Nano-Thin Oxide Layers Formed on Hydrogen Plasma Modified Crystalline Si for Advanced Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Characterization Methods
3. Results
3.1. Electroreflectance Spectroscopy
3.2. Electrical Properties
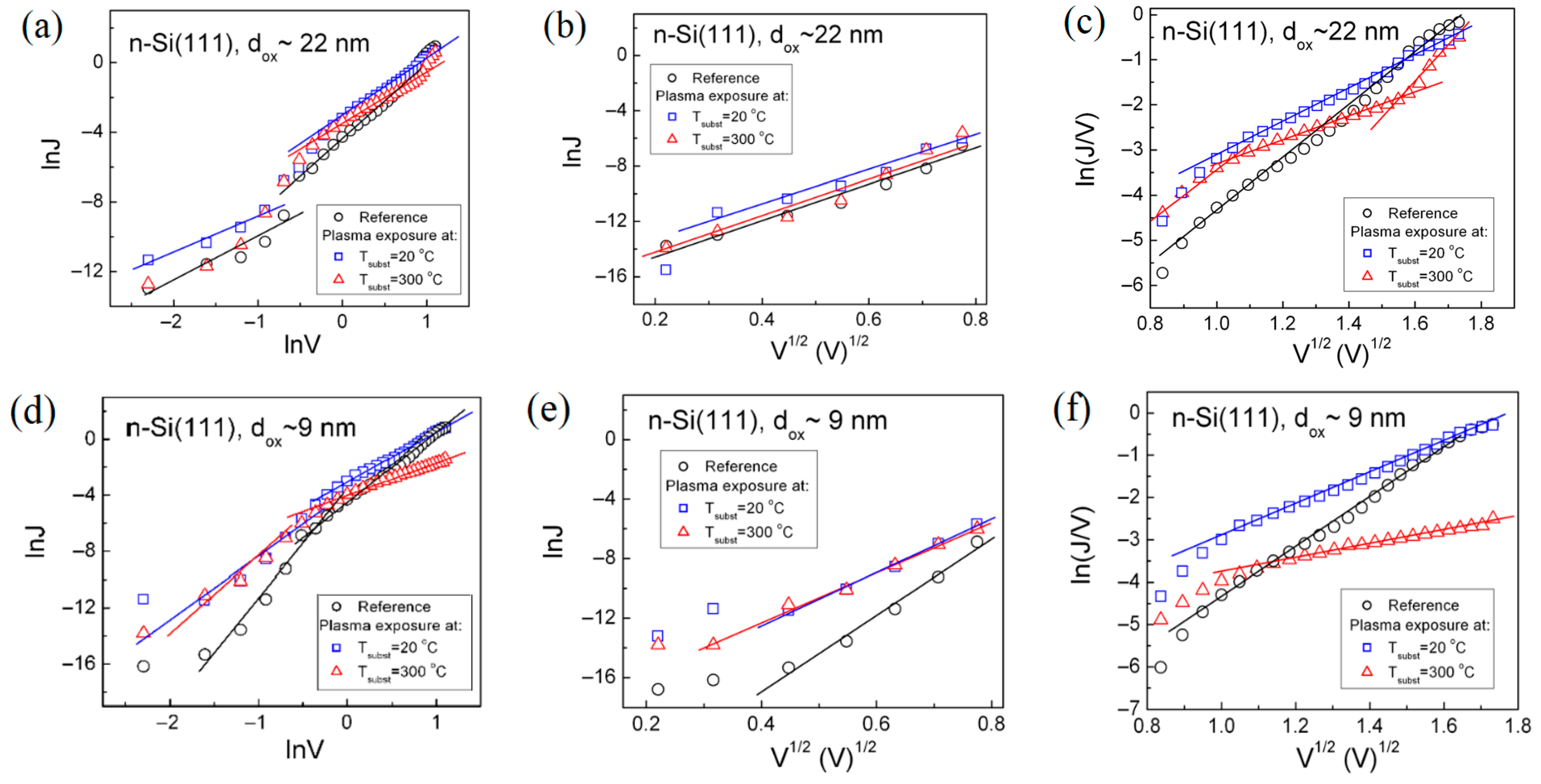
3.2.1. Charge Carrier Transport Through SiO2-Si Structure
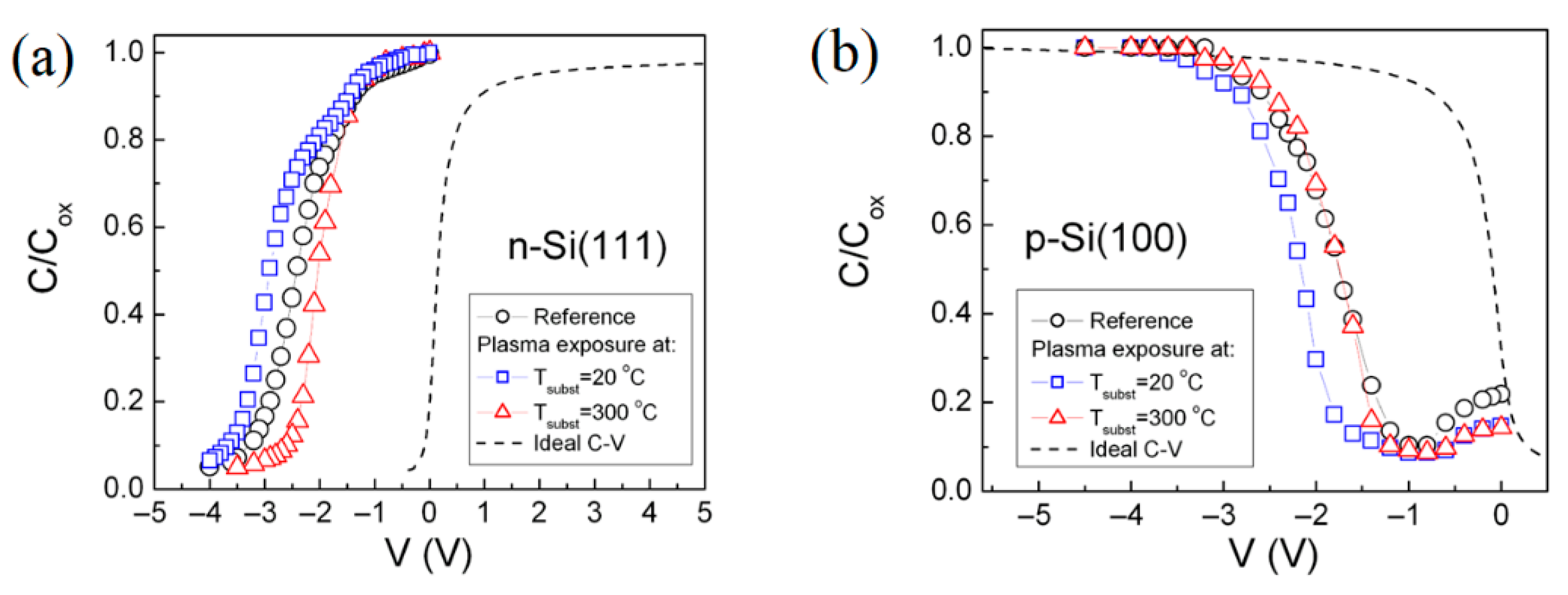
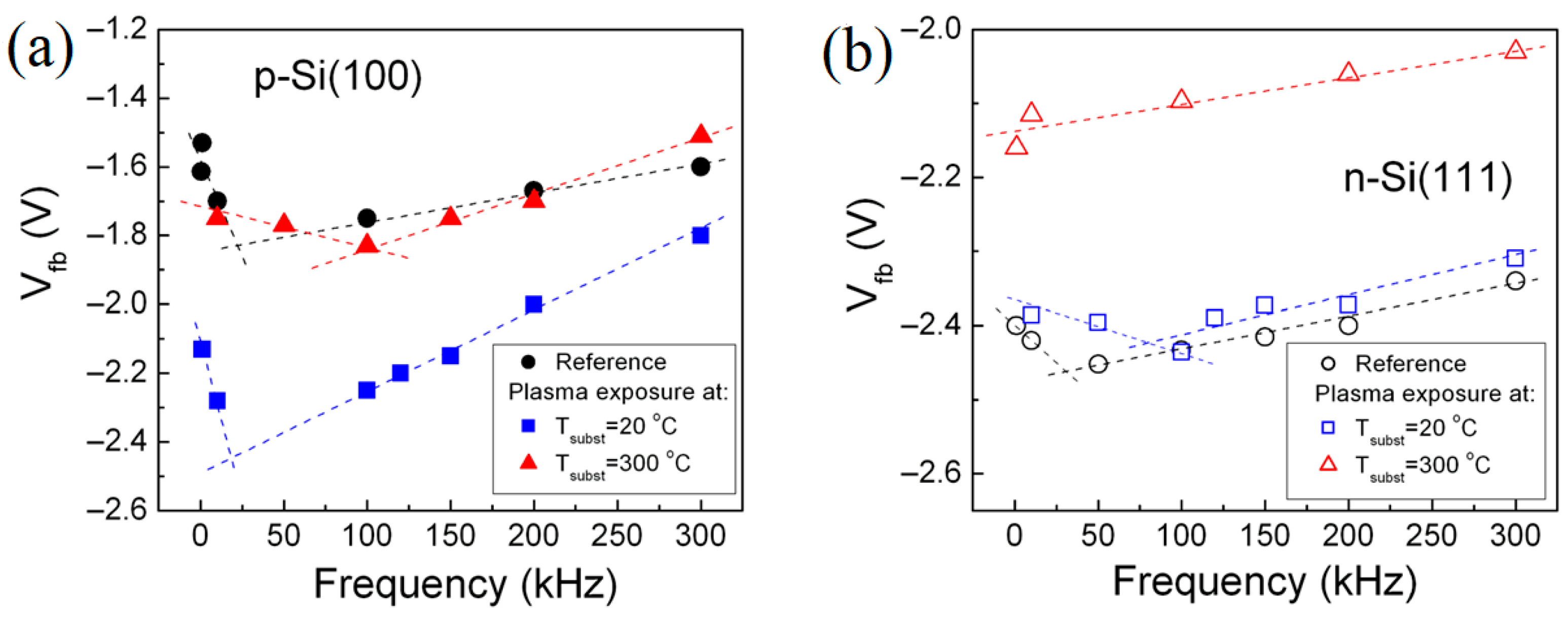
3.2.2. Frequency Dispersion Behavior of the C-V Dependences of the SiO2-Si Structure
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramkumar, K. Charge trapping NVMs with metal oxides in the memory stack. In Metal Oxides for Non-Volatile Memory Materials, Technology and Applications; Dimitrakis, P., Valov, I., Tappertzhofen, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar] [CrossRef]
- Huang, A.P.; Yang, Z.C.; Chu, P.K. Hafnium-based High-k Gate Dielectrics. In Advances in Solid State Circuit Technologies; InTech: Houston, TX, USA, 2010. [Google Scholar] [CrossRef]
- Sharma, U.; Kumar, G.; Mishra, S.; Thomas, R. Advancement of Gate Oxides from SiO2 to High-k Dielectrics in Microprocessor and Memory. J. Phys. Conf. Ser. 2022, 2267, 012142. [Google Scholar] [CrossRef]
- Arul Prishya, A.S.; Chopra, L.; Manikanika. Comprehensive review on uses of silicon dioxide in solar cell. Mater. Today Proc. 2023, 72, 1471–1478. [Google Scholar] [CrossRef]
- Glunz, S.W.; Feldmann, F. SiO2 surface passivation layers–a key technology for silicon solar cells. Sol. Energy Mater. Sol. Cells 2018, 185, 260–269. [Google Scholar] [CrossRef]
- Ferdous, A.R.; Shah, S.S.; Hussain, A.; Mirghni, A.A.; Hardianto, Y.P.; Aziz, M.A. Review on current advancements in silica and their composites for high-performance batteries and supercapacitors. Sustain. Mater. Technol. 2025, 43, e01296. [Google Scholar] [CrossRef]
- Mehonic, A.; Shluger, A.L.; Gao, D.; Valov, I.; Miranda, E.; Ielmini, D.; Bricalli, A.; Ambrosi, E.; Li, C.; Yang, J.J.; et al. Silicon Oxide (SiOx): A Promising Material for Resistance Switching? Adv. Mater. 2018, 30, 1801187. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, M.; Roginskii, E.; Savin, A.; Mazhenov, N.; Pankin, D. Density-Functional Study of the Si/SiO2 Interfaces in Short-Period Superlattices: Structures and Energies. Coatings 2023, 13, 1231. [Google Scholar] [CrossRef]
- Stegemann, B.; Gad, K.M.; Balamou, P.; Sixtensson, D.; Vössing, D.; Kasemann, M.; Angermann, H. Ultra-thin silicon oxide layers on crystalline silicon wafers: Comparison of advanced oxidation techniques with respect to chemically abrupt SiO2/Si interfaces with low defect densities. Appl. Surf. Sci. 2017, 395, 78–85. [Google Scholar] [CrossRef]
- Sze, S.M.; Lee, M.K. Semiconductor Devices: Physics and Technology, 3rd ed.; Wiley: New York, NY, USA, 2012; ISBN 978-0-470-53794-7. [Google Scholar]
- Alexandrova, S.; Szekeres, A. Nano-sized silicon oxide, thermally grown on plasma hydrogenated silicon. J. Optoelectron. Adv. Mater. 2009, 11, 1284–1287. [Google Scholar]
- Szekeres, A.; Alexandrova, S.; Anastasescu, M.; Stroescu, H.; Gartner, M.; Petrik, P. Optical and Morphological Characterization of Nanoscale Oxides Grown in Low-Energy H+-Implanted c-Silicon. Micro 2024, 4, 426–441. [Google Scholar] [CrossRef]
- Turishchev, S.Y.; Terekhov, V.A.; Parinova, E.V.; Korolik, O.V.; Mazanik, A.V.; Fedotov, A.K. Surface modification and oxidation of Si wafers after low energy plasma treatment in hydrogen, helium and argon. Mater. Sci. Semicond. Process. 2013, 16, 1377–1381. [Google Scholar] [CrossRef]
- Zhang, H.; Kumagai, A.; Ge, X.; Ishibashi, K. Low-Temperature Atomic Hydrogen Treatment of SiO2/Si Structures. Jpn. J. Appl. Phys. 2003, 42, 6252–6255. [Google Scholar] [CrossRef]
- Miyagawa, S.; Gotoh, K.; Ogura, S.; Wilde, M.; Kurokawa, Y.; Fukutani, K.; Usami, N. Effect of hydrogen plasma treatment on the passivation performance of TiOx on crystalline silicon prepared by atomic layer deposition. J. Vac. Sci. Technol. A 2020, 38, 022410. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, Y.; Chen, D.; Zhao, T.; Li, D.; Yang, Y. Research on Si/SiO2 Interfaces Characteristics Under Service Conditions. Symmetry 2025, 17, 46. [Google Scholar] [CrossRef]
- Alexandrova, S.; Szekeres, A.; Halova, E. Defects in SiO2/Si Structures Formed by Dry Thermal Oxidation of RF Hydrogen Plasma Cleaned Si. IOP Conf. Ser. Mater. Sci. Eng. 2010, 15, 012037. [Google Scholar] [CrossRef]
- Kern, W.; Puotinen, D. Cleaning Solutions Based on Hydrogen Peroxide for Use in Silicon Semiconductor Technology. RCA Rev. 1970, 31, 187–206. [Google Scholar]
- Misiewicz, J.; Sitarek, P.; Sek, G. Photoreflectance spectroscopy of low-dimensional semiconductor structures. Opto-Electron. Rev. 2000, 8, 1–24. Available online: https://api.semanticscholar.org/CorpusID:116434135 (accessed on 1 January 2006).
- Lewis, E.A.; Irene, E.A. The effect of surface orientation on silicon oxidation kinetics. J. Electrochem. Soc. 1987, 134, 2332–2339. [Google Scholar] [CrossRef]
- Cvitkovich, L.; Waldhör, D.; El-Sayed, A.-M.; Jech, M.; Wilhelmer, C.; Grasser, T. Dynamic modeling of Si (100) thermal oxidation: Oxidation mechanisms and realistic amorphous interface generation. Appl. Surf. Sci. 2023, 610, 155378. [Google Scholar] [CrossRef]
- Grover, J.W.; Handler, P. Electroreflectance of silicon. Phys. Rev. B 1974, 9, 2600–2606. [Google Scholar] [CrossRef]
- Seraphin, B.O. Electroreflectance and the semiconductor surface. Surf. Sci. 1969, 13, 136–150. [Google Scholar] [CrossRef]
- Hong, Z.; Xu, Z. Amorphous SiO2/Si Interface Defects and Mechanism of Passivation/Depassivation Reaction. J. Syst. Simul. 2020, 32, 2362. [Google Scholar] [CrossRef]
- Fleetwood, D.M. Reliability Limiting Defects in MOS Gate Oxides: Mechanisms and Modeling Implications. In Proceedings of the IEEE International Reliability Physics Symposium (IRPS), Monterey, CA, USA, 31 March 2019; pp. 1–10. [Google Scholar] [CrossRef]
- Herzinger, C.M.; Johs, B.; McGahan, W.A.; Woollam, J.A.; Paulson, W. Ellipsometric determination of optical constants for silicon and thermally grown silicon dioxide via a multi-sample, multi-wavelength, multi-angle investigation. J. Appl. Phys. 1998, 83, 3323–3336. [Google Scholar] [CrossRef]
- Fitch, J.T.; Bjorkman, C.H.; Lucovsky, G.; Pollak, F.H.; Yin, X. Intrinsic Stress and Stress Gradients at the SiO2/Si Interface in Structures Prepared by Thermal Oxidation of Si and Subjected to Rapid Thermal Annealing. J. Vac. Sci. Technol. B Microelectron. Process. Phenom. 1989, 7, 775–781. [Google Scholar] [CrossRef]
- Fargeix, A.; Ghibaudo, G. Densification of Thermal SiO2 Due to Intrinsic Oxidation Stressing. J. Phys. D. Appl. Phys. 1984, 17, 2331–2336. [Google Scholar] [CrossRef]
- Gorbach, T.Y.; Holiney, R.Y.; Matiyuk, I.M.; Matveeva, L.A.; Svechnikov, S.V.; Venger, E.F. Electroreflectance spectroscopy and scanning electron microscopy study of microrelief silicon wafer with various surface pretreatments. Semicond. Phys. Quant. Electron. Optoelectron. 1998, 1, 66–70. [Google Scholar] [CrossRef]
- Chiu, F.-C. A Review on Conduction Mechanisms in Dielectric Films. Adv. Mater. Sci. Eng. 2014, 2014, 578168. [Google Scholar] [CrossRef]
- Salas-Rodríguez, S.; Molina-Reyes, J.; Martínez-Castillo, J.; Woo-Garcia, R.M.; Herrera-May, A.L.; López-Huerta, F. Modeling of Conduction Mechanisms in Ultrathin Films of Al2O3 Deposited by ALD. Electronics 2023, 12, 903. [Google Scholar] [CrossRef]
- Alexandrova, S.; Szekeres, A. Oxidation kinetics of hydrogen-enriched Si (100) and Si (111) surfaces. Thin Solid Film. 1999; 343–344, 389–392. [Google Scholar] [CrossRef]
- Kirihara, Y.; Omata, H.; Yasui, A.; Nakagawa, K.; Mitani, Y.; Nohira, H. Evaluation of the effect of hydrogen plasma treatment on the chemical bonding state and distribution of hydrogen in silicon nitride films by angle-resolved hard X-ray photoelectron spectroscopy. Jpn. J. Appl. Phys. 2025, 64, 01SP29. [Google Scholar] [CrossRef]
- Szekeres, A.; Lytvyn, P.; Alexandrova, S. AFM surface imaging of thermally oxidized hydrogenated crystalline silicon. Appl. Surf. Sci. 2002, 191, 148–152. [Google Scholar] [CrossRef]
- Szekeres, A.; Alexandrova, S.; Petrik, P.; Fodor, B.; Bakalova, S. Ellipsometric Study of Crystalline Silicon Hydrogenated by Plasma Immersion Ion Implantation. Appl. Surf. Sci. 2013, 281, 105–108. [Google Scholar] [CrossRef]
- Fedorenko, Y.G. Ion-Beam-Induced Defects in CMOS Technology: Methods of Study. In Ion Implantation-Research and Application; Ahmad, I., Ed.; InTech: Houston, TX, USA, 2017; Chapter 4. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Ono, Y. Charge pumping current from single Si/SiO2 interface traps: Direct observation of Pb centers and fundamental trap-counting by the charge pumping method. Jpn. J. Appl. Phys. 2015, 54, 04DC01. [Google Scholar] [CrossRef]
- Sheikholeslam, S.A.; Manzano, H.; Grecu, C.; Ivanov, A. Hydrogen dissociation and diffusion near the Si/a-SiO2 interface: Understanding degradation in MOSFETs. Superlattices Microstruct. 2018, 120, 561–568. [Google Scholar] [CrossRef]
- Rashkeev, S.N.; Fleetwood, D.M.; Schrimpf, R.D.; Pantelides, S.T. Defect generation by hydrogen at the Si-SiO2 interface. Phys. Rev. Lett. 2001, 87, 165506. [Google Scholar] [CrossRef] [PubMed]
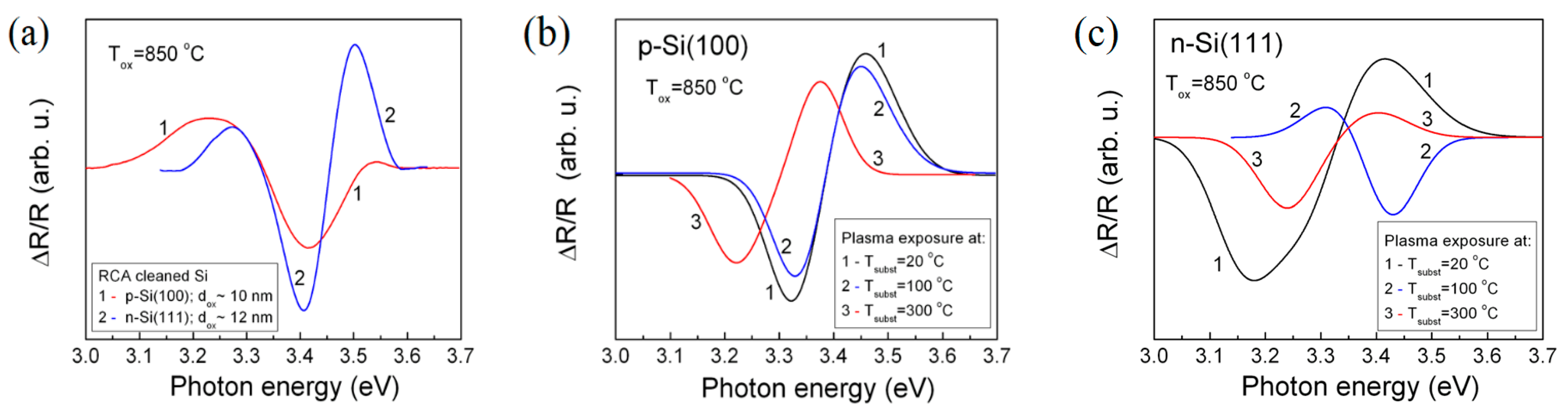
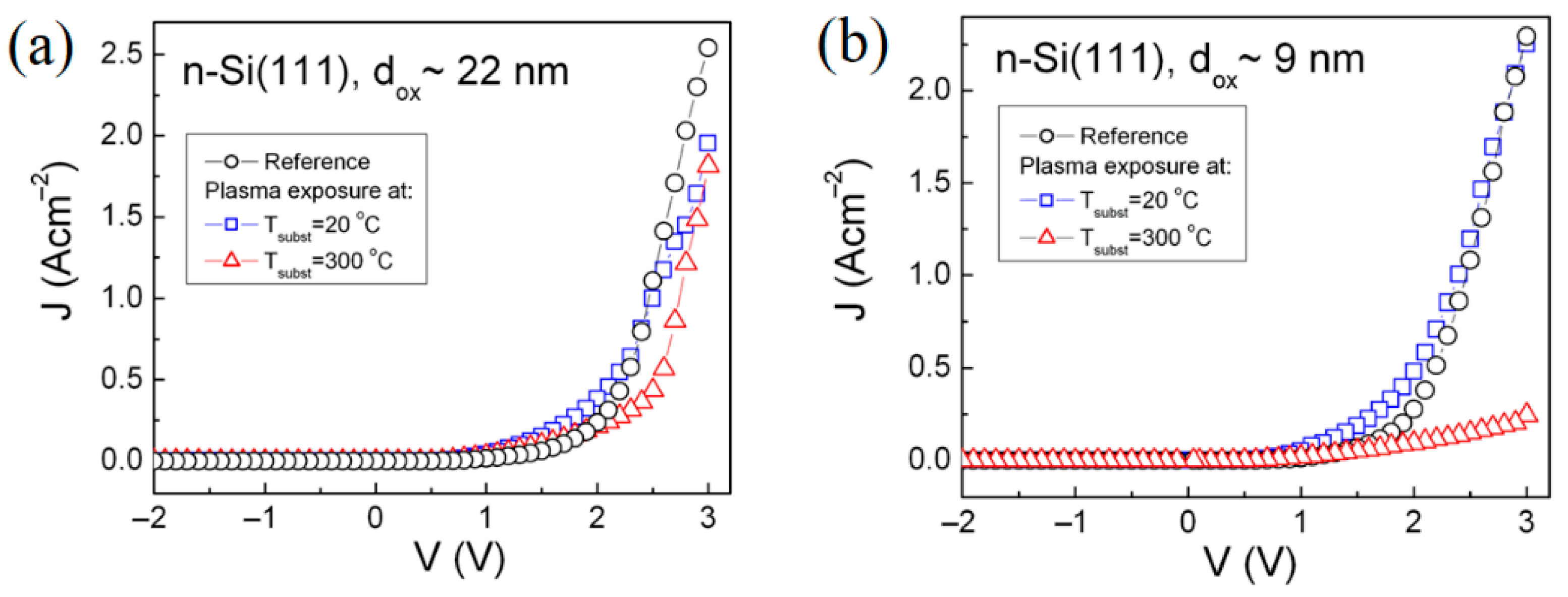

| Type of Substrate | Tsubst (°C) | Polarity | Direct Energy gap, (eV) | Broadening Parameter, Γ (meV) | Internal Stress, (108 Nm−2) | Normalized Mobility, |
|---|---|---|---|---|---|---|
| p-Si(100) | RCA | “+” | 3.3525 | 150 | 9.82 | 1.00 |
| 20 | “−” | 3.3710 | 105 | 6.1 | 1.42 | |
| 100 | “−” | 3.3780 | 110 | 4.6 | 1.36 | |
| 300 | “−” | 3.3600 | 132 | 8.36 | 1.15 | |
| n-Si(111) | RCA | “−” | 3.365 | 164 | 7.3 | 1.00 |
| 20 | “−” | 3.351 | 175 | 10.24 | 0.94 | |
| 100 | “+” | 3.368 | 123 | 6.7 | 1.33 | |
| 300 | “−” | 3.370 | 130 | 6.06 | 1.26 |
| Conduction Mechanism | Analytical Expression | Conduction Plot | References |
|---|---|---|---|
| Schottky Emission (SE) | ln(J) vs. E1/2 | [10,30] | |
| Poole–Frenkel (P-F) Emission | ln(J/E) vs. E1/2 | [10,30] | |
| Fowler–Nordheim (F-N) Tunneling | ln(J/E2) vs. 1/E | [10,30] | |
| Trap Assisted Tunneling (TAT) | ln(JE) vs. 1/E | [30,31] |
| Type of Substrate | Tsubst (°C) | Fixed Oxide Charge Density, (1012 cm−2) | Interface Trap Density, (1011 cm−2) | Density of Slow Interface Traps, (1012 cm−2) |
|---|---|---|---|---|
| p-Si(100) | RCA | 2.83 | 4.40 | 2.30 |
| 20 | 1.44 | 8.83 | 2.52 | |
| 300 | 1.53 | 5.20 | 2.39 | |
| n-Si(111) | RCA | 2.30 | 79 | 2.30 |
| 20 | 2.33 | 86 | 2.14 | |
| 300 | 2.03 | 58 | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexandrova, S.; Szekeres, A.; Valcheva, E. Nano-Thin Oxide Layers Formed on Hydrogen Plasma Modified Crystalline Si for Advanced Applications. Nanomanufacturing 2025, 5, 12. https://doi.org/10.3390/nanomanufacturing5030012
Alexandrova S, Szekeres A, Valcheva E. Nano-Thin Oxide Layers Formed on Hydrogen Plasma Modified Crystalline Si for Advanced Applications. Nanomanufacturing. 2025; 5(3):12. https://doi.org/10.3390/nanomanufacturing5030012
Chicago/Turabian StyleAlexandrova, Sashka, Anna Szekeres, and Evgenia Valcheva. 2025. "Nano-Thin Oxide Layers Formed on Hydrogen Plasma Modified Crystalline Si for Advanced Applications" Nanomanufacturing 5, no. 3: 12. https://doi.org/10.3390/nanomanufacturing5030012
APA StyleAlexandrova, S., Szekeres, A., & Valcheva, E. (2025). Nano-Thin Oxide Layers Formed on Hydrogen Plasma Modified Crystalline Si for Advanced Applications. Nanomanufacturing, 5(3), 12. https://doi.org/10.3390/nanomanufacturing5030012






