Industrial Manufacturing Applications of Zinc Oxide Nanomaterials: A Comprehensive Study
Abstract
1. Introduction
2. Multi-Disciplinary Industrial Applications of ZnO NMs
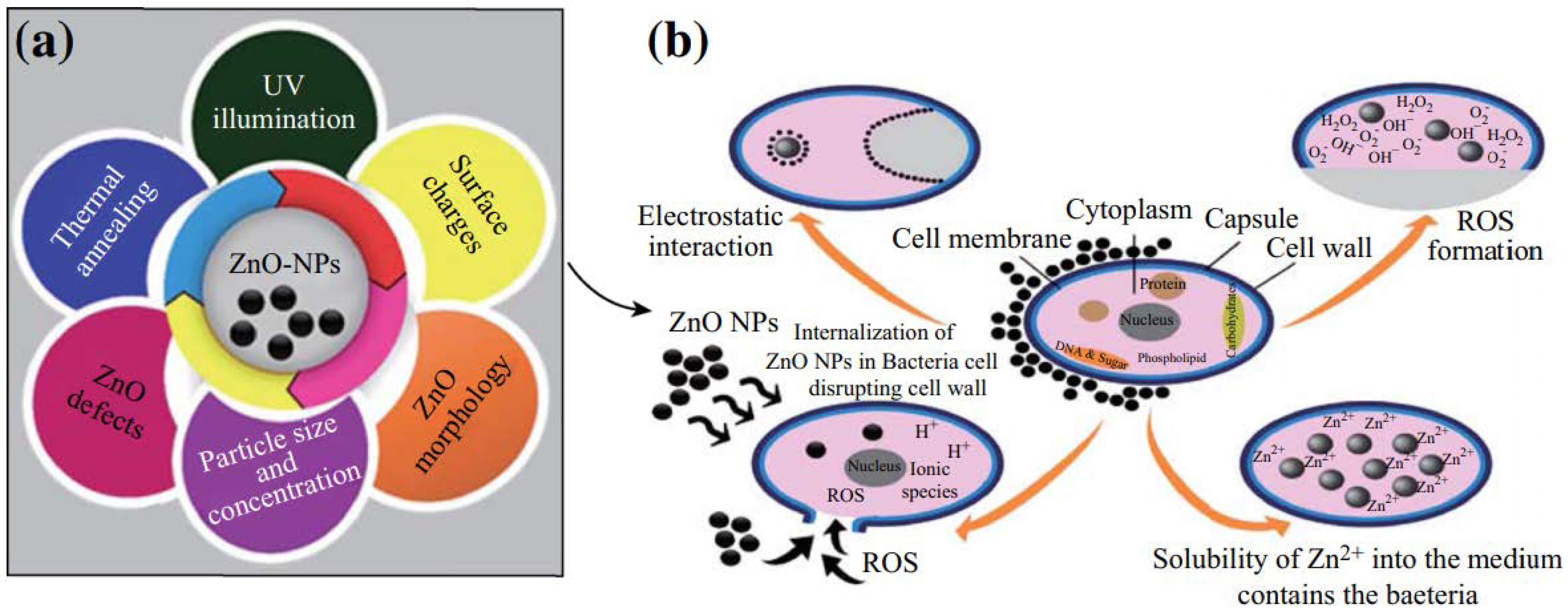
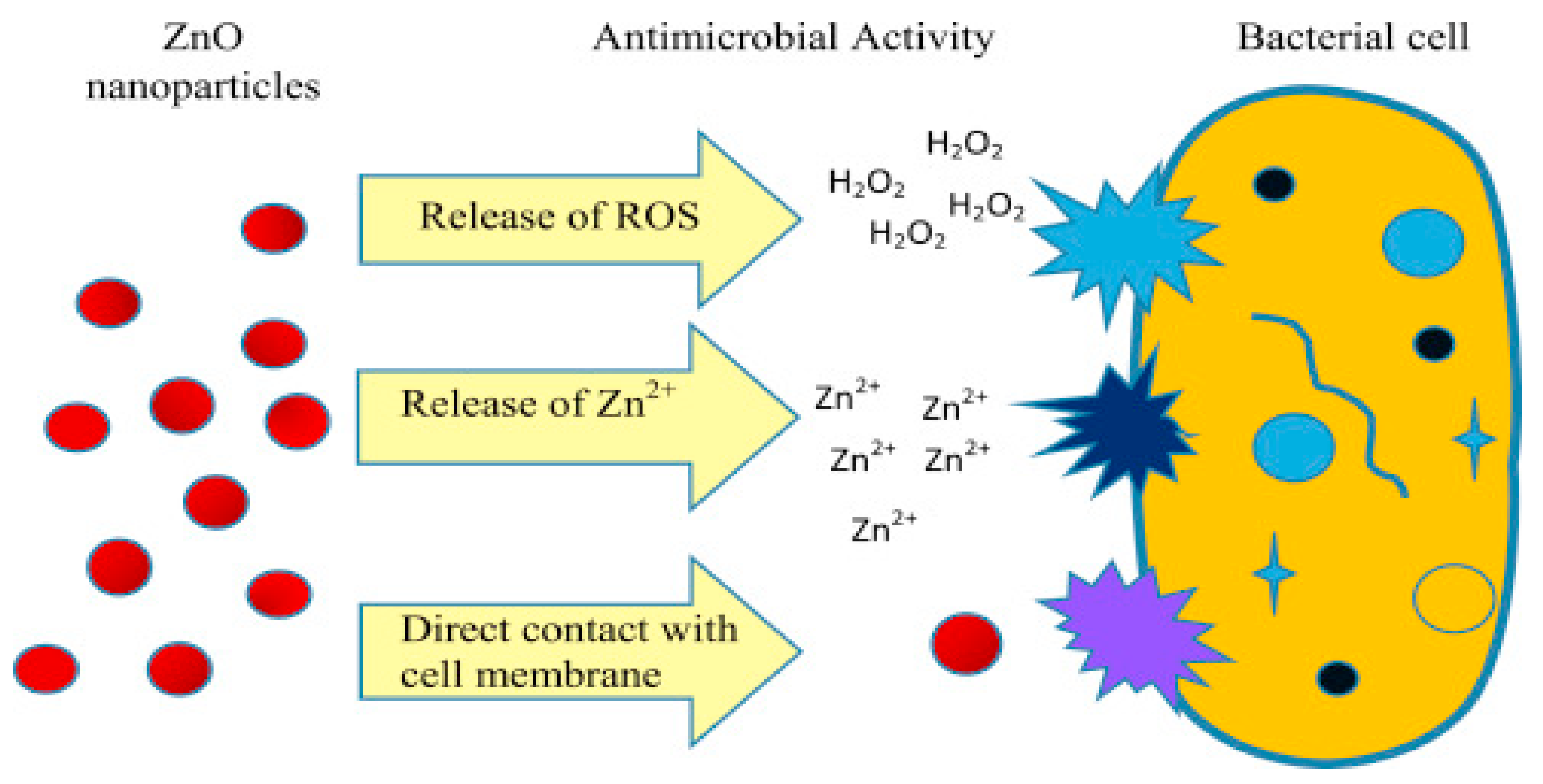
3. Antibacterial and Anti-Fungal Applications of ZnO NPs
| Product | Size (Nanometer) | Species of Bacteria | Mechanism | Ref. |
|---|---|---|---|---|
| ZnO NPs | 30 | E. coli | Damage the membrane’s integrity and the production of ROS. | [40] |
| 8 | S. aureus, E. coli, and B. subtilis | Due to the release of free Zn2+ ions formed in the ZnO suspension for significant growth inhibition of bacteria. | [39] | |
| 10 | L. Plantarum | By reaction between the surface of ZnO and cell surface enzymes of bacteria | [41] | |
| 12, 45 | E. coli | ZnO involves disrupting the membrane of bacteria | [42] | |
| ∼20 | E. coli 11,634 | H2O2 generation | [43] | |
| S. aureus, E. coli | Release of Zn2+ ion | [44] | ||
| ∼80 | V. cholera | Depolarization of the membrane structure, enhanced permeabilization, DNA damage, and ROS production | [45] | |
| 40 | S. pyogenes (MTCC1926), S. mutans (MTCC497), S. flexneri (MTCC1457), V. cholerae (MTCC3906), S. typhi (MTCC1252) | Zn2+ release and ROS production | [46] | |
| 90–100 | enterotoxin E. coli (ETEC), V. cholerae | adenylyl cyclase function inhibition, cAMP levels are reduction | [47] | |
| Ag-ZnO nanocomposite | 64 | GFP E. coli, S. aureus | Release of Ag+ and Zn2+ and ROS production | [48] |
| Phβ-GBP-coated ZnO NPs (Phβ-GBP- ZnO NPs) | 20–50 | P. vulgaris, S. aureus | Changes in the permeability of bacterial cell membranes and a high quantity of reactive oxygen species (ROS) | [49] |
| ZnO nanocatalyst | ∼18 | E. coli, B. subtilis, S. typhimurium, K. pneumonia | OH−, H2O2 generation, ROS generation | [50] |
| ZnO-CdO nanocomposite | 27 | P. aeruginosa, E. coli, K. pneumonia, P. vulgaris, S. aureus, B. spp. | Release Cd2+ and Zn2+ and generate ROS (H2O2, OH−, and O22−) | [51] |
| ZnO QDs | 4 | C. metallidurans CH34, E. coli MG1655 | Released Zn2+ ion generated toxicity | [52] |
| Kaoline-ZnO nanocomposites | E. coli, S. aureus, P. aeruginosa, E. faecalis | Zn2+ release, subsequent diffusion of ions into cytoplasm | [53] | |
| ZnO nanostructures (ZnO NSs) | 70–80 | S. aureus, P. vulgaris, K. pneumoniae, S. typhimurium | Damage to cell membranes by reactive oxygen species (ROS) | [54] |
| ZnO-Ge NPs | 20 | E. faecalis, P. aeruginosa | Bacterial cell death triggered by cell penetration | [55] |
| ZnO-SA composites | S. aureus, E. coli | Reactive Oxygen Species production | [56] | |
| ZnO@GA NPs | 11.5 ± 4.4 | S. aureus, E. coli | Due to GA’s strong affinity for the bacterial cell membrane and the increased lipophilicity that results from its addition. | [57] |
4. Photocatalytic Applications
5. Biosensing Applications
6. Bioimaging Applications
7. Gas Sensing Using ZnO
8. Medicinal Applications of ZnO NPs
9. ZnO in Food Industry
10. Applications of ZnO in Environmental Industry
11. ZnO in Cosmetics and Toiletries Industry
12. Applications of ZnO in Oil and Gas Industry
13. ZnO in Electronics Industry
14. Conclusions
15. Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tamirat, Y. The role of nanotechnology in semiconductor industry: Review article. J. Mater. Sci. Nanotechnol. 2017, 5, 202. [Google Scholar]
- Sobolev, K.; Gutiérrez, M.F. How nanotechnology can change the concrete world. Am. Ceram. Soc. Bull. 2005, 84, 14. [Google Scholar]
- Zhu, W.; Bartos, P.J.; Porro, A. Application of nanotechnology in construction. Mater. Struct. 2004, 37, 649–658. [Google Scholar] [CrossRef]
- Havancsak, K. Nanotechnology at present and its promise for the future. In Materials Science Forum; Trans Tech Publications Ltd.: Wollerau, Switzerland, 2003; Volume 414, pp. 85–94. [Google Scholar]
- Khan, I.; Saeed, K.; Khan, I. NPs: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Natsuki, J.; Natsuki, T.; Hashimoto, Y. A review of silver NPs: Synthesis methods, properties and applications. Int. J. Mater. Sci. Appl. 2015, 4, 325–332. [Google Scholar]
- Mohan, A.C.; Renjanadevi, B. Preparation of ZnO NPs and its characterization using scanning electron microscopy (SEM) and X-ray diffraction (XRD). Procedia Technol. 2016, 24, 761–766. [Google Scholar] [CrossRef]
- Kalpana, V.N.; Devi Rajeswari, V. A review on green synthesis, biomedical applications, and toxicity studies of ZnO NPs. Bioinorg. Chem. Appl. 2018, 2018, 3569758. [Google Scholar] [CrossRef]
- Bala, N.; Saha, S.; Chakraborty, M.; Maiti, M.; Das, S.; Basu, R.; Nandy, P. Green synthesis of ZnO NPs using Hibiscus subdariffa leaf extract: Effect of temperature on synthesis, anti-bacterial activity and anti-diabetic activity. RSC Adv. 2015, 5, 4993–5003. [Google Scholar] [CrossRef]
- Matinise, N.; Fuku, X.G.; Kaviyarasu, K.; Mayedwa, N.; Maaza, M.J.A.S.S. ZnO NPs via Moringa oleifera green synthesis: Physical properties & mechanism of formation. Appl. Surf. Sci. 2017, 406, 339–347. [Google Scholar]
- Aminuzzaman, M.; Ying, L.P.; Goh, W.S.; Watanabe, A. Green synthesis of ZnO NPs using aqueous extract of Garcinia mangostana fruit pericarp and their photocatalytic activity. Bull. Mater. Sci. 2018, 41, 50. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K.; Cumbal, L.; Debut, A. Green approach for fabrication and applications of ZnO NPs. Bioinorg. Chem. Appl. 2014, 2014, 523869. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.K.; Ng, A.; Chen, X.Y.; Ng, Y.H.; Leung, Y.H.; Ho, K.H.; Djurišić, A.B.; Ng, A.M.C.; Chan, W.K.; Yu, L.; et al. Effect of ZnO nanoparticle properties on dye-sensitized solar cell performance. ACS Appl. Mater. Interfaces 2012, 4, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Middya, S.; Layek, A.; Dey, A.; Ray, P.P. Morphological impact of ZnO nanoparticle on MEHPPV: ZnO based hybrid solar cell. J. Mater. Sci. Mater. Electron. 2013, 24, 4621–4629. [Google Scholar] [CrossRef]
- Udawatte, N.; Lee, M.; Kim, J.; Lee, D. Well-defined Au/ZnO nanoparticle composites exhibiting enhanced photocatalytic activities. ACS Appl. Mater. Interfaces 2011, 3, 4531–4538. [Google Scholar] [CrossRef] [PubMed]
- Subhan, M.A.; Saha, P.C.; Rahman, M.M.; Akand, M.A.R.; Asiri, A.M.; Al-Mamun, M. Enhanced photocatalytic activity and chemical sensor development based on ternary B2O3 center dot Zn6Al2O9 center dot ZnO NMs for environmental safety. New J. Chem. 2017, 41, 7220–7231. [Google Scholar] [CrossRef]
- Naveed Ul Haq, A.; Nadhman, A.; Ullah, I.; Mustafa, G.; Yasinzai, M.; Khan, I. Synthesis approaches of ZnO NPs: The dilemma of ecotoxicity. J. Nanomater. 2017, 2017, 8510342. [Google Scholar] [CrossRef]
- Hanley, C.; Thurber, A.; Hanna, C.; Punnoose, A.; Zhang, J.; Wingett, D.G. The influences of cell type and ZnO nanoparticle size on immune cell cytotoxicity and cytokine induction. Nanoscale Res. Lett. 2009, 4, 1409–1420. [Google Scholar] [CrossRef]
- Supraja, N.; Prasad, T.N.V.K.V.; Krishna, T.G.; David, E. Synthesis, characterization, and evaluation of the antimicrobial efficacy of Boswellia ovalifoliolata stem bark-extract-mediated ZnO NPs. Appl. Nanosci. 2016, 6, 581–590. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Otoni, C.G.; Soares, N.F.F. ZnO NPs for food packaging applications. In Antimicrobial Food Packaging; Academic Press: Cambridge, MA, USA, 2016; pp. 425–431. [Google Scholar]
- Kamaraj, P.; Sridharan, M.; Arockiaselvi, J.; Pushpamalini, T.; Vivekanand, P.A. Low cost synthesis of ZnO NPs and evaluation of their photocatalytic activity. Mater. Today Proc. 2021, 36, 873–877. [Google Scholar] [CrossRef]
- Gandhi, S.; Kaur, R.; Sharma, V.; Mandal, S.K. Effect of calcination temperature on the morphology and catalytic properties of ZnO nanostructures fabricated from a chiral precursor for photodegradation of both cationic and anionic dyes. New J. Chem. 2022, 46, 3645–3657. [Google Scholar] [CrossRef]
- Sundrarajan, M.; Ambika, S.; Bharathi, K. Plant-extract mediated synthesis of ZnO NPs using Pongamia pinnata and their activity against pathogenic bacteria. Adv. Powder Technol. 2015, 26, 1294–1299. [Google Scholar] [CrossRef]
- Karnan, T.; Selvakumar, S.A.S. Biosynthesis of ZnO NPs using rambutan (Nephelium lappaceum L.) peel extract and their photocatalytic activity on methyl orange dye. J. Mol. Struct. 2016, 1125, 358–365. [Google Scholar] [CrossRef]
- Ameen, F.; Dawoud, T.; AlNadhari, S. Ecofriendly and low-cost synthesis of ZnO NPs from Acremonium potronii for the photocatalytic degradation of azo dyes. Environ. Res. 2021, 202, 111700. [Google Scholar] [CrossRef]
- Muthayya, S.; Sugimoto, J.D.; Montgomery, S.; Maberly, G.F. An overview of global rice production, supply, trade, and consumption. Ann. N. Y. Acad. Sci. 2014, 1324, 7–14. [Google Scholar] [CrossRef]
- Ahmadpour, G.; Nilforoushan, M.R.; Boroujeny, B.S.; Tayebi, M.; Jesmani, S.M. Effect of substrate surface treatment on the hydrothermal synthesis of ZnO nanostructures. Ceram. Int. 2022, 48, 2323–2329. [Google Scholar] [CrossRef]
- Jumina, J.; Mutmainah, M.; Purwono, B.; Kurniawan, Y.S.; Syah, Y.M. Antibacterial and antifungal activity of three monosaccharide monomyristate derivatives. Molecules 2019, 24, 3692. [Google Scholar] [CrossRef]
- Guo, B.L.; Han, P.; Guo, L.C.; Cao, Y.Q.; Li, A.D.; Kong, J.Z.; Zhai, H.F.; Wu, D. The antibacterial activity of Ta-doped ZnO NPs. Nanoscale Res. Lett. 2015, 10, 336. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Y.; Ding, Y.; Daskalakis, N.; Jeuken, L.; Povey, M.; O’neill, A.J.; York, D.W. Mechanistic investigation into antibacterial behaviour of suspensions of ZnO NPs against E. coli. J. Nanoparticle Res. 2010, 12, 1625–1636. [Google Scholar] [CrossRef]
- Da Silva, B.L.; Caetano, B.L.; Chiari-Andréo, B.G.; Pietro, R.C.L.R.; Chiavacci, L.A. Increased antibacterial activity of ZnO NPs: Influence of size and surface modification. Colloids Surf. B Biointerfaces 2019, 177, 440–447. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A.; Rebezov, M.B.; Semenova, A.A.; Lisitsyn, A.B. A Mini Review of Antibacterial properties of ZnO NPs. Front. Phys. 2021, 9, 641481. [Google Scholar] [CrossRef]
- Sharma, D.; Rajput, J.; Kaith, B.S.; Kaur, M.; Sharma, S. Synthesis of ZnO NPs and study of their antibacterial and antifungal properties. Thin Solid Film. 2010, 519, 1224–1229. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on ZnO NPs: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, K.; Vanegas, P.; Cruzat, C.; Novoa, N.; Arrué, R.; Vanegas, E. Antibacterial and Antifungal Properties of Electrospun Recycled PET Polymeric Fibers Functionalized with ZnO NPs. Polymers 2021, 13, 3763. [Google Scholar] [CrossRef]
- Singh, T.A.; Sharma, A.; Tejwan, N.; Ghosh, N.; Das, J.; Sil, P.C. A state of the art review on the synthesis, antibacterial, antioxidant, antidiabetic and tissue regeneration activities of ZnO NPs. Adv. Colloid Interface Sci. 2021, 295, 102495. [Google Scholar] [CrossRef] [PubMed]
- Chausov, D.N.; Burmistrov, D.E.; Kurilov, A.D.; Bunkin, N.F.; Astashev, M.E.; Simakin, A.V.; Vedunova, M.V.; Gudkov, S.V. New Organosilicon Composite Based on Borosiloxane and Zinc Oxide Nanoparticles Inhibits Bacterial Growth, but Does Not Have a Toxic Effect on the Development of Animal Eukaryotic Cells. Materials 2021, 14, 6281. [Google Scholar] [CrossRef]
- Shi, L.E.; Li, Z.H.; Zheng, W.; Zhao, Y.F.; Jin, Y.F.; Tang, Z.X. Synthesis, antibacterial activity, antibacterial mechanism and food applications of ZnO NPs: A review. Food Addit. Contam. Part A 2014, 31, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Raghupathi, K.R.; Koodali, R.T.; Manna, A.C. Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of ZnO NPs. Langmuir 2011, 27, 4020–4028. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, L.; Wen, D.; Ding, Y. Role of physical and chemical interactions in the antibacterial behavior of ZnO NPs against E. coli. Mater. Sci. Eng. C 2016, 69, 1361–1366. [Google Scholar] [CrossRef]
- Shi, L.E.; Fang, X.J.; Zhang, Z.L.; Zhou, T.; Jiang, D.; Wu, H.H.; Tang, Z.X. Preparation of nano-ZnO using sonication method and its antibacterial characteristics. Int. J. Food Sci. Technol. 2012, 47, 1866–1871. [Google Scholar] [CrossRef]
- Nagarajan, P.; Vijayaraghavan, R. Enhanced bioactivity of ZnO NPs—An antimicrobial study. Sci. Technol. Adv. Mater. 2008, 9, 035004. [Google Scholar]
- Ghule, K.; Ghule, A.V.; Chen, B.J.; Ling, Y.C. Preparation and characterization of ZnO NPs coated paper and its antibacterial activity study. Green Chem. 2006, 8, 1034–1041. [Google Scholar] [CrossRef]
- Reddy, K.M.; Feris, K.; Bell, J.; Wingett, D.G.; Hanley, C.; Punnoose, A. Selective toxicity of ZnO NPs to prokaryotic and eukaryotic systems. Appl. Phys. Lett. 2007, 90, 213902. [Google Scholar] [CrossRef]
- Sarwar, S.; Chakraborti, S.; Bera, S.; Sheikh, I.A.; Hoque, K.M.; Chakrabarti, P. The antimicrobial activity of ZnO NPs against Vibrio cholerae: Variation in response depends on biotype. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1499–1509. [Google Scholar] [CrossRef]
- Soren, S.; Kumar, S.; Mishra, S.; Jena, P.K.; Verma, S.K.; Parhi, P. Evaluation of antibacterial and antioxidant potential of the ZnO NPs synthesized by aqueous and polyol method. Microb. Pathog. 2018, 119, 145–151. [Google Scholar] [CrossRef]
- Babayevska, N.; Przysiecka, Ł.; Iatsunskyi, I.; Nowaczyk, G.; Jarek, M.; Janiszewska, E.; Jurga, S. ZnO size and shape effect on antibacterial activity and cytotoxicity profile. Sci. Rep. 2022, 12, 8148. [Google Scholar] [CrossRef]
- Matai, I.; Sachdev, A.; Dubey, P.; Kumar, S.U.; Bhushan, B.; Gopinath, P. Antibacterial activity and mechanism of Ag–ZnO nanocomposite on S. aureus and GFP-expressing antibiotic resistant E. coli. Colloids Surf. B Biointerfaces 2014, 115, 359–367. [Google Scholar] [CrossRef]
- Iswarya, A.; Vaseeharan, B.; Anjugam, M.; Ashokkumar, B.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Benelli, G. Multipurpose efficacy of ZnO NPs coated by the crustacean immune molecule β-1, 3-glucan binding protein: Toxicity on HepG2 liver cancer cells and bacterial pathogens. Colloids Surf. B Biointerfaces 2017, 158, 257–269. [Google Scholar] [CrossRef]
- Shaban, M.; Mohamed, F.; Abdallah, S. Production and characterization of superhydrophobic and antibacterial coated fabrics utilizing ZnO nanocatalyst. Sci. Rep. 2018, 8, 3925. [Google Scholar] [CrossRef]
- Karthik, K.; Dhanuskodi, S.; Gobinath, C.; Sivaramakrishnan, S. Microwave-assisted synthesis of CdO–ZnO nanocomposite and its antibacterial activity against human pathogens. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 139, 7–12. [Google Scholar] [CrossRef]
- Bellanger, X.; Billard, P.; Schneider, R.; Balan, L.; Merlin, C. Stability and toxicity of ZnO quantum dots: Interplay between NPs and bacteria. J. Hazard. Mater. 2015, 283, 110–116. [Google Scholar] [CrossRef]
- Dědková, K.; Janíková., B.; Matějová, K.; Peikertová, P.; Neuwirthová, L.; Holešinský, J.; Kukutschová, J. Preparation, characterization and antibacterial properties of ZnO/kaoline nanocomposites. J. Photochem. Photobiol. B Biol. 2015, 148, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Ramani, M.; Ponnusamy, S.; Muthamizhchelvan, C.; Cullen, J.; Krishnamurthy, S.; Marsili, E. Morphology-directed synthesis of ZnO nanostructures and their antibacterial activity. Colloids Surf. B Biointerfaces 2013, 105, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Divya, M.; Vaseeharan, B.; Abinaya, M.; Vijayakumar, S.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Benelli, G. Biopolymer gelatin-coated ZnO NPs showed high antibacterial, antibiofilm and anti-angiogenic activity. J. Photochem. Photobiol. B Biol. 2018, 178, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Liu, T.; He, H.; Wu, X.; Cao, X.; Jin, J.; Sun, Q.; Roy, V.A.; Li, R.K. Rhelogical and antibacterial performance of sodium alginate/ZnO composite coating for cellulosic paper. Colloids Surf. B Biointerfaces 2018, 167, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Choi, K.H.; Min, J.; Kim, H.J.; Jee, J.P.; Park, B.J. Functionalized ZnO NPs with gallic acid for antioxidant and antibacterial activity against methicillin-resistant S. aureus. Nanomaterials 2017, 7, 365. [Google Scholar] [CrossRef]
- da Silva, B.L.; Abuçafy, M.P.; Manaia, E.B.; Junior, J.A.O.; Chiari-Andréo, B.G.; Pietro, R.C.R.; Chiavacci, L.A. Relationship between structure and antimicrobial activity of ZnO NPs: An overview. Int. J. Nanomed. 2019, 14, 9395. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; McLean, J.E.; Britt, D.W.; Anderson, A.J. Antifungal activity of ZnO NPs and their interactive effect with a biocontrol bacterium on growth antagonism of the plant pathogen Fusarium graminearum. Biometals 2013, 26, 913–924. [Google Scholar] [CrossRef]
- Jamdagni, P.; Khatri, P.; Rana, J.S. Green synthesis of ZnO NPs using flower extract of Nyctanthes arbortristis and their antifungal activity. J. King Saud Univ. Sci. 2018, 30, 168–175. [Google Scholar] [CrossRef]
- Arciniegas-Grijalba, P.A.; Patiño-Portela, M.C.; Mosquera-Sánchez, L.P.; Guerrero-Vargas, J.A.; Rodríguez-Páez, J.E. ZnO NPs (ZnO-NPs) and their antifungal activity against coffee fungus Erythricium salmonicolor. Appl. Nanosci. 2017, 7, 225–241. [Google Scholar] [CrossRef]
- Erazo, A.; Mosquera, S.A.; Rodríguez-Paéz, J.E. Synthesis of ZnO NPs with different morphology: Study of their antifungal effect on strains of Aspergillus niger and Botrytis cinerea. Mater. Chem. Phys. 2019, 234, 172–184. [Google Scholar] [CrossRef]
- He, L.; Liu, Y.; Mustapha, A.; Lin, M. Antifungal activity of ZnO NPs against Botrytis cinerea and Penicillium expansum. Microbiol. Res. 2011, 166, 207–215. [Google Scholar] [CrossRef]
- Pariona, N.; Paraguay-Delgado, F.; Basurto-Cereceda, S.; Morales-Mendoza, J.E.; Hermida-Montero, L.A.; Mtz-Enriquez, A.I. Shape-dependent antifungal activity of ZnO particles against phytopathogenic fungi. Appl. Nanosci. 2020, 10, 435–443. [Google Scholar] [CrossRef]
- Surendra, T.V.; Roopan, S.M.; Al-Dhabi, N.A.; Arasu, M.V.; Sarkar, G.; Suthindhiran, K. Vegetable Peel Waste for the Production of ZnO NPs and its Toxicological Efficiency, Antifungal, Hemolytic, and Antibacterial Activities. Nanoscale Res. Lett. 2016, 11, 546. [Google Scholar] [CrossRef]
- Gunalan, S.; Sivaraj, R.; Rajendran, V. Green synthesized ZnO NPs against bacterial and fungal pathogens. Prog. Nat. Sci. Mater. Int. 2012, 22, 693–700. [Google Scholar] [CrossRef]
- Jasim, N.O. Antifungal Activity of ZnO NPs on Aspergillus Fumigatus Fungus Candida Albicans Yeast. Citeseer 2015, 5, 23–28. [Google Scholar]
- Decelis, S.; Sardella, D.; Triganza, T.; Brincat, J.-P.; Gatt, R.; Valdramidis, V.P. Assessing the anti-fungal efficiency of filters coated with ZnO NPs. R. Soc. Open Sci. 2017, 4, 1–9. [Google Scholar] [CrossRef]
- Esteban-Tejeda, L.; Prado, C.; Cabal, B.; Sanz, J.; Torrecillas, R.; Moya, J.S. Antibacterial and antifungal activity of ZnO containing glasses. PLoS ONE 2015, 10, e0132709. [Google Scholar] [CrossRef]
- Sierra-Fernandez, A.; De La Rosa-García, S.C.; Gomez-Villalba, L.S.; Gómez-Cornelio, S.; Rabanal, M.E.; Fort, R.; Quintana, P. Synthesis, Photocatalytic, and Antifungal Properties of MgO, ZnO and Zn/Mg Oxide NPs for the Protection of Calcareous Stone Heritage. ACS Appl. Mater. Interfaces 2017, 9, 24873–24886. [Google Scholar] [CrossRef]
- Barad, S.; Roudbary, M.; Omran, N.A.; Daryasari, P.M. Preparation and characterization of ZnO NPs coated by chitosan-linoleic acid; fungal growth and biofilm assay. Bratisl. Med. J. Bratisl. Lek. List. 2017, 118, 169–174. [Google Scholar] [CrossRef]
- Raman, C.D.; Kanmani, S. Textile dye degradation using nano zero valent iron: A review. J. Environ. Manag. 2016, 177, 341–355. [Google Scholar] [CrossRef]
- Yulizar, Y.; Apriandanu, D.O.B.; Surya, R.M. Fabrication of novel SnWO4/ZnO using Muntingia calabura L. leaf extract with enhanced photocatalytic methylene blue degradation under visible light irradiation. Ceram. Int. 2022, 48, 3564–3577. [Google Scholar]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO NPs as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Wu, Y.; Altuner, E.E.; Tiri, R.N.E.H.; Bekmezci, M.; Gulbagca, F.; Aygun, A.; Xia, C.; Van Le, Q.; Sen, F.; Karimi-Maleh, H. Hydrogen generation from methanolysis of sodium borohydride using waste coffee oil modified ZnO NPs and their photocatalytic activities. Int. J. Hydrog. Energy 2022, in press. [Google Scholar]
- Davar, F.; Majedi, A.; Mirzaei, A. Green synthesis of ZnO NPs and its application in the degradation of some dyes. J. Am. Ceram. Soc. 2015, 98, 1739–1746. [Google Scholar] [CrossRef]
- Rahman, Q.I.; Ahmad, M.; Misra, S.K.; Lohani, M. Effective photocatalytic degradation of rhodamine B dye by ZnO NPs. Mater. Lett. 2013, 91, 170–174. [Google Scholar] [CrossRef]
- Hernández-Carrillo, M.A.; Torres-Ricárdez, R.; García-Mendoza, M.F.; Ramírez-Morales, E.; Rojas-Blanco, L.; Díaz-Flores, L.L.; Sepúlveda-Palacios, G.E.; Paraguay-Delgado, F.; Pérez-Hernández, G. Eu-modified ZnO NPs for applications in photocatalysis. Catal. Today 2020, 349, 191–197. [Google Scholar] [CrossRef]
- Chen, M.; Liu, P.; He, J.H.; Wang, H.L.; Zhang, H.; Wang, X.; Chen, R. Nanofiber template-induced preparation of ZnO nanocrystal and its application in photocatalysis. Sci. Rep. 2021, 11, 1–8. [Google Scholar] [CrossRef]
- Kumar, R.; Umar, A.; Kumar, G.; Akhtar, M.S.; Wang, Y.; Kim, S.H. Ce-doped ZnO NPs for efficient photocatalytic degradation of direct red-23 dye. Ceram. Int. 2015, 41, 7773–7782. [Google Scholar] [CrossRef]
- Dar, G.N.; Umar, A.; Zaidi, S.A.; Ibrahim, A.A.; Abaker, M.; Baskoutas, S.; Al-Assiri, M.S. Ce-doped ZnO nanorods for the detection of hazardous chemical. Sens. Actuators B Chem. 2012, 173, 72–78. [Google Scholar] [CrossRef]
- Buasakun, J.; Srilaoong, P.; Rattanakam, R.; Duangthongyou, T. Synthesis of Heterostructure of ZnO@MOF-46(Zn) to Improve the Photocatalytic Performance in Methylene Blue Degradation. Crystals 2021, 11, 1379. [Google Scholar] [CrossRef]
- Gherab, K.; Al-Douri, Y.; Hashim, U.; Ameri, M.; Bouhemadou, A.; Batoo, K.M.; Adil, S.F.; Khan, M.; Raslan, E.H. Fabrication and characterizations of Al NPs doped ZnO nanostructures-based integrated electrochemical biosensor. J. Mater. Res. Technol. 2020, 9, 857–867. [Google Scholar] [CrossRef]
- Tu, W.; Lei, J.; Wang, P.; Ju, H. Photoelectrochemistry of Free-Base-Porphyrin-Functionalized ZnO NPs and Their Applications in Biosensing. Chem. A Eur. J. 2011, 17, 9440–9447. [Google Scholar] [CrossRef]
- Inbasekaran, S.; Senthil, R.; Ramamurthy, G.; Sastry, T.P. Biosensor using ZnO NPs. Int. J. Innov. Res. Sci. Eng. Technol. 2014, 3, 8601–8606. [Google Scholar]
- Napi, M.L.M.; Sultan, S.M.; Ismail, R.; How, K.W.; Ahmad, M.K. Electrochemical-based biosensors on different ZnO nanostructures: A review. Materials 2019, 12, 2985. [Google Scholar] [CrossRef]
- Arya, S.K.; Saha, S.; Ramirez-Vick, J.E.; Gupta, V.; Bhansali, S.; Singh, S.P. Recent advances in ZnO nanostructures and thin films for biosensor applications. Anal. Chim. Acta 2012, 737, 1–21. [Google Scholar] [CrossRef]
- Wang, G.; Tan, X.; Zhou, Q.; Liu, Y.; Wang, M.; Yang, L. Synthesis of highly dispersed ZnO NPs on carboxylic graphene for development a sensitive acetylcholinesterase biosensor. Sens. Actuators B Chem. 2014, 190, 730–736. [Google Scholar] [CrossRef]
- Kim, H.M.; Park, J.H.; Lee, S.K. Fiber optic sensor based on ZnO nanowires decorated by Au nanoparticles for improved plasmonic biosensor. Sci. Rep. 2019, 9, 15605. [Google Scholar] [CrossRef]
- Devi, R.; Yadav, S.; Pundir, C.S. Amperometric determination of xanthine in fish meat by ZnO nanoparticle/chitosan/multiwalled carbon nanotube/polyaniline composite film bound xanthine oxidase. Analyst 2012, 137, 754–759. [Google Scholar] [CrossRef]
- Siddiquee, S.; Yusof, N.A.; Salleh, A.B.; Tan, S.G.; Bakar, F.A. Development of electrochemical DNA biosensor for Trichoderma harzianum based on ionic liquid/ZnO NPs/chitosan/gold electrode. J. Solid State Electrochem. 2012, 16, 273–282. [Google Scholar] [CrossRef]
- Haghighi, B.; Bozorgzadeh, S. Fabrication of a highly sensitive electrochemiluminescence lactate biosensor using ZnO NPs decorated multiwalled carbon nanotubes. Talanta 2011, 85, 2189–2193. [Google Scholar] [CrossRef]
- Supraja, P.; Singh, V.; Vanjari, S.R.K.; Govind Singh, S. Electrospun CNT embedded ZnO nanofiber based biosensor for electrochemical detection of Atrazine: A step closure to single molecule detection. Microsyst. Nanoeng. 2020, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Umar, A.; Rahman, M.M.; Vaseem, M.; Hahn, Y.B. Ultra-sensitive cholesterol biosensor based on low-temperature grown ZnO NPs. Electrochem. Commun. 2009, 11, 118–121. [Google Scholar] [CrossRef]
- Khan, R.; Kaushik, A.; Solanki, P.R.; Ansari, A.A.; Pandey, M.K.; Malhotra, B.D. ZnO NPs-chitosan composite film for cholesterol biosensor. Anal. Chim. Acta 2008, 616, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, S.; Srinivasan, P.; Rayappan, J.B.B.; Solomon, A.P. A photoluminescence biosensor for the detection of N-acyl homoserine lactone using cysteamine functionalized ZnO NPs for the early diagnosis of urinary tract infections. J. Mater. Chem. B 2020, 8, 4228–4236. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, L.; Zhu, Z.; Zhang, J.; Zhu, J. Novel uric acid sensor based on enzyme electrode modified by zno NPs and multiwall carbon nanotubes. Anal. Lett. 2009, 42, 775–789. [Google Scholar] [CrossRef]
- Sadeghi, R.; Karimi-Maleh, H.; Bahari, A.; Taghavi, M. A novel biosensor based on ZnO nanoparticle/1, 3-dipropylimidazolium bromide ionic liquid-modified carbon paste electrode for square-wave voltammetric determination of epinephrine. Phys. Chem. Liq. 2013, 51, 704–714. [Google Scholar] [CrossRef]
- Ali, A.; Ansari, A.A.; Kaushik, A.; Solanki, P.R.; Barik, A.; Pandey, M.K.; Malhotra, B.D. Nanostructured ZnO film for urea sensor. Mater. Lett. 2009, 63, 2473–2475. [Google Scholar] [CrossRef]
- Aydoğdu, G.; Zeybek, D.K.; Pekyardımcı, Ş.; Kılıç, E. A novel amperometric biosensor based on ZnO NPs-modified carbon paste electrode for determination of glucose in human serum. Artif. Cells Nanomed. Biotechnol. 2013, 41, 332–338. [Google Scholar] [CrossRef]
- Aini, B.N.; Siddiquee, S.; Ampon, K.; Rodrigues, K.F.; Suryani, S. Development of glucose biosensor based on ZnO NPs film and glucose oxidase-immobilized eggshell membrane. Sens. Bio-Sens. Res. 2015, 4, 46–56. [Google Scholar] [CrossRef]
- Shukla, S.K.; Deshpande, S.R.; Shukla, S.K.; Tiwari, A. Fabrication of a tunable glucose biosensor based on ZnO/chitosan-graft-poly (vinyl alcohol) core-shell nanocomposite. Talanta 2012, 99, 283–287. [Google Scholar] [CrossRef]
- Chawla, S.; Pundir, C.S. An amperometric hemoglobin A1c biosensor based on immobilization of fructosyl amino acid oxidase onto ZnO NPs–polypyrrole film. Anal. Biochem. 2012, 430, 156–162. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, Y.; Lei, L.; Xu, Z.; Zhang, W. Photoelectrochemical biosensor for acetylcholinesterase activity study based on metal oxide semiconductor nanocomposites. J. Electroanal. Chem. 2016, 781, 377–382. [Google Scholar] [CrossRef]
- Wang, Y.T.; Bao, Y.J.; Lou, L.; Li, J.J.; Du, W.J.; Zhu, Z.Q.; Peng, H.; Zhu, J.Z. A novel L-lactate sensor based on enzyme electrode modified with ZnO NPs and multiwall carbon nanotubes. In SENSORS, 2010 IEEE, Waikoloa, HI, USA, 1–4 November 2010; IEEE: Piscataway, NJ, USA, 2010; pp. 33–37. [Google Scholar]
- Li, X.; Zhao, C.; Liu, X. A paper-based microfluidic biosensor integrating ZnO nanowires for electrochemical glucose detection. Microsyst. Nanoeng. 2015, 1, 15014. [Google Scholar] [CrossRef]
- Danielson, E.; Dhamodharan, V.; Porkovich, A.; Kumar, P.; Jian, N.; Ziadi, Z.; Grammatikopoulos, P.; Sontakke, V.A.; Yokobayashi, Y.; Sowwan, M. Gas-phase ynthesis for label-free biosensors: Zinc-oxide nanowires functionalized with gold NPs. Sci. Rep. 2019, 9, 17370. [Google Scholar] [CrossRef]
- Rong, P.; Ren, S.; Yu, Q. Fabrications and applications of ZnO NMs in flexible functional devices—A review. Crit. Rev. Anal. Chem. 2019, 49, 336–349. [Google Scholar] [CrossRef]
- Salinas, R.A.; Orduña-Díaz, A.; Obregon-Hinostroza, O.; Dominguez, M.A. Biosensors based on ZnO thin-film transistors using recyclable plastic substrates as an alternative for real-time pathogen detection. Talanta 2022, 237, 122970. [Google Scholar] [CrossRef]
- Mansoor, S.; Shahid, S.; Ashiq, K.; Alwadai, N.; Javed, M.; Iqbal, S.; Fatima, U.; Zaman, S.; Sarwar, M.N.; Alshammari, F.H.; et al. Controlled growth of nanocomposite thin layer based on Zn-Doped MgO NPs through Sol-Gel technique for biosensor applications. Inorg. Chem. Commun. 2022, 142, 109702. [Google Scholar] [CrossRef]
- Zhang, X.; Villafuerte, J.; Consonni, V.; Sarigiannidou, E.; Capsal, J.F.; Bruhat, A.; Grinberg, D.; Petit, L.; Cottinet, P.J.; Le, M.Q. Optimization Strategies Used for Boosting Piezoelectric Response of Biosensor Based on Flexible Micro-ZnO Composites. Biosensors 2022, 12, 245. [Google Scholar] [CrossRef]
- Mahmoud, A.; Echabaane, M.; Omri, K.; Boudon, J.; Saviot, L.; Millot, N.; Chaabane, R.B. Cu-Doped ZnO NPs for Non-Enzymatic Glucose Sensing. Molecules 2021, 26, 929. [Google Scholar] [CrossRef]
- Vijayaprasath, G.; Murugan, R.; Narayanan, J.S.; Dharuman, V.; Ravi, G.; Hayakawa, Y. Glucose sensing behavior of cobalt doped ZnO NPs synthesized by co-precipitation method. J. Mater. Sci. Mater. Electron. 2015, 26, 4988–4996. [Google Scholar] [CrossRef]
- Raza, W.; Ahmad, K. A highly selective Fe@ ZnO modified disposable screen printed electrode based non-enzymatic glucose sensor (SPE/Fe@ ZnO). Mater. Lett. 2018, 212, 231–234. [Google Scholar] [CrossRef]
- Guan, H.N.; Chi, D.F.; Yu, J. Photoelectrochemical acetylcholinesterase biosensor incorporating ZnO NPs. In Advanced Materials Research; Trans Tech Publications Ltd.: Wollerau, Switzerland, 2011; Volume 183, pp. 1701–1706. [Google Scholar]
- Singh, S.P.; Arya, S.K.; Pandey, P.; Malhotra, B.D.; Saha, S.; Sreenivas, K.; Gupta, V. Cholesterol biosensor based on rf sputtered ZnO nanoporous thin film. Appl. Phys. Lett. 2007, 91, 063901. [Google Scholar] [CrossRef]
- Solanki, P.R.; Kaushik, A.; Ansari, A.A.; Malhotra, B.D. Nanostructured ZnO platform for cholesterol sensor. Appl. Phys. Lett. 2009, 94, 143901. [Google Scholar] [CrossRef]
- Zhao, Y.; Fang, X.; Gu, Y.; Yan, X.; Kang, Z.; Zheng, X.; Lin, P.; Zhao, L.; Zhang, Y. Gold NPs coated ZnO nanorods as the matrix for enhanced l-lactate sensing. Colloids Surf. B Biointerfaces 2015, 126, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Subhan, M.A.; Saha, P.C.; Hossain, A.; Asiri, A.M.; Alam, M.M.; Al-Mamun, M.; Ghann, W.; Uddin, J.; Raihan, T.; Azad, A.K.; et al. Photocatalytic performance, anti-bacterial activities and 3-chlorophenol sensor fabrication using MnAl2O4·ZnAl2O4 NMs. Nanoscale Adv. 2021, 3, 5872–5889. [Google Scholar] [CrossRef]
- Sruthi, S.; Ashtami, J.; Mohanan, P.V. Biomedical application and hidden toxicity of ZnO NPs. Mater. Today Chem. 2018, 10, 175–186. [Google Scholar] [CrossRef]
- Senthilkumar, K.; Senthilkumar, O.; Yamauchi, K.; Sato, M.; Morito, S.; Ohba, T.; Nakamura, M.; Fujita, Y. Preparation of ZnO NPs for bio-imaging applications. Phys. Status Solidi 2009, 246, 885–888. [Google Scholar] [CrossRef]
- Wu, Y.L.; Lim, C.S.; Fu, S.; Tok, A.I.Y.; Lau, H.M.; Boey, F.Y.C.; Zeng, X.T. Surface modifications of ZnO quantum dots for bio-imaging. Nanotechnology 2007, 18, 215604. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Yuan, Q.; Lu, L. Fluorescence-enhanced gadolinium-doped ZnO quantum dots for magnetic resonance and fluorescence imaging. Biomaterials 2011, 32, 1185–1192. [Google Scholar] [CrossRef]
- Gupta, J.; Hassan, P.A.; Barick, K.C. Core-shell Fe3O4@ ZnO NPs for magnetic hyperthermia and bio-imaging applications. AIP Adv. 2021, 11, 025207. [Google Scholar] [CrossRef]
- Kumawat, A.; Chattopadhyay, S.; Verma, R.K.; Misra, K.P. Eu doped ZnO NPs with strong potential of thermal sensing and bioimaging. Mater. Lett. 2022, 308, 131221. [Google Scholar] [CrossRef]
- Dey, A.; Ray, P.G.; Dhara, S.; Neogi, S. Optically engineered ZnO NPs: Excitable at visible wavelength and lowered cytotoxicity towards bioimaging applications. Appl. Surf. Sci. 2022, 592, 153303. [Google Scholar] [CrossRef]
- Abadjian, M.C.Z.; Choi, J.; Anderson, C.J. NPs for PET imaging of tumors and cancer metastasis. In Design and Applications of NPs in Biomedical Imaging; Springer: Cham, Switzerland, 2017; pp. 229–255. [Google Scholar]
- Li, Y.; Wang, R.; Zheng, W.; Li, Y. Silica-Coated Ga (III)-Doped ZnO: Yb3+, Tm3+ Upconversion NPs for High-Resolution in Vivo Bioimaging using Near-Infrared to Near-Infrared Upconversion Emission. Inorg. Chem. 2019, 58, 8230–8236. [Google Scholar] [CrossRef]
- Eriksson, J.; Khranovskyy, V.; Söderlind, F.; Käll, P.O.; Yakimova, R.; Spetz, A.L. ZnO NPs or ZnO films: A comparison of the gas sensing capabilities. Sens. Actuators B Chem. 2009, 137, 94–102. [Google Scholar] [CrossRef]
- Fan, F.; Feng, Y.; Bai, S.; Feng, J.; Chen, A.; Li, D. Synthesis and gas sensing properties to NO2 of ZnO NPs. Sens. Actuators B Chem. 2013, 185, 377–382. [Google Scholar] [CrossRef]
- Bian, H.; Ma, S.; Sun, A.; Xu, X.; Yang, G.; Yan, S.; Gao, J.; Zhang, Z.; Zhu, H. Improvement of acetone gas sensing performance of ZnO NPs. J. Alloys Compd. 2016, 658, 629–635. [Google Scholar] [CrossRef]
- Tang, H.; Yan, M.; Ma, X.; Zhang, H.; Wang, M.; Yang, D. Gas sensing behavior of polyvinylpyrrolidone-modified ZnO NPs for trimethylamine. Sens. Actuators B Chem. 2006, 113, 324–328. [Google Scholar] [CrossRef]
- Umar, A.; Khan, M.A.; Kumar, R.; Algarni, H. Ag-doped ZnO NPs for enhanced ethanol gas sensing application. J. Nanosci. Nanotechnol. 2018, 18, 3557–3562. [Google Scholar] [CrossRef]
- Yan, H.; Song, P.; Zhang, S.; Yang, Z.; Wang, Q. Facile synthesis, characterization and gas sensing performance of ZnO NPs-coated MoS2 nanosheets. J. Alloys Compd. 2016, 662, 118–125. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, Z.; Li, P.; Zhou, X. Ozone gas sensing properties of metal-organic frameworks-derived In2O3 hollow microtubes decorated with ZnO NPs. Sens. Actuators B Chem. 2019, 301, 127081. [Google Scholar] [CrossRef]
- Jagannathan, M.; Dhinasekaran, D.; Rajendran, A.R.; Subramaniam, B. Selective room temperature ammonia gas sensor using nanostructured ZnO/CuO@ graphene on paper substrate. Sens. Actuators B Chem. 2022, 350, 130833. [Google Scholar] [CrossRef]
- Franco, M.A.; Conti, P.P.; Andre, R.S.; Correa, D.S. A review on chemiresistive ZnO gas sensors. Sens. Actuators Rep. 2022, 4, 100100. [Google Scholar] [CrossRef]
- Ancona, A.; Dumontel, B.; Garino, N.; Demarco, B.; Chatzitheodoridou, D.; Fazzini, W.; Engelke, H.; Cauda, V. Lipid-coated ZnO NPs as innovative ROS-generators for photodynamic therapy in cancer cells. Nanomaterials 2018, 8, 143. [Google Scholar] [CrossRef] [PubMed]
- Sudhagar, S.; Sathya, S.; Pandian, K.; Lakshmi, B.S. Targeting and sensing cancer cells with ZnO nanoprobes in vitro. Biotechnol. Lett. 2011, 33, 1891–1896. [Google Scholar] [CrossRef] [PubMed]
- Bisht, G.; Rayamajhi, S. ZnO NPs: A promising anticancer agent. Nanobiomedicine 2016, 3, 3–9. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Li, Z.; Govindan, R.; Jayakumar, R.; Wang, C.; Gu, F.L. ZnO-quercetin nanocomposite as a smart nano-drug delivery system: Molecular-level interaction studies. Appl. Surf. Sci. 2021, 536, 147741. [Google Scholar] [CrossRef]
- Peng, H.; Cui, B.; Li, G.; Wang, Y.; Li, N.; Chang, Z.; Wang, Y. A multifunctional β-CD-modified Fe3O4@ ZnO: Er3+, Yb3+ nanocarrier for antitumor drug delivery and microwave-triggered drug release. Mater. Sci. Eng. C 2015, 46, 253–263. [Google Scholar] [CrossRef]
- Tan, L.; Liu, J.; Zhou, W.; Wei, J.; Peng, Z. A novel thermal and pH responsive drug delivery system based on ZnO@ PNIPAM hybrid NPs. Mater. Sci. Eng. C 2014, 45, 524–529. [Google Scholar] [CrossRef]
- Zhao, Q.G.; Wang, J.; Zhang, Y.P.; Zhang, J.; Tang, A.N.; Kong, D.M. A ZnO-gated porphyrinic metal–organic framework-based drug delivery system for targeted bimodal cancer therapy. J. Mater. Chem. B 2018, 6, 7898–7907. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Saravanakumar, K.; Malaikozhundan, B.; Divya, M.; Vaseeharan, B.; Durán-Lara, E.F.; Wang, M.H. Biopolymer K-carrageenan wrapped ZnO NPs as drug delivery vehicles for anti MRSA therapy. Int. J. Biol. Macromol. 2020, 144, 9–18. [Google Scholar] [CrossRef]
- Chen, T.; Zhao, T.; Wei, D.; Wei, Y.; Li, Y.; Zhang, H. Core–shell nanocarriers with ZnO quantum dots-conjugated Au nanoparticle for tumor-targeted drug delivery. Carbohydr. Polym. 2013, 92, 1124–1132. [Google Scholar] [CrossRef]
- Afzal, H.; Ikram, M.; Ali, S.; Shahzadi, A.; Aqeel, M.; Haider, A.; Imran, M. Enhanced drug efficiency of doped ZnO–GO (graphene oxide) nanocomposites, a new gateway in drug delivery systems (DDSs). Mater. Res. Express 2020, 7, 015405. [Google Scholar] [CrossRef]
- Yadollahi, M.; Farhoudian, S.; Barkhordari, S.; Gholamali, I.; Farhadnejad, H.; Motasadizadeh, H. Facile synthesis of chitosan/ZnO bio-nanocomposite hydrogel beads as drug delivery systems. Int. J. Biol. Macromol. 2016, 82, 273–278. [Google Scholar] [CrossRef]
- Yuan, Q.; Hein, S.; Misra, R.D.K. New generation of chitosan-encapsulated ZnO quantum dots loaded with drug: Synthesis, characterization and in vitro drug delivery response. Acta Biomater. 2010, 6, 2732–2739. [Google Scholar] [CrossRef]
- Qiu, H.; Cui, B.; Li, G.; Yang, J.; Peng, H.; Wang, Y.; Li, N.; Gao, R.; Chang, Z.; Wang, Y. Novel Fe3O4@ ZnO@ mSiO2 nanocarrier for targeted drug delivery and controllable release with microwave irradiation. J. Phys. Chem. C 2014, 118, 14929–14937. [Google Scholar] [CrossRef]
- Wu, S.; Huang, X.; Du, X. pH-and redox-triggered synergistic controlled release of a ZnO-gated hollow mesoporous silica drug delivery system. J. Mater. Chem. B 2015, 3, 1426–1432. [Google Scholar] [CrossRef]
- Cai, X.; Luo, Y.; Zhang, W.; Du, D.; Lin, Y. pH-Sensitive ZnO quantum dots–doxorubicin NPs for lung cancer targeted drug delivery. ACS Appl. Mater. Interfaces 2016, 8, 22442–22450. [Google Scholar] [CrossRef]
- Xiong, H.M. ZnO NPs applied to bioimaging and drug delivery. Adv. Mater. 2013, 25, 5329–5335. [Google Scholar] [CrossRef]
- Zheng, C.; Wang, Y.; Phua, S.Z.F.; Lim, W.Q.; Zhao, Y. ZnO–DOX@ ZIF-8 core–shell NPs for pH-responsive drug delivery. ACS Biomater. Sci. Eng. 2017, 3, 2223–2229. [Google Scholar] [CrossRef]
- Martínez-Carmona, M.; Gun’Ko, Y.; Vallet-Regí, M. ZnO nanostructures for drug delivery and theranostic applications. Nanomaterials 2018, 8, 268. [Google Scholar] [CrossRef]
- Wang, J.; Lee, J.S.; Kim, D.; Zhu, L. Exploration of Zinc Oxide Nanoparticles as a Multitarget and Multifunctional Anticancer Nanomedicine. ACS Appl. Mater. Interfaces 2017, 9, 39971–39984. [Google Scholar] [CrossRef] [PubMed]
- Vimala, K.; Shanthi, K.; Sundarraj, S.; Kannan, S. Synergistic effect of chemo-photothermal for breast cancer therapy using folic acid (FA) modified zinc oxide nanosheet. J. Colloid Interface Sci. 2017, 488, 92–108. [Google Scholar] [CrossRef] [PubMed]
- Handford, C.E.; Dean, M.; Henchion, M.; Spence, M.; Elliott, C.T.; Campbell, K. Implications of nanotechnology for the agri-food industry: Opportunities, benefits and risks. Trends Food Sci. Technol. 2014, 40, 226–241. [Google Scholar] [CrossRef]
- He, X.; Hwang, H.M. Nanotechnology in food science: Functionality, applicability, and safety assessment. J. Food Drug Anal. 2016, 24, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Sinha, S.; Das, B.; Jayabalan, R.; Suar, M.; Mishra, A.; Tamhankar, A.J.; Stålsby Lundborg, C.; Tripathy, S.K. Disinfection of multidrug resistant Escherichia coli by solar-photocatalysis using Fe-doped ZnO nanoparticles. Sci. Rep. 2017, 7, 104. [Google Scholar] [CrossRef] [PubMed]
- Nesakumar, N.; Thandavan, K.; Sethuraman, S.; Krishnan, U.M.; Rayappan, J.B.B. nanorods an electrochemical biosensor with nanointerface for lactate detection based on lactate dehydrogenase immobilized on ZnO. J. Colloid Interface Sci. 2014, 414, 90–96. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, X.; Kang, Z.; Fang, X.; Zheng, X.; Zhao, L.; Du, H.; Zhang, Y. ZnO nanowires-based electrochemical biosensor for L-lactic acid amperometric detection. J. Nanoparticle Res. 2014, 16, 1–9. [Google Scholar] [CrossRef]
- Tayel, A.A.; El-Tras, W.F.; Moussa, S.; El-Baz, A.F.; Mahrous, H.; Salem, M.F.; Brimer, L. Antibacterial action of ZnO NPs against foodborne pathogens. J. Food Saf. 2011, 31, 211–218. [Google Scholar] [CrossRef]
- Yadav, S.; Mehrotra, G.K.; Dutta, P.K. Chitosan based ZnO NPs loaded gallic-acid films for active food packaging. Food Chem. 2021, 334, 127605. [Google Scholar] [CrossRef]
- Kumar, S.; Thakur, A.; Rangra, V.S.; Sharma, S. Synthesis and use of low-band-gap ZnO NPs for water treatment. Arab. J. Sci. Eng. 2016, 41, 2393–2398. [Google Scholar] [CrossRef]
- Tankhiwale, R.; Bajpai, S.K. Preparation, characterization and antibacterial applications of ZnO-NPs coated polyethylene films for food packaging. Colloids Surf. B Biointerfaces 2012, 90, 16–20. [Google Scholar] [CrossRef]
- Emerald, F.M.E.; Pushpadass, H.A.; Joseph, D.; Jaya, S.V. Impact of Nanotechnology in Beverage Processing; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Khalafi, T.; Buazar, F.; Ghanemi, K. Phycosynthesis and enhanced photocatalytic activity of ZnO NPs toward organosulfur pollutants. Sci. Rep. 2019, 9, 6866. [Google Scholar] [CrossRef]
- Rameshbabu, R.; Kumar, N.; Karthigeyan, A.; Neppolian, B. Visible light photocatalytic activities of ZnFe2O4/ZnO NPs for the degradation of organic pollutants. Mater. Chem. Phys. 2016, 181, 106–115. [Google Scholar] [CrossRef]
- Li, J.; Liu, L.; Liang, Q.; Zhou, M.; Yao, C.; Xu, S.; Li, Z. Core-shell ZIF-8@ MIL-68 (In) derived ZnO NPs-embedded In2O3 hollow tubular with oxygen vacancy for photocatalytic degradation of antibiotic pollutant. J. Hazard. Mater. 2021, 414, 125395. [Google Scholar] [CrossRef]
- Pathakoti, K.; Manubolu, M.; Hwang, H.M. Nanotechnology applications for environmental industry. In Handbook of NMs for Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 894–907. [Google Scholar]
- Saravanan, R.; Gracia, F.; Khan, M.M.; Poornima, V.; Gupta, V.K.; Narayanan, V.; Stephen, A. ZnO/CdO nanocomposites for textile effluent degradation and electrochemical detection. J. Mol. Liq. 2015, 209, 374–380. [Google Scholar] [CrossRef]
- Saravanan, R.; Karthikeyan, N.; Gupta, V.K.; Thirumal, E.; Thangadurai, P.; Narayanan, V.; Stephen, A. ZnO/Ag nanocomposite: An efficient catalyst for degradation studies of textile effluents under visible light. Mater. Sci. Eng. C 2013, 33, 2235–2244. [Google Scholar] [CrossRef] [PubMed]
- Sathishkumar, P.; Sweena, R.; Wu, J.J.; Anandan, S. Synthesis of CuO-ZnO nanophotocatalyst for visible light assisted degradation of a textile dye in aqueous solution. Chem. Eng. J. 2011, 171, 136–140. [Google Scholar] [CrossRef]
- Xie, H.; Ye, X.; Duan, K.; Xue, M.; Du, Y.; Ye, W.; Wang, C. CuAu–ZnO–graphene nanocomposite: A novel graphene-based bimetallic alloy-semiconductor catalyst with its enhanced photocatalytic degradation performance. J. Alloys Compd. 2015, 636, 40–47. [Google Scholar] [CrossRef]
- Danwittayakul, S.; Jaisai, M.; Dutta, J. Efficient solar photocatalytic degradation of textile wastewater using ZnO/ZTO composites. Appl. Catal. B Environ. 2015, 163, 1–8. [Google Scholar] [CrossRef]
- Qian, C.; Yin, J.; Zhao, J.; Li, X.; Wang, S.; Bai, Z.; Jiao, T. Facile preparation and highly efficient photodegradation performances of self-assembled Artemia eggshell-ZnO nanocomposites for wastewater treatment. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125752. [Google Scholar] [CrossRef]
- Campagnolo, L.; Lauciello, S.; Athanassiou, A.; Fragouli, D. Au/ZnO hybrid nanostructures on electrospun polymeric mats for improved photocatalytic degradation of organic pollutants. Water 2019, 11, 1787. [Google Scholar] [CrossRef]
- Akkari, M.; Aranda, P.; Mayoral, A.; García-Hernández, M.; Amara, A.B.H.; Ruiz-Hitzky, E. Sepiolite nanoplatform for the simultaneous assembly of magnetite and ZnO NPs as photocatalyst for improving removal of organic pollutants. J. Hazard. Mater. 2017, 340, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Elmi, F.; Alinezhad, H.; Moulana, Z.; Salehian, F.; Mohseni Tavakkoli, S.; Asgharpour, F.; Fallah, H.; Elmi, M.M. The use of antibacterial activity of ZnO NPs in the treatment of municipal wastewater. Water Sci. Technol. 2014, 70, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Asif, A.K.M.A.H.; Hasan, M.Z. Application of nanotechnology in modern textiles: A review. Int. J. Curr. Eng. Technol. 2018, 8, 227–231. [Google Scholar]
- Yetisen, A.K.; Qu, H.; Manbachi, A.; Butt, H.; Dokmeci, M.R.; Hinestroza, J.P.; Skorobogatiy, M.; Khademhosseini, A.; Yun, S.H. Nanotechnology in textiles. ACS Nano 2016, 10, 3042–3068. [Google Scholar] [CrossRef]
- Dimapilis, E.A.S.; Hsu, C.S.; Mendoza, R.M.O.; Lu, M.C. Zinc oxide nanoparticles for water disinfection. Sustain. Environ. Res. 2018, 28, 47–56. [Google Scholar] [CrossRef]
- Raj, S.; Jose, S.; Sumod, U.S.; Sabitha, M. Nanotechnology in cosmetics: Opportunities and challenges. J. Pharm. Bioallied Sci. 2012, 4, 186. [Google Scholar] [CrossRef]
- Katz, L.M.; Dewan, K.; Bronaugh, R.L. Nanotechnology in cosmetics. Food Chem. Toxicol. 2015, 85, 127–137. [Google Scholar] [CrossRef]
- Garcia-Mesa, J.C.; Montoro-Leal, P.; Rodriguez-Moreno, A.; Guerrero, M.L.; Alonso, E.V. Direct solid sampling for speciation of Zn2+ and ZnO NPs in cosmetics by graphite furnace atomic absorption spectrometry. Talanta 2021, 223, 121795. [Google Scholar] [CrossRef]
- Osmond, M.J.; Mccall, M.J. ZnO NPs in modern sunscreens: An analysis of potential exposure and hazard. Nanotoxicology 2010, 4, 15–41. [Google Scholar] [CrossRef]
- Lu, P.J.; Huang, S.C.; Chen, Y.P.; Chiueh, L.C.; Shih, D.Y.C. Analysis of titanium dioxide and ZnO NPs in cosmetics. J. Food Drug Anal. 2015, 23, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Adebayo-Tayo, B.C.; Borode, S.O.; Olaniyi, O.A. Phytosynthesis of ZnO NPs using methanol extract of Senna alata leaf: Characterization, optimization, antimicrobial properties, and its application in cold cream formulation. Polym. Med. 2020, 50, 5–19. [Google Scholar] [CrossRef]
- Sonia, S.; Ruckmani, K.; Sivakumar, M. Antimicrobial and antioxidant potentials of biosynthesized colloidal ZnO NPs for a fortified cold cream formulation: A potent nanocosmeceutical application. Mater. Sci. Eng. C 2017, 79, 581–589. [Google Scholar]
- Dkhil, M.A.; Diab, M.S.; Aljawdah, H.M.; Murshed, M.; Hafiz, T.A.; Al-Quraishy, S.; Bauomy, A.A. Neuro-biochemical changes induced by ZnO NPs. Saudi J. Biol. Sci. 2020, 27, 2863–2867. [Google Scholar] [CrossRef] [PubMed]
- Norouzzadeh Helali, Z.; Esmailzadeh, M. A comparative study of antibacterial effects of mouthwashes containing Ag/ZnO or ZnO NPs with chlorhexidine and investigation of their cytotoxicity. Nanomed. J. 2018, 5, 102–110. [Google Scholar]
- Soleimani, H.; Baig, M.K.; Yahya, N.; Khodapanah, L.; Sabet, M.; Demiral, B.M.; Burda, M. Synthesis of ZnO NPs for oil–water interfacial tension reduction in enhanced oil recovery. Appl. Phys. A 2018, 124, 1–13. [Google Scholar] [CrossRef]
- Sayyadnejad, M.A.; Ghaffarian, H.R.; Saeidi, M. Removal of hydrogen sulfide by ZnO NPs in drilling fluid. Int. J. Environ. Sci. Technol. 2008, 5, 565–569. [Google Scholar] [CrossRef]
- Alnarabiji, M.S.; Yahya, N.; Bee Abd Hamid, S.; Azizli, K.A.; Shafie, A.; Solemani, H. Microwave synthesis of ZnO NPs for enhanced oil recovery. In Advanced Materials Research; Trans Tech Publications Ltd.: Wollerau, Switzerland, 2014; Volume 1024, pp. 83–86. [Google Scholar]
- Najafi, V.; Zolghadr, S.; Kimiagar, S. Remarkable reproducibility and significant sensitivity of ZnO NPs covered by Chromium (III) oxide as a hydrogen sulfide gas sensor. Optik 2019, 182, 249–256. [Google Scholar] [CrossRef]
- Zhu, B.L.; Zeng, D.W.; Wu, J.; Song, W.L.; Xie, C.S. Synthesis and gas sensitivity of In-doped ZnO NPs. J. Mater. Sci. Mater. Electron. 2003, 14, 521–526. [Google Scholar] [CrossRef]
- Liu, S.; Yu, B.; Zhang, H.; Fei, T.; Zhang, T. Enhancing NO2 gas sensing performances at room temperature based on reduced graphene oxide-ZnO NPs hybrids. Sens. Actuators B Chem. 2014, 202, 272–278. [Google Scholar] [CrossRef]
- Zhu, B.L.; Xie, C.S.; Zeng, D.W.; Song, W.L.; Wang, A.H. Investigation of gas sensitivity of Sb-doped ZnO NPs. Mater. Chem. Phys. 2005, 89, 148–153. [Google Scholar] [CrossRef]
- Tajmiri, M.; Ehsani, M.R. The potential of ZnO NPs to reduce water consuming in iranian heavy oil reservoir. J. Water Environ. Nanotechnol. 2016, 1, 84–90. [Google Scholar]
- Tajmiri, M.; Ehsani, M.R.; Mousavi, S.M.; Roayaei, E.; Emadi, A. The effect of ZnO NPs on improved oil recovery in spontaneous imbibition mechanism of heavy oil production. In AIP Conference Proceedings; AIP Publishing LLC.: Melville, NY, USA, 2015; Volume 1669, p. 020024. [Google Scholar]
- Ismail, W.; Alhamad, N.A.; El-Sayed, W.S.; El Nayal, A.M.; Chiang, Y.R.; Hamzah, R.Y. Bacterial degradation of the saturate fraction of Arabian light crude oil: Biosurfactant production and the effect of ZnO NPs. J. Pet. Environ. Biotechnol. 2013, 4, 163. [Google Scholar]
- Zheng, Z.Q.; Yao, J.D.; Wang, B.; Yang, G.W. Light-controlling, flexible and transparent ethanol gas sensor based on ZnO NPs for wearable devices. Sci. Rep. 2015, 5, 1–8. [Google Scholar]
- Morgenstern, F.S.; Kabra, D.; Massip, S.; Brenner, T.J.; Lyons, P.E.; Coleman, J.N.; Friend, R.H. Ag-nanowire films coated with ZnO NPs as a transparent electrode for solar cells. Appl. Phys. Lett. 2011, 99, 242. [Google Scholar] [CrossRef]
- Suliman, A.E.; Tang, Y.; Xu, L. Preparation of ZnO NPs and nanosheets and their application to dye-sensitized solar cells. Sol. Energy Mater. Sol. Cells 2007, 91, 1658–1662. [Google Scholar] [CrossRef]
- Shaheen, I.; Ahmad, K.S.; Zequine, C.; Gupta, R.K.; Thomas, A.G.; Azad Malik, M. Sustainable synthesis of organic framework-derived ZnO NPs for fabrication of supercapacitor electrode. Environ. Technol. 2020, 43, 605–616. [Google Scholar] [CrossRef]
- Saranya, M.; Ramachandran, R.; Wang, F. Graphene-ZnO (G-ZnO) nanocomposite for electrochemical supercapacitor applications. Adv. Mater. Devices 2016, 1, 454–460. [Google Scholar] [CrossRef]
- Ezeigwe, E.R.; Tan, M.T.; Khiew, P.S.; Siong, C.W. One-step green synthesis of graphene/ZnO nanocomposites for electrochemical capacitors. Ceram. Int. 2015, 41, 715–724. [Google Scholar] [CrossRef]
- Nivin, T.S.; Sindhu, S. Fabrication of novel thin film capacitor based on PVA/ZnO nanocomposites as dielectric material. Bull. Mater. Sci. 2020, 43, 1–6. [Google Scholar] [CrossRef]
- Guo, R.; Yue, W.; An, Y.; Ren, Y.; Yan, X. Graphene-encapsulated porous carbon-ZnO composites as high-performance anode materials for Li-ion batteries. Electrochim. Acta 2014, 135, 161–167. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Zhao, W.; Zou, M.; Chen, Y.; Yang, L.; Xu, L.; Wu, H.; Cao, A. MOF-derived ZnO NPs covered by N-doped carbon layers and hybridized on carbon nanotubes for lithium-ion battery anodes. ACS Appl. Mater. Interfaces 2017, 9, 37813–37822. [Google Scholar] [CrossRef] [PubMed]
- Jing, M.; Li, F.; Chen, M.; Long, F.; Wu, T. Binding ZnO nanorods in reduced graphene oxide via facile electrochemical method for Na-ion battery. Appl. Surf. Sci. 2019, 463, 986–993. [Google Scholar] [CrossRef]
- Faber, H.; Burkhardt, M.; Jedaa, A.; Kälblein, D.; Klauk, H.; Halik, M. Low-temperature solution-processed memory transistors based on ZnO NPs. Adv. Mater. 2009, 21, 3099–3104. [Google Scholar] [CrossRef]
- Cho, S.Y.; Kang, Y.H.; Jung, J.Y.; Nam, S.Y.; Lim, J.; Yoon, S.C.; Choi, D.H.; Lee, C. Novel ZnO inks with ZnO NPs for low-temperature, solution-processed thin-film transistors. Chem. Mater. 2012, 24, 3517–3524. [Google Scholar] [CrossRef]
- Yun, D.Y.; Kwak, J.K.; Jung, J.H.; Kim, T.W.; Son, D.I. Electrical bistabilities and carrier transport mechanisms of write-once-read-many-times memory devices fabricated utilizing ZnO NPs embedded in a polystyrene layer. Appl. Phys. Lett. 2009, 95, 267. [Google Scholar] [CrossRef]
- Hmar, J.J.L. Flexible resistive switching bistable memory devices using ZnO NPs embedded in polyvinyl alcohol (PVA) matrix and poly (3, 4-ethylenedioxythiophene) polystyrene sulfonate (PEDOT: PSS). RSC Adv. 2018, 8, 20423–20433. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, X.; Huang, J.; Bai, J.; Hou, Y.; Wang, C.; Wang, S.; Bai, X. Bistable non-volatile resistive memory devices based on ZnO NPs embedded in polyvinylpyrrolidone. RSC Adv. 2020, 10, 14662–14669. [Google Scholar] [CrossRef]
- Selmi, A.; Fkiri, A.; Bouslimi, J.; Besbes, H.C. Improvement of dielectric properties of ZnO nanoparticles by Cu doping for tunable microwave devices. J. Mater. Sci. Mater. Electron. 2020, 31, 18664–18672. [Google Scholar] [CrossRef]
- Raghunath, A.; Perumal, E. Metal oxide NPs as antimicrobial agents: A promise for the future. Int. J. Antimicrob. Agents 2017, 49, 137–152. [Google Scholar] [CrossRef]
- Wang, S.C.; Wang, X.H.; Qiao, G.Q.; Chen, X.Y.; Wang, X.Z.; Wu, N.N.; Tian, J.; Cui, H.Z. NiO NPs-decorated ZnO hierarchical structures for isopropanol gas sensing. Rare Met. 2022, 41, 960–971. [Google Scholar] [CrossRef]
- San Tang, K. The current and future perspectives of ZnO NPs in the treatment of diabetes mellitus. Life sciences 2019, 239, 117011. [Google Scholar] [CrossRef]
- Patil, V.L.; Dalavi, D.S.; Dhavale, S.B.; Vanalakar, S.A.; Tarwal, N.L.; Kalekar, A.S.; Kim, J.H.; Patil, P.S. Indium doped ZnO nanorods for chemiresistive NO2 gas sensors. New J. Chem. 2022, 46, 7588–7597. [Google Scholar] [CrossRef]
- Kumar, B.; Poddar, S.; Sinha, S.K. Electrochemical cholesterol sensors based on nanostructured metal oxides: Current progress and future perspectives. J. Iran. Chem. Soc. 2022, 19, 4093–4116. [Google Scholar] [CrossRef]
- Kwon, D.H.; Jin, E.H.; Yoo, D.H.; Roh, J.W.; Suh, D.; Commerell, W.; Huh, J.S. Analysis of the Response Characteristics of Toluene Gas Sensors with a ZnO Nanorod Structure by a Heat Treatment Process. Sensors 2022, 22, 4125. [Google Scholar] [CrossRef]
- Sarojini, S.; Jayaram, S. An Impact of Antibacterial Efficacy of Metal Oxide NPs: A Promise for Future. Bio-Manuf. Nanomater. 2021, 393–406. [Google Scholar]
- Cao, P.; Cai, Y.; Pawar, D.; Han, S.; Xu, W.; Fang, M.; Liu, X.; Zeng, Y.; Liu, W.; Lu, Y.; et al. Au@ ZnO/rGO nanocomposite-based ultra-low detection limit highly sensitive and selective NO2 gas sensor. J. Mater. Chem. C 2022, 10, 4295–4305. [Google Scholar] [CrossRef]
- Raliya, R.; Saharan, V.; Dimkpa, C.; Biswas, P. Nanofertilizer for precision and sustainable agriculture: Current state and future perspectives. J. Agric. Food Chem. 2017, 66, 6487–6503. [Google Scholar] [CrossRef]
- Luo, Y.; Ly, A.; Lahem, D.; Martin, J.D.; Romain, A.C.; Zhang, C.; Debliquy, M. Role of cobalt in Co-ZnO nanoflower gas sensors for the detection of low concentration of VOCs. Sens. Actuators B Chem. 2022, 360, 131674. [Google Scholar] [CrossRef]




| Type of Device | Type of Fungi Inhibited | Reference |
|---|---|---|
| ZnO QDs | B. cinerea, P. expansum | [64] |
| A. saloni, S. rolfii | [65] | |
| R. stolonifera, A. nidulans, A. flavus, T. harzianum | [66] | |
| E. salmonicolor | [61] | |
| A. fumigatus, C. albicans | [67] | |
| R. stolonifera, P. expansum | [68] | |
| C. krusei | [69] | |
| Zn/Mg Oxide QDs | A. niger, Paraconiothyrium sp., P. oxalicum, P. maculans | [70] |
| CS-LiA ZnO QDs | C. albicans | [71] |
| ZnO NPs | Biological Compound Sensing | Ref. |
|---|---|---|
| ZnO NRs/TNs | Acetylcholinesterase | [104] |
| ZnO/chitosan-graft-poly(vinyl alcohol)core-shell nanocomposite | Glucose | [102] |
| ZnO/chitosan/MCNT/polyaniline composite film | Xanthine | [90] |
| Ionic liquid/ZnO/chitosan/gold electrode | DNA | [91] |
| ZnO NPs decorated multi-walled carbon nanotubes (MWCNT) | Lactate | [92] |
| MWCNT–ZnO NPs | Cholesterol | [93] |
| Cysteamine functionalized ZnO NPs | N-Acyl Homoserine Lactone | [96] |
| Enzyme electrode modified by ZnO NPs | Uric Acid | [97] |
| ZnO nanoparticle/1, 3-dipropylimidazolium bromide ionic liquid-modified carbon paste electrode | Epinephrine | [98] |
| Nanostructured ZnO film for urea sensor. | Urea | [99] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subhan, M.A.; Neogi, N.; Choudhury, K.P. Industrial Manufacturing Applications of Zinc Oxide Nanomaterials: A Comprehensive Study. Nanomanufacturing 2022, 2, 265-291. https://doi.org/10.3390/nanomanufacturing2040016
Subhan MA, Neogi N, Choudhury KP. Industrial Manufacturing Applications of Zinc Oxide Nanomaterials: A Comprehensive Study. Nanomanufacturing. 2022; 2(4):265-291. https://doi.org/10.3390/nanomanufacturing2040016
Chicago/Turabian StyleSubhan, Md Abdus, Newton Neogi, and Kristi Priya Choudhury. 2022. "Industrial Manufacturing Applications of Zinc Oxide Nanomaterials: A Comprehensive Study" Nanomanufacturing 2, no. 4: 265-291. https://doi.org/10.3390/nanomanufacturing2040016
APA StyleSubhan, M. A., Neogi, N., & Choudhury, K. P. (2022). Industrial Manufacturing Applications of Zinc Oxide Nanomaterials: A Comprehensive Study. Nanomanufacturing, 2(4), 265-291. https://doi.org/10.3390/nanomanufacturing2040016








