DNA and BSA Interaction Studies and Antileukemic Evaluation of Polyaromatic Thiosemicarbazones and Their Copper Complexes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthetic Procedures
2.1.1. General Information
2.1.2. Synthesis of the Ligands
2.1.3. Synthesis of the Complexes
2.2. Measurements
3. Results and Discussion
3.1. Synthetic Comments
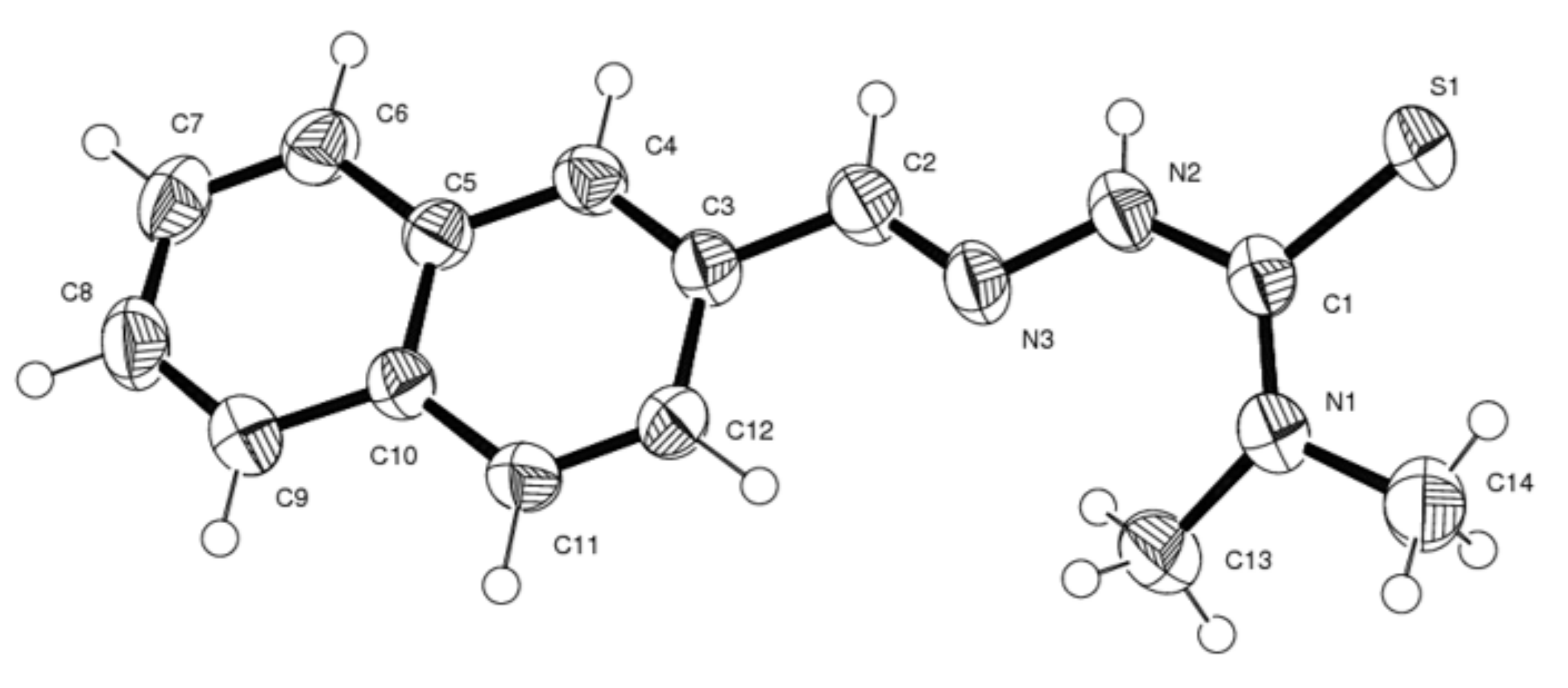
3.2. Description of the Structures
3.3. DNA and BSA Interaction Studies and Cell Viability Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Kelland, L. The Resurgence of Platinum-Based Cancer Chemotherapy. Nat. Rev. Cancer 2007, 7, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Anthony, E.J.; Bolitho, E.M.; Bridgewater, H.E.; Carter, O.W.L.; Donnelly, J.M.; Imberti, C.; Lant, E.C.; Lermyte, F.; Needham, R.J.; Palau, M.; et al. Metallodrugs Are Unique: Opportunities and Challenges of Discovery and Development. Chem. Sci. 2020, 11, 12888–12917. [Google Scholar] [CrossRef] [PubMed]
- Boros, E.; Dyson, P.J.; Gasser, G. Classification of Metal-Based Drugs According to Their Mechanisms of Action. Chem 2020, 6, 41–60. [Google Scholar] [CrossRef]
- More, M.S.; Joshi, P.G.; Mishra, Y.K.; Khanna, P.K. Metal Complexes Driven from Schiff Bases and Semicarbazones for Biomedical and Allied Applications: A Review. Mater. Today Chem. 2019, 14, 100195. [Google Scholar] [CrossRef]
- Denoyer, D.; Clatworthy, S.A.S.; Cater, M.A. Copper Complexes in Cancer Therapy. In Metallo-Drugs: Development and Action of Anticancer Agents; Sigel, A., Sigel, H., Freisinger, E., Sigel, R.K.O., Eds.; De Gruyter: Berlin, Germany; Boston, MA, USA, 2018; Volume 18, pp. 469–506. [Google Scholar]
- Kaim, W.; Rall, J. Copper—A“Modern” Bioelement. Angew. Chem. Int. Edit. 1996, 35, 43–60. [Google Scholar] [CrossRef]
- Pelosi, G. Thiosemicarbazone Metal Complexes: From Structure to Activity. Open Crystallogr. J. 2010, 3, 16–28. [Google Scholar] [CrossRef]
- Kostas, I.D.; Steele, B.R. Thiosemicarbazone Complexes of Transition Metals as Catalysts for Cross-Coupling Reactions. Catalysts 2020, 10, 1107. [Google Scholar] [CrossRef]
- Bisceglie, F.; Pinelli, S.; Alinovi, R.; Tarasconi, P.; Buschini, A.; Mussi, F.; Mutti, A.; Pelosi, G. Copper(II) Thiosemicarbazonate Molecular Modifications Modulate Apoptotic and Oxidative Effects on U937 Cell Line. J. Inorg. Biochem. 2012, 116, 195–203. [Google Scholar] [CrossRef]
- Bisceglie, F.; Tavone, M.; Mussi, F.; Azzoni, S.; Montalbano, S.; Franzoni, S.; Tarasconi, P.; Buschini, A.; Pelosi, G. Effects of Polar Substituents on the Biological Activity of Thiosemicarbazone Metal Complexes. J. Inorg. Biochem. 2018, 179. [Google Scholar] [CrossRef]
- Priyarega, S.; Haribabu, J.; Karvembu, R. Development of Thiosemicarbazone-Based Transition Metal Complexes as Homogeneous Catalysts for Various Organic Transformations. Inorg. Chim. Acta 2022, 532, 120742. [Google Scholar] [CrossRef]
- Egorova, A.; Jackson, M.; Gavrilyuk, V.; Makarov, V. Pipeline of Anti-Mycobacterium Abscessus Small Molecules: Repurposable Drugs and Promising Novel Chemical Entities. Med. Res. Rev. 2021, 41, 2350–2387. [Google Scholar] [CrossRef]
- Bisceglie, F.; Bacci, C.; Vismarra, A.; Barilli, E.; Pioli, M.; Orsoni, N.; Pelosi, G. Antibacterial Activity of Metal Complexes Based on Cinnamaldehyde Thiosemicarbazone Analogues. J. Inorg. Biochem. 2020, 203, 110888. [Google Scholar] [CrossRef]
- Bajaj, K.; Buchanan, R.M.; Grapperhaus, C.A. Antifungal Activity of Thiosemicarbazones, Bis(Thiosemicarbazones), and Their Metal Complexes. J. Inorg. Biochem. 2021, 225, 111620. [Google Scholar] [CrossRef]
- Lin, Y.; Betts, H.; Keller, S.; Cariou, K.; Gasser, G. Recent Developments of Metal-Based Compounds against Fungal Pathogens. Chem. Soc. Rev. 2021, 50, 10346–10402. [Google Scholar] [CrossRef]
- Zani, C.; Bisceglie, F.; Restivo, F.M.; Feretti, D.; Pioli, M.; Degola, F.; Montalbano, S.; Galati, S.; Pelosi, G.; Viola, G.V.C.; et al. A Battery of Assays as an Integrated Approach to Evaluate Fungal and Mycotoxin Inhibition Properties and Cytotoxic/Genotoxic Side-Effects for the Prioritization in the Screening of Thiosemicarbazone Derivatives. Food Chem. Toxicol. 2017, 105, 498–505. [Google Scholar] [CrossRef]
- Degola, F.; Bisceglie, F.; Pioli, M.; Palmano, S.; Elviri, L.; Pelosi, G.; Lodi, T.; Restivo, F.M. Structural Modification of Cuminaldehyde Thiosemicarbazone Increases Inhibition Specificity toward Aflatoxin Biosynthesis and Sclerotia Development in Aspergillus Flavus. Appl. Microbiol. Biotechnol. 2017, 101, 6683–6696. [Google Scholar] [CrossRef]
- Degola, F.; Morcia, C.; Bisceglie, F.; Mussi, F.; Tumino, G.; Ghizzoni, R.; Pelosi, G.; Terzi, V.; Buschini, A.; Restivo, F.M.; et al. In Vitro Evaluation of the Activity of Thiosemicarbazone Derivatives against Mycotoxigenic Fungi Affecting Cereals. Int. J. Food Microbiol. 2015, 200, 104–111. [Google Scholar] [CrossRef]
- Bartoli, J.; Montalbano, S.; Spadola, G.; Rogolino, D.; Pelosi, G.; Bisceglie, F.; Restivo, F.M.; Degola, F.; Serra, O.; Buschini, A.; et al. Antiaflatoxigenic Thiosemicarbazones as Crop-Protective Agents: A Cytotoxic and Genotoxic Study. J. Agric. Food Chem. 2019, 67, 10947–10953. [Google Scholar] [CrossRef]
- Dallabona, C.; Pioli, M.; Spadola, G.; Orsoni, N.; Bisceglie, F.; Lodi, T.; Pelosi, G.; Restivo, F.M.; Degola, F. Sabotage at the Powerhouse? Unraveling the Molecular Target of 2-Isopropylbenzaldehyde Thiosemicarbazone, a Specific Inhibitor of Aflatoxin Biosynthesis and Sclerotia Development in Aspergillus Flavus, Using Yeast as a Model System. Molecules 2019, 24, 2971. [Google Scholar] [CrossRef] [Green Version]
- Rogolino, D.; Gatti, A.; Carcelli, M.; Pelosi, G.; Bisceglie, F.; Restivo, F.M.; Degola, F.; Buschini, A.; Montalbano, S.; Feretti, D.; et al. Thiosemicarbazone Scaffold for the Design of Antifungal and Antiaflatoxigenic Agents: Evaluation of Ligands and Related Copper Complexes. Sci. Rep. 2017, 7, 11214. [Google Scholar] [CrossRef]
- Gupta, O.; Pradhan, T.; Bhatia, R.; Monga, V. Recent Advancements in Anti-Leishmanial Research: Synthetic Strategies and Structural Activity Relationships. Eur. J. Med. Chem. 2021, 223, 113606. [Google Scholar] [CrossRef]
- Moharana, A.K.; Dash, R.N.; Subudhi, B.B. Thiosemicarbazides: Updates on Antivirals Strategy. Mini-Rev. Med. Chem. 2021, 20, 2135–2152. [Google Scholar] [CrossRef]
- Pelosi, G.; Bisceglie, F.; Bignami, F.; Ronzi, P.; Schiavone, P.; Re, M.C.; Casoli, C.; Pilotti, E. Antiretroviral Activity of Thiosemicarbazone Metal Complexes. J. Med. Chem. 2010, 53, 8765–8769. [Google Scholar] [CrossRef]
- Shakya, B.; Yadav, P.N. Thiosemicarbazones as Potent Anticancer Agents and Their Modes of Action. Mini-Rev. Med. Chem. 2020, 20, 638–661. [Google Scholar] [CrossRef]
- Singh, N.K.; Kumbhar, A.A.; Pokharel, Y.R.; Yadav, P.N. Anticancer Potency of Copper(II) Complexes of Thiosemicarbazones. J. Inorg. Biochem. 2020, 210, 111134. [Google Scholar] [CrossRef]
- Chekmarev, J.; Azad, M.G.; Richardson, D.R. The Oncogenic Signaling Disruptor, NDRG1: Molecular and Cellular Mechanisms of Activity. Cells 2021, 10, 2382. [Google Scholar] [CrossRef]
- Babak, M.V.; Ahn, D. Modulation of Intracellular Copper Levels as the Mechanism of Action of Anticancer Copper Complexes: Clinical Relevance. Biomedicines 2021, 9, 852. [Google Scholar] [CrossRef]
- Wijesinghe, T.P.; Dharmasivam, M.; Dai, C.C.; Richardson, D.R. Innovative Therapies for Neuroblastoma: The Surprisingly Potent Role of Iron Chelation in up-Regulating Metastasis and Tumor Suppressors and down-Regulating the Key Oncogene, N-Myc. Pharmacol. Res. 2021, 173, 105889. [Google Scholar] [CrossRef]
- Bisceglie, F.; Orsoni, N.; Pioli, M.; Bonati, B.; Tarasconi, P.; Rivetti, C.; Amidani, D.; Montalbano, S.; Buschini, A.; Pelosi, G. Cytotoxic Activity of Copper(Ii), Nickel(Ii) and Platinum(Ii) Thiosemicarbazone Derivatives: Interaction with DNA and the H2A Histone Peptide. Metallomics 2019, 11, 1729–1742. [Google Scholar] [CrossRef]
- Baruffini, E.; Ruotolo, R.; Bisceglie, F.; Montalbano, S.; Ottonello, S.; Pelosi, G.; Buschini, A.; Lodi, T. Mechanistic Insights on the Mode of Action of an Antiproliferative Thiosemicarbazone-Nickel Complex Revealed by an Integrated Chemogenomic Profiling Study. Sci. Rep. 2020, 10, 10524. [Google Scholar] [CrossRef] [PubMed]
- Baldini, M.; Belicchi-Ferrari, M.; Bisceglie, F.; Capacchi, S.; Pelosi, G.; Tarasconi, P. Zinc Complexes with Cyclic Derivatives of α-Ketoglutaric Acid Thiosemicarbazone: Synthesis, X-ray Structures and DNA Interactions. J. Inorg. Biochem. 2005, 99, 1504–1513. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, A.; Shimer, G.H.; Meehan, T. Polycyclic Aromatic Hydrocarbons Physically Intercalate into Duplex Regions of Denatured DNA. Biochemistry 1987, 26, 6392–6396. [Google Scholar] [CrossRef] [PubMed]
- Kalaivani, P.; Prabhakaran, R.; Poornima, P.; Huang, R.; Hornebecq, V.; Dallemer, F.; Vijaya Padma, V.; Natarajan, K. Synthesis and Structural Characterization of New Ruthenium(Ii) Complexes and Investigation of Their Antiproliferative and Metastatic Effect against Human Lung Cancer (A549) Cells. RSC Adv. 2013, 3, 20363. [Google Scholar] [CrossRef]
- Hernández, W.; Paz, J.; Carrasco, F.; Vaisberg, A.; Spodine, E.; Manzur, J.; Hennig, L.; Sieler, J.; Blaurock, S.; Beyer, L. Synthesis and Characterization of New Palladium(II) Thiosemicarbazone Complexes and Their Cytotoxic Activity against Various Human Tumor Cell Lines. Bioinorg. Chem. Appl. 2013, 2013, 524701. [Google Scholar] [CrossRef] [Green Version]
- Prabhakaran, R.; Kalaivani, P.; Huang, R.; Poornima, P.; Vijaya Padma, V.; Dallemer, F.; Natarajan, K. DNA Binding, Antioxidant, Cytotoxicity (MTT, Lactate Dehydrogenase, NO), and Cellular Uptake Studies of Structurally Different Nickel(II) Thiosemicarbazone Complexes: Synthesis, Spectroscopy, Electrochemistry, and X-ray Crystallography. J. Biol. Inorg. Chem. 2013, 18, 233–247. [Google Scholar] [CrossRef]
- Saswati, S.; Chakraborty, A.; Dash, S.P.; Panda, A.K.; Acharyya, R.; Biswas, A.; Mukhopadhyay, S.; Bhutia, S.K.; Crochet, A.; Patil, Y.P.; et al. Synthesis, X-ray Structure and in Vitro Cytotoxicity Studies of Cu(I/II) Complexes of Thiosemicarbazone: Special Emphasis on Their Interactions with DNA. Dalton Trans. 2015, 44, 6140–6157. [Google Scholar] [CrossRef] [Green Version]
- Qi, J.; Gou, Y.; Zhang, Y.; Yang, K.; Chen, S.; Liu, L.; Wu, X.; Wang, T.; Zhang, W.; Yang, F. Developing Anticancer Ferric Prodrugs Based on the N-Donor Residues of Human Serum Albumin Carrier IIA Subdomain. J. Med. Chem. 2016, 59, 7497–7511. [Google Scholar] [CrossRef]
- Subhashree, G.R.; Haribabu, J.; Saranya, S.; Yuvaraj, P.; Anantha Krishnan, D.; Karvembu, R.; Gayathri, D. In Vitro Antioxidant, Antiinflammatory and in Silico Molecular Docking Studies of Thiosemicarbazones. J. Mol. Struct. 2017, 1145, 160–169. [Google Scholar] [CrossRef]
- Bai, Y.-L.; Zhang, Y.-W.; Xiao, J.-Y.; Guo, H.-W.; Liao, X.-W.; Li, W.-J.; Zhang, Y.-C. Oxovanadium Phenanthroimidazole Derivatives: Synthesis, DNA Binding and Antitumor Activities. Trans. Met. Chem. 2018, 43, 171–183. [Google Scholar] [CrossRef]
- Rajendran, N.; Periyasamy, A.; Kamatchi, N.; Solomon, V. Biological Evaluation of Copper(II) Complexes on N (4)−substituted Thiosemicarbazide Derivatives and Diimine Co-Ligands Using DNA Interaction, Antibacterial and in Vitro Cytotoxicity. J. Coord. Chem. 2019, 72, 1937–1956. [Google Scholar] [CrossRef]
- Lewis, N.A.; Liu, F.; Seymour, L.; Magnusen, A.; Erves, T.R.; Arca, J.F.; Beckford, F.A.; Venkatraman, R.; González-Sarrías, A.; Fronczek, F.R.; et al. Synthesis, Characterisation, and Preliminary In Vitro Studies of Vanadium(IV) Complexes with a Schiff Base and Thiosemicarbazones as Mixed Ligands. Eur. J. Inorg. Chem. 2012, 2012, 664–677. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.; Paul, K.; Singh, I.; Jasinski, J.P.; Smolenski, V.A.; Hotchkiss, E.P.; Kelley, P.T.; Shalit, Z.A.; Kaur, M.; Banerjee, S.; et al. Copper(I) and Silver(I) Complexes of Anthraldehyde Thiosemicarbazone: Synthesis, Structure Elucidation, in Vitro Anti-Tuberculosis/Cytotoxic Activity and Interactions with DNA/HSA. Dalton Trans. 2020, 49, 17350–17367. [Google Scholar] [CrossRef]
- Beckford, F.A.; Leblanc, G.; Thessing, J.; Shaloski, M.; Frost, B.J.; Li, L.; Seeram, N.P. Organometallic Ruthenium Complexes with Thiosemicarbazone Ligands: Synthesis, Structure and Cytotoxicity of [(H6-p-Cymene)Ru(NS)Cl]+ (NS = 9-Anthraldehyde Thiosemicarbazones). Inorg. Chem. Comm. 2009, 12, 1094–1098. [Google Scholar] [CrossRef] [Green Version]
- Beckford, F.A. Reaction of the Anticancer Organometallic Ruthenium Compound, [(η6-p-Cymene)Ru(ATSC)Cl]PF6 with Human Serum Albumin. Int. J. Inorg. Chem. 2010, 2010, 975756. [Google Scholar] [CrossRef] [Green Version]
- Beckford, F.A.; Brock, A.; Gonzalez-Sarrías, A.; Seeram, N.P. Cytotoxic Gallium Complexes Containing Thiosemicarbazones Derived from 9-Anthraldehyde: Molecular Docking with Biomolecules. J. Mol. Struct. 2016, 1121, 156–166. [Google Scholar] [CrossRef] [Green Version]
- Beckford, F.A.; Shaloski, M., Jr.; Leblanc, G.; Thessing, J.; Lewis-Alleyne, L.C.; Holder, A.A.; Li, L.; Seeram, N.P. Microwave Synthesis of Mixed Ligand Diimine–Thiosemicarbazone Complexes of Ruthenium(Ii): Biophysical Reactivity and Cytotoxicity. Dalton Trans. 2009, 48, 10757. [Google Scholar] [CrossRef] [Green Version]
- Beebe, S.J.; Celestine, M.J.; Bullock, J.L.; Sandhaus, S.; Arca, J.F.; Cropek, D.M.; Ludvig, T.A.; Foster, S.R.; Clark, J.S.; Beckford, F.A.; et al. Synthesis, Characterization, DNA Binding, Topoisomerase Inhibition, and Apoptosis Induction Studies of a Novel Cobalt(III) Complex with a Thiosemicarbazone Ligand. J. Inorg. Biochem. 2020, 203, 110907. [Google Scholar] [CrossRef]
- Bruker SAINT; Bruker AXS Inc.: Madison, WI, USA, 2012.
- Sheldrick, G.M. SADABS—Bruker Nonius Area Detector Scaling and Aabsorption Correction—V2016/2; Bruker AXS Inc.: Madison, WI, USA, 2016. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. SHELXL-97—Programs for Crystal Structure Analysis (Release 97-2); Institut fur Anorganishe Chemie der Universitat: Goettingen, Germany, 1998. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea RJ, J.; Howard, A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Johnson, C.K.; Burnett, M.N. ORTEPIII. Report ORNL-6895; Oak Ridge National Laboratory: Oak Ridges, TN, USA, 1996.
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.G.; Shields, P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef] [Green Version]
- Reichmann, M.E.; Rice, S.A.; Thomas, C.A.; Doty, P. A Further Examination of the Molecular Weight and Size of Desoxypentose Nucleic Acid. J. Am. Chem. Soc. 1954, 76, 3047–3053. [Google Scholar] [CrossRef]
- Kalsbeck, W.A.; Thorp, H.H. Determining Binding Constants of Metal Complexes to DNA by Quenching of the Emission of Pt2(pop)44− (pop= P2O5H22−). J. Am. Chem. Soc. 1993, 115, 7146–7151. [Google Scholar] [CrossRef]
- Baldini, M.; Belicchi-Ferrari, M.; Bisceglie, F.; Pelosi, G.; Pinelli, S.; Tarasconi, P. Cu(II) Complexes with Heterocyclic Substituted Thiosemicarbazones: The Case of 5-Formyluracil. Synthesis, Characterization, X-ray Structures, DNA Interaction Studies, and Biological Activity. Inorg. Chem. 2003, 42, 2049–2055. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, T.; Lu, T.; Qu, L.; Zhou, H.; Zhang, Q.; Ji, L. DNA-Binding and Cleavage Studies of Macrocyclic Copper(II) Complexes. J. Inorg. Biohem. 2002, 91, 269–276. [Google Scholar] [CrossRef]
- Mudasir; Yoshioka, N.; Inoue, H. DNA Binding of Iron(II) Mixed-Ligand Complexes Containing 1,10-Phenanthroline and 4,7-Diphenyl-1,10-Phenanthroline. J. Inorg. Biohem. 1999, 77, 239–247. [Google Scholar] [CrossRef]
- Beckford, F.; Dourth, D.; Shaloski, M.; Didion, J.; Thessing, J.; Woods, J.; Crowell, V.; Gerasimchuk, N.; Gonzalez-Sarrías, A.; Seeram, N.P. Half-Sandwich Ruthenium–Arene Complexes with Thiosemicarbazones: Synthesis and Biological Evaluation of [(H6-p-Cymene)Ru(Piperonal Thiosemicarbazones)Cl]Cl Complexes. J. Inorg. Biohem. 2011, 105, 1019–1029. [Google Scholar] [CrossRef] [Green Version]
- Hassan, A.A.; Shawky, A.M.; Shehatta, H.S. Chemistry and Heterocyclization of Thiosemicarbazones. J. Heterocycl. Chem. 2012, 49, 21–37. [Google Scholar] [CrossRef]
- Shaban, M.A.E.; Mostafa, M.A.; Nasr, A.Z. Oxidative Cyclization of D-Fructose Thiosemicarbazones to 2-Amino-5- (d-Arabino-1,2,3,4-Tetrahydroxybut-1-Yl)-1,3,4-Thiadiazoles through Carboncarbon Bond Cleavage of the Sugar Chain. Pharmazie 2003, 58, 367–371. [Google Scholar]
- Buscemi, S.; Gruttadauria, M. Photocyclization Reaction of Some 2-Methyl-4-Phenyl- Substituted Aldehyde Thiosemicarbazones. Mechanistic Aspects. Tetrahedron 2000, 56, 999–1004. [Google Scholar] [CrossRef]
- Meo, P.L.; Gruttadauria, M.; Noto, R. Oxidative Cyclization of Aldehyde Thiosemicarbazones Induced by Potassium Ferricyanide and by Tris(p-Bromophenyl)Amino Hexachloroantimoniate. A Joint Experimental and Computational Study. Arkivoc 2005, 2005, 114–129. [Google Scholar] [CrossRef] [Green Version]
- Noto, R.; Buccheri, F.; Cusmano, G.; Gruttadauria, M.; Werber, G. Substituent Effect on Oxidative Cyclization of Aldehyde Thiosemicarbazones with Ferric Chloride. J. Heterocycl. Chem. 1991, 28, 1421–1427. [Google Scholar] [CrossRef]
- Ivanov, V.I.; Minchenkova, L.E.; Schyolkina, A.K.; Poletayev, A.I. Different Conformations of Double-Stranded Nucleic Acid in Solution as Revealed by Circular Dichroism. Biopolymers 1973, 12, 89–110. [Google Scholar] [CrossRef] [PubMed]
- Nordén, B.; Tjerneld, F. Structure of Methylene Blue-DNA Complexes Studied by Linear and Circular Dichroism Spectroscopy. Biopolymers 1982, 21, 1713–1734. [Google Scholar] [CrossRef]
- Lepecq, J.-B.; Paoletti, C. A Fluorescent Complex between Ethidium Bromide and Nucleic Acids. J. Mol. Biol. 1967, 27, 87–106. [Google Scholar] [CrossRef]
- Song, Y.; Zhong, D.; Luo, J.; Tan, H.; Chen, S.; Li, P.; Wang, L.; Wang, T. Binding Characteristics and Interactive Region of 2-Phenylpyrazolo[1,5-c]Quinazoline with DNA. Luminescence 2014, 29, 1141–1147. [Google Scholar] [CrossRef]
- Sahoo, D.K.; Jena, S.; Dutta, J.; Chakrabarty, S.; Biswal, H.S. Critical Assessment of the Interaction between DNA and Choline Amino Acid Ionic Liquids: Evidences of Multimodal Binding and Stability Enhancement. ACS Cent. Sci. 2018, 4, 1642–1651. [Google Scholar] [CrossRef]
- Byler, D.M.; Susi, H. Examination of the Secondary Structure of Proteins by Deconvolved FTIR Spectra. Biopolymers 1986, 25, 469–487. [Google Scholar] [CrossRef]
- Minafra, L.; di Cara, G.; Albanese, N.N.; Cancemi, P. Proteomic Differentiation Pattern in the U937 Cell Line. Leukemia Res. 2011, 35, 226–236. [Google Scholar] [CrossRef]
- Akiyama, M.; Horiguchi-Yamada, J.; Saito, S.; Hoshi, Y.; Yamada, O.; Mizoguchi, H.; Yamada, H. Cytostatic Concentrations of Anticancer Agents Do Not Affect Telomerase Activity of Leukaemic Cells in Vitro. Eur. J. Cancer 1999, 35, 309–315. [Google Scholar] [CrossRef]












Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelosi, G.; Pinelli, S.; Bisceglie, F. DNA and BSA Interaction Studies and Antileukemic Evaluation of Polyaromatic Thiosemicarbazones and Their Copper Complexes. Compounds 2022, 2, 144-162. https://doi.org/10.3390/compounds2020011
Pelosi G, Pinelli S, Bisceglie F. DNA and BSA Interaction Studies and Antileukemic Evaluation of Polyaromatic Thiosemicarbazones and Their Copper Complexes. Compounds. 2022; 2(2):144-162. https://doi.org/10.3390/compounds2020011
Chicago/Turabian StylePelosi, Giorgio, Silvana Pinelli, and Franco Bisceglie. 2022. "DNA and BSA Interaction Studies and Antileukemic Evaluation of Polyaromatic Thiosemicarbazones and Their Copper Complexes" Compounds 2, no. 2: 144-162. https://doi.org/10.3390/compounds2020011
APA StylePelosi, G., Pinelli, S., & Bisceglie, F. (2022). DNA and BSA Interaction Studies and Antileukemic Evaluation of Polyaromatic Thiosemicarbazones and Their Copper Complexes. Compounds, 2(2), 144-162. https://doi.org/10.3390/compounds2020011







