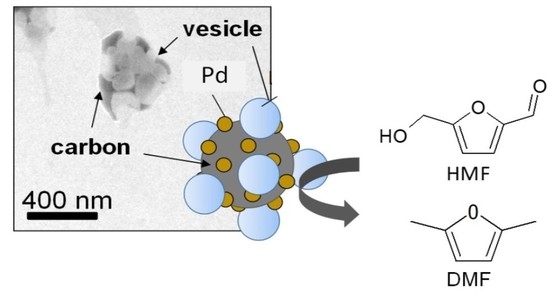
Possible Role of Vesicles on Metallocatalytic Reduction Reaction of 5-Hydroxymethylfurfural to 2,5-Dimethylfuran
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Catalyst
2.3. Preparation of Vesicles
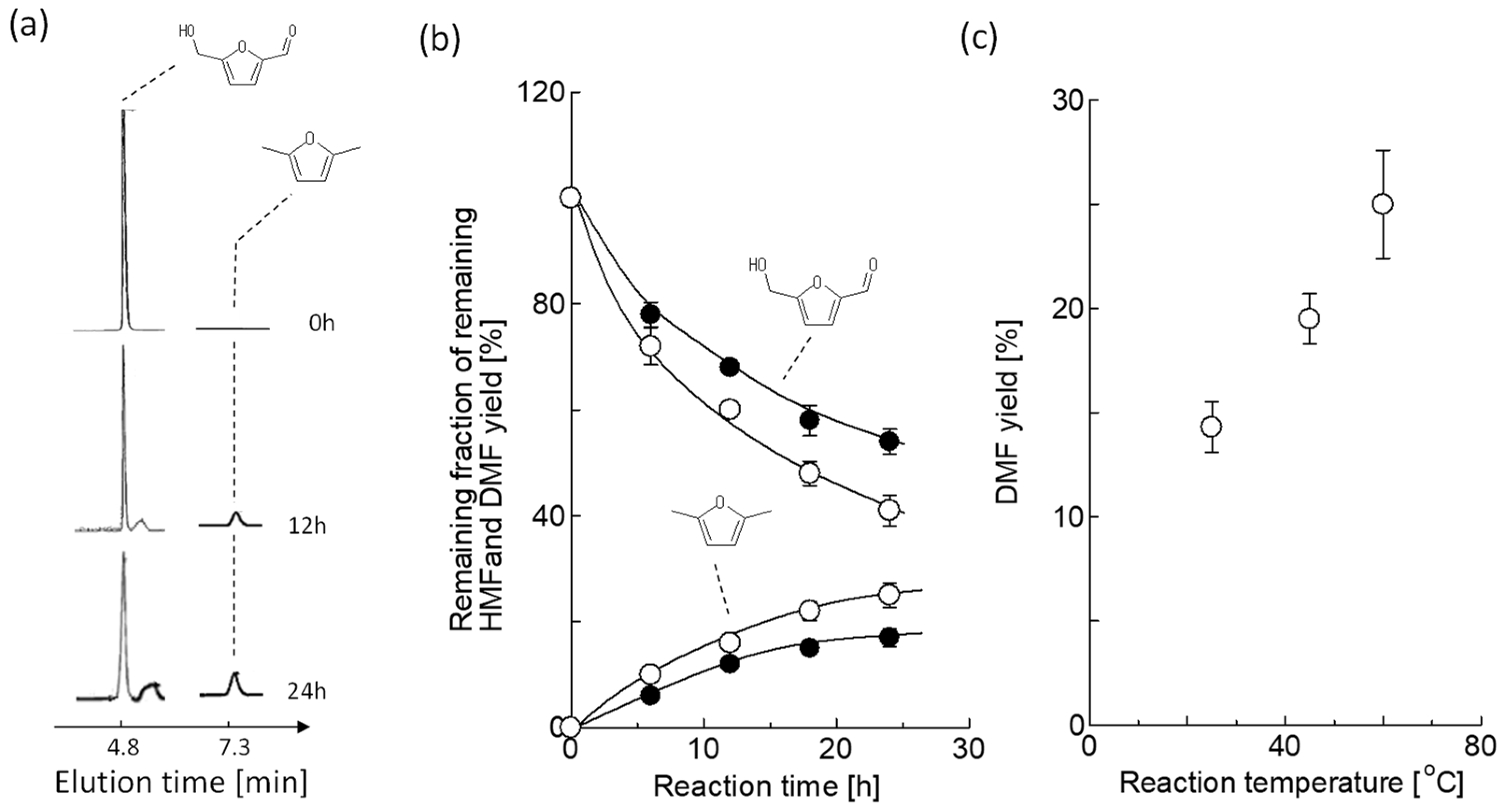
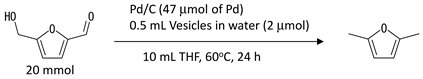
2.4. Synthesis of 2,5-DMF
2.5. Observation of Microscopic Structures
2.6. Calcein Leakage Experiment
2.7. Dielectric Dispersion Analysis
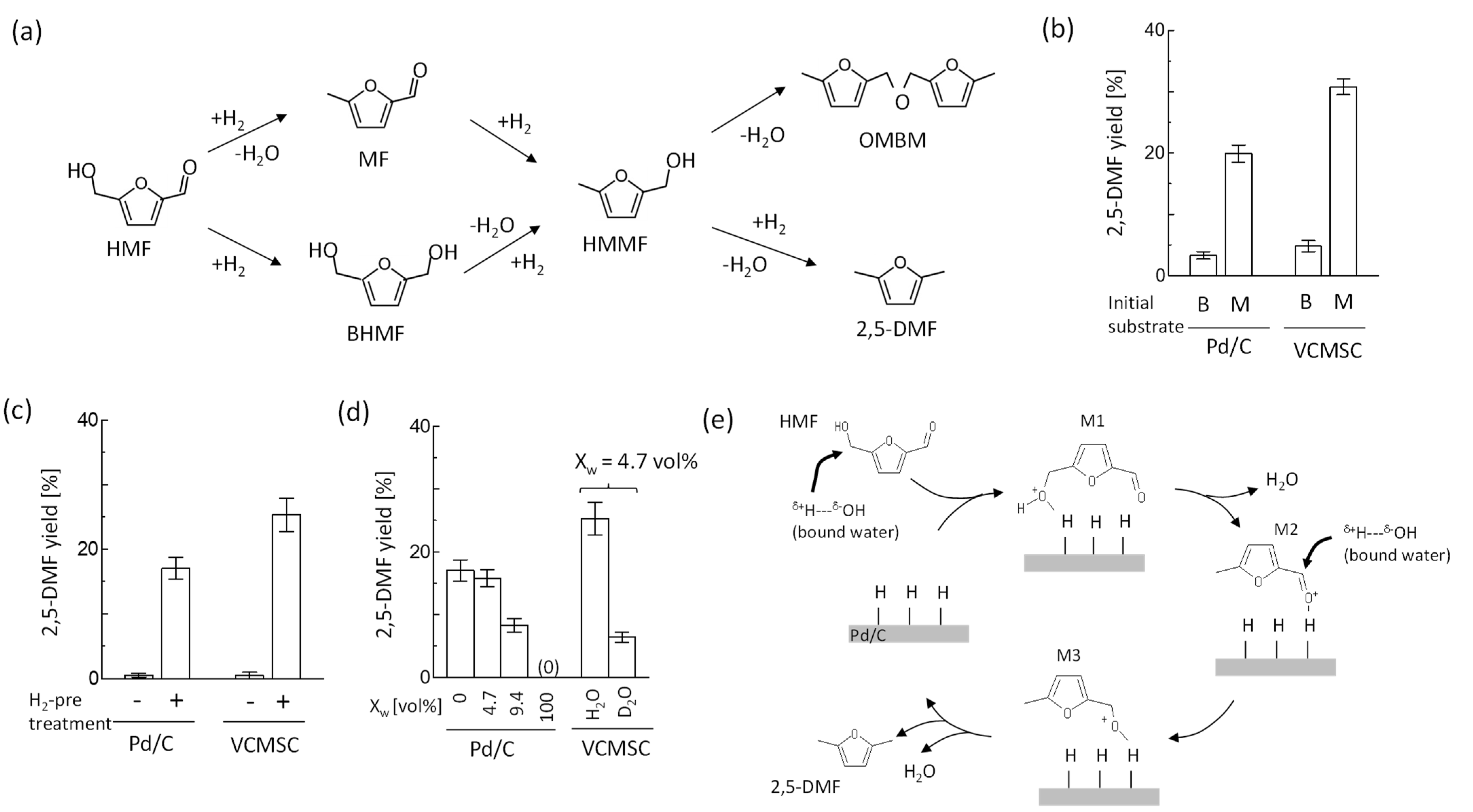
3. Results and Discussion
3.1. Characterization of Metal Catalyst Combined with Vesicles
3.2. 2,5-DMF Synthesis by VCMSCs
3.3. Relationship between Hydration Property of Vesicles and Reactivity
3.4. Possible Reaction Mechanism and Contribution of Vesicles Used in VCMSCs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qian, Y.; Zhu, L.; Wang, Y.; Lu, X. Recent progress in the development of biofuel 2,5-dimethylfuran. Renew. Sustain. Energy Rev. 2015, 41, 633–646. [Google Scholar] [CrossRef]
- Hoang, A.T.; Ölçer, A.I.; Nižetić, S. Prospective review on the application of biofuel 2,5-dimethylfuran to diesel engine. J. Energy Inst. 2021, 94, 360–386. [Google Scholar] [CrossRef]
- Gupta, N.K.; Nishimura, S.; Takagaki, A.; Ebitani, K. Hydrotalcite-supported gold-nanoparticle-catalyzed highly efficient base-free aqueous oxidation of 5-hydroxymethylfurfural into 2,5-furandicarboxylic acid under atmospheric oxygen pressure. Green Chem. 2011, 13, 824–827. [Google Scholar] [CrossRef]
- Lange, J.P.; van der Heide, E.; van Buijtenen, J. Furfural—A Promising Platform for Lignocellulosic Biofuels. ChemSusChem 2012, 5, 150–166. [Google Scholar] [CrossRef]
- Zu, Y.; Yang, P.; Wang, J.; Liu, X.; Ren, J.; Lu, G.; Yang, Y. Efficient production of the liquid fuel 2,5-dimethylfuran from 5-hydroxymethylfurfural over Ru/Co3O4 catalyst. Appl. Cat. B Environ. 2014, 146, 244–248. [Google Scholar] [CrossRef]
- Thananatthanachon, T.; Rauychfuss, T.B. Efficient production of the liquid fuel 2,5-dimethylfuran from fructose using formic acid as a reagent. Angew. Chem. Int. Ed. 2010, 49, 6616–6618. [Google Scholar] [CrossRef]
- Hu, L.; Zhao, G.; Hao, W.; Tang, X.; Sun, Y.; Lin, L.; Liu, S. Catalytic conversion of biomass-derived carbohydrates into fuels and chemicals via furanic aldehydes. RSC Adv. 2012, 2, 11184–11206. [Google Scholar] [CrossRef]
- Chen, S.; Ciotonea, C.; Vigier, K.d.; Jerome, F.; Wojcieszak, R.; Dumeignil, F.; Marceau, E.; Toyer, S. Hydroconversion of 5-hydroxymethylfurfural to 2,5-dimethylfuran and 2,5-dimethyltetrahydrofuran over non-promoted Ni/SBA-15. ChemCatChem 2020, 12, 2050–2059. [Google Scholar] [CrossRef]
- Malley, L.A.; Christoph, G.R.; Stadler, J.C.; Hansen, J.F.; Biesemeier, J.A.; Jasti, S.L. Acute and subchronic neurotoxicological evaluation of tetrahydrofuran by inhalation in rats. Drug Chem. Toxicol. 2001, 24, 201–219. [Google Scholar] [CrossRef]
- Hermida, S.A.; Possari, E.P.; Souza, D.B.; Campos, I.D.; Gomes, O.F.; di Mascio, P.; Medeiros, M.H.; Loureiro, A.P. 20-Deoxyguanosine, 20-deoxycytidine, and 20-deoxyadenosine adducts resulting from the reaction of tetrahydrofuran with DNA bases. Chem. Res. Toxicol. 2006, 19, 927–936. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, C.; Li, D.; Liu, Q.; Tan, J.; Wang, C.; Cai, C.; Ma, L. Recent advances in catalytic conversion of biomass to 5-hydroxymethylfurfural and 2,5-dimethylfuran. Energy Rev. 2019, 103, 227–247. [Google Scholar] [CrossRef]
- Opazo, C.; Huang, X.; Cherny, R.A.; Moir, R.D.; Roher, A.E.; White, A.R.; Cappai, R.; Masters, C.L.; Tanzi, R.E.; Inestosa, N.C.; et al. Metalloenzyme-like activity of Alzheimer’s disease-amyloid. J. Biol. Chem. 2002, 277, 40302–40308. [Google Scholar] [CrossRef] [PubMed]
- Gentry, E.C.; Knowles, R.R. Synthetic applications of proton-coupled electrontransfer. Acc. Chem. Res. 2016, 49, 1546–1556. [Google Scholar] [CrossRef]
- Davis, S.E.; Zope, B.N.; Davis, R.J. On the mechanism of selective oxidation of 5-hydroxymethylfurfural to 2.5-furandicarboxylic acid over supported Pt and Au catalysts. Green Chem. 2012, 14, 143–147. [Google Scholar] [CrossRef]
- Zhang, J.H.; Lin, L.; Liu, S.J. Efficient Production of Furan Derivatives from a Sugar Mixture by Catalytic Process. Energy Fuels 2012, 26, 4560–4567. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Q.; Li, D.; Tan, J.; Zhang, Q.; Wang, C.; Ma, L. Selective hydrodeoxygenation of 5-hydroxymethylfurfural to 2,5-dimethylfuran on Ru–MoOx/C catalysts. RSC Adv. 2017, 7, 16311–16318. [Google Scholar] [CrossRef]
- Shimanouchi, T.; Tasaki, M.; Vu, H.T.; Ishii, H.; Yoshimoto, N.; Umakoshi, H. Aβ/Cu-oxidation of cholesterol on liposome membrane. J. Bioschi. Bioeng. 2009, 109, 145–148. [Google Scholar] [CrossRef]
- Vu, H.T.; Shimanouchi, T.; Ishikawa, D.; Matsumoto, T.; Yagi, H.; Goto, Y.; Umakoshi, H.; Kuboi, R. Effect of liposome membranes on disaggregation of amyloid β fibrils by dopamine. Biochem. Eng. J. 2013, 71, 118–126. [Google Scholar] [CrossRef]
- Nagami, H.; Yoshimoto, N.; Umakoshi, H.; Shimanouchi, T.; Kuboi, R. Liposome-assisted activity of superoxide dismutase under oxidative stress. J. Biosci. Bioeng. 2005, 99, 423–428. [Google Scholar] [CrossRef]
- Bach, D.; Wachtel, E. Phospholipid/cholesterol model membranes: Formation of cholesterol crystallites. Biochim. Biophys. Acta 2003, 1610, 187–197. [Google Scholar] [CrossRef]
- Hayashi, K.; Shimanouchi, T.; Kato, K.; Miyazaki, T.; Nakamura, A.; Umakoshi, H. Fluid, Flexible, and “Wet” Surface of Span80 Vesicle, Compared with Phospholipid Liposomes. Colloids Surf. B 2011, 87, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Iwai, H.; Shimanouchi, T.; Umakoshi, H.; Iwasaki, T.; Kato, A.; Nakamura, H. Formation of Lens-like Vesicles Induced via Microphase Separation on a Sorbitan Monoester Membrane with Different Headgroups. Colloids Surf. B 2015, 135, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Kadomura, Y.; Yamamoto, N.; Tominaga, K. Broadband dielectric spectroscopy from sub GHz to THz of hydrated lipid bilayer of DMPC. Eur. Phys. J. E 2019, 42, 139. [Google Scholar] [CrossRef] [PubMed]
- Penkov, N.V.; Yashin, V.A.; Belosludtsev, K.N. Hydration Shells of DPPC Liposomes from the Point of View of Terahertz Time-Domain Spectroscopy. Appl. Spectrosc. 2021, 75, 189–198. [Google Scholar] [CrossRef]
- Gironi, B.; Lapini, A.; Ragnoni, E.; Calvagna, C.; Paolantoni, M.; Morresi, A.; Sassi, P. Free volume and dynamics in a lipid bilayer. Phys. Chem. Chem. Phys. 2019, 41, 23169–23178. [Google Scholar] [CrossRef]
- Shimanouchi, T.; Kitagawa, Y.; Kimura, Y. Application of liposome membrane as the reaction field: A case study using the Horner–Wadsworth–Emmons reaction. J. Biosci. Bioeng. 2019, 128, 198–202. [Google Scholar] [CrossRef]
- Hirose, M.; Fujiwara, S.; Ishigami, T.; Suga, K.; Okamoto, Y.; Umakoshi, H. Liposome Membranes Assist the l-Proline-catalyzed Aldol Reaction of Acetone and p-Nitrobenzaldehyde in Water. Chem. Lett. 2018, 47, 931–934. [Google Scholar] [CrossRef]
- Chern, M.S.; Watanabe, N.; Suga, K.; Okamoto, Y.; Umakoshi, H. Modulation of the Belousov–Zhabotinsky Reaction with Lipid Bilayers: Effects of Lipid Head Groups and Membrane Properties. Langmuir 2021, 37, 6811–6818. [Google Scholar] [CrossRef]
- Shimanouchi, T.; Sasaki, M.; Hiroiwa, A.; Yoshimoto, N.; Miyagawa, K.; Umakoshi, H.; Kuboi, R. Relationship between the mobility of phosphocholine headgroups of liposomes and the hydrophobicity at the membrane interface: A characterization with spectrophotometric measurements. Colloids Surf. B 2011, 88, 221–230. [Google Scholar] [CrossRef]
- Kato, K.; Walde, P.; Koine, N.; Ichikawa, S.; Ishikawa, T.; Nagahama, R.; Ishihara, T.; Tsujii, T.; Shudou, M.; Omokawa, Y.; et al. Temperature-Sensitive Nonionic Vesicles Prepared from Span 80 (Sorbitan Monooleate). Langmuir 2008, 24, 10762–10770. [Google Scholar] [CrossRef]
- Shimanouchi, T.; Kawasaki, H.; Fuse, M.; Umakoshi, H.; Kuboi, R. Membrane fusion mediated by phospholipase C under endosomal pH conditions. Colloids Surf. B 2013, 103, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Klosgen, B.; Reichle, C.; Kohlsmann, S.; Kramer, K.D. Dielectric spectroscopy as a sensor of membrane headgroup mobility and hydration. Biophys. J. 1996, 71, 3251–3260. [Google Scholar] [CrossRef][Green Version]
- Sun, H.; Fan, L.; Zou, K.; Zhu, H.; Du, J. Decoration of homopolymer vesicles by antibacterial ultrafine silver nanoparticles. RSC Adv. 2014, 4, 41331–41335. [Google Scholar] [CrossRef]
- van den Bogaart, G.; Guzman, J.V.; Mika, J.T.; Poolman, B. On the mechanism of pore formation by melittin. J. Biol. Chem. 2008, 283, 33854–33857. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, D.; Azuma, T.; Noguchi, H.; Uosaki, K.; Takai, M. Role of Interfacial Water in Protein Adsorption onto Polymer Brushes as Studied by SFG Spectroscopy and QCM. J. Phys. Chem. C 2015, 119, 17193–17201. [Google Scholar] [CrossRef]
- Ho, C.; Slater, S.J.; Stubbs, C.D. Hydration and order in lipid bilayers. Biochemistry 1995, 34, 6188–6195. [Google Scholar] [CrossRef]
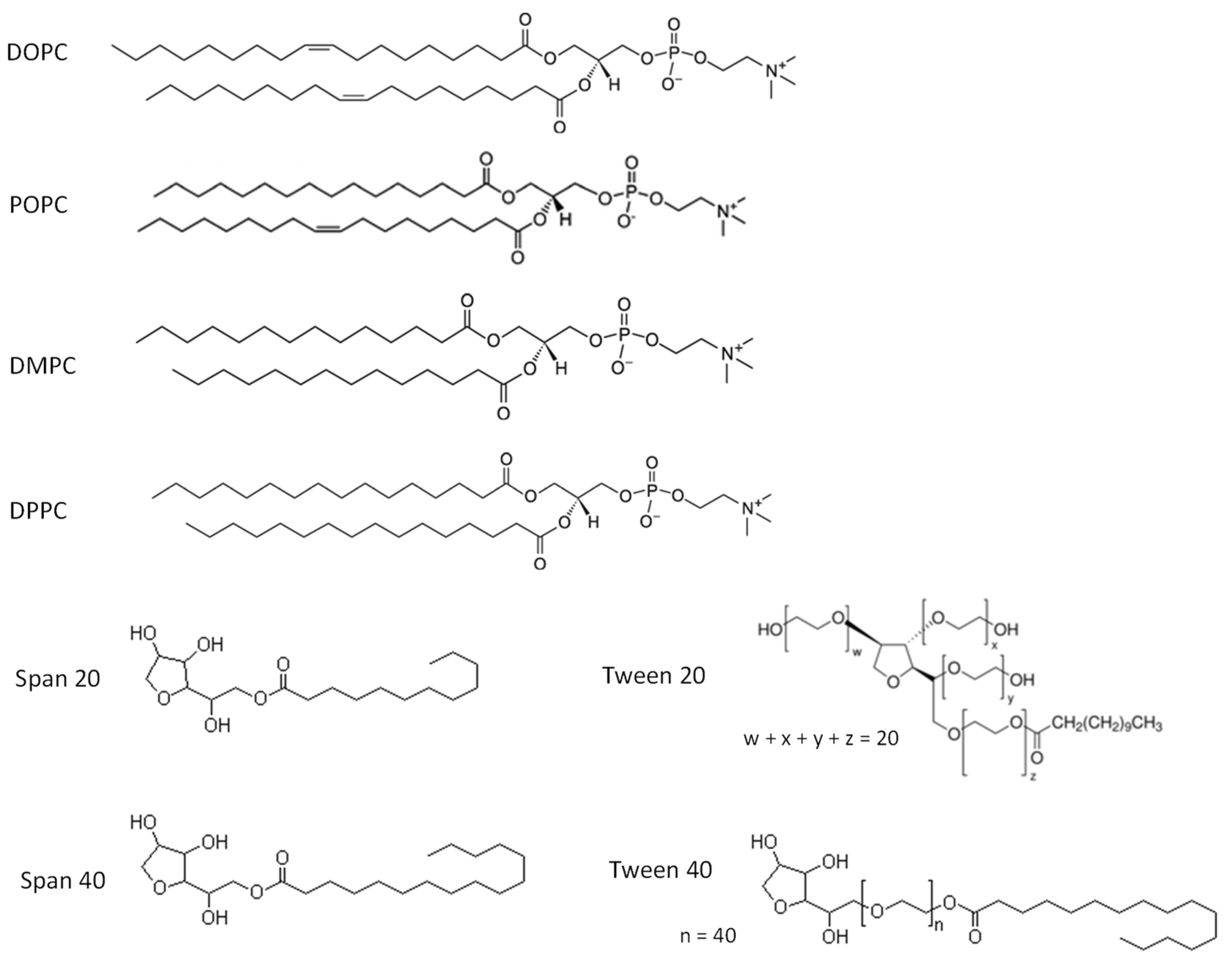

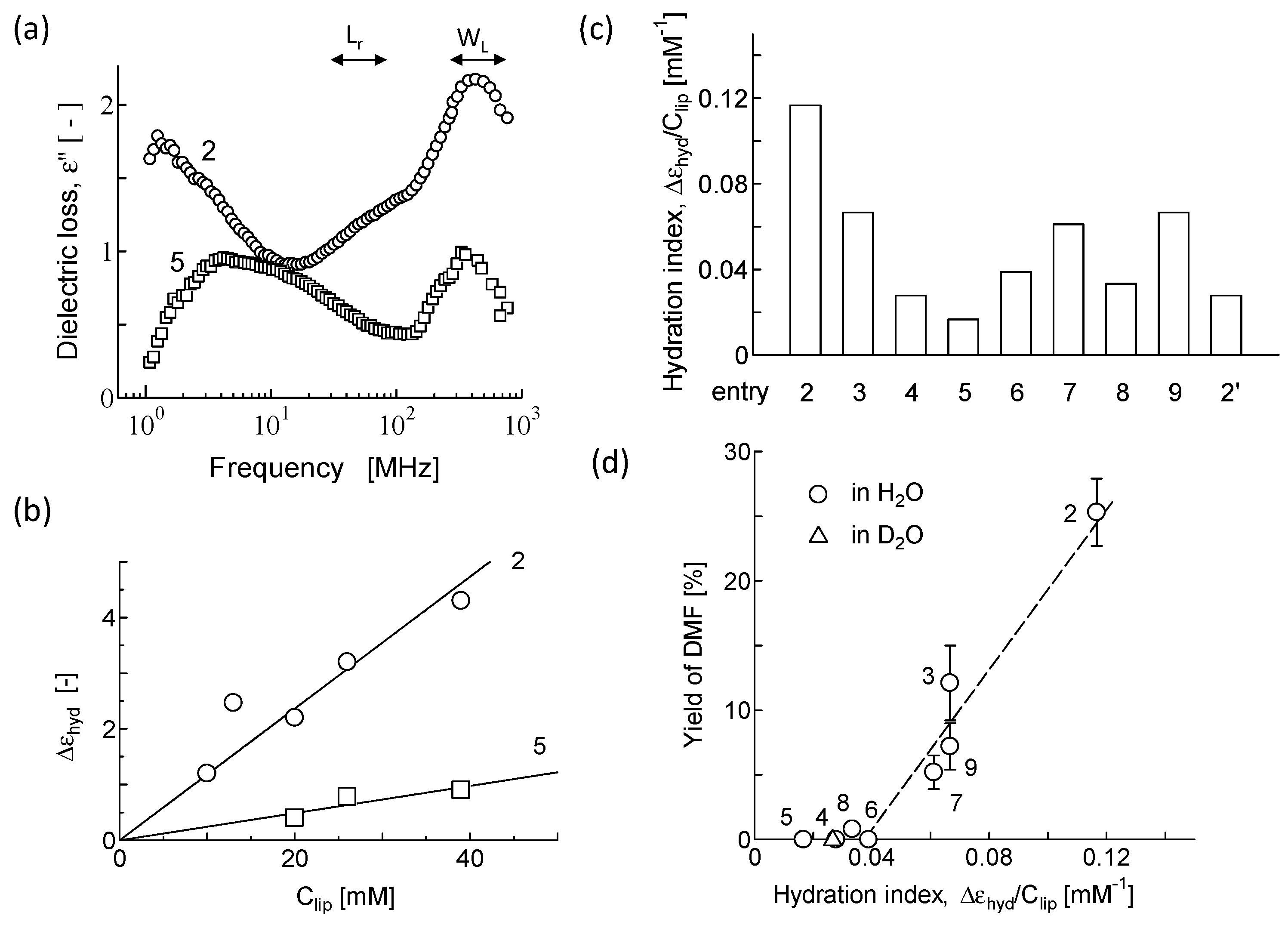
| Entry | Composition * | Water Content [vol%] | HMF Conversion # [%] | 2,5-DMF Yield # [%] |
|---|---|---|---|---|
| 1 | None | 0 | 54.3 ± 2.3 | 17.1 ± 1.7 |
| 2 | DOPC | 4.7 | 41.2 ± 2.9 | 25.3 ± 2.6 |
| 3 | POPC | 4.7 | 55.2 ± 2.5 | 12.1 ± 2.9 |
| 4 | DMPC | 4.7 | 0 | 0 |
| 5 | DPPC | 4.7 | 0 | 0 |
| 6 | Span40/Tween40(50/50) | 4.7 | 55.5 ± 2.6 | 0 |
| 7 | Span40/Tween40(75/25) | 4.7 | 55.5 ± 3.2 | 5.2 ± 1.3 |
| 8 | Span20/Tween20(50/50) | 4.7 | 64.2 ± 2.9 | 0.8 ± 0.2 |
| 9 | Span20/Tween20(75/25) | 4.7 | 61.7 ± 2.2 | 7.2 ± 1.8 |
 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimanouchi, T.; Takahashi, Y.; Hayashi, K.; Yasuhara, K.; Kimura, Y. Possible Role of Vesicles on Metallocatalytic Reduction Reaction of 5-Hydroxymethylfurfural to 2,5-Dimethylfuran. Compounds 2022, 2, 321-333. https://doi.org/10.3390/compounds2040027
Shimanouchi T, Takahashi Y, Hayashi K, Yasuhara K, Kimura Y. Possible Role of Vesicles on Metallocatalytic Reduction Reaction of 5-Hydroxymethylfurfural to 2,5-Dimethylfuran. Compounds. 2022; 2(4):321-333. https://doi.org/10.3390/compounds2040027
Chicago/Turabian StyleShimanouchi, Toshinori, Yuki Takahashi, Keita Hayashi, Kazuma Yasuhara, and Yukitaka Kimura. 2022. "Possible Role of Vesicles on Metallocatalytic Reduction Reaction of 5-Hydroxymethylfurfural to 2,5-Dimethylfuran" Compounds 2, no. 4: 321-333. https://doi.org/10.3390/compounds2040027
APA StyleShimanouchi, T., Takahashi, Y., Hayashi, K., Yasuhara, K., & Kimura, Y. (2022). Possible Role of Vesicles on Metallocatalytic Reduction Reaction of 5-Hydroxymethylfurfural to 2,5-Dimethylfuran. Compounds, 2(4), 321-333. https://doi.org/10.3390/compounds2040027