Abstract
To date, many studies have been published, aiming to extract bioactive compounds from plants. Lately, research focuses on maximizing the extraction yield, using environmentally friendly techniques and solvents. In this study, the extraction of polyphenolic compounds from Cistus creticus is discussed. Extraction of the compounds has been carried out with water and ethanol, employing the most common approach. To further enhance the extraction yield, the usage of four deep eutectic solvents (DESs) has also been examined. In an effort to further enhance the extraction yield, pulsed electric fields have also been employed. According to the results, it was evident that the use of DESs made of glycerol: choline chloride (2:1) increased the extraction yield by 75%, compared to extraction with water. Moreover, the use of pulsed electric fields enhanced the extraction yield of the common approach, by up to 70%. When pulsed electric fields and DESs were combined, the extraction yield increased by 14%, compared to the use of DESs only. Finally, the extract was analyzed for its content in volatile compounds, and it was found that new compounds could be extracted with the use of DESs.
1. Introduction
Cistus is a genus of flowering plants found in the Mediterranean region. Cistus plants grow in rocky areas, sandy plains, and on mountain peaks. They have distinctive-looking flowers that are yellow, red, white, or violet in color [1]. They contain many bioactive compounds and due to these, cistus plants have been used, since ancient times, to prepare herbal remedies. One major class of bioactive compounds in Cistus plants is polyphenols [2]. Polyphenols are a class of naturally occurring plant metabolites that have attracted much attention, due to their wide range of biological activities, which include antioxidant, anti-inflammatory, and anticarcinogenic effects. The health-promoting effects of polyphenols have been attributed to their ability to scavenge free radicals and modulate signaling pathways involved in cell proliferation, cell death, and inflammation [3,4].
Generally, the most popular methods for the extraction of phenolic compounds are based on the use of organic solvents, such as methanol, ethanol, acetone, and ethyl acetate. However, the use of these solvents has some drawbacks, such as the high cost and the need for special disposal methods as they are hazardous and environmentally unfriendly [5,6]. Some methods use water as the extraction solvent, albeit with lower extraction yields [5,6]. To date, there are a few reports focusing on the extraction of polyphenols from Cistus plants [7,8,9,10,11,12].
In recent years, deep eutectic solvents (DESs) have emerged as a promising alternative to organic solvents for the extraction of polyphenols from plants. DESs comprise of a hydrogen bond acceptor and a hydrogen bond donor, resulting in a mixture with alternate properties from that of the individual components [13,14]. They exhibit a series of advantageous properties, such as negligible vapor pressure, low toxicity, and biocompatibility, while they are non-flammable. Moreover, their physicochemical properties, and as a result, their extractive potential, can be tuned, either by altering the components, or their ratio [15]. Several studies have shown that DESs can be a more efficient and less expensive extraction method than organic solvents [16,17,18,19].
Another important parameter for the extraction of polyphenols from plants is the extraction technique employed. The conventional techniques include percolation, decoction, Soxhlet extraction, etc. [5,6]. However, other, non-conventional techniques (e.g., ultrasound-assisted extraction, microwave-assisted extraction, etc.) have been developed, with each technique exhibiting different pros and cons [5,6]. One of the latest techniques that has been developed and used for polyphenols extraction is pulsed electric fields (PEFs). The PEF method is a technique that electroporates cell membranes, resulting in the maximization of the extraction yield [20]. Moreover, PEFs use a minimal amount of energy, rendering the method environmentally friendly. It is noteworthy that the PEF method is a non-thermal technique. As such, many polyphenolic compounds that are thermally unstable are kept intact in the resulting extracts [20]. Due to the multiple favorable characteristics that PEFs exhibit, the number of reports on the method’s usage for the extraction of bioactive compounds from plants is increasing [21,22,23,24,25]. Furthermore, it can be comparable in terms of efficiency with other methods, such as ultrasound treatment [23].
In this study, the extraction of polyphenols from Cistus creticus was examined. Four DESs were prepared and examined for their potential to extract polyphenols and they were compared to water and a hydroethanolic mixture. Moreover, the effect of the PEF method on the extraction process was evaluated. Finally, the composition of the extract in volatile components was also evaluated, so as to gain a better overview of the volatile compounds that potentiate the extract for additional uses.
2. Materials and Methods
2.1. Chemicals and Reagents
Glycerol anhydrous, trisodium citrate, choline chloride, lactic acid, glycine, sodium butyrate, and sodium carbonate anhydrous came from Penta (Prague, Czech Republic). The Folin–Ciocalteu regent, gallic acid monohydrate, and sodium chloride were obtained from Panreac (Barcelona, Spain). The SPME fiber coated with a layer of divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) was obtained from Supelco (Supelco, Bellefonte, PA, USA). All solvents used were at least of analytical grade. Deionized water was used for all experiments. Luteolin-7-O-glucoside, rutin hydrate, myricetin and quercetin 3-β-D glycoside were purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Plant Material
Fresh leaves from Cistus creticus were collected near the Styrfakas (Moschokaryas) area (according to Google Earth version 7.3.2.5776 Latitude: 38.97 and Longitude: 22.31), Greece. The leaves, while transferred to the lab, were stored at 4 °C. Then, the leaves were washed thoroughly with tap water and dried with paper towels. Next, the leaves were cut into smaller pieces (~1 × 1 cm) and used immediately for the extraction of the compounds. In order to express the results in dry weight (dw), the moisture of the leaves was calculated to be 74 ± 3%.
2.3. Instrumentation
Full details about the PEF system used are given in our previous studies [23,26]. In brief, a high-voltage current generator (Leybold, LD Didactic GmbH, Hürth, Germany), a digital oscilloscope, a function/arbitrary waveform generator, and two stainless steel plates (10 cm long, 10 cm high) with a 1 cm Teflon layer between them, for insulation used. The pulse duration used to process the sample was 1000 μs, the pulse frequency was 1 Hz, the electric field strength was 0.85 kV cm−1, and a square waveform was employed. Furthermore, the maximum delay was set at 20 ns.
The absorbance measurements were recorded on a Shimadzu spectrophotometer (UV-1700, Shimadzu Europa GmbH, Duisburg, Germany).
A Finnigan AQA mass spectrometer (San Jose, CA, USA), a P4000 pump, and a UV6000LP diode array detector were used for the chromatographic separation and detection of the compounds. The stationary phase was a Phenomenex Luna C18 column (5 μm, 4.6 mm × 250 mm) (Phenomenex Inc., Torrance, CA, USA), which was placed in an oven, set at 40 °C. The mobile phase consisted of (A) water containing 0.5% v/v formic acid and (B) a mixture of acetonitrile: water (6:4) containing 0.5% v/v formic acid. The following gradient elution program was employed for the separation of the compounds: 5% B to 40% B in 40 min, then to 50% B in 10 min, and finally to 70% B in 10 min and kept constant for 10 more min. The flow rate of the mobile phase was 1 mL min−1. The injection volume was 20 μL, and injections were made using a rheodyne injector. The spectra were recorded between 220 and 360 nm. The electrospray ionization (ESI), in a positive ion mode, was used to acquire the mass spectra.
An Agilent Technologies (Santa Clara, CA, USA) gas chromatograph model 7890A, equipped with a mass detector (5975C), and a capillary column Agilent J&W DB-1 (30 m × 320 µm × 0.25 µm) (Agilent Technologies Inc., Santa Clara, CA, USA) was used for the analysis of the volatile compounds. Helium (flow rate 1.5 mL min−1) was used as carrier gas. The injector was set at 240 °C. The samples were inserted in the column using a splitless mode. The temperature program was as follows: 40 °C for 5 min, increased to 140 °C with a rate of 2 °C per min, and, finally, increased to 240 °C with a rate of 10 °C per min. The volatile compounds were identified by comparing their mass spectra with data from the integrated NIST 11 library (National Institute of Standards and Technology, Gaithersburg, MD, USA). The peaks were assigned when the similarity was above 80%. The percentages were expressed as mean values from the duplicate analyses.
2.4. Preparation of the DESs
Selection of the DESs was based on previous studies [27,28,29,30]. For the preparation of the DESs, appropriate amounts of the hydrogen bond donor and the hydrogen bond acceptor were transferred to glass bottles. DES-1 was prepared by mixing glycerol: trisodium citrate (15:1 w/w). DES-2 was prepared by mixing glycerol: choline chloride (2:1 w/w). DES-3 was prepared by mixing lactic acid: glycine (5:1 w/w). DES-4 was prepared by mixing glycerol: sodium butyrate (6:1 w/w). The mixtures were heated at 70 °C using a magnetic stirrer (400 rpm) until a transparent liquid was formed. The prepared DES was left to cool naturally to room temperature and stored in the dark. All DESs were stored for seven days, during which they were inspected daily for their stability (no formation of crystals denoted a stable DES). Finally, water (20% v/v) was added to the DESs and the resulting mixture was used for the extraction.
2.5. Conventional and the PEF-Based Extraction of the Polyphenols
For the preparation of the extracts without the PEF pretreatment, 1 g of the plant material was mixed with 10 mL of the examined solvent and placed in the PEF treatment chamber, and left intact for 20 min. Next, the mixture was transferred to a screw-capped, amber glass bottle and stirred with a magnetic stirrer (500 rpm), and heated at 50 °C (in an oil bath) for 3 h. For the extracts prepared with the PEF pretreatment, the abovementioned process was followed, but while the mixture was initially placed in the treatment chamber, the PEF method was applied.
2.6. Measurement of the Total Polyphenol Content (TPC)
The Folin–Ciocalteu assay was used, in order to determine the TPC of the extracts [26]. In an Eppendorf tube, 0.1 mL of diluted extract and 0.1 mL Folin–Ciocalteu reagent were transferred and the solution was left for incubation for 2 min. Next, 0.8 mL of 5% (w/v) sodium carbonate solution was added and the new solution was further incubated for 20 min at 40 °C. Finally, the absorbance was measured at 740 nm. The results were expressed as gallic acid equivalents (GAEs) per g of dry weight (dw), using a calibration curve prepared with gallic acid.
2.7. HPLC-Based Identification and the Quantification of the Polyphenols
For the identification of the polyphenols contained in the extracts, the abovementioned LC–DAD–MS (ESI+) system was employed. The compounds were identified by matching the retention time and the absorption spectrum with that of the standard compounds. The rest of the compounds were tentatively annotated, based on their mass spectra and previous reports [31]. The quantification was carried out using calibration curves (prepared using the standard compounds).
2.8. Determination of the Volatile Components
For the determination of the volatile components of the extracts, a solid phase microextraction (SPME) procedure was employed. The SPME fiber was initially preconditioned for 30 min at 270 °C. In a glass vial (100 mL volume), 10 mL of the examined extract was transferred, along with 3 g of sodium chloride. The vial was sealed and the mixture was heated at 40 °C, under continuous stirring. Ten min later, the fiber was placed in the headspace and the extraction was carried out for 50 min. Next, the fiber was transferred to the GC–MS injection system.
2.9. Statistical Analysis
All extracts were prepared three times and each analysis was carried out three times from each extract, resulting in a total of nine measurements per condition. The variability was expressed with the standard deviation of the nine measurements. The results were expressed as mean values ± standard deviation. The normality of the distribution of the results was examined with the Shapiro–Wilk test. The statistically significant differences between the samples were examined with the Mann–Whitney U test and the Kruskal–Wallis test. All statistical analyses were carried out using SPSS (version 26) (SPSS Inc., Chicago, IL, USA) software.
3. Results and Discussion
3.1. Effect of the DESs and the PEF Usage on the TPC of the Extracts
In order to maximize the extraction yield of the polyphenols, the use of DESs was evaluated. The selection of DESs was based on previous studies [27,28,29,30]. The selected DESs achieved the maximum extraction recoveries, compared to other types of DESs.
The results from the extracts prepared using different solvents are presented in Figure 1. When water was used as an extraction solvent the TPC was found to be 108.2 ± 0.7 mg GAE/g dw. All four tested DESs resulted in extracts with an increased TPC. More specifically, DES-4 caused a 52% increase in the TPC, compared to the extract prepared with water, DES-1 and DES-3 caused a ~64% increase, while the maximum increase was achieved when DES-2 was used (75%). In many published reports, it is mentioned that hydroethanolic solutions are superior, compared to water, for polyphenol extraction, with 60% (v/v) ethanol-water mixtures, exhibiting a much better performance [32,33,34]. To this end, an extraction with this solvent was carried out, so as to compare the DES-based extracts with the hydroethanolic extract, in terms of the TPC, serving as a positive control. As can be seen in Figure 1, the hydroethanolic solution achieved a 47% increase in the TPC, compared to plain water. DES-4 achieved nearly the same extraction yield (no statistically significant differences were recorded), whereas DES-1, DES-3, and DES-2 resulted in extracts with an 11–19% increased TPC, compared to the hydroethanolic solution. It is evident that DES-4 can be used as an alternative option to extract polyphenols from Cistus creticus, yielding extracts with a comparable TPC with hydroethanolic extracts, whereas the other three DESs increase notably the extracted polyphenols. In order for the extraction yield to increase, the extraction medium needs to match as closely as possible, the polarity of the target compounds [35]. Since all tested DESs resulted in extracts with an increased TPC, the polarities of the tested DESs may be more favorable to extract polyphenols, compared to water [36]. According to a previous study, the main mechanism that governs the increased efficiencies of DESs for polyphenol extraction is the formation of an extended network of hydrogen bonds that not only helps solubilize the increased amounts of polyphenols, but also stabilize them [37].
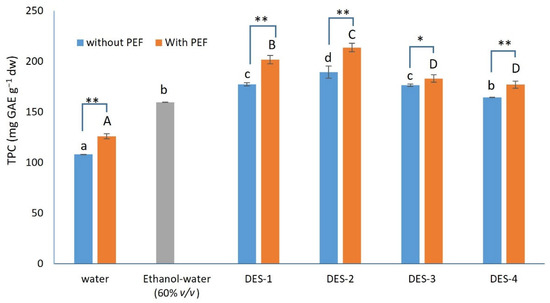
Figure 1.
TPC (mg GAE g−1 dw) in Cistus creticus extracts prepared with water, an ethanol-water mixture, and the four examined DESs, with and without the PEF pretreatment; Statistically significant differences between the same solvent used are denoted with one asterisk (*) for p < 0.05 and two asterisks (**) for p < 0.01. Statistically significant differences (p < 0.05) for samples without the PEF treatment are denoted with small letters, and differences for samples prepared with the PEF pretreatment are denoted with capital letters; Ethanol-water mixture was used as a positive control.
In a previous study, it was reported that the TPC of Cistus monspeliensis and Cistus salviifolius was nearly 55 mg GAE/g dw [38]. Moreover, it was reported that the TPC from the Syrian and Bulgarian Cistus creticus was 70 and 115 mg GAE/g dw, respectively [7,39]. In the previous cases, the polyphenols were extracted using hydroethanolic solutions. The slightly increased TPC in our case, when the ethanol-water mixture was used, can be attributed to the environmental conditions [11]. The increased TPC, when DESs were employed, was also found in previous studies. For instance, in the study of Bakirtzi et al. [36], it was described that lactic acid: glycine: water (3:1:3) was the most efficient DES, compared to other lactic acid-based DESs for the extraction of polyphenols from dittany, fennel, and sage, while this was not the case for mint (all tested DESs achieved similar extraction yields). This may be due to the physicochemical characteristics of the polyphenols that can be extracted from each plant, which can be affected by the properties of the DESs. The increased extraction yield from the glycine-based DESs was also reported in previous studies [40,41].
To date, there are many reports that examine the use of different DESs for the extraction of polyphenols from plant materials [40,41]. Furthermore, there are a few reports that examine the combination of DESs with other extraction techniques (such as microwave-assisted extraction and ultrasound-assisted extraction) [42,43,44,45]. Despite the fact that there are quite a few reports on the use of PEFs for polyphenol extraction [21,46], the combination of DESs with PEFs is an underdeveloped field with relevant reports being scanty and sparse [47]. In an effort to further enhance the TPC of the extracts, we examined whether the use of PEFs could be beneficial. The results are presented in Figure 1. As regards the use of PEFs, it is obvious that in all cases, the DES-PEF prepared extracts contained more polyphenols, compared to the water-PEF extracts, as occurred in the case without the PEF treatment. When the PEF method was used as a pretreatment step, the TPC of the extract prepared with water was increased by 16%, compared to the extract prepared without the PEF treatment. The extracts prepared with DES-1 and DES-2 and the use of PEFs, exhibited a 12 and 14% increase, respectively, in their TPC, compared to the extracts prepared without PEFs. Finally, the extracts prepared with DES-3 and DES-4 and the PEF pretreatment, contained 4–8% more polyphenols, compared to the extracts prepared without PEFs. According to the results, it can be concluded that the use of PEFs was beneficial in all cases, and with a short (20 min) treatment step, a notable increase was achieved. A ~20% increase in the flavonoid content of the extracts prepared from noni-processing wastes, using DESs and PEFs was also reported by Li et al. [47]. The increase in the extraction yield can be attributed to the electroporation that takes place, making it more feasible for the DESs to come in contact with the polyphenols, resulting in an increase. Moreover, the pH of each DES may further affect the extraction process. The pH of DES-1, DES-2, DES-3, and DES-4 were 5.2, 4.3, 2.2, and 10.0, respectively. It can therefore be inferred that the highly acidic and alkaline pH of DES-3 and DES-4 are less suitable for the extraction of polyphenols, since the compounds may be more unstable at that pH. Therefore, DES-1 and DES-2, which have moderate acidic pH values, are more suitable.
3.2. HPLC-Based Quantification of the Polyphenols
As can be seen in Table 1, a total of nine compounds were identified in the extracts prepared using water and DES-2, with or without the PEF treatment. For all detected compounds, except for the luteolin glucoside derivative, an increase in the concentration was recorded when DES-2 was used as a solvent. The decrease in the concentration of luteolin glucoside may be due to its polarity. Since the examined DES is highly polar, it may be better extracted by water, than DES-2 [48]. Moreover, for both examined solvents, when the PEF method was employed, a notable increase in the extraction yield was recorded. Thus, both the selected DESs and the use of the PEF treatment increase the extraction of all identified polyphenols.

Table 1.
Identified polyphenols in the extracts prepared with and without PEFs, using water or DES-2; Results are expressed as mg of the compounds per g of dw; Values are expressed as the mean values (±SD) of triplicate determinations and means within each row (polyphenol compound), with different superscript letters as significantly (p < 0.05) different.
3.3. Volatile Profile of the Extract
The volatile profiles of the extracts prepared with DES-2 with and without PEFs were examined. The results are presented in Table 2. As can be seen, a total of 51 compounds were identified in both extracts. The extract prepared without PEFs contained 38 compounds, while when the PEF method was employed, a total of 41 compounds could be identified. In many cases, the percentage of each volatile compound in the extract prepared with PEFs was higher, compared to the extract without PEFs. The profile of the volatile compounds determined is similar to the profiles determined in other plants of the Cistus family by Morales-Soto et al. [49]. From the results, it can be concluded that the use of PEFs can also affect the volatile profile of the extract.

Table 2.
Volatile compounds identified in the extracts prepared with DES-2 with and without PEFs, by HS–SPME/GC–MS. Values represent the % area of each compound over the total area of the identified compounds; nd: not detected; * denote the compounds with the highest concentration.
4. Conclusions
In this study, the extraction of polyphenols from Cistus creticus was discussed. In order to increase the extraction yield, DESs were examined and it was found that glycerol: choline chloride (2:1) can increase the extraction yield by 75%, compared to extraction with water. Moreover, the use of the PEF method as a pretreatment step was also examined, and it was found to benefit the extraction of polyphenols. When the use of DESs was combined with PEFs, a 14% increase was recorded. Moreover, the use of PEFs was found to affect the composition of the extract in the volatile compounds. From the above, it can be inferred that the combination of the alternative solvents, such as DESs, with new pretreatment techniques, such as PEFs, not only can maximize the extraction yield of the polyphenols from Cistus creticus, but can further maximize the extraction of other classes of compounds.
Author Contributions
Conceptualization, D.P., D.P.M. and S.I.L.; methodology, V.A. and T.C.; software, V.A.; validation, D.P. and V.A.; formal analysis, D.P., V.A. and T.C.; investigation, D.P. and E.B.; resources, S.I.L.; data curation, D.P. and E.B.; writing—original draft preparation, V.A. and T.C.; writing—review and editing, V.A., T.C., D.P., E.B., D.P.M. and S.I.L.; visualization, V.A.; supervision, D.P.M. and S.I.L.; project administration, S.I.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zalegh, I.; Akssira, M.; Bourhia, M.; Mellouki, F.; Rhallabi, N.; Salamatullah, A.M.; Alkaltham, M.S.; Khalil Alyahya, H.; Mhand, R.A. A review on Cistus sp.: Phytochemical and antimicrobial activities. Plants 2021, 10, 1214. [Google Scholar] [CrossRef] [PubMed]
- Papaefthimiou, D.; Papanikolaou, A.; Falara, V.; Givanoudi, S.; Kostas, S.; Kanellis, A.K. Genus Cistus: A model for exploring labdane-type diterpenes’ biosynthesis and a natural source of high value products with biological, aromatic, and pharmacological properties. Front. Chem. 2014, 2, 35. [Google Scholar] [CrossRef] [PubMed]
- LIczbiński, P.; Bukowska, B. Tea and coffee polyphenols and their biological properties based on the latest in vitro investigations. Ind. Crops Prod. 2022, 175, 114265. [Google Scholar] [CrossRef]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and beyond. Am. J. Clin. Nutr. 2005, 81, 215S–217S. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, A.; Ponnuchamy, M.; Kumar, P.S.; Kapoor, A.; Vo, D.V.N.; Prabhakar, S. Techniques and modeling of polyphenol extraction from food: A review. Environ. Chem. Lett. 2021, 19, 3409–3443. [Google Scholar] [CrossRef]
- Dimcheva, V.; Karsheva, M. Cistus incanus from strandja mountain as a source of bioactive antioxidants. Plants 2018, 7, 8. [Google Scholar] [CrossRef]
- Haida, S.; Bakkouche, K.; Kribii, A.R.; Kribii, A. Chemical Composition of Essential Oil, Phenolic Compounds Content, and Antioxidant Activity of Cistus monspeliensis from Northern Morocco. Biochem. Res. Int. 2021, 2021, 6669877. [Google Scholar] [CrossRef]
- Dimcheva, V.; Karsheva, M. Antioxidant activity and polyphenolic content of the Bulgarian wild herb Cistus incanus L. stored under different conditions. J. Chem. Technol. Metall. 2017, 52, 781–790. [Google Scholar]
- Rebaya, A.; Belghith, S.I.; Cherif, J.K.; Trabelsi-Ayadi, M. Total phenolic compounds and antioxidant potential of rokrose (Cistus salviifolius) leaves and flowers grown in Tunisia. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 327–331. [Google Scholar]
- Lukas, B.; Bragagna, L.; Starzyk, K.; Labedz, K.; Stolze, K.; Novak, J. Polyphenol diversity and antioxidant activity of european Cistus creticus L. (cistaceae) compared to six further, partly sympatric Cistus species. Plants 2021, 10, 615. [Google Scholar] [CrossRef]
- Gori, A.; Ferrini, F.; Marzano, M.C.; Tattini, M.; Centritto, M.; Baratto, M.C.; Pogni, R.; Brunetti, C. Characterisation and antioxidant activity of crude extract and polyphenolic rich fractions from C. Incanus leaves. Int. J. Mol. Sci. 2016, 17, 1344. [Google Scholar] [CrossRef]
- Trivedi, T.J.; Lee, J.H.; Lee, H.J.; Jeong, Y.K.; Choi, J.W. Deep eutectic solvents as attractive media for CO2 capture. Green Chem. 2016, 18, 2834–2842. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 2003, 70–71. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Ozturk, B.; Parkinson, C.; Gonzalez-Miquel, M. Extraction of polyphenolic antioxidants from orange peel waste using deep eutectic solvents. Sep. Purif. Technol. 2018, 206, 1–13. [Google Scholar] [CrossRef]
- Choudhary, P.; Guleria, S.; Sharma, N.; Salaria, K.H.; Chalotra, R.; Ali, V.; Vyas, D. Comparative phenolic content and antioxidant activity of some medicinal plant extracts prepared by choline chloride based green solvents and methanol. Curr. Res. Green Sustain. Chem. 2021, 4, 100224. [Google Scholar] [CrossRef]
- Wan Mahmood, W.M.A.; Lorwirachsutee, A.; Theodoropoulos, C.; Gonzalez-Miquel, M. Polyol-Based Deep Eutectic Solvents for Extraction of Natural Polyphenolic Antioxidants from Chlorella vulgaris. ACS Sustain. Chem. Eng. 2019, 7, 5018–5026. [Google Scholar] [CrossRef]
- Ruesgas-Ramón, M.; Figueroa-Espinoza, M.C.; Durand, E. Application of Deep Eutectic Solvents (DES) for Phenolic Compounds Extraction: Overview, Challenges, and Opportunities. J. Agric. Food Chem. 2017, 65, 3591–3601. [Google Scholar] [CrossRef]
- Ranjha, M.M.A.N.; Kanwal, R.; Shafique, B.; Arshad, R.N.; Irfan, S.; Kieliszek, M.; Kowalczewski, P.Ł.; Irfan, M.; Khalid, M.Z.; Roobab, U.; et al. A critical review on pulsed electric field: A novel technology for the extraction of phytoconstituents. Molecules 2021, 26, 4893. [Google Scholar] [CrossRef]
- Frontuto, D.; Carullo, D.; Harrison, S.M.; Brunton, N.P.; Ferrari, G.; Lyng, J.G.; Pataro, G. Optimization of Pulsed Electric Fields-Assisted Extraction of Polyphenols from Potato Peels Using Response Surface Methodology. Food Bioprocess Technol. 2019, 12, 1708–1720. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Pappas, V.M.; Palaiogiannis, D.; Chatzimitakos, T.; Bozinou, E.; Makris, D.P.; Lalas, S.I. Pulsed Electric Field-Based Extraction of Total Polyphenols from Sideritis raiseri Using Hydroethanolic Mixtures. Oxygen 2022, 2, 8. [Google Scholar] [CrossRef]
- Ntourtoglou, G.; Drosou, F.; Chatzimitakos, T.; Athanasiadis, V.; Bozinou, E.; Dourtoglou, V.G.; Elhakem, A.; Sami, R.; Ashour, A.A.; Shafie, A.; et al. Combination of Pulsed Electric Field and Ultrasound in the Extraction of Polyphenols and Volatile Compounds from Grape Stems. Appl. Sci. 2022, 12, 6219. [Google Scholar] [CrossRef]
- Carpentieri, S.; Režek Jambrak, A.; Ferrari, G.; Pataro, G. Pulsed Electric Field-Assisted Extraction of Aroma and Bioactive Compounds from Aromatic Plants and Food By-Products. Front. Nutr. 2022, 8, 792203. [Google Scholar] [CrossRef] [PubMed]
- Peiró, S.; Luengo, E.; Segovia, F.; Raso, J.; Almajano, M.P. Improving Polyphenol Extraction from Lemon Residues by Pulsed Electric Fields. Waste Biomass Valorization 2019, 10, 889–897. [Google Scholar] [CrossRef]
- Ntourtoglou, G.; Drosou, F.; Dourtoglou, V.G.; Athanasiadis, V.; Chatzimitakos, T.; Bozinou, E.; Lalas, S.I. Hyphenated Extraction of Valuable Compounds from Aesculus carnea: Ultrasound Extraction with Pulsed Electric Field Pretreatment. AgriEngineering 2022, 4, 54. [Google Scholar] [CrossRef]
- Mouratoglou, E.; Malliou, V.; Makris, D.P. Novel Glycerol-Based Natural Eutectic Mixtures and Their Efficiency in the Ultrasound-Assisted Extraction of Antioxidant Polyphenols from Agri-Food Waste Biomass. Waste Biomass Valorization 2016, 7, 1377–1387. [Google Scholar] [CrossRef]
- Jancheva, M.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Optimised extraction of antioxidant polyphenols from Satureja thymbra using newly designed glycerol-based natural low-transition temperature mixtures (LTTMs). J. Appl. Res. Med. Aromat. Plants 2017, 6, 31–40. [Google Scholar] [CrossRef]
- Kaltsa, O.; Lakka, A.; Grigorakis, S.; Karageorgou, I.; Batra, G.; Bozinou, E.; Lalas, S.; Makris, D.P. A green extraction process for polyphenols from elderberry (Sambucus nigra) flowers using deep eutectic solvent and ultrasound-assisted pretreatment. Molecules 2020, 25, 921. [Google Scholar] [CrossRef]
- Slim, Z.; Jancheva, M.; Grigorakis, S.; Makris, D.P. Polyphenol extraction from Origanum dictamnus using low-transition temperature mixtures composed of glycerol and organic salts: Effect of organic anion carbon chain length. Chem. Eng. Commun. 2018, 205, 1494–1506. [Google Scholar] [CrossRef]
- Abdoun, R.; Grigorakis, S.; Kellil, A.; Loupassaki, S.; Makris, D.P. Process Optimization and Stability of Waste Orange Peel Polyphenols in Extracts Obtained with Organosolv Thermal Treatment Using Glycerol-Based Solvents. ChemEngineering 2022, 6, 35. [Google Scholar] [CrossRef]
- Fadjare Frempong, T.; Owusu Boadi, N.; Badu, M. Optimization of extraction conditions for polyphenols from the stem bark of Funtumia elastica (Funtum) utilizing response surface methodology. AAS Open Res. 2021, 4, 46. [Google Scholar] [CrossRef]
- Zhumakanova, B.S.; Korona-Głowniak, I.; Skalicka-Woźniak, K.; Ludwiczuk, A.; Baj, T.; Wojtanowski, K.K.; Józefczyk, A.; Zhaparkulova, K.A.; Sakipova, Z.B.; Malm, A. Phytochemical fingerprinting and in vitro antimicrobial and antioxidant activity of the aerial parts of Thymus marschallianus willd. And thymus seravschanicus klokov growing widely in Southern Kazakhstan. Molecules 2021, 26, 3193. [Google Scholar] [CrossRef]
- Bonifácio-Lopes, T.; Vilas-Boas, A.; Machado, M.; Costa, E.M.; Silva, S.; Pereira, R.N.; Campos, D.; Teixeira, J.A.; Pintado, M. Exploring the bioactive potential of brewers spent grain ohmic extracts. Innov. Food Sci. Emerg. Technol. 2022, 76, 102943. [Google Scholar] [CrossRef]
- Skarpalezos, D.; Detsi, A. Deep eutectic solvents as extraction media for valuable flavonoids from natural sources. Appl. Sci. 2019, 9, 4169. [Google Scholar] [CrossRef]
- Bakirtzi, C.; Triantafyllidou, K.; Makris, D.P. Novel lactic acid-based natural deep eutectic solvents: Efficiency in the ultrasound-assisted extraction of antioxidant polyphenols from common native Greek medicinal plants. J. Appl. Res. Med. Aromat. Plants 2016, 3, 120–127. [Google Scholar] [CrossRef]
- Akli, H.; Grigorakis, S.; Kellil, A.; Loupassaki, S.; Makris, D.P.; Calokerinos, A.; Mati, A.; Lydakis-Simantiris, N. Extraction of Polyphenols from Olive Leaves Employing Deep Eutectic Solvents: The Application of Chemometrics to a Quantitative Study on Antioxidant Compounds. Appl. Sci. 2022, 12, 831. [Google Scholar] [CrossRef]
- Mahmoudi, H.; Aouadhi, C.; Kaddour, R.; Gruber, M.; Zargouni, H.; Zaouali, W.; Ben Hamida, N.; Ben Nasri, M.; Ouerghi, Z.; Hosni, K. Comparison of antioxidant and antimicrobial activities of two cultivated cistus species from Tunisia. Biosci. J. 2016, 32, 226–237. [Google Scholar] [CrossRef]
- Ghalia, S.; Adawia, K.; Waed, A. Evaluation of radical scavenging activity, total phenolics and total flavonoids contents of Cistus species in Syria. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 1071–1077. [Google Scholar]
- Saini, A.; Kumar, A.; Panesar, P.S.; Thakur, A. Potential of deep eutectic solvents in the extraction of value-added compounds from agro-industrial by-products. Appl. Food Res. 2022, 2, 100211. [Google Scholar] [CrossRef]
- Makris, D.P.; Lalas, S. Glycerol and glycerol-based deep eutectic mixtures as emerging green solvents for polyphenol extraction: The evidence so far. Molecules 2020, 25, 5842. [Google Scholar] [CrossRef] [PubMed]
- Lanjekar, K.; Gokhale, S.; Rathod, V. Utilization of Waste Mango Peels for Extraction of Polyphenolic Antioxidants by Ultrasound-Assisted Natural Deep Eutectic Solvent (Ua-Nades). SSRN Electron. J. 2022, 18, 101074. [Google Scholar] [CrossRef]
- Bubalo, M.C.; Ćurko, N.; Tomašević, M.; Ganić, K.K.; Redovnikovic, I.R. Green extraction of grape skin phenolics by using deep eutectic solvents. Food Chem. 2016, 200, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Bener, M.; Şen, F.B.; Önem, A.N.; Bekdeşer, B.; Çelik, S.E.; Lalikoglu, M.; Aşçı, Y.S.; Capanoglu, E.; Apak, R. Microwave-assisted extraction of antioxidant compounds from by-products of Turkish hazelnut (Corylus avellana L.) using natural deep eutectic solvents: Modeling, optimization and phenolic characterization. Food Chem. 2022, 385, 132633. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Peng, X.; Yao, X.H.; Wei, Z.F.; Luo, M.; Wang, W.; Zhao, C.J.; Fu, Y.J.; Zu, Y.G. Deep eutectic solvent-based microwave-assisted extraction of genistin, genistein and apigenin from pigeon pea roots. Sep. Purif. Technol. 2015, 150, 63–72. [Google Scholar] [CrossRef]
- Liu, Z.; Esveld, E.; Vincken, J.P.; Bruins, M.E. Pulsed Electric Field as an Alternative Pre-treatment for Drying to Enhance Polyphenol Extraction from Fresh Tea Leaves. Food Bioprocess Technol. 2019, 12, 183–192. [Google Scholar] [CrossRef]
- Li, J.; Chen, W.; Niu, D.; Wang, R.; Xu, F.Y.; Chen, B.R.; Lin, J.W.; Tang, Z.S.; Zeng, X.A. Efficient and green strategy based on pulsed electric field coupled with deep eutectic solvents for recovering flavonoids and preparing flavonoid aglycones from noni-processing wastes. J. Clean. Prod. 2022, 368, 133019. [Google Scholar] [CrossRef]
- Cicci, A.; Bravi, M. Leveraging novel green solvents to drive conceptual and practical biorefinery innovation. Stud. Surf. Sci. Catal. 2019, 179, 243–259. [Google Scholar]
- Morales-Soto, A.; Oruna-Concha, M.J.; Elmore, J.S.; Barrajón-Catalán, E.; Micol, V.; Roldán, C.; Segura-Carretero, A. Volatile profile of Spanish Cistus plants as sources of antimicrobials for industrial applications. Ind. Crops Prod. 2015, 74, 425–433. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).