Abstract
Human subcutaneous dirofilariasis caused by Dirofilaria repens is an emerging zoonotic parasitic infection increasingly observed in southern Europe. We report a case of a woman from western Sicily, who likely acquired the infection locally through a mosquito bite. The high prevalence of D. repens in the local canine population (up to 20.4%) suggests a role of dogs as a probable reservoir for transmission to humans in the local population. With ultrasound analysis being inconclusive, only after surgical removal was the presence of an adult worm evident, and only after molecular analysis through DNA sequencing of the cytochrome oxidase subunit I gene (COI) could a definitive D. repens infection diagnosis be obtained. This case highlights the need for enhanced vector control, animal health surveillance and public awareness to limit the transmission to humans of D. repens. A correct parasitological diagnosis would be imperative for the recognition of human cases.
1. Introduction
Human dirofilariasis is caused by a nematode parasite belonging to the family Onchocercidae and genus Dirofilaria, usually infecting dogs, foxes, cats and accidentally humans through the bites of mosquitos of the genera Aedes, Anopheles and Culex [1]. Mosquito-borne infections are emerging worldwide as a result of climatic change, as observed for West Nile virus, Chikungunya and other arboviruses [2]. Incidental transmission to humans occurs by arthropod inoculation of infective third-stage larvae (L3), and subcutaneous, ocular-periocular, pulmonary and cardiac lesions have been reported [3,4]. Dirofilaria repens and Dirofilaria immitis are the prevalent species in Europe, with the Mediterranean region being an endemic area with numerous human cases in France, Greece and Spain. In Italy, canine filariasis caused by D. repens is more prevalent in southern regions, while D. immitis, is more prevalent in northern regions [5]. D. immitis is more involved in invasive filariasis, while D. repens frequently causes localized subcutaneous infestations, which in most cases are controlled and resolved by the immune response [6]. Consequently, humans are generally considered ‘hopeless hosts’ for this parasite. In some cases, a single larva may survive and develop into a pre-adult or adult worm. Symptoms are generally mild and resolve quickly after surgical removal of the worm. In this study, the authors report a case of human dirofilariasis caused by D. repens and the diagnostic pathway undertaken.
2. Materials and Methods
2.1. Case Report
A 34-year-old woman presented to the Vittorio Emanuele II Hospital in Castelvetrano, Sicily (Italy), for outpatient evaluation of a subcutaneous lump in the right hemithorax measuring approximately 3 cm. The patient reported that the lesion had been present for at least two years. Clinical examination revealed a firm, immobile, non-erythematous nodule with characteristics suggestive of a lipoma or sebaceous cyst. No oral or topical antimicrobial therapy was prescribed; only ultrasound imaging and blood tests were performed.
Ultrasound, performed with a high-frequency probe, showed a non-vascularized subcutaneous cystic formation (maximum diameter 5 × 5 cm), hypoechoic in echostructure, with posterior wall thickening. Hematological analysis was unremarkable, with no eosinophilia. Surgical excision of the lesion revealed a white filamentous structure consistent with a parasitic nematode. The parasite was sent to the Microbiology Laboratory of the University Hospital “P. Giaccone,” Palermo, Italy, for species identification via molecular analysis. The excised tissue was forwarded to the Department of Pathology and Cytodiagnostics, S. Antonio Abate Hospital, Provincial Health Authority of Trapani (ASP Trapani), Sicily, Italy, for histopathological evaluation.
2.2. Parasitological and Morphological Analysis
Immediately after surgical removal, the nematode was placed in sterile saline to prevent desiccation and preserve morphological features. Upon arrival at the laboratory, the specimen was measured, photographed and examined macroscopically and microscopically.
Microscopic examination was performed under a compound microscope (low and high magnification) using saline-mounted preparations. The nematode measured ~14 cm, consistent with an adult female. Morphological features included a cylindrical body, smooth cuticle, clearly defined anterior and posterior ends and absence of lateral alae, supporting presumptive identification as an adult female Dirofilaria spp. (Figure 1).
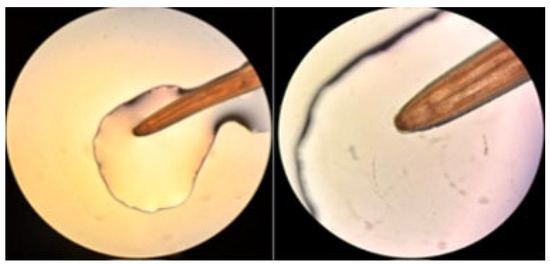
Figure 1.
Microscopic examination of the nematode identified as Dirofilaria repens (40× magnification). The caudal region is visible on the right, while the cephalic region is shown on the left.
2.3. DNA Extraction, PCR Amplification and Sequencing
Genomic DNA was extracted from the nematode using a DNeasy Blood and Tissue Kit (Qiagen, Hilden, Germany) following the manufacturer’s protocol. DNA concentration and purity were measured spectrophotometrically (NanoDrop, Thermo Fisher Scientific, Waltham, MA, USA). Approximately 20 ng of DNA served as the template for amplification of a partial cytochrome c oxidase subunit I (COI) gene fragment using species-specific primers described by Nazar et al., 2017; [1]. The forward primer was DR COI-F1 (5′-AGTGTTGATGGTCAACCTGAATTA-3′), and the reverse primer was DR COI-R1 (5′-GCCAAAACAGGAACAGATAAAACT-3′), yielding an expected amplicon size of approximately 200 bp. PCR was performed in a final reaction volume of 25 µL, consisting of 12.5 µL of 2 × PCR Master Mix (Thermo Fisher Scientific), 1 µL of each primer (10 µM), 2 µL of genomic DNA (20 ng) and nuclease-free water to volume. A positive control (D. repens DNA previously confirmed in our laboratory) and a negative control (reaction mixture without template DNA) were included in each run. Amplifications were carried out in a thermal cycler (Applied Biosystems, Thermo Fisher Scientific, Waltham, MA, USA) under the following cycling conditions: initial denaturation at 95 °C for 5 min, 35 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s and extension at 72 °C for 45 s, followed by a final extension at 72 °C for 7 min. PCR products were visualized by electrophoresis on a 1.5% agarose gel stained with ethidium bromide and examined under UV illumination. PCR products were purified and subjected to Sanger sequencing (Applied Biosystems 3500 Genetic Analyzer, Thermo Fisher Scientific, Waltham, MA, USA). The resulting sequences were edited and aligned and then analyzed using BLAST (NCBI available at: https://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 19 September 2025) to confirm species identification, followed by phylogenetic analysis to compare them with reference sequences from GenBank.
2.4. Phylogenetic Analysis
The amplified COI gene fragment was edited and aligned using ClustalW. The resulting sequence was compared with reference sequences retrieved from GenBank using BLAST (NCBI) to confirm species identity. Phylogenetic reconstruction was performed in MEGA X (version 10.2.6) following internationally accepted procedures. The evolutionary history was inferred using the UPGMA method, and the robustness of the inferred topology was assessed by the bootstrap test with 1000 replicates [7,8]. The percentage of replicate trees in which the associated taxa clustered together is shown next to the branches in the final tree. Evolutionary distances were computed using the Maximum Composite Likelihood (MCL) method and are expressed as the number of base substitutions per site [9]. This analysis involved 15 nucleotide sequences: the sequence generated in this study (GenBank accession number PX380488.1) and reference D. repens sequences from Europe. Onchocerca lupi was used as an outgroup to root the tree and provide a clear topological orientation. This approach provides a robust phylogenetic framework, confirming the clustering of our isolate within the D. repens clade [9].
3. Results
The patient presented with a subcutaneous nodule located on the right hemithorax. Ultrasonographic evaluation was inconclusive, and the lesion was initially presumed to be a lipoma. Surgical excision was performed for diagnostic and therapeutic purposes. Upon incision, a worm was identified within a cystic structure. Histopathological analysis of the excised tissue revealed fibro-adipose fragments containing numerous ectatic and congested blood vessels, accompanied by mild lymphocytic infiltrate. Granulomatous areas were also present, characterized by fibrocollagenous stroma and dense inflammatory infiltrates composed of eosinophils, lymphocytes and histiocytes. No parasitic structures were identified in histological sections, and findings were consistent with a chronic inflammatory reaction. Parasitological macroscopic examination confirmed the presence of a single adult nematode, approximately 14 cm in length. Direct microscopic observation showed morphological characteristics consistent with Dirofilaria spp., including a slender cylindrical body with clearly defined anterior and posterior ends. Based on size and morphology, the nematode was presumptively identified as an adult female Dirofilaria (Figure 1).
Molecular analysis confirmed species identification as Dirofilaria repens. Sequencing of a fragment of the cytochrome c oxidase subunit I (COI) gene revealed 100% identity with D. repens sequences deposited in GenBank. Phylogenetic analysis of the COI sequence clustered the isolate with D. repens isolates from Europe and Italy (Figure 2).
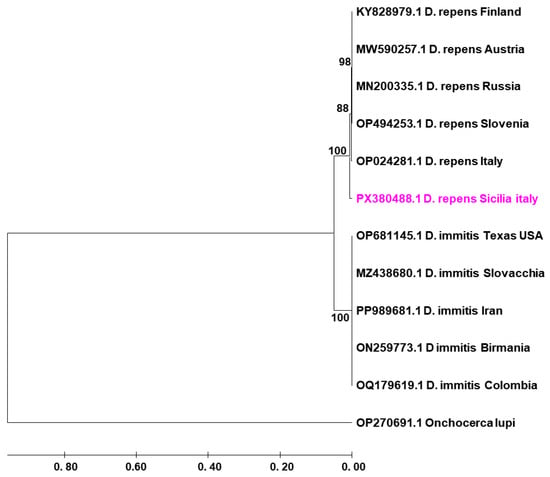
Figure 2.
UPGMA phylogenetic tree based on partial COI gene sequences of Dirofilaria repens and D. immitis, with Onchocerca lupi as outgroup. The sequence obtained in this study (PX380488.1, D. repens, Sicilia, Italy) is highlighted in pink.
4. Discussion
Human subcutaneous dirofilariasis caused by Dirofilaria repens has increasingly been recognized as an emerging zoonosis in Europe over the last few decades. Traditionally confined to Mediterranean countries such as Italy, Greece and France, the infection is now well documented in central and even northern regions, including Austria, Slovakia, Hungary and Serbia. This epidemiological shift is strongly associated with environmental and climatic changes that favor the expansion of competent mosquito vectors, alongside increased mobility of pets and limited awareness among clinicians. Recent comprehensive reviews have highlighted D. repens as the leading causative agent of human dirofilariasis in Europe, with thousands of cases reported since the 1970s and a continuing upward trend [10]. In Italy, which remains one of the most affected countries, multiple case reports and series confirm both the high canine reservoir prevalence and the human health impact. Gabrielli et al., 2021, described eight human infections over a two-year period, all confirmed molecularly, with both subcutaneous and ocular presentations [11]. Additional reports from central and southern Italy reinforce the endemic nature of the parasite, while surveys in Sicily historically documented canine prevalence rates exceeding 20%, corroborating the hypothesis of autochthonous human acquisition. Similarly, in France, particularly in southeastern regions, combined veterinary and human surveys have shown clear evidence of circulation of D. repens in local dog populations and occasionally in humans [12]. Central and eastern Europe provide further confirmation of the parasite’s expansion. In Austria, 39 human cases were documented between 1978 and 2020, with a marked increase after 1998, and several confirmed autochthonous cases have been documented, including a subcutaneous inguinal nodule recently diagnosed through PCR [13,14]. In Slovakia, at least twelve autochthonous infections have been recorded since the first case in 2007, involving subcutaneous, ocular and even pulmonary forms [15]. Serbia has contributed valuable evidence, including cases of subcutaneous dirofilariasis with detectable microfilaremia in peripheral blood—an unusual finding in humans, but one that raises questions about parasite adaptation and transmission [16].
Human cases have also been reported in Hungary and Greece, confirming the establishment of local transmission cycles even in regions previously considered marginal for this parasite [17]. Clinically, our case is consistent with most European presentations, in which patients typically develop subcutaneous nodules frequently misdiagnosed as benign lesions such as lipomas, cysts, or tumors. As documented in Italy, Austria and Slovakia, imaging techniques including ultrasonography often fail to provide a definitive diagnosis, yielding only nonspecific evidence of nodular structures [14]. Therefore, surgical excision followed by laboratory examination remains the mainstay for definitive identification. Ocular forms, more frequently reported in Italy and France, represent an important differential diagnosis because of their potential impact on vision, whereas the rare instances of microfilaremia documented in Serbia illustrate a unique clinical scenario distinct from the otherwise abortive infections observed in most humans [16]. Definitive confirmation relies on parasitological and molecular methods. Histopathological and morphological characterization of the extracted worm allow for presumptive identification, but species confirmation increasingly depends on molecular approaches. Sequencing of the mitochondrial cytochrome c oxidase subunit I (COI) gene is considered the gold standard, enabling precise species-level resolution and phylogenetic comparison with reference isolates from other geographic areas [11]. This approach not only confirms the diagnosis but also provides epidemiological insights into the likely geographic origin of the infection. Several European studies, including those conducted in Italy and Austria, have highlighted the utility of COI sequencing in differentiating D. repens from other filarial nematodes and linking human isolates to local canine reservoirs [18]. The epidemiological implications of these findings are substantial. The expansion of D. repens across Europe is facilitated by climate change, which prolongs the seasonal activity of vectors, increases mosquito density and allows for transmission in regions previously unsuitable for filarial development [10]. Competent vectors include widely distributed species such as Culex pipiens, Aedes albopictus and Anopheles spp., which are highly abundant in the Mediterranean basin and beyond [19]. The coexistence of dense canine reservoirs and abundant vector populations creates an ideal setting for zoonotic transmission, particularly in rural and peri-urban areas where human–animal–vector contact is frequent [17]. From a public health perspective, D. repens must now be regarded as an emerging zoonosis in Europe. The likely underestimation of cases—resulting from misdiagnosis or the failure to submit excised nodules for parasitological examination—remains a major concern. Strengthening integrated surveillance within a One Health framework, which connects veterinary, medical and entomological data, is essential to better monitor and control the spread of infection [19]. Control measures should focus on routine prophylaxis and management of domestic dogs, systematic vector monitoring and control and improved public awareness campaigns, especially in high-risk areas. Finally, molecular tools such as COI sequencing should be incorporated into routine diagnostic workflows to accelerate and improve the accuracy of Dirofilaria species identification.
5. Conclusions
This case adds further evidence of the endemic circulation of Dirofilaria repens in Sicily and reinforces its relevance as an emerging zoonosis in Europe. Unlike many previously reported ocular cases, the present infection manifested as a subcutaneous nodule, underscoring the wide clinical spectrum and the frequent misinterpretation of such lesions as benign tumors or cysts. The failure of ultrasonography to provide a conclusive diagnosis confirms that surgical excision followed by morphological and molecular examination remains the diagnostic gold standard in human dirofilariasis. The application of COI gene sequencing not only ensured definitive identification but also highlighted the importance of molecular tools in linking human isolates with regional epidemiological patterns.
From an epidemiological perspective, the case reflects the continuing expansion of D. repens across Europe, a phenomenon driven by climate change, the abundance of competent mosquito vectors (Culex, Aedes, Anopheles spp.) and high canine reservoir prevalence. This emphasizes the urgent need to update regional prevalence data and to strengthen surveillance programs. From a public health standpoint, the infection remains underestimated and probably underdiagnosed in both humans and animals. Enhanced awareness among physicians and veterinarians, routine submission of excised nodules for parasitological investigation and the integration of veterinary, medical and entomological data within a One Health framework are crucial. Preventive strategies should prioritize vector control, responsible dog management and molecular confirmation of suspected cases.
Overall, the present case contributes not only to the clinical documentation of human dirofilariasis but also to the broader understanding of its diagnostic pitfalls, epidemiological drivers and public health implications. It therefore supports the need for sustained research, stronger surveillance networks and coordinated preventive efforts to mitigate the impact of this emerging zoonotic infection in the Mediterranean and beyond.
Author Contributions
Conceptualization, C.C. and V.G.; methodology, R.V.; software, V.G.; validation, G.M.G., F.S. and S.R.; formal analysis, V.G., W.R. and T.F.; investigation, R.V., V.G., T.F. and C.C.; resources, V.G.; data curation, G.M.G.; writing—original draft preparation, C.C. and V.G.; writing—review and editing, G.M.G.; visualization, R.I. and A.V.; supervision, G.M.G.; project administration, R.V. and G.M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical approval was not required for this case report because the tests and procedures described were part of the patient’s routine clinical care and did not deviate from standard practice or constitute research interventions.
Informed Consent Statement
Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
Data supporting the findings of this study are available from the corresponding author upon reasonable request. No publicly archived datasets were generated or analyzed in this study.
Acknowledgments
The authors sincerely thank the patient who, with remarkable altruism, consented to the anonymous use of her clinical data. The authors acknowledge the use of ChatGPT (OpenAI, GPT-5; https://openai.com), employed exclusively to improve the clarity and scientific writing style of the manuscript. No AI-generated data, analyses, or experimental design were used. The authors take full responsibility for all interpretations, results, and conclusions presented.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| COI | Cytochrome c oxidase subunit I |
References
- Nazar, N.; Lakshmanan, B.; Jayavardhanan, K.K. Molecular Characterization of Human Dirofilaria Isolates from Kerala. Indian J. Med. Res. 2017, 146, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Franco, J.G.; Fernández-Santos, N.A.; Adebiyi, A.A.; López-López, M.D.J.; Aguilar-Durán, J.A.; Hernández-Triana, L.M.; Prosser, S.W.J.; Hebert, P.D.N.; Fooks, A.R.; Hamer, G.L.; et al. Vertebrate-Aedes aegypti and Culex quinquefasciatus (Diptera)-Arbovirus Transmission Networks: Non-Human Feeding Revealed by Meta-Barcoding and next-Generation Sequencing. PLoS Neglected Trop. Dis. 2020, 14, e0008867. [Google Scholar] [CrossRef] [PubMed]
- Noack, S.; Harrington, J.; Carithers, D.S.; Kaminsky, R.; Selzer, P.M. Heartworm Disease—Overview, Intervention, and Industry Perspective. Int. J. Parasitol. Drugs Drug Resist. 2021, 16, 65–89. [Google Scholar] [CrossRef] [PubMed]
- Otranto, D.; Deplazes, P. Zoonotic Nematodes of Wild Carnivores. Int. J. Parasitol. Parasites Wildl. 2019, 9, 370–383. [Google Scholar] [CrossRef] [PubMed]
- Morchón, R.; Carretón, E.; González-Miguel, J.; Mellado-Hernández, I. Heartworm Disease (Dirofilaria immitis) and Their Vectors in Europe—New Distribution Trends. Front. Physio. 2012, 3, 196. [Google Scholar] [CrossRef] [PubMed]
- Simón, F.; Siles-Lucas, M.; Morchón, R.; González-Miguel, J.; Mellado, I.; Carretón, E.; Montoya-Alonso, J.A. Human and Animal Dirofilariasis: The Emergence of a Zoonotic Mosaic. Clin. Microbiol. Rev. 2012, 25, 507–544. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The Neighbor-Joining Method: A New Method for Reconstructing Phylogenetic Trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Confidence Limits on Phylogenies: An Approach Using the Bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular Evolutionary Genetics Analysis Using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Capelli, G.; Genchi, C.; Baneth, G.; Bourdeau, P.; Brianti, E.; Cardoso, L.; Danesi, P.; Fuehrer, H.-P.; Giannelli, A.; Ionică, A.M.; et al. Recent Advances on Dirofilaria repens in Dogs and Humans in Europe. Parasites Vectors 2018, 11, 663. [Google Scholar] [CrossRef] [PubMed]
- Gabrielli, S.; Mangano, V.; Furzi, F.; Oliva, A.; Vita, S.; Poscia, R.; Fazii, P.; Di Paolo, J.; Marocco, R.; Mastroianni, C.M.; et al. Molecular Identification of New Cases of Human Dirofilariosis (Dirofilaria repens) in Italy. Pathogens 2021, 10, 251. [Google Scholar] [CrossRef] [PubMed]
- Younes, S.; Al-Jighefee, H.; Shurrab, F.; Al-Sadeq, D.W.; Younes, N.; Dargham, S.R.; Al-Dewik, N.; Qotba, H.; Syed, M.; Alnuaimi, A.; et al. Diagnostic Efficiency of Three Fully Automated Serology Assays and Their Correlation with a Novel Surrogate Virus Neutralization Test in Symptomatic and Asymptomatic SARS-COV-2 Individuals. Microorganisms 2021, 9, 245. [Google Scholar] [CrossRef] [PubMed]
- Fuehrer, H.-P.; Auer, H.; Leschnik, M.; Silbermayr, K.; Duscher, G.; Joachim, A. Dirofilaria in Humans, Dogs, and Vectors in Austria (1978–2014)—From Imported Pathogens to the Endemicity of Dirofilaria repens. PLoS Neglected Trop. Dis. 2016, 10, e0004547. [Google Scholar] [CrossRef] [PubMed]
- Schatz, C.; Füßl, M.; Caf, Y.; Schmitz, K.; Kresse, D.; Ludwig, W.; Walochnik, J.; Knabl, L. A Rare Case Report of a Human Dirofilaria repens Infection. Microorganisms 2025, 13, 476. [Google Scholar] [CrossRef] [PubMed]
- Zadravec, M.; Slavec, B.; Krapež, U.; Gombač, M.; Švara, T.; Poljšak-Prijatelj, M.; Gruntar, I.; Račnik, J. Trichomonosis Outbreak in a Flock of Canaries (Serinus canaria f. Domestica) Caused by a Finch Epidemic Strain of Trichomonas gallinae. Vet. Parasitol. 2017, 239, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Tasić-Otasevic, S.; Golubović, M.; Trichei, S.; Zdravkovic, D.; Jordan, R.; Gabrielli, S. Microfilaremic Dirofilaria repens Infection in Patient from Serbia. Emerg. Infect. Dis. 2023, 29, 2548–2550. [Google Scholar] [CrossRef] [PubMed]
- Miterpáková, M.; Valentová, D.; Hurníková, Z. Dirofilaria immitis Conquering the Regions in Slovakia Previously Endemic for D. Repens. Parasitol. Res. 2023, 122, 2945–2950. [Google Scholar] [CrossRef] [PubMed]
- Riebenbauer, K.; Weber, P.B.; Walochnik, J.; Karlhofer, F.; Winkler, S.; Dorfer, S.; Auer, H.; Valencak, J.; Laimer, M.; Handisurya, A. Human Dirofilariosis in Austria: The Past, the Present, the Future. Parasites Vectors 2021, 14, 227. [Google Scholar] [CrossRef] [PubMed]
- Laidoudi, Y.; Otranto, D.; Stolowy, N.; Amrane, S.; Santhakumari Manoj, R.R.; Polette, L.; Watier-Grillot, S.; Mediannikov, O.; Davoust, B.; L’Ollivier, C. Human and Animal Dirofilariasis in Southeast of France. Microorganisms 2021, 9, 1544. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).