Humic Acids Aggregates as Microheterogeneous Reaction Media: Alkaline Hydrolysis Reactions
Abstract
:1. Introduction
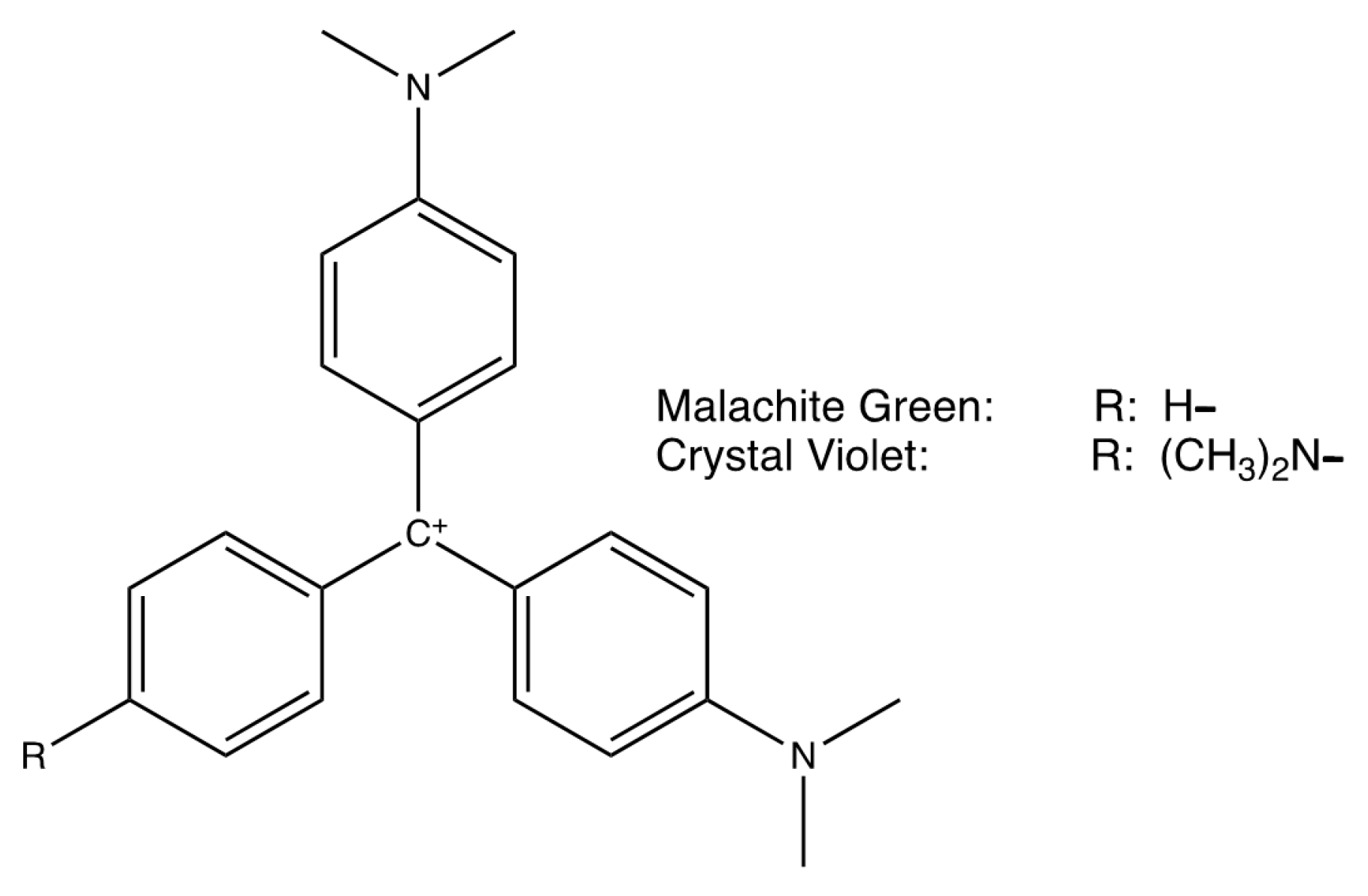
2. Hydrolysis of Carbocations
3. Hydrolysis of N-Methyl-N-nitroso-p-toluene Sulfonamide
4. Hydrolysis of Carbofuran and Derivatives, Iprodione, and Vinclozolin
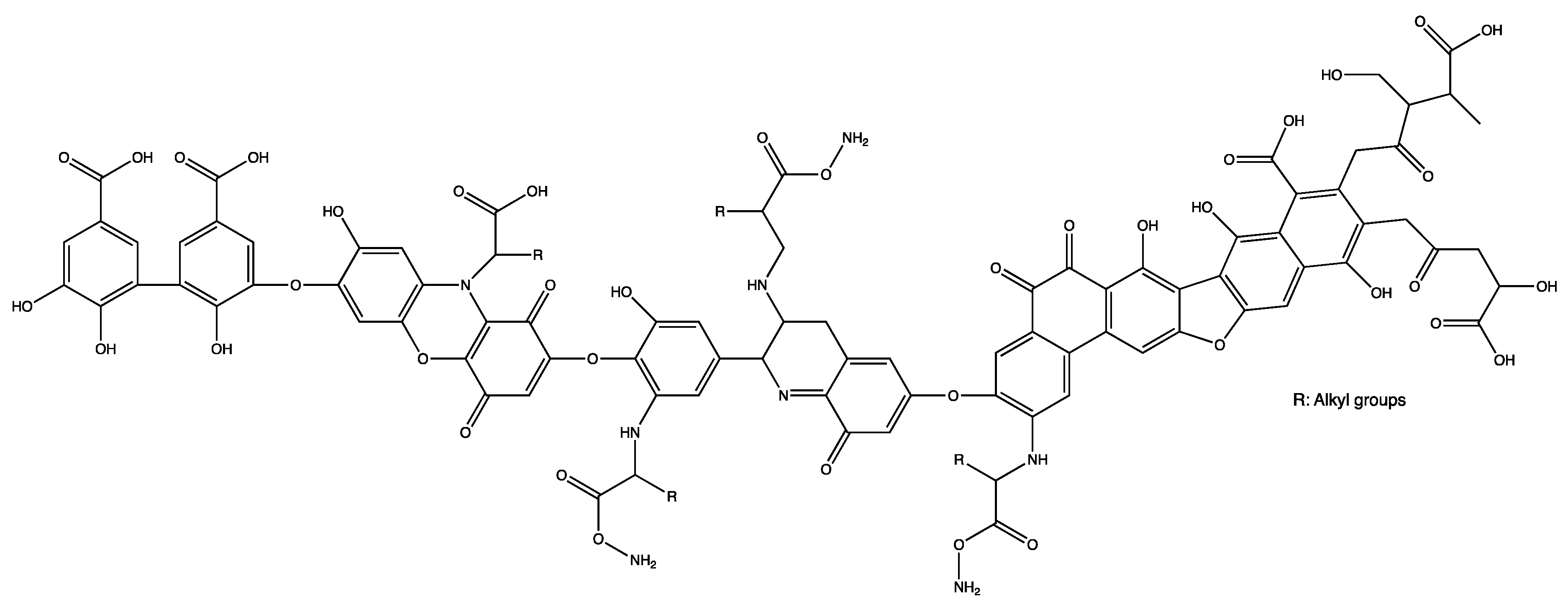
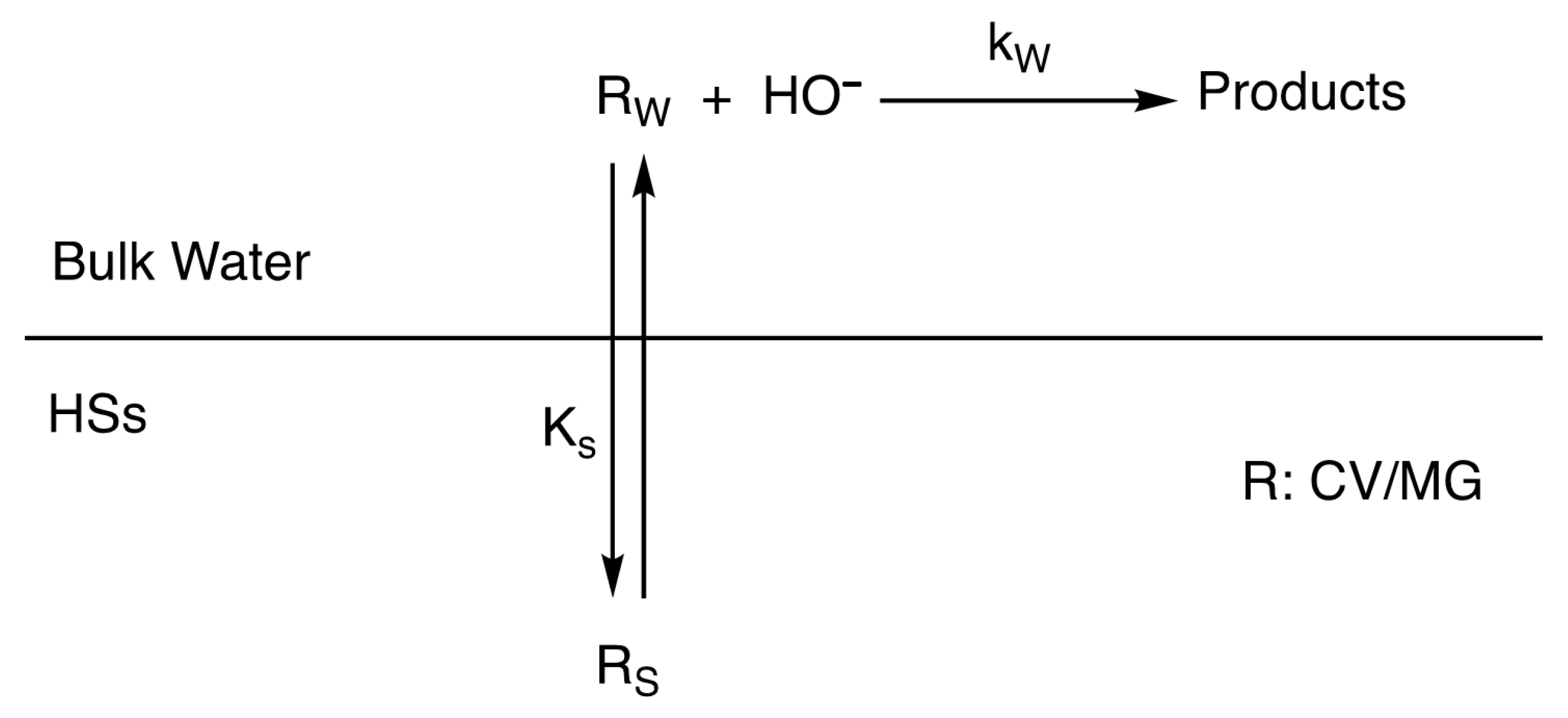
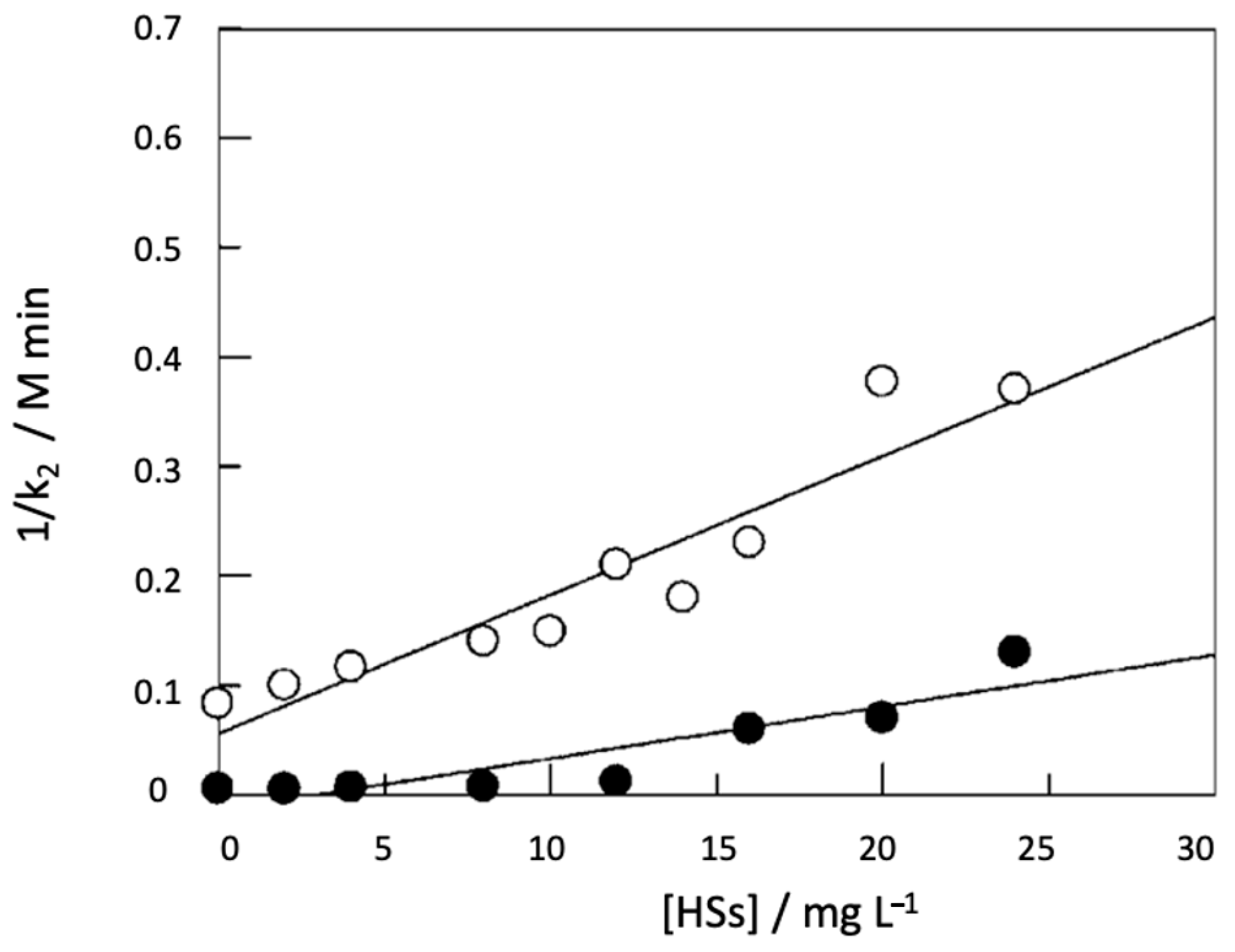
5. Binding Constants and Hydrophobicity of HSs Core
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CEC | ionized charge capacity |
| CF | carbofuran |
| CV | crystal violet |
| HCF | 3-hydroxy-carbofuran |
| HSs | humic substances |
| IP | iprodione |
| KCF | 3-keto-carbofuran |
| MG | malachite green |
| MNTS | N-methyl-N-nitroso-p-toluenesulfonamide |
| OTACl | octadecyl trimethyl ammonium chloride |
| SDS | sodium dodecyl sulfate |
| VI | vinclozolin |
References
- García-Río, L.; Hervés, P.; Mejuto, J.C.; Pérez-Juste, J.; Rodríguez-Dafonte, P. Comparative study of nitroso group transfer in colloidal aggregates: Micelles, vesicles and microemulsions. New J. Chem. 2003, 27, 372–380. [Google Scholar] [CrossRef]
- García-Río, L.; Hervés, P.; Mejuto, J.C.; Pérez-Juste, J.; Rodríguez-Dafonte, P. Pseudophase Approach to Reactivity in Microemulsions: Quantitative Explanation of the Kinetics of the Nitroso Group Transfer Reactions between N-methyl-N-nitroso-p-toluenesulfonamide and Secondary Alkylamines in Water/AOT/Isooctane Microemulsions. Ind. Eng. Chem. Res. 2003, 42, 5450–5456. [Google Scholar] [CrossRef]
- García-Río, L.; Hervés, P.; Mejuto, J.C.; Rodríguez-Dafonte, P. Nitrosation reactions in water/AOT/xylene microemulsions. Ind. Eng. Chem. Res. 2006, 45, 2. [Google Scholar] [CrossRef]
- Bunton, C.A.; Savelli, G. Organic Reactivity in Aqueous Micelles and Similar Assemblies. Adv. Phys. Org. Chem. 1986, 22, 213–309. [Google Scholar]
- García-Río, L.; Hervés, P.; Leis, J.R.; Mejuto, J.C.; Rodríguez-Dafonte, P. Reactive micelles: Nitroso group transfer from N-methyl-N-nitroso-p- toluenesulfonamide to amphiphilic amines. J. Phys. Org. Chem. 2004, 17, 1067–1072. [Google Scholar] [CrossRef]
- Huang, X.; Xue, L. Recent Progress in Enzyme Catalysis in Reverse Micelles. In Bridging Heterogeneous and Homogeneous Catalysis: Concepts, Strategies, and Applications; Wiley-VCH: Weinheim, Germany, 2014; ISBN 9783527675906. [Google Scholar]
- Bunton, C.A. Micellar rate effects-assumptions and approximations. Arkivoc 2011, 2011, 490–504. [Google Scholar] [CrossRef] [Green Version]
- Bunton, C.A.; Yatsimirsky, A.K. Comparisons and Analyses of Theoretical Treatments of Micellar Effects upon Ion−Molecule Reactions. Relevance to Amide Exchange. Langmuir 2000, 16, 5921–5931. [Google Scholar] [CrossRef]
- Bunton, C.A. The dependence of micellar rate effects upon reaction mechanism. Adv. Colloid Interface Sci. 2006, 123–126, 333–343. [Google Scholar] [CrossRef]
- Bunton, C.A. Reactivity in aqueous association colloids. Descriptive utility of the pseudophase model. J. Mol. Liq. 1997, 72, 231–249. [Google Scholar] [CrossRef]
- Tian, S.; Tan, W.; Wang, X.; Li, T.; Song, F.; Huang, N.; Bai, Y. Surface Activity of Humic Acid and Its Sub-Fractions from Forest Soil. Sustainability 2021, 13, 8122. [Google Scholar] [CrossRef]
- Arias, M.; García-Río, L.; Mejuto, J.C.; Rodríguez-Dafonte, P.; Simal-Gándara, J. Influence of Micelles on the Basic Degradation of Carbofuran. J. Agric. Food Chem. 2005, 53, 7172–7178. [Google Scholar] [CrossRef] [PubMed]
- Flis-Bujak, M.; Ksiezopolska, A.; Stawinski, J.; Turski, R. The surface properties of humic acids extracted from chernozem and grey-brown podzolic soils. Int. Agrophisics 1995, 9, 115–123. [Google Scholar]
- Mendez, J.C.; Hiemstra, T.; Koopmans, G.F. Assessing the Reactive Surface Area of Soils and the Association of Soil Organic Carbon with Natural Oxide Nanoparticles Using Ferrihydrite as Proxy. Environ. Sci. Technol. 2020, 54, 11990–12000. [Google Scholar] [CrossRef] [PubMed]
- Chiou, C.T.; Lee, J.F.; Boyd, S.A. The surface area of soil organic matter. Environ. Sci. Technol. 1990, 24, 1164–1166. [Google Scholar] [CrossRef]
- De Melo, B.A.G.; Motta, F.L.; Santana, M.H.A. Humic acids: Structural properties and multiple functionalities for novel technological developments. Mater. Sci. Eng. C 2016, 62, 967–974. [Google Scholar] [CrossRef]
- Conte, P.; Piccolo, A. Conformational arrangement of dissolved humic substances. Influence of solution composition on association of humic molecules. Environ. Sci. Technol. 1999, 33, 1682–1690. [Google Scholar] [CrossRef]
- Clapp, C.E.; Hayes, M.H.B. Sizes and shapes of humic substances. Soil Sci. 1999, 164, 777–789. [Google Scholar] [CrossRef]
- He, W.; Bai, Z.; Li, Y.; Kong, X.; Liu, W.; Yang, C.; Yang, B.; Xu, F. Advances in environmental behaviors and effects of dissolved organic matter in aquatic ecosystems. Sci. China Earth Sci. 2016, 59, 746–759. [Google Scholar] [CrossRef]
- Arias-Estévez, M.; López-Periago, E.; Martínez-Carballo, E.; Simal-Gándara, J.; Mejuto, J.C.; García-Río, L. The mobility and degradation of pesticides in soils and the pollution of groundwater resources. Agric. Ecosyst. Environ. 2008, 123, 247–260. [Google Scholar] [CrossRef]
- Rutherford, D.W.; Chiou, C.T.; Klle, D.E. Influence of soil organic matter composition on the partition of organic compounds. Environ. Sci. Technol. 1992, 26, 336–340. [Google Scholar] [CrossRef]
- Leboeuf, E.J.; Weber, W.J., Jr. Macromolecular characteristics of natural organic matter. 2. Sorption and desorption behavior. Environ. Sci. Technol. 2000, 34, 3632–3640. [Google Scholar] [CrossRef]
- Arias-Estévez, M.; Fernandez-Gandara, D.; Garcia-Falcon, M.S.; Garcia-Rio, L.; Mejuto, J.C.; Simal-Gandara, J. Sorption of PAHs to colloid dispersions of humic substances in water. Bull. Environ. Contam. Toxicol. 2007, 79, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Prosen, H.; Zupančič-Kralj, L. The interaction of triazine herbicides with humic acids. Chromatographia 2000, 51, S155–S164. [Google Scholar] [CrossRef]
- Martin-Neto, L.; Traghetta, D.G.; Vaz, C.M.P.; Crestana, S.; Sposito, G. On the interaction mechanisms of atrazine and hydroxyatrazine with humic substances. J. Environ. Qual. 2001, 30, 520–525. [Google Scholar] [CrossRef]
- Loiseau, L.; Barriuso, E. Characterization of the atrazine’s bound (nonextractable) residues using fractionation techniques for soil organic matter. Environ. Sci. Technol. 2002, 36, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Kopinke, F.-D.; Ramus, K.; Poerschmann, J.; Georgi, A. Kinetics of desorption of organic compounds from dissolved organic matter. Environ. Sci. Technol. 2011, 45, 10013–10019. [Google Scholar] [CrossRef] [PubMed]
- Arias, M.; Barral, M.T.; da Silva–Carvalhal, J.; Mejuto, J.C.; Rubinos, D. Interaction of Hg(II) with kaolin-humic acid complexes. Clay Miner. 2004, 39, 35–45. [Google Scholar] [CrossRef]
- Chiou, C.T.; McGroddy, S.E.; Kile, D.E. Partition Characteristics of Polycyclic Aromatic Hydrocarbons on Soils and Sediments. Environ. Sci. Technol. 1998, 32, 264–269. [Google Scholar] [CrossRef]
- Kile, D.E.; Chiou, C.T. Water solubility enhancements of DDT and trichlorobenzene by some surfactants below and above the critical micelle concentration. Environ. Sci. Technol. 1989, 23, 832–838. [Google Scholar] [CrossRef]
- Prosen, H.; Zupančič-Kralj, L. Evaluation of photolysis and hydrolysis of atrazine and its first degradation products in the presence of humic acids. Environ. Pollut. 2005, 133, 517–529. [Google Scholar] [CrossRef]
- Noblet, J.A.; Smith, L.A.; Suffet, I.H. Influence of Natural Dissolved Organic Matter, Temperature, and Mixing on the Abiotic Hydrolysis of Triazine and Organophosphate Pesticides. J. Agric. Food Chem. 1996, 44, 3685–3693. [Google Scholar] [CrossRef]
- Maurya, P.K.; Malik, D.S. Bioaccumulation of xenobiotics compound of pesticides in riverine system and its control technique: A critical review. J. Ind. Pollut. Control 2016, 32, 580–594. [Google Scholar]
- Vigneshwaran, S.; Sirajudheen, P.; Karthikeyan, P.; Nabeena, C.P.; Meenakshi, S. Remediation of Persistent Organic Pesticides from Wastewater Matrices—Present and Future Conceptions. In Energy, Environment, and Sustainability; Springer: Singapore, 2021. [Google Scholar] [CrossRef]
- Ramus, K.; Kopinke, F.-D.; Georgi, A. Influence of dissolved humic substances on the mass transfer of organic compounds across the air-water interface. Chemosphere 2012, 86, 138–143. [Google Scholar] [CrossRef]
- Ramus, K.; Kopinke, F.-D.; Georgi, A. Sorption-induced effects of humic substances on mass transfer of organic pollutants through aqueous diffusion boundary layers: The example of water/air exchange. Environ. Sci. Technol. 2012, 46, 2196–2203. [Google Scholar] [CrossRef] [PubMed]
- Chiou, C.T.; Kile, D.E.; Brinton, T.I.; Malcolm, R.L.; Leenheer, J.A.; MacCarthy, P. A comparison of water solubility enhancements of organic solutes by aquatic humic materials and commercial humic acids. MacCarthy. Environ. Sci. Technol. 1987, 21, 1231–1234. [Google Scholar] [CrossRef]
- Georgi, A.; Reichl, A.; Trommler, U.; Kopinke, F.-D. Influence of sorption to dissolved humic substances on transformation reactions of hydrophobic organic compounds in water. I. Chlorination of PAHs. Environ. Sci. Technol. 2007, 41, 7003–7009. [Google Scholar] [CrossRef]
- Georgi, A.; Trommler, U.; Reichl, A.; Kopinke, F.-D. Influence of sorption to dissolved humic substances on transformation reactions of hydrophobic organic compounds in water. Part II: Hydrolysis reactions. Chemosphere 2008, 71, 1452–1460. [Google Scholar] [CrossRef]
- Chiou, C.T.; Malcolm, R.L.; Brinton, T.I.; Kile, D.E. Water solubility enhancement of some organic pollutants and pesticides by dissolved humic and fulvic acids. Environ. Sci. Technol. 1986, 20, 502–508. [Google Scholar] [CrossRef]
- Chiou, C.T. Roles of Organic Matter, Minerals, and Moisture in Sorption of Nonionic Compounds and Pesticides by Soil; Humic Substances in Soil and Crop Sciences: Selected Readings; American Society of Agronomy and Soil Science Society of America: Madison, WI, USA, 2015; pp. 111–160. [Google Scholar]
- Chiou, C.T.; Shoup, T.D.; Porter, P.E. Mechanistic roles of soil humus and minerals in the sorption of nonionic organic compounds from aqueous and organic solutions. Org. Geochem. 1985, 8, 9–14. [Google Scholar] [CrossRef]
- Hung, W.-N.; Lin, T.-F.; Chiu, C.-H.; Chiou, C.T. On the use of a freeze-dried versus an air-dried soil humic acid as a surrogate of soil organic matter for contaminant sorption. Environ. Pollut. 2012, 160, 125–129. [Google Scholar] [CrossRef]
- Chiou, C.T.; Kile, D.E.; Rutherford, D.W.; Sheng, G.; Boyd, S.A. Sorption of Selected Organic Compounds from Water to a Peat Soil and Its Humic-Acid and Humin Fractions: Potential Sources of the Sorption nonlinearity. Environ. Sci. Technol. 2000, 34, 1254–1258. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Singh, J. Kinetic study of the biodegradation of glyphosate by soil bacterial isolates in presence of humic acid, Fe(III) and Cu(II) ions. J. Envion. Chem. Eng. 2019, 7, 103098. [Google Scholar] [CrossRef]
- Salvestrini, S.; Capasso, S.; Iovino, P. Catalytic effect of dissolved humic acids on the chemical degradation of phenylurea herbicides. Pest. Manag. Sci. 2008, 64, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Georgi, A.; Kopinke, F.-D. Validation of a modified flory-huggins concept for description of hydrophobic organic compound sorption on dissolved humic substances. Environ. Toxicol. Chem. 2002, 21, 1766–1774. [Google Scholar]
- Xie, Y.; Guan, Z.; Cheng, J.; Zhou, Y.; Yan, M. Competition between Al(III) and Fe(III) for binding onto natural organic matter: In situ monitoring by UV–Vis absorbance spectroscopy. Chemosphere 2021, 270, 128655. [Google Scholar] [CrossRef]
- Qu, C.; Chen, W.; Hu, X.; Cai, P.; Chen, C.; Yu, X.-Y.; Huang, Q. Heavy metal behaviour at mineral-organo interfaces: Mechanisms, modelling and influence factors. Environ. Int. 2019, 131, 104995. [Google Scholar] [CrossRef]
- Marsac, R.; Banik, N.; Lützenkirchen, J.; Catrouillet, C.; Marquardt, C.; Johannesso, K. Modeling metal ion-humic substances complexation in highly saline conditions. Appl. Geochem. 2017, 79, 52–64. [Google Scholar] [CrossRef] [Green Version]
- Marsac, R.; Davranche, M.; Gruau, G.; Dia, A.; Bouhnik-Le Coz, M. Aluminium competitive effect on rare earth elements binding to humic acid. Geochim. Et Cosmochim. Acta 2012, 89, 1–9. [Google Scholar] [CrossRef]
- Tipping, E.; Rey-Castro, C.; Bryan, S.E.; Hamilton-Taylor, J. Al(III) and Fe(III) binding by humic substances in freshwaters, and implications for trace metal speciation. Geochim. Cosmochim. Acta 2002, 66, 3211–3224. [Google Scholar] [CrossRef] [Green Version]
- Esfandiar, N.; Suri, R.; McKenzie, E.R. Competitive sorption of Cd, Cr, Cu, Ni, Pb and Zn from stormwater runoff by five low-cost sorbents; Effects of co-contaminants, humic acid, salinity and pH. J. Hazard. Mater. 2022, 423, 126938. [Google Scholar] [CrossRef]
- Zhou, P.; Yan, H.; Gu, B. Competitive complexation of metal ions with humic substances. Chemosphere 2005, 58, 1327–1337. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Michel, F.M.; Choi, Y.; Eng, P.J.; Levard, C.; Siebner, H.; Gu, B.; Bargar, J.R.; Brown, G.E., Jr. Pb, Cu, and Zn distributions at humic acid-coated metal-oxide surfaces. Geochim. Cosmochim. Acta 2016, 188, 407–423. [Google Scholar] [CrossRef] [Green Version]
- Arias, M.; Barral, M.T.; Mejuto, J.C. Enhancement of copper and cadmium adsorption on kaolin by the presence of humic acids. Chemosphere 2002, 48, 1081–1088. [Google Scholar] [CrossRef]
- Klavins, M.; Babre, K. Decarboxylation and alkaline colour fading reactions in presence of humic substances. Chemosphere 2002, 49, 685–689. [Google Scholar] [CrossRef]
- Kaviš, M.; Dipne, J.; Babre, K. Humic substances as catalysts in condensation reactions. Chemosphere 2001, 44, 737–742. [Google Scholar]
- Morales, J.; Cid, A.; Mejuto, J.C. Alkaline hydrolysis of vinclozolin: Effect of humic acids aggregates in water. J. Mol. Catal. A Chem. 2015, 401, 13–17. [Google Scholar] [CrossRef]
- Arias-Estevez, M.; Astray, G.; Cid, A.; Fernández-Gándara, D.; García-Río, L.; Mejuto, J.C. Influence of colloid suspensions of humic acids upon the alkaline fading of carbocations. J. Phys. Org. Chem. 2008, 21, 555–560. [Google Scholar] [CrossRef]
- Morales, J.; Manso, J.A.; Cid, A.; Mejuto, J.C. Stability study of Iprodione in alkaline media in the presence of humic acids. Chemosphere 2013, 92, 1536–1541. [Google Scholar] [CrossRef] [Green Version]
- Morales, J.; Manso, J.A.; Cid, A.; Mejuto, J.C. Degradation of carbofuran and carbofuran-derivatives in presence of humic substances under basic conditions. Chemosphere 2012, 89, 1267–1271. [Google Scholar] [CrossRef]
- Astray, G.; García-Río, L.; Lodeiro, C.; Mejuto, J.C.; Moldes, O.; Morales, J.; Moyano, F. Influence of colloid suspensions of humic acids on the alkaline hydrolysis of N-methyl-N-nitroso-p-toluene sulfonamide. Int. J. Chem. Kinet. 2010, 42, 316–322. [Google Scholar] [CrossRef]
- Klute, A.; Page, A.L. Methods of Soil Analysis; American Society of Agronomy: Madison, WI, USA, 1986. [Google Scholar]
- Ritchie, C.D. Nucleophilic Reactivities toward Cations. Acc. Chem. Res. 1972, 5, 348–354. [Google Scholar] [CrossRef]
- Ritchie, C.D.; Virtanen, P.O.I. Cation–Anion Combination Reactions. V. Correlation of Reactivities of Nucleophiles with Diazonium Ions and with Triarylmethyl Cations. J. Am. Chem. Soc. 1972, 94, 1589–1594. [Google Scholar] [CrossRef]
- Mayr, H.; Ofial, A.R. The reactivity-selectivity principle: An imperishable myth in organic chemistry. Angew. Chem. Int. Ed. 2006, 45, 1844–1854. [Google Scholar] [CrossRef] [PubMed]
- Bentley, T.W. Additivity rules using similarity models for chemical reactivity: Calculation and interpretation of electrofugality and nucleofugality. Chem. A Eur. J. 2006, 12, 6514–6520. [Google Scholar] [CrossRef] [PubMed]
- Duynstee, E.F.J.; Grunwald, E. Organic Reactions Occurring in or on Micelles. II. Kinetic and Thermodynamic Analysis of the Alkaline Fading of Triphenylmethane Dyes in the Presence of Detergent Salts. J. Am. Chem. Soc. 1959, 81, 4542–4548. [Google Scholar] [CrossRef]
- Duynstee, E.F.J.; Grunwald, E. Organic Reactions Occurring in or on Micelles. I. Reaction Rate Studies of the Alkaline Fading of Triphenylmethane Dyes and Sulfonphthalein Indicators in the Presence of Detergent Salts. J. Am. Chem. Soc. 1959, 81, 4540–4542. [Google Scholar] [CrossRef]
- Valiente, M.; Rodenas, E. Benzene, toluene, and cyclohexane incorporation into hexadecyltrimethylammonium bromide micelles: Effect on the basic hydrolysis of crystal violet. J. Colloid Interface Sci. 1990, 138, 299–306. [Google Scholar] [CrossRef]
- Leis, J.R.; Mejuto, J.C.; Peña, M.E. Comparison between the Kinetics of the Alkaline Fading of Carbocation Dyes in Water/Sodium Bis(2-ethylhexyl) Sulfosuccinate/Isooctane Microemulsions and in Homogeneous Media. Langmuir 1993, 9, 889–893. [Google Scholar] [CrossRef]
- Cañadas, O.; Valiente, M.; Rodenas, E. Study of the cetyltrimethylammonium bromide/1,6-hexanediol/water system. J. Colloid Interface Sci. 1998, 203, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, L.; Mitra, N.; Bhattacharya, P.K.; Moulik, S.P. Kinetics in Microemulsion Medium. 4. Alkaline Fading of Crystal Violet in Aqueous (H2O/Aerosol OT/Isooctane and H2O/Aerosol OT/Decane) and Nonaqueous (Ethylene Glycol/Aerosol OT/Isooctane) Microemulsions. Langmuir 1995, 11, 2866–2871. [Google Scholar] [CrossRef]
- García-Río, L.; Leis, J.R.; Mejuto, J.C.; Navarro-Vázquez, A.; Pérez-Juste, J.; Rodríguez-Dafonte, P. Basic hydrolysis of crystal violet in β-cyclodextrin/surfactant mixed systems. Langmuir 2004, 20, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Rakitin, A.R.; Pack, G.R. Monte Carlo calculations of ion distributions around micelles. Colloids Surf. A Physicochem. Eng. Asp. 2003, 218, 265–276. [Google Scholar] [CrossRef]
- García-Río, L.; Leis, J.R.; Mejuto, J.C.; Pérez-Juste, J. Basic hydrolysis of m-nitrophenyl acetate in micellar media containing β-cyclodextrins. J. Phys. Chem. B 1998, 102, 4581–4587. [Google Scholar] [CrossRef]
- Rodenas, E.; Perez-Benito, E.; Valiente, M. Effect of the location of reactants in the L2 phase on the chemical reactivity. Tenside Surfactants Deterg. 1994, 31, 168–170. [Google Scholar] [CrossRef]
- Schoental, R. Carcinogenic action of diazomethane and of nitroso-N-methyl urethane. Nature 1960, 188, 420–421. [Google Scholar] [CrossRef] [PubMed]
- Astray, G.; Cid, A.; García-Río, L.; Hervella, P.; Mejuto, J.C.; Pérez-Lorenzo, M. Organic reactivity in AOT-stabilized microemulsions. Prog. React. Kinet. Mech. 2008, 33, 81. [Google Scholar] [CrossRef]
- García-Río, L.; Mejuto, J.C.; Pérez-Lorenzo, M.; Rodríguez-Dafonte, P. Nitroso group transfer reactions between N-methyl-N-nitroso-P-toluene sulfonamide and N-alkylamines in CTAC1 micellar aggregates. Prog. React. Kinet. Mech. 2006, 31, 129–138. [Google Scholar] [CrossRef]
- Fernández, I.; García-Río, L.; Hervés, P.; Mejuto, J.C. β-Cyclodextrin-micelle mixed systems as a reaction medium. Denitrosation of N-ethyl-N-nitrososo-p-toluene-sulfonamide. J. Phys. Org. Chem. 2000, 13, 664–669. [Google Scholar] [CrossRef]
- García-Río, L.; Hervés, P.; Mejuto, J.C.; Pérez-Juste, J.; Rodríguez-Dafonte, P. Pseudophase approach to the transfer of the nitroso group in water/AOT/SDS/isooctane quaternary microemulsions. Langmuir 2000, 16, 9716–9721. [Google Scholar] [CrossRef]
- García-Río, L.; Hervés, P.; Leis, J.R.; Mejuto, J.C.; Perez-Juste, J. Hydrolysis of N-methyl-N-nitroso-p-toluenesulphonamide in micellar media. J. Phys. Org. Chem. 1998, 11, 584–588. [Google Scholar] [CrossRef]
- García-Río, L.; Leis, J.R.; Mejuto, J.C.; Pérez-Juste, J. Investigation of micellar media containing β-cyclodextrins by means of reaction kinetics: Basic hydrolysis of N-methyl-N-nitroso-p-toluenesulfonamide. J. Phys. Chem. B 1997, 101, 38. [Google Scholar] [CrossRef]
- Bravo, C.; Hervés, P.; Leis, J.R.; Peña, M.E. Micellar effects in the acid denitrosation of N-Nitroso-N-methyl-p-toluenesulfonamide. J. Phys. Chem. 1990, 94, 8816–8820. [Google Scholar] [CrossRef]
- García-Rio, L.; Hervés, P.; Mejuto, J.C.; Parajó, M.; Perez-Juste, J. Association Constant of Crystal Violet in Micellar Aggregates: Determination by Spectroscopic Techniques. J. Chem. Res. Part S 1998, 11, 716–717. [Google Scholar] [CrossRef]
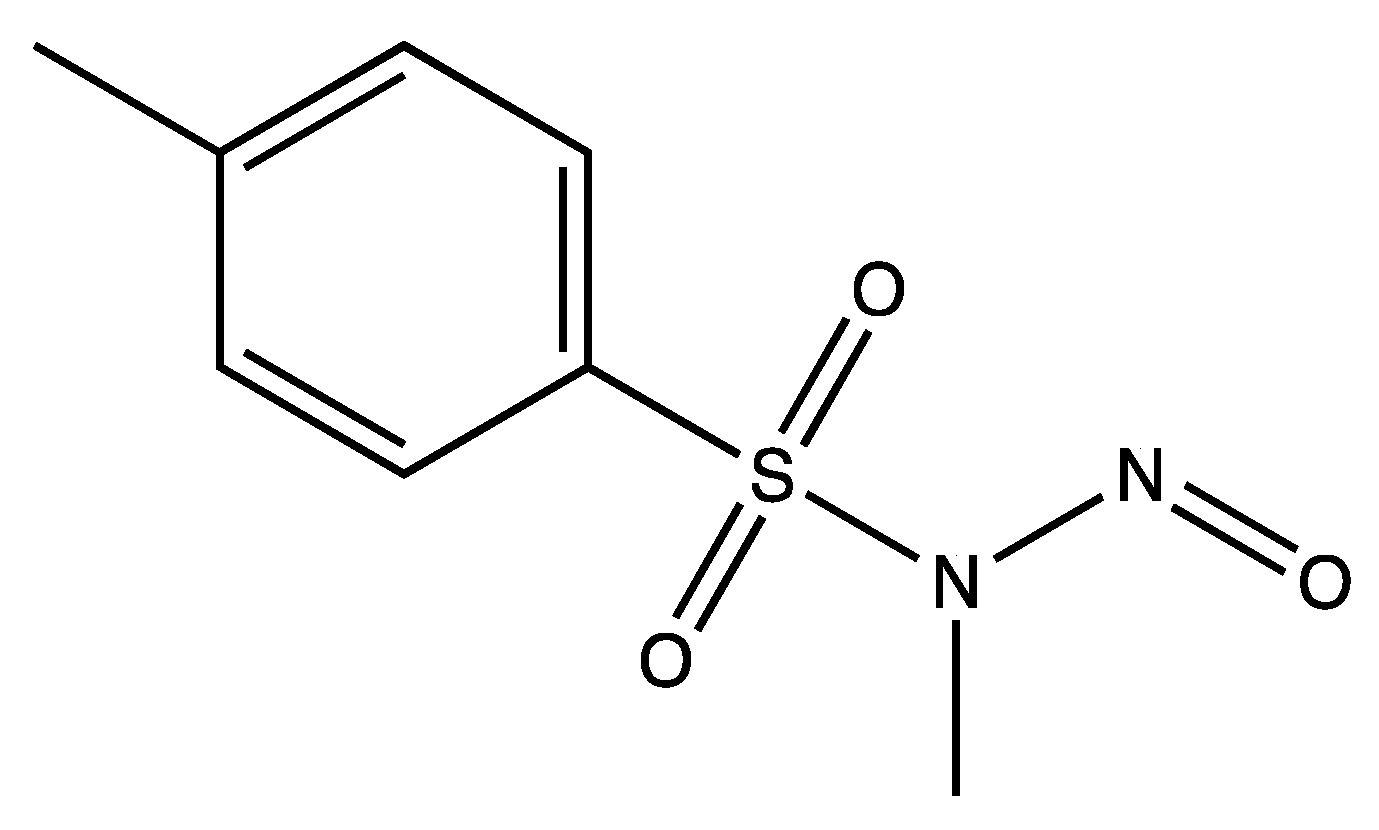
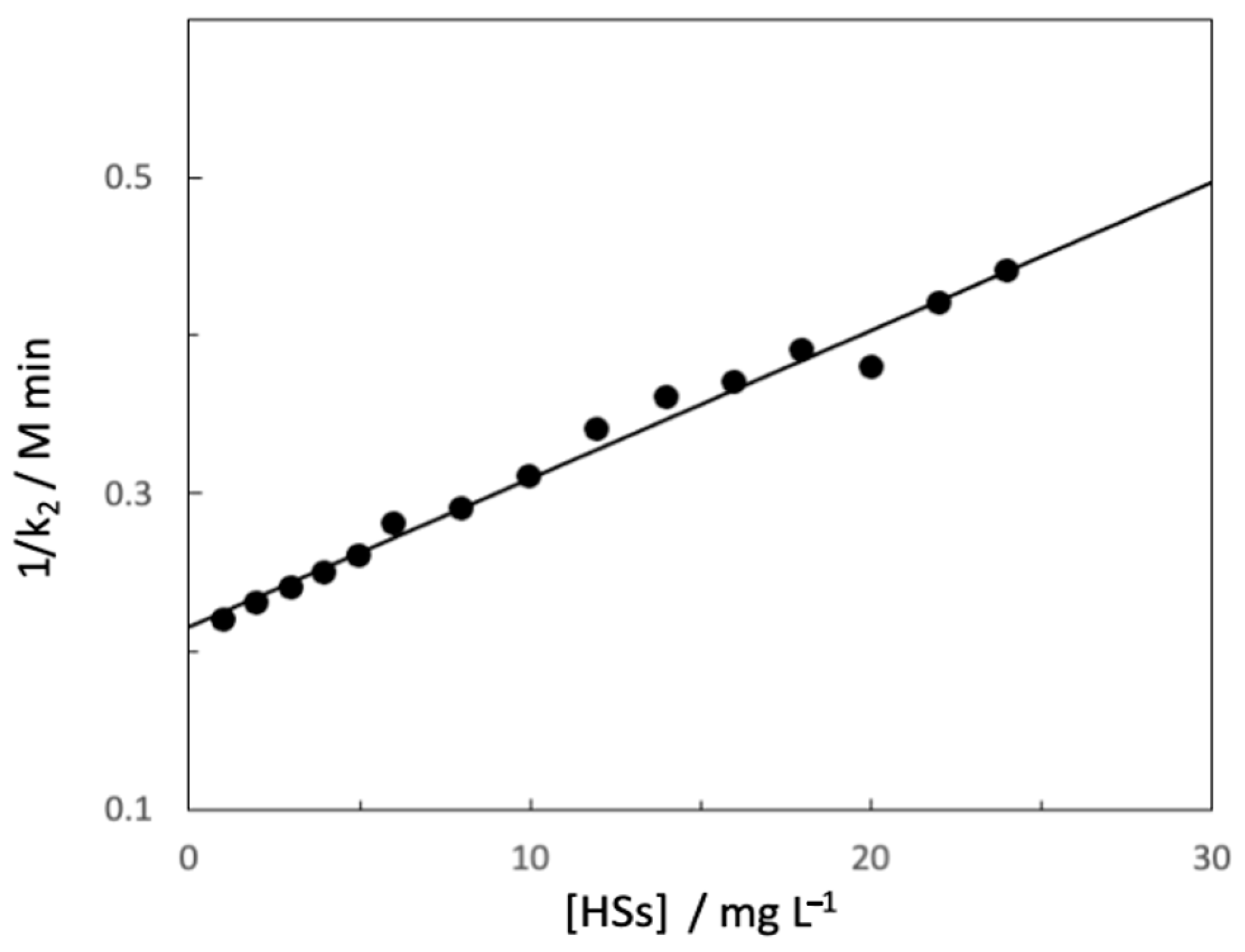
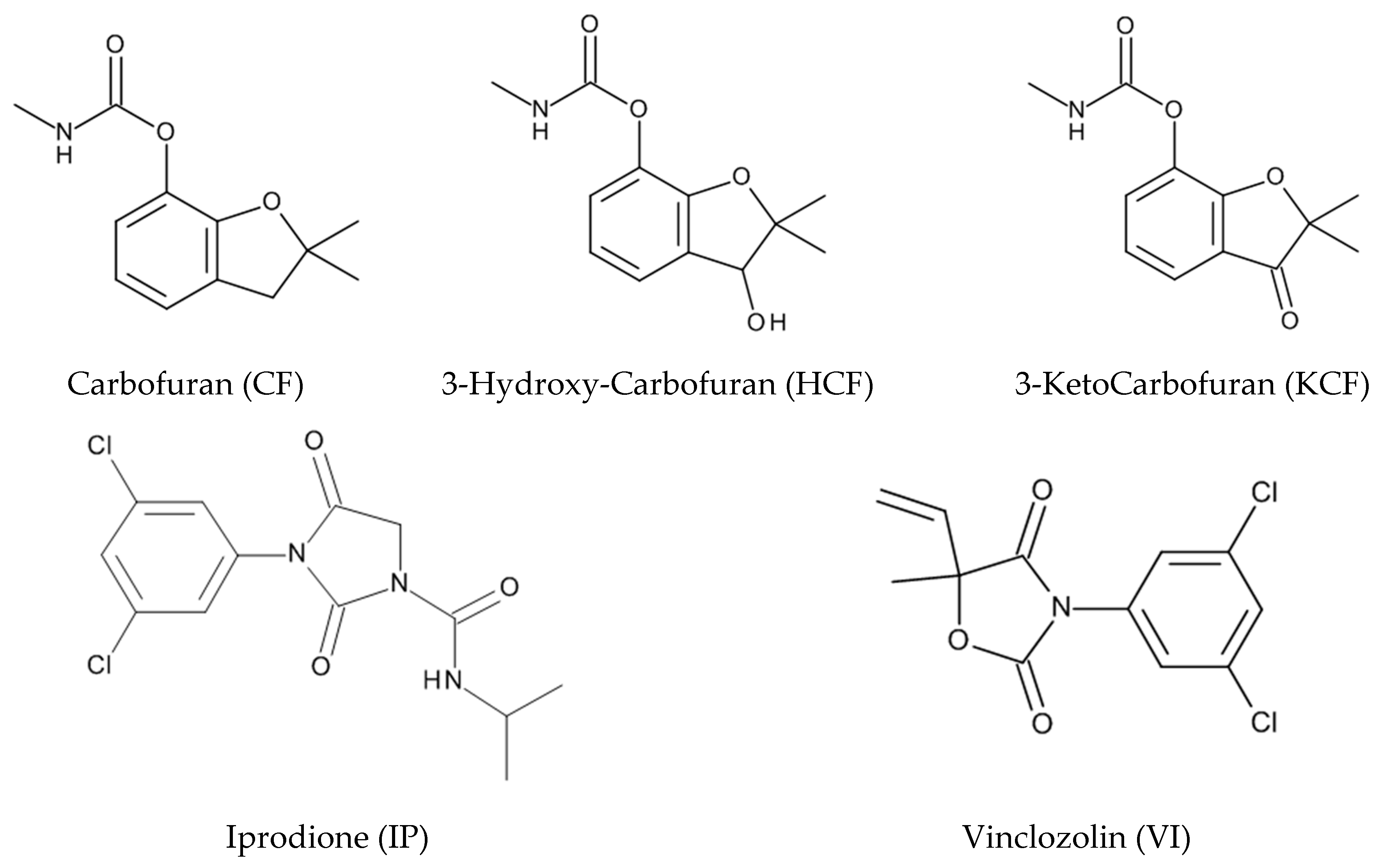
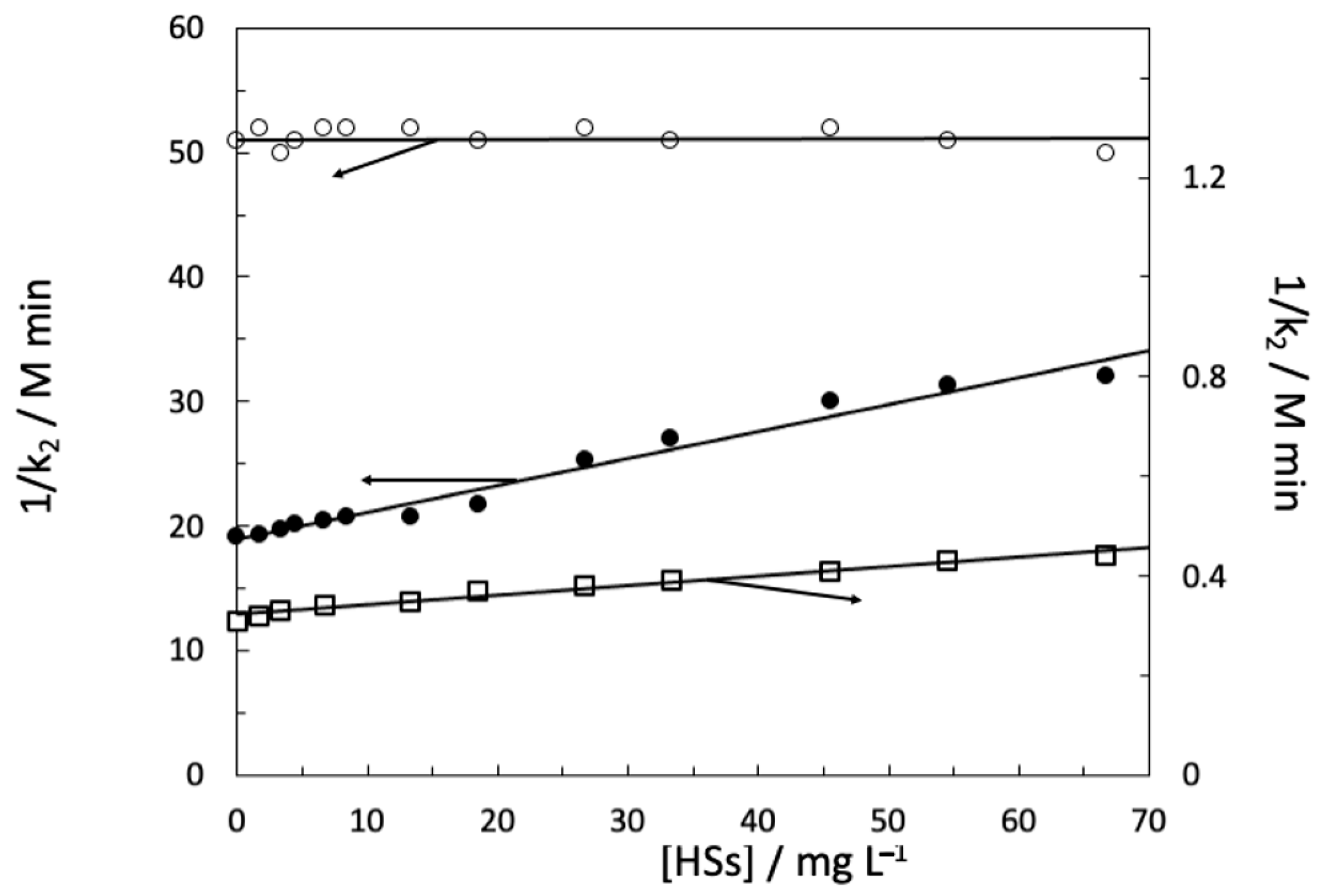
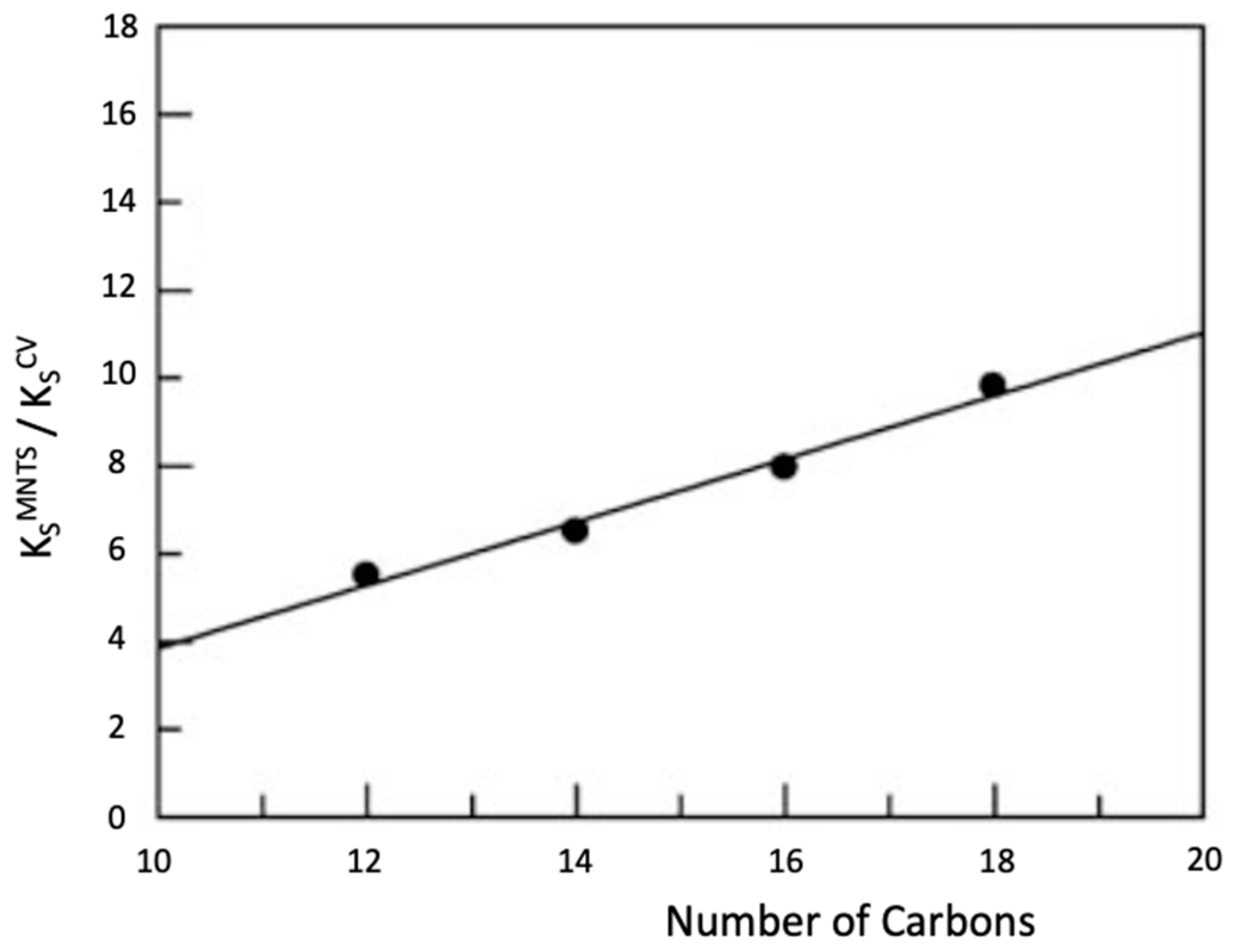
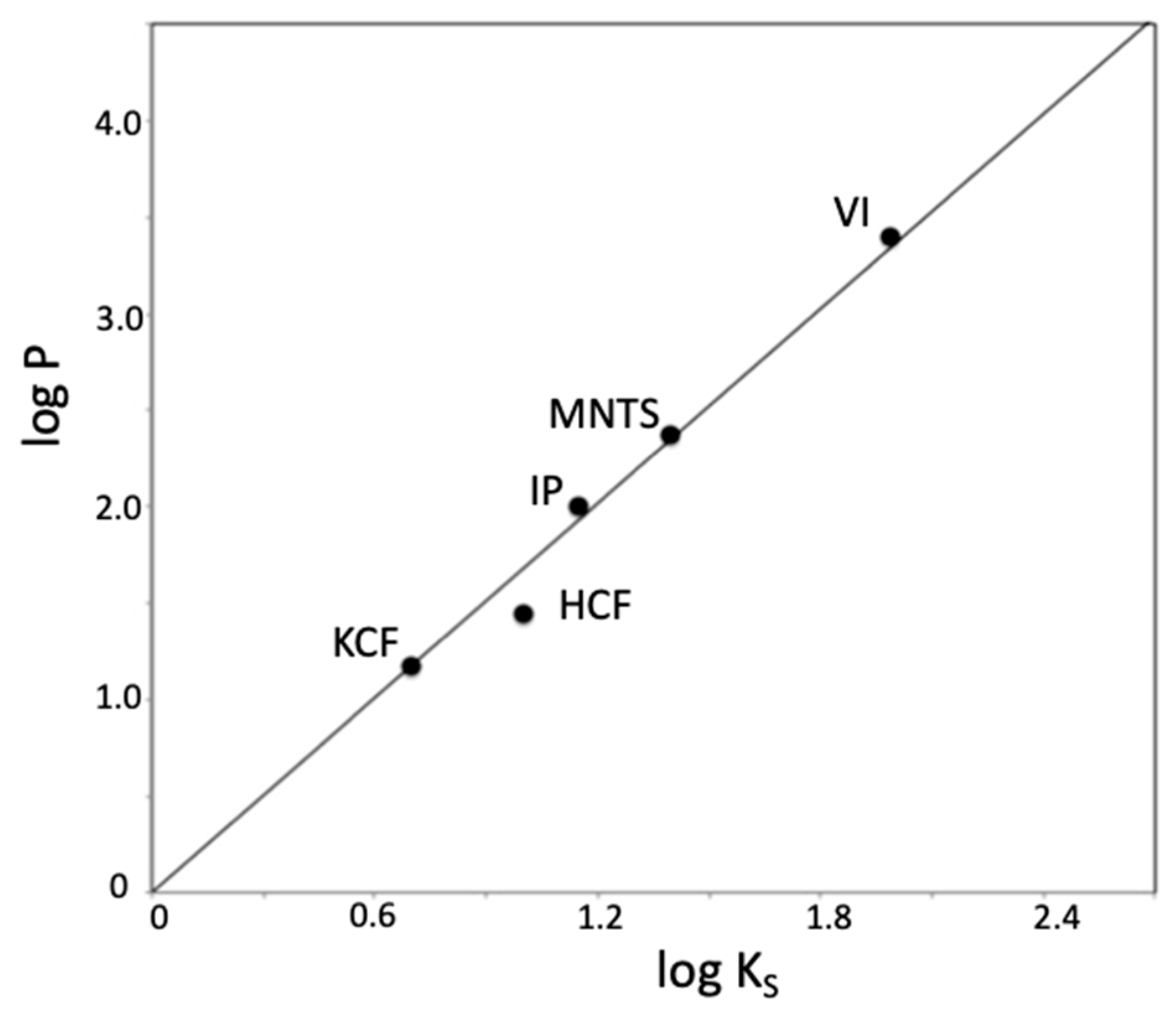
| Substrate | kw/M−1·min−1 | Ks/mg−1·L |
|---|---|---|
| CV [61] | 1.23 ± 0.02 | 0.13 ± 0.01 |
| MG [61] | 181 ± 6 | 0.65 ± 0.05 |
| Substrate | kw/M−1·min−1 | Ks/mg−1·L |
|---|---|---|
| CF [62] | (0.66 ± 0.06) × 102 | 0 |
| HCF [62] | (1.86 ± 0.06) × 102 | (1.0 ± 0.1) × 10−2 |
| KCF [62] | (11.40 ± 0.60) × 103 | (5.0 ± 1.0) × 10−3 |
| IP [61] | (1.88 ± 0.01) × 103 | (1.4 ± 0.1) × 10−2 |
| VI [59] | (8.20 ± 0.40) × 102 | (9.7 ± 0.1) × 10−2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cid-Samamed, A.; García-Río, L.; Mejuto, J.C.; Saavedra, A. Humic Acids Aggregates as Microheterogeneous Reaction Media: Alkaline Hydrolysis Reactions. Compounds 2022, 2, 131-143. https://doi.org/10.3390/compounds2020010
Cid-Samamed A, García-Río L, Mejuto JC, Saavedra A. Humic Acids Aggregates as Microheterogeneous Reaction Media: Alkaline Hydrolysis Reactions. Compounds. 2022; 2(2):131-143. https://doi.org/10.3390/compounds2020010
Chicago/Turabian StyleCid-Samamed, Antonio, Luis García-Río, Juan C. Mejuto, and Alejandro Saavedra. 2022. "Humic Acids Aggregates as Microheterogeneous Reaction Media: Alkaline Hydrolysis Reactions" Compounds 2, no. 2: 131-143. https://doi.org/10.3390/compounds2020010
APA StyleCid-Samamed, A., García-Río, L., Mejuto, J. C., & Saavedra, A. (2022). Humic Acids Aggregates as Microheterogeneous Reaction Media: Alkaline Hydrolysis Reactions. Compounds, 2(2), 131-143. https://doi.org/10.3390/compounds2020010








