Abstract
Mixed micelles from copolymers in aqueous media have emerged as a valuable tool for producing functional polymer nanostructures with applications in nanomedicine, including drug delivery and bioimaging. In this review, we discuss the basics of mixed copolymer micelles’ design, structure, and physicochemical properties. We also focus on their utilization in biomedical applications using examples from recent literature.
1. Introduction
Nanotechnology is a field of study in which structures that consist of at least one dimension on the nanoscale (1–100 nm) are implemented in various applications [1,2,3]. The previous Horizon initiative (Horizon 2020) allocated around 2 billion euros in financing for projects in the booming field of nanotechnology [4]. Nanomedicine is a branch of medicine that employs a wide range of nanotechnology tools and aspects for therapy and diagnosis applications. Amongst a variety of nanomedicine formulations, biopolymer and synthetic polymer nanostructures are commonly explored owing to their potential as drug carriers [5]. Micelles, a subgroup of these nanostructures, are generated above a particular concentration (referred to as the critical micelle concentration (CMC)) and take on a sphere-like shape in most cases [6]. These structures are made up of a hydrophobic core that is ideal for retaining non-soluble substances and a hydrophilic corona that serves as a protective barrier from the aqueous environment [7,8]. In addition to enabling the solubilization of various valuable hydrophobic drugs, polymeric micelles offer a plethora of properties in terms of bio-applications induced by the functionalization of the normally hydrophilic outer shell, such as targeted drug delivery [9], or triggered drug release [10]. Polymeric micelles generated from a single type of copolymer may be deficient in several aspects. Given that these micelles have a finite total of building blocks, they are restricted in chemical and compositional variety and nanoscopic structure. An established but underutilized technique to address these issues is pairing different polymers to produce mixed micelles. There is the possibility to further improve the existing optimal features and eliminate some of the anticipated limitations of standard polymeric micelles with a minimum of effort in terms of synthetic techniques. Mixed micelles have often been shown to have considerable advantages, such as enhancing micelle stability and drug encapsulation effectiveness [11,12,13,14]. In the past, several studies on mixed micellar systems that achieved clinical stage research status have been presented in an excellent review by Cagel et al. [14]. Recently, an overview of different reports by Manjappa et al. highlighted the recurring merits of mixed micelles over single polymer micelles towards anticancer efficacy [12]. This review is mainly focused on the exploration of mixed micelle systems with a common denominator, which is that the combination of different types of polymers or the combination of a polymer and a biopolymer subserves the delivery of bioactive compounds or even operates as a prodrug system.
2. Inclusivity of the Term
Discussion around mixed micelles has been lately focused on binary mixtures of amphiphilic block copolymers [12,13]. Due to their well-defined macromolecular structure combined with versatility and ease in preparation, amphiphilic block copolymers have surpassed amphiphilic surfactants in molecular self-assembly research areas associated with nanocarrier design for bio-applications, since the former commonly present lower CMC values [15]. Conceivably, many of the thoroughly investigated block copolymers in single copolymer-type micelles are beneficially utilized in the exploration of distinct polymer co-assembly. Pluronics are probably the most commonly employed commercially available polymers for the preparation of mixed micelles systems in the field of nanomedicine [16]. These are a class of amphiphilic and temperature-responsive [17] triblock copolymers consisting of poly (propylene oxide) and poly (ethylene oxide) blocks, PEOX-PPOY-PEOX. The PEO, also known as PEG in the pharmaceutical industry [18], blocks form a hydrophilic corona that shields the hydrophobic core formed by the PPO blocks, offering stealth properties to the system, and the hydrophobic drug is encapsulated into the hydrophobic core, where it is preserved for a particular time frame until it is released. As a result, the water solubility of the hydrophobic drug significantly increases in aqueous solutions, thus increasing its bioavailability. Although Pluronic copolymers cannot be degraded into the human body, the kidneys usually filter out these systems, which are then removed through micturition [18]. Pluronics combination usually includes a mix of the species with similar PPO moieties, since they present more cooperative aggregation than that of species with differing PPO units [19,20]. Recently, Lee et al. conducted a comparative study regarding the effect of molecular weight and hydrophilic–lipophilic balance (HLB) of different combinations of distinct Pluronics on the temperature-dependent co-micellization process and drug solubility using hydrophobic ibuprofen as a model drug. The study demonstrated that all the mixed Pluronic systems form unimodal systems of mixed micelles by cooperative binding, except for the combination of Pluronic P123 and F68, with distinct PPO moieties (i.e., a difference of 37 PO units), whereas the addition of ibuprofen in the system enhanced the cooperative binding [17].
Mixed micelles can be formed by a number of other combinations including block copolymer/homopolymer mixtures [21], copolymer mixtures [22], polymer/biopolymerliposome, enzyme, protein, peptide mixtures [23], and polymer/surfactant mixtures [24], to name the most prominent ones. Regarding the design of biocompatible micelle nanoplatforms for medicinal applications, mixing complex copolymer topologies or architectures is more seldomly applied, due to expected irregular co-assembly. Lo et al. examined the self-assembly of a micelle structure from a graft poly (N-isopropyl acrylamide-co-methacrylic acid)-g-poly (d,l-lactide) (P(NIPAM-co-MAAc)-g-PLA) copolymer and two diblock copolymers, PEG-b-PLA and poly (2-ethyl-2-oxazoline)-b-PLA. The variation between the CMCs of the copolymer components was used to manipulate the size of the self-assemblies. Intracellular drug delivery and cytotoxicity studies displayed a significant correlation of drug release results with the designed functionality induced by the screening feature of the negative charges in the mixed micelles structure, which resulted in higher drug activity and lower material cytotoxicity when compared with micelles from plain P(NIPAM-co-MAAc)-g-PLA copolymer [25]. An example of mixed micelles from the combination of two branched copolymers has been reported by Zhang et al., wherein two polyglycerols grafted with β-cyclodextrin and lactobionic acid, respectively, were employed as hepatocellular carcinoma-targeted delivery nanocarriers for the hydrophobic antitumor drug paclitaxel, with in vitro experiments demonstrating positive results [26]. In terms of macromolecular topology, an intriguing study by Jiang and researchers has described blended assemblies of amphiphilic random and block copolymers. A series of amphiphilic random methacrylate copolymers composed of hydrophobic pyridyl disulfide (PDS) derivatives and hydrophilic PEGs (PDS-r-PEG) were synthesized and blended with a PEG and poly(pyridyl disulfide ethyl methacrylate) (PDS-b-PEG) block copolymer. Even though the research does not refer to the co-assemblies as micelles, the emerged nanoformulations presented low hydrodynamic diameters with hydrophobic cores, while hydrophobic cargo loading and release was achieved following disulfide linkages between the PDS moieties [27].
3. Stabilizing Factors
Micelles are formed as the outcome of one or more stabilizing mechanisms that take place in an aqueous solution. The most frequent type of interaction involves hydrophobic groups, which aggregate or cluster as a result of an equilibrium between hydrophilic and hydrophobic components [28]. Walter Kauzmann first observed that nonpolar substances in a water medium appeared to aggregate rather than distribute uniformly, allowing the hydrophobic entities to have little interaction with the hydrophilic environment [29]. Amphiphilic copolymer mixtures exhibit the same principle in an aqueous media. Hydrogen bonds are another type of self-assembly interaction, which can arise when functional groups are in close proximity. In contrast to hydrophobic interactions, the creation of hydrogen bonds is energetically unfavored in aqueous media. It is generally accepted that the formation of micelles from amphiphilic copolymers is the result of the ordering of hydrophobic moieties in the core which then may be stabilized by hydrogen bonds [12,30]. The electrostatic interactions between two copolymers with opposite charges are what primarily cause the development of polyion complex (PIC) micelles, another type of mixed micelle. PIC micelles are synthesized in an aqueous solution, which eliminates the presence of residual organic solvents, making them an ideal option as an excipient for sensitive cargo, e.g., nucleic acids, peptides, and proteins. PIC micelles provide the additional benefit of encapsulating ionic substances, in contrast to amphiphilic-based mixed micelles [31,32,33]. Another method of stabilizing micelles is chemical crosslinking, which offers the opportunity to reinforce the structure of these types of nanoparticles and make it permanent or disintegrable at will. Disulfide bridges are a common type of crosslinking and, considering that tumor cells have high levels of glutathione (GSH) and GSH’s capacity to destabilize disulfide bonds, many researchers have been driven to create nanostructures with these bonds for the purpose of actively targeting anticancer drugs accompanied with target-specific drug release due to disintegration of disulfide bonds [34,35].
4. Preparation Methods
Many techniques may be employed to produce mixed micelles. The thin film hydration method, also referred to as the solvent evaporation method, involves dissolving the polymers and the active pharmaceutical ingredient (API) in a volatile organic solvent and allowing the solvent to entirely evaporate, employing rotary vacuum evaporation. The thin film is rehydrated using sonication, a vortex, or simply by stirring [36,37]. In the dialysis method, the polymers and the API are dispersed in an organic solvent and left to be dialyzed in deionized water. The gradual replacement of the organic solvent triggers the formation of micelles [38,39]. In the oil/water (o/w) emulsion method, the API is first dissolved in a water-immiscible organic solvent, then the organic phase is incorporated into the aqueous phase while being vigorously stirred. Either phase can be used to dissolve the copolymers. Evaporation is then used to get rid of the organic solvent [40]. In the co-solvent approach, an organic solvent is used to disperse the copolymers. The organic phase is then added rapidly or dropwise to an aqueous solution in agitation, triggering self-assembly. The solvent is then eliminated through evaporation [41].
5. Mixed Micelles for Diabetes Therapy
Worldwide, type 1 diabetes cases are increasing at a rate of roughly 2–3% per year. Insulin management, which involves frequent, uncomfortable injections, is still the primary method. As a result, new forms of treatment are required. In the interest of safeguarding insulin in the bloodstream, nano excipients with the ability to control insulin release and respond quickly to glucose levels are required, and Liu et al. produced a glucose-responsive complex polymeric mixed micelle comprised of PEG-b-poly(aspartic acid-co-aspartamidophenylboronic acid) (PEG-b-P(Asp-co-AspPBA)) and PNIPAM-b-P(Asp-co-AspPBA). Due to the reversible expansion of the PEG channels enmeshed in the PNIPAM membrane, the stimuli-sensitive core was able to create reversible swelling, which resulted in the reproducible on–off management of the secretion of insulin. The delicate cargo was also shielded by the PNIPAM corona [42,43].
Esculetin (EC) is a hydrophobic drug that has shown promising results in combating hyperglycemia. Taking into account its low bioavailability, mixed micelles could improve its effectiveness. Li et al. produced mixed micelles by combining phospholipid, sodium cholate, polyvinyl caprolactam–polyvinyl acetate–PEG graft copolymer (soluplus®), and 1, 2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy PEG−2000] (DSPE-mPEG2000). The thin-film hydration method was utilized to obtain these structures. The mixed micelles outperformed free EC in the various media when assessing drug release at varied pH levels to mimic in vivo conditions. The highest readings, at pH 6.8, indicate increased bioavailability in the intestinal environment. Moreover, drug stability was increased, and the mixed micelles were superior to free EC in treating mice with hyperglycemia [44].
6. Mixed Micelles for Cancer Therapy
A chemotherapeutic drug with a strong propensity for several types of cancers is doxorubicin (DOX). Its capacity to intercept into the DNA helix and/or form covalent bonds to proteins essential for DNA replication and transcription is the basis for its function. Apoptosis will eventually result from these interactions [45]. Active targeting is needed for optimal drug tumor accumulation, although the addition of targeting agents on a nanocarrier can often lead to phagocytosis. To address these limitations, Gao et al. developed pH-responsive mixed micelles composed of PEG-b-poly (ɛ-caprolactone) (PEG-b-PCL) and PCL-b-poly (β-amino ester) conjugated with cyclic peptide RGDfK, which operates as a targeting group (PCL-b-PAE-c(RGDfK). The pH-responsive shell was composed of the biodegradable positively charged polymer PAE, which has a value of pKa ≈ 6.5. When exposed to the bloodstream, it converts to a hydrophobic state, extending blood circulation, minimizing explosion from reticuloendothelial system (RES) organs, and promoting avoidance of the premature release of DOX (Figure 1). To avoid opsonization and immunological reactions, the active targeting component of this nanocarrier was “hidden” in the hydrophobic core, which could extend out to the interface for binding. Significant enhancement of the therapeutic impact was successfully achieved in animal trials, which could be attributable to the acidic conditions found in tumors that encouraged the appropriate release of the drug [46].
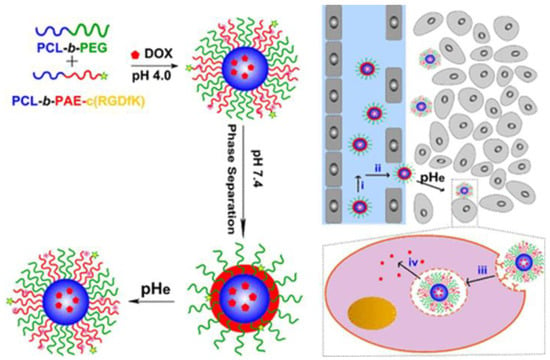
Figure 1.
Self-assembly of DOΧ loaded mixed micelles composed of PEG-b-PCL and PCL-b-PAE-c(RGDfK) [46].
Tumor sites are more acidic as a result of cancer cells’ propensity to engage in aerobic glycolysis. Similarly, making use of this phenomenon, Jafarzadeh-Holagh et al. produced Dextran-Stearic Acid (Dex-SA) and Dextran-Histidine (Dex-His) mixed micelles for DOX delivery. Because of the presence of an imidazole ring on its structure, histidine was chosen to provide pH sensitivity to the system. Dextran, a non-ionic polymer, was used to provide biocompatibility and prevent the opsonization of the emergent nanocarrier. When compared to free drug and Dex-SA, drug-loaded mixed micelles of Dex-SA/Dex-His demonstrated increased cytotoxicity on the U87MG cell line and cell internalization. This may have occurred because dextran hindered the effusing of DOX by p-glycoprotein [47].
Curcumin is a natural compound that is extracted from the rhizome of turmeric, a perennial plant with the botanical name Curcuma longa. Research has shown that curcumin has numerous therapeutic and preventative uses, including antioxidant, anti-inflammatory, and antitumor properties. Curcumin has been utilized extensively in cancer treatment. The class of curcuminoids that includes bisdemethoxycurcumin (BDMC) can be found in standard curcumin at a concentration of 17%. The enhanced anticancer activity of BDMC combined with its increased hydrophobicity makes it a prime choice for mixed micelle encapsulation. Polymeric micelles from Pluronics® F68 and F127 were produced by Mehanny et al. by applying the thin-film hydration method. The CMC value of the 20:80 ratio (F68:F127) was 9.9457 × 10−6 g/mL, indicating that this system is more durable and capable of retaining integrity even when subjected to high dilution in the bloodstream. Data over a six-month period revealed no significant structural changes, demonstrating the mixed micelle stability and capacity to tolerate storage in typical drug settings. The HepG-2 cell line’s IC50 values for neat micelles were 12.2 g/mL, 0.85 g/mL for the free drug BDMC, and 0.74 g/mL for BDMC-loaded micelles, indicating that the drug’s entrapment did not have a negative impact on its cytotoxic effects [18].
Another natural drug with anticancer, anti-inflammatory, and antioxidant properties is quercetin (3′,3′4′,5,7-pentahydroxyflavone; Que). In an attempt to combat non-small cell lung cancer, Li et al. generated Que-loaded mixed micelles (Que-MMICs) by applying the thin-film method, combining DSPE-PEG-biotin and poly (ethylene glycol) methyl ether methacrylate-poly [2-(dimethylamino) ethyl acrylate]–PCL (PEGMA–PDMAEA–PCL). In this mixed micelle system, the hydrophilic PEGMA and PDMAEA form the protective corona. The hydrophobic interactions between Que and PCL units would cause the water-insoluble drug Que to migrate towards the center of the structure. The addition of Que resulted in a considerable decrease in size when compared to the neat micelles, which is most plausibly explained because of the amplifying effect of the interactions between the hydrophobic segments. All systems, nevertheless, were below the 200 nm threshold required for greater tumor accumulation. Data confirmed that biotin conjugation greatly boosted 1.2-fold cellular uptake in A549 cells, further illustrating the value of adding an active targeting agent to mixed micelles. Whereas neat nanostructures displayed minor cytotoxicity, promoting the excipient’s biocompatibility, mixed micelles significantly outperformed the free drug in terms of cytotoxicity values. Animal testing produced similar findings [48].
Photodynamic treatment (PDT) is a type of cancer therapy that involves the process whereby a photosensitizer is excited by a light source, and the effect of the irradiation is the excitation of oxygen, forming reactive oxygen species (ROS) in the body, causing apoptosis in tumor cells through a cascade of processes [49]. In an effort to treat multi-drug resistant breast cancer, Li et al. prepared by the co-solvent method MIT-PCL-pluronic F68-PCL/poly (LA-co-glycolide)–PEG–poly(LA-co-glycolide) (MIT-PFP/PPP) mixed micelles. The antineoplastic drug Mitoxantrone (MIT) is a photosensitive agent used in photodynamic therapy. The resistance to this drug stems from the abundance of ATP-binding cassettes (ABC), which include P-glycoprotein (P-gp). The mechanism underlying MIT suppression is drug expulsion from cancer cells mediated from the transporter; reversing this activity can result in a larger tumor accumulation of MIT. The encapsulation of the photosensitive substance was put to the test since the foundation of photothermic therapy is optimal laser irradiation, and the results indicated that absorptivity was not affected. The free drug and/or irradiation did not exhibit a significant cytotoxic effect, according to data collected from the MTT test of MCF-7/ADR cells. MIT-PFP/PPP micelles, on the other hand, were able to accelerate cell death following irradiation by 60%. Results concluded that with irradiation, cells treated with MIT and MIT-PFP/PPP mixed micelles showed decreased P-gp activity and elevated reactive oxygen species levels [50]. Another anticancer drug with therapeutic value for a variety of malignancies is paclitaxel. Cell death results from its function, which is the polymerization of tubulin proteins into microtubules, and that of a stabilizing factor. To combat this drug’s poor aqueous solubility, lack of targeting abilities, and high rate of degradation in physiological conditions, it would be advantageous to encapsulate it in a nanocarrier [16,51]. Phosphatidylserine (PS) is typically located in the inner cellular layer of healthy tissue. Guan et al. took advantage of the presence of PS on the surface of malignancies, coupling it to pH-sensitive mixed micelles through PS binding peptide (PSBP-6). The drug-loaded mixed system was prepared using a dialysis technique combining PEG-b-PLA) and PEG-b-poly(L-histidine). The system’s pH sensitivity can be linked to the presence of the imidazole group, which at a pH of 6.5, as a result of protonation, led to swelling because electrostatic interactions were more prevalent than hydrophobic interactions. This phenomenon will inhibit the premature release of the drug, taking into account the neutral pH conditions in healthy tissue. In vitro cell studies confirmed the protein-conjugated mixed micelles were more efficient than unbound mixed micelles and the free drug. Biodistribution animal studies revealed the high concentration and retention of the drug within the tumor sites, which provided further confirmation of the mixed systems’ ability to evade the RES system and sinking effect (accumulation in the lymphatic organs). This could be attributed to the poor lymphatic drainage of tumor sites and the binding capabilities of the active targeting agent [52].
7. Mixed Micelles for Bioimaging
The typical image of a micelle is that of a sphere [53], despite the fact that filamentous morphologies often have many advantages over their spherical counterparts, including the ability to evade the RES system and a larger drug loading capacity. In their research, Kim et al. encapsulated the indocyanine green dye (ICG) in mixed micelles of PEO-b-poly [(R)-3-hydroxybutyrate]-b-PEO (PEO-PHB-PEO) and Pluronic F127, prepared through the co-solvent method (Figure 2). Indocyanine green (ICG) is a biocompatible near-infrared (NIR) fluorescent dye that has been approved by the FDA. Nonetheless, because of its amphiphilic nature, ICG binds almost entirely (98%) to the majority of the primary plasma proteins in circulation. After opsonization, ICG is rapidly eliminated by the liver. Furthermore, it lacks target specificity, like the majority of chemical dyes [54,55,56]. F127 offers stimuli sensitivity to nanoformulations, although it lacks loading capacity. The PEO-PHB-PEO copolymer produced stable micelles with high drug loading capabilities, according to research by the same group. As a result of the highly polyhydroxybutyrate hydrophobic moiety present in the complementary triblock copolymer (due to the existence of methyl groups on the pendant chains), the combination of the two triblock copolymers produced stable structures at room temperature. Further evidence for the durability of the emergent structures came from CMC studies, which demonstrated that raising the PHB copolymer concentration decreased CMC values and hinted at micelle durability following significant dilution in the bloodstream. It was established in a mice case study that mixed micelle formulations boosted tumor accumulation and increased ICG stability in vivo [53].
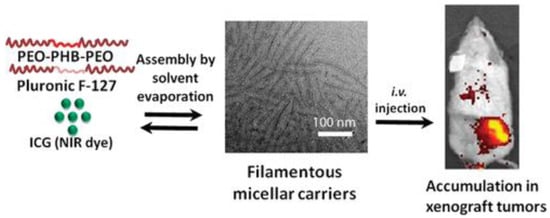
Figure 2.
Preparation of mixed micelles composed of PEO-PHB-PEO and Pluronic F127 for imaging applications [53].
In a similar manner, ICG was encapsulated in pH-sensitive mixed micelles composed of PCL-b-poly (methoxytri(ethylene glycol) methacrylate-co-N-(2-methacrylamido) ethyl folatic amide) (PCL-b-P(TEGMA-co-NMFA) and PCL-b-poly(diethylene glycol monomethyl ether methacrylate) (PCL-b-PDEGMA). The LSCT was evaluated for the emerging micelles since both copolymers contained a thermosensitive component (PDEGMA and PTEGMA). Interestingly, the 1:1 copolymer ratio (M1) exhibited a lower critical solution temperature (LCST) value of 36.6 °C at pH = 5.3. These findings imply that M1 would release the dye in a tumor site rather than prematurely into the bloodstream. Similar outcomes were attained in acidic environments using the dye-loaded micelles’ ICG. Within a 24 h period, 84% of the contrasting agent was released from the vector, compared to 50% under physiological conditions. Additionally, the size and surface charge of ICG-M1 remained constant over time. HeLa and HT-29 cells were evaluated for cytotoxicity. It is essential to remember that HeLa cell lines exhibit a high level of surface folate receptors. It was discovered that the neat mixed micelles were biocompatible. Notably, the phototoxicity tests yielded positive outcomes. The selective internalization of the micelles by receptor-mediated endocytosis led to ICG-M1 exhibiting greater affinity to He-La cancer cells after irradiation, as was anticipated. This mixed system could serve as a theragnostic formulation for NIR imaging-guided photochemotherapy, based on these results [57].
8. Mixed Micelles for Alzheimer’s Disease and Central Nervous System (CNS) Disorders
Alzheimer’s disease (AD) is a neurodegenerative disorder characterized by high complexity as well as intricacy in diagnostic and therapeutic methods due to the limited bioavailability and ability of AD-associated drugs to surpass the blood–brain barrier [58,59]. A combination of different Pluronic copolymers has been studied as a micellar nanocarrier system for morin hydrate, a mild hydrophobic substance operating as an inhibitor of amyloid-β (AΒ) peptide hyperproduction aggregates and hyperphosphorylated tau protein neurofibrillary tangles, which are believed to be the main causes of AD. Specifically, considering the disadvantages of the administration of plain morin hydrate via the parenteral route, Singh and his group developed a mixed micellar system composed of Pluronic P127 and Pluronic F123 block copolymers to study morin hydrate oral delivery and effectiveness in improving the memory performance of AlCl3-administrated Wistar rats with decreased locomotor activity. The group combined the selected copolymers because of lipophilic chain length and overall structure resemblance. Different ratios of the respective copolymers allowed optimization based on micelle size and size distribution, among other characteristics. Pharmacokinetic studies of the optimized loaded micellar nanocarriers resulted in much higher brain drug concentration when compared with plain morin hydrate solutions, whereas chronic treatment remarkably increased the memory of the AD Wistar rats control group [60]. Combining different Pluronics or Pluronics with other polymers is a common strategy to facilitate novel medicinal nanoplatforms in respect to CNS disorders, owing to the tunable properties imposed by their characteristic HLB. Other studied platforms include the delivery of various therapeutics such as trans-resveratrol [61], silymarin [62], vinpocetine [63], or sodium dodecyl sulfate [64].
Another binary mixture of block copolymers studied to accommodate a therapeutic agent for the management of AD is PCL-b-PEG PCL-b-poly (β-amino ester) (PCL-b-PAE). Huang et al. utilized these copolymers to fabricate complex micelles (SAMC) of a PCL hydrophobic core, able to host low-water-soluble γ-Secretase inhibitor (GSI) DAPT, which is known for limiting AB generation, and a mixed shell composed of hydrophilic stabilizing PEG and pH-responsive PAE chains (Figure 3). Via immunoblot assay, it was proven that GSI-loaded micelles indeed present a decreasing effect on the γ-Secretase activity, and therefore AB expression, with concentration dependency, whereas a Thioflavin-T fluorescence assay demonstrated that both GSI-loaded and neat mixed micelles treatment could reduce AB fibril formation. The last was attributed to the collapse of PAE chains onto the micellar core resulting in the emergence of hydrophobic regions that attract AB species and antagonize aggregation, due to the surface microphase separation under physiological pH. Additionally, in an MTT assay utilizing rat pheochromocytoma PC12 cells as the neuronal model, cell viability reached up to 78% for both loaded and plain micelles, indicating that the designed mixed nanocarrier could offer protection from toxicity induced by AB aggregation, whereas neuron targeting by surface functionalization was also achieved. In comparison with other AB-targeted therapeutic strategies, this system originally demonstrated a synchronous effect on AB homeostasis by both blocking source and interrupting the aggregation of AB [65].
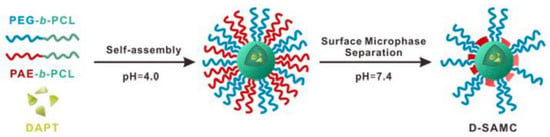
Figure 3.
Preparation of DAPT-loaded SAMC mixed micelles [65].
The same group of researchers had previously reported on a similar complex micelle nanoplatform designed in order to frustrate AB aggregation, wherein instead of pH-responsive PAE, temperature-responsive PNIPAM was employed as the key factor in AB aggregation suppression, since body temperature enforces hydrophobic interactions and causes the collapse of PNIPAM chains. Interestingly, in this study, wherein several ratios of PNIPAM consistency were examined, the researchers concluded that hydrophobic–hydrophilic balance played a crucial role in the inhibition efficiency. Nonetheless, the importance of the synergistic effect of the mixed copolymers of various chemical compositions is denoted [66].
9. Mixed Micelles for Human Immunodeficiency Virus (HIV)
Chiappetta et al. have reported on mixed polymeric micelles from the combination of Pluronic F127 and branched hydrophobic poloxamine counterparts (Tetronic T304 and T904) aiming at the incorporation of the anti-HIV drug efavirenz (EFV) in their micellar core. Having previously investigated pure T904 micelles loaded with EFV, which presented low physical stability, co-micellization with a highly hydrophilic polymer was considered as a solution. Testing different weight ratios of the combined copolymer components revealed that CMC and micelle size are controllable, with both T304 and T904 presenting favorable mixed self-assembly in micelles of higher hydrophobicity than plain F127 micelles, even though T304 displayed low EFV solubilization capacity. Additionally, increased stability was observed in F127 in a concentration-dependent manner, highlighting the importance of its presence in the mixed nanostructure [67].
Towards a more in vivo approach, Patil et al. composed a mixed nanoplatform from a binary mixture of Pluronic F127 and D-α-tocopherol PEG 1000 succinate to improve the oral bioavailability of Nelfinavir mesylate, an HIV-1 protease inhibitor. In vitro release studies demonstrated sustained release for up to four days, whereas in vivo pharmacokinetic investigation suggested enhanced bioavailability, attributed to the size of the obtained micelles [68].
10. Mixed Micelles for Gene Delivery
Gene therapy alternative approaches are essential for dealing with vascular diseases since the absence of effective targeting functions remains consistent. Li et al. investigated blended assemblies of cationic graft poly (lactide-co-3(S)-methyl-morpholine-2,5-dione)-g-polyethylenimine (PLMD-g-PEI) and comb-like PLMD-g-poly(poly(ethylene glycol)monomethacrylate) linked to Cys-Arg-Glu-Asp-Val-Trp endothelial cell recognizer peptide (CREDVW) (PLMD-PPEGMA-CREDVW) copolymers, and their complexation with pEGFP-ZNF580, a gene known for assisting proliferation and migration of endothelial cells. This particular pattern was based on the purpose of producing a gene carrier able to excel the hindrance of inflexible single copolymer carriers towards multifunctionality. Accordingly, the peptide modification was carried out using the PEG chain ends, and not PEI direct linking, aiming at a structure with a more pliant architecture. The mixed micelles were prepared in different mixing ratios by a dialysis method, and later complexes with several plasmid DNA (pDNA) to copolymer fractions were studied. In vitro cytotoxicity assay of the complexes revealed a >80% cell viability, while transfection efficiency, cell migration, and wound healing ability were demonstrated [69].
Unlike traditional vaccinations for viral infections, cancer vaccines have more of a therapeutic than a preventive effect. They are a type of immunotherapy in which cancer cells are eradicated by an immune reaction [70]. The issue is that adjuvants produce immunological reactions that are antibody-mediated instead of cell-mediated. These nanocarriers would need to actively target the cytosols of lymph-node-dwelling dendritic cells to boost cross-presentation, which would subsequently release a significant number of cytotoxic T-lymphocytes, leading to apoptosis. Sui et al. generated PCL-PEG and PCL-PEI mixed micelles employing the thin-film hydration method. Due to its pH sensitivity, PEI is frequently used in gene transfer applications. By means of the use of disulfide bonds (-ss-), ovalbumin (OVA) was cross-linked to PEG-PCL, and then Cytosine-phosphorothioate-guanine oligodeoxynucleotides (CpG-ODNs) were incorporated into the system as a second step. As tumor growth inhibitors, CpG-ODNs work by stimulating TLR9-bearing cells, which in turn triggers the emergence of T-cells. The emergent mixed micelles’ CMC value was 2.35 × 10−4 g/mL, which demonstrated that the micelles had acceptable dilution tolerance. As PEI-PCL has a pKb of 5.5, the mixed system has the potential to remain in circulation until it enters the endo/lysosome environment. As previously mentioned, glutathione disrupts disulfide bonds, prompting OVA to be released intracellularly. The adjuvant did not appear to have a cytotoxic effect on dendritic cells generated from bone marrow. Interestingly, there was greater cell uptake, which was probably brought about by the structures’ cationic charge density, which relates to PEI-PCL. Most crucially, when the nanoparticles were evaluated to see if they could prevent lung cancer metastasis, there were 1.7 or 2.3 times more lung metastatic nodules present after the introduction of nanostructures conjugated with amide bonds than in the case where crosslinking was accomplished using disulfide bonds. This highlights how crucial a role this kind of crosslinking plays in cancer suppression. Finally, animal studies, comparing the survival rate following vaccination with the PP-SS-OVA/CpG group to the adjuvant and the free protein, showed that the survival rate was increased by up to 50% [71,72].
Mixed micelles that are fabricated from polyion complexation between pDNA and (PEG)-b-polycation block copolymers frequently exhibit poor bloodstream durability. The addition of a hydrophobic moiety to strengthen hydrophobic interactions is a typical technique to increase the stability of electrostatic interaction-based systems. While increasing the PEG fraction can produce similar outcomes, cellular absorption is hampered. Li et al. developed the ternary polyplex micelle system (TPM) by combining pDNA with the mixture of block copolymers PEG-b-poly [N-(2- aminoethyl)-2-aminoethyl]aspartamide (PEG-b-PAsp(DET)) and PNIPAM-b-PAsp(DET) in consideration of the aforementioned. When compared to binary polyplex micelles of PEG-b-PAsp(DET) (BPMs), both systems at standard ambient temperature generated the same findings in terms of nuclease activity protection because PNIPAM units act as hydrophilic shells at 25 °C. TPMs, however, demonstrated noticeably superior protective capabilities to BPMs at body temperature. This is owing to the thermoresponsive nature of PNIPAM, which acts as a hydrophobic intermediate layer in these conditions. Blood studies corroborated those features; the half-life of TPMs (29.3 ± 1.8 min) was over two times the duration of that of BPMs (12.8 ± 0.96 min). To evaluate whether polyplex micelles accumulate at tumor sites with the inclusion of Cy5, animal investigations were conducted. In mice possessing a xenografted H22 tumor, it was found that TPMs accumulated immensely, amounting to 3.8-fold higher values than BPMs. These findings support the efficacy of the introduction of PNIPAM in gene therapy [73].
When compared to pDNA, messenger RNA (mRNA) has gained traction as it does not need to penetrate the nuclear envelope and excludes integration into the host cell’s genetic material. Nevertheless, the need for novel vectors remains, as it experiences rapid degradation as a result of nuclease activity, similar to its counterparts. Yang et al. created mRNA-loaded PIC micelles utilizing cis-aconitic anhydride-modified PEG-poly (L-lysine) (PEG-pLL(CAA)) block copolymers. The pLL(CAA) segment’s side chains of amino groups can facilitate electrostatic interactions with the phosphate groups of mRNA, and pH-sensitive covalent bonding between amino groups and the CAA moieties is capable of stabilizing the core. The mean diameter of the PEG-pLL(CAA) micelles was 94 ± 1 nm, but more significantly, the encapsulation efficiency was roughly 100% (Figure 4). The micelles were stable at physiological pH (pH 7.4) and released their sensitive cargo, mRNA, in acidic conditions in the range of endosomal pH (pH 5.5–4.5). Fortunately, PEG-pLL(CAA) was superior to PEG-pLL micelles at preventing mRNA from decaying, by nucleases and enhancing cellular uptake. This was most likely brought on by the increased stability of the PEG-pLL(CAA) against polyanion exchange, as the negatively charged acidic proteoglycans found on cellular walls can destabilize such types of nanostructures. Comparing PEG-pLL(CAA) to PEI polyplexes, which are frequently used for gene delivery, revealed that it produced higher levels of mRNA transfection in mice bearing the CT26 tumor [74].
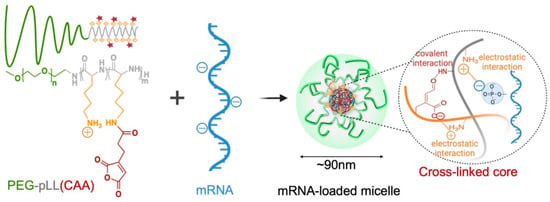
Figure 4.
The formation of PEG-pLL(CAA) micelles via cross-linking and electrostatic interactions [74].
11. Mixed Micelles for Protein Delivery
The biocompatibility and binding abilities of proteins have contributed to their increase in prevalence in medicine for the treatment of a variety of illnesses. However, they experience poor cellular absorption and circulatory integrity, as do the majority of biomolecules [75]. Several types of vectors have attracted attention; among them are polyphenols. These amphiphilic compounds, such as tannic acid (TA), contain functional groups such as hydroxyl groups and aromatic rings, which aid in the encapsulation of biomolecules via hydrophobic interactions and hydrogen bonding. TA lacks targeting abilities mainly because of its propensity to interact unintentionally with numerous biological elements. Honda et al. combined phenylboronic-acid-conjugated PEG10000-poly(L-lysine) block copolymer, TA, and green fluorescent protein (GFP) in an aqueous solution to assemble a ternary complex (Figure 5). To simulate the conditions the complex would come across in vivo, FBS was utilized. The hydrodynamic radius of TA/GFP complexes increased, pointing to the creation of a protein corona. On the other hand, because of the protective qualities of PEG moieties, the ternary complex maintained its size even in an environment of 50% FBS. It is essential to note that TA has a strong affinity for proteins, including albumin. Similar results were obtained with glucose, suggesting this nanocarrier would evade opsonization. The interaction of the polyphenol with cell membranes in TA/GFP complexes produced the greatest results in terms of cellular internalization. Just as was reported in prior studies, the PEG corona for the ternary complex led to only restricted cellular adsorption. The ternary complex performed far more effectively than the free protein and the protein/TA complex when tested in tumor accumulation studies in subcutaneously implanted murine colorectal carcinoma-bearing mice. This was accomplished through passive targeting and sustained blood circulation [76].
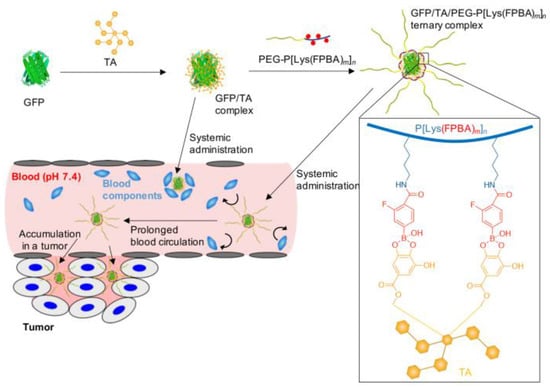
Figure 5.
An illustration depicting how the ternary complex of TA, GFP, and PEG10000-poly(L-lysine) block copolymer forms in an aqueous medium [76].
A wide spectrum of conditions may benefit considerably from the use of antisense oligonucleotides (ASO). The aforementioned group of synthetic polymers can be applied for reducing the expression level of RNA structures through RNase H-mediated cleavage or by restricting transcriptional protein entry through steric inhibition. One of its main drawbacks is its rapid elimination via opsonization, primarily by albumin. However, a significant portion of ASOs, as with most small-sized polymers, are removed from the circulatory system through renal filtration, with a relatively short half-life. In order to enhance bioavailability and more efficient accumulation in tissues of concern, such as malignancies, vectors must be created. Kim et al. developed ASO-Loaded PICs consisting of a triblock copolymer based on the combination of poly (2-ethyl-2-oxazoline), poly(2-n-propyl-2-oxazoline), and poly(L-lysine). While poly (2-n-propyl-2-oxazoline) (PnPrOx) provided thermosensitivity to the system and poly (2-ethyl-2-oxazoline) (PEtOx) served as a hydrophilic corona, poly (L-lysine) (PLL) enabled electrostatic interactions between the ASO and the nanocarrier. This research revealed that when comparing to diblock-based poly (PEtOx-b-PnPrOx) micelles, the presence of the PnPrOx component significantly enhanced the durability of mixed micelles in FBS and caused more effective cellular accumulation of the oligonucleotide, which led to higher suppression of gene expression in a model of androgen-independent prostate cancer cells [77].
12. Conclusions and Future Perspectives
The global market for nanomedicine is expected to continue to grow to USD 350.8 billion in the upcoming years [78]. In this rapidly expanding field of research and technology, mixed micelles are anticipated to play an integral role. The emergent micelles outperformed their precursor copolymers in the majority of study cases, demonstrating the advantages of synergistic effects. Studies confirm that mixed micelle systems provide significant benefits to drug entrapment effectiveness by increasing drug loading capacity, micelle durability by increasing hydrophobic interactions, H-bonding, and other stabilizing factors, and by providing better thermodynamic aspects, with a decrease in critical micelle concentration and the simplicity of adding multiple stimuli-sensitive properties (e.g., pH sensitivity and temperature responsiveness). In light of this, it is critical that more of these nanoformulations be studied in a clinical setting in order to provide standard information on how such vectors with this kind of chemical profile would behave in humans, and, furthermore, confirm their ability to surpass the more costly and less practical design of novel polymeric materials. Additionally, in upcoming conventional nanoformulations, polymeric mixed micelles are expected to include a wider variety of already studied copolymers in terms of chemical composition and architecture. This review provides a good starting point for discussion and further research in mixed systems.
Author Contributions
Writing—original draft preparation, A.M.G. and A.B.; writing—review and editing, A.M.G., A.B. and S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No experimental data were produced.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ferrari, M. Cancer Nanotechnology: Opportunities and Challenges. Nat. Rev. Cancer 2005, 5, 161–171. [Google Scholar] [CrossRef]
- Farokhzad, O.C.; Langer, R. Impact of Nanotechnology on Drug Delivery. ACS Nano 2009, 3, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, B. Introduction to Nanotechnology. In Springer Handbook of Nanotechnology; Springer Handbooks; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–19. [Google Scholar]
- European Observatory for Nanomaterials. EU Research Projects. Available online: https://euon.echa.europa.eu/eu-research-projects (accessed on 24 February 2023).
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Nanomedicine: Current Status and Future Prospects. FASEB J. 2005, 19, 311–330. [Google Scholar] [CrossRef] [PubMed]
- Kunitake, T.; Shinkai, S. Catalysis by Micelles, Membranes and Other Aqueous Aggregates as Models of Enzyme Action. Adv. Phys. Org. Chem. 1980, 17, 435–487. [Google Scholar] [CrossRef]
- Kumar, C.; Balasubramanlan, D. Structural Features of Water-in-OII Microemulsions. J. Phys. Chem. 1980, 84, 1895–1899. [Google Scholar] [CrossRef]
- Haider, M.S.; Lübtow, M.M.; Endres, S.; Forster, S.; Flegler, V.J.; Böttcher, B.; Aseyev, V.; Pöppler, A.C.; Luxenhofer, R.; Luxenhofer, R. Think beyond the Core: Impact of the Hydrophilic Corona on Drug Solubilization Using Polymer Micelles. ACS Appl. Mater. Interfaces 2020, 12, 24531–24543. [Google Scholar] [CrossRef]
- Manzari, M.T.; Shamay, Y.; Kiguchi, H.; Rosen, N.; Scaltriti, M.; Heller, D.A. Targeted Drug Delivery Strategies for Precision Medicines. Nat. Rev. Mater. 2021, 6, 351–370. [Google Scholar] [CrossRef]
- Wells, C.M.; Harris, M.; Choi, L.; Murali, V.P.; Guerra, F.D.; Jennings, J.A. Stimuli-Responsive Drug Release from Smart Polymers. J. Funct. Biomater. 2019, 10, 34. [Google Scholar] [CrossRef]
- Saxena, V.; Hussain, M.D. Polymeric Mixed Micelles for Delivery of Curcumin to Multidrug Resistant Ovarian Cancer. J. Biomed. Nanotechnol. 2013, 9, 1146–1154. [Google Scholar] [CrossRef]
- Manjappa, A.S.; Kumbhar, P.S.; Patil, A.B.; Disouza, J.I.; Patravale, V.B. Polymeric Mixed Micelles: Improving the Anticancer Efficacy of Single-Copolymer Micelles. Crit. Rev. Ther. Drug Carr. Syst. 2019, 36, 1–58. [Google Scholar] [CrossRef]
- Ebrahim Attia, A.B.; Ong, Z.Y.; Hedrick, J.L.; Lee, P.P.; Ee, P.L.R.; Hammond, P.T.; Yang, Y.-Y. Mixed Micelles Self-Assembled from Block Copolymers for Drug Delivery. Curr. Opin. Colloid Interface Sci. 2011, 16, 182–194. [Google Scholar] [CrossRef]
- Cagel, M.; Tesan, F.C.; Bernabeu, E.; Salgueiro, M.J.; Zubillaga, M.B.; Moretton, M.A.; Chiappetta, D.A. Polymeric Mixed Micelles as Nanomedicines: Achievements and Perspectives. Eur. J. Pharm. Biopharm. 2017, 113, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Kapse, A.; Anup, N.; Patel, V.; Saraogi, G.K.; Mishra, D.K.; Tekade, R.K. Polymeric Micelles: A Ray of Hope among New Drug Delivery Systems. In Drug Delivery Systems; Academic Press: Cambridge, MA, USA, 2020; pp. 235–289. [Google Scholar] [CrossRef]
- Wei, Z.; Hao, J.; Yuan, S.; Li, Y.; Juan, W.; Sha, X.; Fang, X. Paclitaxel-Loaded Pluronic P123/F127 Mixed Polymeric Micelles: Formulation, Optimization and in Vitro Characterization. Int. J. Pharm. 2009, 376, 176–185. [Google Scholar] [CrossRef]
- Lee, C.F.; Yang, C.H.; Lin, T.L.; Bahadur, P.; Chen, L.J. Role of Molecular Weight and Hydrophobicity of Amphiphilic Tri-Block Copolymers in Temperature-Dependent Co-Micellization Process and Drug Solubility. Colloids Surf. B Biointerfaces 2019, 183, 110461. [Google Scholar] [CrossRef] [PubMed]
- Mehanny, M.; Hathout, R.M.; Geneidi, A.S.; Mansour, S. Bisdemethoxycurcumin Loaded Polymeric Mixed Micelles as Potential Anti-Cancer Remedy: Preparation, Optimization and Cytotoxic Evaluation in a HepG-2 Cell Model. J. Mol. Liq. 2016, 214, 162–170. [Google Scholar] [CrossRef]
- Chaibundit, C.; Ricardo, N.M.P.S.; Costa, F.D.M.L.L.; Yeates, S.G.; Booth, C. Micellization and Gelation of Mixed Copolymers P123 and F127 in Aqueous Solution. Langmuir 2007, 23, 9229–9236. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.Y.; Zhang, W.M. Recent Progress in Drug Delivery of Pluronic P123: Pharmaceutical Perspectives. J. Drug Target. 2017, 25, 471–484. [Google Scholar] [CrossRef]
- Kinning, D.J.; Thomas, E.L.; Fetters, L.J. Morphological Studies of Micelle Formation in Block Copolymer/Homopolymer Blends. J. Chem. Phys. 1998, 90, 5806. [Google Scholar] [CrossRef]
- Singh, V.; Khullar, P.; Dave, P.N.; Kaur, N. Micelles, Mixed Micelles, and Applications of Polyoxypropylene (PPO)-Polyoxyethylene (PEO)-Polyoxypropylene (PPO) Triblock Polymers. Int. J. Ind. Chem. 2013, 4, 12. [Google Scholar] [CrossRef]
- Mu, L.; Elbayoumi, T.A.; Torchilin, V.P. Mixed Micelles Made of Poly(Ethylene Glycol)–Phosphatidylethanolamine Conjugate and d-α-Tocopheryl Polyethylene Glycol 1000 Succinate as Pharmaceutical Nanocarriers for Camptothecin. Int. J. Pharm. 2005, 306, 142–149. [Google Scholar] [CrossRef]
- Kancharla, S.; Bedrov, D.; Tsianou, M.; Alexandridis, P. Structure and Composition of Mixed Micelles Formed by Nonionic Block Copolymers and Ionic Surfactants in Water Determined by Small-Angle Neutron Scattering with Contrast Variation. J. Colloid Interface Sci. 2022, 609, 456–468. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.L.; Lin, K.M.; Huang, C.K.; Hsiue, G.H. Self-Assembly of a Micelle Structure from Graft and Diblock Copolymers: An Example of Overcoming the Limitations of Polyions in Drug Delivery. Adv. Funct. Mater. 2006, 16, 2309–2316. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Yu, P.; Han, Y.; Li, Y.; Li, C. Hydrotropic Polymeric Mixed Micelles Based on Functional Hyperbranched Polyglycerol Copolymers as Hepatoma-Targeting Drug Delivery System. J. Pharm. Sci. 2013, 102, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Liu, H.; He, H.; Ribbe, A.E.; Thayumanavan, S. Blended Assemblies of Amphiphilic Random and Block Copolymers for Tunable Encapsulation and Release of Hydrophobic Guest Molecules. Macromolecules 2020, 53, 2713–2723. [Google Scholar] [CrossRef]
- Meyer, E.E.; Rosenberg, K.J.; Israelachvili, J. Recent Progress in Understanding Hydrophobic Interactions. Proc. Natl. Acad. Sci. USA 2006, 103, 15739–15746. [Google Scholar] [CrossRef]
- Chemistry LibreTexts. Hydrophobic Interactions. Available online: https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Physical_Properties_of_Matter/Atomic_and_Molecular_Properties/Intermolecular_Forces/Hydrophobic_Interactions (accessed on 19 March 2023).
- Baglioni, P.; Berti, D. Self Assembly in Micelles Combining Stacking and H-Bonding. Curr. Opin. Colloid Interface Sci. 2003, 8, 55–61. [Google Scholar] [CrossRef]
- Chen, F.; Stenzel, M.H. Polyion Complex Micelles for Protein Delivery. Aust. J. Chem. 2018, 71, 768–780. [Google Scholar] [CrossRef]
- Harada, A.; Kataoka, K. Novel Polyion Complex Micelles Entrapping Enzyme Molecules in the Core. 2. Characterization of the Micelles Prepared at Nonstoichiometric Mixing Ratios. Langmuir 1999, 15, 4208–4212. [Google Scholar] [CrossRef]
- Li, G.; Guo, L.; Meng, Y.; Zhang, T. Self-Assembled Nanoparticles from Thermo-Sensitive Polyion Complex Micelles for Controlled Drug Release. Chem. Eng. J. 2011, 174, 199–205. [Google Scholar] [CrossRef]
- Kamenova, K.; Grancharov, G.; Kortenova, V.; Petrov, P.D. Redox-Responsive Crosslinked Mixed Micelles for Controllable Release of Caffeic Acid Phenethyl Ester. Pharmaceutics 2022, 14, 679. [Google Scholar] [CrossRef]
- Petrov, P.; Tsvetanov, C.B.; Jérôme, R. Two-Component “Onionlike” Micelles with a PPO Core, a PDMAEMA Shell and a PEO Corona: Formation and Crosslinking. Polym. Int. 2008, 57, 1258–1264. [Google Scholar] [CrossRef]
- Zhao, L.; Du, J.; Duan, Y.; Zang, Y.; Zhang, H.; Yang, C.; Cao, F.; Zhai, G. Curcumin Loaded Mixed Micelles Composed of Pluronic P123 and F68: Preparation, Optimization and in Vitro Characterization. Colloids Surf. B Biointerfaces 2012, 97, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Dou, J.; Zhang, H.; Liu, X.; Zhang, M.; Zhai, G. Preparation and Evaluation in Vitro and in Vivo of Docetaxel Loaded Mixed Micelles for Oral Administration. Colloids Surf. B Biointerfaces 2014, 114, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.K.; Lo, C.L.; Chen, H.H.; Hsiue, G.H. Multifunctional Micelles for Cancer Cell Targeting, Distribution Imaging, and Anticancer Drug Delivery. Adv. Funct. Mater. 2007, 17, 2291–2297. [Google Scholar] [CrossRef]
- Wu, H.; Zhu, L.; Torchilin, V.P. PH-Sensitive Poly(Histidine)-PEG/DSPE-PEG Co-Polymer Micelles for Cytosolic Drug Delivery. Biomaterials 2013, 34, 1213–1222. [Google Scholar] [CrossRef]
- Aliabadi, H.M.; Lavasanifar, A. Polymeric Micelles for Drug Delivery. Expert Opin. Drug Deliv. 2006, 3, 139–162. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, Z.; Chen, T.; Guo, X.; Zhou, S. Preparation and Characterization of Thermosensitive Pluronic F127-b-Poly(ɛ-Caprolactone) Mixed Micelles. Colloids Surf. B Biointerfaces 2011, 86, 45–57. [Google Scholar] [CrossRef]
- DiMeglio, L.A.; Evans-Molina, C.; Oram, R.A. Type 1 Diabetes. Lancet 2018, 391, 2449–2462. [Google Scholar] [CrossRef]
- Liu, G.; Ma, R.; Ren, J.; Li, Z.; Zhang, H.; Zhang, Z.; An, Y.; Shi, L. A Glucose-Responsive Complex Polymeric Micelle Enabling Repeated on–off Release and Insulin Protection. Soft Matter 2013, 9, 1636–1644. [Google Scholar] [CrossRef]
- Li, X.; Xia, X.; Zhang, J.; Adu-Frimpong, M.; Shen, X.; Yin, W.; He, Q.; Rong, W.; Shi, F.; Cao, X.; et al. Preparation, Physical Characterization, Pharmacokinetics and Anti-Hyperglycemic Activity of Esculetin-Loaded Mixed Micelles. J. Pharm. Sci. 2023, 112, 148–157. [Google Scholar] [CrossRef]
- Carvalho, C.; Santos, R.X.; Cardoso, S.; Correia, S.; Oliveira, P.J.; Santos, M.S.; Moreira, P.I. Doxorubicin: The Good, the Bad and the Ugly Effect. Curr. Med. Chem. 2009, 16, 3267–3285. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Cheng, T.; Liu, J.; Liu, J.; Yang, C.; Chu, L.; Zhang, Y.; Ma, R.; Shi, L. Self-Regulated Multifunctional Collaboration of Targeted Nanocarriers for Enhanced Tumor Therapy. Biomacromolecules 2014, 15, 3634–3642. [Google Scholar] [CrossRef] [PubMed]
- Jafarzadeh-Holagh, S.; Hashemi-Najafabadi, S.; Shaki, H.; Vasheghani-Farahani, E. Self-Assembled and PH-Sensitive Mixed Micelles as an Intracellular Doxorubicin Delivery System. J. Colloid Interface Sci. 2018, 523, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zang, X.; Meng, X.; Li, Y.; Xie, Y.; Chen, X. Targeted Delivery of Quercetin by Biotinylated Mixed Micelles for Non-Small Cell Lung Cancer Treatment. Drug Deliv. 2022, 29, 970–985. [Google Scholar] [CrossRef]
- Manyak, M.J.; Russo, A.; Smith, P.D.; Glatstein, E. Photodynamic Therapy. J. Clin. Oncol. 2016, 6, 380–391. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Cai, Y.; Zhao, Y.; Yu, H.; Zhou, H.; Chen, M. Polymeric Mixed Micelles Loaded Mitoxantrone for Overcoming Multidrug Resistance in Breast Cancer via Photodynamic Therapy. Int. J. Nanomed. 2017, 12, 6595. [Google Scholar] [CrossRef]
- Yang, H.; Khan, A.R.; Liu, M.; Fu, M.; Ji, J.; Chi, L.; Zhai, G. Stimuli-Responsive Polymeric Micelles for the Delivery of Paclitaxel. J. Drug Deliv. Sci. Technol. 2020, 56, 101523. [Google Scholar] [CrossRef]
- Guan, S.; Zhang, Q.; Bao, J.; Duan, T.; Hu, R.; Czech, T.; Tang, J. Phosphatidylserine Targeting Peptide-Functionalized PH Sensitive Mixed Micelles for Enhanced Anti-Tumor Drug Delivery. Eur. J. Pharm. Biopharm. 2020, 147, 87–101. [Google Scholar] [CrossRef]
- Kim, T.H.; Mount, C.W.; Dulken, B.W.; Ramos, J.; Fu, C.J.; Khant, H.A.; Chiu, W.; Gombotz, W.R.; Pun, S.H. Filamentous, Mixed Micelles of Triblock Copolymers Enhance Tumor Localization of Indocyanine Green in a Murine Xenograft Model. Mol. Pharm. 2012, 9, 135–143. [Google Scholar] [CrossRef]
- Wu, L.; Fang, S.; Shi, S.; Deng, J.; Liu, B.; Cai, L. Hybrid Polypeptide Micelles Loading Indocyanine Green for Tumor Imaging and Photothermal Effect Study. Biomacromolecules 2013, 14, 3027–3033. [Google Scholar] [CrossRef]
- Kirchherr, A.K.; Briel, A.; Mäder, K. Stabilization of Indocyanine Green by Encapsulation within Micellar Systems. Mol. Pharm. 2009, 6, 480–491. [Google Scholar] [CrossRef]
- Kim, T.H.; Chen, Y.; Mount, C.W.; Gombotz, W.R.; Li, X.; Pun, S.H. Evaluation of Temperature-Sensitive, Indocyanine Green-Encapsulating Micelles for Noninvasive Near-Infrared Tumor Imaging. Pharm. Res. 2010, 27, 1900–1913. [Google Scholar] [CrossRef]
- Chien, Y.Y.; Wang, T.Y.; Liao, P.W.; Wu, W.C.; Chen, C.Y. Folate-Conjugated and Dual Stimuli-Responsive Mixed Micelles Loading Indocyanine Green for Photothermal and Photodynamic Therapy. Macromol. Biosci. 2018, 18, 1700409. [Google Scholar] [CrossRef]
- Hajipour, M.J.; Santoso, M.R.; Rezaee, F.; Aghaverdi, H.; Mahmoudi, M.; Perry, G. Advances in Alzheimer’s Diagnosis and Therapy: The Implications of Nanotechnology. Trends Biotechnol. 2017, 35, 937–953. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, M.J.; Andrade, S.; Loureiro, J.A.; do Carmo Pereira, M. Nanotechnology to Improve the Alzheimer’s Disease Therapy with Natural Compounds. Drug Deliv. Transl. Res. 2020, 10, 380–402. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Thakur, V.; Deshmukh, R.; Sharma, A.; Rathore, M.S.; Kumar, A.; Mishra, N. Development and Characterization of Morin Hydrate-Loaded Micellar Nanocarriers for the Effective Management of Alzheimer’s Disease. J. Microencapsul. 2018, 35, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Katekar, R.; Thombre, G.; Riyazuddin, M.; Husain, A.; Rani, H.; Praveena, K.S.; Gayen, J.R. Pharmacokinetics and Brain Targeting of Trans-Resveratrol Loaded Mixed Micelles in Rats Following Intravenous Administration. Pharm. Dev. Technol. 2019, 25, 300–307. [Google Scholar] [CrossRef]
- Singh, A.; Ujjwal, R.R.; Kumar, A.; Verma, R.K.; Shukla, R. Formulation and Optimization of Silymarin-Encapsulated Binary Micelles for Enhanced Amyloid Disaggregation Activity. Drug Dev. Ind. Pharm. 2022, 47, 1775–1785. [Google Scholar] [CrossRef]
- Ding, J.; Sun, Y.; Li, J.; Wang, H.; Mao, S. Enhanced Blood–Brain Barrier Transport of Vinpocetine by Oral Delivery of Mixed Micelles in Combination with a Message Guider. J. Drug Target. 2017, 25, 532–540. [Google Scholar] [CrossRef]
- Mondal, R.; Ghosh, N.; Paul, B.K.; Mukherjee, S. Triblock-Copolymer-Assisted Mixed-Micelle Formation Results in the Refolding of Unfolded Protein. Langmuir 2018, 34, 896–903. [Google Scholar] [CrossRef]
- Huang, F.; Qu, A.; Yang, H.; Zhu, L.; Zhou, H.; Liu, J.; Long, J.; Shi, L. Self-Assembly Molecular Chaperone to Concurrently Inhibit the Production and Aggregation of Amyloid β Peptide Associated with Alzheimer’s Disease. ACS Macro Lett. 2018, 7, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Wang, J.; Qu, A.; Shen, L.; Liu, J.; Liu, J.; Zhang, Z.; An, Y.; Shi, L. Maintenance of Amyloid β Peptide Homeostasis by Artificial Chaperones Based on Mixed-Shell Polymeric Micelles. Angew. Chem. Int. Ed. 2014, 53, 8985–8990. [Google Scholar] [CrossRef]
- Chiappetta, D.A.; Facorro, G.; Rubin de Celis, E.; Sosnik, A. Synergistic Encapsulation of the Anti-HIV Agent Efavirenz within Mixed Poloxamine/Poloxamer Polymeric Micelles. Nanomedicine 2011, 7, 624–637. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.H.; Mahajan, H.S. Mixed Micelles for Bioavailability Enhancement of Nelfinavir Mesylate: In Vitro Characterisation and In Vivo Pharmacokinetic Study. Mater. Technol. 2018, 33, 793–802. [Google Scholar] [CrossRef]
- Li, Q.; Hao, X.; Lv, J.; Ren, X.; Zhang, K.; Ullah, I.; Feng, Y.; Shi, C.; Zhang, W. Mixed Micelles Obtained by Co-Assembling Comb-like and Grafting Copolymers as Gene Carriers for Efficient Gene Delivery and Expression in Endothelial Cells. J. Mater. Chem. B 2017, 5, 1673–1687. [Google Scholar] [CrossRef]
- Guo, C.; Manjili, M.H.; Subjeck, J.R.; Sarkar, D.; Fisher, P.B.; Wang, X.Y. Therapeutic Cancer Vaccines: Past, Present, and Future. Adv. Cancer Res. 2013, 119, 421–475. [Google Scholar] [CrossRef] [PubMed]
- Sherbet, G.V. Notable Approaches to Cancer Immunotherapy. In Molecular Approach to Cancer Management; Academic Press: Cambridge, MA, USA, 2017; pp. 223–244. [Google Scholar] [CrossRef]
- Sui, Y.; Li, J.; Qu, J.; Fang, T.; Zhang, H.; Zhang, J.; Wang, Z.; Xia, M.; Dai, Y.; Wang, D. Dual-Responsive Nanovaccine for Cytosolic Delivery of Antigens to Boost Cellular Immune Responses and Cancer Immunotherapy. Asian J. Pharm. Sci. 2022, 17, 583–595. [Google Scholar] [CrossRef]
- Li, J.; Chen, Q.; Zha, Z.; Li, H.; Toh, K.; Dirisala, A.; Matsumoto, Y.; Osada, K.; Kataoka, K.; Ge, Z. Ternary Polyplex Micelles with PEG Shells and Intermediate Barrier to Complexed DNA Cores for Efficient Systemic Gene Delivery. J. Control. Release 2015, 209, 77–87. [Google Scholar] [CrossRef]
- Yang, W.; Chen, P.; Boonstra, E.; Hong, T.; Cabral, H. Polymeric Micelles with PH-Responsive Cross-Linked Core Enhance In Vivo MRNA Delivery. Pharmaceutics 2022, 14, 1205. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, Y.; Zhang, J.; Gao, H.; Liu, G.; Ma, R.; An, Y.; Kong, D.; Shi, L. PH/Sugar Dual Responsive Core-Cross-Linked PIC Micelles for Enhanced Intracellular Protein Delivery. Biomacromolecules 2013, 14, 3434–3443. [Google Scholar] [CrossRef]
- Honda, Y.; Nomoto, T.; Matsui, M.; Takemoto, H.; Kaihara, Y.; Miura, Y.; Nishiyama, N. Sequential Self-Assembly Using Tannic Acid and Phenylboronic Acid-Modified Copolymers for Potential Protein Delivery. Biomacromolecules 2020, 21, 3826–3835. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Kim, H.J.; Osawa, S.; Hayashi, K.; Toh, K.; Naito, M.; Min, H.S.; Yi, Y.; Kwon, I.C.; Kataoka, K.; et al. Dually Stabilized Triblock Copolymer Micelles with Hydrophilic Shell and Hydrophobic Interlayer for Systemic Antisense Oligonucleotide Delivery to Solid Tumor. ACS Biomater. Sci. Eng. 2019, 5, 5770–5780. [Google Scholar] [CrossRef] [PubMed]
- Kumbhar, P.S.; Nadaf, S.; Manjappa, A.S.; Jha, N.K.; Shinde, S.S.; Chopade, S.S.; Shete, A.S.; Disouza, J.I.; Sambamoorthy, U.; Kumar, S.A. D-ɑ-Tocopheryl Polyethylene Glycol Succinate: A Review of Multifarious Applications in Nanomedicines. OpenNano 2022, 6, 100036. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).