Abstract
Thiazolidinediones (TZDs), also known as Glitazones, have anti-diabetic, anti-inflammatory and anti-cancer properties. A simple, efficient and cost-effective synthesis of a thiazolidinedione compound library was developed. The synthesis is facilitated by microwave irradiation in three of the four steps followed by reduction under pressurized hydrogen gas using palladium hydroxide. All reactions, except one, were completed within an hour and provided desired products in moderate to good yields after a simple work-up.
1. Introduction
The occurrence of diabetes and other diseases contracted around the globe has vastly increased over the past few decades driven by the global rise in the prevalence of obesity. Worldwide there is a projected increase in the frequency of diabetes from 285 million in 2010 to 439 million in 2030. Estimates in developing countries show marked increases, particularly in areas where populations are rapidly adopting Western lifestyles [1]. The increase in the occurrence of childhood obesity has led to the development of type II diabetes in children, and young adults, particularly those in high susceptible ethnic groups [1]. For this reason, the availability of drugs and therapeutics aimed at diabetes must increase, most specifically type II diabetes mellitus, which accounts for approximately 90–95% of all diagnosed cases of diabetes [2,3]. Along with diabetes treatments comes the necessity to treat other associated cardiovascular diseases such as hypertension, atherosclerosis, dyslipidemia, coagulation abnormalities, heart disease and many more [4].
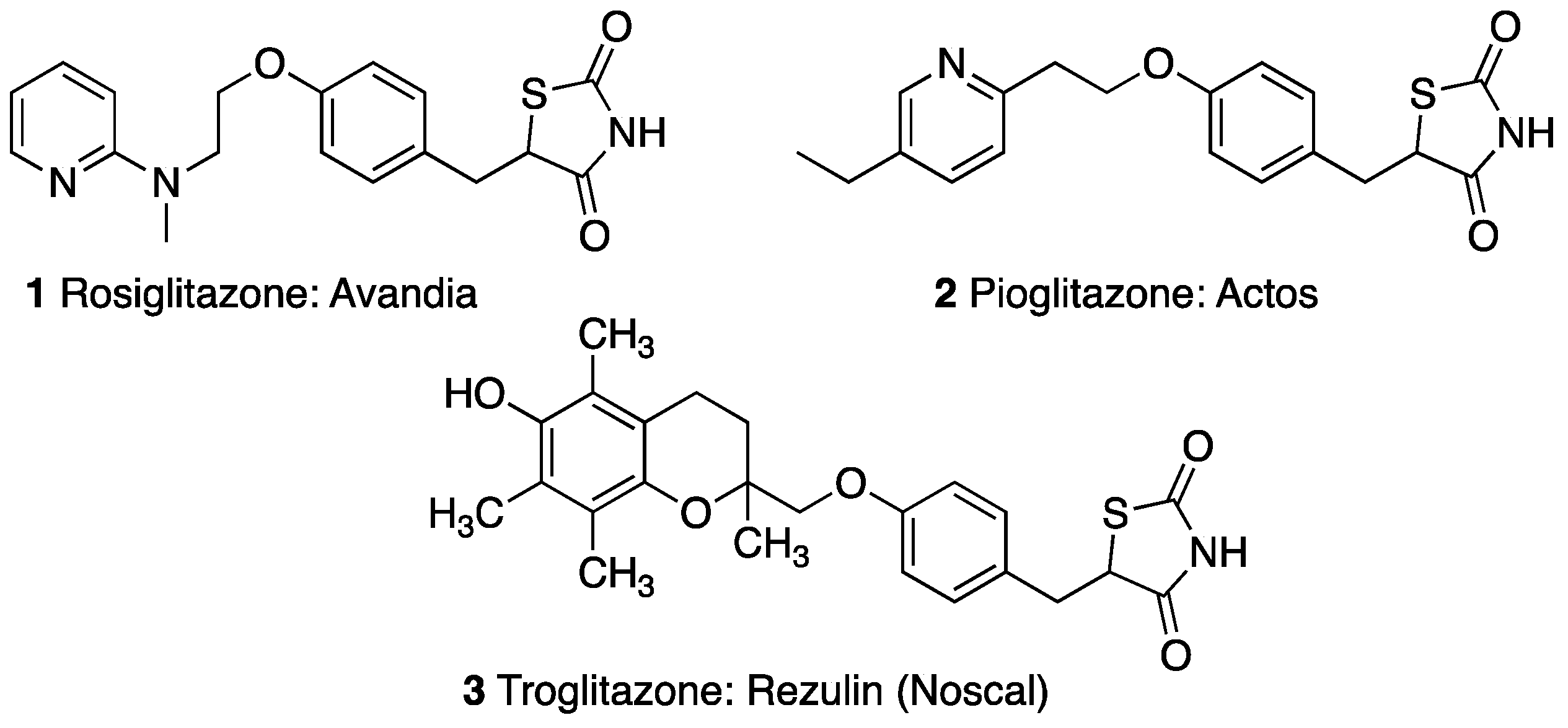
Thiazolidinediones (TZDs), also known as Glitazones, are a class of insulin-sensitizing agents, which are used in the oral therapy of type II diabetes mellitus [5,6,7]. TZDs were introduced in the late 1990s and have been widely used since due to their clinical advantages of treating insulin resistance and sustaining glycemic control [8,9]. The first thiazolidinedione drug approved by the FDA, called troglitazone, was withdrawn from the market within three years due to severe liver damage in some patients [10,11]. The only thiazolidinedione drugs currently in use and on the market are rosiglitazone and pioglitazone (Figure 1) [12].
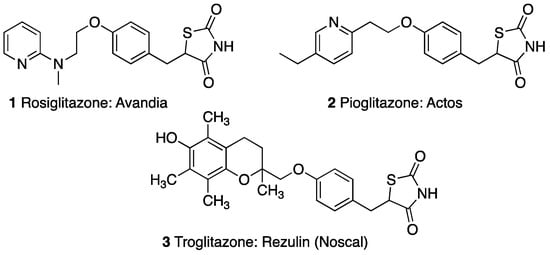
Figure 1.
Previous and current TZD drugs on the U.S. market.
After its release, it was found that rosiglitazone was associated with an increased risk of myocardial infarction, and in November of 2011, the FDA began restricting access, granting the drug only to patients with no cardiovascular risk, and whose diabetes is not well controlled with other medications [8,13,14]. However, in November of 2013, the FDA removed the restriction due to recent findings of no risk of heart failure from the use of rosiglitazone [15,16].
The mechanism in which TZDs work is relatively well known. Treatment of type II diabetes is achieved from the thiazolidinedione ring binding to, and activating the peroxisome proliferators-activated receptor γ (PPAR-γ), which promotes glucose utilization, primarily in adipose tissue [17,18]. Peroxisome proliferator-activated receptor γ is a nuclear receptor that modulates the transcription of insulin-responsive genes involved in the control of glucose lipid metabolism, as well as the gene involved in inflammatory responses [19,20]. Thiazolidinediones were also shown to have significant anti-inflammatory effects, which would be beneficial in patients that suffer from both diabetes and atherosclerosis [11,21]. Furthermore, the discovery of anti-cancer properties, and the suggestion that TZDs may improve cognitive abilities in patients with Alzheimer’s disease and dementia, add other possibilities for the potential uses of thiazolidinediones [15,22,23].
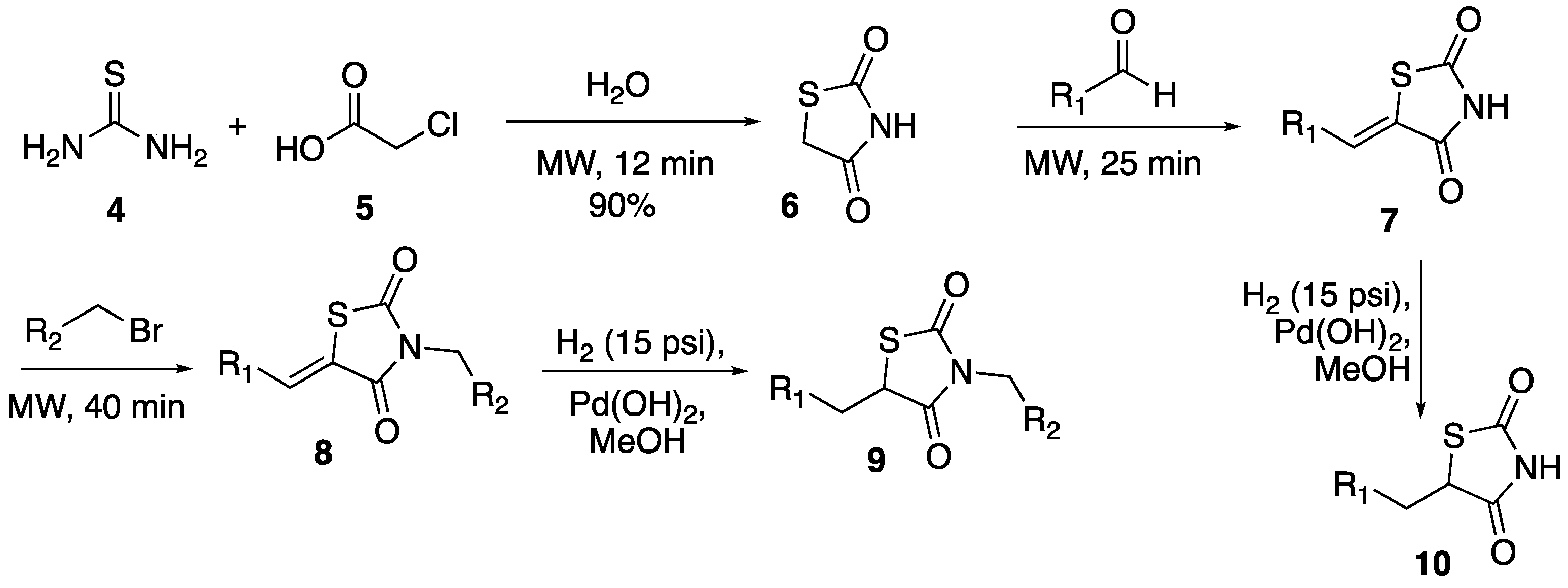
Given the side effects of previously marketed TZDs, and increasing potential effectiveness in many diseases, the necessity for newly developed TZD compounds is high. The goal of this study was to develop an efficient synthesis of a thiazolidinedione compound library over four steps, three of which were accomplished in less than an hour using microwave heating. Given that all thiazolidinediones previously and currently on the market rely solely upon the addition of substituents to the methylene of the TZD ring (Scheme 1), this project focused on the effects of adding substituents to the nitrogen of the TZD ring as well. This was accomplished through microwave-assisted N-benzylation reactions and subsequent reduction of the olefin for decreased rigidity. It is known that N-alkylation of TZDs lowers the antidiabetic activity, however, we are also interested in a new mode of action with TZD derivatives. Unlike conventional heating, microwave radiation causes a uniform increase in temperature throughout the sample, which allows for shorter reaction times, increased yields, and less side product formation [24].
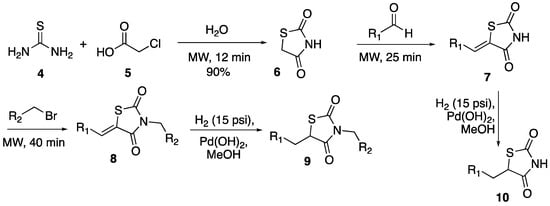
Scheme 1.
Synthesis of TZD library.
2. Materials and Methods
2.1. General Information
Reactions using microwave irradiation were performed using a Milestone Start S dual-move microwave synthesizer (Milestone, Sorisole, Italy) and contained in a Synthware pressure vial. Reactions under H2 gas were carried out using a Parr pressure apparatus.
All chemicals were purchased from Acros Organics (Morris Plains, NJ, USA), Aldrich (Springfield, MO, USA) and Alfa Aesar (Tewksbury, MA, USA), and were used without further purification.
1H and 13C NMR spectra were recorded on Varian UNITY I Nova 300 MHz, Bruker Ultrashield 300 MHz and Bruker Ascend 500 MHz. Dimethyl Sulfoxide-d6 and Chloroform-d were used as the reference point in 1H and 13C NMR spectra (2.50, 39.5 and 7.24, 77.23, respectively). Coupling constants (J values) are given in hertz (Hz). Spin multiplicities are indicated by the following symbols: s (singlet), d (doublet), t (triplet), q (quartet), p (pentet), sx (sextet), sp (septet), o (octet), br (broad), dd (doublet of doublets), td (triplet of dublets), m (multiplet).
High-resolution mass spectra were collected using JEOL AccuTOF mass spectrometer (JEOL, Tokyo, Japan).
IR spectra were collected using neat samples on a Nicolet iS5 infrared spectrometer (ThermoFisher Scientific, Waltham, MA, USA). Band positions are given in reciprocal centimeters (cm−1) and relative intensities are listed as s (strong), m (medium), w (weak) or br (broad).
Melting points were taken in soft glass capillary tubes using an uncalibrated Mel-Temp II capillary melting point apparatus (Barnstead International, Dubuque, IA, USA).
Of the total, 38 compounds were tested for antimicrobial activity towards E. coli, Bacillus subtilis, Staphyloccus aueus, Salmonella typhimurium, Sinomonas atrocyanea and Rhodococcus erythropolis using the paper diffusion method. The selection of compounds for testing was made based on grouping compounds according to their functional groups.
Supplementary Material includes all new compound spectral data.
2.2. Experimental Procedure for Thiazolidine-2,4-dione (6)
A mixture of thiourea (4, 3.34 g, 43.4 mmol) and monochloroacetic acid (5, 4.16 g, 44.0 mmol) in 8 mL of water was added to a 15 mL pressure vial equipped with a stir bar. The reaction mixture was allowed to stir for 1 h at room temperature and microwave irradiated at 110 °C and 350 W for 12 min. (2 min. ramp, 10 min. sustain). The resulting solution was cooled and stirred at room temperature for 1 h. The precipitate was recrystallized from water to produce the product as a white crystalline solid (4.57 g) in 90% yield.
2.3. General Experimental Procedure for Compound 7
A mixture of substituted aryl aldehyde (1.00 mmol), thiazolidine-2,4-dione (6, 1.50 mmol), silica gel (200 mg), 5 drops (~0.25 mL) of both acetic acid and piperidine in 2 mL toluene were added to a Synthware pressure vial equipped with a stir bar. The mixture was microwave irradiated for 25 min at 110 °C and 300 W (5 min. ramp at 500 W, 20 min. sustain). The resulting mixture was diluted with 4 mL of water and precipitated on ice for 15 min. Silica gel was removed by vacuum filtration and washed with hot methanol and the filtrate was concentrated under reduced pressure. The resulting solid was recrystallized using ethanol and dried in vacuo to give the products as colored solids in 35–75% yield.
2.4. General Experimental Procedure for Compound 8
A mixture of monosubstituted thiazolidine-2,4-dione (1.00 mmol), substituted benzyl bromide (1.00 mmol) potassium hydroxide (100 mg, 1.78 mmol), tertbutylammonium hydrogen sulfate (110 mg, 0.324 mmol) in 2 mL of water and 3 mL of toluene were added to a Synthware pressure vial equipped with a stir bar. The reaction mixture was microwave irradiated at 85 °C and 250 W for 45 min while pausing every 2 min and the reaction vial shaken to obtain sufficient agitation (5 min ramp, 40 min sustain). The resulting reaction mixture was diluted with 5 mL of water, extracted twice with 30 mL of ethyl acetate, washed with 20 mL of water, and dried with magnesium sulfate. The magnesium sulfate was filtered, the filtrate concentrated under reduced pressure, rinsed with 20 mL of ethanol and the solid dried in vacuo resulting in the products as colored, textured solids in 27–97% yield.
2.5. General Experimental Procedure for Compounds 9 and 10
A mixture of disubstituted thiazolidine-2,4-dione (100 mg) and 20% palladium hydroxide on activated carbon (120 mg, 0.855 mmol) in 20 mL of methanol was added to a 30 psi pressure vial and shaken by a pressurized reaction apparatus at 15 psi under hydrogen atmosphere for 15 h. The resulting mixture was filtered using celite, dried with silica gel and concentrated under reduced pressure to give the products as solids in 43–98% yield.
3. Results and Discussion
Overall, 76 thiazolidinedione compounds were synthesized by the synthetic pathway shown in Scheme 1, with the utilization of microwave irradiation. The synthesis of thiazolidinedione-2,4-dione (6) was accomplished by following an established literature procedure using water as the solvent and reagent [22,23,24]. The synthesis of TZD ring 6 was easily scaled-up to provide four grams of the product without any reduction in the yield. Next, Knoevangel condensation of an aldehyde with thiazolidine-2,4-dione 6 was performed resulting in the formation (Scheme 1) of 1-(benzylidene)-3-thiazolidine-2,4-dione (7), by following a modified literature procedure (at a lower temperature and using different work-up procedure) (Table 1) [22]. Ten different aldehydes were chosen to react with TZD 6 based on their electronic (electron-rich and -poor), steric (ortho-substituted), and hydrogen bond donor (containing OH group) properties. Overall, the reaction provided the desired coupled derivatives of 7 in moderate to good yields. In general, electron-poor aldehyde derivatives (Table 1, 7E–G) led to slightly better results compared to electron-rich derivatives (Table 1, 7B–D). Both hydroxyl-containing aldehydes worked under the conditions, however, 2-hydroxybenzaldehyde (Table 1, 7J) resulted in a lesser yield compared to 4-hydroxybenzaldehyde (Table 1, 7I).

Table 1.
Knoevangel condensation of thiazolidine-2,4-dione.
Seven out of ten derivatives of TZD 7 were successfully carried further in derivatization efforts for N-benzylation with various benzyl bromides resulting in the formation of thiazolidine-2,4-dione derivative 8 (Table 2).

Table 2.
N-benzylated derivatives of Compound 7.
TZD 7H–J in Table 1 gave extremely poor yields in reacting with benzyl bromide derivatives, and it was difficult to purify their reaction mixtures. Thus, these results do not appear in Table 2. N-benzylation of the TZD ring with benzyl bromides proved to be the critical step due to the fact that excessive agitation of the reaction mixture was required in order to achieve acceptable yields. We were able to synthesize 33 variations of compound 8 by using a phase transfer catalyst in a biphasic reaction mixture under microwave heating. Except for a few (Table 2, 8C-5, 8E-6, and 8G-4), most of the derivatives were obtained in good to very good yields. The reaction did not appear to depend upon the electronic or steric nature of the benzyl bromide derivatives, since it provided mixed results with various derivatives. For example, sterically hindered and electron-poor 2-chlorobenzyl bromide provided good yields in the synthesis of derivatives 8A-4, 8E-4, and 8F-3 (71–86% yield) while providing moderate yields in the synthesis of derivatives 8C-4, 8D-4, and 8G-4 (41–56% yield). N-benzylation using phase transfer catalysis either provided pure products after a simple work-up or provided a complex mixture of products and starting materials with very low yield.
Finally, in order to test the importance of rigidity around C1 of the TZD ring, the benzylidene double bond of compound 8 was reduced to give racemic mixtures of fully functionalized compound 9 (Table 3). The reduction reaction was initially performed using magnesium in methanol by following a literature procedure [22], which resulted in no products even after several optimization efforts of reaction conditions. However, reduction of the olefin using palladium hydroxide in methanol under hydrogen pressure led to the desired product with very good yields. In addition, the same procedure was tested in the reduction of select derivatives of 7, which successfully provided compounds 10 in very good yields (Table 4).

Table 3.
Olefin reduction of Compound 8 derivatives.

Table 4.
Olefin reduction of 1-(sub. benzylidene)-3-thiazolidine-2,4-dione (7).
Of the 76 compounds synthesized, 39 were tested for antimicrobial activity against E. coli, Bacillus subtilis, Staphylococcus aureus, Salmonella typhimurium, Sinomonas atrocyanea and Rhodococcus erythropolis (Table 5). Compounds 7A and 7E were found to have antimicrobial properties toward Bacillus subtilis, Staphylococcus aureus, Sinomonas atrocyanea and Rhodococcus erythropolis. Microorganisms were chosen based on what was available to hand at the time of testing with no further reasoning. Further activity tests will be conducted on the synthesized derivatives in the future.

Table 5.
The results of antimicrobial testing using paper disk method [25] 1.
4. Conclusions
In conclusion, we developed a simple, efficient, and cost-effective synthesis of a thiazolidinedione compound library with the use of microwave irradiation and phase transfer catalysis. It should be noted all products were obtained with a simple work-up without column chromatography. Future studies will entail expansion of the compound library, improvements in yield and further biological evaluation of the compounds.
Supplementary Materials
https://www.mdpi.com/article/10.3390/compounds2030013/s1, Table S1: 1H and 13C-NMR spectral data for compound 7 derivatives; Table S2: 1H and 13C-NMR spectral data for compound 8 derivatives; Table S3: 1H and 13C-NMR spectral data for compound 9 derivatives; Table S4: 1H and 13C-NMR spectral data for compound 10 derivatives.
Author Contributions
Conceptualization, F.D.; methodology development, F.D., A.A.S. and J.P.B.; performing reactions and formal analysis, A.A.S., J.P.B. and R.C.; writing—original draft preparation, J.P.B.; writing—review and editing, F.D.; supervision and project administration, F.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors gratefully acknowledge SUNY at Oswego for providing the Student Scholarly Creativity and Activity Grant and the Department of Chemistry for providing additional funds. Special thanks to Michael Knopp (The University of Maine at Presque Isle) and to Wataru Kitagawa of AIST Hokkaido for performing the antimicrobial cultures.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Forouhi, N.G.; Wareham, N.J. Epidemiology of diabetes. Medicine 2010, 38, 602–606. [Google Scholar] [CrossRef]
- Shaw, J.E.; Sicree, R.A.; Zimmet, P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Statistics Report|Data & Statistics|Diabetes|CDC. Available online: http://www.cdc.gov/diabetes/data/statistics/2014statisticsreport.html (accessed on 7 July 2015).
- Sowers, J.R.; Epstein, M.; Frohlich, E.D. Diabetes, hypertension, and cardiovascular disease: An update. Hypertension 2001, 37, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Chinthala, Y.; Kumar Domatti, A.; Sarfaraz, A.; Singh, S.P.; Kumar Arigari, N.; Gupta, N.; Satya, S.K.V.N.; Kotesh Kumar, J.; Khan, F.; Tiwari, A.K.; et al. Synthesis, biological evaluation and molecular modeling studies of some novel thiazolidinediones with triazole ring. Eur. J. Med. Chem. 2013, 70, 308–314. [Google Scholar] [CrossRef]
- Millioni, R.; Puricelli, L.; Iori, E.; Arrigoni, G.; Tessari, P. The effects of rosiglitazone and high glucose on protein expression in endothelial cells. J. Proteome Res. 2010, 9, 578–584. [Google Scholar] [CrossRef]
- Flowers, E.; Aouizerat, B.E.; Abbasi, F.; Lamendola, C.; Grove, K.M.; Fukuoka, Y.; Reaven, G.M. Circulating MicroRNA-320a and MicroRNA-486 Predict Thiazolidinedione Response: Moving Towards Precision Health for Diabetes Prevention. Metabolism 2015, 64, 1051–1059. [Google Scholar] [CrossRef]
- Hsu, J.C.; Ross-Degnan, D.; Wagner, A.K.; Zhang, F.; Lu, C.Y. How Did Multiple FDA Actions Affect the Utilization and Reimbursed Costs of Thiazolidinediones in US Medicaid? Clin. Ther. 2015, 37, 1420–1432. [Google Scholar] [CrossRef][Green Version]
- Zhu, Z.-N.; Jiang, Y.-F.; Ding, T. Risk of fracture with thiazolidinediones: An updated meta-analysis of randomized clinical trials. Bone 2014, 68, 115–123. [Google Scholar] [CrossRef]
- Helal, M.A.; Darwish, K.M.; Hammad, M.A. Homology modeling and explicit membrane molecular dynamics simulation to delineate the mode of binding of thiazolidinediones into FFAR1 and the mechanism of receptor activation. Bioorg. Med. Chem. Lett. 2014, 24, 5330–5336. [Google Scholar] [CrossRef]
- Mohanty, P.; Aljada, A.; Ghanim, H.; Hofmeyer, D.; Tripathy, D.; Syed, T.; Al-Hadad, W.; Dhindsa, S.; Dandona, P. Evidence for a Potent Antiinflammatory Effect of Rosiglitazone. J. Clin. Endocrinol. Metab. 2004, 89, 2728–2735. [Google Scholar] [CrossRef]
- Keil, A.M.; Frederick, D.M.; Jacinto, E.Y.; Kennedy, E.L.; Zauhar, R.J.; West, N.M.; Tchao, R.; Harvison, P.J. Cytotoxicity of Thiazolidinedione-, Oxazolidinedione- and Pyrrolidinedione-Ring Containing Compounds in HepG2 Cells. Toxicol. In Vitro 2015, 29, 1887–1896. [Google Scholar] [CrossRef]
- Hoffmann, B.R.; El-Mansy, M.F.; Sem, D.S.; Greene, A.S. Chemical Proteomics-Based Analysis of Off-Target Binding Profiles for Rosiglitazone and Pioglitazone: Clues for Assessing Potential for Cardiotoxicity. J. Med. Chem. 2012, 55, 8260–8271. [Google Scholar] [CrossRef]
- Park, K.W.; Kang, J.; Park, J.J.; Yang, H.-M.; Kwon, Y.-W.; Lee, H.-Y.; Kang, H.-J.; Koo, B.-K.; Oh, B.-H.; Park, Y.-B.; et al. Thiazolidinedione usage is associated with decreased response to clopidogrel in DM patients. Int. J. Cardiol. 2013, 168, 608–610. [Google Scholar] [CrossRef]
- Mughal, A.; Kumar, D.; Vikram, A. Effects of Thiazolidinediones on metabolism and cancer: Relative influence of PPARgamma and IGF-1 signaling. Eur. J. Pharmacol. 2015, 768, 217–225. [Google Scholar] [CrossRef]
- Roussel, R.; Hadjadj, S.; Pasquet, B.; Wilson, P.W.F.; Smith, S.C., Jr.; Goto, S.; Tubach, F.; Marre, M.; Porath, A.; Krempf, M.; et al. Thiazolidinedione use is not associated with worse cardiovascular outcomes: A study in 28,332 high risk patients with diabetes in routine clinical practice. Int. J. Cardiol. 2013, 167, 1380–1384. [Google Scholar] [CrossRef]
- Sotiriou, A.; Blaauw, R.H.; Meijer, C.; Gijsbers, L.H.; van der Burg, B.; Vervoort, J.; Rietjens, I.M.C.M. Correlation between activation of PPARγ and resistin downregulation in a mouse adipocyte cell line by a series of thiazolidinediones. Toxicol. Vitr. 2013, 27, 1425–1432. [Google Scholar] [CrossRef]
- He, J.; Xu, C.; Kuang, J.; Liu, Q.; Jiang, H.; Mo, L.; Geng, B.; Xu, G. Thiazolidinediones attenuate lipolysis and ameliorate dexamethasone-induced insulin resistance. Metabolism 2015, 64, 826–836. [Google Scholar] [CrossRef]
- Sinagra, T.; Tamburella, A.; Urso, V.; Siarkos, I.; Drago, F.; Bucolo, C.; Salomone, S. Reversible inhibition of vasoconstriction by thiazolidinediones related to PI3K/Akt inhibition in vascular smooth muscle cells. Biochem. Pharmacol. 2013, 85, 551–559. [Google Scholar] [CrossRef]
- Lecca, D.; Nevin, D.K.; Mulas, G.; Casu, M.A.; Diana, A.; Rossi, D.; Sacchetti, G.; Carta, A.R. Neuroprotective and anti-inflammatory properties of a novel non-thiazolidinedione PPARγ agonist in vitro and in MPTP-treated mice. Neuroscience 2015, 302, 23–35. [Google Scholar] [CrossRef]
- Molero, J.C.; Lee, S.; Leizerman, I.; Chajut, A.; Cooper, A.; Walder, K. Effects of rosiglitazone on intramyocellular lipid accumulation in Psammomys obesus. Biochim. Biophys. Acta 2010, 1802, 235–239. [Google Scholar] [CrossRef]
- Gaonkar, S.L.; Shimizu, H. Microwave-assisted synthesis of the antihyperglycemic drug rosiglitazone. Tetrahedron 2010, 66, 3314–3317. [Google Scholar] [CrossRef]
- Seaquist, E.R.; Miller, M.E.; Fonseca, V.; Ismail-Beigi, F.; Launer, L.J.; Punthakee, Z.; Sood, A. Effect of Thiazolidinediones and Insulin on Cognitive Outcomes in ACCORD-MIND. J. Diabetes Complicat. 2013, 27, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Lidström, P.; Tierney, J.; Wathey, B.; Westman, J. Microwave Assisted Organic Synthesis—A Review. Tetrahedron 2001, 57, 9225–9283. [Google Scholar] [CrossRef]
- Hudzicki, J. Kirby-Bauer Disk Diffusion Susceptibility Test Protocol; American Society for Microbiology: Washington, DC, USA, 2009; Available online: https://asm.org/getattachment/2594ce26-bd44-47f6-8287-0657aa9185ad/Kirby-Bauer-Disk-Diffusion-Susceptibility-Test-Protocol-pdf.pdf (accessed on 17 May 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).