The Nano4XX Nanotechnology Platform: The Triumph of Nanotechnology
Funding
Data Availability Statement
Conflicts of Interest
References
- Efthimiadou, E.K.; Fragogeorgi, E.; Palamaris, L.; Karampelas, T.; Lelovas, P.; Loudos, G.; Tamvakopoulos, C.; Kostomitsopoulos, N.; Kordas, G. Versatile quarto stimuli nanostructure based on Trojan Horse approach for cancer therapy: Synthesis, characterization, in vitro and in vivo studies. Mater. Sci. Eng. C 2017, 79, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Kordas, G. Nanotechnology in Cancer Treatment as a Trojan Horse: From the Bench to Preclinical Studies; Nanostruct.; Sarat Kumar Swain, M.J., Ed.; Elsevier Inc.: London, UK, 2019; ISBN 9780128167717. [Google Scholar]
- Tapeinos, C.; Efthimiadou, E.K.; Boukos, N.; Kordas, G. Sustained release profile of quatro stimuli nanocontainers as a multi sensitive vehicle exploiting cancer characteristics. Colloids Surf. B Biointerfaces 2016, 148, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Lelovas, P.; Efthimiadou, E.K.; Mantziaras, G.; Siskos, N.; Kordas, G.; Kostomitsopoulos, N. In vivo toxicity study of quatro stimuli nanocontainers in pregnant rats: Gestation, parturition and offspring evaluation. Regul. Toxicol. Pharmacol. 2018, 98, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Efthimiadou, E.K.; Lelovas, P.; Fragogeorgi, E.; Boukos, N.; Balafas, V.; Loudos, G.; Kostomitsopoulos, N.; Theodosiou, M.; Tziveleka, A.L.; Kordas, G. Folic acid mediated endocytosis enhanced by modified multi stimuli nanocontainers for cancer targeting and treatment: Synthesis, characterization, in-vitro and in-vivo evaluation of therapeutic efficacy. J. Drug Deliv. Sci. Technol. 2020, 55, 101481. [Google Scholar] [CrossRef]
- Bilalis, P.; Boukos, N.; Kordas, G.C. Novel PEGylated pH-sensitive polymeric hollow microspheres. Mater. Lett. 2012, 67, 180–183. [Google Scholar] [CrossRef]
- Efthimiadou, E.K.; Tapeinos, C.; Bilalis, P.; Kordas, G. New approach in synthesis, characterization and release study of pH-sensitive polymeric micelles, based on PLA-Lys-b-PEGm, conjugated with Doxorubicin. J. Nanopart. Res. 2011, 13, 6725–6736. [Google Scholar] [CrossRef]
- Kordas, G. Adjustable Quarto Stimuli (T, pH, Redox, Hyperthermia) Targeted Nanocontainers (Nano4Dox and Nano4Cis) for Cancer Therapy Based on Trojan Horse Approach. Arch. Pharm. Pharmacol. Res. 2018, 1, 1–7. [Google Scholar] [CrossRef]
- Kordas, G. WO2015074762A1.pdf 2015. George KORDAS, Eleni EFTHIMIADOU, “Multi—Responsive targeting drug delivery systems for controlled-release pharmaceutical formulation”, WO2015074762A1, 2015-09-24. Available online: https://patents.google.com/?inventor=George+KORDAS (accessed on 11 May 2023).
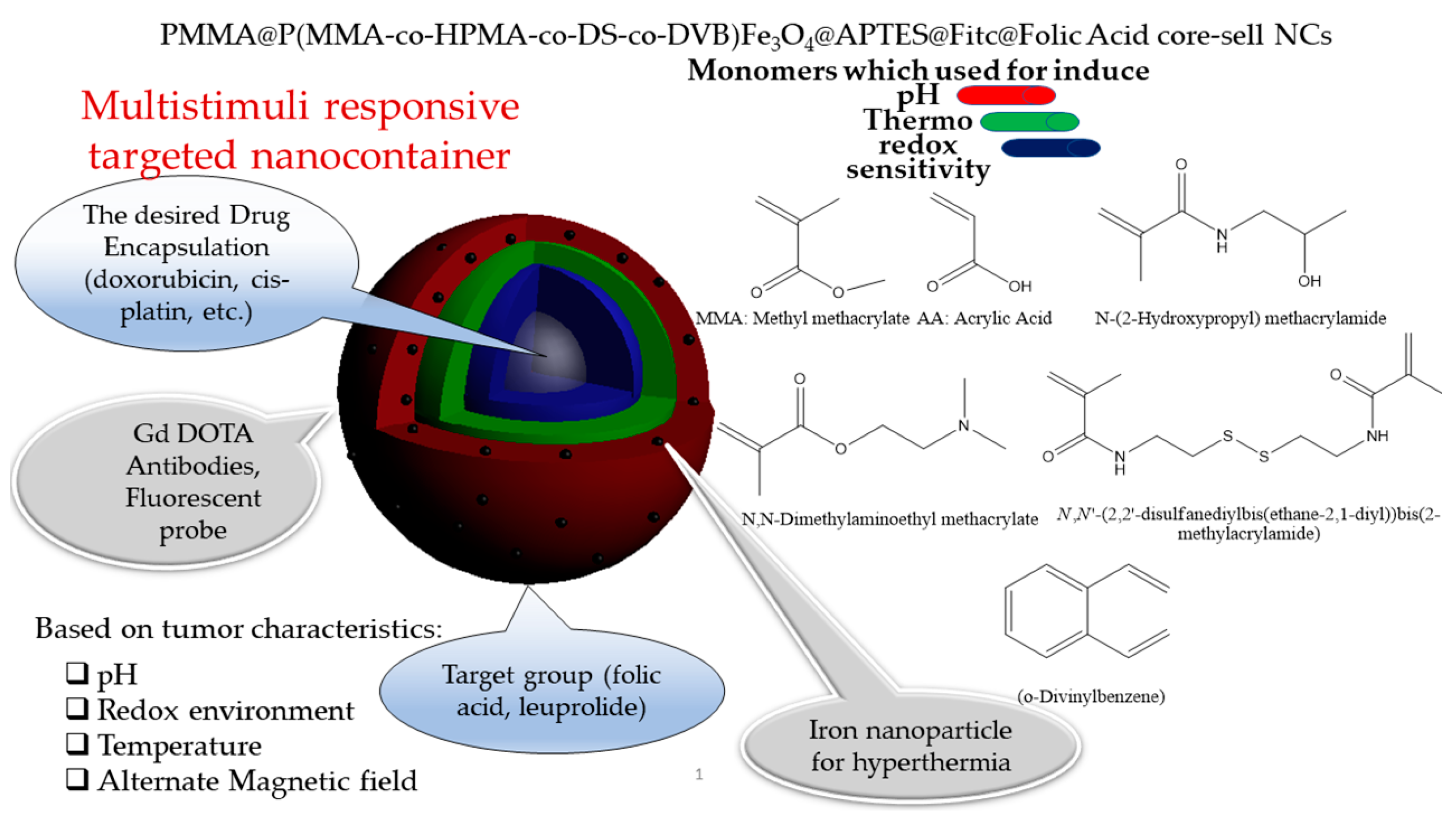
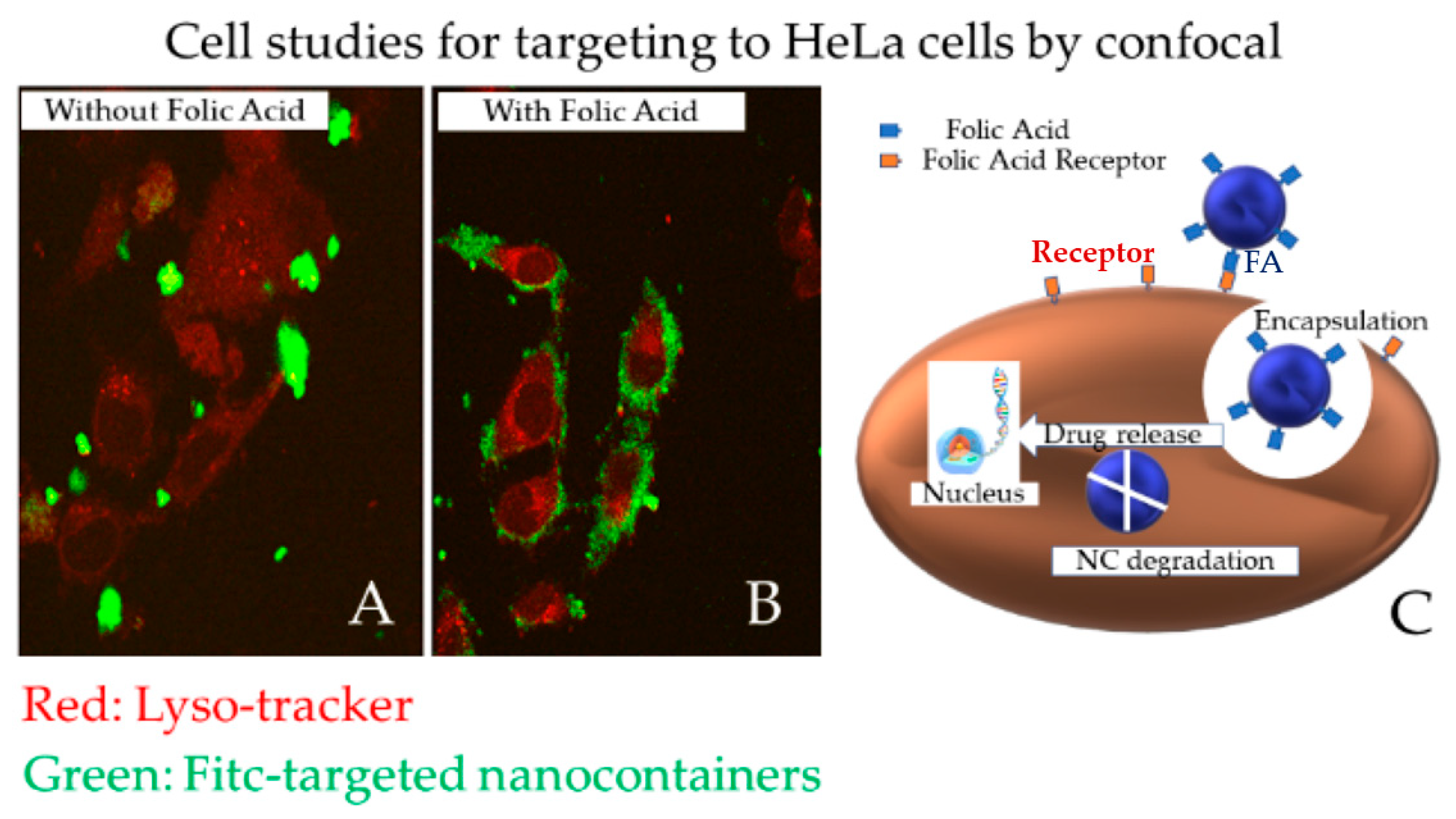
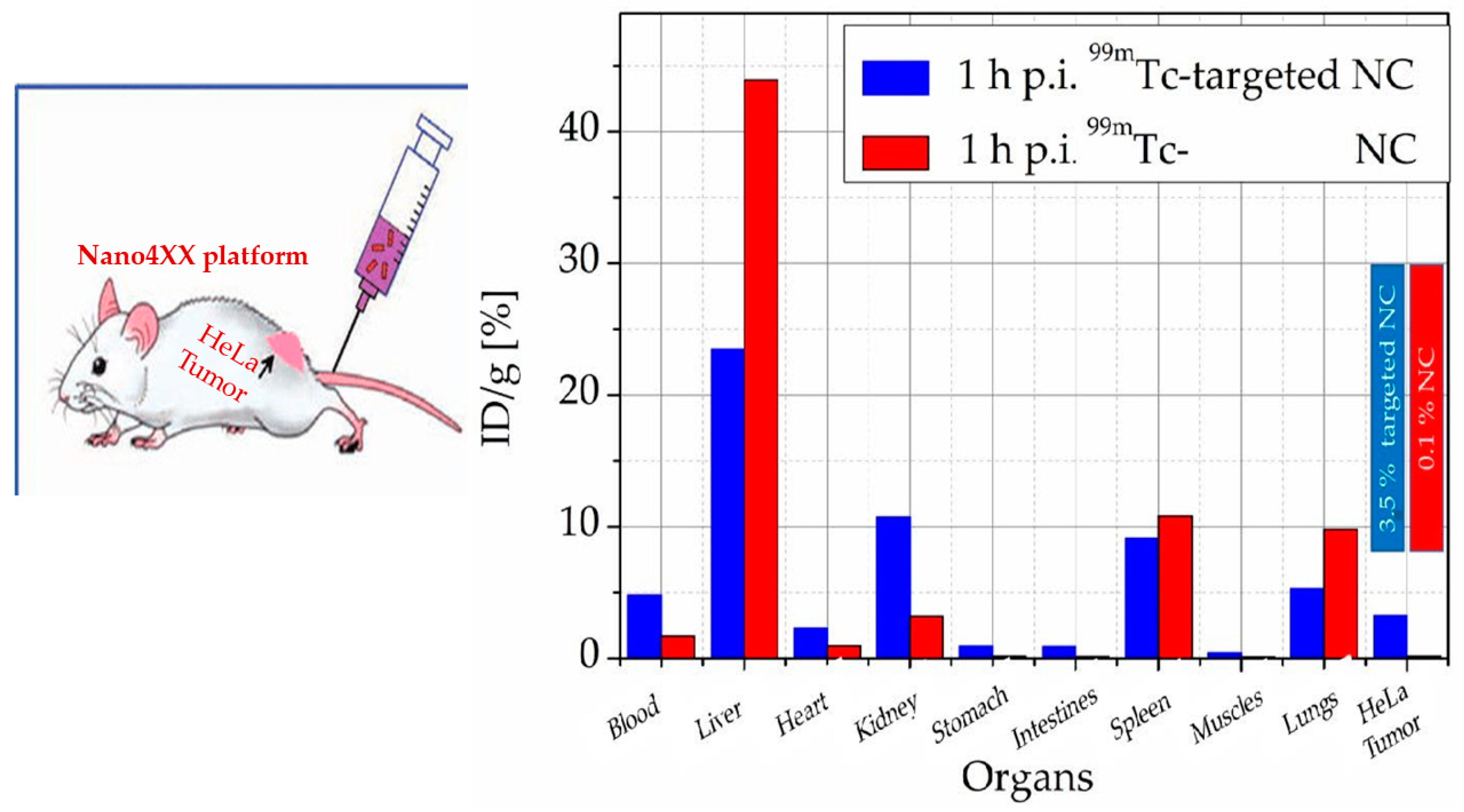
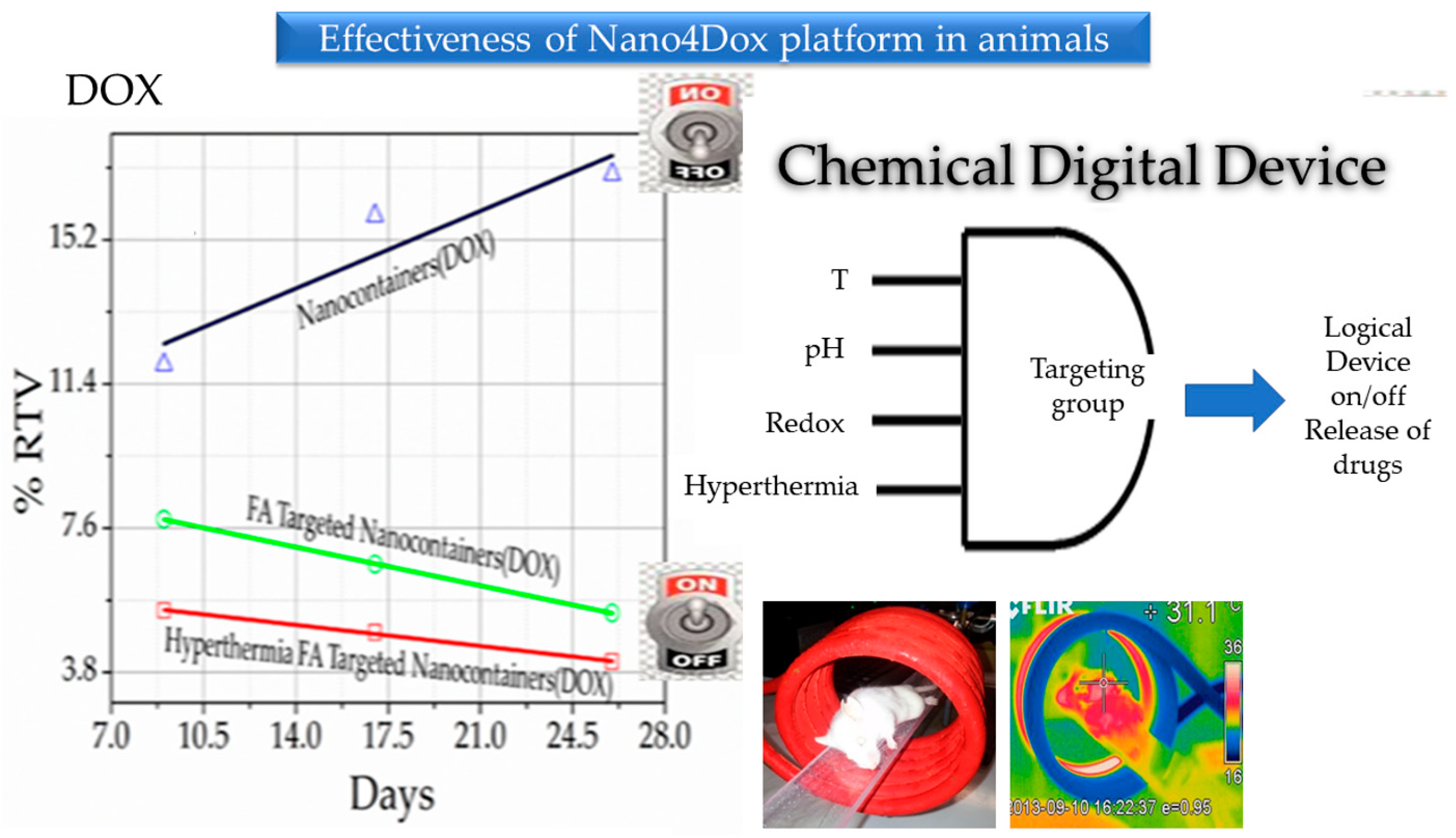
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kordas, G. The Nano4XX Nanotechnology Platform: The Triumph of Nanotechnology. Nanomanufacturing 2023, 3, 228-232. https://doi.org/10.3390/nanomanufacturing3020014
Kordas G. The Nano4XX Nanotechnology Platform: The Triumph of Nanotechnology. Nanomanufacturing. 2023; 3(2):228-232. https://doi.org/10.3390/nanomanufacturing3020014
Chicago/Turabian StyleKordas, George. 2023. "The Nano4XX Nanotechnology Platform: The Triumph of Nanotechnology" Nanomanufacturing 3, no. 2: 228-232. https://doi.org/10.3390/nanomanufacturing3020014
APA StyleKordas, G. (2023). The Nano4XX Nanotechnology Platform: The Triumph of Nanotechnology. Nanomanufacturing, 3(2), 228-232. https://doi.org/10.3390/nanomanufacturing3020014




