Monthly and Daily Dynamics of Stomoxys calcitrans (Linnaeus, 1758) (Diptera: Muscidae) in Livestock Farms of the Batna Region (Northeastern Algeria)
Abstract
1. Background
2. Methods
2.1. Animals and Study Area
2.2. Flies Trapping
2.3. Flies Identification and Data Analysis
2.4. Climatic Data
3. Results
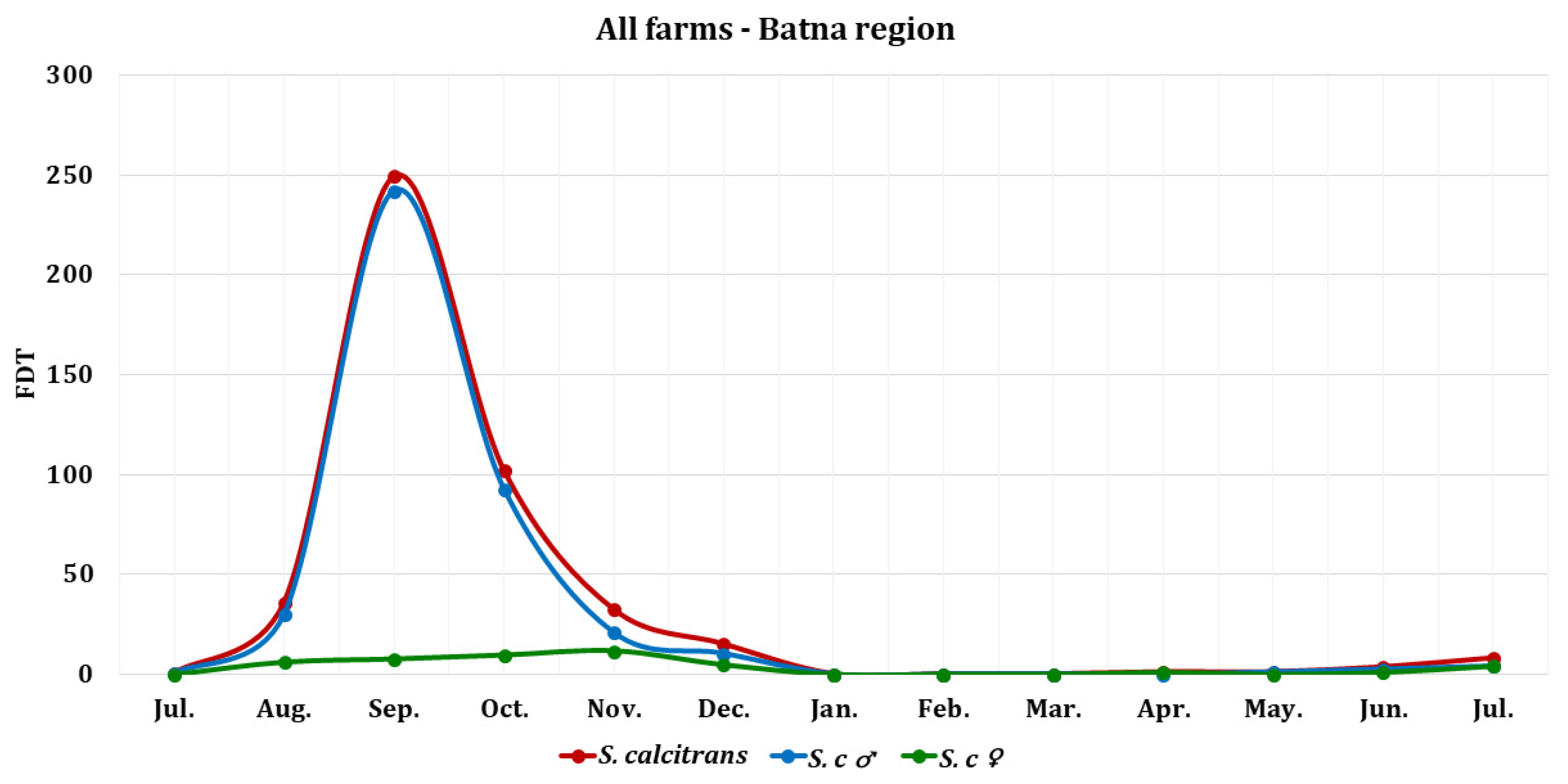
3.1. S.calcitrans Abundance
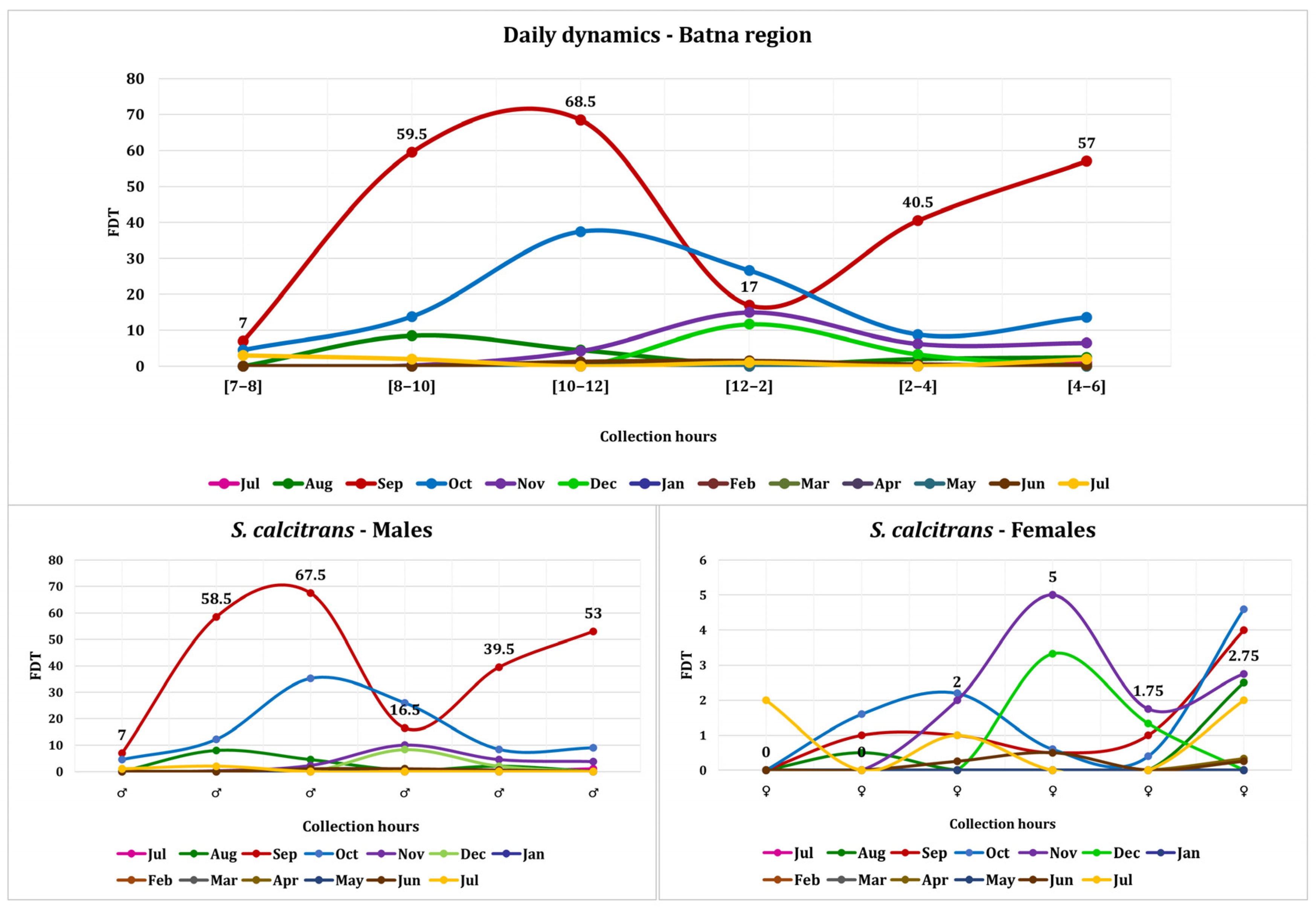
3.2. S. calcitrans Activity
3.3. S. calcitrans Abundance Among Farms
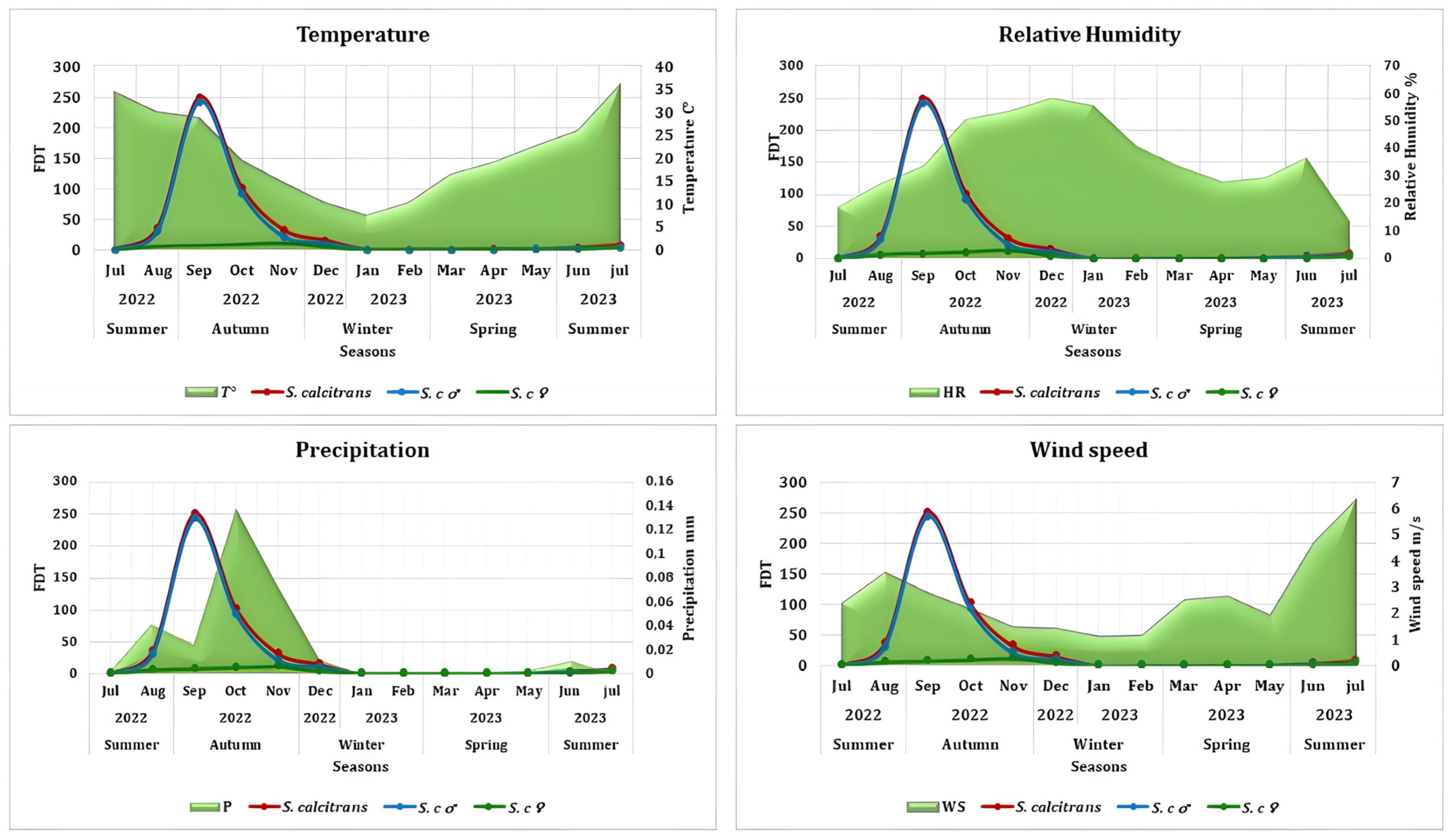
3.4. Influence of Climatic Factors
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moreki, J.C.; Tjinyeka, K.; Makore, J.; Tlotleng, K.; Moseki, M.I. The impact of stable flies (Stomoxys calcitrans L.) on small stock production in Bodibeng, Bothatogo and Sehithwa in the North West district, Botswana; a survey study. Online J. Anim. Feed Res. 2022, 12, 73–80. [Google Scholar] [CrossRef]
- Chalchisa, A.; Kumsa, B.; Gutema Wegi, F. Biting Flies and Associated Pathogens in Camels in Amibara District of Afar Region, Ethiopia. Vet. Med. Int. 2024, 2024, 5407898. [Google Scholar] [CrossRef] [PubMed]
- Rochon, K.; Hogsette, J.; Kaufman, P.; Olafson, P.; Swiger, S.; Taylor, D. Stable fly (Diptera: Muscidae)—Biology, management, and research needs. J. Integr. Pest Manag. 2021, 12, 38. [Google Scholar] [CrossRef]
- Duvallet, G.; Hogsette, J.A. Global Diversity, Distribution, and Genetic Studies of Stable Flies (Stomoxys sp.). Diversity 2023, 15, 600. [Google Scholar] [CrossRef]
- Dsouli-Aymes, N.; Michaux, J.; De Stordeur, E.; Couloux, A.; Veuille, M.; Duvallet, G. Global population structure of the stable fly (Stomoxys calcitrans) inferred by mitochondrial and nuclear sequence data. Infect. Genet. Evol. 2011, 11, 334–342. [Google Scholar] [CrossRef]
- Abbas, K.H.; Alfatlawi, M.; Ali, M. Pests of Livestock: I-Stomoxys calcitrans (Insecta: Diptera: Muscidae) (Stable fly). HIV Nurs. 2022, 22, 1237–1240. [Google Scholar]
- Parravani, A.; Chivers, C.A.; Bell, N.; Long, S.; Burden, F.; Wall, R. Seasonal abundance of the stable fly Stomoxys calcitrans in southwest England. Med. Vet. Entomol. 2019, 33, 485–490. [Google Scholar] [CrossRef]
- Campbell, J.; Skoda, S.; Berkebile, D.; Boxler, D.; Thomas, G.; Adams, D.; Davis, R. Effects of stable flies (Diptera: Muscidae) on weight gains of grazing yearling cattle. J. Econ. Entomol. 2001, 94, 780–783. [Google Scholar] [CrossRef]
- González, M.A.; Bravo-Barriga, D.; Fernández, E.B.; Frontera, E.; Ruiz-Arrondo, I. Severe Skin lesions caused by persistent bites of the stable fly Stomoxys calcitrans (Diptera: Muscidae) in a donkey sanctuary of Western Spain. J. Equine Vet. Sci. 2022, 116, 104056. [Google Scholar] [CrossRef]
- Semelbauer, M.; Mangová, B.; Barta, M.; Kozánek, M. The factors influencing seasonal dynamics and spatial distribution of stable fly Stomoxys calcitrans (Diptera, Muscidae) within stables. Insects 2018, 9, 142. [Google Scholar] [CrossRef]
- Lendzele, S.S.; François, M.J.; Roland, Z.-K.C.; Armel, K.A.; Duvallet, G. Factors influencing seasonal and daily dynamics of the genus Stomoxys Geoffroy, 1762 (Diptera: Muscidae), in the Adamawa Plateau, Cameroon. Int. J. Zool. 2019, 2019, 3636943. [Google Scholar] [CrossRef]
- Khalifa, A.; Nasr, Z.; Errouissi, F. First data on the daily and seasonal activity patterns of Stomoxys calcitrans (Diptera: Muscidae) under Mediterranean semiarid climate in a dairy cattle farm in Tunisia. Int. J. Trop. Insect Sci. 2022, 42, 1437–1447. [Google Scholar] [CrossRef]
- Schofield, S.; Brady, J. Circadian activity pattern in the stable fly, Stomoxys calcitrans. Physiol. Entomol. 1996, 21, 159–163. [Google Scholar] [CrossRef]
- Showler, A.T.; Osbrink, W.L. Stable fly, Stomoxys calcitrans (L.), dispersal and governing factors. Int. J. Insect Sci. 2015, 7, 19–25. [Google Scholar] [CrossRef]
- Olafson, P.U.; Aksoy, S.; Attardo, G.M.; Buckmeier, G.; Chen, X.; Coates, C.J.; Davis, M.; Dykema, J.; Emrich, S.J.; Friedrich, M. The genome of the stable fly, Stomoxys calcitrans, reveals potential mechanisms underlying reproduction, host interactions, and novel targets for pest control. BMC Biol. 2021, 19, 41. [Google Scholar] [CrossRef]
- Makhahlela, N.B.; Liebenberg, D.; Van Hamburg, H.; Taioe, M.O.; Onyiche, T.; Ramatla, T.; Thekisoe, O.M. Detection of pathogens of veterinary importance harboured by Stomoxys calcitrans in South African feedlots. Sci. Afr. 2022, 15, e01112. [Google Scholar] [CrossRef]
- Levchenko, M.A.; Silivanova, E.A. Stomoxys calcitrans (Diptera: Muscidae): Value for Veterinary Medicine. Review. Russ. J. Parasitol. 2020, 14, 40–52. [Google Scholar] [CrossRef]
- Patra, G.; Behera, P.; Das, S.K.; Saikia, B.; Ghosh, S.; Biswas, P.; Kumar, A.; Alam, S.S.; Kawlni, L.; Lalnunpuia, C. Stomoxys calcitrans and its importance in livestock. Int. J. Adv. Agric. Res. 2018, 6, 30–37. [Google Scholar]
- Baldacchino, F.; Muenworn, V.; Desquesnes, M.; Desoli, F.; Charoenviriyaphap, T.; Duvallet, G. Transmission of pathogens by Stomoxys flies (Diptera, Muscidae): A review. Parasite 2013, 20, 26. [Google Scholar] [CrossRef] [PubMed]
- Araújo, T.R.; Júnior, M.A.L.M.; Vilela, T.S.; Bittecourt, A.J.; Santos, H.A.; Fampa, P. First report of the presence of Anaplasma marginale in different tissues of the stable-fly Stomoxys calcitrans (Linnaeus, 1758) in Rio de Janeiro state, Brazil. Vet. Parasitol. Reg. Stud. Rep. 2021, 23, 100515. [Google Scholar] [CrossRef]
- Hornok, S.; Takács, N.; Szekeres, S.; Szőke, K.; Kontschán, J.; Horváth, G.; Sugár, L. DNA of Theileria orientalis, T. equi and T. capreoli in stable flies (Stomoxys calcitrans). Parasites Vectors 2020, 13, 186. [Google Scholar] [CrossRef]
- Sharif, S.; Jacquiet, P.; Prevot, F.; Grisez, C.; Raymond-Letron, I.; Semin, M.; Geffré, A.; Trumel, C.; Franc, M.; Bouhsira, É. Stomoxys calcitrans, mechanical vector of virulent Besnoitia besnoiti from chronically infected cattle to susceptible rabbit. Med. Vet. Entomol. 2019, 33, 247–255. [Google Scholar] [CrossRef]
- Cook, C.G.; Munyanduki, H.; Fay, P.C.; Wijesiriwardana, N.; Moffat, K.; Gubbins, S.; Armstrong, S.; Batten, C.; Dietrich, I.; Greaves, D.R. The mechanical arthropod vector Stomoxys calcitrans influences the outcome of lumpy skin disease virus infection in cattle. bioRxiv 2023. [Google Scholar] [CrossRef]
- González, M.A.; Ruiz-Arrondo, I.; Bravo-Barriga, D.; Cervera-Acedo, C.; Santibañez, P.; Oteo, J.A.; Miranda, M.Á.; Barceló, C. Surveillance and screening in Stomoxyinae flies from Mallorca Island (Spain) reveal the absence of selected pathogens but confirms the presence of the endosymbiotic bacterium Wolbachia pipientis. Res. Vet. Sci. 2024, 171, 105206. [Google Scholar] [CrossRef] [PubMed]
- Haegeman, A.; Sohier, C.; Mostin, L.; De Leeuw, I.; Van Campe, W.; Philips, W.; De Regge, N.; De Clercq, K. Evidence of Lumpy Skin Disease Virus Transmission from Subclinically Infected Cattle by Stomoxys calcitrans. Viruses 2023, 15, 1285. [Google Scholar] [CrossRef]
- Cook, D. A historical review of management options used against the stable fly (Diptera: Muscidae). Insects 2020, 11, 313. [Google Scholar] [CrossRef] [PubMed]
- Leesombun, A.; Sungpradit, S.; Boonmasawai, S.; Weluwanarak, T.; Klinsrithong, S.; Ruangsittichai, J.; Ampawong, S.; Masmeatathip, R.; Changbunjong, T. Insecticidal activity of Plectranthus amboinicus essential oil against the stable fly Stomoxys calcitrans (Diptera: Muscidae) and the horse fly Tabanus megalops (Diptera: Tabanidae). Insects 2022, 13, 255. [Google Scholar] [CrossRef]
- Akash; Shilpa; Ravindra, B.G.; Halmandge, S.; Kumar, R.T.; Kasaralikar, V.R.; Kumar, D.D. Evaluation of neem oil and lemon grass oil as natural fly repellant on lumpy skin disease vector in buffalo farms. Pharma Innov. J. 2022, 11, 389–392. [Google Scholar]
- González, M.A.; Duvallet, G.; Morel, D.; de Blas, I.; Barrio, E.; Ruiz-Arrondo, I. An Integrated Pest Management Strategy Approach for the Management of the Stable Fly Stomoxys calcitrans (Diptera: Muscidae). Insects 2024, 15, 222. [Google Scholar] [CrossRef]
- Beresford, D.; Sutcliffe, J. Evidence for sticky-trap avoidance by stable fly, Stomoxys calcitrans (Diptera: Muscidae), in response to trapped flies. J. Am. Mosq. Control Assoc. 2017, 33, 250–252. [Google Scholar] [CrossRef]
- Jacquiet, P.; Rouet, D.; Bouhsira, E.; Salem, A.; Lienard, E.; Franc, M. Population dynamics of Stomoxys calcitrans (L.) (Diptera: Muscidae) in southwestern France. Rev. Med. Vet. 2014, 165, 267–271. [Google Scholar]
- Phasuk, J.; Prabaripai, A.; Chareonviriyaphap, T. Seasonality and daily flight activity of stable flies (Diptera: Muscidae) on dairy farms in Saraburi Province, Thailand. Parasite 2013, 20, 17. [Google Scholar] [CrossRef]
- Rodríguez-Batista, Z.; Leite, R.; Oliveira, P.; Lopes, C.; Borges, L. Populational dynamics of Stomoxys calcitrans (Linneaus) (Diptera: Muscidae) in three biocenosis, Minas Gerais, Brazil. Vet. Parasitol. 2005, 130, 343–346. [Google Scholar] [CrossRef]
- Taylor, D.B.; Moon, R.D.; Mark, D.R. Economic impact of stable flies (Diptera: Muscidae) on dairy and beef cattle production. J. Med. Entomol. 2012, 49, 198–209. [Google Scholar] [CrossRef]
- Gilles, J.; David, J.F.; Duvallet, G.; De La Rocque, S.; Tillard, E. Efficiency of traps for Stomoxys calcitrans and Stomoxys niger niger on Reunion Island. Med. Vet. Entomol. 2007, 21, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Machtinger, E.; Leppla, N.; Hogsette, J. House and stable fly seasonal abundance, larval development substrates, and natural parasitism on small equine farms in Florida. Neotrop. Entomol. 2016, 45, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Boucheikhchoukh, M.; Mechouk, N.; Benakhla, A.; Raoult, D.; Parola, P. Molecular evidence of bacteria in Melophagus ovinus sheep keds and Hippobosca equina forest flies collected from sheep and horses in northeastern Algeria. Comp. Immunol. Microbiol. Infect. Dis. 2019, 65, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Zeghouma, D.; Bouslama, Z.; Duvallet, G.; Amr, Z.S. Horse flies and their seasonal abundance in El Tarf Province of northeastern Algeria. J. Vector Ecol. 2018, 43, 305–311. [Google Scholar] [CrossRef]
- Cherairia, M.; Adler, P.H.; Samraoui, B. Biodiversity and bionomics of the black flies (Diptera: Simuliidae) of northeastern Algeria. Zootaxa 2014, 3796, 166–174. [Google Scholar] [CrossRef]
- Lafri, I.; Bitam, I. Phlebotomine sandflies and associated pathogens in Algeria: Update and comprehensive overview. Vet. Ital. 2021, 57, 175–180. [Google Scholar]
- Kadjoudj, N.; Bounamous, A.; Kouba, Y.; Dik, B.; Zeroual, S.; Amira, A.; Chenchouni, H. Composition and diversity of Culicoides biting midges (Diptera: Ceratopogonidae) in rural and suburban environments of Algeria. Acta Trop. 2022, 234, 106588. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Laveissière, C. Les glossines vectrices de la trypanosomiase africaine. In Biologie et Contröle; WHO: Geneva, Switzerland; UBC: Vancouver, BC, Canada, 1988. [Google Scholar]
- Zumpt, F. The Stomoxyine Biting Flies of the World: Diptera, Muscidae; Taxonomy, Biology, Economic Importance and Control Measures; Fischer: Stuttgart, Germany, 1973. [Google Scholar]
- Malaithong, N.; Duvallet, G.; Ngoen-Klan, R.; Bangs, M.J.; Chareonviriyaphap, T. Stomoxyinae flies in Thailand: A precis, with abridged taxonomic key to the adult species. Vector-Borne Zoonotic Dis. 2019, 19, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Power, N. Data Access Viewer. 2022. Available online: https://power.larc.nasa.gov/data-access-viewer/ (accessed on 1 July 2022).
- Kahana-Sutin, E.; Klement, E.; Lensky, I.; Gottlieb, Y. High relative abundance of the stable fly Stomoxys calcitrans is associated with lumpy skin disease outbreaks in Israeli dairy farms. Med. Vet. Entomol. 2017, 31, 150–160. [Google Scholar] [CrossRef]
- Zohra, L.; Ferroudja, M.-B.; Bahia, D.-M.; Salaheddine, D. Bioecology of culicidae (Diptera: Nematocera) in north central Algeria. Bull. Pure Appl. Sci.-Zool. 2022, 41, 86–100. [Google Scholar] [CrossRef]
- Adler, P.H.; Haouchine, S.; Belqat, B.; Lounaci, A. North African Endemism: A New Species of Black Fly (Diptera: Simuliidae) from the Djurdjura Mountains of Algeria. Insects 2024, 15, 150. [Google Scholar] [CrossRef]
- Tunnakundacha, S.; Desquesnes, M.; Masmeatathip, R. Comparison of Vavoua, Malaise and Nzi traps with and without attractants for trapping of Stomoxys spp.(Diptera: Muscidae) and tabanids (Diptera: Tabanidae) on cattle farms. Agric. Nat. Resour. 2017, 51, 319–323. [Google Scholar] [CrossRef]
- Mihok, S.; Kang’Ethe, E.K.; Kamau, G.K. Trials of traps and attractants for Stomoxys spp.(Diptera: Muscidae). J. Med. Entomol. 1995, 32, 283–289. [Google Scholar] [CrossRef]
- Murchie, A.K.; Hall, C.E.; Gordon, A.W.; Clawson, S. Black Border Increases Stomoxys calcitrans Catch on White Sticky Traps. Insects 2018, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Malaithong, N.; Duvallet, G.; Nararak, J.; Ngoen-Klan, R.; Tainchum, K.; Chareonviriyaphap, T. Comparison of stable fly (Diptera: Muscidae) population dynamics on a cattle farm and at an open zoo in Thailand. Agric. Nat. Resour. 2021, 55, 359–366. [Google Scholar]
- Warnes, M.; Finlayson, L. Effect of host behaviour on host preference in Stomoxys calcitrans. Med. Vet. Entomol. 1987, 1, 53–57. [Google Scholar] [CrossRef]
- Khwanket, N.; Tainchum, K.; Chareonviriyaphap, T.; Ngoen-Klan, R.; Noosidum, A. Preferences for livestock bedding as a development substrate of the stable fly, Stomoxys calcitrans L.(Diptera: Muscidae), and potential application of entomopathogenic nematodes for controlling stable fly larvae. Med. Vet. Entomol. 2024, 38, 429–439. [Google Scholar] [CrossRef]
- Nayani, S.A.; Meraj, S.; Mohr, E.; Gries, R.; Kovacs, E.; Devireddy, A.; Gries, G. Staphylococcus microbes in the bovine skin microbiome attract blood-feeding stable flies. Front. Ecol. Evol. 2023, 11, 1212222. [Google Scholar] [CrossRef]
- Kovacs, E.M.; Pinard, C.; Gries, R.; Manku, A.; Gries, G. Carbon Dioxide, Methane, and Synthetic Cattle Breath Volatiles Attract Host-Seeking Stable Flies, Stomoxys calcitrans. J. Chem. Ecol. 2024, 50, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.C.; Moon, R.D.; Endres, M.I.; Pereira, G.M.; Heins, B.J. The Defensive Behaviors and Milk Production of Pastured Dairy Cattle in Response to Stable Flies, Horn Flies, and Face Flies. Animals 2023, 13, 3847. [Google Scholar] [CrossRef]
- Baleba, S.B.; Torto, B.; Masiga, D.; Getahun, M.N.; Weldon, C.W. Stable flies, Stomoxys calcitrans L.(Diptera: Muscidae), improve offspring fitness by avoiding oviposition substrates with competitors or parasites. Front. Ecol. Evol. 2020, 8, 5. [Google Scholar] [CrossRef]
- Lysyk, T. Seasonal abundance of stable flies and house flies (Diptera: Muscidae) in dairies in Alberta, Canada. J. Med. Entomol. 1993, 30, 888–895. [Google Scholar] [CrossRef]
- Karam, G. Population Dynamics and Overwintering Capabilities of the Stable Fly Stomoxys calcitrans (L.) (Diptera: Muscidae) and Their Pupal Parasitoids (Hymenoptera: Pteromalidae, Ichneumonidae) on Dairy Operations in Southern Manitoba. Master’s Thesis, Department of Entomology, University of Manitoba, Winnipeg, MB, Canada, 2020. [Google Scholar]
- Skovgård, H.; Nachman, G. Population dynamics of stable flies Stomoxys calcitrans (Diptera: Muscidae) at an organic dairy farm in Denmark based on mark-recapture with destructive sub-sampling. Environ. Entomol. 2012, 41, 20–29. [Google Scholar] [CrossRef]
- Masmeatathip, R.; Gilles, J.; Ketavan, C.; Duvallet, G. First survey of seasonal abundance and daily activity of Stomoxys spp.(Diptera: Muscidae) in Kamphaengsaen Campus, Nakornpathom Province, Thailand. Parasite 2006, 13, 245–250. [Google Scholar] [CrossRef]
- Issimov, A.; Taylor, D.B.; Zhugunissov, K.; Kutumbetov, L.; Zhanabayev, A.; Kazhgaliyev, N.; Akhmetaliyeva, A.; Nurgaliyev, B.; Shalmenov, M.; Absatirov, G. The combined effects of temperature and relative humidity parameters on the reproduction of Stomoxys species in a laboratory setting. PLoS ONE 2020, 15, e0242794. [Google Scholar] [CrossRef] [PubMed]
- Skovgård, H.; Nachman, G. Modeling the temperature-and age-dependent survival, development, and oviposition rates of stable flies (Stomoxys calcitrans) (Diptera: Muscidae). Environ. Entomol. 2017, 46, 1130–1142. [Google Scholar] [CrossRef]
- Lysyk, T. Relationships between temperature and life-history parameters of Stomoxys calcitrans (Diptera: Muscidae). J. Med. Entomol. 1998, 35, 107–119. [Google Scholar] [CrossRef]
- Gilles, J.; David, J.-F.; Duvallet, G. Temperature effects on development and survival of two stable flies, Stomoxys calcitrans and Stomoxys niger niger (Diptera: Muscidae), in La Reunion Island. J. Med. Entomol. 2005, 42, 260–265. [Google Scholar] [CrossRef]
- Beresford, D.; Sutcliffe, J. The effect of Macrocheles muscaedomesticae and M. subbadius (Acarina: Macrochelidae) phoresy on the dispersal of Stomoxys calcitrans (Diptera: Muscidae). Syst. Appl. Acarol. Spec. Publ. 2009, 23, 1–30. [Google Scholar] [CrossRef][Green Version]
- Azevedo, L.H.; Ferreira, M.P.; Castilho, R.d.C.; Cançado, P.H.D.; de Moraes, G.J. Potential of Macrocheles species (Acari: Mesostigmata: Macrochelidae) as control agents of harmful flies (Diptera) and biology of Macrocheles embersoni Azevedo, Castilho and Berto on Stomoxys calcitrans (L.) and Musca domestica L. (Diptera: Muscidae). Biol. Control 2018, 123, 1–8. [Google Scholar] [CrossRef]
- Dawit, L.; Addis, M.; Gari, G. Distribution, seasonality and relative abundance of Stomoxys flies in selected districts of central Ethiopia. World Appl. Sci. J. 2012, 19, 998–1002. [Google Scholar]
- Taylor, D.B.; Berkebile, D.R.; Scholl, P.J. Stable fly population dynamics in eastern Nebraska in relation to climatic variables. J. Med. Entomol. 2007, 44, 765–771. [Google Scholar] [CrossRef][Green Version]
- Nayani, S.; Gries, R.; Blake, A.; Gries, G. Abiotic characteristics and organic constituents of oviposition sites as oviposition attractants and stimulants for gravid female stable flies, Stomoxys calcitrans. Entomol. Exp. Appl. 2024, 172, 96–110. [Google Scholar] [CrossRef]
- Muenworn, V.; Duvallet, G.; Thainchum, K.; Tuntakom, S.; Tanasilchayakul, S.; Prabaripai, A.; Akratanakul, P.; Sukonthabhirom, S.; Chareonviriyaphap, T. Geographic Distribution of Stomoxyine Flies (Diptera: Muscidae) and Diurnal Activity of Stomoxys calcitrans in Thailand. J. Med. Entomol. 2010, 47, 791–797. [Google Scholar] [CrossRef]
- Mihok, S.; Clausen, P.H. Feeding habits of Stomoxys spp. stable flies in a Kenyan forest. Med. Vet. Entomol. 1996, 10, 392. [Google Scholar] [CrossRef]
- Berry, I.; Campbell, J. Time and weather effects on daily feeding patterns of stable flies (Diptera: Muscidae). Environ. Entomol. 1985, 14, 336–342. [Google Scholar] [CrossRef]
- Müller, G.; Hogsette, J.; Beier, J.; Traore, S.; Toure, M.; Traore, M.; Bah, S.; Doumbia, S.; Schlein, Y. Attraction of Stomoxys sp. to various fruits and flowers in Mali. Med. Vet. Entomol. 2012, 26, 178–187. [Google Scholar] [CrossRef] [PubMed]



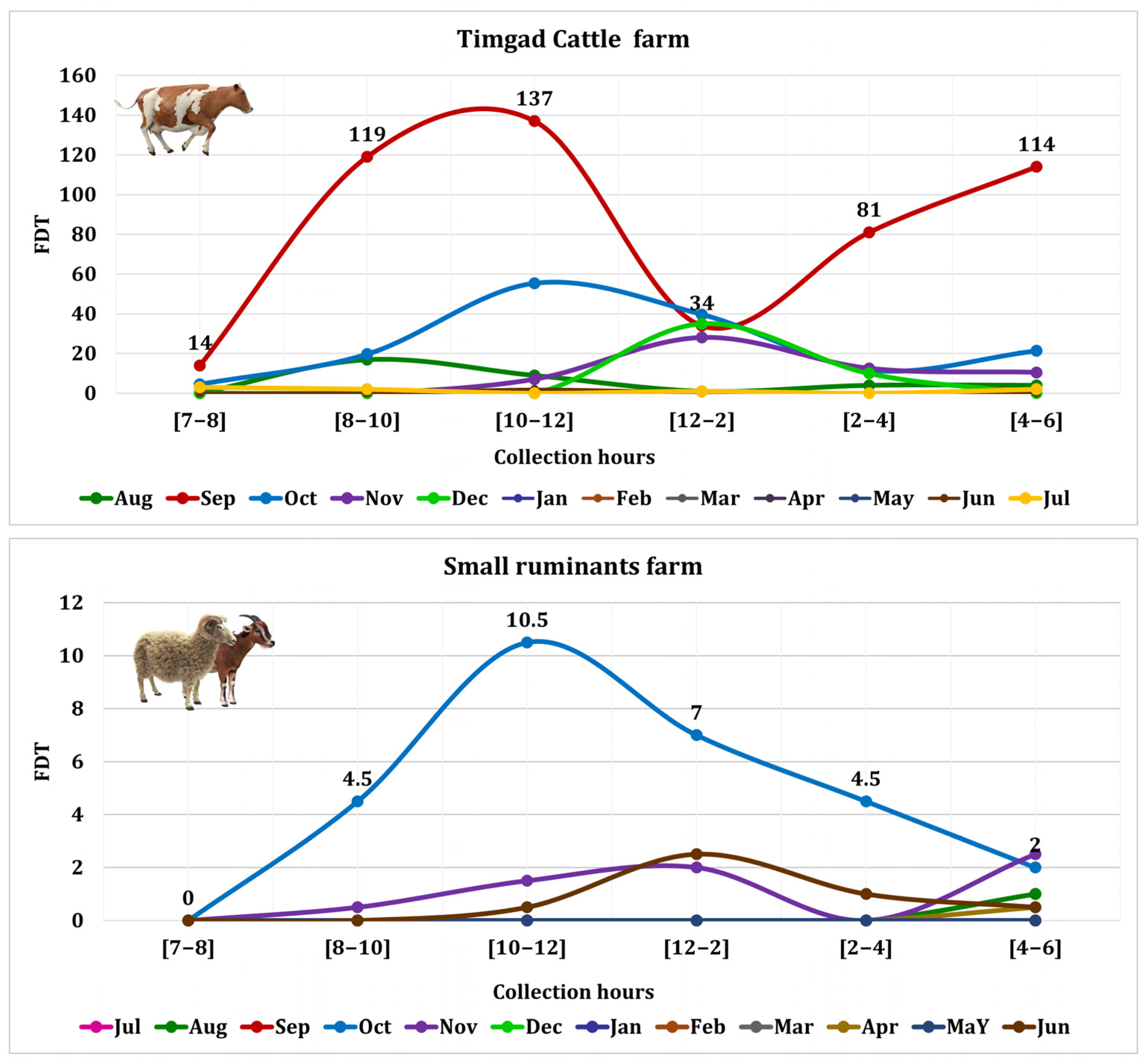
| Cattle | Small Ruminants | |||
|---|---|---|---|---|
| N | FDT | N | FDT | |
| S. calcitrans | 1162 | 68.35 | 82 | 5.85 |
| S. calcitrans ♂ | 1030 | 60.58 | 74 | 5.29 |
| S. calcitrans ♀ | 132 | 7.76 | 8 | 0.57 |
| Temperature | Precipitation | Wind Speed | Relative Humidity | ||
|---|---|---|---|---|---|
| Stomoxys calcitrans | Correlation coefficient | 0.290 | 0.153 | 0.034 | −0.035 |
| Spearman’s p value | 0.000 | 0.034 | 0.637 | 0.625 | |
| Males S. calcitrans | Correlation coefficient | 0.250 | 0.153 | 0.047 | −0.022 |
| Spearman’s p value | 0.000 | 0.034 | 0.520 | 0.760 | |
| Females S. calcitrans | Correlation coefficient | 0.165 | 0.144 | −0.052 | 0.015 |
| Spearman’s p value | 0.022 | 0.046 | 0.469 | 0.837 |
| Summer | Automn | Winter | Spring | Summer | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2022 | 2023 | ||||||||||||||||
| July | Aug | Sept | Oct | Nov | Dec | Jan | Feb | Mar | Apr | May | Jun | July | |||||
| Stomoxys calcitrans | 0.5 | 36 | 249.5 | 102 | 32.25 | 15 | 0 | 0 | 0 | 1 | 1 | 3.5 | 8 | ||||
| S. calcitrans ♂ | 0.5 | 30 | 242 | 92.6 | 20.75 | 10.33 | 0 | 0 | 0 | 0 | 1 | 2.5 | 4 | ||||
| S. calcitrans ♀ | 0 | 6 | 7.5 | 9.4 | 11.5 | 4.67 | 0 | 0 | 0 | 1 | 0 | 1 | 4 | ||||
| Temperature (°C) | 34.41 | 30.04 | 28.81 | 19.65 | 14.83 | 10.35 | 7.77 | 10.59 | 16.64 | 19.04 | 22.73 | 26.16 | 35.96 | ||||
| Maximum Temperature (°C) | 39.14 | 36.73 | 36.52 | 28.06 | 23.63 | 19.89 | 16.97 | 19.82 | 25.03 | 30.72 | 28.42 | 34.39 | 39.63 | ||||
| Minimum temperature (°C) | 14.46 | 14.54 | 8.17 | 8.56 | −0.12 | −0.34 | −4.9 | −5.91 | −3.32 | −1.88 | 3.39 | 9.96 | 17.29 | ||||
| Relative Humidity (%) | 18.67 | 27.24 | 33.6 | 50.66 | 53.94 | 58.39 | 55.79 | 40.88 | 33.68 | 27.97 | 29.2 | 36.5 | 13.71 | ||||
| Precipitation (mm) | 0.0015 | 0.041 | 0.024 | 0.137 | 0.073 | 0.012 | 0 | 0 | 0.0008 | 0 | 0.003 | 0.011 | 0 | ||||
| Wind speed (m/s) | 2.37 | 3.54 | 2.8 | 2.17 | 1.49 | 1.44 | 1.12 | 1.17 | 2.52 | 2.66 | 1.94 | 4.67 | 6.28 | ||||
| Maximum wind speed (m/s) | 5.34 | 7.29 | 6.25 | 4.2 | 7.23 | 5.52 | 6.31 | 5.79 | 6.01 | 5.74 | 6.43 | 5.62 | 6.00 | ||||
| Minimum wind speed (m/s) | 0.12 | 0.05 | 0.09 | 0.19 | 0.14 | 0.15 | 0.08 | 0.1 | 0.16 | 0.17 | 0.14 | 0.04 | 0.12 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azzouzi, C.; Boucheikhchoukh, M.; Mechouk, N.; Sedraoui, S.; Zenia, S. Monthly and Daily Dynamics of Stomoxys calcitrans (Linnaeus, 1758) (Diptera: Muscidae) in Livestock Farms of the Batna Region (Northeastern Algeria). Parasitologia 2025, 5, 52. https://doi.org/10.3390/parasitologia5040052
Azzouzi C, Boucheikhchoukh M, Mechouk N, Sedraoui S, Zenia S. Monthly and Daily Dynamics of Stomoxys calcitrans (Linnaeus, 1758) (Diptera: Muscidae) in Livestock Farms of the Batna Region (Northeastern Algeria). Parasitologia. 2025; 5(4):52. https://doi.org/10.3390/parasitologia5040052
Chicago/Turabian StyleAzzouzi, Chaimaa, Mehdi Boucheikhchoukh, Noureddine Mechouk, Scherazad Sedraoui, and Safia Zenia. 2025. "Monthly and Daily Dynamics of Stomoxys calcitrans (Linnaeus, 1758) (Diptera: Muscidae) in Livestock Farms of the Batna Region (Northeastern Algeria)" Parasitologia 5, no. 4: 52. https://doi.org/10.3390/parasitologia5040052
APA StyleAzzouzi, C., Boucheikhchoukh, M., Mechouk, N., Sedraoui, S., & Zenia, S. (2025). Monthly and Daily Dynamics of Stomoxys calcitrans (Linnaeus, 1758) (Diptera: Muscidae) in Livestock Farms of the Batna Region (Northeastern Algeria). Parasitologia, 5(4), 52. https://doi.org/10.3390/parasitologia5040052







