Abstract
A simple method is developed to produce free-standing films of polypyrrole (PPy) in one step. It consists of the interfacial polymerization (without surfactants) of pyrrole (dissolved in chloroform) with an oxidant (ammonium persulfate, dissolved in water). It is observed that the area of the formed film only depends on the size of the interface, achieving the manufacture of PPy films of up to 300 cm2, with a thickness of 200 microns. Transmission electron microscopy (TEM) images show the presence of superimposed nanoplatelets of ca. 100 nm main axis. These nanoparticles seem to aggregate in two dimensions to form the free-standing film. Scanning electron microscopy (SEM) shows a compact surface with nanowires decorating the surface. PPy films show an electrical conductivity of 63 (±3) S cm−1. PPy conductive films are then applied in the construction of an antenna that shows a response in two bands: at 1.52 GHz (−13.85 dB) and at 3.50 GHz (−33.55 dB). The values are comparable to those of other antennas built with different PPy films. The simple synthesis of large-area PPy films in a single step would allow the fabrication of large quantities of electronic elements (e.g., sensors) with uniform properties in a short time.
1. Introduction
Conductive polymer films have various technological applications. These include sensors of volatiles [1]; electromagnetic shielding (Faraday cage) [2]; thermoelectric [3], antistatic [4], and electrochemical muscles [5]; flexible electrodes for LCD [6]; surface heating [7], etc.
Polypyrrole (PPy) is very attractive due to its wide range of technological applications: protective coatings [8], anstistatic films [9], charge storage (supercapacitors) electrodes [10], sensors [11], drug delivery devices [12], electrodialysis systems [13], and photothermal therapy in cancer [14]. The material can be deposited electrochemically as a thin film on conductive substrates [15,16] or produced as a powder [17] by chemical synthesis. PPy exhibits the optical and electrical properties of doped metals or semiconductors with the chemical properties of conventional polymers [18]. The conduction mechanism in PPy is related to the motion of charge carriers (polarons and bipolarons) along the conjugated chain [19]. The doping degree of the polymer (directly related to the oxidation level), approaches 0.25–0.32 per pyrrole ring [20], depending on the type and charge of the anion. This means that one (monocharged) anion binds three to four pyrrole units. The type and size of the ion have a profound effect on the electronic [21], optical [22], and mechanical properties of PPy [23]. While several conducting polymers (e.g., polypyrrole) can be deposited on dielectric polymers by in situ polymerization, the films tend to be thin (<500 nm) [24], and therefore, the surface resistance is very high. On the other hand, the poor adhesion of the conducting polymer film to the substrate can lead to conduction loss, especially under bending.
To the best of our knowledge, free-standing films have not been obtained by simple interfacial polymerization (organic solvent/water interface without surfactive agents). However, structured free-standing films have been obtained using interfacial polymerization with the addition of a surfactant [25,26], affecting the nano/micromorphology. On the other hand, in a recent review, it was shown that the interfacial polymerization of pyrrole renders a dispersion of PPy nanofibers, in a fashion similar to that of polyaniline [27]. Thin free-standing films of polypyrrole have been fabricated through the self-assembly of nanostructures [28,29,30], or by oxidation with air (catalyzed by Cu+2) at the ionic liquid/air interface. Free-standing films of PPy are usually obtained by electrochemical polymerization and delamination [31]. In the present work, we show that large (>300 cm2) free-standing polymer films, made of nanoplatelets, could be easily produced through the interfacial oxidative polymerization of pyrrole.
Since PPy is conductive, it interacts strongly with electromagnetic radiation [32]; therefore, it can be used to build a passive antenna. The passive antenna can be made from different electrically conductive materials. However, not all electrically conductive materials are suitable for the production of lightweight antennas with high flexibility. Currently, microstrip antennas are widely used. Copper materials with varying shapes (foils, strips, films) are mainly used as conductive material. Microstrip antennas can also be built with electrically conductive microstrips of polymer-based composites [33,34]. A microstrip of free-standing PPy film was used to build an antenna, and its properties were measured.
2. Materials and Methods
2.1. Materials
Pyrrole (98%) and ammonium persulfate (APS, 98%) were reagent-grade (Sigma-Aldrich, St. Louis, MI, USA). Chloroform (99.9%) and hydrochloric acid (37%) were of analytical-grade (Mach Chemikalie, Slezská Ostrava, Czech Republic). The water used was purified by inverse osmosis, with a resistivity of 18 Mohms.
2.2. Fabrication of the Free-Staing Films
A solution of pyrrole (0.32 M) in CHCl3 was placed in a container, and a solution of APS (0.08 M in 1 M HCl/H2O) was gently poured on top of it, avoiding the mixing of the solutions. The volume of each phase was calculated, using the interface area, so that each solution formed a uniform liquid layer of at least 3 mm in thickness. For example, the fabrication of a PPy film of ca. 300 cm2 of area required the use of 100 cm3 of solution of both pyrrole and APS. A vessel with the two phases was placed in a fume hood, with a glass lid on the container to prevent evaporation, and left undisturbed for at least 6 h. Subsequently, the solutions were removed, and the film was gently washed with water and ethanol and dried at 60 °C with air circulation.
2.3. Characterization
Optical photographs were taken with the camera (200 Mpixels) of a smartphone (Redmi 15 G, Xiaomi, Beijing, China). Electrical conductivity was measured using the four-point method with a precision mustimeter (Agilent, Santa Clara, CA, USA, 34401A). Scanning electron microscopy (SEM) images were obtained with a Carl Zeiss EVO MA10 microscope in high-vacuum mode. Since the material was an electrical conductor, metallization was not required. Transmission electron microscopy images were obtained with a JEOL-1400 120 kV microscope. The film was disaggregated by ultrasound in ethanol, and a small drop of the dispersion was applied to a Formvar grid.
2.4. Building the Antenna
A piece of polypyrrole free-standing film (fsPPyf) was glued onto the substrate made of an electrically non-conductive material: Polymethylmethacrylate (PMMA) as shown in Scheme 1.
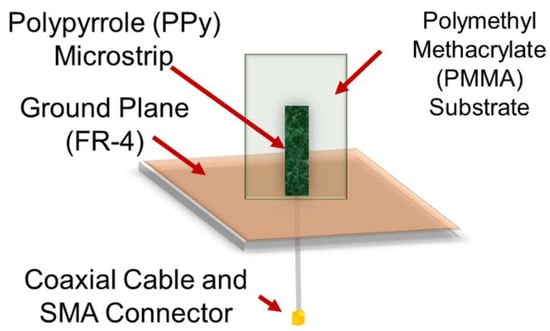
Scheme 1.
Schematic of set-up of antenna made of polypyrrole (PPy) microstrip, ground plane, and coaxial cable.
A strip of fsPPyf with a length of 40 mm and a width of 10 mm was glued to a PMMA support, which consisted of a rectangular piece with a length of 50 mm and a width of 40 mm, anchored to a ground plane made of an epoxy (Flame Retardant 4) layer on one side plated with copper. The PMMA piece with the attached PPy strip was at 90° of the ground plane. The thickness of the polypyrrole (PPy) layer was 200 µm.
The main part of the antenna (PPy strip) was connected using conductive silver paste to the gold-plated micro-SMA connector of a semi-rigid coaxial cable (50 Ω impedance). The main function of the silver paste is to minimize signal loss during transmission between the coaxial line and the microstrip antenna. This joining method was chosen because PPy/PMMA substrates do not allow welding with molten metal alloys.
2.5. Characterization of Antenna Properties
The antenna measurement was performed using a spectrum analyzer type N9912A FieldFox Handheld RF with a measuring range within 2 MHz to 4 GHz. With this spectrum analyzer, parameter S11 was measured; this parameter determines the best frequencies of impedance matching for the antenna.
3. Results and Discussion
3.1. Free-Standing Film Fabrication
The oxidative interfacial polymerization of pyrrole was performed by contacting a solution of pyrrole in CHCl3 with a solution of an oxidant solution (ammonium persulfate) in water. The interface darkens slowly, indicating the formation of a PPy film. The aim was the formation of nanofibers, as occurs in the case of the interfacial polymerization of aniline, but a darkish film was obtained instead. It is noteworthy that other monomers (aniline, N-methylaniline, o-phenylenediamine) of conducting polymers were similarly tested. In all cases, dispersions of nanoparticles in the water phase were obtained, without the formation of a film at the interphase.
However, in the case of pyrrole, the polymer (insoluble in both phases) was deposited at the CHCl3/H2O interface and formed a mechanically stable film (Figure 1), which could be removed by extracting the liquids after completion. The size of the film directly depends on the size of the interface (Figure 1), defined by the diameter of the vessel containing the interface, as can be seen in Figure 2.

Figure 1.
Photograph of the two-phase polymerization set-up, at an advanced state of film formation.

Figure 2.
Photograps of FEPPyF of increasing sizes.
The film disks have diameters of 9, 7, and 5 cm (from left to right), corresponding approximately to the internal sizes of the containers used. The ripples observed in the films (most noticeable in the larger one (Figure 2, left) probably originate from waves in the liquids created at the interface during the slow (>6 h) film manufacture, related to mechanical vibrations in the fumehood. However, the film can be made flat by holding it tightly between two glass plates during drying (at 60 °C). It seems that the wet film has enough plasticity to flatten out.
In each case, the volume of both liquid phases must be adjusted to obtain continuous phases covering the entire surface. Since there does not seem to be a limit to the area of fePPyf, polymerization was attempted in a much larger, rectangular vessel.
In this way, the fsPPyf shown in Figure 3 was obtained, which has dimensions of 22 × 14 cm, i.e., ca. 300 cm2. To the best of our knowledge, this is the largest free-standing film of PPy (or any conducting polymer) manufactured.

Figure 3.
Photograph of large free-standing film of polypyrrole (fsPPyf).
3.2. Film Characterization
SEM images of a typical film (Figure 4) show a continuous surface with ribbon-like structures. This is in contrast to solution-formed PPy, which exhibits a granular structure [32]. Since the film is produced by polymerization and not by evaporation from solution, film formation could occur through an intermediate state of nanofibers or other large aspect ratio nanostructures that can interact with each other.
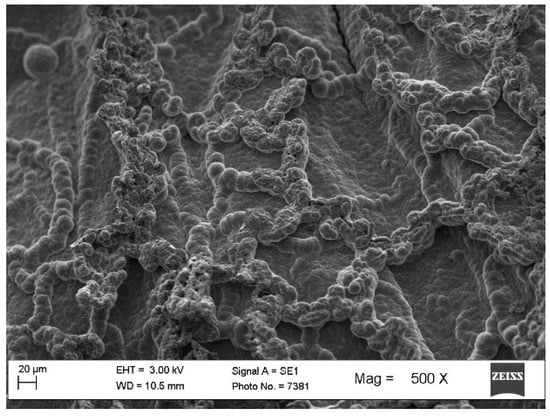
Figure 4.
Scanning electron microscopy image of a typical fsPPy film surface.
From the SEM micrograph, a free volume of ca. 0.65 can be calculated (using ImageJ 1.54p 17 [35]). However, such a value reflects only the surface of the film, of which its topography is measured by SEM.
On the other hand, the TEM image of the ultrasound-disintegrated film in ethanol shows the presence of superimposed nanoplatelets. The observation of the uppermost nanoplatelet suggests a size of ca. 100 nm along the long axis, with ca. 55 nm along the short axis (Figure 5). The aggregates of those platelets could form ribbons/cords (as shown in the SEM and TEM) but also superimpose evenly to form flat areas, as also observed in the SEM (Figure 4).
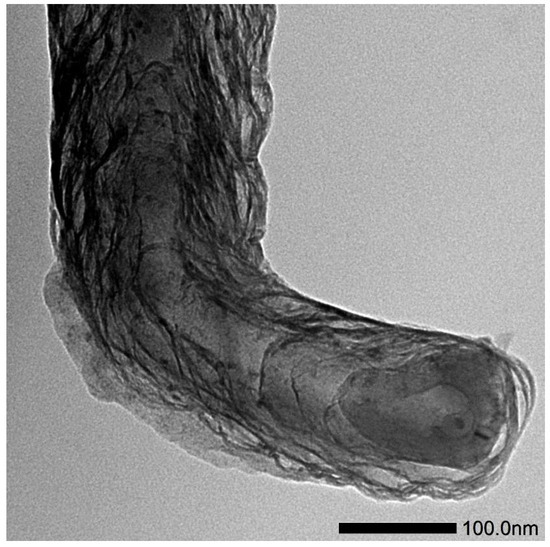
Figure 5.
Transmission electron microscopy (TEM) of fsPPyF fragment.
Polypyrrole was oxidized to a radical cation using a persulfate ion (Scheme 2), and this attacks a neutral molecule, generating a dimer that continues to grow until a polymeric chain is formed. In addition to the reaction in positions 2 and 5, it is possible to generate branches by attack in positions 3 and 4. These branches can crosslink neighboring chains forming nanoribbons or, in connecting several chains, nanoplatelets.
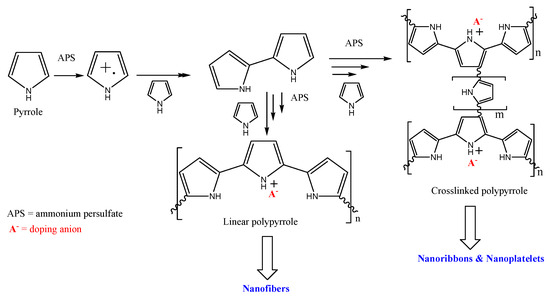
Scheme 2.
Mechanism of pyrrole polymerization and nanostructure formation.
The film is continuous, with a typical thickness of 200 μm. It exhibits electrical conductivity between all its points (even in the large film). The average conductivity is 63 (±3) Scm−1.
The measured conductivity values of the PPy films seem quite high. Indeed, the comparison with the data reviewed by Ling Pang et al. [36] shows that only ca. 4% of the reported conductivity values are equal or higher than the one measured here (Figure 6).
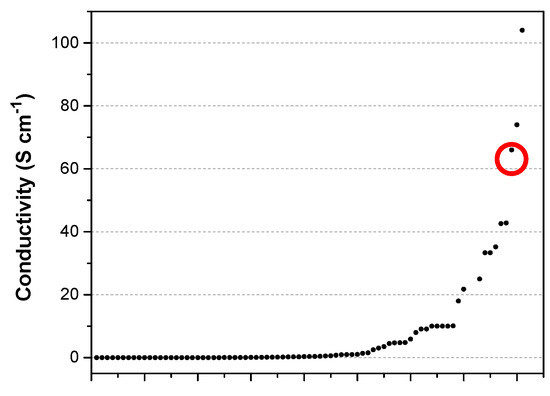
Figure 6.
Plot of conductivity values obtained from table 3 of ref. [36]. The red circle shows the conductivity of the free-standing films formed in this work.
3.3. Measuring Antenna Properties
One application of conductive films is the creation of antennas for electromagnetic radiation [37]. A sample of fePPyf was mounted in a suitable configuration (Figure 7), and its properties were measured. The antenna operates in a perpendicular configuration that allows us to measure the properties of the conducting polymer film [37].

Figure 7.
Photograph of the antenna containing a free-standing polypyrrole film.
The antenna response shows peaks at frequencies of 1.52 GHz and 3.50 GHz (Figure 8). The return loss was determined to be −13.85 dB for 1.52 GHz and −33.55 dB for 3.50 GHz.
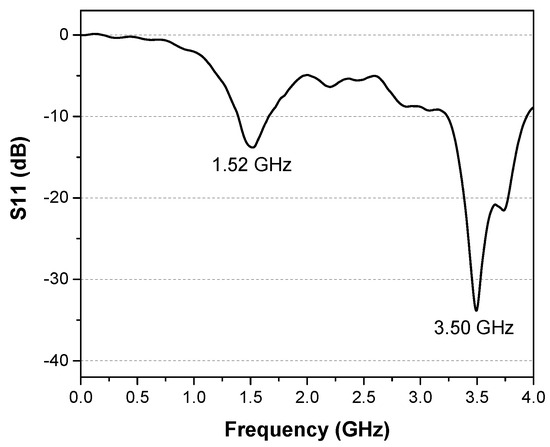
Figure 8.
Measured return loss of the PPy antenna.
Signal values for other antennas built with PPy films are depicted in Table 1.

Table 1.
Comparison of antenna parameters with data from the literature.
Micro-strip patch antennas differ in their structural design, as they have a differently designed ground plane. The antenna described in our research paper has a ground plane on which the PPy antenna radiator is placed perpendicularly. The antennas described in previous research papers are therefore comparable in terms of their parameters; however, it is evident that the antenna design and also the material composition and orientation of the ground plane bring different results in all cases, especially in frequency bands. The antenna described here is a dual-band antenna. Compared to the antennas described in Table 1, it has a similar return loss but at different frequencies.
4. Conclusions
A simple, surfactant-free interfacial polymerization of pyrrole produces free-standing films of polypyrrole. The films are homogeneous and have the same dimensions as the reaction vessel. In this way, films of up to ca. 300 cm2 can be obtained. The electrical conductivity of the films is 63 ± 3 S/cm.
The simple assembly of a rectangular strip of the film, attached perpendicularly to a Cu ground plane, acts as a radio frequency antenna showing large return loss for 1.52 (13.85 dB) and 3.50 GHz (33.55 dB). Therefore, the antenna made using the PPy film acts as a two-band antenna. The frequency of the maximum signal could be tailored to a more useful value (e.g., 2.4 GHz) by changing the geometry of the antenna.
Since it is possible to fabricate films of large dimensions, these films can be used for antennas, radio frequency isolation (Faraday’s cage), pressure sensors, etc. Furthermore, the one-step manufacturing of large areas allows us to produce many pieces of the material with similar properties in one step. For example, from the film with an area of 300 cm2, 300 gas sensors with an area of 1 × 1 cm [42] can be manufactured. This can be achieved by cutting the strips mechanically or with a laser. Besides the higher reproducibility of the sensing strips, the fabrication time decreases by at least two orders of magnitude, making it amenable to industrial production.
The simplicity of the procedure makes it amenable to teaching a polymer chemistry lab, where a conducting film (easily checked) can be produced in a short amount of time.
Interfacial polymerization using other monomers of conducting polymers (aniline, o-phenylenediamine, or N-methylaniline always produce nanofiber dispersions and not free-standing films. It is likely that the formation of PPy nanoplatelets by chain-extended crosslinking makes the structures grow in two dimensions, creating a solid film.
Author Contributions
Conceptualization, C.A.B. and P.S.; methodology, C.A.B., R.O., and J.M.; validation, C.A.B. and P.S.; investigation, C.A.B. and P.S.; resources, C.A.B. and P.S.; data curation, C.A.B., R.O. and J.M.; writing—original draft preparation, C.A.B. and P.S.; writing—review and editing, C.A.B.; visualization, C.A.B.; supervision, C.A.B.; project administration, P.S.; funding acquisition, P.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Education, Youth and Sports of the Czech Republic—DKRVO (RP/CPS/2024-28/005), SECYT-UNRC (PPI), CONICET (PUE of IITEMA), and Williams Foundation (Argentina). C.A. Barbero is a permanent researcher of CONICET.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The TEM measurements by M. Fernandez and SEM measurements by L. Tamborini are gratefully acknowledged. The suggestion by a referee regarding the use of the procedure in teaching is gratefully acknowledged.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Abel, S.B.; Olejnik, R.; Rivarola, C.R.; Slobodian, P.; Saha, P.; Acevedo, D.F.; Barbero, C.A. Resistive Sensors for Organic Vapors Based on Nanostructured and Chemically Modified Polyanilines. IEEE Sens. J. 2018, 18, 6510–6516. [Google Scholar] [CrossRef]
- Li, P.; Du, D.; Guo, L.; Guo, Y.; Ouyang, J. Stretchable and Conductive Polymer Films for High-Performance Electromagnetic Interference Shielding. J. Mater. Chem. C 2016, 4, 6525–6532. [Google Scholar] [CrossRef]
- Song, E.; Liu, P.; Yifan, L.; Wang, E.; Guo, C.-Y. Conductive Polymer-Based Thermoelectric Composites: Preparation, Properties, and Applications. J. Compos. Sci. 2024, 8, 308. [Google Scholar] [CrossRef]
- Goyal, M.; Singh, K.; Bhatnagar, N. Conductive Polymers: A Multipurpose Material for Protecting Coating. Prog. Org. Coat. 2024, 187, 108083. [Google Scholar] [CrossRef]
- Otero, T.F.; Beaumont, S. The Cooperative Actuation of Polypyrrole Electrochemical Machines Senses the Chemical Conditions as Muscles Sense Their Fatigue State. Sens. Actuators B Chem. 2018, 263, 493–501. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Sohn, D.; Kim, E.-R. Polymer-Based Multi-Layer Conductive Electrode Film for Plastic LCD Applications. Appl. Phys. A 2001, 72, 699–704. [Google Scholar] [CrossRef]
- Koncar, V.; Cochrane, C.; Lewandowski, M.; Boussu, F.; Dufour, C. Electro-Conductive Sensors and Heating Elements Based on Conductive Polymer Composites. Int. J. Cloth. Sci. Technol. 2009, 21, 82–92. [Google Scholar] [CrossRef]
- Yin, Y.; Prabhakar, M.; Ebbinghaus, P.; da Silva, C.; Rohwerder, M. Neutral inhibitor molecules entrapped into polypyrrole network for corrosion protection. Chem. Eng. J. 2022, 440, 135739–135753. [Google Scholar] [CrossRef]
- Ismael, A.; Guessasma, S.; Bozek, A.; Durand, S.; Papineau, P.; Le Duigou, A.; Castro, M.; Beaugrand, J.; Villares, A. Conductive and Thermoactivated Flax Yarns Developed by in Situ Polypyrrole Polymerizations: Interactions with Carbohydrate Polymers. Prog. Org. Coat. 2024, 198, 108894. [Google Scholar] [CrossRef]
- Chen, Y.; Kang, G.; Xu, H.; Kang, L. PPy doped with different metal sulphate as electrode materials for supercapacitors. Russ. J. Electrochem. 2017, 53, 359–365. [Google Scholar] [CrossRef]
- El Guerraf, A.; Ben Jadi, S.; Karadas Bakirhan, N.; Eylul Kiymaci, M.; Bazzaoui, M.; Aysil Ozkan, S.; Arbi Bazzaoui, E. Antibacterial activity and volatile organic compounds sensing property of polypyrrole-coated cellulosic paper for food packaging purpose. Polym. Bull. 2022, 79, 11543–11566. [Google Scholar] [CrossRef]
- Krukiewicz, K.; Gniazdowska, B.; Jarosz, T.; Herman, A.P.; Boncel, S.; Turczyn, R. Effect of immobilization and release of ciprofloxacin and quercetin on electrochemical properties of poly(3,4-ethylenedioxypyrrole) matrix. Synt. Met. 2019, 249, 52–62. [Google Scholar] [CrossRef]
- Ashu Tufa, R.; Piallat, T.; Hnát, J.; Fontananova, E.; Paidar, M.; Chanda, D.; Curcio, E.; di Profio, G.; Bouzek, K. Salinity gradient power reverse electrodialysis: Cation exchange membrane design based on polypyrrole-chitosan composites for enhanced monovalent selectivity. Chem. Eng. J. 2020, 380, 122461. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, J.; Miao, Z.; Qian, H.; Zha, Z. dl-Menthol Loaded Polypyrrole Nanoparticles as a Controlled Diclofenac Delivery Platform for Sensitizing Cancer Cells to Photothermal Therapy. ACS Appl. Bio Mater. 2019, 2, 848–855. [Google Scholar] [CrossRef]
- Kahvazi Zadeh, M.; Yeganeh, M.; Tavakoli Shoushtari, M.; Esmaeilkhanian, A. Corrosion performance of polypyrrole-coated metals: A review of perspectives and recent advances. Synt. Met. 2021, 274, 116723. [Google Scholar] [CrossRef]
- Golba, S.; Loskot, J. The Alphabet of Nanostructured Polypyrrole. Materials 2023, 16, 7069. [Google Scholar] [CrossRef]
- Kausaite-Minkstimiene, A.; Mazeiko, V.; Ramanaviciene, A.; Ramanavicius, A. Evaluation of Chemical Synthesis of Polypyrrole Particles. Colloids Surf. A Physicochem. Eng. Asp. 2015, 483, 224–231. [Google Scholar] [CrossRef]
- Xiao, H.-M.; Fu, S.-Y. Synthesis and Physical Properties of Electromagnetic Polypyrrole Composites via Addition of Magnetic Crystals. CrystEngComm 2013, 16, 2097–2112. [Google Scholar] [CrossRef]
- Zotti, G.; Schiavon, G. Spin and Spinless Conductivity in Polypyrrole. Evidence for Mixed-Valence Conduction. Synth. Met. 1991, 41, 445–448. [Google Scholar] [CrossRef]
- Vernitskaya, T.V.; Efimov, O.N. Polypyrrole: A conducting polymer; its synthesis, properties and applications. Russ. Chem. Rev. 1997, 66, 443–457. [Google Scholar] [CrossRef]
- Ouyang, J.; Li, Y. Effect of Electrolyte Solvent on the Conductivity and Structure of As-Prepared Polypyrrole Films. Polymer 1997, 38, 1971–1976. [Google Scholar] [CrossRef]
- Thombare, J.V.; Shinde, S.K.; Lohar, G.M.; Chougale, U.M.; Dhasade, S.S.; Dhaygude, H.D.; Relekar, B.P.; Fulari, V.J. Optical Properties of Electrochemically Synthesized Polypyrrole Thin Films: The Electrolyte Effect. J. Semicond. 2014, 35, 063001. [Google Scholar] [CrossRef]
- Baek, S.; Green, R.A.; Poole-Warren, L.A. Effects of dopants on the biomechanical properties of conducting polymer films on platinum electrodes. J. Biomed. Mater. Res. Part. A 2014, 102A, 2743–2754. [Google Scholar] [CrossRef] [PubMed]
- Stejskal, J.; Sapurina, I. Polyaniline: Thin Films and Colloidal Dispersions (IUPAC Technical Report). Pure Appl. Chem. 2005, 77, 815–826. [Google Scholar] [CrossRef]
- Yang, Q.; Hou, Z.; Huang, T. Self-Assembled Polypyrrole Film by Interfacial Polymerization for Supercapacitor Applications. J. Appl. Polym. Sci. 2014, 132, 41615. [Google Scholar] [CrossRef]
- Hou, Z.; Lu, H.; Yang, Q.; Zhao, Q.; Liu, J. Micromorphology-Controlled Synthesis of Polypyrrole Films by Using Binary Surfactant of Span80/OP10 via Interfacial Polymerization and Their Enhanced Electrochemical Capacitance. Electrochim. Acta 2018, 265, 601–608. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, F. Synthesis and Application of Polypyrrole Nanofibers: A Review. Nanoscale Adv. 2023, 5, 3606–3618. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Kim, J.-T.; Song, E.-A.; Min, Y.-K.; Hamaguchi, H. Polypyrrole Nanostructures Self-Assembled in Magnetic Ionic Liquid as a Template. Macromolecules 2008, 41, 2886–2889. [Google Scholar] [CrossRef]
- Newman, M.; Doan-Nguyen, V. Electrochemical Delamination for Free-Standing Polypyrrole Doped with Dodecylbenzenesulfonate Films and the Effect of Substrate. J. Electrochem. Soc. 2024, 171, 055503. [Google Scholar] [CrossRef]
- Rani, P.; Malik, R.S. Electromagnetic Interference Shielding Behavior of Polypyrrole-Impregnated Poly (Ether Imide)/Sulfonated Poly (Ether Ether Ketone) Composites. Mater. Chem. Phys. 2023, 307, 128187. [Google Scholar] [CrossRef]
- Huang, J.; Virji, S.; Weiller, B.H.; Kaner, R.B. Polyaniline Nanofibers: Facile Synthesis and Chemical Sensors. J. Am. Chem. Soc. 2002, 125, 314–315. [Google Scholar] [CrossRef] [PubMed]
- Yussuf, A.; Al-Saleh, M.; Al-Enezi, S.; Abraham, G. Synthesis and Characterization of Conductive Polypyrrole: The Influence of the Oxidants and Monomer on the Electrical, Thermal, and Morphological Properties. Int. J. Polym. Sci. 2018, 2018, 4191747. [Google Scholar] [CrossRef]
- Matyas, J.; Munster, L.; Olejnik, R.; Vlcek, K.; Slobodian, P.; Krcmar, P.; Urbanek, P.; Kuritka, I. Antenna of Silver Nanoparticles Mounted on a Flexible Polymer Substrate Constructed Using Inkjet Print Technology. Jpn. J. Appl. Phys. 2016, 55, 02BB13. [Google Scholar] [CrossRef]
- Olejnik, R.; Matyas, J.; Slobodian, P.; Vlcek, K. The Multifunctional Composite on the Base of Carbon Nanotubes Network and Its Use as a Passive Antenna and Gas Sensing Element. Key Eng. Mater. 2014, 605, 322–325. [Google Scholar] [CrossRef]
- Available online: https://imagej.net/ij/ (accessed on 16 February 2025).
- Pang, A.L.; Arsad, A.; Ahmadipour, M. Synthesis and Factor Affecting on the Conductivity of Polypyrrole: A Short Review. Polym. Adv. Technol. 2020, 32, 1428–1454. [Google Scholar] [CrossRef]
- Singh, S.; Dahima, V.; Mishra, R. Exploring the Dynamics of Microstrip Antenna and Radiation Mechanism of Dipole Antennas; CRC Press: Boca Raton, FL, USA, 2024; pp. 102–133. [Google Scholar] [CrossRef]
- Verma, A.; Weng, B.; Shepherd, R.; Christophe, F.; Truong, V.-T.; Wallace, G.G.; Bates, B.D. 6 GHz Microstrip Patch Antennas with PEDOT and Polypyrrole Conducting Polymers. In Proceedings of the 2010 International Conference on Electromagnetics in Advanced Applications, Sydney, Australia, 20–24 September 2010; pp. 329–332. [Google Scholar] [CrossRef]
- Verma, A.; Fumeaux, C.; Truong, V.-T.; Bates, B.D. A 2 GHz Polypyrrole Microstrip Patch Antenna on PlexiglasTM Substrate. In Proceedings of the 2009 Asia Pacific Microwave Conference. In Proceedings of the 2009 Asia Pacific Microwave Conference, Singapore, 7–10 December 2009; pp. 36–39. [Google Scholar] [CrossRef]
- Varghese, L.; Choudhury, B. Conducting Polymer-Based Antennas. In Multiscale Modelling of Advanced Materials; Kumari, R., Choudhury, B., Eds.; Springer Nature: Berlin/Heidelberg, Germany, 2020; ISBN 9789811522673. [Google Scholar]
- Verma, A.; Fumeaux, C.; Truong, V.-T.; Bates, B.D. Effect of Film Thickness on the Radiation Efficiency of a 4.5 GHz Polypyrrole Conducting Polymer Patch Antenna. In Proceedings of the 2010 Asia-Pacific Microwave Conference, Yokohama, Japan, 7–10 December 2010; pp. 95–98. [Google Scholar]
- Navale, S.T.; Mane, A.T.; Chougule, M.A.; Sakhare, R.D.; Nalage, S.R.; Patil, V.B. Highly Selective and Sensitive Room Temperature NO2 Gas Sensor Based on Polypyrrole Thin Films. Synth. Met. 2014, 189, 94–99. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).