Preparation and Characterizations of Curcumin Protection and Delivery System Using Linear Dextrin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Linear Dextrin-Curcumin Nanocomplexes
2.3. Determination of Curcumin Loading and Encapsulation Rate
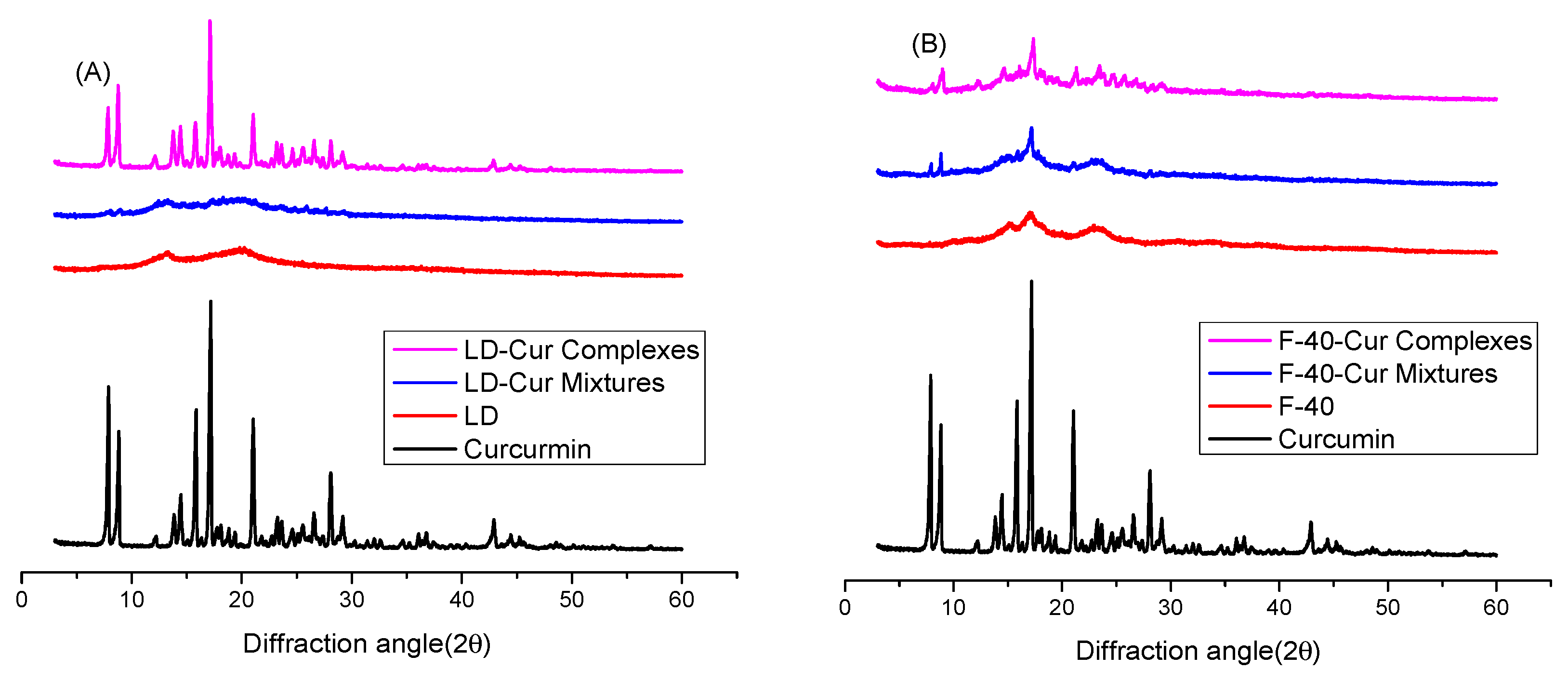
2.4. XRD Analysis
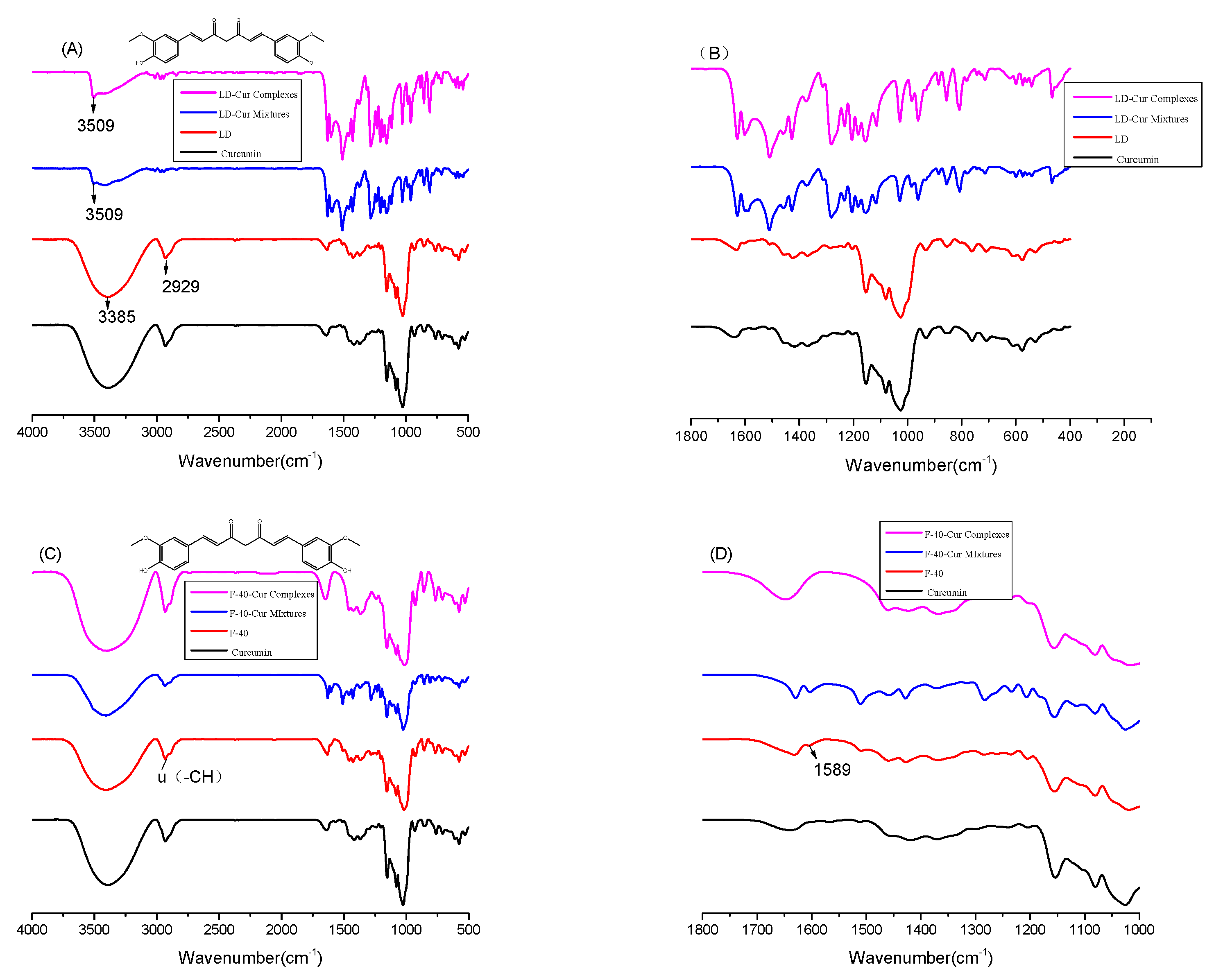
2.5. FT-IR Analysis
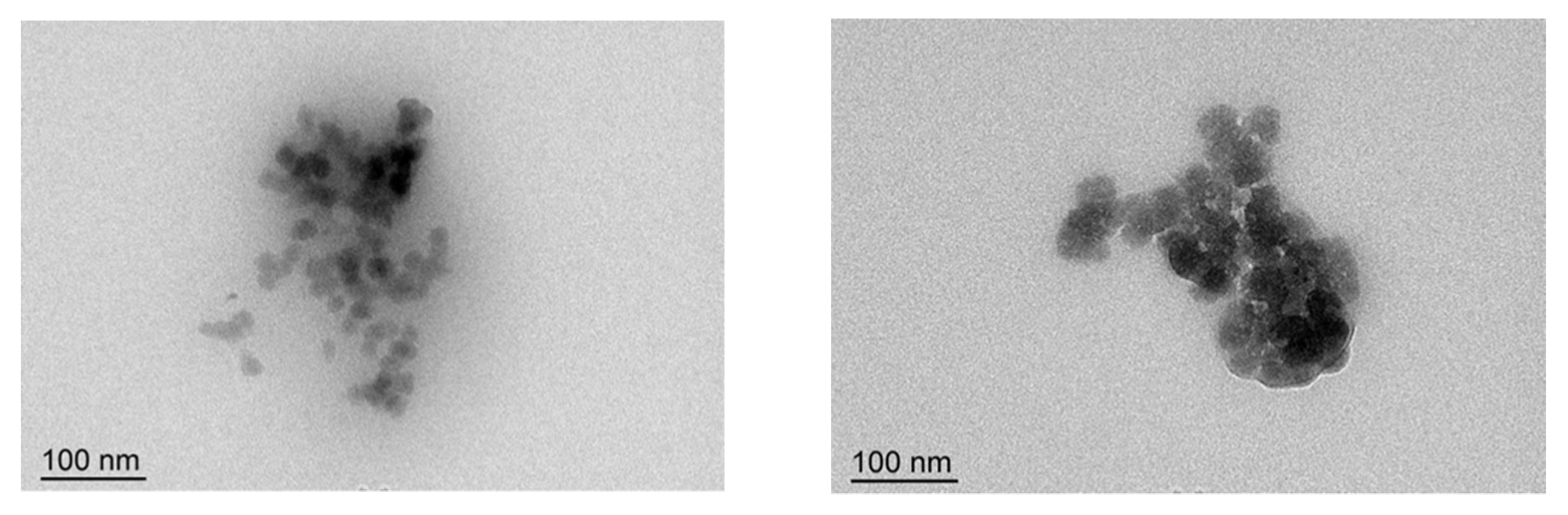
2.6. TEM Analysis
2.7. Thermal Stability Analysis
2.8. Photochemical Stability Analysis
2.9. In Vitro Simulation of Gastrointestinal Digestion Characterization
2.10. Statistical Analysis
3. Results and Discussion
3.1. XRD Results
3.2. FT-IR Results
3.3. TEM Results
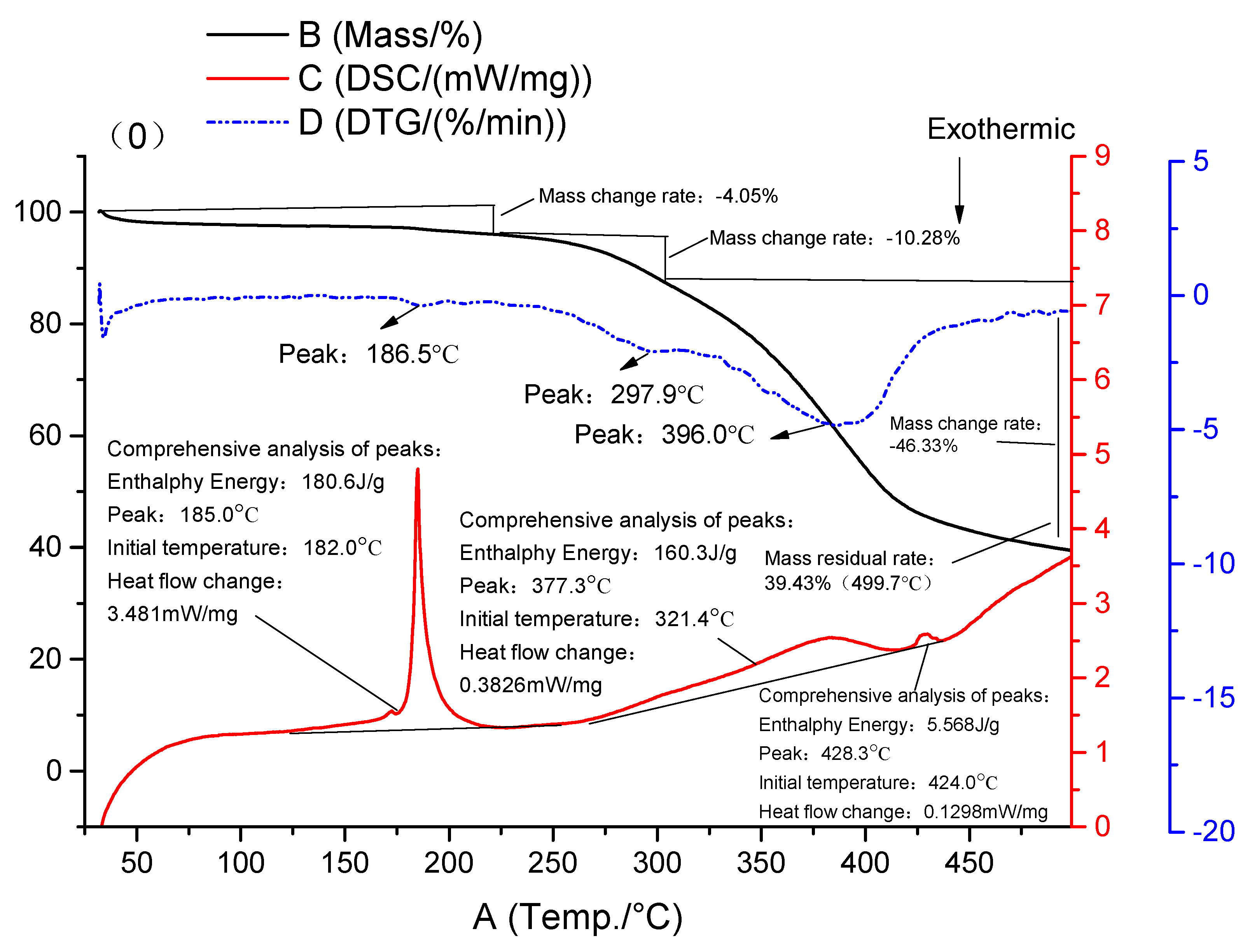
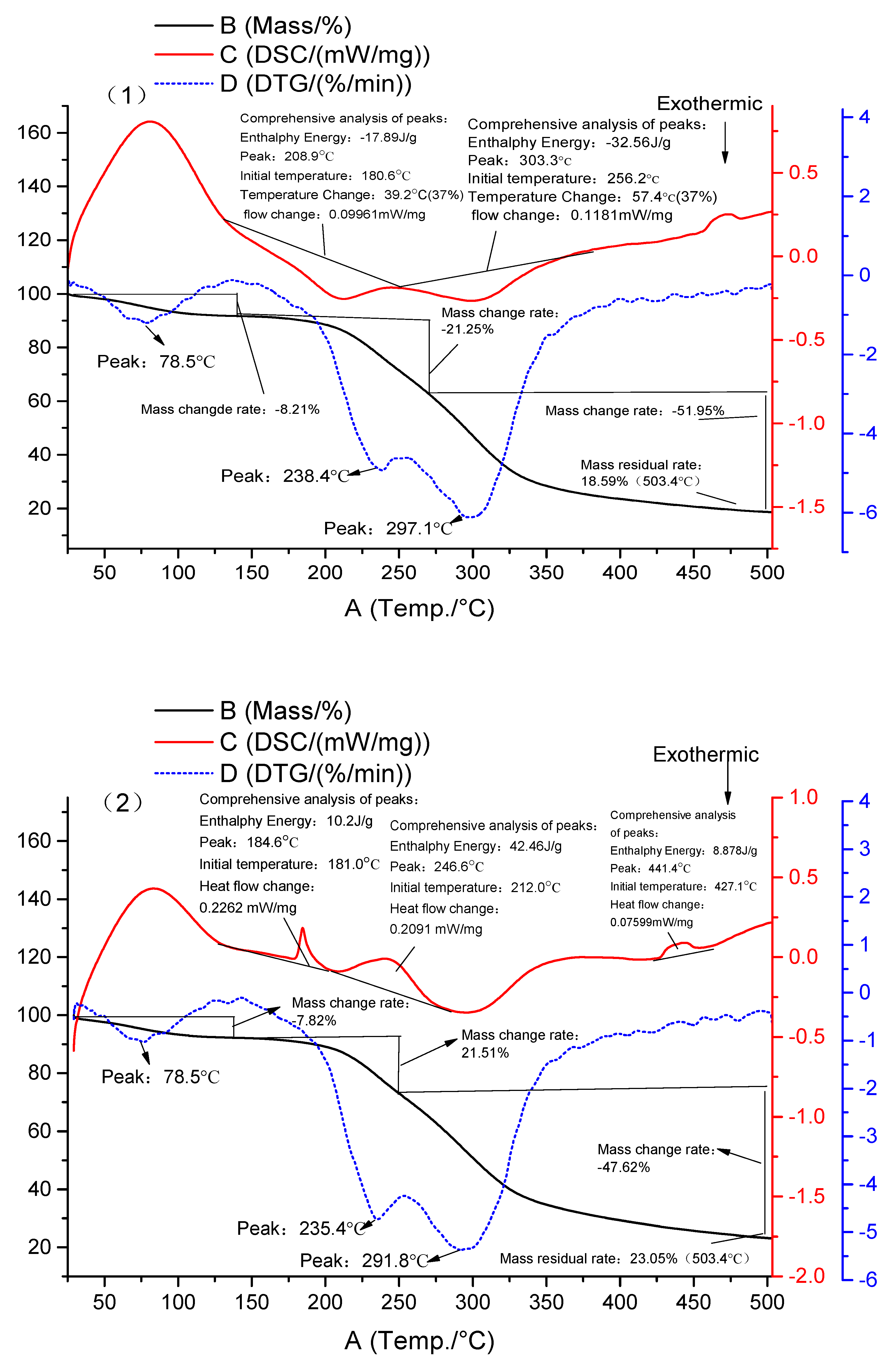
3.4. DSC-TG Results
3.5. Light Stability Analysis
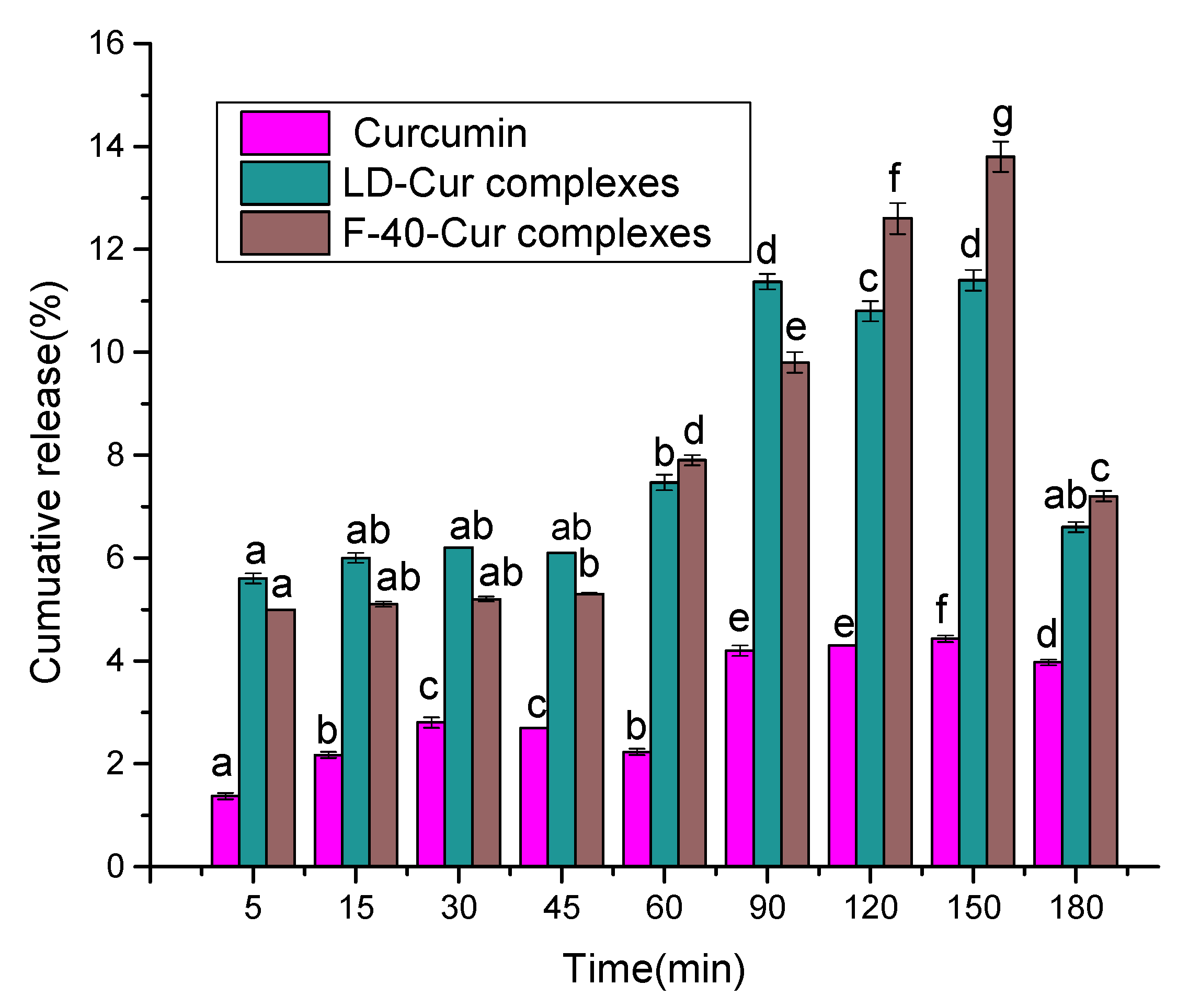
3.6. In Vitro Simulation of Gastrointestinal Digestion Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lestari, M.L.; Indrayanto, G. Curcumin. Profiles Drug. Subst. Excip. Relat. Methodol. 2014, 39, 113–204. [Google Scholar] [CrossRef] [PubMed]
- Mangolim, C.S.; Moriwaki, C.; Nogueira, A.C.; Sato, F.; Baesso, M.L.; Neto, A.M.; Matioli, G. Curcumin-β-cyclodextrin inclusion complex: Stability, solubility, characterisation by FT-IR, FT-Raman, X-ray diffraction and photoacoustic spectroscopy, and food application. Food Chem. 2014, 153, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Mahady, G.B.; Pendland, S.L.; Yun, G.; Lu, Z.Z. Turmeric (Curcuma longa) and curcumin inhibit the growth of Helicobacter pylori, a group 1 carcinogen. Anticancer Res. 2002, 22, 4179–4181. [Google Scholar] [PubMed]
- Reddy, R.C.; Vatsala, P.G.; Keshamouni, V.G.; Padmanaban, G.; Rangarajan, P.N. Curcumin for malaria therapy. Biochem Biophys Res. Commun. 2005, 326, 472–474. [Google Scholar] [CrossRef]
- Wright, L.E.; Frye, J.B.; Gorti, B.; Timmermann, B.N.; Funk, J.L. Bioactivity of turmeric-derived curcuminoids and related metabolites in breast cancer. Curr. Pharm. Des. 2013, 19, 6218–6225. [Google Scholar] [CrossRef]
- Vera-Ramirez, L.; Pérez-Lopez, P.; Varela-Lopez, A.; Ramirez-Tortosa, M.; Battino, M.; Quiles, J.L. Curcumin and liver disease. Biofactors 2013, 39, 88–100. [Google Scholar] [CrossRef]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic roles of curcumin: Lessons learned from clinical trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Harikumar, K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef]
- Sun, A.; Shoji, M.; Lu, Y.J.; Liotta, D.C.; Snyder, J.P. Synthesis of EF24-tripeptide chloromethyl ketone: A novel curcumin-related anticancer drug delivery system. J. Med. Chem. 2006, 49, 3153–3158. [Google Scholar] [CrossRef]
- Vemula, P.K.; Cruikshank, G.A.; Karp, J.M.; John, G. Self-assembled prodrugs: An enzymatically triggered drug-delivery platform. Biomaterials 2009, 30, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Namazi, H.; Dadkhah, A. Convenient method for preparation of hydrophobically modified starch nanocrystals with using fatty acids. Carbohydr. Polym. 2010, 79, 731–737. [Google Scholar] [CrossRef]
- Angellier, H.; Molina-Boisseau, S.; Dole, P.; Dufresne, A. Thermoplastic starch-waxy maize starch nanocrystals nanocomposites. Biomacromolecules 2006, 7, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ding, W.; Liu, J.; Li, Y.; Kennedy, J.F.; Gu, Q.; Shao, S. Preparation and characterization of organic-soluble acetylated starch nanocrystals. Carbohydr. Polym. 2010, 80, 1078–1084. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Tiyaboonchai, W.; Tungpradit, W.; Plianbangchang, P. Formulation and characterization of curcuminoids loaded solid lipid nanoparticles. Int. J. Pharm. 2007, 337, 299–306. [Google Scholar] [CrossRef]
- Sun, B. Preparation, Phase Behavior and Application of Linear Dextrin; Jiangnan University: Wuxi, China, 2018. [Google Scholar]
- Xie, H.; Ma, X.; Lin, W.; Dong, S.; Liu, Q.; Chen, Y.; Gao, Q. Linear Dextrin as Potential Insulin Delivery System: Effect of Degree of Polymerization on the Physicochemical Properties of Linear Dextrin-Insulin Inclusion Complexes. Polymers 2021, 13, 4187. [Google Scholar] [CrossRef]
- Marcolino, V.A.; Zanin, G.M.; Durrant, L.R.; Benassi Mde, T.; Matioli, G. Interaction of curcumin and bixin with β-cyclodextrin: Complexation methods, stability, and applications in food. J. Agric. Food Chem. 2011, 59, 3348–3357. [Google Scholar] [CrossRef]
- Patel, A.; Hu, Y.; Tiwari, J.K.; Velikov, K.P. Synthesis and characterisation of zein-curcumin colloidal particles. Soft Matter. 2010, 6, 6192–6199. [Google Scholar] [CrossRef]
- Pereira, A.G.B.; Fajardo, A.R.; Nocchi, S.; Nakamura, C.V.; Rubira, A.F.; Muniz, E.C. Starch-based microspheres for sustained-release of curcumin: Preparation and cytotoxic effect on tumor cells. Carbohydr. Polym. 2013, 98, 711–720. [Google Scholar] [CrossRef]
- Kasapoglu-Calik, M.; Ozdemir, M. Synthesis and controlled release of curcumin-β-cyclodextrin inclusion complex from nanocomposite poly (N-isopropylacrylamide/sodium alginate) hydrogels. J. Appl. Polym. Sci. 2019, 136, 47554. [Google Scholar] [CrossRef]
- Zeng, F.; Ma, F.; Gao, Q.; Yu, S.; Kong, F.; Zhu, S. Debranching and temperature-cycled crystallization of waxy rice starch and their digestibility. Carbohydr. Polym. 2014, 113, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.; Schwartz, B.; Peri, I.; Shimoni, E. Improving bioavailability and stability of genistein by complexation with high-amylose corn starch. J. Agric. Food Chem. 2011, 59, 7932–7938. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shin, G.H.; Lee, I.W.; Chen, X.; Park, H.J. Soluble starch formulated nanocomposite increases water solubility and stability of curcumin. Food Hydrocoll. 2016, 56, 41–49. [Google Scholar] [CrossRef]
- Chai, Y.; Wang, M.; Zhang, G. Interaction between amylose and tea polyphenols modulates the postprandial glycemic response to high-amylose maize starch. J. Agric. Food Chem. 2013, 61, 8608–8615. [Google Scholar] [CrossRef]
- Tang, B.; Ma, L.; Wang, H.-y.; Zhang, G.-y. Study on the Supramolecular Interaction of Curcumin and β-cyclodextrin by Spectrophotometry and Its Analytical Application. J. Agric. Food Chem. 2002, 50, 1355–1361. [Google Scholar] [CrossRef]
- Pang, S.C.; Tay, S.H.; Chin, S.F. Facile synthesis of curcumin-loaded starch-maleate nanoparticles. J. Nanomater. 2014, 2014, 22. [Google Scholar] [CrossRef]
- Klaochanpong, N.; Puttanlek, C.; Rungsardthong, V.; Puncha-arnon, S.; Uttapap, D. Physicochemical and structural properties of debranched waxy rice, waxy corn and waxy potato starches. Food Hydrocoll. 2015, 45, 218–226. [Google Scholar] [CrossRef]
- Schick, C. Differential scanning calorimetry (DSC) of semicrystalline polymers. Anal. Bioanal. Chem. 2009, 395, 1589–1611. [Google Scholar] [CrossRef]
- Sousdaleff, M.; Baesso, M.L.; Neto, A.M.; Nogueira, A.C.; Marcolino, V.A.; Matioli, G. Microencapsulation by freeze-drying of potassium norbixinate and curcumin with maltodextrin: Stability, solubility, and food application. J. Agric. Food Chem. 2013, 61, 955–965. [Google Scholar] [CrossRef]
- Cabrespine-Faugeras, A.; Bayet-Robert, M.; Bay, J.-O.; Chollet, P.; Barthomeuf, C. Possible Benefits of Curcumin Regimen in Combination With Taxane Chemotherapy for Hormone-Refractory Prostate Cancer Treatment. Nutr. Cancer 2010, 62, 148–153. [Google Scholar] [CrossRef] [PubMed]
 ) representing hydrogen bonding.
) representing hydrogen bonding.
 ) representing hydrogen bonding.
) representing hydrogen bonding.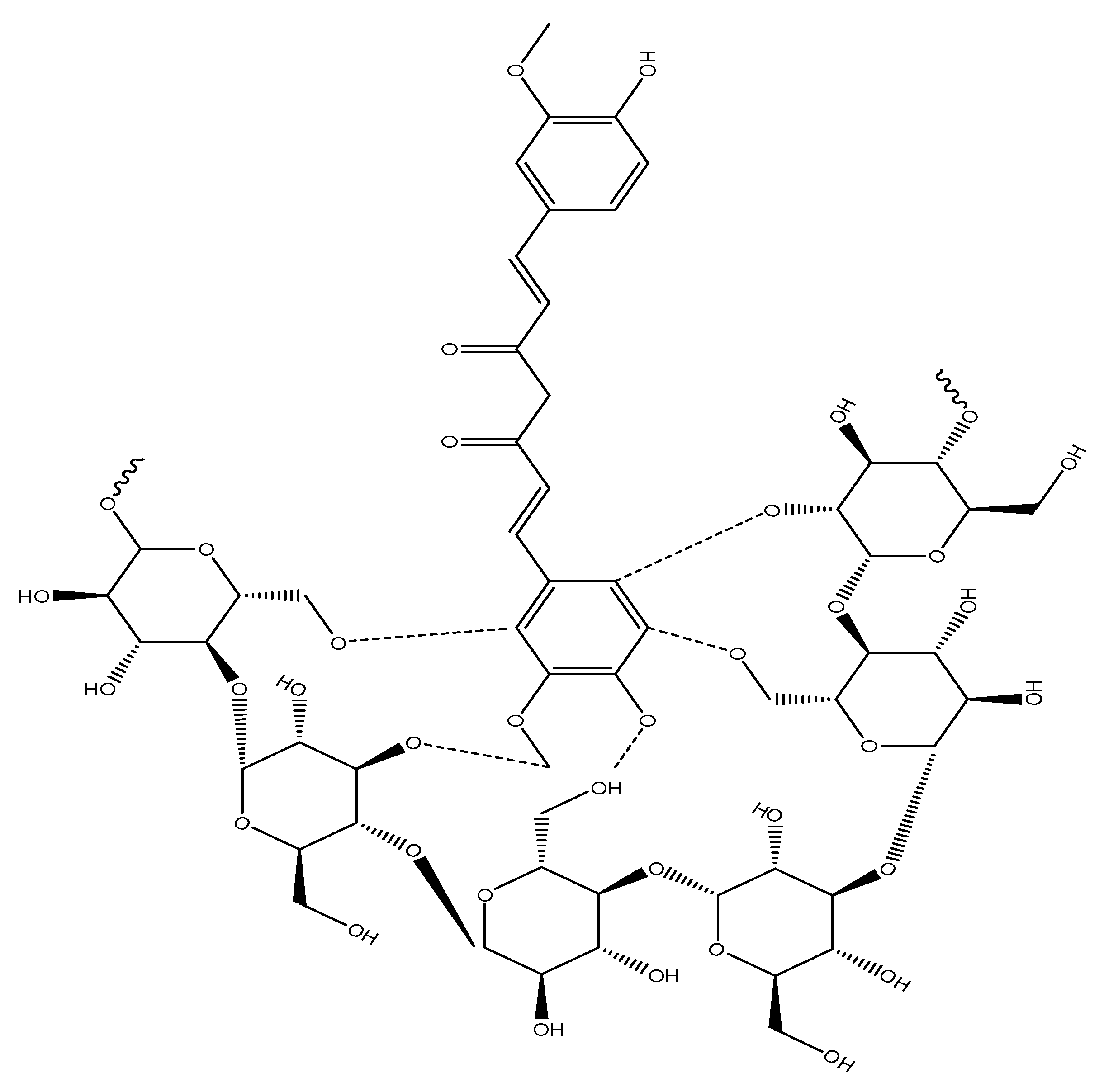



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, H.; Ma, L.; Li, Y.; Fu, J.; Li, Z.; Yu, X.; Gao, Q. Preparation and Characterizations of Curcumin Protection and Delivery System Using Linear Dextrin. Compounds 2022, 2, 353-366. https://doi.org/10.3390/compounds2040029
Xie H, Ma L, Li Y, Fu J, Li Z, Yu X, Gao Q. Preparation and Characterizations of Curcumin Protection and Delivery System Using Linear Dextrin. Compounds. 2022; 2(4):353-366. https://doi.org/10.3390/compounds2040029
Chicago/Turabian StyleXie, Huifang, Litao Ma, Yanan Li, Jun Fu, Zhongxian Li, Xuejun Yu, and Qunyu Gao. 2022. "Preparation and Characterizations of Curcumin Protection and Delivery System Using Linear Dextrin" Compounds 2, no. 4: 353-366. https://doi.org/10.3390/compounds2040029
APA StyleXie, H., Ma, L., Li, Y., Fu, J., Li, Z., Yu, X., & Gao, Q. (2022). Preparation and Characterizations of Curcumin Protection and Delivery System Using Linear Dextrin. Compounds, 2(4), 353-366. https://doi.org/10.3390/compounds2040029





