Nanoimprinted Polymeric Structured Surfaces for Facilitating Biofilm Formation of Beneficial Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Structure Design
2.2. Nanoimprint Master Fabrication
2.2.1. UV Lithography
2.2.2. Si Etching and Smoothing
2.2.3. Anti-Sticking Layer Coating
2.3. Nanoimprint
2.3.1. Polymer Material
2.3.2. Nanoimprint Parameters
2.4. Characterization of Polymer Surfaces
2.4.1. Laser Confocal Microscope
2.4.2. SEM
2.5. Bacterial Assays
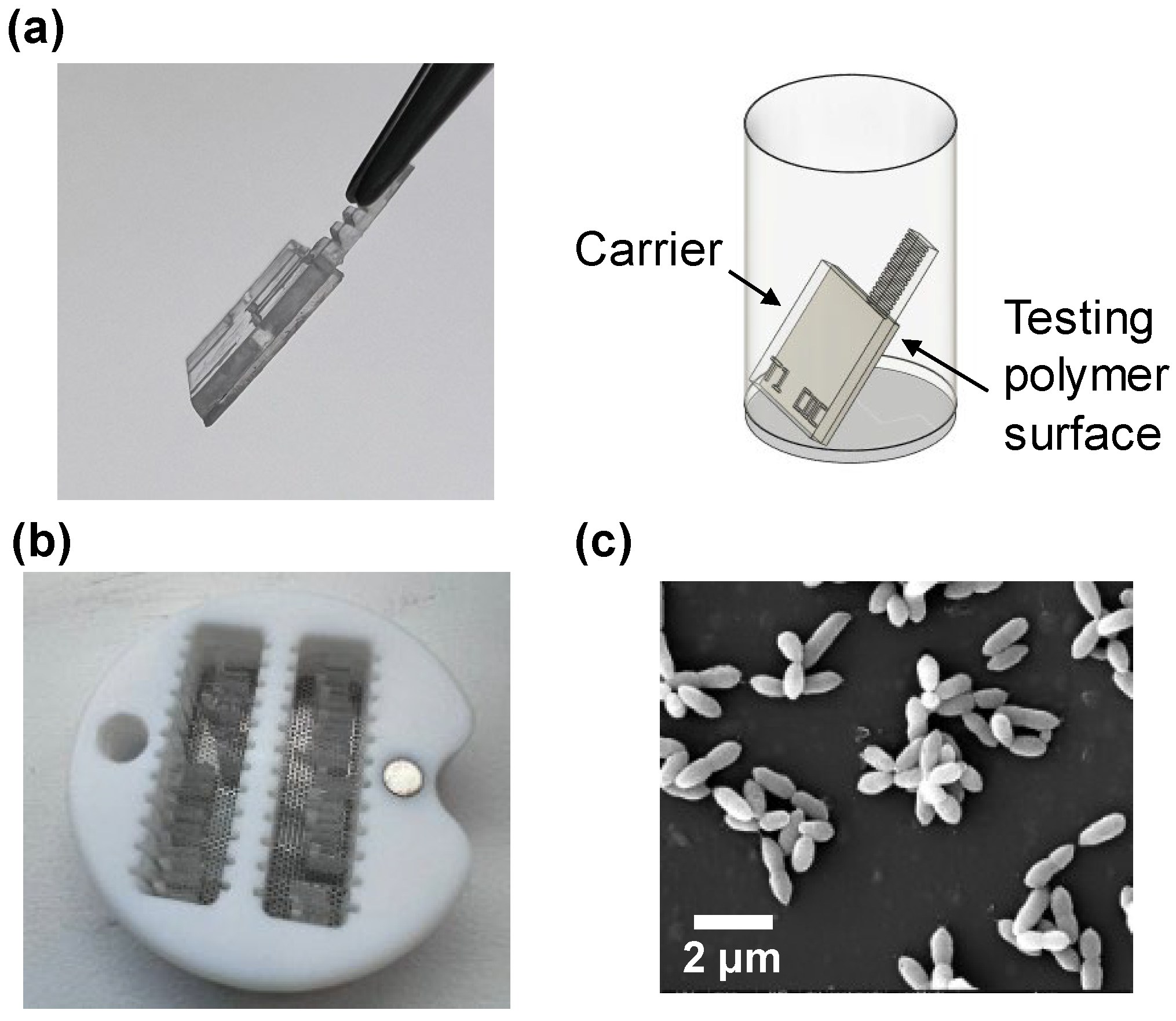
2.5.1. Sample Preparation
2.5.2. Bacterial Strains and Culture Conditions
2.5.3. Biofilm Formation Assay
2.5.4. Bacterial Characterization
3. Results
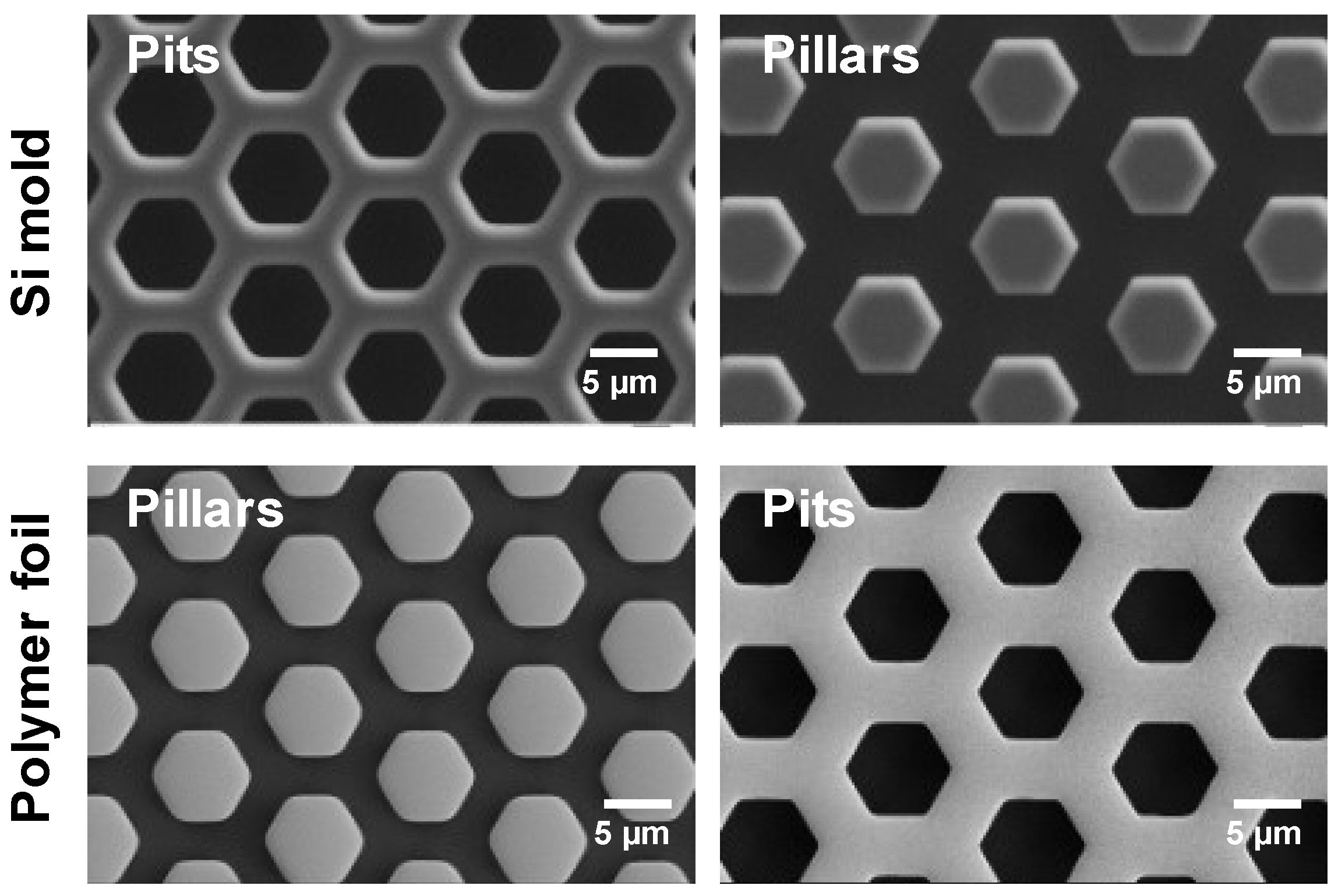
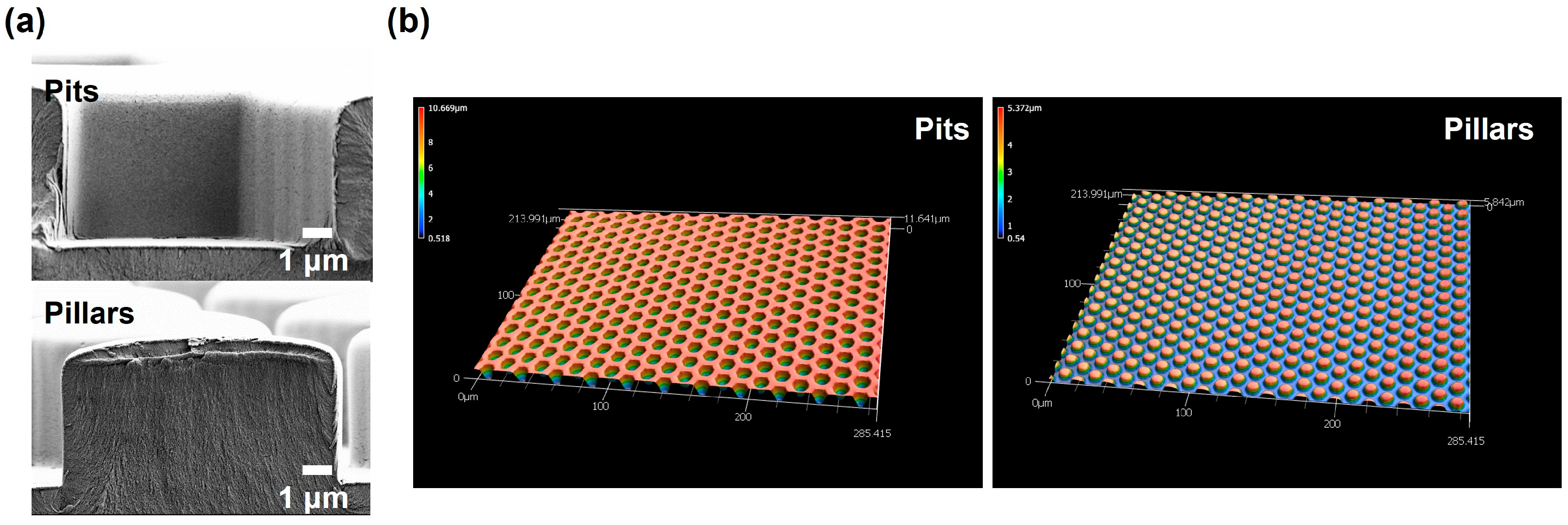
3.1. Mold Characterization
3.2. Characterization of Polymer Foils
3.2.1. Fabricated Almost-Perfect Print
3.2.2. Failure Modes
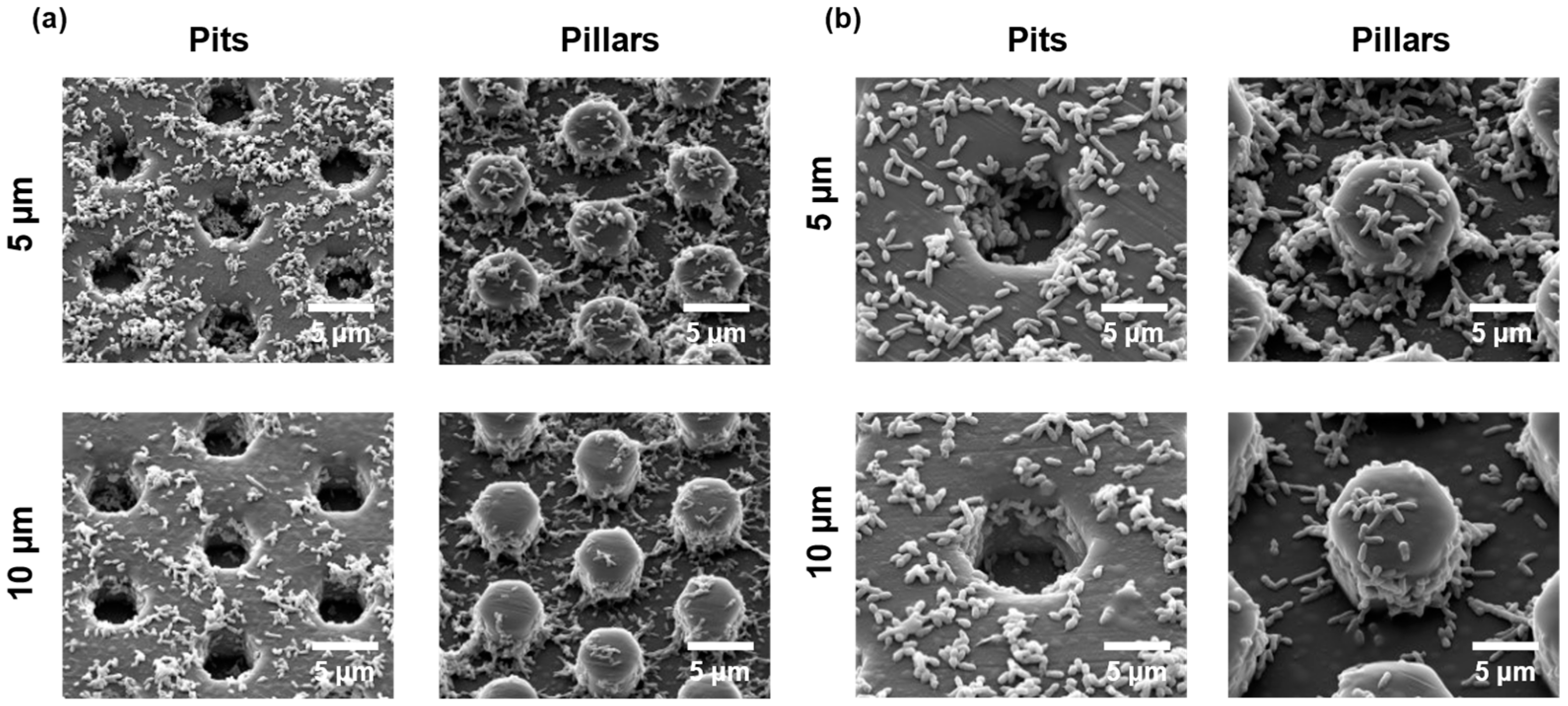
3.3. Bacterial Growth
4. Discussion
4.1. Nanoimprinting
4.2. Bacterial Growth
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vert, M.; Doi, Y.; Hellwich, K.-H.; Hess, M.; Hodge, P.; Kubisa, P.; Rinaudo, M.; Schué, F. Terminology for biorelated polymers and applications (IUPAC Recommendations 2012). Pure Appl. Chem. 2012, 84, 377–410. [Google Scholar] [CrossRef]
- Xu, L.-C.; Siedlecki, C.A. Submicron-textured biomaterial surface reduces staphylococcal bacterial adhesion and biofilm formation. Acta Biomater. 2012, 8, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Cloutier, M.; Mantovani, D.; Rosei, F. Antibacterial Coatings: Challenges, Perspectives, and Opportunities. Trends Biotechnol. 2015, 33, 637–652. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.C.; Fang, J.; Borca-Tasciuc, D.A.; Worobo, R.W.; Moraru, C.I. Effect of Micro- and Nanoscale Topography on the Adhesion of Bacterial Cells to Solid Surfaces. Appl. Environ. Microbiol. 2013, 79, 2703–2712. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Leng, Y.; Lu, X.; Ren, F.; Wang, K.; Ding, Y.; Yang, M. Bacterial Responses to Periodic Micropillar Array. J. Biomed. Mater. Res. Part A 2015, 103, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Ghilini, F.; Pissinis, D.E.; Miñán, A.G.; Schilardi, P.L.; Diaz, C. How Functionalized Surfaces Can Inhibit Bacterial Adhesion and Viability. ACS Biomater. Sci. Eng. 2019, 5, 4920–4936. [Google Scholar] [CrossRef] [PubMed]
- Linklater, D.P.; Baulin, V.A.; Juodkazis, S.; Crawford, R.J.; Stoodley, P.; Ivanova, E.P. Mechano-bactericidal actions of nanostructured surfaces. Nat. Rev. Microbiol. 2021, 19, 8–22. [Google Scholar] [CrossRef]
- Shah, M.P. Bioremediation-Waste Water Treatment. J. Bioremediation Biodegrad. 2018, 9, 427. [Google Scholar] [CrossRef]
- Gude, V.G. Wastewater treatment in microbial fuel cells—An overview. J. Clean. Prod. 2016, 122, 287–307. [Google Scholar] [CrossRef]
- Kiran, V.; Gaur, B. Microbial fuel cell: Technology for harvesting energy from biomass. Rev. Chem. Eng. 2013, 29, 189–203. [Google Scholar] [CrossRef]
- Ercan, D.; Demirci, A. Current and future trends for biofilm reactors for fermentation processes. Crit. Rev. Biotechnol. 2015, 35, 1–14. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, Y.; Zhang, X.; Gao, H.; Mou, L.; Wu, M.; Zhang, W.; Xin, F.; Jiang, M. Biofilm application in the microbial biochemicals production process. Biotechnol. Adv. 2021, 48, 107724. [Google Scholar] [CrossRef] [PubMed]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the Natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. The State of World Fisheries and Aquaculture—2006; Food and Agriculture Organization of the United Nations: Rome, Italy, 2007; Available online: https://openknowledge.fao.org/items/b4669c98-6f0c-4553-91d6-1ea6646a9b02 (accessed on 19 November 2024).
- Defoirdt, T.; Sorgeloos, P.; Bossier, P. Alternatives to antibiotics for the control of bacterial disease in aquaculture. Curr. Opin. Microbiol. 2011, 14, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Sommerset, I.; Krossøy, B.; Biering, E.; Frost, P. Vaccines for fish in aquaculture. Expert Rev. Vaccines 2005, 4, 89–101. [Google Scholar] [CrossRef]
- Brock, N.L.; Nikolay, A.; Dickschat, J.S. Biosynthesis of the antibiotic tropodithietic acid by the marine bacterium Phaeobacter inhibens. Chem. Commun. 2014, 50, 5487–5489. [Google Scholar] [CrossRef]
- D’Alvise, P.W.; Magdenoska, O.; Melchiorsen, J.; Nielsen, K.F.; Gram, L. Biofilm formation and antibiotic production in Ruegeria mobilis are influenced by intracellular concentrations of cyclic dimeric guanosinmonophosphate: C-di-GMP signalling in Ruegeria mobilis. Environ. Microbiol. 2014, 16, 1252–1266. [Google Scholar] [CrossRef]
- Fruleux, T.; Sauleau, P.; Caudal, F.; Champion, M.; Chauvin, L.; Castro, M.; Le Duigou, A. Marine biofilm formation on flax fibre reinforced biocomposites. Biofouling 2024, 40, 415–430. [Google Scholar] [CrossRef]
- De-La-Pinta, I.; Cobos, M.; Ibarretxe, J.; Montoya, E.; Eraso, E.; Guraya, T.; Quindós, G. Effect of biomaterials hydrophobicity and roughness on biofilm development. J. Mater. Sci. Mater. Med. 2019, 30, 77. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Levänen, E. Superhydrophobic surfaces for the reduction of bacterial adhesion. RSC Adv. 2013, 3, 12003–12020. [Google Scholar] [CrossRef]
- Carniello, V.; Peterson, B.W.; van der Mei, H.C.; Busscher, H.J. Physico-chemistry from initial bacterial adhesion to surface-programmed biofilm growth. Adv. Colloid Interface Sci. 2018, 261, 1–14. [Google Scholar] [CrossRef]
- Yang, K.; Shi, J.; Wang, L.; Chen, Y.; Liang, C.; Yang, L.; Wang, L.-N. Bacterial anti-adhesion surface design: Surface patterning, roughness and wettability: A review. J. Mater. Sci. Technol. 2022, 99, 82–100. [Google Scholar] [CrossRef]
- Droumpali, A.; Liu, Y.; Ferrer-Florensa, X.; Sternberg, C.; Dimaki, M.; Andersen, A.J.C.; Strube, M.L.; Kempen, P.J.; Gram, L.; Taboryski, R. Biosynthesis enhancement of tropodithietic acid (TDA) antibacterial compound through biofilm formation by marine bacteria Phaeobacter inhibens on micro-structured polymer surfaces. RSC Adv. 2023, 13, 33159–33166. [Google Scholar] [CrossRef] [PubMed]
- Matteucci, M.; Triches, M.; Nava, G.; Kristensen, A.; Pollard, M.R.; Berg-Sørensen, K.; Taboryski, R.J. Fiber-Based, Injection-Molded Optofluidic Systems: Improvements in Assembly and Applications. Micromachines 2015, 6, 1971–1983. [Google Scholar] [CrossRef]
- Murthy, S.; Matschuk, M.; Huang, Q.; Mandsberg, N.K.; Feidenhans’L, N.A.; Johansen, P.; Christensen, L.; Pranov, H.; Kofod, G.; Pedersen, H.C.; et al. Fabrication of Nanostructures by Roll-to-Roll Extrusion Coating. Adv. Eng. Mater. 2016, 18, 484–489. [Google Scholar] [CrossRef]
- Friedlander, R.S.; Vlamakis, H.; Kim, P.; Khan, M.; Kolter, R.; Aizenberg, J. Bacterial flagella explore microscale hummocks and hollows to increase adhesion. Proc. Natl. Acad. Sci. USA 2013, 110, 5624–5629. [Google Scholar] [CrossRef]
- Grotkjær, T.; Bentzon-Tilia, M.; D’alvise, P.; Dourala, N.; Nielsen, K.F.; Gram, L. Isolation of TDA-producing Phaeobacter strains from sea bass larval rearing units and their probiotic effect against pathogenic Vibrio spp. in Artemia cultures. Syst. Appl. Microbiol. 2016, 39, 180–188. [Google Scholar] [CrossRef][Green Version]
- Chang, B.; Leussink, P.; Jensen, F.; Hübner, J.; Jansen, H. DREM: Infinite etch selectivity and optimized scallop size distribution with conventional photoresists in an adapted multiplexed Bosch DRIE process. Microelectron. Eng. 2018, 191, 77–83. [Google Scholar] [CrossRef]
- Shuo, W.; Jingyang, R.; Lai, Y.; Fashun, Y.; Kui, M. Improving Sidewall Flatness of Through Silicon Via by Thermal Oxidation. In Proceedings of the 2021 IEEE 32nd International Conference on Microelectronics (MIEL), Nis, Serbia, 12–14 September 2021; pp. 197–201. [Google Scholar]
- Worgull, M.; Heckele, M.; Hétu, J.F.; Kabanemi, K.K. Modeling and optimization of the hot embossing process for micro- and nanocomponent fabrication. J. Micro/Nanolithogr. MEMS MOEMS 2006, 5, 011005. [Google Scholar] [CrossRef]
- Heckele, M.; Schomburg, W.K. Review on micro molding of thermoplastic polymers. J. Micromech. Microeng. 2004, 14, R1–R14. [Google Scholar] [CrossRef]
- Scheer, H.-C.; Mayer, A.; Dhima, K.; Wang, S.; Steinberg, C. Challenges with high aspect ratio nanoimprint. Microsyst. Technol. 2014, 20, 1891–1898. [Google Scholar] [CrossRef]
- Schift, H. Nanoimprint lithography: An old story in modern times? A review. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. 2008, 26, 458–480. [Google Scholar] [CrossRef]
- Fernandez-Cuesta, I.; Borrisé, X.; Retolaza, A.; Merino, S.; Mendels, D.-A.; Hansen, O.; Kristensen, A.; Pérez-Murano, F. Determination of stress build-up during nanoimprint process in triangular polymer structures. Microelectron. Eng. 2008, 85, 838–841. [Google Scholar] [CrossRef]
- Okada, Y.; Tokumaru, Y. Precise determination of lattice parameter and thermal expansion coefficient of silicon between 300 and 1500 K. J. Appl. Phys. 1984, 56, 314–320. [Google Scholar] [CrossRef]
- Agha, A.; Waheed, W.; Alamoodi, N.; Mathew, B.; Alnaimat, F.; Abu-Nada, E.; Abderrahmane, A.; Alazzam, A. A Review of Cyclic Olefin Copolymer Applications in Microfluidics and Microdevices. Macromol. Mater. Eng. 2022, 307, 2200053. [Google Scholar] [CrossRef]
- Available online: https://www.engineeringtoolbox.com/thermal-expansion-metals-d_859.html (accessed on 19 November 2024).
- Park, S.; Song, Z.; Brumfield, L.; Amirsadeghi, A.; Lee, J. Demolding temperature in thermal nanoimprint lithography. Appl. Phys. A 2009, 97, 395–402. [Google Scholar] [CrossRef]
- Trabadelo, V.; Schift, H.; Merino, S.; Bellini, S.; Gobrecht, J. Measurement of demolding forces in full wafer thermal nanoimprint. Microelectron. Eng. 2008, 85, 907–909. [Google Scholar] [CrossRef]
- Cadarso, V.J.; Chidambaram, N.; Jacot-Descombes, L.; Schift, H. High-aspect-ratio nanoimprint process chains. Microsyst. Nanoeng. 2017, 3, 17017. [Google Scholar] [CrossRef]
- Bruhn, J.B.; Nielsen, K.F.; Hjelm, M.; Hansen, M.; Bresciani, J.; Schulz, S.; Gram, L. Ecology, Inhibitory Activity, and Morphogenesis of a Marine Antagonistic Bacterium Belonging to the Roseobacter Clade. Appl. Environ. Microbiol. 2005, 71, 7263–7270. [Google Scholar] [CrossRef]
- Mappes, T.; Worgull, M.; Heckele, M.; Mohr, J. Submicron polymer structures with X-ray lithography and hot embossing. Microsyst. Technol. 2008, 14, 1721–1725. [Google Scholar] [CrossRef]


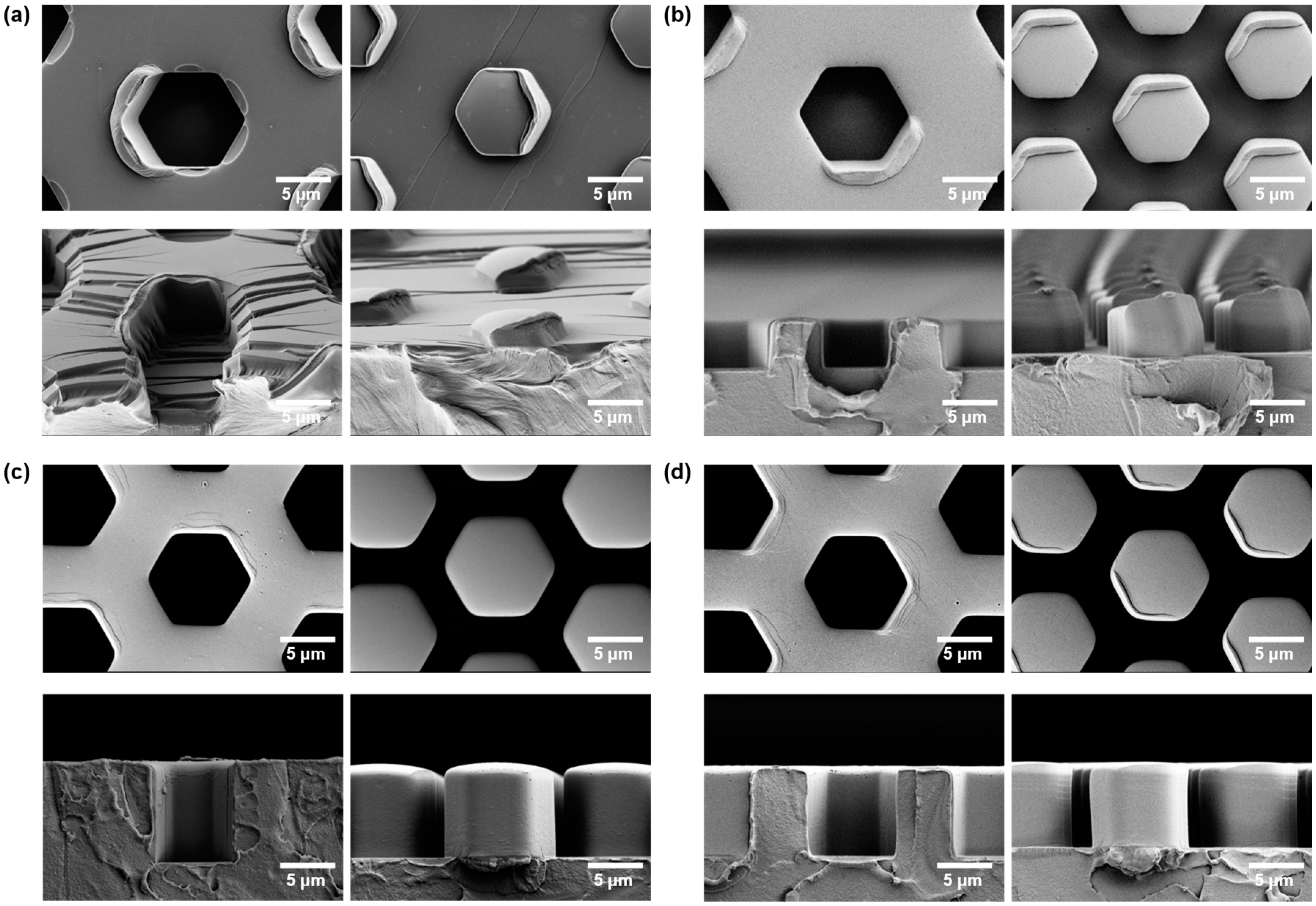
| Imprinting Process | Imprint Temperature (°C) | Cooling Time (min) | Demold Temperature (°C) |
|---|---|---|---|
| Insufficient imprint temperature | 135 | 10 | 80 |
| Poor demold | 190 | 20 | RT * |
| Demold with assists | 190 | 20 | RT |
| Demold at high temperature | 190 | 20 | 140 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Ferrer-Florensa, X.; Sternberg, C.; Kempen, P.; Schift, H.; Gram, L.; Taboryski, R. Nanoimprinted Polymeric Structured Surfaces for Facilitating Biofilm Formation of Beneficial Bacteria. Nanomanufacturing 2024, 4, 202-213. https://doi.org/10.3390/nanomanufacturing4040014
Liu Y, Ferrer-Florensa X, Sternberg C, Kempen P, Schift H, Gram L, Taboryski R. Nanoimprinted Polymeric Structured Surfaces for Facilitating Biofilm Formation of Beneficial Bacteria. Nanomanufacturing. 2024; 4(4):202-213. https://doi.org/10.3390/nanomanufacturing4040014
Chicago/Turabian StyleLiu, Yuyan, Xavier Ferrer-Florensa, Claus Sternberg, Paul Kempen, Helmut Schift, Lone Gram, and Rafael Taboryski. 2024. "Nanoimprinted Polymeric Structured Surfaces for Facilitating Biofilm Formation of Beneficial Bacteria" Nanomanufacturing 4, no. 4: 202-213. https://doi.org/10.3390/nanomanufacturing4040014
APA StyleLiu, Y., Ferrer-Florensa, X., Sternberg, C., Kempen, P., Schift, H., Gram, L., & Taboryski, R. (2024). Nanoimprinted Polymeric Structured Surfaces for Facilitating Biofilm Formation of Beneficial Bacteria. Nanomanufacturing, 4(4), 202-213. https://doi.org/10.3390/nanomanufacturing4040014







