plastiras, O.-E.; Andreasidou, E.; Samanidou, V. Microextraction techniques with deep eutectic solvents. Molecules 2020, 25, 6026. [Google Scholar] [CrossRef]
- Farajzadeh, M.A.; Afshar Mogaddam, M.R.; Aghanassab, M. Deep eutectic solvent-based dispersive liquid-liquid microextraction. Anal. Methods 2016, 8, 2576–2583. [Google Scholar] [CrossRef]
- Sprunger, L.; Proctor, A.; Acree, W.E., Jr.; Abraham, M.H. Characterization of the sorption of gaseous and organic solutes onto polydimethyl siloxane solid-phase microextraction surfaces using the Abraham model. J. Chromatogr. A 2007, 1175, 162–173. [Google Scholar] [CrossRef]
- Zhu, T.; Tao, C. Prediction models with multiple machine learning algorithms for POPs: The calculation of PDMS-air partition coefficient from molecular descriptor. J. Hazard. Mater. 2022, 423, 127037. [Google Scholar] [CrossRef]
- Endo, S.; Hale, S.E.; Goss, K.-U.; Arp, H.P.H. Equilibrium partition coefficients of diverse polar and nonpolar organic compounds to polyoxymethylene (POM) passive sampling devices. Environ. Sci. Technol. 2011, 45, 10124–10132. [Google Scholar] [CrossRef]
- Reppas-Chrysovitsinos, E.; Sobek, A.; MacLeod, M. Screening-level models to estimate partition ratios of organic chemicals between polymeric materials, air and water. Environ. Sci. Process. Impacts 2016, 18, 667–676. [Google Scholar] [CrossRef]
- Jiang, B.; Horton, M.Y.; Acree, W.E., Jr.; Abraham, M.H. Ion-specific equation coefficient version of the Abraham model for ionic liquid solvents: Determination of coefficients for tributylethylphosphonium, 1-butyl-1-methylmorpholinium, 1-allyl-3-methylimidazolium and octyltriethylammonium cations. Phys. Chem. Liq. 2017, 55, 358–385. [Google Scholar] [CrossRef]
- Stephens, T.W.; Chou, V.; Quay, A.N.; Shen, C.; Dabadge, N.; Tian, A.; Loera, M.; Willis, B.; Wilson, A.; Acree, W.E., Jr.; et al. Thermochemical investigations of solute transfer into ionic liquid solvents: Updated Abraham model equation coefficients for solute activity coefficient and partition coefficient predictions. Phys. Chem. Liq. 2014, 52, 488–518. [Google Scholar] [CrossRef]
- Poole, C.F.; Atapattu, S.N. Determination of physicochemical properties of ionic liquids by gas chromatography. J. Chromatogr. A 2021, 1644, 461964. [Google Scholar] [CrossRef] [PubMed]
- Poole, C.F.; Poole, S.K. Ionic liquid stationary phases for gas chromatography. J. Sep. Sci. 2011, 34, 888–900. [Google Scholar] [CrossRef] [PubMed]
- Twu, P.; Zhao, Q.; Pitner, W.R.; Acree, W.E., Jr.; Baker, G.A.; Anderson, J.L. Evaluating the solvation properties of functionalized ionic liquids with varied cation/anion composition using the solvation parameter model. J. Chromatogr. A 2011, 1218, 5311–5318. [Google Scholar] [CrossRef]
- Kim, K.; Shanmugam, N.; Xu, A.; Varadharajan, A.; Cai, S.K.; Huang, E.; Acree, W.E., Jr. Abraham model correlations for describing solute transfer into anisole based on measured activity coefficients and molar solubilities. Phys. Chem. Liq. 2022, 60, 452–462. [Google Scholar] [CrossRef]
- Sinha, S.; Yang, C.; Wu, E.; Acree, W.E., Jr. Abraham solvation parameter model: Examination of possible intramolecular hydrogen-bonding using calculated solute descriptors. Liquids 2022, 2, 131–146. [Google Scholar] [CrossRef]
- Longacre, L.; Wu, E.; Yang, C.; Zhang, M.; Sinha, S.; Varadharajan, A.; Acree, W.E., Jr. Development of Abraham model correlations for solute transfer into the tert-butyl acetate mono-solvent and updated equations for both ethyl acetate and butyl acetate. Liquids 2022, 2, 258–288. [Google Scholar] [CrossRef]
- Varadharajan, A.; Sinha, S.; Xu, A.; Daniel, A.; Kim, K.; Shanmugam, N.; Wu, E.; Yang, C.; Zhang, M.; Acree, W.E., Jr. Development of Abraham model correlations for describing solute transfer into transcutol based on molar solubility ratios for pharmaceutical and other organic compounds. J. Solut. Chem. 2022, 52, 70–90. [Google Scholar] [CrossRef]
- Daniel, A.; Kim, K.; Shanmugam, N.; Sinha, S.; Varadharajan, A.; Acree, W.E., Jr. Abraham model correlations for solute transfer into cyclopentanone. Phys. Chem. Liq. 2022, 60, 964–976. [Google Scholar] [CrossRef]
- Sprunger, L.M.; Proctor, A.; Acree, W.E., Jr.; Abraham, M.H.; Benjelloun-Dakhama, N. Correlation and prediction of partition coefficient between the gas phase and water, and the solvents dry methyl acetate, dry and wet ethyl acetate, and dry and wet butyl acetate. Fluid Phase Equilibr. 2008, 270, 30–44. [Google Scholar] [CrossRef]
- Hierlemann, A.; Zellers, E.T.; Ricco, A.J. Use of linear solvation energy relationships for modeling responses from polymer-coated acoustic-wave vapor sensors. Anal. Chem. 2001, 73, 3458–3466. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.R.; Baynes, R.E.; Monteiro-Riviere, N.A.; Riviere, J.E. A system coefficient approach for quantitative assessment of the solvent effects on membrane absorption from chemical mixtures. SAR QSAR Environ. Res. 2007, 18, 579–593. [Google Scholar] [CrossRef]
- Yap, C.W. PaDEL-descriptor: An open source software to calculate molecular descriptors and fingerprints. J. Comput. Chem. 2011, 32, 1466–1474. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Chen, W.; Singh, R.P.; Chen, W.; Singh, R.P.; Cui, Y. Versatile in silico modeling of partition coefficients of organic compounds in polydimethylsiloxane using linear and nonlinear methods. J. Hazard. Mater. 2020, 399, 123012. [Google Scholar] [CrossRef]
- Boscaini, E.; Alexander, M.L.; Prazeller, P.; Märk, T.D. Investigation of fundamental physical properties of a polydimethylsiloxane (PDMS) membrane using a proton transfer reaction mass spectrometer (PTRMS). Int. J. Mass Spectrom. 2004, 239, 179–186. [Google Scholar] [CrossRef]
- Isidorov, V.; Purzyńska, A.; Modzelewska, A.; Serowiecka, M. Distribution coefficients of aliphatic alcohols, carbonyl compounds and esters between air and Carboxen/polydimethylsiloxane fiber coating. Anal. Chim. Acta 2006, 560, 103–109. [Google Scholar] [CrossRef]
- Isidorov, V.A.; Vinogorova, V.T. Experimental determination and calculation of distribution coefficients between air and fiber with polydimethylsiloxane coating for some groups of organic compounds. J. Chromatogr. A 2005, 1077, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, T.; Larsen, T.O.; Montanarella, L.; Madsen, J.Ø. Application of head-space solid-phase microextraction for the analysis of volatile metabolites emitted by Penicillium species. J. Microbiol. Methods 1996, 25, 245–255. [Google Scholar] [CrossRef]
- Martos, P.A.; Saraullo, A.; Pawliszyn, J. Estimation of air/coating distribution coefficients for solid phase microextraction using retention indexes from linear temperature-programmed capillary gas chromatography. application to the sampling and analysis of total petroleum hydrocarbons in air. Anal. Chem. 1997, 69, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Chao, K.-P.; Lu, Y.-T.; Yang, H.-W. Prediction of partition coefficients of organic compounds between SPME/PDMS and aqueous solution. Int. J. Mol. Sci. 2014, 15, 2585–2595. [Google Scholar] [CrossRef]
- Ulrich, N.; Endo, S.; Brown, T.N.; Watanabe, N.; Bronner, G.; Abraham, M.H.; Goss, K.-U. UFZ-LSER Database v 3.2.1 [Internet], Leipzig, Germany, Helmholtz Centre for Environmental Research-UFZ. 2017. Available online: http://www.ufz.de/lserd (accessed on 1 September 2022).
- Platts, J.A.; Butina, D.; Abraham, M.H.; Hersey, A. Estimation of molecular linear free energy relation descriptors using a group contribution approach. J. Chem. Inf. Comput. Sci. 1999, 39, 835–845. [Google Scholar] [CrossRef]
- Platts, J.A.; Abraham, M.H.; Butina, D.; Hersey, A. Estimation of molecular linear free energy relationship descriptors by a group contribution approach. 2. Prediction of partition coefficients. J. Chem. Inf. Comput. Sci. 2000, 40, 71–80. [Google Scholar] [CrossRef]
- Chung, Y.; Vermeire, F.H.; Wu, H.; Walker, P.J.; Abraham, M.H.; Green, W.H. Group contribution and machine learning approaches to predict Abraham solute parameters, solvation free energy, and solvation enthalpy. J. Chem. Inf. Model. 2022, 62, 433–446. [Google Scholar] [CrossRef]
- Ulrich, N.; Ebert, A. Can deep learning algorithms enhance the prediction of solute descriptors for linear solvation energy relationship approaches? Fluid Phase Equilib. 2022, 555, 113349. [Google Scholar] [CrossRef]
- Sinha, S.; Varadharajan, A.; Xu, A.; Wu, E.; Acree, W.E., Jr. Determination of Abraham model solute descriptors for hippuric acid from measured molar solubilities in several organic mono-solvents of varying polarity and hydrogen-bonding ability. Phys. Chem. Liq. 2022, 60, 563–571. [Google Scholar] [CrossRef]

| Solute | E | S | A | B | V | Log P | Ref. |
|---|---|---|---|---|---|---|---|
| Methane | 0.000 | 0.000 | 0.000 | 0.000 | 0.2495 | 1.160 | [10] |
| Ethane | 0.000 | 0.000 | 0.000 | 0.000 | 0.3904 | 1.710 | [10] |
| Propane | 0.000 | 0.000 | 0.000 | 0.000 | 0.5313 | 2.320 | [10] |
| Butane | 0.000 | 0.000 | 0.000 | 0.000 | 0.6722 | 2.930 | [10] |
| 2-Methylpropane | 0.000 | 0.000 | 0.000 | 0.000 | 0.6722 | 2.880 | [10] |
| Pentane | 0.000 | 0.000 | 0.000 | 0.000 | 0.8131 | 3.470 | [10] |
| 2,2-Dimethylpropane | 0.000 | 0.000 | 0.000 | 0.000 | 0.8131 | 3.230 | [10] |
| Hexane | 0.000 | 0.000 | 0.000 | 0.000 | 0.9540 | 4.040 | [10] |
| 2-Methylpentane | 0.000 | 0.000 | 0.000 | 0.000 | 0.9540 | 3.508 | [33] |
| 3-Methylpentane | 0.000 | 0.000 | 0.000 | 0.000 | 0.9540 | 3.515 | [33] |
| 2,3-Dimethylbutane | 0.000 | 0.000 | 0.000 | 0.000 | 0.9540 | 3.373 | [33] |
| Heptane | 0.000 | 0.000 | 0.000 | 0.000 | 1.0949 | 4.610 | [10] |
| 2,2-Dimethylpentane | 0.000 | 0.000 | 0.000 | 0.000 | 1.0949 | 3.866 | [33] |
| 2,3-Dimethylpentane | 0.000 | 0.000 | 0.000 | 0.000 | 1.0949 | 4.116 | [33] |
| 3,3-Dimethylpentane | 0.000 | 0.000 | 0.000 | 0.000 | 1.0949 | 4.040 | [33] |
| 2,2,3-Trimethylbutane | 0.000 | 0.000 | 0.000 | 0.000 | 1.0949 | 3.991 | [33] |
| 2-Methylhexane | 0.000 | 0.000 | 0.000 | 0.000 | 1.0949 | 4.009 | [33] |
| 3-Methylhexane | 0.000 | 0.000 | 0.000 | 0.000 | 1.0949 | 4.047 | [33] |
| Octane | 0.000 | 0.000 | 0.000 | 0.000 | 1.2358 | 5.282 | [33] |
| 2,3-Dimethylpentane | 0.000 | 0.000 | 0.000 | 0.000 | 1.0949 | 3.954 | [33] |
| 2-Methylheptane | 0.000 | 0.000 | 0.000 | 0.000 | 1.2358 | 4.412 | [33] |
| 4-Methylheptane | 0.000 | 0.000 | 0.000 | 0.000 | 1.2358 | 4.436 | [33] |
| 3-Methylheptane | 0.000 | 0.000 | 0.000 | 0.000 | 1.2358 | 4.503 | [33] |
| 3,5-Dimethylheptane | 0.000 | 0.000 | 0.000 | 0.000 | 1.3767 | 4.897 | [33] |
| 3,4-Dimethylheptane | 0.000 | 0.000 | 0.000 | 0.000 | 1.3767 | 4.970 | [33] |
| 2,2,3-Trimethylpentane | 0.000 | 0.000 | 0.000 | 0.000 | 1.2358 | 4.820 | [33] |
| 2,5-Dimethylhexane | 0.000 | 0.000 | 0.000 | 0.000 | 1.2358 | 4.371 | [33] |
| 2,2-Dimethylhexane | 0.000 | 0.000 | 0.000 | 0.000 | 1.2358 | 4.389 | [33] |
| 2,3-Dimethylhexane | 0.000 | 0.000 | 0.000 | 0.000 | 1.2358 | 4.528 | [33] |
| 2,4-Dimethylhexane | 0.000 | 0.000 | 0.000 | 0.000 | 1.2358 | 4.614 | [33] |
| 3-Ethylhexane | 0.000 | 0.000 | 0.000 | 0.000 | 1.2358 | 4.452 | [33] |
| Nonane | 0.000 | 0.000 | 0.000 | 0.000 | 1.3767 | 5.400 | [10] |
| 3,3-Diethylpentane | 0.000 | 0.000 | 0.000 | 0.000 | 1.3767 | 4.980 | [33] |
| 2,5-Dimethylheptane | 0.000 | 0.000 | 0.000 | 0.000 | 1.3767 | 4.925 | [33] |
| 3,3-Dimethylheptane | 0.000 | 0.000 | 0.000 | 0.000 | 1.3767 | 4.881 | [33] |
| 2,3-Dimethylheptane | 0.000 | 0.000 | 0.000 | 0.000 | 1.3767 | 4.837 | [33] |
| 3-Methyloctane | 0.000 | 0.000 | 0.000 | 0.000 | 1.3767 | 4.824 | [33] |
| Decane | 0.000 | 0.000 | 0.000 | 0.000 | 1.5176 | 5.820 | [10] |
| 2-Methylnonane | 0.000 | 0.000 | 0.000 | 0.000 | 1.5176 | 6.100 | [33] |
| Undecane | 0.000 | 0.000 | 0.000 | 0.000 | 1.6585 | 6.270 | [10] |
| Dodecane | 0.000 | 0.000 | 0.000 | 0.000 | 1.7994 | 6.820 | [10] |
| Tridecane | 0.000 | 0.000 | 0.000 | 0.000 | 1.9402 | 7.270 | [10] |
| Tetradecane | 0.000 | 0.000 | 0.000 | 0.000 | 2.0810 | 7.480 | [10] |
| Cyclopropane | 0.408 | 0.230 | 0.000 | 0.000 | 0.4227 | 1.430 | [10] |
| Cyclopentane | 0.263 | 0.100 | 0.000 | 0.000 | 0.7045 | 2.853 | [34] |
| Methylcyclopentane | 0.225 | 0.100 | 0.000 | 0.000 | 0.8454 | 3.132 | [34] |
| Cyclohexane | 0.305 | 0.100 | 0.000 | 0.000 | 0.8454 | 3.520 | [10] |
| Methylcyclohexane | 0.244 | 0.060 | 0.000 | 0.000 | 0.9863 | 3.668 | [34] |
| Ethene | 0.107 | 0.100 | 0.000 | 0.070 | 0.3474 | 1.343 | [10] |
| Propene | 0.103 | 0.080 | 0.000 | 0.070 | 0.4883 | 1.800 | [10] |
| 1-Butene | 0.100 | 0.080 | 0.000 | 0.070 | 0.6292 | 2.310 | [10] |
| 2-Methyl-1-propene | 0.120 | 0.080 | 0.000 | 0.080 | 0.6292 | 2.160 | [10] |
| 1,3-Butadiene | 0.320 | 0.230 | 0.000 | 0.100 | 0.5862 | 1.780 | [10] |
| Trichloromethane | 0.430 | 0.490 | 0.150 | 0.020 | 0.6167 | 1.620 | [10] |
| Trichloromethane | 0.430 | 0.490 | 0.150 | 0.020 | 0.6167 | 1.710 | [10] |
| Tetrachloromethane | 0.460 | 0.380 | 0.000 | 0.000 | 0.7391 | 2.840 | [10] |
| 1,1,1-Trichloroethane | 0.370 | 0.410 | 0.000 | 0.090 | 0.7576 | 2.750 | [10] |
| 1,1,1,2-Tetrachloroethane | 0.540 | 0.630 | 0.100 | 0.080 | 0.8800 | 2.660 | [10] |
| 1,1,2,2-Tetrachloroethane | 0.600 | 0.760 | 0.160 | 0.120 | 0.8800 | 2.170 | [10] |
| 1,2-Dichloropropane | 0.370 | 0.630 | 0.000 | 0.170 | 0.7761 | 2.100 | [10] |
| cis 1,2-Dichloroethene | 0.436 | 0.610 | 0.110 | 0.050 | 0.5922 | 1.840 | [11] |
| Trichloroethylene | 0.524 | 0.370 | 0.080 | 0.030 | 0.7146 | 2.240 | [10] |
| Trichloroethylene | 0.524 | 0.370 | 0.080 | 0.030 | 0.7146 | 2.410 | [10] |
| Tetrachloroethylene | 0.639 | 0.440 | 0.000 | 0.000 | 0.8370 | 3.270 | [10] |
| Dibromochloromethane | 0.775 | 0.680 | 0.120 | 0.100 | 0.7219 | 2.160 | [10] |
| Trifluoromethane | −0.430 | 0.180 | 0.110 | 0.030 | 0.3026 | 0.600 | [10] |
| Propanone | 0.179 | 0.700 | 0.040 | 0.490 | 0.5470 | −0.670 | [10] |
| Butanone | 0.166 | 0.700 | 0.000 | 0.510 | 0.6879 | −0.320 | [10] |
| Pentan-2-one | 0.143 | 0.680 | 0.000 | 0.510 | 0.8288 | 0.410 | [10] |
| Pentan-3-one | 0.154 | 0.660 | 0.000 | 0.510 | 0.8288 | 1.290 | [11] |
| Hexan-2-one | 0.136 | 0.680 | 0.000 | 0.510 | 0.9697 | 0.860 | [10] |
| Hexan-3-one | 0.136 | 0.660 | 0.000 | 0.510 | 0.9697 | 0.980 | [10] |
| Heptan-2-one | 0.123 | 0.680 | 0.000 | 0.510 | 1.1106 | 1.350 | [10] |
| Cyclohexanone | 0.403 | 0.860 | 0.000 | 0.560 | 0.8611 | 0.070 | [10] |
| Acetophenone | 0.818 | 1.010 | 0.000 | 0.480 | 1.0140 | 1.040 | [10] |
| 4-Chloroacetophenone | 0.955 | 1.090 | 0.000 | 0.440 | 1.1360 | 1.640 | [10] |
| Ethyl acetate | 0.106 | 0.620 | 0.000 | 0.450 | 0.7466 | 0.271 | [10] |
| Isobutyl acetate | 0.052 | 0.570 | 0.000 | 0.470 | 1.0284 | 1.660 | [10] |
| Phenyl acetate | 0.661 | 1.130 | 0.000 | 0.540 | 1.0726 | 0.860 | [10] |
| Methyl benzoate | 0.733 | 0.850 | 0.000 | 0.460 | 1.0726 | 1.650 | [10] |
| Ethyl benzoate | 0.689 | 0.850 | 0.000 | 0.460 | 1.2135 | 2.120 | [10] |
| Methyl 2-methylbenzoate | 0.772 | 0.870 | 0.000 | 0.430 | 1.2135 | 2.150 | [10] |
| Ethanol | 0.246 | 0.420 | 0.370 | 0.480 | 0.4491 | −1.410 | [10] |
| Propan-1-ol | 0.236 | 0.420 | 0.370 | 0.480 | 0.5900 | −1.160 | [10] |
| Propan-2-ol | 0.212 | 0.360 | 0.330 | 0.560 | 0.5900 | −1.210 | [10] |
| 2-Butanol | 0.217 | 0.360 | 0.330 | 0.560 | 0.7309 | −0.630 | [10] |
| 2-Methylpropan-1-ol | 0.217 | 0.390 | 0.370 | 0.480 | 0.7309 | −0.390 | [10] |
| 3-Methyl-1-butanol | 0.192 | 0.390 | 0.370 | 0.480 | 0.8718 | −0.100 | [10] |
| Benzene | 0.610 | 0.520 | 0.000 | 0.140 | 0.7176 | 1.849 | [33] |
| Benzene | 0.610 | 0.520 | 0.000 | 0.140 | 0.7176 | 1.990 | [10] |
| Toluene | 0.601 | 0.520 | 0.000 | 0.140 | 0.8573 | 2.263 | [33] |
| Toluene | 0.601 | 0.520 | 0.000 | 0.140 | 0.8573 | 2.580 | [10] |
| Ethylbenzene | 0.613 | 0.510 | 0.000 | 0.150 | 0.9982 | 2.736 | [33] |
| 1,2-Dimethylbenzene | 0.663 | 0.560 | 0.000 | 0.160 | 0.9982 | 2.802 | [33] |
| 1,3-Dimethylbenzene | 0.623 | 0.520 | 0.000 | 0.160 | 0.9982 | 2.710 | [33] |
| 1,4-Dimethylbenzene | 0.613 | 0.520 | 0.000 | 0.160 | 0.9982 | 2.808 | [33] |
| Propylbenzene | 0.604 | 0.500 | 0.000 | 0.150 | 1.1391 | 3.312 | [33] |
| 1-Methyl-4-ethylbenzene | 0.630 | 0.510 | 0.000 | 0.180 | 1.1391 | 3.199 | [33] |
| Isopropylbenzene | 0.602 | 0.490 | 0.000 | 0.160 | 1.1391 | 3.250 | [10] |
| Isobutylbenzene | 0.580 | 0.470 | 0.000 | 0.150 | 1.2800 | 4.040 | [33] |
| sec-Butylbenzene | 0.603 | 0.480 | 0.000 | 0.160 | 1.2800 | 3.623 | [33] |
| 1-Methyl-3-isopropylbenzene | 0.621 | 0.490 | 0.000 | 0.190 | 1.2800 | 3.516 | [33] |
| 1-Methyl-3-propylbenzene | 0.624 | 0.500 | 0.000 | 0.180 | 1.2800 | 3.577 | [33] |
| 1,2-Dimethyl-4-ethylbenzene | 0.685 | 0.560 | 0.000 | 0.190 | 1.2800 | 3.600 | [33] |
| 1,3-Dimethyl-2-ethylbenzene | 0.757 | 0.600 | 0.000 | 0.190 | 1.2800 | 3.638 | [33] |
| 1,2-Dimethyl-3-ethylbenzene | 0.742 | 0.610 | 0.000 | 0.190 | 1.2800 | 3.800 | [33] |
| (2-Methylbutyl)benzene | 0.630 | 0.480 | 0.000 | 0.170 | 1.4209 | 3.959 | [33] |
| 1,2,4-Trimethylbenzene | 0.677 | 0.560 | 0.000 | 0.190 | 1.1391 | 2.940 | [10] |
| 1,3,5-Trimethylbenzene | 0.649 | 0.520 | 0.000 | 0.190 | 1.1391 | 3.250 | [10] |
| 1,2,4,5-Tetramethylbenzene | 0.739 | 0.600 | 0.000 | 0.190 | 1.2800 | 3.860 | [33] |
| 1-tert-Butyl-2-methylbenzene | 0.670 | 0.570 | 0.000 | 0.220 | 1.4209 | 3.670 | [33] |
| Pentylbenzene | 0.594 | 0.510 | 0.000 | 0.150 | 1.4209 | 3.913 | [33] |
| 1-tert-Butyl-4-ethylbenzene | 0.640 | 0.500 | 0.000 | 0.220 | 1.5618 | 4.200 | [33] |
| 1,3,5-Triethylbenzene | 0.672 | 0.500 | 0.000 | 0.190 | 1.5618 | 4.268 | [33] |
| 1,2,4-Triethylbenzene | 0.714 | 0.530 | 0.000 | 0.210 | 1.5618 | 4.211 | [33] |
| Styrene | 0.849 | 0.650 | 0.000 | 0.160 | 0.9552 | 2.860 | [10] |
| Chlorobenzene | 0.718 | 0.650 | 0.000 | 0.070 | 0.8388 | 2.400 | [10] |
| 1,2-Dichlorobenzene | 0.872 | 0.780 | 0.000 | 0.040 | 0.9612 | 2.870 | [10] |
| 1,3-Dichlorobenzene | 0.847 | 0.730 | 0.000 | 0.020 | 0.9612 | 3.290 | [10] |
| 1,4-Dichlorobenzene | 0.825 | 0.750 | 0.000 | 0.020 | 0.9612 | 2.930 | [10] |
| 1,2,3-Trichlorobenzene | 1.030 | 0.860 | 0.000 | 0.000 | 1.0836 | 3.450 | [10] |
| 1,2,4-Trichlorobenzene | 0.980 | 0.810 | 0.000 | 0.000 | 1.0836 | 3.480 | [10] |
| 1,3,5-Trichlorobenzene | 0.980 | 0.730 | 0.000 | 0.000 | 1.0836 | 3.640 | [10] |
| 1,2,3,4-Tetrachlorobenzene | 1.180 | 0.920 | 0.000 | 0.000 | 1.2060 | 3.900 | [10] |
| 1,2,3,5-Tetrachlorobenzene | 1.160 | 0.850 | 0.000 | 0.000 | 1.2060 | 4.180 | [10] |
| 1,2,4,5-Tetrachlorobenzene | 1.160 | 0.860 | 0.000 | 0.000 | 1.2060 | 4.090 | [10] |
| Pentachlorobenzene | 1.330 | 0.960 | 0.000 | 0.000 | 1.3284 | 4.620 | [10] |
| Pentachlorobenzene | 1.330 | 0.960 | 0.000 | 0.000 | 1.3284 | 4.420 | [10] |
| Hexachlorobenzene | 1.490 | 0.990 | 0.000 | 0.000 | 1.4508 | 5.010 | [10] |
| 2-Chlorotoluene | 0.762 | 0.650 | 0.000 | 0.070 | 0.9797 | 3.070 | [10] |
| 4-Chlorotoluene | 0.705 | 0.740 | 0.000 | 0.050 | 0.9797 | 2.870 | [10] |
| 2,4,5-Trichlorotoluene | 1.060 | 0.850 | 0.000 | 0.000 | 1.2250 | 4.170 | [10] |
| Bromobenzene | 0.882 | 0.730 | 0.000 | 0.090 | 0.8914 | 2.510 | [10] |
| Iodobenzene | 1.188 | 0.820 | 0.000 | 0.120 | 0.9750 | 2.730 | [10] |
| Phenyl methyl ether | 0.708 | 0.750 | 0.000 | 0.290 | 0.9160 | 1.705 | [10] |
| 4-Chloroanisole | 0.838 | 0.860 | 0.000 | 0.240 | 1.0380 | 2.370 | [10] |
| Aniline | 0.955 | 0.960 | 0.260 | 0.410 | 0.8162 | 0.010 | [10] |
| 3,4-Dimethylaniline | 0.960 | 0.970 | 0.200 | 0.490 | 1.0980 | 1.070 | [10] |
| 2-Chloroaniline | 1.033 | 0.920 | 0.250 | 0.310 | 0.9386 | 1.040 | [10] |
| 4-Chloroaniline | 1.060 | 1.130 | 0.300 | 0.310 | 0.9386 | 0.840 | [10] |
| 2,4-Dichloroaniline | 1.140 | 1.150 | 0.300 | 0.220 | 1.0610 | 1.690 | [10] |
| 3,4-Dichloroaniline | 1.160 | 1.240 | 0.350 | 0.240 | 1.0610 | 1.390 | [10] |
| Nitrobenzene | 0.871 | 1.110 | 0.000 | 0.280 | 0.8906 | 1.210 | [10] |
| Phenol | 0.805 | 0.890 | 0.600 | 0.300 | 0.7751 | −0.530 | [10] |
| 3-Methylphenol | 0.822 | 0.880 | 0.570 | 0.340 | 0.9160 | −0.030 | [10] |
| 3,5-Dimethylphenol | 0.820 | 0.840 | 0.570 | 0.360 | 1.0569 | 0.420 | [10] |
| 4-Ethylphenol | 0.800 | 0.900 | 0.550 | 0.360 | 1.0569 | 0.600 | [10] |
| 3-Bromophenol | 1.060 | 1.150 | 0.700 | 0.160 | 0.9501 | 0.460 | [10] |
| 2-Chlorophenol | 0.853 | 0.880 | 0.320 | 0.310 | 0.8975 | 0.560 | [10] |
| 3-Chlorophenol | 0.909 | 1.060 | 0.690 | 0.150 | 0.8975 | 0.310 | [10] |
| Pentachlorophenol | 1.217 | 0.860 | 0.610 | 0.090 | 1.3870 | 2.650 | [10] |
| 4-Fluorophenol | 0.670 | 0.970 | 0.630 | 0.230 | 0.7930 | −0.280 | [10] |
| Biphenyl | 1.360 | 0.990 | 0.000 | 0.260 | 1.3240 | 3.370 | [10] |
| Naphthalene | 1.340 | 0.920 | 0.000 | 0.200 | 1.0854 | 2.830 | [10] |
| 1-Methylnaphthalene | 1.337 | 0.940 | 0.000 | 0.220 | 1.2263 | 3.260 | [10] |
| 2-Methylnaphthalene | 1.304 | 0.810 | 0.000 | 0.250 | 1.2263 | 3.170 | [10] |
| 1,2-Dimethylnaphthalene | 1.431 | 0.970 | 0.000 | 0.250 | 1.3672 | 3.470 | [10] |
| 2,6-Dimethylnaphthalene | 1.347 | 0.820 | 0.000 | 0.250 | 1.3672 | 3.590 | [10] |
| Acenaphthene | 1.604 | 1.050 | 0.000 | 0.220 | 1.2586 | 3.630 | [10] |
| Fluorene | 1.588 | 1.060 | 0.000 | 0.250 | 1.3565 | 3.720 | [10] |
| Phenanthrene | 2.055 | 1.290 | 0.000 | 0.260 | 1.4544 | 4.000 | [10] |
| Anthracene | 2.290 | 1.340 | 0.000 | 0.280 | 1.4544 | 3.840 | [10] |
| Fluoranthene | 2.377 | 1.550 | 0.000 | 0.240 | 1.5846 | 4.260 | [10] |
| Benz[a]anthracene | 2.992 | 1.700 | 0.000 | 0.330 | 1.8234 | 4.770 | [10] |
| Pyrene | 2.808 | 1.710 | 0.000 | 0.280 | 1.5846 | 4.320 | [10] |
| Chrysene | 3.027 | 1.730 | 0.000 | 0.330 | 1.8234 | 4.690 | [10] |
| Benzo[b]fluoranthene | 3.194 | 1.820 | 0.000 | 0.400 | 1.9536 | 5.160 | [10] |
| Benzo[k]fluoranthene | 3.190 | 1.910 | 0.000 | 0.330 | 1.9536 | 5.330 | [10] |
| Benzo[a]pyrene | 3.625 | 1.980 | 0.000 | 0.440 | 1.9536 | 5.240 | [10] |
| Benzo[ghi]perylene | 4.073 | 1.900 | 0.000 | 0.480 | 2.0838 | 5.500 | [10] |
| Dibenz[a,h]anthracene | 4.000 | 2.040 | 0.000 | 0.440 | 2.1924 | 6.200 | [10] |
| 1-Methylphenanthrene | 2.055 | 1.250 | 0.000 | 0.260 | 1.5953 | 4.500 | [10] |
| Perylene | 3.256 | 1.760 | 0.000 | 0.400 | 1.9536 | 4.980 | [10] |
| Benzonitrile | 0.742 | 1.110 | 0.000 | 0.330 | 0.8711 | 1.040 | [10] |
| Dimethyl sulfide | 0.404 | 0.430 | 0.000 | 0.270 | 0.5539 | 0.820 | [10] |
| Helium | 0.000 | 0.000 | 0.000 | 0.000 | 0.0680 | 0.470 | [10] |
| Neon | 0.000 | 0.000 | 0.000 | 0.000 | 0.0850 | 0.580 | [10] |
| Argon | 0.000 | 0.000 | 0.000 | 0.000 | 0.1900 | 0.820 | [10] |
| Krypton | 0.000 | 0.000 | 0.000 | 0.000 | 0.2460 | 0.980 | [10] |
| Xenon | 0.000 | 0.000 | 0.000 | 0.000 | 0.3290 | 1.253 | [10] |
| Hydrogen | 0.000 | 0.000 | 0.000 | 0.000 | 0.1086 | 0.420 | [10] |
| Oxygen | 0.000 | 0.000 | 0.000 | 0.000 | 0.1830 | 1.150 | [10] |
| Nitrogen | 0.000 | 0.000 | 0.000 | 0.000 | 0.2222 | 0.850 | [10] |
| Nitrous Oxide | 0.068 | 0.350 | 0.000 | 0.100 | 0.2809 | 0.510 | [10] |
| Carbon Dioxide | 0.000 | 0.280 | 0.050 | 0.100 | 0.2809 | 0.240 | [10] |
| Tetrafluoromethane | −0.580 | −0.260 | 0.000 | 0.000 | 0.3203 | 1.570 | [10] |
| Sulfur hexafluoride | −0.600 | −0.200 | 0.000 | 0.000 | 0.4643 | 2.100 | [10] |
| Benzyl alcohol | 0.803 | 0.870 | 0.330 | 0.560 | 0.9160 | −0.350 | [10] |
| Phenethyl alcohol | 0.784 | 0.830 | 0.300 | 0.660 | 1.0569 | 0.120 | [10] |
| 3-Methylbenzyl alcohol | 0.815 | 0.900 | 0.330 | 0.590 | 1.0569 | 0.170 | [10] |
| 2-Chlorobiphenyl | 1.480 | 1.070 | 0.000 | 0.200 | 1.4466 | 3.970 | [10] |
| 4,4′-Dichlorobiphenyl | 1.640 | 1.180 | 0.000 | 0.160 | 1.5690 | 4.590 | [10] |
| 2,4,4′-Trichlorobiphenyl | 1.760 | 1.330 | 0.000 | 0.150 | 1.6914 | 4.700 | [10] |
| 2,4,4′-Trichlorobiphenyl | 1.760 | 1.330 | 0.000 | 0.150 | 1.6914 | 5.030 | [10] |
| 2,4′,6′-Trichlorobiphenyl | 1.740 | 1.350 | 0.000 | 0.170 | 1.6914 | 5.000 | [10] |
| 2,2′,4,5,5′-Pentachlorobiphenyl | 2.040 | 1.610 | 0.000 | 0.130 | 1.9362 | 5.710 | [10] |
| 2,2′,5,5′-Tetrachlorobiphenyl | 1.900 | 1.480 | 0.000 | 0.150 | 1.8138 | 5.300 | [10] |
| Limonene | 0.488 | 0.280 | 0.000 | 0.210 | 1.3230 | 4.140 | [10] |
| Hexafluoroethane | −0.690 | −0.410 | 0.000 | 0.000 | 0.4966 | 2.400 | [10] |
| Hydrogen sulfide | 0.350 | 0.310 | 0.100 | 0.070 | 0.2721 | 0.300 | [10] |
| Camphor | 0.500 | 0.690 | 0.000 | 0.710 | 1.3161 | 1.480 | [10] |
| Acridine | 2.536 | 1.320 | 0.000 | 0.580 | 1.4133 | 3.170 | [10] |
| 2,3,3′,4,4′-Pentachlorobiphenyl | 2.040 | 1.590 | 0.000 | 0.110 | 1.9362 | 5.890 | [10] |
| 2,2′,3,4,4′,5′-Hexachlorobiphenyl | 2.180 | 1.740 | 0.000 | 0.110 | 2.0586 | 6.200 | [10] |
| 2,3,3′,4,4′,5-Hexachlorobiphenyl | 2.210 | 1.720 | 0.000 | 0.090 | 2.0586 | 6.280 | [10] |
| 2,2′,3,4,4′,5,5′-Heptachlorobiphenyl | 2.290 | 1.870 | 0.000 | 0.090 | 2.1810 | 6.400 | [10] |
| 2,3′,4,4′,5-Pentachlorobiphenyl | 2.060 | 1.590 | 0.000 | 0.110 | 1.9362 | 5.870 | [10] |
| Bromoform | 0.974 | 0.680 | 0.150 | 0.060 | 0.7745 | 1.870 | [10] |
| 2,4,5-Trichloroaniline | 1.240 | 1.150 | 0.300 | 0.140 | 1.1834 | 2.080 | [10] |
| 2,3,3′,5,6-Pentachlorobiphenyl | 2.050 | 1.610 | 0.000 | 0.130 | 1.9362 | 5.710 | [10] |
| 2,2′,4,4′,5,5′-Hexachlorobiphenyl | 2.180 | 1.740 | 0.000 | 0.110 | 2.0586 | 6.160 | [10] |
| 2,2′,4,4′,5,6′-Hexachlorobiphenyl | 2.150 | 1.740 | 0.000 | 0.110 | 2.0586 | 6.170 | [10] |
| 2,2′,4,4′,6,6′-Hexachlorobiphenyl | 2.120 | 1.740 | 0.000 | 0.110 | 2.0586 | 6.030 | [10] |
| Propionaldehyde | 0.196 | 0.650 | 0.000 | 0.450 | 0.5470 | −0.867 | [10] |
| Butyraldehyde | 0.187 | 0.650 | 0.000 | 0.450 | 0.6879 | −0.289 | [10] |
| Pyridine | 0.631 | 0.840 | 0.000 | 0.520 | 0.6753 | −0.454 | [10] |
| Thiophene | 0.687 | 0.570 | 0.000 | 0.150 | 0.6411 | 1.748 | [10] |
| 1,2-Dichloroethane | 0.420 | 0.640 | 0.100 | 0.110 | 0.6352 | 1.161 | [10] |
| Benzonitrile | 0.742 | 1.110 | 0.000 | 0.330 | 0.8711 | 0.859 | [10] |
| Diethyl ether | 0.041 | 0.250 | 0.000 | 0.450 | 0.7309 | 0.664 | [10] |
| Ethanethiol | 0.392 | 0.420 | 0.000 | 0.200 | 0.5539 | 1.115 | [10] |
| 2,3,5,6-Tetrachlorobiphenyl | 1.890 | 1.480 | 0.000 | 0.150 | 1.8138 | 5.340 | [10] |
| Linalool | 0.398 | 0.510 | 0.200 | 0.650 | 1.4903 | 1.490 | [11] |
| Acetophenone | 0.818 | 1.010 | 0.000 | 0.480 | 1.0139 | 1.040 | [11] |
| Myrcene | 0.483 | 0.290 | 0.000 | 0.320 | 1.3886 | 3.560 | [11] |
| 3-Carene | 0.492 | 0.220 | 0.000 | 0.140 | 1.2574 | 4.520 | [11] |
| Terpinolene | 0.564 | 0.290 | 0.000 | 0.200 | 1.3230 | 4.370 | [11] |
| 3-Chlorobiphenyl | 1.051 | 1.050 | 0.000 | 0.180 | 1.4466 | 4.190 | [28] |
| 4-Chlorobiphenyl | 1.500 | 1.050 | 0.000 | 0.180 | 1.4466 | 4.190 | [28] |
| 3,3′,4,4′-Tetrahchlorobiphenyl | 1.940 | 1.440 | 0.000 | 0.110 | 1.8138 | 5.640 | [28] |
| 2,2′,5,5′-Tetrachlorobiphenyl | 1.900 | 1.480 | 0.000 | 0.150 | 1.8138 | 5.370 | [28] |
| Pentachlorophenol | 1.220 | 0.910 | 0.660 | 0.060 | 1.3871 | 2.650 | [28] |
| 4-Chloroanisole | 0.838 | 0.860 | 0.000 | 0.210 | 1.0284 | 2.370 | [28] |
| 2,4,6-Trichlorobiphenyl | 1.740 | 1.350 | 0.000 | 0.170 | 1.6914 | 5.180 | [28] |
| 2,2′,4-Trichlorobiphenyl | 1.740 | 1.350 | 0.000 | 0.170 | 1.6914 | 4.850 | [28] |
| 2,2′,3-Trichlorobiphenyl | 1.750 | 1.350 | 0.000 | 0.170 | 1.6914 | 5.120 | [28] |
| 2,6-Dichlorobiphenyl | 1.660 | 1.220 | 0.000 | 0.200 | 1.5690 | 4.500 | [28] |
| 2,4′,6-Trichlorobiphenyl | 1.740 | 1.350 | 0.000 | 0.170 | 1.6914 | 5.120 | [28] |
| 3,3′,4-Trichlorobiphenyl | 1.790 | 1.310 | 0.000 | 0.130 | 1.6914 | 5.270 | [28] |
| 2,4′-DDT | 1.850 | 1.700 | 0.000 | 0.250 | 2.2180 | 5.820 | [28] |
| 2,4′-DDE | 1.900 | 1.500 | 0.000 | 0.180 | 2.0526 | 5.940 | [28] |
| 2,4′-DDD | 1.800 | 1.730 | 0.100 | 0.260 | 2.0956 | 5.080 | [28] |
| 2,2′,3,3′,4,4′-Hexachlorobiphenyl | 2.180 | 1.740 | 0.000 | 0.110 | 2.0586 | 6.380 | [28] |
| 2,2′,3,4′,5′-Pentachlorobiphenyl | 2.040 | 1.610 | 0.000 | 0.130 | 1.9362 | 6.100 | [28] |
| Solute | E | S | A | B | L | log K | Ref. |
|---|---|---|---|---|---|---|---|
| Methane | 0.000 | 0.000 | 0.000 | 0.000 | −0.323 | −0.300 | [10] |
| Ethane | 0.000 | 0.000 | 0.000 | 0.000 | 0.492 | 0.370 | [10] |
| Propane | 0.000 | 0.000 | 0.000 | 0.000 | 1.050 | 0.880 | [10] |
| Butane | 0.000 | 0.000 | 0.000 | 0.000 | 1.615 | 1.410 | [10] |
| 2-Methylpropane | 0.000 | 0.000 | 0.000 | 0.000 | 1.409 | 1.180 | [10] |
| Pentane | 0.000 | 0.000 | 0.000 | 0.000 | 2.162 | 1.770 | [10] |
| 2,2-Dimethylpropane | 0.000 | 0.000 | 0.000 | 0.000 | 1.820 | 1.390 | [10] |
| Hexane | 0.000 | 0.000 | 0.000 | 0.000 | 2.668 | 2.200 | [10] |
| 3-Methylpentane | 0.000 | 0.000 | 0.000 | 0.000 | 2.581 | 2.200 | [31] |
| Heptane | 0.000 | 0.000 | 0.000 | 0.000 | 3.173 | 2.650 | [10] |
| 2,4-Dimethylpentane | 0.000 | 0.000 | 0.000 | 0.000 | 2.809 | 2.420 | [33] |
| 2,2,3-Trimethylbutane | 0.000 | 0.000 | 0.000 | 0.000 | 2.918 | 2.450 | [33] |
| 2-Methylhexane | 0.000 | 0.000 | 0.000 | 0.000 | 3.001 | 2.590 | [33] |
| Octane | 0.000 | 0.000 | 0.000 | 0.000 | 3.677 | 3.170 | [33] |
| 2,3-Dimethylpentane | 0.000 | 0.000 | 0.000 | 0.000 | 3.016 | 2.610 | [33] |
| 2-Methylheptane | 0.000 | 0.000 | 0.000 | 0.000 | 3.480 | 3.000 | [33] |
| 4-Methylheptane | 0.000 | 0.000 | 0.000 | 0.000 | 3.483 | 3.030 | [33] |
| 3-Methylheptane | 0.000 | 0.000 | 0.000 | 0.000 | 3.510 | 3.040 | [33] |
| 3,5-Dimethylheptane | 0.000 | 0.000 | 0.000 | 0.000 | 3.826 | 3.290 | [33] |
| 3,4-Dimethylheptane | 0.000 | 0.000 | 0.000 | 0.000 | 3.935 | 3.380 | [33] |
| 2,2,3-Trimethylpentane | 0.000 | 0.000 | 0.000 | 0.000 | 3.325 | 2.760 | [33] |
| 2,5-Dimethylhexane | 0.000 | 0.000 | 0.000 | 0.000 | 3.308 | 2.770 | [33] |
| 2,2-Dimethylhexane | 0.000 | 0.000 | 0.000 | 0.000 | 3.261 | 2.830 | [33] |
| 2,3-Dimethylhexane | 0.000 | 0.000 | 0.000 | 0.000 | 3.451 | 2.990 | [33] |
| 3-Ethylhexane | 0.000 | 0.000 | 0.000 | 0.000 | 3.519 | 3.000 | [33] |
| Nonane | 0.000 | 0.000 | 0.000 | 0.000 | 4.182 | 3.250 | [10] |
| 3,3-Diethylpentane | 0.000 | 0.000 | 0.000 | 0.000 | 4.065 | 3.420 | [33] |
| 2,5-Dimethylheptane | 0.000 | 0.000 | 0.000 | 0.000 | 3.822 | 3.290 | [33] |
| 3,3-Dimethylheptane | 0.000 | 0.000 | 0.000 | 0.000 | 3.833 | 3.320 | [33] |
| 2,3-Dimethylheptane | 0.000 | 0.000 | 0.000 | 0.000 | 3.925 | 3.380 | [33] |
| 2-Methyloctane | 0.000 | 0.000 | 0.000 | 0.000 | 3.966 | 3.410 | [33] |
| 3-Methyloctane | 0.000 | 0.000 | 0.000 | 0.000 | 3.998 | 3.460 | [33] |
| Decane | 0.000 | 0.000 | 0.000 | 0.000 | 4.686 | 3.500 | [10] |
| 2,2-Dimethyloctane | 0.000 | 0.000 | 0.000 | 0.000 | 4.225 | 3.640 | [33] |
| 3,3-Dimethyloctane | 0.000 | 0.000 | 0.000 | 0.000 | 4.307 | 3.700 | [33] |
| 2,3-Dimethyloctane | 0.000 | 0.000 | 0.000 | 0.000 | 4.401 | 3.790 | [33] |
| 2-Methylnonane | 0.000 | 0.000 | 0.000 | 0.000 | 4.453 | 3.830 | [33] |
| 3-Ethyloctane | 0.000 | 0.000 | 0.000 | 0.000 | 4.467 | 3.840 | [33] |
| 3-Methylnonane | 0.000 | 0.000 | 0.000 | 0.000 | 4.486 | 3.850 | [33] |
| Undecane | 0.000 | 0.000 | 0.000 | 0.000 | 5.191 | 3.890 | [10] |
| Dodecane | 0.000 | 0.000 | 0.000 | 0.000 | 5.696 | 4.290 | [10] |
| Cyclopropane | 0.408 | 0.230 | 0.000 | 0.000 | 1.314 | 0.880 | [10] |
| Cyclopentane | 0.263 | 0.100 | 0.000 | 0.000 | 2.477 | 1.973 | [34] |
| Methylcyclopentane | 0.225 | 0.100 | 0.000 | 0.000 | 2.907 | 1.962 | [34] |
| Cyclohexane | 0.305 | 0.100 | 0.000 | 0.000 | 2.964 | 2.620 | [10] |
| Methylcyclohexane | 0.244 | 0.060 | 0.000 | 0.000 | 3.319 | 2.418 | [34] |
| Alpha-pinene | 0.438 | 0.200 | 0.000 | 0.140 | 4.256 | 3.650 | [11] |
| Limonene | 0.500 | 0.310 | 0.000 | 0.230 | 4.688 | 4.040 | [10] |
| d-Limonene | 0.500 | 0.310 | 0.000 | 0.230 | 4.688 | 4.010 | [11] |
| gamma-Terpinene | 0.522 | 0.290 | 0.000 | 0.220 | 4.840 | 4.140 | [11] |
| Ethene | 0.107 | 0.100 | 0.000 | 0.070 | 0.289 | 0.403 | [10] |
| Propene | 0.103 | 0.080 | 0.000 | 0.070 | 0.946 | 0.830 | [10] |
| 1-Butene | 0.100 | 0.080 | 0.000 | 0.070 | 1.529 | 1.300 | [10] |
| 2-Methyl-1-propene | 0.120 | 0.080 | 0.000 | 0.080 | 1.579 | 1.300 | [10] |
| 1,3-Butadiene | 0.320 | 0.230 | 0.000 | 0.100 | 1.543 | 1.330 | [10] |
| cis 2-Butene | 0.140 | 0.080 | 0.000 | 0.050 | 1.737 | 1.460 | [10] |
| trans 2-Butene | 0.126 | 0.080 | 0.000 | 0.050 | 1.664 | 1.400 | [10] |
| Trichloromethane | 0.430 | 0.490 | 0.150 | 0.020 | 2.480 | 2.410 | [10] |
| Trichloromethane | 0.430 | 0.490 | 0.150 | 0.020 | 2.480 | 2.500 | [10] |
| Tetrachloromethane | 0.460 | 0.380 | 0.000 | 0.000 | 2.823 | 2.650 | [10] |
| 1,1,1-Trichloroethane | 0.370 | 0.410 | 0.000 | 0.090 | 2.733 | 2.870 | [10] |
| 1,1,1,2-Tetrachloroethane | 0.540 | 0.630 | 0.100 | 0.080 | 3.641 | 3.600 | [10] |
| 1,1,2,2-Tetrachloroethane | 0.600 | 0.760 | 0.160 | 0.120 | 3.803 | 3.980 | [10] |
| 1,2-Dichloropropane | 0.370 | 0.630 | 0.000 | 0.170 | 2.836 | 3.070 | [10] |
| cis 1,2-Dichloroethene | 0.436 | 0.610 | 0.110 | 0.050 | 2.439 | 2.700 | [11] |
| Trichloroethylene | 0.524 | 0.370 | 0.080 | 0.030 | 2.997 | 2.560 | [10] |
| Trichloroethylene | 0.524 | 0.370 | 0.080 | 0.030 | 2.997 | 2.730 | [10] |
| Tetrachloroethylene | 0.639 | 0.440 | 0.000 | 0.000 | 3.584 | 3.200 | [10] |
| Dibromochloromethane | 0.775 | 0.680 | 0.120 | 0.100 | 3.304 | 3.440 | [10] |
| Trifluoromethane | −0.430 | 0.180 | 0.110 | 0.030 | −0.274 | 0.000 | [10] |
| Propanone | 0.179 | 0.700 | 0.040 | 0.490 | 1.696 | 2.160 | [10] |
| Butanone | 0.166 | 0.700 | 0.000 | 0.510 | 2.287 | 2.400 | [10] |
| Pentan-2-one | 0.143 | 0.680 | 0.000 | 0.510 | 2.755 | 2.990 | [10] |
| Pentan-3-one | 0.154 | 0.660 | 0.000 | 0.510 | 2.811 | 3.790 | [11] |
| Hexan-2-one | 0.136 | 0.680 | 0.000 | 0.510 | 3.286 | 3.270 | [10] |
| Hexan-3-one | 0.136 | 0.660 | 0.000 | 0.510 | 3.271 | 3.250 | [10] |
| Heptan-2-one | 0.123 | 0.680 | 0.000 | 0.510 | 3.760 | 3.600 | [10] |
| Octan-3-one | 0.117 | 0.660 | 0.000 | 0.510 | 4.264 | 3.910 | [11] |
| Cyclohexanone | 0.403 | 0.860 | 0.000 | 0.560 | 3.792 | 3.670 | [10] |
| Acetophenone | 0.818 | 1.010 | 0.000 | 0.480 | 4.501 | 4.400 | [10] |
| 4-Chloroacetophenone | 0.955 | 1.090 | 0.000 | 0.440 | 5.404 | 4.860 | [10] |
| Ethyl acetate | 0.106 | 0.620 | 0.000 | 0.450 | 2.314 | 2.431 | [10] |
| Isobutyl acetate | 0.052 | 0.570 | 0.000 | 0.470 | 3.161 | 3.390 | [10] |
| Phenyl acetate | 0.661 | 1.130 | 0.000 | 0.540 | 4.414 | 4.120 | [10] |
| Methyl benzoate | 0.733 | 0.850 | 0.000 | 0.460 | 4.704 | 4.530 | [10] |
| Ethyl benzoate | 0.689 | 0.850 | 0.000 | 0.460 | 5.075 | 4.790 | [10] |
| Ethanol | 0.246 | 0.420 | 0.370 | 0.480 | 1.485 | 2.260 | [10] |
| Propan-1-ol | 0.236 | 0.420 | 0.370 | 0.480 | 2.031 | 2.400 | [10] |
| Propan-2-ol | 0.212 | 0.360 | 0.330 | 0.560 | 1.764 | 2.270 | [10] |
| 2-Butanol | 0.217 | 0.360 | 0.330 | 0.560 | 2.338 | 2.760 | [10] |
| 2-Methylpropan-1-ol | 0.217 | 0.390 | 0.370 | 0.480 | 2.413 | 2.910 | [10] |
| Pentan-1-ol | 0.219 | 0.420 | 0.370 | 0.480 | 3.106 | 3.347 | [10] |
| 3-Methyl-1-butanol | 0.192 | 0.390 | 0.370 | 0.480 | 3.011 | 3.140 | [10] |
| 3-Octanol | 0.176 | 0.360 | 0.330 | 0.560 | 4.290 | 4.060 | [11] |
| Benzene | 0.610 | 0.520 | 0.000 | 0.140 | 2.786 | 2.479 | [33] |
| Toluene | 0.601 | 0.520 | 0.000 | 0.140 | 3.325 | 2.913 | [33] |
| Ethylbenzene | 0.613 | 0.510 | 0.000 | 0.150 | 3.778 | 3.316 | [33] |
| 1,2-Dimethylbenzene | 0.663 | 0.560 | 0.000 | 0.160 | 3.939 | 3.462 | [33] |
| 1,3-Dimethylbenzene | 0.623 | 0.520 | 0.000 | 0.160 | 3.839 | 3.320 | [33] |
| 1,4-Dimethylbenzene | 0.613 | 0.520 | 0.000 | 0.160 | 3.839 | 3.398 | [33] |
| Propylbenzene | 0.604 | 0.500 | 0.000 | 0.150 | 4.230 | 3.702 | [33] |
| 1-Methyl-3-ethylbenzene | 0.630 | 0.510 | 0.000 | 0.180 | 4.275 | 3.680 | [33] |
| 1-Methyl-4-ethylbenzene | 0.630 | 0.510 | 0.000 | 0.180 | 4.289 | 3.790 | [33] |
| 1-Methyl-2-ethylbenzene | 0.680 | 0.550 | 0.000 | 0.180 | 4.346 | 3.820 | [33] |
| Isopropylbenzene | 0.602 | 0.490 | 0.000 | 0.160 | 4.084 | 3.690 | [10] |
| Isobutylbenzene | 0.580 | 0.470 | 0.000 | 0.150 | 4.500 | 3.920 | [33] |
| sec-Butylbenzene | 0.603 | 0.480 | 0.000 | 0.160 | 4.506 | 3.930 | [33] |
| 1-Methyl-3-isopropylbenzene | 0.621 | 0.490 | 0.000 | 0.190 | 4.556 | 4.000 | [33] |
| 1-Methyl-4-isopropylbenzene | 0.607 | 0.490 | 0.000 | 0.190 | 4.590 | 4.010 | [33] |
| 1-Methyl-2-isopropylbenzene | 0.669 | 0.530 | 0.000 | 0.190 | 4.622 | 4.080 | [33] |
| 1-Methyl-3-propylbenzene | 0.624 | 0.500 | 0.000 | 0.180 | 4.710 | 4.120 | [33] |
| 1-Methyl-2-propylbenzene | 0.664 | 0.540 | 0.000 | 0.180 | 4.766 | 4.170 | [33] |
| 1,3-Dimethyl-5-ethylbenzene | 0.653 | 0.520 | 0.000 | 0.190 | 4.760 | 4.180 | [33] |
| 1,4-Dimethyl-2-ethylbenzene | 0.693 | 0.550 | 0.000 | 0.190 | 4.824 | 4.200 | [33] |
| 1,2-Dimethyl-4-ethylbenzene | 0.685 | 0.560 | 0.000 | 0.190 | 4.873 | 4.240 | [33] |
| 1,3-Dimethyl-2-ethylbenzene | 0.757 | 0.600 | 0.000 | 0.190 | 4.866 | 4.260 | [33] |
| 1,2-Dimethyl-3-ethylbenzene | 0.742 | 0.610 | 0.000 | 0.190 | 4.946 | 4.300 | [33] |
| (2-Methylbutyl)benzene | 0.630 | 0.480 | 0.000 | 0.170 | 5.128 | 4.380 | [33] |
| 1,2,4-Trimethylbenzene | 0.677 | 0.560 | 0.000 | 0.190 | 4.441 | 3.570 | [10] |
| 1,3,5-Trimethylbenzene | 0.649 | 0.520 | 0.000 | 0.190 | 4.344 | 3.910 | [10] |
| 1,2,4,5-Tetramethylbenzene | 0.739 | 0.600 | 0.000 | 0.190 | 5.029 | 4.390 | [33] |
| 1-tert-Butyl-2-methylbenzene | 0.670 | 0.570 | 0.000 | 0.220 | 4.998 | 4.420 | [33] |
| Pentylbenzene | 0.594 | 0.510 | 0.000 | 0.150 | 5.230 | 4.540 | [33] |
| 1-tert-Butyl-4-ethylbenzene | 0.640 | 0.500 | 0.000 | 0.220 | 5.450 | 4.640 | [33] |
| 1-tert-Butyl-3,5-dimethylbenzene | 0.665 | 0.550 | 0.000 | 0.220 | 5.575 | 4.660 | [33] |
| 1,3,5-Triethylbenzene | 0.672 | 0.500 | 0.000 | 0.190 | 5.510 | 4.830 | [33] |
| 1,2,4-Triethylbenzene | 0.714 | 0.530 | 0.000 | 0.210 | 5.586 | 4.880 | [33] |
| Hexylbenzene | 0.591 | 0.500 | 0.000 | 0.150 | 5.720 | 4.950 | [33] |
| Styrene | 0.849 | 0.650 | 0.000 | 0.160 | 3.856 | 3.770 | [10] |
| Chlorobenzene | 0.718 | 0.650 | 0.000 | 0.070 | 3.657 | 3.220 | [10] |
| 1,2-Dichlorobenzene | 0.872 | 0.780 | 0.000 | 0.040 | 4.518 | 3.770 | [10] |
| 1,3-Dichlorobenzene | 0.847 | 0.730 | 0.000 | 0.020 | 4.410 | 4.010 | [10] |
| 1,4-Dichlorobenzene | 0.825 | 0.750 | 0.000 | 0.020 | 4.435 | 3.670 | [10] |
| 1,2,3-Trichlorobenzene | 1.030 | 0.860 | 0.000 | 0.000 | 5.419 | 4.360 | [10] |
| 1,2,4-Trichlorobenzene | 0.980 | 0.810 | 0.000 | 0.000 | 5.248 | 4.300 | [10] |
| 1,3,5-Trichlorobenzene | 0.980 | 0.730 | 0.000 | 0.000 | 5.045 | 4.210 | [10] |
| 1,2,3,5-Tetrachlorobenzene | 1.160 | 0.850 | 0.000 | 0.000 | 5.922 | 5.370 | [10] |
| 1,2,4,5-Tetrachlorobenzene | 1.160 | 0.860 | 0.000 | 0.000 | 5.926 | 5.070 | [10] |
| Pentachlorobenzene | 1.330 | 0.920 | 0.060 | 0.000 | 6.633 | 5.860 | [10] |
| Pentachlorobenzene | 1.330 | 0.920 | 0.060 | 0.000 | 6.633 | 5.660 | [10] |
| Hexachlorobenzene | 1.490 | 0.990 | 0.000 | 0.000 | 7.390 | 6.510 | [10] |
| 2-Chlorotoluene | 0.762 | 0.650 | 0.000 | 0.070 | 4.173 | 3.680 | [10] |
| 4-Chlorotoluene | 0.705 | 0.740 | 0.000 | 0.050 | 4.205 | 3.550 | [10] |
| Bromobenzene | 0.882 | 0.730 | 0.000 | 0.090 | 4.041 | 3.580 | [10] |
| Iodobenzene | 1.188 | 0.820 | 0.000 | 0.120 | 4.502 | 4.010 | [10] |
| Phenyl methyl ether | 0.708 | 0.750 | 0.000 | 0.290 | 3.890 | 3.505 | [10] |
| Aniline | 0.955 | 0.960 | 0.260 | 0.410 | 3.934 | 4.300 | [10] |
| 2-Chloroaniline | 1.033 | 0.920 | 0.250 | 0.310 | 4.674 | 4.640 | [10] |
| 4-Chloroaniline | 1.060 | 1.130 | 0.300 | 0.310 | 4.889 | 5.170 | [10] |
| Nitrobenzene | 0.871 | 1.110 | 0.000 | 0.280 | 4.557 | 4.230 | [10] |
| Phenol | 0.805 | 0.890 | 0.600 | 0.300 | 3.766 | 4.320 | [10] |
| 3-Methylphenol | 0.822 | 0.880 | 0.570 | 0.340 | 4.310 | 4.570 | [10] |
| 3,5-Dimethylphenol | 0.820 | 0.840 | 0.570 | 0.360 | 4.856 | 5.020 | [10] |
| 4-Ethylphenol | 0.800 | 0.900 | 0.550 | 0.360 | 4.737 | 5.100 | [10] |
| 2-Chlorophenol | 0.853 | 0.880 | 0.320 | 0.310 | 4.178 | 3.900 | [10] |
| 3-Chlorophenol | 0.909 | 1.060 | 0.690 | 0.150 | 4.773 | 5.160 | [10] |
| 4-Fluorophenol | 0.670 | 0.970 | 0.630 | 0.230 | 3.844 | 4.260 | [10] |
| Biphenyl | 1.360 | 0.990 | 0.000 | 0.260 | 6.014 | 5.320 | [10] |
| Naphthalene | 1.340 | 0.920 | 0.000 | 0.200 | 5.161 | 4.560 | [10] |
| 1-Methylnaphthalene | 1.337 | 0.940 | 0.000 | 0.220 | 5.802 | 5.050 | [10] |
| 2-Methylnaphthalene | 1.304 | 0.810 | 0.000 | 0.250 | 5.617 | 5.000 | [10] |
| 2,6-Dimethylnaphthalene | 1.347 | 0.820 | 0.000 | 0.250 | 6.146 | 5.520 | [10] |
| Acenaphthene | 1.604 | 1.050 | 0.000 | 0.220 | 6.469 | 5.990 | [10] |
| Fluorene | 1.588 | 1.060 | 0.000 | 0.250 | 6.922 | 6.180 | [10] |
| Phenanthrene | 2.055 | 1.290 | 0.000 | 0.260 | 7.632 | 6.800 | [10] |
| Anthracene | 2.290 | 1.340 | 0.000 | 0.280 | 7.568 | 6.870 | [10] |
| Fluoranthene | 2.377 | 1.550 | 0.000 | 0.240 | 8.827 | 7.700 | [10] |
| Pyrene | 2.808 | 1.710 | 0.000 | 0.280 | 8.833 | 7.820 | [10] |
| Chrysene | 3.027 | 1.730 | 0.000 | 0.330 | 10.334 | 9.480 | [10] |
| Benzo[a]pyrene | 3.625 | 1.980 | 0.000 | 0.440 | 11.736 | 10.780 | [10] |
| Perylene | 3.256 | 1.760 | 0.000 | 0.400 | 12.053 | 10.720 | [10] |
| Benz[a]anthracene | 2.992 | 1.700 | 0.000 | 0.350 | 10.291 | 9.360 | [10] |
| Benzo[ghi]perylene | 4.073 | 1.900 | 0.000 | 0.450 | 13.447 | 12.080 | [10] |
| Benzonitrile | 0.742 | 1.110 | 0.000 | 0.330 | 4.039 | 4.130 | [10] |
| Dimethyl sulfide | 0.404 | 0.430 | 0.000 | 0.270 | 2.037 | 1.960 | [10] |
| Helium | 0.000 | 0.000 | 0.000 | 0.000 | −1.741 | −1.550 | [10] |
| Neon | 0.000 | 0.000 | 0.000 | 0.000 | −1.575 | −1.380 | [10] |
| Argon | 0.000 | 0.000 | 0.000 | 0.000 | −0.688 | −0.650 | [10] |
| Krypton | 0.000 | 0.000 | 0.000 | 0.000 | −0.211 | −0.230 | [10] |
| Xenon | 0.000 | 0.000 | 0.000 | 0.000 | 0.378 | 0.283 | [10] |
| Hydrogen | 0.000 | 0.000 | 0.000 | 0.000 | −1.200 | −1.300 | [10] |
| Oxygen | 0.000 | 0.000 | 0.000 | 0.000 | −0.723 | −0.360 | [10] |
| Nitrogen | 0.000 | 0.000 | 0.000 | 0.000 | −0.978 | −0.950 | [10] |
| Nitrous Oxide | 0.068 | 0.350 | 0.000 | 0.100 | 0.164 | 0.280 | [10] |
| Carbon Dioxide | 0.000 | 0.280 | 0.050 | 0.100 | 0.058 | 0.160 | [10] |
| Tetrafluoromethane | −0.580 | −0.260 | 0.000 | 0.000 | −0.817 | −0.720 | [10] |
| Sulfur hexafluoride | −0.600 | −0.200 | 0.000 | 0.000 | −0.120 | −0.120 | [10] |
| Benzyl alcohol | 0.803 | 0.870 | 0.330 | 0.560 | 4.221 | 4.510 | [10] |
| Phenethyl alcohol | 0.784 | 0.830 | 0.300 | 0.660 | 4.628 | 5.100 | [10] |
| 2,4,4′-Trichlorobiphenyl | 1.760 | 1.330 | 0.000 | 0.150 | 7.904 | 6.800 | [10] |
| 2,2′,4,5,5′-Pentachlorobiphenyl | 2.040 | 1.610 | 0.000 | 0.130 | 8.868 | 8.140 | [10] |
| 2,2′,5,5′-Tetrachlorobiphenyl | 1.900 | 1.480 | 0.000 | 0.150 | 8.144 | 7.500 | [10] |
| Hydrogen sulfide | 0.350 | 0.310 | 0.100 | 0.070 | 0.723 | 0.700 | [10] |
| Camphor | 0.500 | 0.690 | 0.000 | 0.710 | 5.084 | 4.630 | [10] |
| 2,2′,3,4,4′,5′-Hexachlorobiphenyl | 2.180 | 1.740 | 0.000 | 0.110 | 9.772 | 8.630 | [10] |
| 2,3,3′,4,4′,5-Hexachlorobiphenyl | 2.210 | 1.720 | 0.000 | 0.090 | 10.200 | 9.180 | [10] |
| 2,2′,3,4,4′,5,5′-Heptachlorobiphenyl | 2.290 | 1.870 | 0.000 | 0.090 | 10.415 | 9.440 | [10] |
| 2,3′,4,4′,5-Pentachlorobiphenyl | 2.060 | 1.590 | 0.000 | 0.110 | 9.396 | 8.360 | [10] |
| Bromoform | 0.974 | 0.680 | 0.150 | 0.060 | 3.784 | 3.430 | [10] |
| 2,2′,4,4′,5,5′-Hexachlorobiphenyl | 2.180 | 1.740 | 0.000 | 0.110 | 9.587 | 8.378 | [10] |
| 2,2′,4,4′,6,6′-Hexachlorobiphenyl | 2.120 | 1.740 | 0.000 | 0.110 | 8.715 | 8.360 | [10] |
| Propionaldehyde | 0.196 | 0.650 | 0.000 | 0.450 | 1.815 | 1.653 | [10] |
| Butyraldehyde | 0.187 | 0.650 | 0.000 | 0.450 | 2.270 | 2.041 | [10] |
| Pyridine | 0.631 | 0.840 | 0.000 | 0.520 | 3.022 | 2.986 | [10] |
| Thiophene | 0.687 | 0.570 | 0.000 | 0.150 | 2.819 | 2.778 | [10] |
| 1,2-Dichloroethane | 0.420 | 0.640 | 0.100 | 0.110 | 2.573 | 2.431 | [10] |
| Benzonitrile | 0.742 | 1.110 | 0.000 | 0.330 | 4.039 | 3.949 | [10] |
| Diethyl ether | 0.041 | 0.250 | 0.000 | 0.450 | 2.015 | 1.954 | [10] |
| Ethanethiol | 0.392 | 0.420 | 0.000 | 0.200 | 2.079 | 1.955 | [10] |
| Linalool | 0.398 | 0.510 | 0.200 | 0.650 | 4.975 | 4.580 | [11] |
| Acetophenone | 0.818 | 1.010 | 0.000 | 0.480 | 4.501 | 4.400 | [11] |
| Myrcene | 0.483 | 0.290 | 0.000 | 0.320 | 4.513 | 3.930 | [11] |
| 3-Carene | 0.492 | 0.220 | 0.000 | 0.140 | 4.679 | 3.940 | [11] |
| Terpinolene | 0.564 | 0.290 | 0.000 | 0.200 | 5.029 | 4.250 | [11] |
| 3-Bromophenol | 1.060 | 1.130 | 0.700 | 0.160 | 5.144 | 5.620 | [10] |
| 3-Chlorobiphenyl | 1.051 | 1.050 | 0.000 | 0.180 | 6.667 | 6.190 | [28] |
| 4-Chlorobiphenyl | 1.500 | 1.050 | 0.000 | 0.180 | 6.718 | 6.210 | [28] |
| 3,3′,4,4′-Tetrahchlorobiphenyl | 1.940 | 1.440 | 0.000 | 0.110 | 9.205 | 8.290 | [28] |
| 2,2′,5,5′-Tetrachlorobiphenyl | 1.900 | 1.480 | 0.000 | 0.150 | 8.144 | 7.560 | [28] |
| Pentachlorophenol | 1.220 | 0.910 | 0.660 | 0.060 | 6.805 | 6.310 | [28] |
| Diphenylamine | 1.470 | 1.130 | 0.310 | 0.319 | 7.094 | 7.030 | [28] |
| 2,4,6-Trichlorobiphenyl | 1.740 | 1.350 | 0.000 | 0.170 | 7.286 | 6.840 | [28] |
| 2,2′,4-Trichlorobiphenyl | 1.740 | 1.350 | 0.000 | 0.170 | 7.521 | 6.640 | [28] |
| 2,2′,3-Trichlorobiphenyl | 1.750 | 1.350 | 0.000 | 0.170 | 7.647 | 7.030 | [28] |
| 2,6-Dichlorobiphenyl | 1.660 | 1.220 | 0.000 | 0.200 | 6.765 | 6.060 | [28] |
| 2,4′,6-Trichlorobiphenyl | 1.740 | 1.350 | 0.000 | 0.170 | 7.667 | 7.050 | [28] |
| 3,3′,4-Trichlorobiphenyl | 1.790 | 1.310 | 0.000 | 0.130 | 8.392 | 7.630 | [28] |
| 2,2′,3,3′,4,4′-Hexachlorobiphenyl | 2.180 | 1.740 | 0.000 | 0.110 | 9.957 | 9.020 | [28] |
| 2,2′,3,4′,5′-Pentachlorobiphenyl | 2.040 | 1.610 | 0.000 | 0.130 | 9.033 | 8.340 | [28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, A.; Longacre, L.; Motati, R.; Acree, W.E., Jr. Abraham Solvation Parameter Model: Revised Predictive Expressions for Solute Transfer into Polydimethylsiloxane Based on Much Larger and Chemically Diverse Datasets. Compounds 2023, 3, 205-223. https://doi.org/10.3390/compounds3010017
Zhou A, Longacre L, Motati R, Acree WE Jr. Abraham Solvation Parameter Model: Revised Predictive Expressions for Solute Transfer into Polydimethylsiloxane Based on Much Larger and Chemically Diverse Datasets. Compounds. 2023; 3(1):205-223. https://doi.org/10.3390/compounds3010017
Chicago/Turabian StyleZhou, Amy, Laine Longacre, Ramya Motati, and William E. Acree, Jr. 2023. "Abraham Solvation Parameter Model: Revised Predictive Expressions for Solute Transfer into Polydimethylsiloxane Based on Much Larger and Chemically Diverse Datasets" Compounds 3, no. 1: 205-223. https://doi.org/10.3390/compounds3010017
APA StyleZhou, A., Longacre, L., Motati, R., & Acree, W. E., Jr. (2023). Abraham Solvation Parameter Model: Revised Predictive Expressions for Solute Transfer into Polydimethylsiloxane Based on Much Larger and Chemically Diverse Datasets. Compounds, 3(1), 205-223. https://doi.org/10.3390/compounds3010017







