Prevalence of Human and Animal African Trypanosomiasis in Nigeria: A Scoping Review
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Eligibility Criteria
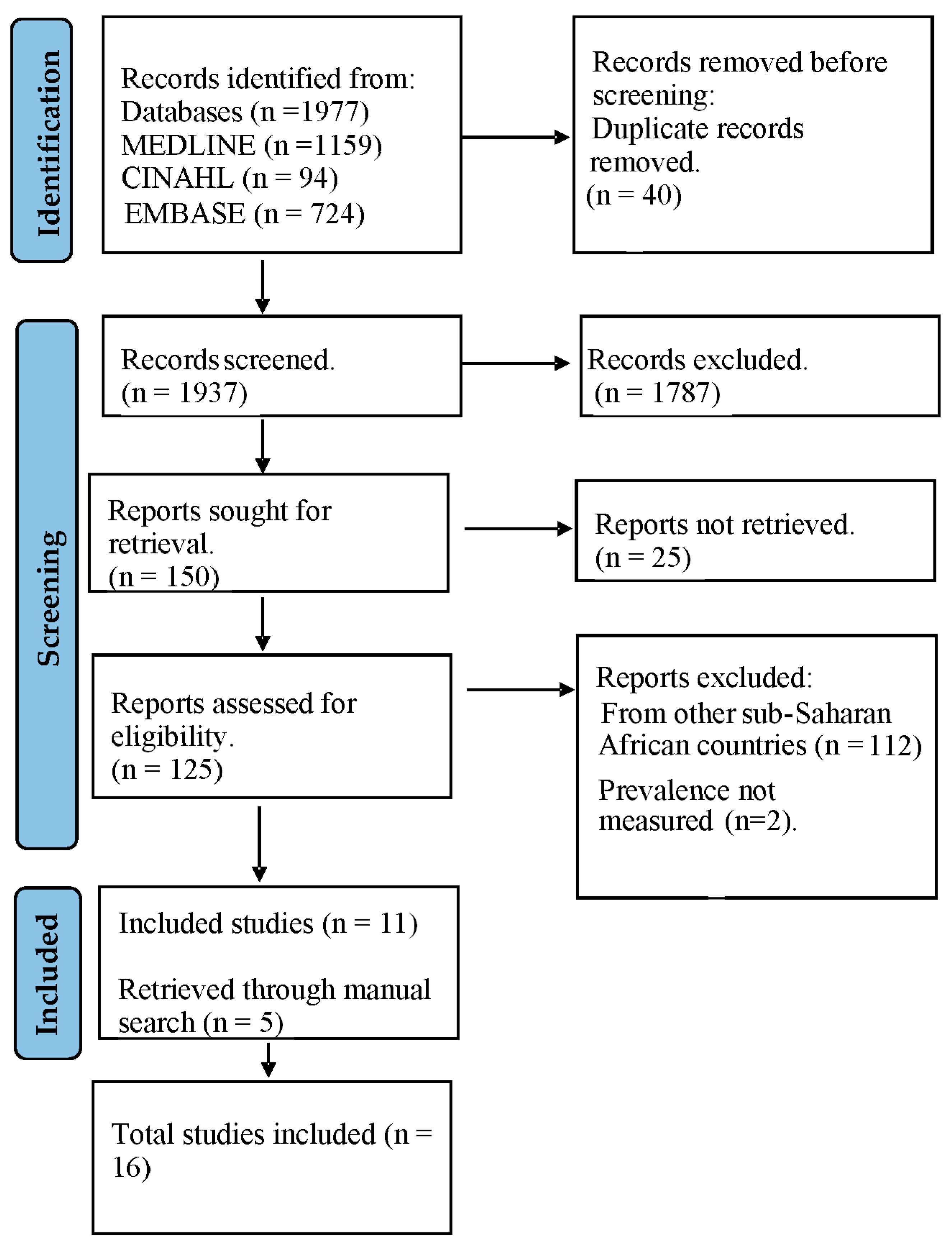
2.3. Study Selection
2.4. Data Extraction
2.5. Data Synthesis
3. Results
3.1. Description of Eligible Studies
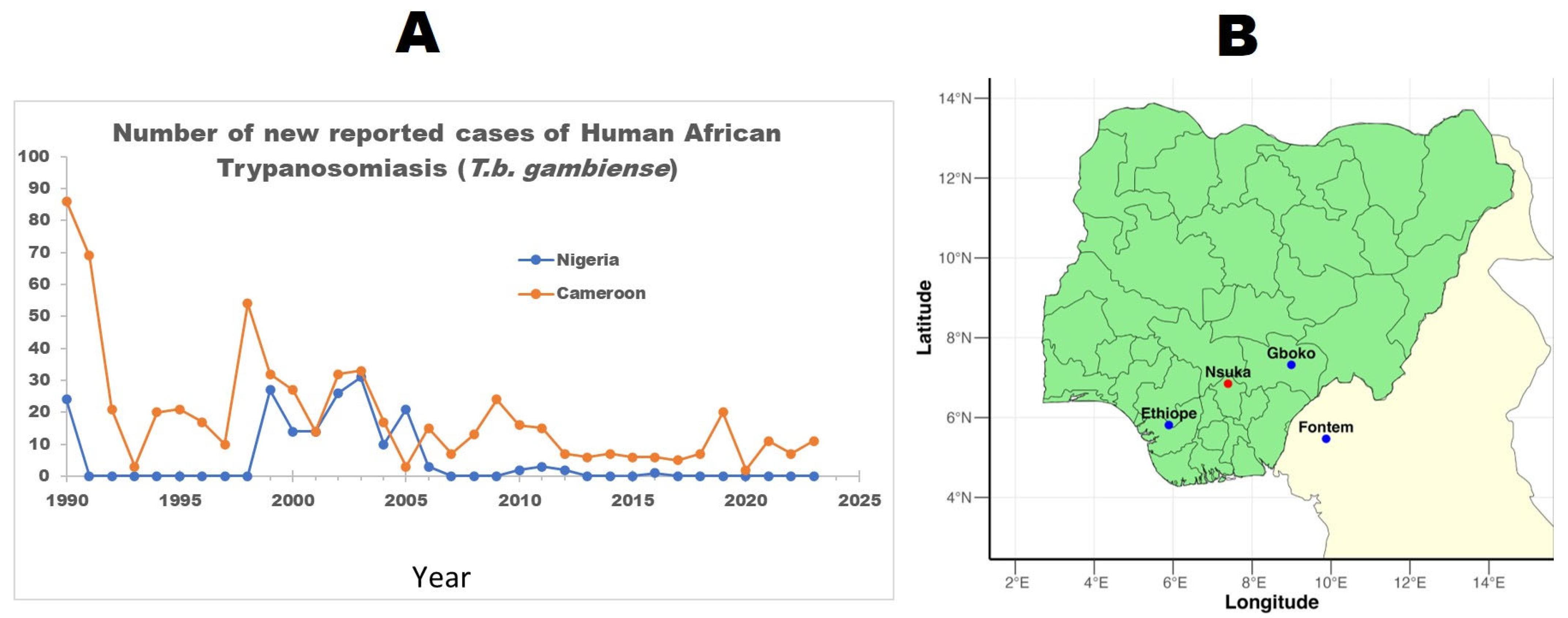
3.2. Prevalence of HAT
3.3. Prevalence of AAT
3.4. Diagnostic Techniques
4. Discussion
4.1. The Prevalence of Human African Trypanosomiasis in Nigeria
4.2. Prevalence of AAT in Nigeria
4.3. The Diagnosis of Trypanosomiasis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- The Disease|Programme Against African Trypanosomosis (PAAT)|Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/paat/the-programme/the-disease/en/ (accessed on 15 August 2025).
- Papagni, R.; Novara, R.; Minardi, M.L.; Frallonardo, L.; Panico, G.G.; Pallara, E.; Cotugno, S.; Bartoli, T.A.; Guido, G.; Vita, E.; et al. Human African Trypanosomiasis (Sleeping Sickness): Current Knowledge and Future Challenges. Front. Trop. Dis. 2023, 4, 1087003. [Google Scholar] [CrossRef]
- Balasegaram, M.; Balasegaram, S.; Malvy, D.; Millet, P. Neglected Diseases in the News: A Content Analysis of Recent International Media Coverage Focussing on Leishmaniasis and Trypanosomiasis. PLoS Negl. Trop. Dis. 2008, 2, e234. [Google Scholar] [CrossRef]
- Simarro, P.P.; Diarra, A.; Postigo, J.A.R.; Franco, J.R.; Jannin, J.G. The Human African Trypanosomiasis Control and Surveillance Programme of the World Health Organization 2000–2009: The Way Forward. PLoS Negl. Trop. Dis. 2011, 5, e1007. [Google Scholar] [CrossRef]
- About Sleeping Sickness|Sleeping Sickness (African Trypanosomiasis)|CDC. Available online: https://www.cdc.gov/sleeping-sickness/about/index.html (accessed on 18 August 2025).
- Ortiz-Martínez, Y.; Kouamé, M.G.; Bongomin, F.; Lakoh, S.; Henao-Martínez, A.F. Human African Trypanosomiasis (Sleeping Sickness)—Epidemiology, Clinical Manifestations, Diagnosis, Treatment, and Prevention. Curr. Trop. Med. Rep. 2023, 10, 222. [Google Scholar] [CrossRef] [PubMed]
- Trypanosomiasis, Human African (Sleeping Sickness). Available online: https://www.who.int/news-room/fact-sheets/detail/trypanosomiasis-human-african-(sleeping-sickness) (accessed on 18 August 2025).
- Franco, J.R.; Simarro, P.P.; Diarra, A.; Jannin, J.G. Epidemiology of Human African Trypanosomiasis. Clin. Epidemiol. 2014, 6, 257–275. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.R.; Brisse, S. Systematics of Trypanosomes of Medical and Veterinary Importance. In The Trypanosomiases; Cabi Publishing: Wallingford, UK, 2004; pp. 1–23. [Google Scholar] [CrossRef]
- Steverding, D. The History of African Trypanosomiasis. Parasit. Vectors 2008, 1, 3. [Google Scholar] [CrossRef]
- Desquesnes, M.; Gonzatti, M.; Sazmand, A.; Thévenon, S.; Bossard, G.; Boulangé, A.; Gimonneau, G.; Truc, P.; Herder, S.; Ravel, S.; et al. A Review on the Diagnosis of Animal Trypanosomoses. Parasit. Vectors 2022, 15, 64. [Google Scholar] [CrossRef]
- Ponte-Sucre, A. An Overview of Trypanosoma Brucei Infections: An Intense Host-Parasite Interaction. Front. Microbiol. 2016, 7, 232783. [Google Scholar] [CrossRef]
- Brun, R.; Blum, J.; Chappuis, F.; Burri, C. Human African Trypanosomiasis. Lancet 2010, 375, 148–159. [Google Scholar] [CrossRef]
- Lindner, A.K.; Priotto, G. The Unknown Risk of Vertical Transmission in Sleeping Sickness—A Literature Review. PLoS Negl. Trop. Dis. 2010, 4, e783. [Google Scholar] [CrossRef]
- Fèvre, E.M.; Wissmann, B.V.; Welburn, S.C.; Lutumba, P. The Burden of Human African Trypanosomiasis. PLoS Negl. Trop. Dis. 2008, 2, e333. [Google Scholar] [CrossRef]
- Ilemobade, A.A. Tsetse and Trypanosomosis in Africa: The Challenges, the Opportunities. Onderstepoort J. Vet. Res. 2009, 76, 35–40. [Google Scholar] [CrossRef]
- Accelerating Work to Overcome the Global Impact of Neglected Tropical Diseases—A Roadmap for Implementation. Available online: https://www.who.int/publications/i/item/WHO-HTM-NTD-2012.1 (accessed on 15 August 2025).
- Franco, J.R.; Cecchi, G.; Priotto, G.; Paone, M.; Diarra, A.; Grout, L.; Simarro, P.P.; Zhao, W.; Argaw, D. Monitoring the Elimination of Human African Trypanosomiasis at Continental and Country Level: Update to 2018. PLoS Negl. Trop. Dis. 2020, 14, e0008261. [Google Scholar] [CrossRef]
- Luintel, A.; Lowe, P.; Cooper, A.; MacLeod, A.; Büscher, P.; Brooks, T.; Brown, M. Case of Nigeria-Acquired Human African Trypanosomiasis in the United Kingdom, 2016. Emerg. Infect. Dis. 2016, 23, 1225–1227. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Estarli, M.; Barrera, E.S.A.; et al. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 Statement. Rev. Esp. Nutr. Humana Diet. 2016, 20, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Munn, Z.; MClinSc, S.M.; Lisy, K.; Riitano, D.; Tufanaru, C. Methodological Guidance for Systematic Reviews of Observational Epidemiological Studies Reporting Prevalence and Cumulative Incidence Data. Int. J. Evid. Based Healthc. 2015, 13, 147–153. [Google Scholar] [CrossRef]
- Nmorsi, O.P.G.; Isaac, C.; Igbinosa, I.B.; Umukoro, D.O.; Aitaikuru, D.P. Human African Trypanosomiasis in Endemic Focus of Abraka, Nigeria. Asian Pac. J. Trop. Med. 2010, 3, 448–450. [Google Scholar] [CrossRef]
- Karshima, S.N.; Lawal, I.A.; Okubanjo, O.O. Silent Human Trypanosoma Brucei Gambiense Infections around the Old Gboko Sleeping Sickness Focus in Nigeria. J. Parasitol. Res. 2016, 2016, 2656121. [Google Scholar] [CrossRef]
- Uba, B.V.; Aliyu, A.; Abubakar, A.; Uba, S.A.; Gidado, S.; Edukugho, A.; Anagbogu, I.; Kalejaiye, J.; Nguku, P. Knowledge and Prevalence of Human African Trypanosomiasis among Residents of Kachia Grazing Reserve, Kachia Local Government Area, Kaduna State, Nigeria, 2012. Pan Afr. Med. J. 2016, 23, 89. [Google Scholar] [CrossRef]
- Daniel, A.D.; Dadah, A.J.; Kalejaiye, J.O.; Dalhatu, A.D. Prevalence of Bovine Trypanosomiasis in Gongola State of Northern Nigeria. Rev. Elev. Med. Vet. Pays. Trop. 1993, 46, 571–574. Available online: https://pubmed.ncbi.nlm.nih.gov/7915428/ (accessed on 15 August 2025). [CrossRef]
- Enwezor, F.N.C.; Umoh, J.U.; Esievo, K.A.N.; Halid, I.; Zaria, L.T.; Anere, J.I. Survey of Bovine Trypanosomosis in the Kachia Grazing Reserve, Kaduna State, Nigeria. Vet. Parasitol. 2009, 159, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Habeeb, I.F.; Chechet, G.D.; Kwaga, J.K.P. Molecular Identification and Prevalence of Trypanosomes in Cattle Distributed within the Jebba Axis of the River Niger, Kwara State, Nigeria. Parasit. Vectors 2021, 14, 560. [Google Scholar] [CrossRef] [PubMed]
- Majekodunmi, A.O.; Fajinmi, A.; Dongkum, C.; Picozzi, K.; Thrusfield, M.V.; Welburn, S.C. A Longitudinal Survey of African Animal Trypanosomiasis in Domestic Cattle on the Jos Plateau, Nigeria: Prevalence, Distribution and Risk Factors. Parasit. Vectors 2013, 6, 239. [Google Scholar] [CrossRef] [PubMed]
- Takeet, M.I.; Fagbemi, B.O.; De Donato, M.; Yakubu, A.; Rodulfo, H.E.; Peters, S.O.; Wheto, M.; Imumorin, I.G. Molecular Survey of Pathogenic Trypanosomes in Naturally Infected Nigerian Cattle. Res. Vet. Sci. 2013, 94, 555–561. [Google Scholar] [CrossRef]
- Omeke, B.C. Pig trypanosomosis: Prevalence and significance in the endemic Middle Belt zone of southern Nigeria. Rev. Elev. Med. Vet. Pays. Trop. 1994, 47, 381–386. Available online: https://pubmed.ncbi.nlm.nih.gov/7770662/ (accessed on 15 August 2025). [CrossRef] [PubMed]
- Onah, D.N.; Ebenebe, O.O. Isolation of a Human Serum-Resistant Trypanosoma Brucei from a Naturally Infected Pig in the Nsukka Area of Enugu State. Niger. Vet. J. 2004, 24, 37–43. [Google Scholar] [CrossRef][Green Version]
- Daniel, A.D.; Joshua, R.A.; Kalejaiye, J.O.; Dada, A.J. Prevalence of Trypanosomiasis in Sheep and Goats in a Region of Northern Nigeria. Rev. D’élevage Médecine Vétérinaire Pays Trop. 1994, 47, 295–297. [Google Scholar] [CrossRef] [PubMed]
- Kalu, A.U.; Lawani, F.A. Observations on the Epidemiology of Ruminant Trypanosomosis in Kano State, Nigeria. Rev. D’élevage Médecine Vétérinaire Pays Trop. 1996, 49, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Wayo, B.; Samdi, S.M.; Fajinmi, A.O.; Bizi, R.; Dauda, H.; Muhammad, A.A.; Kalejaiye, J.O. Prevalence of Trypanosomiasis in Sheep in the Kachia Grazing Reserve, Kachia, Kaduna State, Nigeria. Afr. J. Clin. Exp. Microbiol. 2017, 18, 120. [Google Scholar] [CrossRef]
- Umeakuana, P.U.; Gibson, W.; Ezeokonkwo, R.C.; Anene, B.M. Identification of Trypanosoma Brucei Gambiense in Naturally Infected Dogs in Nigeria. Parasit. Vectors 2019, 12, 420. [Google Scholar] [CrossRef] [PubMed]
- Karshima, S.N.; Lawal, I.A.; Bata, S.I.; Barde, I.J.; Adamu, P.V.; Salihu, A.; Dross, P.N.; Obalisa, A. Animal Reservoirs of Trypanosoma Brucei Gambiense around the Old Gboko Sleeping Sickness Focus in Nigeria. J. Parasitol. Vector Biol. 2016, 8, 47–54. [Google Scholar] [CrossRef]
- Enwezor, F.N.C.; Emmanuel, R.; Olanrewaju, T.O.; Yarnap, J.E.; Bizi, R.L.; David, K.; Ezebuiro, O.G.C.; Yusuf, R.J.; Kugama, M.A.; Abubakar, S.; et al. Investigation of livestock for presence of Trypanosoma brucei gambiense in Tafa local government area of Niger state, Nigeria. Sci. World J. 2019, 14, 39–44. [Google Scholar]
- Human African Trypanosomiasis (T.b. Gambiense), Cases, Reported Number. Available online: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/hat-tb-gambiense (accessed on 18 August 2025).
- Mwiinde, A.M.; Simuunza, M.; Namangala, B.; Chama-Chiliba, C.M.; Machila, N.; Anderson, N.E.; Atkinson, P.M.; Welburn, S.C. Healthcare Management of Human African Trypanosomiasis Cases in the Eastern, Muchinga and Lusaka Provinces of Zambia. Trop. Med. Infect. Dis. 2022, 7, 270. [Google Scholar] [CrossRef]
- Selby, R.; Wamboga, C.; Erphas, O.; Mugenyi, A.; Jamonneau, V.; Waiswa, C.; Torr, S.J.; Lehane, M. Gambian Human African Trypanosomiasis in North West Uganda. Are We on Course for the 2020 Target? PLoS Negl. Trop. Dis. 2019, 13, e0007550. [Google Scholar] [CrossRef]
- Koné, M.; Kaba, D.; Kaboré, J.; Thomas, L.F.; Falzon, L.C.; Koffi, M.; Kouamé, C.M.; Ahouty, B.; Compaoré, C.F.A.; N’gouan, E.K.; et al. Passive Surveillance of Human African Trypanosomiasis in Côte d’ivoire: Understanding Prevalence, Clinical Symptoms and Signs, and Diagnostic Test Characteristics. PLoS Negl. Trop. Dis. 2021, 15, e0009656. [Google Scholar] [CrossRef]
- Odeniran, P.O.; Ademola, I.O. A Meta-Analysis of the Prevalence of African Animal Trypanosomiasis in Nigeria from 1960 to 2017. Parasit. Vectors 2018, 11, 280. [Google Scholar] [CrossRef]
- Boundenga, L.; Mombo, I.M.; Augustin, M.O.; Barthélémy, N.; Nzassi, P.M.; Moukodoum, N.D.; Rougeron, V.; Prugnolle, F. Molecular Identification of Trypanosome Diversity in Domestic Animals Reveals the Presence of Trypanosoma Brucei Gambiense in Historical Foci of Human African Trypanosomiasis in Gabon. Pathogens 2022, 11, 992. [Google Scholar] [CrossRef]
- Simo, G.; Asonganyi, T.; Nkinin, S.W.; Njiokou, F.; Herder, S. High Prevalence of Trypanosoma Brucei Gambiense Group 1 in Pigs from the Fontem Sleeping Sickness Focus in Cameroon. Vet. Parasitol. 2006, 139, 57–66. [Google Scholar] [CrossRef]
- Njiokou, F.; Nimpaye, H.; Simo, G.; Njitchouang, G.R.; Asonganyi, T.; Cuny, G.; Herder, S. Domestic Animals as Potential Reservoir Hosts of Trypanosoma Brucei Gambiense in Sleeping Sickness Foci in Cameroon. Parasite 2010, 17, 61–66. [Google Scholar] [CrossRef]
- Franco, J.R.; Simarro, P.P.; Diarra, A.; Ruiz-Postigo, J.A.; Jannin, J.G. The Journey towards Elimination of Gambiense Human African Trypanosomiasis: Not Far, nor Easy. Parasitology 2014, 141, 748–760. [Google Scholar] [CrossRef]
- Mehlitz, D.; Molyneux, D.H. The Elimination of Trypanosoma Brucei Gambiense? Challenges of Reservoir Hosts and Transmission Cycles: Expect the Unexpected. Parasite Epidemiol. Control 2019, 6, e00113. [Google Scholar] [CrossRef]
- Molyneux, D.H.; Hopkins, D.R.; Zagaria, N. Disease Eradication, Elimination and Control: The Need for Accurate and Consistent Usage. Trends Parasitol. 2004, 20, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Büscher, P.; Bart, J.M.; Boelaert, M.; Bucheton, B.; Cecchi, G.; Chitnis, N.; Courtin, D.; Figueiredo, L.M.; Franco, J.R.; Grébaut, P.; et al. Do Cryptic Reservoirs Threaten Gambiense-Sleeping Sickness Elimination? Trends Parasitol. 2018, 34, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Geerts, M.; Chen, Z.; Bebronne, N.; Savill, N.J.; Schnaufer, A.; Büscher, P.; Van Reet, N.; Van den Broeck, F. Deep Kinetoplast Genome Analyses Result in a Novel Molecular Assay for Detecting Trypanosoma Brucei Gambiense-Specific Minicircles. NAR Genom. Bioinform. 2022, 4, lqac081. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.K.; Kea, B.; Wang, R. Recognising Bias in Studies of Diagnostic Tests Part 1: Patient Selection. Emerg. Med. J. 2019, 36, 431–434. [Google Scholar] [CrossRef]
- Shreffler, J.; Huecker, M.R. Diagnostic Testing Accuracy: Sensitivity, Specificity, Predictive Values and Likelihood Ratios; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Okello, I.; Mafie, E.; Eastwood, G.; Nzalawahe, J.; Mboera, L.E.G. African Animal Trypanosomiasis: A Systematic Review on Prevalence, Risk Factors and Drug Resistance in Sub-Saharan Africa. J. Med. Entomol. 2022, 59, 1099–1143. [Google Scholar] [CrossRef]
- Picozzi, K.; Tilley, A.; Fèvre, E.M.; Coleman, P.G.; Welburn, S.C. The Diagnosis of Trypanosome Infections: Applications of Novel Technology for Reducing Disease Risk. Adv. J. Microbiol. Res. 2002, 1, 39–45. [Google Scholar]
- Contreras Garcia, M.; Walshe, E.; Steketee, P.C.; Paxton, E.; Lopez-Vidal, J.; Pearce, M.C.; Matthews, K.R.; Ezzahra-Akki, F.; Evans, A.; Fairlie-Clark, K.; et al. Comparative Sensitivity and Specificity of the 7SL SRNA Diagnostic Test for Animal Trypanosomiasis. Front. Vet. Sci. 2022, 9, 868912. [Google Scholar] [CrossRef]
- Inojosa, W.O.; Augusto, I.; Bisoffi, Z.; Josenado, T.; Abel, P.M.; Stich, A.; Whitty, C.J.M. Diagnosing Human African Trypanosomiasis in Angola Using a Card Agglutination Test: Observational Study of Active and Passive Case Finding Strategies. Br. Med. J. 2006, 332, 1479–1481. [Google Scholar] [CrossRef]
- Dukes, P.; Gibson, W.; Gashumba, J.; Hudson, K.; Bromidge, T.; Kaukus, A.; Asonganyi, T.; Magnus, E. Absence of the LiTat 1.3 (CATT Antigen) Gene in Trypanosoma Brucei Gambiense Stocks from Cameroon. Acta Trop. 1992, 51, 123–134. [Google Scholar] [CrossRef]
- Van Meirvenne, N.; Magnus, E.; Büscher, P. Evaluation of Variant Specific Trypanolysis Tests for Serodiagnosis of Human Infections with Trypanosoma Brucei Gambiense. Acta Trop. 1995, 60, 189–199. [Google Scholar] [CrossRef]
- Chappuis, F.; Loutan, L.; Simarro, P.; Lejon, V.; Büscher, P. Options for Field Diagnosis of Human African Trypanosomiasis. Clin. Microbiol. Rev. 2005, 18, 133–146. [Google Scholar] [CrossRef]
| Author/Year | Title | Aim | City/Area | Study Design | Study Population | Sample Size | Outcome Measured | Result | Prevalence Rate | Diagnostic Method | Species Identified |
| Daniel et al., 1993 [26] | Prevalence of bovine trypanosomiasis in Gongola State of Northern Nigeria | To assess the prevalence of bovine trypanosomosis in Karim Lamido and Numan local government areas of Gongola State. | Adamawa: Formerly Gongola (Karim Lamido and Numan LGAs) | Cross-sectional Survey | Cattle | 1065 | Bovine trypanosomiasis | A total of 42 (3.9%) Cattle were found to be infected with trypanosomes. Out of which 27 (64.3%) were due to T. vivax, 13 (31%) to T. congolense and 2 (4.8%) to T. brucei. | 3.90% | Microscopy | T. vivax T. congolense T. brucei |
| Daniel et al., 1994 [33] | Prevalence of trypanosomiasis in sheep and goats in a region of northern Nigeria | To investigate the sensitivity of four techniques currently in use for the parasitological diagnosis of trypanosomosis. | Bauchi (Alkaleri and Gombe LGAs) | Cross-sectional Survey | Sheep Goat | 615 (258 sheep and 357 goats) | Animal African trypanosomiasis | A total of 19 (7.4%) sheep and 18 (5.0%) goats were positive giving a total infection rate of 37 (6.0%), 22 being positive with T vivax, 9 with T. congolense and 6 with T. brucei | 6% | Microscopy | T. vivax T. congolense T. brucei |
| Enwezor et al., 2009 [27] | Survey of bovine trypanosomosis in the Kachia Grazing Reserve, Kaduna State, Nigeria | To assess the prevalence of trypanosomes in cattle at the Kachia Grazing Reserve (KGR) | Kaduna | Cross-sectional Survey | Cattle | 1293 | Bovine trypanosomiasis | A total of 109 cattle were infected with trypanosomes giving an overall prevalence of 8.4%. Out of these, 105 (96.3%) was due to T. vivax, 2 (1.9%) to T. congolense, 1 (0.9%) to T. b. brucei and a mixed infection of T. congolense and T. vivax 1 (0.9%). | 8.40% | Microscopy | T. vivax T. congolense T. brucei brucei |
| Enwezor et al., 2019 [38] | Investigation of livestock for presence of Trypanosoma brucei gambiense In Tafa Local Government Area of Niger State, Nigeria | Aimed at screening livestock for possible presence of T. b. gambiense within Tafa Local Government Area (LGA) of Niger State, Nigeria. | Niger | Cross-sectional Survey | Cattle Sheep Goats Local dogs Monkey | 460 animals (177 cattle, 209 sheep, 61 goats, 12 local dogs and 1 monkey) | Animal African trypanosomiasis | A total of 10 animals were positive for trypanosomes with an overall prevalence of 2.17%. The morphological identification indicated the presence of T. brucei, T. vivax and a mixed infection of T. brucei and T. congolense. Details by animal species showed 3 cattle (1.69%), 6 sheep (3.02%) and 1 goat (1.64%) infected with trypanosomes. Neither the dogs nor the monkeys were positive for trypanosomes. | 2.17% | Microscopy | T. brucei T. congolense T. vivax |
| Habeeb et al., 2021 [28] | Molecular identification and prevalence of trypanosomes in cattle distributed within the Jebba axis of the River Niger, Kwara state, Nigeria | To investigate the prevalence of trypanosome infection in cattle, and molecularly identified the species of trypanosomes in infected cattle and the spatial distribution of trypanosome-infected herds along the Jebba axis of the River Niger. | Kwara | Cross-sectional Survey | Cattle | 398 | Bovine trypanosomiasis | A total of 3 Cattle were positive by microscopy, representing 0.8% prevalence, while 12 samples representing 3.0% tested positive by nested PCR. With T. congolense more prevalent (50.0%). | Microscopy: 0.8% PCR: 3.0% | PCR Microscopy | T. theileri T. evansi T. simiae T. congolense T. brucei T. vivax |
| Kalu et al., 1996 [34] | Observations on the epidemiology of ruminant trypanosomosis in Kano State, Nigeria | To evaluate the prevalence of trypanosomosis among ruminants in Kano State and to elucidate aspects of the disease transmission in the area | Kano | Cross-sectional Survey | Ruminants (Cattle, sheep, goat) | 1424 (1106 cattle and 318 small ruminants) | Animal African trypanosomiasis | 59 cattle were infected with trypanosomes giving a prevalence of 5.3% with a confidence interval of ±1.3. Three out of 318 small ruminants sampled were infected (prevalence 0.9% ± 1.0). | Cattle: 5.3% (95% CI: 4.0–6.6%) Small ruminants (95% CI: −0.1–1.9%) | Microscopy | T. vivax T. congolense T. brucei |
| Karshima et al., 2016 [24] | Silent Human Trypanosoma brucei gambiense Infections around the Old Gboko Sleeping Sickness Focus in Nigeria | To conduct an active screening of T. b. gambiense in humans in the old Gboko sleeping sickness focus in Nigeria and characterized isolates using TgsGP-polymerase chain reaction. | Gboko | Cross-sectional Survey | Humans | 1200 | Human African trypanosomiasis | Out of sampled people, a total of 28 were found positive. The CATT revealed an overall infection rate of 1.8% of the 1200 samples studied. PCR revealed an overall infection rate of 0.6% of the 1200 samples analyzed. Trypanosomes (TbG) were isolated from 7 of the samples | CATT: 1.8% Parasite isolation/PCR: 0.6% | CATT PCR | T. b. gambiense |
| Karshima et al., 2016 [37] | Animal reservoirs of Trypanosoma brucei gambiense around the old Gboko sleeping sickness focus in Nigeria | To ascertain the possible role of animal reservoirs in the epidemiology of the parasite in the old Gboko sleeping sickness focus in Nigeria and characterized isolates using TgsGP polymerase chain reaction. | Gboko | Cross-sectional Survey | Cattle Pigs | Animal African trypanosomiasis | A total of 118 animals were positive (46 cattle, 72 pigs). The overall infection rates for the CATT and TgsGP-PCR were 8.9 and 0.9%, respectively. Trypanosomes of animal origin identified by ITS 1 PCR were T. brucei (4.2%), T. congolense forest (3.2%), T. congolense savannah (2.0%), T. vivax (2.2%) and mixed infections (1.5%) in cattle as well as T. brucei (4.8%), T. congolense forest (1.8%), T. congolense savannah (1.0%) and mixed infections (1.2%) in pigs. T. brucei gambiense and other animal trypanosomes were identified among animals in the focus, indicating the existence of animal reservoirs of human infective T. b. gambiense. | CATT: 8.9% PCR: 0.9% | CATT PCR | T. brucei (T. b. gambiense) T. congolense forest T. congolense savannah T. vivax | |
| Majekodunmi et al., 2013 [29] | A longitudinal survey of African animal trypanosomiasis in domestic cattle on the Jos Plateau, Nigeria: Prevalence, distribution, and risk factors | To determine seasonal variations in the prevalence of AAT across the Jos Plateau | Jos | Cross-sectional Survey | Cattle | 7143 | Bovine trypanosomiasis | 3, 342 cattle were found positive. The prevalence of bovine trypanosomiasis was found to be high at 46.8% (39.0–54.5%) and significant, seasonal variation was observed between the dry and the end of the wet season. T. b. brucei was observed at a prevalence of 3.2% (1–5.5%); T. congolense at 27.7% (21.8–33.6%) and T. vivax at 26.7% (18.2–35.3%). | 46.8% (39.0–54.5%) | PCR | T. congolense T. vivax T. b. brucei |
| Nmorsi et al., 2010 [23] | Human African trypanosomiasis in endemic focus of Abraka, Nigeria | To investigate the prevalence of human African trypanosomiasis (HAT), caused by Trypanosoma brucei gambiense in an endemic focus of Nigeria, as it relates to age, sex and occupational differences. | Abraka, Delta | Cross-sectional Survey | Humans | 474 | Human African trypanosomiasis | Of the 474 screened, 44(9.3%) were seropositive with seroprevalence of 22(9.6%) in Urhouka, 14(9.5%) in Umeghe and 8(7.9%) for Ugonu. | CATT: 9.30% Microscopy (blood): 3.4% Microscopy (CSF): 0.8% | Microscopy CATT | T. b. gambiense |
| Omeke, 1994 [31] | Pig trypanosomosis: prevalence and significance in the endemic Middle Belt zone of southern Nigeria | To determine the prevalence of trypanosome species pathogenic to pigs and the significance of pig trypanosomosis in the Middle Belt zone of Southern Nigeria. | Anambra and Benue states | Cross-sectional Survey | Pigs | 1954 | Porcine trypanosomiasis | 524 (26.8%) were positive for trypanosome infections, 348 (66.5%) of which had a mixed T. brucei and T. congolense burden, while 125 (23.9%) and 43 (8.2%) others had single T. brucei and T. congolense infections, respectively. | 26.80% | Microscopy | T. congolense T. brucei |
| Onah and Ebenebe 2004 [32] | Isolation of a human serum-resistant Trypanosoma brucei from a naturally infected pig in the Nsukka area of Enugu State | To re-evaluate the role of domestic pig as a reservoir for T. b. gambiense | Enugu | Cross-sectional Survey | Pigs | 85 | Porcine trypanosomiasis | 19 positive cases were identified from the 85 sampled pigs. 15 (78.96%) were identified as single infection caused by T. brucei. While the remaining 4 (21.05%) were due to mixed infections of T. brucei and T. congolense. | 22.35% | Microscopy | T. brucei T. congolense T. brucei gambiense |
| Takeet et al., 2013 [30] | Molecular survey of pathogenic trypanosomes in naturally infected Nigerian cattle | To determine the prevalence and characteristics of trypanosome species and strains in Nigerian cattle using PCR for the first time. | Ogun and Kaduna states | Cross-sectional Survey | Cattle | 411 | Bovine trypanosomiasis | Parasite detection by microscopy observation showed 62 samples infected by one or more species of Trypanosomes, for a prevalence of 15.1% (95% CI, 12–18%). PCR detection showed 262 samples infected by one or more species of Trypanosoma, for an overall prevalence of 63.7% (95% CI, 59.4–68.8%) and T congolense was the most prevalent 48.7% (95% CI, 4.2–54.3), followed by T. vivax 26.0% (95% CI, 21.8–31.1%) and T. brucei 4.4% (95% CI, 3.3–7.1%). Prevalence of mixed infections was 13.9% (95% CI, 10.6–17.4%) being co-infection by T. congolense and T. vivax. | Microscopy: 15.1% (95% CI, 12–18%). PCR: 63.7% (95% CI, 59.4–68.8%) | Microscopy PCR | T. brucei T. vivax T. congolense |
| Uba et al., 2016 [25] | Knowledge and prevalence of human african trypanosomiasis among residents of kachia grazing reserve, Kachia local government area, Kaduna state, Nigeria, 2012 | To determine the knowledge, practices and prevalence of HAT among residents of the grazing reserve. | Kaduna | Cross-sectional Survey | Humans | 300 | Human African Trypanosomiasis | Of the 300 respondents that were examined and screened for HAT, none had palpable cervical lymph nodes enlargement, and none tested positive on CATT; hence HAT prevalence was zero. | 0.00% | CATT | |
| Umeakuana et al., 2019 [36] | Identification of Trypanosoma brucei gambiense in naturally infected dogs in Nigeria | To determine which trypanosome species, cause canine trypanosomosis in the Nsukka area of Nigeria and whether any dogs harbor the human-infective trypanosome, Tbg1. | Nsukka | Cross-sectional Survey | Dogs | 19 | Canine African Trypanosomiasis (CAT) | All 19 dogs sampled had canine trypanosomosis caused by trypanosomes of the T. brucei group. Two of the dogs were shown to be infected with the human pathogen T. b. gambiense Group 1 (Tbg1). | Tbg: 10.52% | PCR | T. b. gambiense T. brucei T. congolense |
| Wayo, 2017 [35] | Prevalence of trypanosomiasis in sheep in the Kachia grazing reserve, Kachia, Kaduna State, Nigeria | To update information on the prevalence of trypanosomiasis in small ruminants in the area, to allow for proper planning of control activities and serve as valuable scientific data. | Kaduna | Cross-sectional Survey | Sheep | 110 | Animal African trypanosomiasis | A total of 45 (40.9%) animals were found positive. The trypanosomes observed were T. congolense (40.0%), T. Brucei (28.8%), T. vivax (17.7%) and mixed infections (13.3%) | 40.9 | Microscopy | T. brucei T. vivax T. congolense |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chukwudi, C.; Odebunmi, E.; Ibeachu, C. Prevalence of Human and Animal African Trypanosomiasis in Nigeria: A Scoping Review. Parasitologia 2025, 5, 53. https://doi.org/10.3390/parasitologia5040053
Chukwudi C, Odebunmi E, Ibeachu C. Prevalence of Human and Animal African Trypanosomiasis in Nigeria: A Scoping Review. Parasitologia. 2025; 5(4):53. https://doi.org/10.3390/parasitologia5040053
Chicago/Turabian StyleChukwudi, Chinwe, Elizabeth Odebunmi, and Chukwuemeka Ibeachu. 2025. "Prevalence of Human and Animal African Trypanosomiasis in Nigeria: A Scoping Review" Parasitologia 5, no. 4: 53. https://doi.org/10.3390/parasitologia5040053
APA StyleChukwudi, C., Odebunmi, E., & Ibeachu, C. (2025). Prevalence of Human and Animal African Trypanosomiasis in Nigeria: A Scoping Review. Parasitologia, 5(4), 53. https://doi.org/10.3390/parasitologia5040053







