Abstract
The second most significant cause of cancer-related mortality and morbidity in the United States is colorectal cancer (CRC), the third most diagnosed malignancy. People over 50 have an increased risk of CRC everywhere in the world. Genetic and environmental risk factors significantly influence CRC development. Early detection is critical in the treatment and prevention of CRC. The population’s incidence rate of CRC is currently reduced by screening techniques and medicines, although recurrence of the disease may result from the cancer’s ability to spread locally. Consequently, the difficulty is in finding a different treatment for CRC. Nanotechnology is crucial for cancer treatment because it allows for the delivery of targeted chemotherapies to cancer cells directly and with greater therapeutic potency. Nanoemulsions have broad application in pharmaceutics, cosmetics, and food; their outstanding properties include enhanced dispersion of active hydrophobic components, small size, high surface area per unit volume, and improved absorption in cancer treatment. The present review highlights formulation aspects, preparation methods, and characterization techniques. We also provide a critical analysis of recent developments in nanoemulsions in colorectal cancer treatment that hold promise in delivering nanoemulsions in colorectal treatment.
1. Introduction
The dispersion phase and the continuous phase are two immiscible phases that make up an emulsion. In the beginning, Schulman coined the phrase “microemulsion”. After more investigation into microemulsion, researchers discovered a kinetically stable emulsion known as nanoemulsion [1]. The definition of a nanoemulsion by the International Union of Pure and Applied Chemistry (IUPAC) is a dispersion made up of water, oil, and surfactant with a dispersed domain diameter and is isotropic and thermodynamically stable. In practice, the nanoscale emulsion is created using mechanical force. Nanoemulsions are emulsions that are smaller than tens to hundreds of nanometers in size. As the system strives to reach a state of minimal Gibbs free energy, surfactants work at the interface between the two immiscible phases to reduce surface tension and stabilize the emulsion [2]. Because they can quickly solubilize hydrophobic pharmaceuticals and lessen severe side effects, nanoemulsions have much potential [3]. In this article, numerous emulsion systems are introduced, their technique and stability are discussed, and their characterization is covered, along with various surfactants and factors to consider when choosing a surfactant before application.
1.1. Advantages of Nanoemulsion
Nanoemulsions are safe and will not cause irritation; they can be applied to the skin [4]. The nanosized droplets would influence the drug’s transport properties, a critical factor in targeted and sustained drug delivery [5]. Because of the small droplet size, there is some sedimentation and no creaming during storage. The small droplet size reduces gravity force, and Brownian motion induction may be sufficient to overcome gravity. Peptide drugs are hydrolyzed enzymatically in the G.I. tract. However, nanoemulsions have the potential to deliver peptides [6]. They are tiny droplets with a larger surface area for enhanced absorption with lower energy requirements [7]. Cell culture technology makes it feasible to study the toxicity of oil-soluble medicines and enhances cell proliferation in certain diseases. Vesicles can be used in place of liposomes to boost medication bioavailability. They come in several forms, such as lotions, liquids, sprays, and foams. Oral administration is possible if the formulation includes biocompatible surfactants, which is therapeutically beneficial for humans and animals because it has no toxic effects on healthy tissue [8].
1.2. Disadvantages
Nanoemulsifier stability could be affected by environmental conditions, including temperature and pH. This is problematic for compounds with high melting points because of their poor solubility. Nanodroplet stabilization requires a more excellent surfactant and cosurfactant concentration in nanoemulsifiers [8].
2. Types, Composition, and Characterization of the Nanoemulsion System
Emulsions are a mixture of two or more phases, one of which is hydrophilic and the other hydrophobic, scattered throughout each other. When tiny oil droplets are scattered throughout water, the emulsion is called an oil-in-water (O/W) emulsion [9]. The homogenization method employed during the creation of nanoemulsions and traditional O/W emulsions determines the droplet sizes of the final products [9,10]. When water droplets are mixed with oil, water-in-oil (W/O) emulsions are created. In microreactors, W/O nanoemulsions regulate nanoparticle formation, including Cd [cadmium] nanoparticles and titania–silica nanoparticles [11,12]. As reaction media, different W/O emulsions are employed to produce ceramic nanoparticles. W/O emulsions are used in the pharmaceutical business as adjuvants for vaccines containing uncommon antigens, such as synthetic peptides, DNA, or recombinant proteins. However, by enclosing an emulsion within an emulsion, resulting in oil-in-water-in-oil (O/W/O) or water-in-oil-in-water (W/O/W) emulsions, these straightforward systems can be made more complex.
Double emulsions are often created in two steps: An initial internal emulsion is created, and then a second emulsion is created to cover the initial emulsion. Double emulsions present difficulties in their formation and stabilization, including the need to preserve the integrity of the first emulsion when creating the second one, the need for both a hydrophilic surfactant and a lipophilic surfactant to stabilize each oil–water interface, and a higher propensity for coalescence and degradation because of diffusion between each phase [13]. Microfluidic devices have sparked interest in studying uniform double emulsions for use in food science and as templates for particle synthesis or microreactors [14,15]. In contrast to most emulsions, in which the internal phase only accounts for a negligible fraction (10%) of the overall emulsion volume, high internal phase emulsions contain an internal phase higher than 74% of the total emulsion volume [16].
Pickering emulsions are oil-in-water or water-in-oil emulsions in which minute particles operate as a steric barrier to coalescence at the oil–water interface as opposed to small molecules that alter the interfacial tension [17]. Stabilizing particles by moistening pickering emulsions with both emulsion phases is possible. This property makes them more effective than conventional surfactants at increasing emulsion stability by allowing them to sit at the oil–water interface [18]. Pickering emulsions have been investigated as an alternative to small molecule surfactants for drug delivery applications because of their increased stability and biocompatibility.
2.1. Composition of Nanoemulsion
Nanoemulsion formulation consists of the following components.
2.1.1. Oils
The oil phase influences the choice of other nanoemulsion ingredients. The appropriate oil phase must be chosen to achieve the desired properties of a nanoemulsion [19]. The ability of the oil to solubilize the chosen drug candidate and permit the creation of a nanoemulsion with appropriate qualities will determine which oil is used. Combining fixed oil and medium-chain triglycerides can balance drug loading and emulsification. It is possible to select oil components resilient to oxidative deterioration [20]. Medium-chain triglycerides (MCT) have better liquid ability and oxidation resistance than lengthy triglycerides (LCT) because of their high hydrophilic nature and solvent capacity for medicines (LCT) [21]. More recently, semi-synthetic medium-chain triglycerides have developed as a viable alternative to traditional MCTs. Olive oil, corn oil, soybean oil, palm oil, oleic acid, sesame oil, peanut oil, hydrogenated soybean oil, and beeswax are all examples of oils and fats used in the oil phase along with other substances, such as modified vegetable oils [21].
2.1.2. Surfactants
Surfactants with a high hydrophilic–lipophilic balance (HLB) value (more than 10) are hydrophilic and are used to prepare O/W nanoemulsions, and hydrophobic surfactants (HLB value 10) are utilized to make W/O nanoemulsions. To create nanoemulsions, it is sometimes necessary to use both low and high HLB surfactants [22].
Types of surfactants are as follows:
- Small molecule surfactants;
- Particle as surfactants;
- Phospholipids surfactants;
- Bioinspired peptide surfactants;
- Bioinspired protein surfactants.
Small Molecule Surfactants
The hydrophobic tail and hydrophilic head structure characteristic of small molecule surfactants are referred to as “head-tail morphology”. Their hydrophilic–lipophilic balance describes the hydrophobicity and hydrophilicity of these surfactants. According to the primary group’s electric charge, small molecule surfactants are classified as anionic, cation, or nonionic [23].
- Anionic surfactants
Sodium salts of carboxylate and sulfate ester derivatives, such as sodium oleate and sodium dodecyl sulfate, have a negatively charged polar terminal functional group. Compared to nonionic and cationic surfactants, anionic surfactants have superior foaming capacity and foam stability. To fabricate them, saponification and sulfonation with inexpensive resources, such as cooking oil, are required [24].
- Cationic surfactants
They belong to the functional group with a positive charge at the polar head composed of charged quaternary amine salts, such as cetrimonium bromide, which is used to purify DNA extracts, and cetylpyridinium chloride (CPC), a quaternary ammonium compound, which was primarily used in oral products, such as mouthwashes and dentifrices. Mouthwash is a solution, often containing antiseptic, astringent, and breath-sweetening agents, used for cleansing the mouth and teeth and gargling [25].
- Nonionic surfactants
Nonionic surfactants consist of a hydrophilic head functional group (usually PEG-based) and a fatty acid tail; their heads are uncharged. Nonionic surfactants, such as polysorbates, sorbitan esters, and Brij polyoxyethylene alkyl ethers, can be identified if the group is broken down further. Compared to cationic and anionic small molecule surfactants, nonionic surfactants are preferred for use in biological applications because of their lower toxicity potential: Tween 80 [26].
Particles as Surfactants
Silica, clay, iron oxide, hydroxyapatite, and graphene oxide are all examples of particles with surface-active characteristics. Particle forms, such as spheres, sheets, and rods, significantly impact the thermodynamic barrier to coalescence by affecting the self-assembly of particles at the interface [27].
Phospholipids
They are the main structural component of cell membranes and have a head–tail structure similar to novel molecular surfactants with a positive charge phosphate head and two triglyceride tails. Phospholipids serve as a steric barrier, increase the thermodynamic energy needed for droplets to coalesce, and provide repulsive forces between droplets, which helps stabilize emulsions. These are derived from agricultural sources, such as soy lecithin and egg yolks, and can provide good long-term stability to the emulsion [28].
2.1.3. Bioinspired Peptide Surfactants
Peptide surfactants share structural similarities with other surfactants, including the presence of both hydrophilic and hydrophobic domains. They are divided into three categories based on their distinct structural patterns: end-by-end peptide surfactants, side-by-side peptide surfactants, and end-by-side peptide surfactants. Similar to natural phospholipids, lipid-like or surfactant-like peptides (SLPs) consist of a hydrophilic head and a hydrophobic tail. Proteins, chemicals, and medicines can benefit from the stabilization provided by many SLPs’ ability to self-assemble into micelles, vesicles, or nanotube structures [29]. The side-by-side peptide surfactant is another example. The peptide has specific hydrophilic and hydrophobic amino acid sequences that allow the formation of α-helices or β-sheets.
2.1.4. Bioinspired Protein Surfactants
Proteins can be used as surfactants to stabilize emulsions due to their amphiphilic nature, which allows them to contain both hydrophilic and hydrophobic amino acid sequences that function at the oil–water interface. Proteins cause an early onset of droplet coalescence during the emulsion formation because they adsorb to oil–water surfaces at a slower rate than small molecule surfactants. However, after the proteins adsorb onto the droplet surface, the protein surfactant provides a significantly more significant stabilizing impact due to the formation of vast protein networks that exhibit solid electrostatic repulsion and steric stability. When transporting drugs that are not water-soluble, protein-based surfactants are often utilized [30].
2.1.5. Cosurfactants
The addition of a cosurfactant is expected to work when a surfactant alone cannot sufficiently reduce the oil–water interfacial tension to create a nanoemulsion—liquid crystalline phases form when a surfactant coating becomes too rigid. By penetrating the surfactant monolayer and agitating the liquid crystalline phases, cosurfactants increase the fluidity of the interfacial film. The oil/water interface can be lowered, and nanoemulsions can be formed spontaneously with the help of hydrophilic cosurfactants, particularly those with an intermediate chain length [31].
2.2. Physicochemical Characterization of Nanoemulsion
2.2.1. Phase Behavior
To ascertain the nanoemulsion phase, dispersibility, and ingredient optimization, research on phase behavior is essential for nanoemulsions created by the phase inversion temperature and self-emulsification method (surfactant, oil phase, and aqueous phase). The various nanoemulsion components are analyzed by changing their concentration in glass ampoules. After reaching equilibrium at a set temperature, the ingredients are mixed vigorously until completely uniform. To identify an isotropic phase, polarized light is used [32,33].
2.2.2. Droplet Size and Polydispersity Index
The polydispersity index (PDI), mean particle diameter (Z-averages), and particle size distribution are important markers of nanoemulsion quality, stability, homogeneity, and dispersibility over time [34]. Since the release rate and the amount of active ingredient absorbed are affected by droplet size, it is crucial to the performance of a nanoemulsion. Droplet size in a nanoemulsion is often measured using light scattering and photon correlation spectroscopy (PCS) techniques, including static light scattering (SLS) and dynamic light scattering (DLS) [35]. Generally, to measure the droplet sizes of nanoemulsions, the polydispersity index calculated using DLS is defined as the square of the standard deviation divided by the mean droplet diameter (Figure 1). The PDI quantifies differences in size from the norm. The droplets in a nanoemulsion are considered well-dispersed and free of adhesion and aggregation if their PDI is less than 0.22. A PDI above 0.25 implies strong emulsion stability, while a PDI above 0.5 suggests an unfavorable attribute of less uniform droplet size. Most notably, a PDI near one (one) suggests a wide variety of droplet sizes, while a PDI around zero indicates a monodisperse droplet population [36].
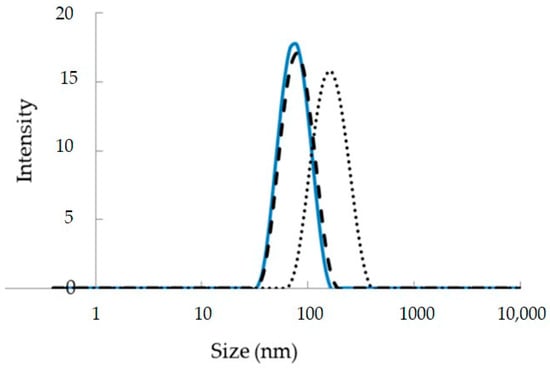
Figure 1.
Droplet size distribution of empty and curcumin-loaded nanoemulsions fabricated using low energy O/W nanoemulsion techniques adapted from [37].
2.2.3. Zeta Potential
This parameter, often measured using a zeta sizer, describes the role of surface charge on droplets. Once the nanoemulsion sample has been prepared, it is measured in a zeta cuvette by measuring the droplet potential in millivolts [38]. A dividing line between stable and unstable aqueous dispersions is generally taken at either +30 or −30 mV. Globules with zeta potentials greater than +30 mV and lower than −30 mV are usually considered stable [39]. Zeta potentials greater than either +60 mV or −60 mV characterize exceptionally stable nanoemulsions [40].
2.2.4. Viscosity
A preparation’s viscosity is crucial in maintaining its consistency as a liquid or semi-solid. The surfactant, water, and oil contents in an emulsion all significantly determine the emulsion’s viscosity. In most cases, the viscosity can be lowered by increasing the water content during formulation. However, the interfacial tension between the water and oil can be increased by reducing the amount of surfactant and cosurfactant, leading to a more viscous emulsion. A Brookfield-type rotational viscometer can be submerged in a 37 ± 0.2 °C thermo-bath, and the nanoemulsion’s viscosity can be evaluated at varying shear rates and temperatures [41].
2.2.5. Conductivity and Dielectric Measurement
Conductivity readings reveal details regarding oil-continuous or water-continuous phases and phase inversion occurrences. Greater ion mobility is possible because of the higher water content reflected in nanoemulsion conductivity. Whether or not a formulation undergoes phase inversion can be determined by measuring whether its conductivity changes after being stored at room temperature for one month. The structure and behavior of a nanoemulsion system can be demonstrated via dielectric measurements. A formulation is stable and free of phase inversion if its conductivity has not changed after being stored at room temperature for one month [42].
2.2.6. Entrapment Efficiency
The capacity of a nanocarrier to entrap the drug/active component and transport an effective dose to the treatment site is referred to as its entrapment efficiency (E.E.). Essential aspects that might significantly affect E.E. include the manner of formulation, ingredients, and the nature of the encapsulated bioactive molecule in the vesicles. We show that the microdialysis method may accurately estimate E.E. in nanocapsules, nanospheres, and nanoemulsions. Gel filtration, ultrafiltration, dialysis bag diffusion, and ultracentrifugation are different methods for calculating the E.E. of various nanocarriers [7,43].
The following is a general formula for calculating the E.E.
where W1 represents the amount of active ingredient added to the formulation and W2 represents the amount of active ingredient in the supernatant.
2.2.7. Fourier-Transform Infrared Spectroscopy (FTIR) Spectral Analysis
Drug and excipient interactions, polymerization, crosslinking, and drug content can be evaluated by FTIR analysis. It is also helpful for determining the molecular fingerprint and the functional groups attached to a molecule [44].
2.2.8. Morphological Study Using SEM and TEM Techniques
Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) are methods for analyzing the morphology of nanoemulsions. The globules may be seen in three dimensions thanks to SEM. The samples are examined at various magnifications and an appropriate accelerating voltage, typically 20 kV. SEM provides a good examination of the surface morphology of the formulation’s dispersed phase. Image analysis tools might be used to analyze shape and surface morphology automatically [25,35]. When creating samples for TEM, a holey film grid is coated with a few drops of nanoemulsion or a suspension of lyophilized nanoparticles in double-distilled water. Any residual solution must be rinsed away and colored after immobilizing the grid. The dyed nanoparticle sample is then subjected to a particular voltage. The SEM and TEM analysis images are shown in Figure 2 and Figure 3. Understanding the surface morphology of nanoemulsion formulations using an atomic force microscope (AFM) or cryo-electron microscopy is a cutting-edge technique [45,46].
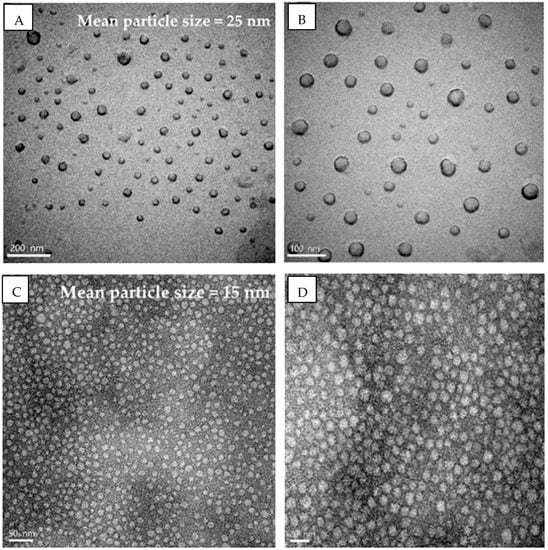
Figure 2.
Mean globule size of xanthone (A,B) and anthocyanin (C,D) nanoemulsions using transmission electron microscopy [47].
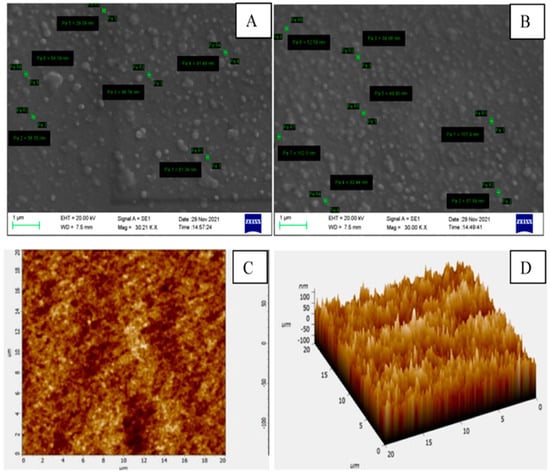
Figure 3.
Mean globule size of essential oil encapsulated chitosan nanoemulsions using scan electron microscopy (A,B) and atomic force microscopy (C,D) [48].
2.2.9. In Vitro Drug Release Study
With the aid of in vitro drug release studies, the efficacy of therapeutic formulations in vivo can be predicted. A medicine’s in vitro release rate can be measured with USP dissolution equipment II. Dialysis membrane pouches containing a drug equivalent to 10 mg were introduced into a buffer flask after the medication had been distributed in nanoemulsion or dried nanoparticles at 37 ± 0.5 °C with a stirring speed of 75 rpm. At regular intervals, a sample was removed, and the same volume of new dissolving liquid was added. After the samples were adequately diluted, their absorbance was measured spectrophotometrically at a predetermined wavelength. By comparing the absorbance of the collected sample to a calibration curve, we may calculate the percentage of drugs released at various times [49].
2.2.10. In Vitro Skin Permeation Studies
The Keshary-Chien diffusion cell is used for both in vitro and ex vivo permeation experiments. Commonly utilized in permeation tests is the abdomen skin of adult male rats weighing between 250 and 300 gm. The rat’s skin was between the donor and receiver chambers of the diffusion cell. Fresh water and 20% ethanol were stored in receiver chambers heated to 37 degrees Celsius and swirled at 300 revolutions per minute. The donor space housed all formulas. At regular intervals (e.g., 2, 4, 6, 8 h), 0.5 mL of solution was taken from the receiver chamber and analyzed by gas chromatography; adding a new volume of the solution followed this. Each sampling procedure was repeated three times. Cumulative adjustments were used to determine the total amount of medication that penetrated rat skins at each time interval, and these data were then shown as a function of time. The plot’s slope can determine drug penetration rates in a steady state [50] (Table 1).

Table 1.
Physical and chemical characterization of nanoemulsions.
2.3. Stability Studies
Stability studies focus on drug stability in different conditions, including temperature, humidity, and light. Nanoemulsion stability tests are undertaken only after the formulation has been stored for 24 months in a dispersed and freeze-dried state as the International Council for Harmonization (ICH) recommends. The shelf life of a nanoemulsion formulation was also determined after performing accelerated stability testing (40 °C ± 2 °C and 75% ± 5% RH) along with an intermediate stability study (30 °C ± 2 °C/65% RH ± 5% RH) [61]. The optimized nanoemulsion was stored in airtight glass containers. Parameters, such as globule size, loading, entrapment efficiency, and in vitro drug release profile, assessed by taking samples at regular intervals [62].
2.3.1. Stability Study of Nanoemulsion
It is important to make sure nanoemulsions are appropriately made and stored at appropriate conditions. This can be performed by finding the mechanisms of emulsion instability and stabilization to make nanoemulsions thermodynamically unstable systems that bifurcate over time. By producing a kinetically stable emulsion with surfactants, a well-designed emulsion can keep its original qualities for months or even years after it has been made [41,63]. Nanoemulsion versatility relies on it, as even slight formulation changes over time can severely impact patients. Emulsion stability is a product of electrostatic double-layer repulsion and attractive van der Waal interactions, as proposed by the Derjaguin−Landau−Verwey−Overbeek (DLVO) hypothesis [64]. The estimated stability within 5 nm is calculated by adding the attractive and repulsive forces at given distances, as the two forces are believed to be unrelated (Equation (1)) [65]:
F.T. = F.A. + F.R.
Colloid stability is maximized when droplets are kept at a safe distance from one another where repulsive forces have a more significant impact on the interaction energy than attractive ones. This instability manifests in four types: Ostwald ripening, coalescence, flocculation, creaming, and sedimentation.
2.3.2. Coalescence
Two or more droplets can coalesce into one giant droplet. When droplets collide and distort, a continuous phase film is created [66]. Moving a continuous phase and surfactant along a film’s surface may regulate the film’s thickness. Rupture occurs when a film’s thickness decreases below a specific value [67]. Thin films are responsible for the Marangoni effect, which explains mass transport across an interface regarding interfacial tension gradients. As the droplets combine, their combined surface area drops, resulting in a decrease in the interfacial and Gibbs energies of the system.
2.3.3. Flocculation
During flocculation, a continuous phase forms a thin film that separates clusters of dispersed, unbroken droplets. The electrostatic repulsion created by the film delays emulsion coalescence, making the film stable; nevertheless, stability against coalescence must be regarded independently from stability against flocculation since emulsions can readily flocculate and then slowly coalesce and vice versa [68].
2.3.4. Creaming and Sedimentation
Emulsion droplets cream and settle when their densities are not the same as those of the scattered and separate phases, respectively. The frequency of droplet interaction is raised during creaming/sedimentation; hence, droplets are more likely to coalesce [69].
2.3.5. Ostwald Ripening
Due to the diffusion of the dispersed phase through the continuous phase, larger droplets form at the expense of smaller droplets during the Ostwald ripening process. These little droplets serve as a source of nutrition for the larger ones. This process is induced by the Kelvin effect, which claims that particles with smaller diameters have superior solubility in solution and decrease the total surface area of the dispersed phase, resulting in a reduction in the Gibbs free energy. By ensuring a monodispersed size distribution of droplets and employing a dispersed phase with very low solubility in the continuous phase, Ostwald ripening can be avoided [70].
2.3.6. Nanoemulsion Stability Enhancement
The DLVO hypothesis postulates that there are two separate mechanisms at work to dampen droplet contact and, in turn, emulsion instability [71]. The first is electrostatic repulsion, which occurs when two drops with the same electric charge collide. The second type, steric stabilization, occurs when a substantial surface layer between droplets creates an essential energy barrier, preventing the droplets from interacting and coalescing. Ionic surfactants stabilize an electrostatic emulsion by attracting counter ions from the solution and generating an electrical double layer [16,66]. The charged surfaces of the emulsion make up the first layer of an electrical double layer followed by a layer of tightly bound counter ions and finally a layer of low-concentration material. When the diffuse layer stops moving in tandem with the emulsion droplets, you have reached the slip plane. The zeta potential, determined by the charge at the slip plane, is a crucial indicator of emulsion stability, with highly positive or negative emulsions being more stable [72]. DLVO theory suggests droplet interaction is necessary for emulsion destabilization, inhibited by the stearic hindrance by increasing surface layer thickness and complexity [73]. Nonionic surfactants with long side chains, pickering particles, and conjugating/coating polymers can all be used to modify the droplet surface in this way [74].
2.4. Formulation Method of Nanoemulsion
Nanoemulsions are challenging to prepare spontaneously due to their nonequilibrium nature [75]. But by providing sufficient energy with mechanical equipment, they can be prepared. Alternatively, spontaneous techniques are used to prepare nanoemulsions. Higher energy is required to formulate the smaller droplets (i.e., sizeable interfacial area) of a nanoemulsion, which the following equation can calculate:
where W is the work required to increase the interface;
W = ⊗A γ
- ⊗A is the increasing total interfacial area;
- γ is the interfacial tension.
According to this correlation, increased efforts are required when ⊗A is large (i.e., when the droplet size is tiny or when the interfacial tension is high). Formulations for nanoemulsions can be broken down into two categories, one based on energy consumption and the other on the type of energy used [76]:
- Low-energy methods;
- High-energy methods.
2.4.1. Low Energy Methods
The “Low energy” method involves the spontaneous emulsification of nanoemulsions without using any machine or energy. These techniques for creating nanodroplets rely on the physicochemical qualities of the components themselves. To create a uniform liquid at room temperature, a lipophilic phase must be combined with a hydrophilic surfactant, which is then solubilized. After the oily phase’s hydrophilic species (i.e., the surfactants) are solubilized in an aqueous phase, the unmixing of the oil begins, and nanodroplets get produced. In most cases, water alone makes up the aqueous phase. The formation of nanoemulsions can be achieved with minimal energy expenditure through the application of techniques such as phase inversion composition (PIC) and phase inversion temperature (PIT) [77].
Phase Inversion Temperature (PIT)
An essential part of this technique is the phase transition caused by the surfactant’s spontaneous curvature during emulsification. Additionally, characteristics such as temperature can be altered to alter the surfactant’s spontaneous curvature. In the PIT technique, the spontaneous curvature of the surfactant is reversed by adjusting the temperature. A nonionic surfactant dehydrates the polyoxyethylene groups of a polyethoxylated surfactant. This alters the molecular structure of the polyethoxylated surfactant, making it more lipophilic (Figure 4). Due to phase inversion, nanoemulsion is generated [78,79].
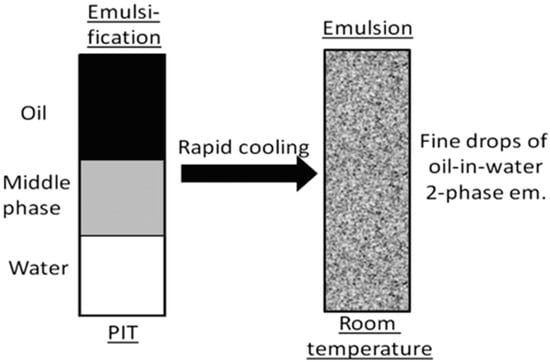
Figure 4.
The image shows the formation of nanoemulsion by the phase inversion temperature technique (adapted with permission from [80]).
Phase Inversion Composition (PIC)
By changing the composition, the PIC method can counteract a surfactant’s spontaneous curvature. A nonionic surfactant dehydrates the polyoxyethylene groups of a polyethoxylated surfactant. The polyethoxylated surfactant’s molecular structure is modified, increasing its lipophilicity. In a process known as phase inversion, nanoemulsion is produced [79]. An example of a component that can be added to a PIC is water followed by an oil-surfactant combination or oil added to the water-surfactant combination. Most nanoemulsions are made using the PIC technique and nonionic polyoxyethylene surfactants. Slowly adding water to the oil phase and increasing the water percentage volume causes the surfactant’s polyoxyethylene chain to hydrate. At the HLB temperature, the surfactant’s hydrophilic and lipophilic properties in the water phase are balanced, and the surfactant’s spontaneous curvature changes to zero, just as it does in the PIT method (Figure 5). During this change, a bi-continuous or lamellar structure is created. When the transition composition is exceeded, the zero-curvature structures of the surfactant layer transform into highly positive-curvature structures as more water is introduced. Curvature transition leads to phase inversion and the subsequent production of nanosized droplets [81,82].
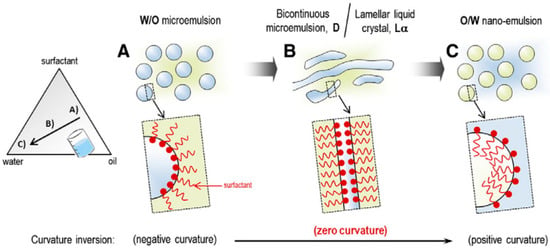
Figure 5.
Schematic representation of the formation of nano-emulsions by the PIC method (A) W/O emulsion, (B) Bicontinuous microemulsion, (C) O/W nano-emulsion (Adapted with permission [79]).
2.4.2. High-Energy Methods
“High energy” nanoemulsions require specialized equipment to manufacture nanodroplets and expand the water/oil interface [83,84]. It typically takes two stages of a high-energy technique to obtain nanoemulsions ready for use: deformation and disruption of micrometric droplets into smaller droplets and surfactant adsorption at their interface (to ensure steric stability).
Stirring at High Shear Using a Rotor/Stator System
This rapid stirring method is propelled by hydrodynamic shear. There are two stages to the emulsification process: Firstly, the high shear stress deforms the droplets, increasing their specific surface area for disruption, and then, second, emulsifiers maintain the new interface. Reynolds numbers represent the shear forces produced by rapid stirring and are used to quantify shear forces. To be more precise, a more significant Reynolds number encourages the production of smaller particles. Nanoemulsions with typical diameters of around 100 nm require shear rates in the 108 s−1 range to produce nanoemulsions [85].
High-Pressure Homogenization (HPH)
High-pressure homogenization is the most used technique for making nanoemulsions (HPH). Cavitation, a robust phenomenon, breaks up oil droplets and creates smaller ones. Significant variations in mean droplet size and particle distributions can also result from changes in other variables, such as homogenization pressure and cycle number. Oil, water, and surfactant or cosurfactant are combined in a high-pressure homogenizer and subjected to high pressure (Figure 6). HPH loses productivity and experiences component deterioration because of high temperatures. Only O/W liquid nanoemulsions with an oil phase concentration of less than 20% should be prepared using HPH. Nanoemulsions with mean droplet sizes of less than 200 nm, such as those with high viscosity or creamy consistency, cannot be effectively formulated using this method [86,87].
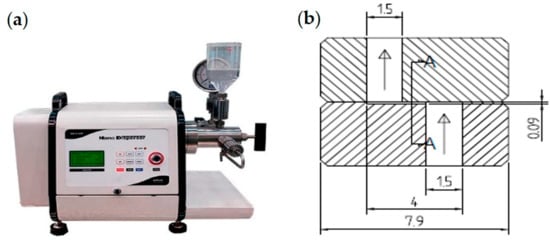
Figure 6.
High-pressure homogenization technique showing the formation of nanoemulsion (a) electric motor-driven high-pressure homogenizer, and (b) Diacell® Z-type nozzle (unit in mm) (adapted with permission from [86]).
Ultrasonication
Due to its low production cost, high energy efficiency, and minimal mixing instrument needs, ultrasonication emulsification is quickly rising in popularity. Ultrasonic emulsification uses an acoustic field to mix two immiscible liquids [88]. Canselier proposes a two-stage technique for ultrasonic-assisted emulsification. The droplets of the dispersed phase rupture into the continuous phase due to interfacial waves and system instability, and then the droplets are further broken up by cavitation near the interface [89]. Cavitation, the rapid generation of vapor bubbles in a liquid at room temperature, and decreased pressure are the primary consequences of ultrasound. Shock waves are produced when the bubbles quickly collapse. As a result, high-velocity liquid jets are produced as the liquid undergoes intense localized turbulence and shear stresses. When a bubble bursts, it causes nearby droplets to scatter, which stimulates emulsion mixing. This technique for preparing nanoemulsions is currently only applicable in the lab due to the small batch sizes required (Figure 7).
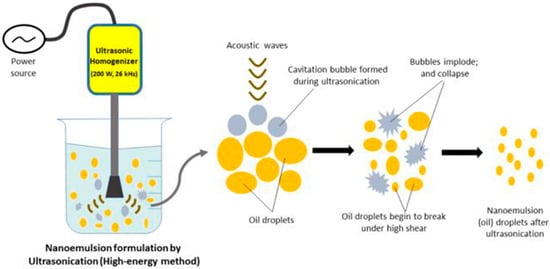
Figure 7.
Image shows the formation of nanoemulsion by ultrasonication method (adapted with permission from [90]).
Microfluidization
This method utilizes high-powered equipment and abundant energy inputs to create ultrafine emulsions with a surfactant-to-oil ratio (SOR) <0.1. Shear cavitation and impact create an emulsifying environment [91]. A coarse emulsion is created by forcing the water–oil mixture through an inline homogenizer. Finally, a high-pressure positive displacement pump drives the newly created coarse emulsion into a microchannel-lined interaction chamber (3.447 MPa). After passing through an impingement region, an emulsion’s viscous mixture is transformed into very thin submicron or nanosized droplets, leading to a stable nanoemulsion [92]. To create a nanoemulsion, the pressure must be raised to around 700 MPa (Figure 8).
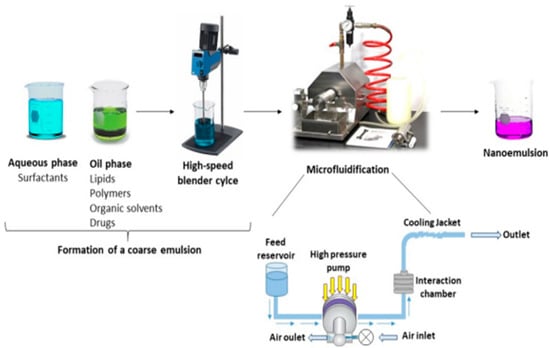
Figure 8.
Formulation of nanoemulsion by micro fluidification technique (adapted with permission [93]).
3. Nanoemulsion for Colorectal Cancer Delivery
A large fraction of cancer-related deaths worldwide is caused by colon cancer. Treatment options for colon cancer typically include surgery, herbal remedies, immunotherapy, radiation, and chemotherapy. Since nanotechnology has been proven effective for treating cancer, researchers have concentrated their efforts on this area. The most prevalent types of nanoemulsions for different types of cancer are summarized in Table 2, a summary of some recent nanoemulsions developed and applications for colorectal and other cancer.
Worldwide, colon cancer is responsible for a disproportionate number of cancer deaths. This section classifies the various subtypes of colon cancer, including familial adenomatous polyposis, hereditary nonpolyposis colon cancer, cancer that develops independently, and cancer linked to colitis [94]. Surgery is the primary treatment option for colon cancer; other possibilities include chemotherapy, radiation therapy, immunotherapy, and alternative medicine. However, survival rates due to metastasis and reoccurrence continue to decline for around five years after surgery, suggesting that the tumor is not the primary cause of death [95]. Tomatoes contain lycopene, which possesses several potential health benefits if it were more stable and bioavailable, including protection from chronic diseases, antiproliferation activity against leukemia and colon cancer cells, and the induction of cell cycle arrest in some tumor cells [96,97,98]. In the described research, a nanoemulsion was created using lycopene to address the issue.
Additionally, gold nanoparticles could be encased in a nanoemulsion formulation (AN). Rather than merely serving as a medication carrier, AN could also comprise cell receptor ligands to facilitate precise cell targeting [79]. Combining lycopene-derived molecules with liposomes, polymeric substances, or other lipid-based assemblies can mitigate this effect [99]. In this formulation, Tween 80® serves as the emulsifier, oil containing lycopene is the oil phase, and an aqueous AN solution is the water phase. The HT-29 human colon cancer cell line is suitable for its application. The formulation’s effectiveness was demonstrated by testing the effects of AN alone, lycopene alone, and combining the two on a cell line [100]. Using a nanoemulsion preparation method, the author of this study investigated the anticancer and apoptotic effects of S. aromaticum L. bud essential oil (SABE) on human HT-29 colon cancer cells. Apoptosis (Cas3 overexpression and higher SubG1 peaks) and cell death (reduced HT-29 viability) were seen in response to a wavelength of 131.2 nm in this study [97]. In a phase inversion process, Sabna Kotta created a nanoemulsion of resveratrol (low energy technique). Grapes, berries, and other fruits contain resveratrol, a polyphenolic phytoalexin. Human colorectal cancer cell lines (HCT-166) were employed to test the antiproliferative effects of a resveratrol nanoemulsion. The research also showed that nanoemulsion had a concentration-dependent killing effect on HCT-166 colon cancer cells [101].
The author investigated the Lycium barbarum L. plant’s carotenoid extracts. Nine carotenoids, including neoxanthin, all-trans-zeaxanthin and its cis-isomers, all-trans—cryptoxanthin), all-trans—carotene and its cis-isomers, were analyzed by high-performance liquid chromatography-mass spectrometry with the highest extraction yield achieved using a solvent system of hexane–ethanol–acetone (1:1:1); the IC50 values for inhibiting the development of HT-29 colon cancer cells were reported to be 4.5 and 4.9 µg mL−1 for carotenoid extracts and their nanoemulsion, respectively. G2/M phase arrest was caused by the extract and nanoemulsion, which also raised p53 and p21 expression and lowered CDK2, CDK1, cyclin A, and cyclin B expression [102].
The Spirulina platensis microalgae were used to prepare cerium oxide nanoparticles (CeO2-NPs) by Seyedeh and Asoodeh. Additionally, the ultrasonic emulsification process created nanoemulsions (CeO2-NE) by encapsulating cerium oxide nanoparticles (CeO2-NPs) inside nanoporous polymer shells. Quantitative PCR analysis of apoptotic and antioxidant-related gene expression in HT-29 cells treated with CeO2-NE revealed that CeO2-NE had a more potent effect on anticancer activities. Apoptosis was validated by finding overexpressed caspase-3, caspase-8, and caspase-9 in CeO2-NE. A correlation was found between the overexpression of superoxide dismutase and the redox potential of CeO2-NE in HT-29 colon cells [103].
To test the cytotoxic activity of Carum carvi oil nanoemulsions (CCONE), the researchers used the MTT assay on human HT29 colon cells. They found that increasing the nanoemulsion (CCONE) concentration significantly elevated caspase3 gene expression. These data demonstrate that CCONE promotes apoptosis in human colon cancer cells with minimal toxicity. However, further research is required both in vivo and in vitro [104].
Due to its serious adverse effects, oxaliplatin (Oxa) has had a limited therapeutic role in the management of colon cancer. Many distinct mutational patterns of p53 are seen in colon cancer, affecting the effectiveness of chemotherapeutic drugs such as Oxa. For this reason, it is crucial to discover an alternate therapeutic strategy that mitigates the side effects of Oxa but also enhances its efficacy in the fight against colon malignancies. In this study, Al-Otaibi and Al-Motwaa created a nanoemulsion of Teucrium polium L. essential oil (TPO-NANO). They tested its efficacy in treating colon cancer cells that differed in their sensitivity to the chemotherapeutic agent oxaliplatin based on their p53 status (HCT116 wild-type and HT-29 mutant-type). According to results from an anticancer screen, Oxa+TPO-NANO inhibited the development of both p53 wild-type HCT116 and p53 mutant-type HT-29 colon cancer cells and generated more morphological changes and apoptotic cell death than did Oxa-NS alone. Put another way, the essential oil nanoemulsion of Teucrium polium L. has a synergistic anticancer impact [105].
The antitumor activity of the combination formulas of DOX and SIM, either loaded in water (DOX-SIM-Solution) or nanoemulsions (N.E.s) (DOX-SIM-NE), was evaluated in a Swiss albino mouse model of Ehrlich ascites carcinoma. The study showed that incorporating SIM into the DOX-loaded-NE formulation remarkably improved its efficiency and reduced adverse effects [106]. Moreover, resiquimod-fortified nanoemulsion demonstrated significant antitumor immunity by TLR7/8 and TLR9 activations with induced PD-L1 upregulation in tumors. In addition, a pronounced immunostimulatory effect against polarized macrophage cells treated with test and control nanoemulsions was displayed (Figure 9) [107]. Another safety evaluation of quercetin nanoemulsions on Wistar rat’s organ histopathology, including the liver, spleen, kidney, heart, and brain, indicated insignificant signs of atrophy, hyperplasia, necrosis, or inflammations (Figure 10) [108]. The recent patents on nanoemulsions for cancer treatment are tabulated in Table 3.



Table 2.
Summary of some recent nanoemulsions developed and applications for colorectal and other cancer.
Table 2.
Summary of some recent nanoemulsions developed and applications for colorectal and other cancer.
| Nanoemulsion Constituents | Active Compound | Production Technique | Type of Cancer | References |
|---|---|---|---|---|
| Carotenoid; nanoemulsions; CapryolTM 90; Transcutol®HP; Tween 80 | Carotenoid extract from Lycium barbarism | Ultrasonication method | Colon cancer | [100] |
| Nanoemulsions carrying gold nanoparticles Tween 80 | Lycopene | Ultrasonication method | Colon cancer | [102] |
| Transcutol-HP (surfactant), IPA (cosurfactant), water (aqueous phase), and castor oil (oil phase) | 5-Fluorouracil (5-FU) | Oil phase titration method (PIT) | Colon cancer (HT-29) | [109] |
| Tween 80 (T80) and propylene glycol (P.G.) as surfactant and cosurfactant | Teucrium polium L. essential oil | High-pressure homogenization method | Colon cancer HCT116 wild-type and HT-29 mutant-type | [105] |
| Capryol 90, Surfactant (Tween 20) m andcosurfactant (PEG 400) | Resveratrol | Phase inversion composition | Colorectal cancer | [110] |
| DSPE-PEG2000 | Irinotecan | Phase inversion composition | Colorectal cancer | [111] |
| Salvia (SAL) essential oil | Ifosfamide | Oil phase titration method (PIT) | Colorectal cancer | [112] |
| Tween 80 as a surfactant, tocopheryl polyethylene glycol 1000 succinate (TPGS) as a cosurfactant, and Kollisolv MCT 70 as the oil phase | Curcumin | Oil phase titration method (PIT) | Pituitary and colon cancer | [113] |
| Anethole (57.9%), terpinolene (13.8%), G-terpinene (8.1%), myrcene (6.8%), hexyl butyrate (5.2%), octylbu-tanoate (4.5%), and octyl acetate (3.7%) | Heracleum persicum | Phase inversion composition | Breast cancer | [114] |
| linalyl acetate, limonene, and α-terpineol | Citrus aurantium L. | Phase inversion composition | Human lung (A549 cells) | [115] |

Table 3.
Recent patents on nanoemulsions for cancer treatment.
Table 3.
Recent patents on nanoemulsions for cancer treatment.
| Year | Patented Nanoemulsion | Patent No. | Used for | References |
|---|---|---|---|---|
| 2019 | An oil-in-water nanoemulsion (N.E.) drug delivery that encapsulates omega-3 polyunsaturated fatty acid (PUFA)-taxoid | US10206875 | Anticancer | [116] |
| 2021 | A nanoemulsion consisting of an oil phase, a surfactant, and an aqueous component and an aqueous phase containing a water-soluble active ingredient, a polysaccharide, and hyaluronic acid | US11103600 | Anticancer | [117] |
| 2022 | A nanoemulsion prepared using oxysterol or oxysterol-like compound | US11332494 | Anticancer | [118] |
| 2022 | A water-in-oil nanoemulsion containing an oil phase ingredient, a surfactant, and an aqueous phase ingredient which includes a cancer cell fluorescence-inducing substance and a polysaccharide that targets cancer cells, and it is dispersed in water to remove the oil phase ingredient, resulting in the formation of the nanocarrier, which also contains the aqueous phase ingredient | US11298428 | Anticancer | [119] |
| 2022 | The O/W nanoemulsion that contains 8–12% w/v essential oil, 1–5% w/v polysorbate 80 surfactants, 2–6% w/v glyceryl citrate/lactate/linoleate/oleate co-surfactant, and 1–5% w/v glycerol monocaprylate, type I, wherein a ratio of the surfactant and cosurfactant to essential oil is from 1:1.1 to 1:1.6 | US11364199 | Transdermal delivery | [120] |
| 2022 | Ionic liquid-based nanoemulsion consisting of hydrophobic ionic liquid comprises a dication comprising two monocationic groups linked by a bridging group wherein the bridging group provides an at least partially hydrophobic character | US11464738 | Hydrophobic drug delivery | [121] |
| 2022 | An oil-in-water nanoemulsion that contains clobetasol, one or more oil components, one or more surfactants, and one or more pharmaceutically acceptable excipients or carriers, as well as a continuous aqueous phase and dispersed oil droplets | US10857160 | Prophylaxis or treatment of inflammatory diseases or conditions | [122] |
| 2022 | A fluorocarbon nanoemulsion prepared by using perfluorohexane and one or more surfactants selected from perfluoro-n-hexyl-oligo ethylene oxy-alcohols | US11304899 | For enhanced oxygen delivery | [123] |
| 2022 | An oil-in-water emulsion consisting of an internal oil phase includes lauric oil, an external aqueous phase (water or glycerol), and surfactants, preferably anionic surfactants, amphoteric surfactants | US11266580 | [124] | |
| 2022 | An aqueous, transparent nanoemulsion composition includes at least two different bilayer water-core liposome components and at least one monolayer surfactant-bound particle component | US11304900 | For delivering oil- and water-soluble components of a vitamin supplement | [125] |
| 2022 | An injection fluid nanoemulsion is prepared by dispersing the oil phase in an aqueous phase, and the formed oil nanodroplets that have a diameter of from 20–1000 nanometers and dispersion of the oil phase in the aqueous phase stabilized by surfactant and nonsuperparamagnetic magnetic nanoparticles encapsulated in the formed oil nanodroplets | US11506049 | - | [126] |
| 2022 | The nanoemulsion composition consists of a lipid nanoparticle with an inorganic nanoparticle-based hydrophobic core | US11534497 | For delivering RNA | [127] |
| 2022 | A nanoemulsion composition comprising nanoemulsion particles that contain a hydrophobic core (mixture of liquid oil and one or more inorganic nanoparticles or one or more lipids, such as a cationic lipid) with one or more surfactants and a bioactive agent complexed with the nanoemulsion particles | US11376335 | For delivering RNA | [128] |
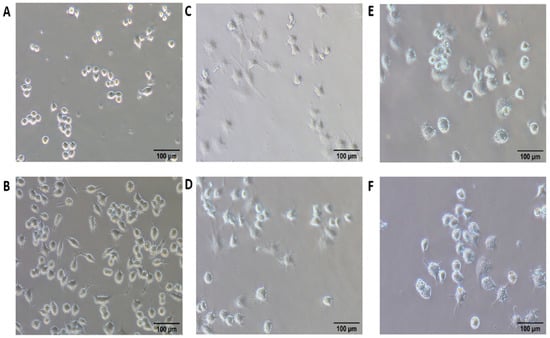
Figure 9.
Bright-field image of macrophage untreated (A), control nanoemulsions (B), free resiquimod (C), SD-101 a TLR9 agonist containing cytidine-phospho-guanosine dinucleotides (D), resiquimod fortified nanoemulsions (E), and the combined effect of resiquimod fortified nanoemulsions with SD-101 (F) after 12 h (adapted with permission from [107]).
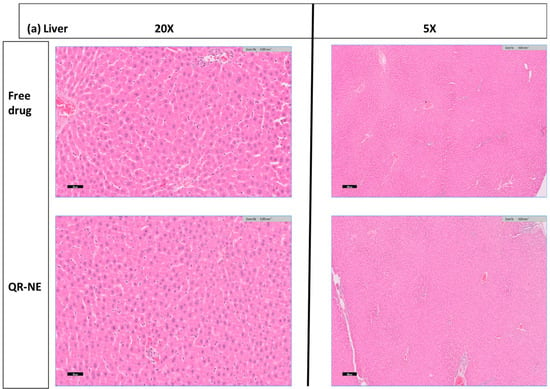
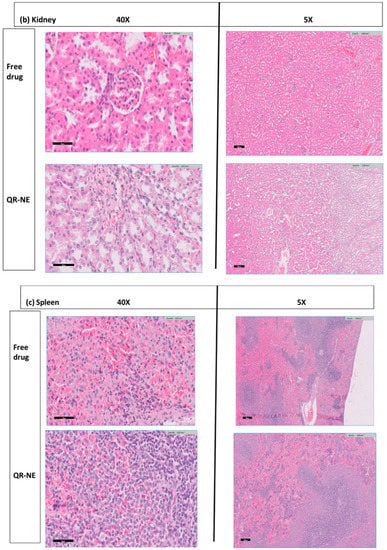
Figure 10.
Safety evaluation of quercetin nanoemulsions on Wistar rat’s organ histopathology (H and E hematoxylin and eosin sections), including (a) liver, (b) kidney, and (c) spleen (adapted with permission from [108]).
4. Ongoing Clinical Trials on Nanoemulsions for Cancer Therapy
Nanoemulsions can be an excellent alternative to other anticancer medication to ensure better cancer treatment. There are just a few trials reported on nanoemulsions. In an ongoing clinical experiment, nanoemulsions have been employed for photodynamic treatment on superficial basal carcinoma cells. Three photosensitizers were compared using a randomized, prospective, double-blind approach (phase 2). Methyl aminolevulinate (MAL/Metvix®), hexyl amino levulinate (HAL/Hexvix®), and aminolevulinic acid nanoemulsion (BF-200 ALA/Ameluz®) was used to treat basal cell carcinomas.
Curcumin has been extensively researched for cancer, as was previously mentioned. Curcumin is a naturally occurring polyphenolic substance isolated from the rhizomes of Curcuma longa. It exhibits various biological properties, including antioxidant and anti-inflammatory properties [129]. No clinical study specifically designed to treat cancer has been conducted yet. Instead, a randomized, double-blinded phase one controlled research trial (ClinicalTrial.gov ID: NCT03865992) evaluated curcumin-loaded nanoemulsions.
Curcumin nanoemulsions have been the subject of a second clinical trial reported to treat obese women at a high risk of breast cancer (ClinicalTrial.gov ID: NCT01975363). It was a randomized pilot trial that examined the safety, tolerability, and compliance of various doses (50 or 100 mg) of nanoemulsion curcumin in overweight women with a greater chance of developing breast cancer. Curcumin’s anti-inflammatory properties in breast tissue and fat can reduce breast cancer risk (Table 4) [130].

Table 4.
Ongoing clinical trials in nanoemulsions [131] *.
Nanoemulsions can be an excellent alternative to other anticancer medication to ensure better cancer treatment. There are just a few trials reported on nanoemulsions. In an ongoing clinical experiment, nanoemulsions have been employed for photodynamic treatment on superficial basal carcinoma cells.
5. Limitations and Challenges of Nanoemulsions
5.1. Nanoemulsion Stability
In the new approaches, such as in the gas and oil sectors, nanoemulsions still require lab- and pilot-scale research to fully understand the relationship between the chemical composition and properties of nanoemulsions. There will be phase separation in nanoemulsions since they are kinetically stable (although slowly). When used in long-term field applications, this destabilization may occur during on-site storage, ground transportation, or even on-site transportation in the reservoir. It is currently unclear how extreme reservoir conditions, such as high temperature, high salinity, and complex chemical interactions, affect the stability of nanoemulsions. Ex situ research could, on the one hand, explain the phase separation mechanisms of nanoemulsions in reservoir-like circumstances and investigate desirable strategies [130].
5.2. Economic Concerns about Nanoemulsion
To overcome economic concerns, we must consider two elements: (1) nanoemulsion manufacturing and (2) required facilities. To create a nanoemulsion in large quantities, many different chemicals are required. These compounds include polymers, surfactants, and solvents. Similar to other high-energy techniques, emulsification relies on intense heat or pressure to break up the interfaces and spread the droplets. Secondly, a wide range of facilities and equipment are required in nanoemulsion formulation, which may significantly increase the cost, particularly when large-scale mixing and homogenization processes require special equipment, which also affects the formulation cost.
Herbal bioactives have gained popularity worldwide for their variety of health benefits. However, limitations remain, such as low solubility, permeability, and bioavailability. A total of 40% of the active principle molecules made using high-throughput methods are poorly soluble. These provide significant challenges to formulating suitable herbal remedies for health treatment. Research into novel carriers for the transport of herbal bioactive components is required to obtain the optimal blood concentration level in the body [132,133].
6. Future Perspective
The scientific community’s interest in nanoemulsions has been prompted by their potential uses in the healthcare, food, agricultural, and cosmetics industries. In the food and pesticide sectors, they can carry phytochemicals and other bioactive components. Through size reduction, encapsulation, and stability, nanocarrier manufacturing aims to enhance the pharmacokinetics and absorption of marginally accessible herbal bioactives. Improved therapeutic efficacy at the target site can only be achieved with a high drug delivery level. The main rate-limiting factors for biomembrane permeability in the case of herbal bioactives are sizable molecular size and lipid solubilization. Overcoming the membrane with an enhanced pharmacokinetic profile is a significant challenge in producing herbal medicines. Nanocarriers made of oils or lipids could be the solution to these issues. They can increase solubility, permeability, and gastrointestinal tract (GIT) absorption, minimize the effect of food on the absorption rate of highly lipophilic substances, and boost bioavailability.
Nanoemulsions can be utilized to deliver allopathic and plant-derived medicines, which have been demonstrated on numerous platforms for use in pharmaceuticals. Compared to other delivery techniques, it has a higher encapsulation effectiveness, which may be beneficial for dissolving hydrophobic moieties and other weakly soluble molecules. Future perspectives on herbal medication delivery using nanoemulsions include numerous application types, including vaccination, protein and peptide drug targeting, topical, transdermal, ophthalmic, and oral. This method could benefit the delivery of nutritional supplements for various health benefits. With the help of nanoemulsions, the drug therapeutic levels can be maintained by a prolonged and sustained release action.
7. Conclusions
Nanoemulsions are a novel and potentially practical approach to treating cancer. One of the primary issues with cancer therapy pharmaceuticals is that the body poorly absorbs them, but using a hydrophobic core permits the encapsulation of lipophilic drugs, providing a solution to this issue. Emulsifying agents and GRAS excipients allows for developing a reliable and secure substitute. Due to their small particle size, they can remain in circulation for a considerable time. Nanoemulsions have an advantage over conventional drug carriers because they can be engineered to selectively target tumor cells while avoiding the development of acquired resistance. Since the failure of most anticancer medications is mainly attributable to their significant toxicity to healthy cells/tissues or even to the cancer cells’ discovery of mechanisms to resist treatment, this is an important development in cancer therapy. The ERP effect, prevalent in tumor tissues, is exploited for passive targeted delivery.
The case studies shown here show the various approaches to designing nanoemulsions for therapeutic use across various cancer types. These benefits would be for naught if the manufacturing process and the metabolism of the medicine and excipients were not adequately studied. These two factors are the primary stumbling blocks in nanoemulsion research and development. To formulate such a medicine, see it through all the necessary clinical studies, and get it approved, researchers will need to think outside the box and explore every possible option for making anticancer drugs that are both effective and safe. Given that cancer is a multifactorial disease responsible for a substantial proportion of deaths and that no entirely viable medication has been discovered to date, the creation of this formulation is crucial in cancer therapy.
Author Contributions
Conceptualization, P.M.; data curation, P.M. and A.S.; writing of original draft preparation, T.R., R.P. and A.S.; review and editing, P.M., R.P., T.R., A.S., S.S. and B.G.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to acknowledge Prakruti Tulajashankar Joshi and Shreya of Ganpat University, Shree S. K. Patel College of Pharmaceutical Education and Research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schulman, J.H.; Stoeckenius, W.; Prince, L.M. Mechanism of Formation and Structure of Micro Emulsions by Electron Microscopy. J. Phys. Chem. 1959, 63, 1677–1680. [Google Scholar] [CrossRef]
- Jin, Y.; Tian, S.; Guo, J.; Ren, X.; Li, X.; Gao, S. Synthesis, Characterization and Exploratory Application of Anionic Surfactant Fatty Acid Methyl Ester Sulfonate from Waste Cooking Oil. J. Surfactants Deterg. 2016, 19, 467–475. [Google Scholar] [CrossRef]
- Jaiswal, M.; Dudhe, R.; Sharma, P.K. Nanoemulsion: An advanced mode of drug delivery system. 3 Biotech 2015, 5, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.J.; Li, Y.; Yang, G.; Zhao, C.-X. Nanoemulsions for drug delivery. Particuology 2022, 64, 85–97. [Google Scholar] [CrossRef]
- Constantinides, P.P. Lipid Microemulsions for Improving Drug Dissolution and Oral Absorption: Physical and Biopharmaceutical Aspects. Pharm. Res. 1995, 12, 1561–1572. [Google Scholar] [CrossRef]
- Jumaa, M.; Müller, B.W. Formulating and stability of benzodiazepines in a new lipid emulsion formulation. Die Pharm. 2002, 57, 740–743. [Google Scholar]
- Gurpret, K.; Singh, S.K. Review of Nanoemulsion Formulation and Characterization Techniques. Indian J. Pharm. Sci. 2018, 80, 781–789. [Google Scholar] [CrossRef]
- Lovelyn, C.; Attama, A.A. Current State of Nanoemulsions in Drug Delivery. J. Biomater. Nanobiotechnol. 2011, 2, 626–639. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Chen, F.; Liang, L.; Zhang, Z.; Deng, Z.; Decker, E.A.; McClements, D.J. Inhibition of lipid oxidation in nanoemulsions and filled microgels fortified with omega-3 fatty acids using casein as a natural antioxidant. Food Hydrocoll. 2017, 63, 240–248. [Google Scholar] [CrossRef]
- Uskoković, V.; Drofenik, M. Synthesis of Materials within Reverse Micelles. Surf. Rev. Lett. 2005, 12, 239–277. [Google Scholar] [CrossRef]
- Peng, L.-C.; Liu, C.-H.; Kwan, C.-C.; Huang, K.-F. Optimization of water-in-oil nanoemulsions by mixed surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2010, 370, 136–142. [Google Scholar] [CrossRef]
- Leister, N.; Karbstein, H.P. Evaluating the Stability of Double Emulsions—A Review of the Measurement Techniques for the Systematic Investigation of Instability Mechanisms. Colloids Interfaces 2020, 4, 8. [Google Scholar] [CrossRef]
- Middelberg, A.P.J.; Dimitrijev-Dwyer, M. A Designed Biosurfactant Protein for Switchable Foam Control. ChemPhysChem 2011, 12, 1426–1429. [Google Scholar] [CrossRef]
- Zhao, C.; Dwyer, M.D.; Yu, L.; Middelberg, A.P.J. From Folding to Function: Design of a New Switchable Biosurfactant Protein. ChemPhysChem 2017, 18, 488–492. [Google Scholar] [CrossRef]
- Pulko, I.; Krajnc, P. High Internal Phase Emulsion Templating—A Path To Hierarchically Porous Functional Polymers. Macromol. Rapid Commun. 2012, 33, 1731–1746. [Google Scholar] [CrossRef]
- Pickering, S.U. CXCVI.—Emulsions. J. Chem. Soc. Trans. 1907, 91, 2001–2021. [Google Scholar] [CrossRef]
- Xiao, M.; Xu, A.; Zhang, T.; Hong, L. Tailoring the Wettability of Colloidal Particles for Pickering Emulsions via Surface Modification and Roughness. Front. Chem. 2018, 6, 225. [Google Scholar] [CrossRef]
- Dasgupta, N.; Ranjan, S.; Gandhi, M. Nanoemulsion ingredients and components. Environ. Chem. Lett. 2019, 17, 917–928. [Google Scholar] [CrossRef]
- Rao, J.; McClements, D.J. Lemon oil solubilization in mixed surfactant solutions: Rationalizing microemulsion & nanoemulsion formation. Food Hydrocoll. 2012, 26, 268–276. [Google Scholar] [CrossRef]
- Kim, D.S.; Cho, J.H.; Park, J.H.; Kim, J.S.; Song, E.S.; Kwon, J.; Giri, B.R.; Jin, S.G.; Kim, K.S.; Choi, H.-G. Self-microemulsifying drug delivery system (SMEDDS) for improved oral delivery and photostability of methotrexate. Int. J. Nanomed. 2019, 14, 4949–4960. [Google Scholar] [CrossRef] [PubMed]
- Azeem, A.; Rizwan, M.; Ahmad, F.J.; Iqbal, Z.; Khar, R.K.; Aqil, M.; Talegaonkar, S. Nanoemulsion Components Screening and Selection: A Technical Note. AAPS PharmSciTech 2009, 10, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Salem, A.M.; Ezzat, M.S. Nanoemulsions in Food Industry. In Some New Aspects of Colloidal Systems in Foods; Milani, M.J., Ed.; IntechOpen: London, UK, 2019; ISBN 978-1-78985-781-8. [Google Scholar]
- Liu, D.; Xu, J.; Zhao, H.; Zhang, X.; Zhou, H.; Wu, D.; Liu, Y.; Yu, P.; Xu, Z.; Kang, W.; et al. Nanoemulsions stabilized by anionic and non-ionic surfactants for enhanced oil recovery in ultra-low permeability reservoirs: Performance evaluation and mechanism study. Colloids Surf. A Physicochem. Eng. Asp. 2022, 637, 128235. [Google Scholar] [CrossRef]
- Fernandes, A.R.; Sanchez-Lopez, E.; dos Santos, T.; Garcia, M.L.; Silva, A.M.; Souto, E.B. Development and Characterization of Nanoemulsions for Ophthalmic Applications: Role of Cationic Surfactants. Materials 2021, 14, 7541. [Google Scholar] [CrossRef] [PubMed]
- Koroleva, M.; Nagovitsina, T.; Yurtov, E. Nanoemulsions stabilized by non-ionic surfactants: Stability and degradation mechanisms. Phys. Chem. Chem. Phys. 2018, 20, 10369–10377. [Google Scholar] [CrossRef]
- Schreiner, T.B.; Santamaria-Echart, A.; Ribeiro, A.; Peres, A.M.; Dias, M.M.; Pinho, S.P.; Barreiro, M.F. Formulation and Optimization of Nanoemulsions Using the Natural Surfactant Saponin from Quillaja Bark. Molecules 2020, 25, 1538. [Google Scholar] [CrossRef]
- Isailović, T.M.; Todosijević, M.N.; Đorđević, S.M.; Savić, S.D. Natural Surfactants-Based Micro/Nanoemulsion Systems for NSAIDs—Practical Formulation Approach, Physicochemical and Biopharmaceutical Characteristics/Performances. In Microsized and Nanosized Carriers for Nonsteroidal Anti-Inflammatory Drugs; Elsevier: Amsterdam, The Netherlands, 2017; pp. 179–217. ISBN 978-0-12-804017-1. [Google Scholar]
- Wang, X.; Corin, K.; Baaske, P.; Wienken, C.J.; Jerabek-Willemsen, M.; Duhr, S.; Braun, D.; Zhang, S. Peptide surfactants for cell-free production of functional G protein-coupled receptors. Porc. Natl. Acad. Sci. USA 2011, 108, 9049–9054. [Google Scholar] [CrossRef]
- Lam, R.S.H.; Nickerson, M.T. Food proteins: A review on their emulsifying properties using a structure—Function approach. Food Chem. 2013, 141, 975–984. [Google Scholar] [CrossRef]
- Date, A.A.; Nagarsenker, M.S. Parenteral microemulsions: An overview. Int. J. Pharm. 2008, 355, 19–30. [Google Scholar] [CrossRef]
- Zainol, S.; Basri, M.; Basri, H.B.; Shamsuddin, A.F.; Abdul-Gani, S.S.; Karjiban, R.A.; Abdul-Malek, E. Formulation Optimization of a Palm-Based Nanoemulsion System Containing Levodopa. Int. J. Mol. Sci. 2012, 13, 13049–13064. [Google Scholar] [CrossRef]
- Forgiarini, A.; Esquena, J.; González, C.; Solans, C. Studies of the relation between phase behavior and emulsification methods with nanoemulsion formation. In Trends in Colloid and Interface Science XIV; Buckin, V., Ed.; Progress in Colloid and Polymer Science; Springer: Berlin/Heidelberg, Germany, 2000; Volume 115, pp. 36–39. ISBN 978-3-540-67128-2. [Google Scholar]
- Mason, T.G.; Wilking, J.N.; Meleson, K.; Chang, C.B.; Graves, S.M. Nanoemulsions: Formation, structure, and physical properties. J. Phys. Condens. Matter 2006, 18, R635–R666. [Google Scholar] [CrossRef]
- Chime, S.A.; Kenechukwu, F.C.; Attama, A.A. Nanoemulsions—Advances in Formulation, Characterization and Applications in Drug Delivery. In Application of Nanotechnology in Drug Delivery; Sezer, A.D., Ed.; InTech: Hong Kong, China, 2014; ISBN 978-953-51-1628-8. [Google Scholar]
- Kumar, M.; Pathak, K.; Misra, A. Formulation and Characterization of Nanoemulsion-Based Drug Delivery System of Risperidone. Drug Dev. Ind. Pharm. 2009, 35, 387–395. [Google Scholar] [CrossRef]
- Demisli, S.; Mitsou, E.; Pletsa, V.; Xenakis, A.; Papadimitriou, V. Development and Study of Nanoemulsions and Nanoemulsion-Based Hydrogels for the Encapsulation of Lipophilic Compounds. Nanomaterials 2020, 10, 2464. [Google Scholar] [CrossRef] [PubMed]
- Honary, S.; Zahir, F. Effect of Zeta Potential on the Properties of Nano-Drug Delivery Systems—A Review (Part 2). Trop. J. Pharm. Res. 2013, 12, 265–273. [Google Scholar] [CrossRef]
- Kadu, P.J.; Kushare, S.S.; Thacker, D.D.; Gattani, S.G. Enhancement of oral bioavailability of atorvastatin calcium by self-emulsifying drug delivery systems (SEDDS). Pharm. Dev. Technol. 2011, 16, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Kurpiers, M.; Wolf, J.D.; Steinbring, C.; Zaichik, S.; Bernkop-Schnürch, A. Zeta potential changing nanoemulsions based on phosphate moiety cleavage of a PEGylated surfactant. J. Mol. Liq. 2020, 316, 113868. [Google Scholar] [CrossRef]
- Da Costa, S.; Basri, M.; Shamsudin, N.; Basri, H. Stability of Positively Charged Nanoemulsion Formulation Containing Steroidal Drug for Effective Transdermal Application. J. Chem. 2014, 2014, 748680. [Google Scholar] [CrossRef]
- Malik, A.; Magisetty, R.; Kumar, V.; Shukla, A.; Kandasubramanian, B. Dielectric and conductivity investigation of polycarbonate-copper phthalocyanine electrospun nonwoven fibres for electrical and electronic application. Polym. Plast. Technol. Mater. 2020, 59, 154–168. [Google Scholar] [CrossRef]
- Masoumi, H.R.F.; Basri, M.; Samiun, W.S.; Izadiyan, Z.; Lim, C.J. Enhancement of encapsulation efficiency of nanoemulsion-containing aripiprazole for the treatment of schizophrenia using mixture experimental design. Int. J. Nanomed. 2015, 10, 6469–6476. [Google Scholar] [CrossRef]
- Dinache, A.; Tozar, T.; Smarandache, A.; Andrei, I.R.; Nistorescu, S.; Nastasa, V.; Staicu, A.; Pascu, M.-L.; Romanitan, M.O. Spectroscopic Characterization of Emulsions Generated with a New Laser-Assisted Device. Molecules 2020, 25, 1729. [Google Scholar] [CrossRef]
- Kaur, G.; Singh, P.; Sharma, S. Physical, morphological, and storage studies of cinnamon based nanoemulsions developed with Tween 80 and soy lecithin: A comparative study. J. Food Meas. Charact. 2021, 15, 2386–2398. [Google Scholar] [CrossRef]
- Kumari, S.; Kumaraswamy, R.V.; Choudhary, R.C.; Sharma, S.S.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Thymol nanoemulsion exhibits potential antibacterial activity against bacterial pustule disease and growth promotory effect on soybean. Sci. Rep. 2018, 8, 6650. [Google Scholar] [CrossRef]
- Li, R.; Inbaraj, B.S.; Chen, B.-H. Quantification of Xanthone and Anthocyanin in Mangosteen Peel by UPLC-MS/MS and Preparation of Nanoemulsions for Studying Their Inhibition Effects on Liver Cancer Cells. Int. J. Mol. Sci. 2023, 24, 3934. [Google Scholar] [CrossRef]
- Prasad, J.; Das, S.; Maurya, A.; Soni, M.; Yadav, A.; Singh, B.; Dwivedy, A.K. Encapsulation of Cymbopogon khasiana × Cymbopogon pendulus Essential Oil (CKP-25) in Chitosan Nanoemulsion as a Green and Novel Strategy for Mitigation of Fungal Association and Aflatoxin B1 Contamination in Food System. Foods 2023, 12, 722. [Google Scholar] [CrossRef] [PubMed]
- Gulati, N.; Chellappan, D.K.; Tambuwala, M.M.; Aljabali, A.A.A.; Prasher, P.; Singh, S.K.; Anand, K.; Sharma, A.; Jha, N.K.; Gupta, G.; et al. Oral Nanoemulsion of Fenofibrate: Formulation, Characterization, and In Vitro Drug Release Studies. ASSAY Drug Dev. Technol. 2021, 19, 246–261. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Won, M.; Yang; Lee, K.M.; Kim, C.S. In Vitro Permeation Studies of Nanoemulsions Containing Ketoprofen as a Model Drug. Drug Deliv. 2008, 15, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Mohd Izham, M.N.; Hussin, Y.; Aziz, M.N.M.; Yeap, S.K.; Rahman, H.S.; Masarudin, M.J.; Mohamad, N.E.; Abdullah, R.; Alitheen, N.B. Preparation and Characterization of Self Nano-Emulsifying Drug Delivery System Loaded with Citraland Its Antiproliferative Effect on Colorectal Cells In Vitro. Nanomaterials 2019, 9, 1028. [Google Scholar] [CrossRef] [PubMed]
- Tscharnuter, W. Photon Correlation Spectroscopy in Particle Sizing. In Encyclopedia of Analytical Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Sharifi, F.; Jahangiri, M.; Nazir, I.; Asim, M.H.; Ebrahimnejad, P.; Hupfauf, A.; Gust, R.; Bernkop-Schnürch, A. Zeta potential changing nanoemulsions based on a simple zwitterion. J. Colloid Interface Sci. 2021, 585, 126–137. [Google Scholar] [CrossRef]
- Iacob-Tudose, E.T.; Mamaliga, I.; Iosub, A.V. TES Nanoemulsions: A Review of Thermophysical Properties and Their Impact on System Design. Nanomaterials 2021, 11, 3415. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Martín-Belloso, O.; McClements, D. Excipient Nanoemulsions for Improving Oral Bioavailability of Bioactives. Nanomaterials 2016, 6, 17. [Google Scholar] [CrossRef]
- Agnish, S.; Sharma, A.D.; Kaur, I. Nanoemulsions (O/W) containing Cymbopogon pendulus essential oil: Development, characterization, stability study, and evaluation of in vitro anti-bacterial, anti-inflammatory, anti-diabetic activities. Bionanoscience 2022, 12, 540–554. [Google Scholar] [CrossRef] [PubMed]
- Klang, V.; Matsko, N.B.; Valenta, C.; Hofer, F. Electron microscopy of nanoemulsions: An essential tool for characterisation and stability assessment. Micron 2012, 43, 85–103. [Google Scholar] [CrossRef] [PubMed]
- de Marques, T.Z.S.; Santos-Oliveira, R.; Betzler de Oliveira de Siqueira, L.; da Silva Cardoso, V.; de Freitas, Z.M.F.; Barros, R.C.S.A.; Villa, A.L.V.; Monteiro, M.S.S.B.; Pereira dos Santos, E.; Ricci-Junior, E. Development and characterization of a nanoemulsion containing propranolol for topical delivery. Int. J. Nanomed. 2018, 13, 2827–2837. [Google Scholar] [CrossRef]
- Malik, M.R.; Al-Harbi, F.F.; Nawaz, A.; Amin, A.; Farid, A.; Al Mohaini, M.; Alsalman, A.J.; Al Hawaj, M.A.; Alhashem, Y.N. Formulation and Characterization of Chitosan-Decorated Multiple Nanoemulsion for Topical Delivery In Vitro and Ex Vivo. Molecules 2022, 27, 3183. [Google Scholar] [CrossRef]
- Ledet, G.; Pamujula, S.; Walker, V.; Simon, S.; Graves, R.; Mandal, T.K. Development and in vitro evaluation of a nanoemulsion for transcutaneous delivery. Drug Dev. Ind. Pharm. 2014, 40, 370–379. [Google Scholar] [CrossRef]
- Ali, M.S. Accelerated Stability Testing of a Clobetasol Propionate Loaded Nanoemulsion as per ICH Guideline. Sci. Pharm. 2013, 81, 1089–1100. [Google Scholar] [CrossRef]
- Ali, M.S.; Alam, M.S.; Alam, N.; Siddiqui, M.R. Preparation, Characterization and Stability Study of Dutasteride Loaded Nanoemulsion for Treatment of Benign Prostatic Hypertrophy. Iran. J. Pharm. Res. IJPR 2014, 13, 1125–1140. [Google Scholar] [CrossRef] [PubMed]
- Romes, N.B.; Wahab, R.A.; Hamid, M.A.; Oyewusi, H.A.; Huda, N.; Kobun, R. Thermodynamic stability, in-vitro permeability, and in-silico molecular modeling of the optimal Elaeis guineensis leaves extract water-in-oil nanoemulsion. Sci. Rep. 2021, 11, 2085. [Google Scholar] [CrossRef] [PubMed]
- Derjaguin, B.; Landau, L. Theory of the stability of strongly charged lyophobic sols and of the adhesion of strongly charged particles in solutions of electrolytes. Prog. Surf. Sci. 1993, 43, 30–59. [Google Scholar] [CrossRef]
- Kontogeorgis, G.M.; Kiil, S. Introduction to Colloid and Surface Chemistry. In Introduction to Applied Colloid and Surface Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2016; pp. 1–10. ISBN 978-1-118-88119-4. [Google Scholar]
- Aswathanarayan, J.B.; Vittal, R.R. Nanoemulsions and Their Potential Applications in Food Industry. Front. Sustain. Food Syst. 2019, 3, 95. [Google Scholar] [CrossRef]
- Ivanov, I.B.; Danov, K.D.; Kralchevsky, P.A. Flocculation and coalescence of micron-size emulsion droplets. Colloids Surf. A Physicochem. Eng. Asp. 1999, 152, 161–182. [Google Scholar] [CrossRef]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, properties and applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef]
- Tadros, T.F. Creaming/Sedimentation of Emulsions and Its Prevention. In Emulsions; De Gruyter: Berlin, Germany, 2016; pp. 95–112. ISBN 978-3-11-045224-2. [Google Scholar]
- Khurana, V.; Kwatra, D.; Shah, S.; Mandal, A.; Mitra, A.K. Emerging Nanotechnology for Stem Cell Therapy. In Emerging Nanotechnologies for Diagnostics, Drug Delivery and Medical Devices; Elsevier: Amsterdam, The Netherlands, 2017; pp. 85–103. ISBN 978-0-323-42978-8. [Google Scholar]
- Tadros, T.; Izquierdo, P.; Esquena, J.; Solans, C. Formation and stability of nano-emulsions. Adv. Colloid Interface Sci. 2004, 108–109, 303–318. [Google Scholar] [CrossRef]
- Delgado, A.V.; González-Caballero, F.; Hunter, R.J.; Koopal, L.K.; Lyklema, J. Measurement and interpretation of electrokinetic phenomena. J. Colloid Interface Sci. 2007, 309, 194–224. [Google Scholar] [CrossRef]
- Tambe, D.E.; Sharma, M.M. Factors Controlling the Stability of Colloid-Stabilized Emulsions. J. Colloid Interface Sci. 1994, 162, 1–10. [Google Scholar] [CrossRef]
- Claesson, P.M.; Kjellin, M.; Rojas, O.J.; Stubenrauch, C. Short-range interactions between non-ionic surfactant layers. Phys. Chem. Chem. Phys. 2006, 8, 5501. [Google Scholar] [CrossRef]
- Safaya, M.; Rotliwala, Y.C. Nanoemulsions: A review on low energy formulation methods, characterization, applications and optimization technique. Mater. Today Proc. 2020, 27, 454–459. [Google Scholar] [CrossRef]
- Shaikh, N.M.; Swamy, S.M.V.; Narsing, N.S.; Kulkarni, K.B. Formulation and evaluation of nanoemulsion for topical application. J. Drug Deliv. Ther. 2019, 9, 370–375. [Google Scholar] [CrossRef]
- Wang, L.; Guan, X.; Zheng, C.; Wang, N.; Lu, H.; Huang, Z. New Low-Energy Method for Nanoemulsion Formation: pH Regulation Based on Fatty Acid/Amine Complexes. Langmuir 2020, 36, 10082–10090. [Google Scholar] [CrossRef]
- de Morais, J.M.; dos Santos, O.D.H.; Delicato, T.; Gonçalves, R.A.; da Rocha-Filho, P.A. Physicochemical Characterization of Canola Oil/Water Nano-emulsions Obtained by Determination of Required HLB Number and Emulsion Phase Inversion Methods. J. Dispers. Sci. Technol. 2006, 27, 109–115. [Google Scholar] [CrossRef]
- Solans, C.; Solé, I. Nano-emulsions: Formation by low-energy methods. Curr. Opin. Colloid Interface Sci. 2012, 17, 246–254. [Google Scholar] [CrossRef]
- Friberg, S.E.; Corkery, R.W.; Blute, I.A. Phase Inversion Temperature (PIT) Emulsification Process. J. Chem. Eng. Data 2011, 56, 4282–4290. [Google Scholar] [CrossRef]
- Kumar, A.; Li, S.; Cheng, C.-M.; Lee, D. Recent Developments in Phase Inversion Emulsification. Ind. Eng. Chem. Res. 2015, 54, 8375–8396. [Google Scholar] [CrossRef]
- Feng, J.; Rodríguez-Abreu, C.; Esquena, J.; Solans, C. A Concise Review on Nano-emulsion Formation by the Phase Inversion Composition (PIC) Method. J. Surfactants Deterg. 2020, 23, 677–685. [Google Scholar] [CrossRef]
- Håkansson, A.; Rayner, M. General Principles of Nanoemulsion Formation by High-Energy Mechanical Methods. In Nanoemulsions; Elsevier: Amsterdam, The Netherlands, 2018; pp. 103–139. ISBN 978-0-12-811838-2. [Google Scholar]
- Jasmina, H.; Džana, O.; Alisa, E.; Edina, V.; Ognjenka, R. Preparation of nanoemulsions by high-energy and low energy emulsification methods. In CMBEBIH 2017; Badnjevic, A., Ed.; IFMBE Proceedings; Springer: Singapore, 2017; Volume 62, pp. 317–322. ISBN 978-981-10-4165-5. [Google Scholar]
- Scholz, P.; Keck, C.M. Nanoemulsions produced by rotor–stator high speed stirring. Int. J. Pharm. 2015, 482, 110–117. [Google Scholar] [CrossRef]
- Hidajat, M.J.; Jo, W.; Kim, H.; Noh, J. Effective Droplet Size Reduction and Excellent Stability of Limonene Nanoemulsion Formed by High-Pressure Homogenizer. Colloids Interfaces 2020, 4, 5. [Google Scholar] [CrossRef]
- Sharma, S.; Sahni, J.K.; Ali, J.; Baboota, S. Effect of high-pressure homogenization on formulation of TPGS loaded nanoemulsion of rutin—Pharmacodynamic and antioxidant studies. Drug Deliv. 2015, 22, 541–551. [Google Scholar] [CrossRef]
- Tang, S.Y.; Shridharan, P.; Sivakumar, M. Impact of process parameters in the generation of novel aspirin nanoemulsions—Comparative studies between ultrasound cavitation and microfluidizer. Ultrason. Sonochem. 2013, 20, 485–497. [Google Scholar] [CrossRef]
- Canselier, J.P.; Delmas, H.; Wilhelm, A.M.; Abismaïl, B. Ultrasound Emulsification—An Overview. J. Dispers. Sci. Technol. 2002, 23, 333–349. [Google Scholar] [CrossRef]
- Ali, G.S.; Hambali, E.; Fahma, F. Potential of nanoemulsion process and method using agro-industrial based materials in skincare formulations: A review. IOP Conf. Ser. Earth Environ. Sci. 2022, 1034, 012029. [Google Scholar] [CrossRef]
- Kale, S.N.; Deore, S.L. Emulsion Micro Emulsion and Nano Emulsion: A Review. Syst. Rev. Pharm. 2016, 8, 39–47. [Google Scholar] [CrossRef]
- Hussain, A.; Singh, V.K.; Singh, O.P.; Shafaat, K.; Kumar, S.; Ahmad, F.J. Formulation and optimization of nanoemulsion using antifungal lipid and surfactant for accentuated topical delivery of Amphotericin B. Drug Deliv. 2016, 23, 3101–3110. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, E.; Guerra, M.; Dias-Ferreira, J.; Lopez-Machado, A.; Ettcheto, M.; Cano, A.; Espina, M.; Camins, A.; Garcia, M.L.; Souto, E.B. Current Applications of Nanoemulsions in Cancer Therapeutics. Nanomaterials 2019, 9, 821. [Google Scholar] [CrossRef]
- Yu, H.; Xu, Z.; Chen, X.; Xu, L.; Yin, Q.; Zhang, Z.; Li, Y. Reversal of Lung Cancer Multidrug Resistance by pH-Responsive Micelleplexes Mediating Co-Delivery of siRNA and Paclitaxel: Reversal of Lung Cancer Multidrug Resistance. Macromol. Biosci. 2014, 14, 100–109. [Google Scholar] [CrossRef]
- Chen, B.-H.; Huang, R.-F.S.; Wei, Y.-J.; Inbaraj, B.S. Inhibition of colon cancer cell growth by nanoemulsion carrying gold nanoparticles and lycopene. Int. J. Nanomed. 2015, 10, 2823–2846. [Google Scholar] [CrossRef]
- Ahsan, H. Family History of Colorectal Adenomatous Polyps and Increased Risk for Colorectal Cancer. Ann. Intern. Med. 1998, 128, 900. [Google Scholar] [CrossRef]
- Thiery, J.P.; Sleeman, J.P. Complex networks orchestrate epithelial-mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 2006, 7, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Mein, J.R.; Lian, F.; Wang, X.-D. Biological activity of lycopene metabolites: Implications for cancer Prevention: Nutrition Reviews©. Nutr. Rev. 2008, 66, 667–683. [Google Scholar] [CrossRef]
- Lu, W.; Zhang, G.; Zhang, R.; Flores, L.G.; Huang, Q.; Gelovani, J.G.; Li, C. Tumor Site–Specific Silencing of NF-κB p65 by Targeted Hollow Gold Nanosphere–Mediated Photothermal Transfection. Cancer Res. 2010, 70, 3177–3188. [Google Scholar] [CrossRef]
- Hu, C.-M.J.; Zhang, L. Nanoparticle-based combination therapy toward overcoming drug resistance in cancer. Biochem. Pharmacol. 2012, 83, 1104–1111. [Google Scholar] [CrossRef]
- Abadi, A.V.M.; Karimi, E.; Oskoueian, E.; Mohammad, G.R.K.S.; Shafaei, N. Chemical investigation and screening of anti-cancer potential of Syzygium aromaticum L. bud (clove) essential oil nanoemulsion. 3 Biotech 2022, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.J.; Huang, R.F.; Kao, T.H.; Inbaraj, B.S.; Chen, B.H. Preparation of carotenoid extracts and nanoemulsions from Lycium barbarum L. and their effects on growth of HT-29 colon cancer cells. Nanotechnology 2017, 28, 135103. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Cai, T.; Liu, Q.; Whitney, J.; Du, M.; Ma, Q.; Zhang, R.; Yang, L.; Cole, S.; Cai, Y. Preparation and evaluation of spirulina polysaccharide nanoemulsions. Int. J. Mol. Med. 2018, 42, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Khatamian, N.; Tabrizi, M.H.; Ardalan, P.; Yadamani, S.; Maragheh, A.D. Synthesis of Carum Carvi essential oil nanoemulsion, the cytotoxic effect, and expression of caspase 3 gene. J. Food Biochem. 2019, 43, e12956. [Google Scholar] [CrossRef]
- Al-Otaibi, W.A.; AlMotwaa, S.M. Oxaliplatin-loaded nanoemulsion containing Teucrium polium L. essential oil induces apoptosis in Colon cancer cell lines through ROS-mediated pathway. Drug Deliv. 2022, 29, 2190–2205. [Google Scholar] [CrossRef]
- Alkreathy, H.M.; Alkhatib, M.H.; Al Musaddi, S.A.; Balamash, K.S.A.; Osman, N.N.; Ahmad, A. Enhanced antitumour activity of doxorubicin and simvastatin combination loaded nanoemulsion treatment against a Swiss albino mouse model of Ehrlich ascites carcinoma. Clin. Exp. Pharmacol. Physiol. 2019, 46, 496–505. [Google Scholar] [CrossRef]
- Zhang, Z.; Kuo, J.C.-T.; Zhang, C.; Huang, Y.; Zhou, Z.; Lee, R.J. A Squalene-Based Nanoemulsion for Therapeutic Delivery of Resiquimod. Pharmaceutics 2021, 13, 2060. [Google Scholar] [CrossRef]
- Enin, H.A.A.; Alquthami, A.F.; Alwagdani, A.M.; Yousef, L.M.; Albuqami, M.S.; Alharthi, M.A.; Alsaab, H.O. Utilizing TPGS for Optimizing Quercetin Nanoemulsion for Colon Cancer Cells Inhibition. Colloids Interfaces 2022, 6, 49. [Google Scholar] [CrossRef]
- Ahmad, N.; Albassam, A.A.; Khan, M.F.; Ullah, Z.; Buheazah, T.M.; AlHomoud, H.S.; Al-Nasif, H.A. A novel 5-Fluorocuracil multiple-nanoemulsion used for the enhancement of oral bioavailability in the treatment of colorectal cancer. Saudi J. Biol. Sci. 2022, 29, 3704–3716. [Google Scholar] [CrossRef]
- Kotta, S. Formulation of Resveratrol Nanoemulsion by Phase Inversion Technique and Evaluation of Anti-Cancer Activity on Human Colon Cancer Cell Lines. Indian J. Pharm. Educ. Res. 2021, 55, S623–S629. [Google Scholar] [CrossRef]
- Patel, R.; Singh, S.K.; Singh, S.; Sheth, N.R.; Gendle, R. Development and characterization of curcumin loaded transfersome for transdermal delivery. J. Pharm. Sci. Res. 2009, 1, 65–71. [Google Scholar]
- Almotwaa, S.M.; Alkhatib, M.H.; Alkreathy, H.M. Incorporating ifosfamide into salvia oil-based nanoemulsion diminishes its nephrotoxicity in mice inoculated with tumor. Bioimpacts 2019, 10, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.D.; Tamane, P.K.; Khante, S.N.; Pokharkar, V.B. QbD Based Optimization of Curcumin Nanoemulsion: DoE and Cytotoxicity Studies. Indian J. Pharm. Educ. Res. 2020, 54, 329–336. [Google Scholar] [CrossRef]
- Bashlouei, S.G.; Karimi, E.; Zareian, M.; Oskoueian, E.; Shakeri, M. Heracleum persicum Essential Oil Nanoemulsion: A Nanocarrier System for the Delivery of Promising Anticancer and Antioxidant Bioactive Agents. Antioxidants 2022, 11, 831. [Google Scholar] [CrossRef] [PubMed]
- Shoorvarzi, S.N.; Shahraki, F.; Shafaei, N.; Karimi, E.; Oskoueian, E. Citrus aurantium L. bloom essential oil nanoemulsion: Synthesis, characterization, cytotoxicity, and its potential health impacts on mice. J. Food Biochem. 2020, 44, e13181. [Google Scholar] [CrossRef] [PubMed]
- James, E.E.; Iwao, O.; Botchkina, M.M.; Ivanovna, G. Nanoemulsion Compositions of Taxoid Drugs, and Methods for the Use Thereof to Target Cancer Cells and Cancer Stem Cells. US10206875, February 2019. Available online: https://www.freepatentsonline.com/result.html?sort=relevance&srch=top&query_txt=10206875&submit=&patents_us=on (accessed on 10 February 2023).
- Won, L.K.; Sara, Y.L. Hydrogel-Based Nanoemulsion for Selectively Labelling Cancer Lesion, and Preparation Method Therefor United States Seoul National University R&db Foundation. US11103600, 2021. Available online: https://www.freepatentsonline.com/11103600.html (accessed on 10 February 2023).
- Thome, K.F.; Paulo, B.S.; Debora, L. Nanoemulsions and Methods for Cancer Therapy United States Fernando Thome Kreutz. US11332494, 2022. Available online: https://www.freepatentsonline.com/11332494.html (accessed on 10 February 2023).
- Won, L.K.; Yoon, J.; Sara, L. Nanocarrier for Selective Fluorescence Labeling of Cancer Cell and Preparation Method Therefor United Statesseoul National University R&Db Foundation. US11298428, 2022. Available online: https://www.freepatentsonline.com/11298428.html (accessed on 10 February 2023).
- Tayeb, H.H.; Felimban, R.; Hasaballah, N.; Mahfouz, J.B.; Chaudhary, A.; Felemban, M.; Alnadwi, F.; Rizg, W. Essential Oil Nanoemulsion and Methods of Use Thereof United States King Abdulaziz University. US11364199, 2022. Available online: https://www.freepatentsonline.com/11364199.html (accessed on 10 February 2023).
- Moira, M.S. Ionic Liquid-Based Nanoemulsion Formulation for the Efficient Delivery of Hydrophilic and Hydrophobic Therapeutic Agents United States Wisconsin Alumni Research Foundation. US11464738, 2022. Available online: https://www.freepatentsonline.com/11464738.html (accessed on 10 February 2023).
- Aquilue, S.; Gris, J.L.; Del Carmen, M.; Gañán, D.; Isabel, M. Oil-in-Water Nanoemulsion Composition of Clobetasol United States Laboratorios Salvat, S.A. US10857160, 2020. Available online: https://www.freepatentsonline.com/10857160.html (accessed on 10 February 2023).
- Evan, C.U.; Edmund, R.M. Compositions of Fluorocarbon Nanoemulsion, and Methods of Preparation and Use Thereof United States NuvOx Pharma LLC (Tucson, AZ, US). US11304899, 2022. Available online: https://www.freepatentsonline.com/11304899.html (accessed on 10 February 2023).
- Congling, Q. Transparent Nanoemulsions Comprising Lauric Oil United States Conopco, Inc. (Trumbull, CT, US). US11266580, 2022. Available online: https://www.freepatentsonline.com/11266580.html (accessed on 10 February 2023).
- Christopher, W.S.; Steven, T. Transparent Colloidal Vitamin Supplement Blend United States Quicksilver Scientific, Inc. (Lafayette, CO, US). US11304900, 2022. Available online: https://www.freepatentsonline.com/11304900.html (accessed on 10 February 2023).
- Felix, S.J.M.; Nan, S.; Amr, I.A.-F. Magnetic Emulsions as Contrast Agents for Subsurface Applications United States SAUDI ARABIAN OIL COMPANY (Dhahran, SA). US11506049, 2022. Available online: https://www.freepatentsonline.com/11506049.html (accessed on 10 February 2023).
- Amit, K.; Steven, R.; Malcolm, D.; Jesse, E.; Darrick, C.; Bryan, J.B. Compositions and Methods for Delivery of RNA United States HDT Bio Corp. (Seattle, WA, USA). Available online: https://www.freepatentsonline.com/11534497.htmlus11534497 (accessed on 10 February 2023).
- Amit, K.; Duthie, R.S.; Erasmus, M.; Carter, J.; Bryan, B.; Seattle, J. RNA-Lipid Nanoparticle Complexes of Viral RNA Polymerase Region and SARS-CoV-2 Spike Protein Region United States HDT Bio Corp (Seattle, WA, US). US11376335, 2022. Available online: https://www.freepatentsonline.com/11376335.html (accessed on 10 February 2023).
- Luan, L.; Chi, Z.; Liu, C. Chinese White Wax Solid Lipid Nanoparticles as a Novel Nanocarrier of Curcumin for Inhibiting the Formation of Staphylococcus aureus Biofilms. Nanomaterials 2019, 9, 763. [Google Scholar] [CrossRef]
- Aljabri, N.M.; Shi, N.; Cavazos, A. Nanoemulsion: An emerging technology for oilfield applications between limitations and potentials. J. Pet. Sci. Eng. 2021, 208, 109306. [Google Scholar] [CrossRef]
- Available online: https://clinicaltrials.gov (accessed on 12 February 2023).
- Chittasupho, C.; Chaobankrang, K.; Sarawungkad, A.; Samee, W.; Singh, S.; Hemsuwimon, K.; Okonogi, S.; Kheawfu, K.; Kiattisin, K.; Chaiyana, W. Antioxidant, anti-inflammatory and attenuating intracellular reactive oxygen species activities of Nicotiana tabacum var. Virginia leaf extract phytosomes and shape memory gel Formulation. Gels 2023, 9, 78. [Google Scholar] [CrossRef]
- Chittasupho, C.; Ditsri, S.; Singh, S.; Kanlayavattanakul, M.; Duangnin, N.; Ruksiriwanich, W.; Athikomkulchai, S. Ultraviolet radiation protective and anti-inflammatory effects of Kaempferia galanga L. rhizome oil and microemulsion: Formulation, characterization, and hydrogel preparation. Gels 2022, 8, 639. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).