Spatial Distribution of Microsporidia MB Along Clinal Gradient and the Impact of Its Infection on Pyrethroid Resistance in Anopheles gambiae s.l. Mosquitoes from Nigeria and Niger Republic
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Anopheles Mosquitoes
2.2. Morphological and Molecular Identification of Anopheles Mosquitoes to Species Level
2.3. Insecticide Susceptibility Bioassays
2.4. Investigation of Spatial Distribution of MB Infection in Anopheles Mosquitoes
2.5. Investigation of the Association of Insecticide Resistance and MB Infection
2.6. Data Analysis
3. Results
3.1. Microsporidia MB Infects Anopheles Mosquitoes from Contrasting Ecological Settings
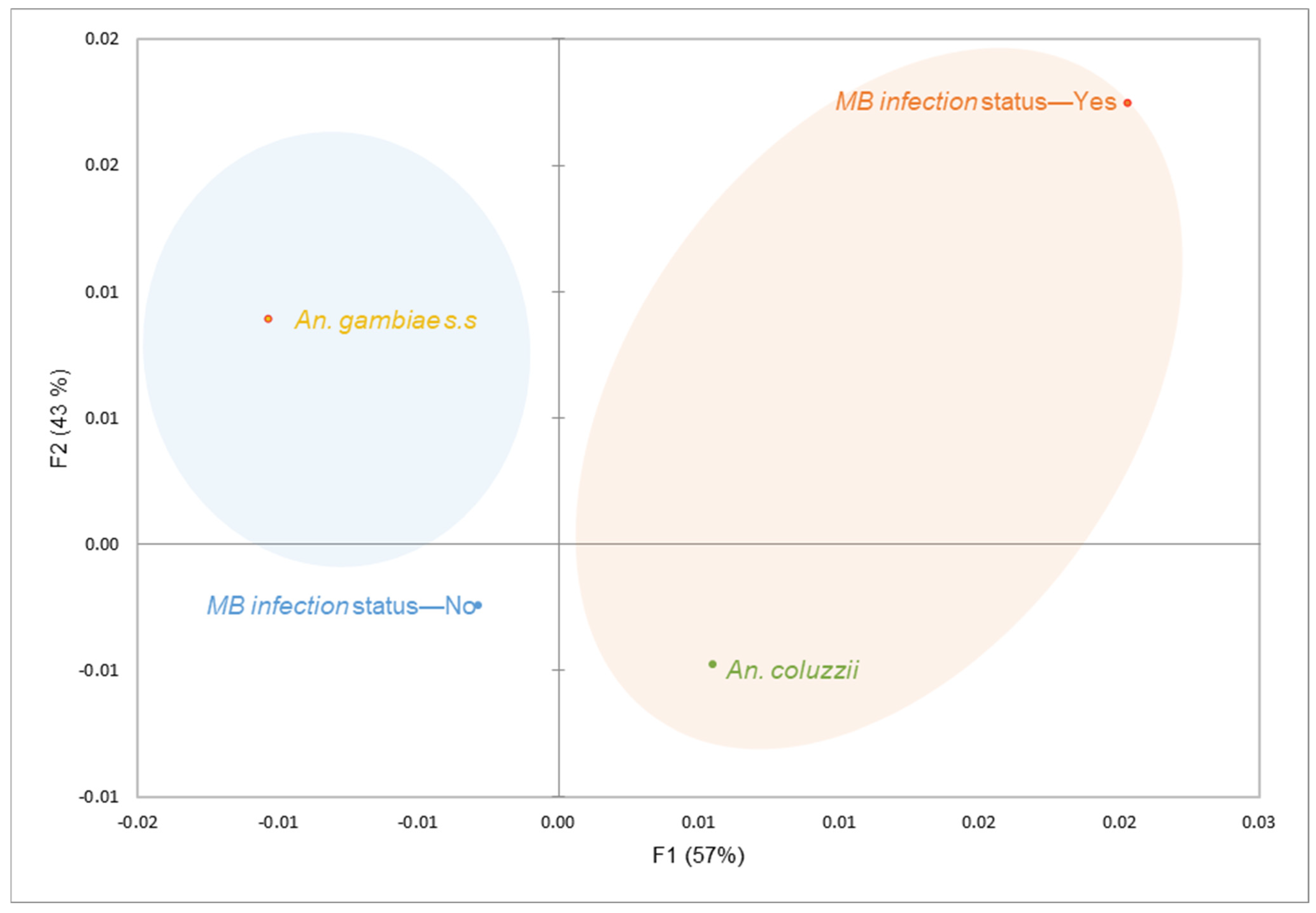
3.2. Microsporidia MB Infection Probably Correlates with Pyrethroid Resistance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. World Malaria Report 2024: Addressing Inequity in the Global Malaria Response; WHO: Geneva, Switzerland, 2024. [Google Scholar]
- Ibrahim, S.S.; Manu, Y.A.; Tukur, Z.; Irving, H.; Wondji, C.S. High Frequency of Kdr L1014F Is Associated with Pyrethroid Resistance in Anopheles coluzzii in Sudan Savannah of Northern Nigeria. BMC Infect. Dis. 2014, 14, 441. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ibrahim, S.S.; Mukhtar, M.M.; Datti, J.A.; Irving, H.; Kusimo, M.O.; Tchapga, W.; Lawal, N.; Sambo, F.I.; Wondji, C.S. Temporal Escalation of Pyrethroid Resistance in the Major Malaria Vector Anopheles coluzzii from Sahelo-Sudanian Region of Northern Nigeria. Sci. Rep. 2019, 9, 7395. [Google Scholar] [CrossRef]
- Ibrahim, S.S.; Mukhtar, M.M.; Irving, H.; Riveron, J.M.; Fadel, A.N.; Tchapga, W.; Hearn, J.; Muhammad, A.; Sarkinfada, F.; Wondji, C.S. Exploring the Mechanisms of Multiple Insecticide Resistance in a Highly Plasmodium-Infected Malaria Vector Anopheles funestus Sensu Stricto from Sahel of Northern Nigeria. Genes 2020, 11, 454. [Google Scholar] [CrossRef]
- Lamidi, T.B.; Alo, E.B.; Naphtali, R. Distribution and Abundance of Anopheles Mosquito Species in Three Selected Areas of Taraba State, North-Eastern Nigeria. Anim. Res. Int. 2017, 14, 2730–2740. [Google Scholar]
- Moustapha, L.M.; Sadou, I.M.; Arzika, I.I.; Maman, L.I.; Gomgnimbou, M.K.; Konkobo, M.; Diabate, A.; Bilgo, E. First Identification of Microsporidia MB in Anopheles coluzzii from Zinder City, Niger. Parasit. Vectors 2024, 17, 39. [Google Scholar] [CrossRef]
- Oduola, A.O.; Adelaja, O.J.; Aiyegbusi, Z.O.; Tola, M.; Obembe, A.; Ande, A.T.; Awolola, S. Dynamics of Anopheline Vector Species Composition and Reported Malaria Cases during Rain and Dry Seasons in Two Selected Communities of Kwara State. Niger. J. Parasitol. 2016, 37, 157. [Google Scholar] [CrossRef]
- Adeogun, A.; Babalola, A.S.; Okoko, O.O.; Oyeniyi, T.; Omotayo, A.; Izekor, R.T.; Adetunji, O.; Olakiigbe, A.; Olagundoye, O.; Adeleke, M.; et al. Spatial Distribution and Ecological Niche Modeling of Geographical Spread of Anopheles gambiae Complex in Nigeria Using Real Time Data. Sci. Rep. 2023, 13, 13679. [Google Scholar] [CrossRef]
- Ebenezer, A.; Okiwelu, S.; Agi, P.; Noutcha, M.E.; Awolola, T.; Oduola, A. Species Composition of the Anopheles gambiae Complex across Eco-Vegetational Zones in Bayelsa State, Niger Delta Region, Nigeria. J. Vector Borne Dis. 2012, 49, 164. [Google Scholar] [CrossRef]
- Thabet, H.S.; TagEldin, R.A.; Fahmy, N.T.; Diclaro, J.W.; Alaribe, A.A.; Ezedinachi, E.; Nwachuku, N.S.; Odey, F.O.; Arimoto, H. Spatial Distribution of PCR-Identified Species of Anopheles Gambiae senu lato (Diptera: Culicidae) Across Three Eco-Vegetational Zones in Cross River State, Nigeria. J. Med. Entomol. 2022, 59, 576–584. [Google Scholar] [CrossRef]
- Ibrahim, S.S.; Mukhtar, M.M.; Irving, H.; Labbo, R.; Kusimo, M.O.; Mahamadou, I.; Wondji, C.S. High Plasmodium Infection and Multiple Insecticide Resistance in a Major Malaria Vector Anopheles coluzzii from Sahel of Niger Republic. Malar. J. 2019, 18, 181. [Google Scholar] [CrossRef]
- Soumaila, H.; Hamani, B.; Arzika, I.I.; Soumana, A.; Daouda, A.; Daouda, F.A.; Iro, S.M.; Gouro, S.; Zaman-Allah, M.S.; Mahamadou, I.; et al. Countrywide Insecticide Resistance Monitoring and First Report of the Presence of the L1014S Knock down Resistance in Niger, West Africa. Malar. J. 2022, 21, 385. [Google Scholar] [CrossRef] [PubMed]
- Tchouakui, M.; Assatse, T.; Tazokong, H.R.; Oruni, A.; Menze, B.D.; Nguiffo-Nguete, D.; Mugenzi, L.M.J.; Kayondo, J.; Watsenga, F.; Mzilahowa, T.; et al. Detection of a Reduced Susceptibility to Chlorfenapyr in the Malaria Vector Anopheles gambiae Contrasts with Full Susceptibility in Anopheles funestus across Africa. Sci. Rep. 2023, 13, 2363. [Google Scholar] [CrossRef] [PubMed]
- Kouassi, B.L.; Edi, C.; Tia, E.; Konan, L.Y.; Akré, M.A.; Koffi, A.A.; Ouattara, A.F.; Tanoh, A.M.; Zinzindohoue, P.; Kouadio, B.; et al. Susceptibility of Anopheles gambiae from Côte d’Ivoire to Insecticides Used on Insecticide-Treated Nets: Evaluating the Additional Entomological Impact of Piperonyl Butoxide and Chlorfenapyr. Malar. J. 2020, 19, 454. [Google Scholar] [CrossRef] [PubMed]
- Fouet, C.; Ashu, F.A.; Ambadiang, M.M.; Tchapga, W.; Wondji, C.S.; Kamdem, C. Clothianidin-Resistant Anopheles gambiae Adult Mosquitoes from Yaoundé, Cameroon, Display Reduced Susceptibility to SumiShield® 50WG, a Neonicotinoid Formulation for Indoor Residual Spraying. BMC Infect. Dis. 2024, 24, 133. [Google Scholar] [CrossRef]
- Ranson, H.; Lissenden, N. Insecticide Resistance in African Anopheles Mosquitoes: A Worsening Situation That Needs Urgent Action to Maintain Malaria Control. Trends Parasitol. 2016, 32, 187–196. [Google Scholar] [CrossRef]
- Maiga, A.-A.; Sombié, A.; Zanré, N.; Yaméogo, F.; Iro, S.; Testa, J.; Sanon, A.; Koita, O.; Kanuka, H.; McCall, P.J.; et al. First Report of V1016I, F1534C and V410L Kdr Mutations Associated with Pyrethroid Resistance in Aedes aegypti Populations from Niamey, Niger. PLoS ONE 2024, 19, e0304550. [Google Scholar] [CrossRef]
- Busari, L.O.; Raheem, H.O.; Iwalewa, Z.O.; Fasasi, K.A.; Adeleke, M.A. Investigating Insecticide Susceptibility Status of Adult Mosquitoes against Some Class of Insecticides in Osogbo Metropolis, Osun State, Nigeria. PLoS ONE 2023, 18, e0285605. [Google Scholar] [CrossRef]
- Omotayo, A.I.; Ande, A.T.; Oduola, A.O.; Adelaja, O.J.; Adesalu, O.; Jimoh, T.R.; Ghazali, A.I.; Awolola, S.T. Multiple Insecticide Resistance Mechanisms in Urban Population of Anopheles coluzzii (Diptera: Culicidae) from Lagos, South-West Nigeria. Acta Trop. 2022, 227, 106291. [Google Scholar] [CrossRef]
- Awolola, T.S.; Adeogun, A.; Olakiigbe, A.K.; Oyeniyi, T.; Olukosi, Y.A.; Okoh, H.; Arowolo, T.; Akila, J.; Oduola, A.; Amajoh, C.N. Pyrethroids Resistance Intensity and Resistance Mechanisms in Anopheles gambiae from Malaria Vector Surveillance Sites in Nigeria. PLoS ONE 2018, 13, e0205230. [Google Scholar] [CrossRef]
- Ibrahim, S.S.; Muhammad, A.; Hearn, J.; Weedall, G.D.; Nagi, S.C.; Mukhtar, M.M.; Fadel, A.N.; Mugenzi, L.J.; Patterson, E.I.; Irving, H.; et al. Molecular Drivers of Insecticide Resistance in the Sahelo-Sudanian Populations of a Major Malaria Vector Anopheles coluzzii. BMC Biol. 2023, 21, 125. [Google Scholar] [CrossRef]
- Muhammad, A.; Ibrahim, S.S.; Mukhtar, M.M.; Irving, H.; Abajue, M.C.; Edith, N.M.A.; Da’u, S.S.; Paine, M.J.I.; Wondji, C.S. High Pyrethroid/DDT Resistance in Major Malaria Vector Anopheles coluzzii from Niger-Delta of Nigeria Is Probably Driven by Metabolic Resistance Mechanisms. PLoS ONE 2021, 16, e0247944. [Google Scholar] [CrossRef] [PubMed]
- Soumaila, H.; Idrissa, M.; Akgobeto, M.; Habi, G.; Jackou, H.; Sabiti, I.; Abdoulaye, A.; Daouda, A.; Souleymane, I.; Osse, R. Multiple Mechanisms of Resistance to Pyrethroids in Anopheles gambiae s.l Populations in Niger. Méd. Mal. Infect. 2017, 47, 415–423. [Google Scholar] [CrossRef]
- Mitchell, S.N.; Rigden, D.J.; Dowd, A.J.; Lu, F.; Wilding, C.S.; Weetman, D.; Dadzie, S.; Jenkins, A.M.; Regna, K.; Boko, P.; et al. Metabolic and Target-Site Mechanisms Combine to Confer Strong DDT Resistance in Anopheles gambiae. PLoS ONE 2014, 9, e92662. [Google Scholar] [CrossRef]
- Djouaka, R.F.; Bakare, A.A.; Coulibaly, O.N.; Akogbeto, M.C.; Ranson, H.; Hemingway, J.; Strode, C. Expression of the Cytochrome P450s, CYP6P3 and CYP6M2 Are Significantly Elevated in Multiple Pyrethroid Resistant Populations of Anopheles gambiae s.s. from Southern Benin and Nigeria. BMC Genom. 2008, 9, 538. [Google Scholar] [CrossRef] [PubMed]
- Weetman, D.; Wilding, C.S.; Neafsey, D.E.; Müller, P.; Ochomo, E.; Isaacs, A.T.; Steen, K.; Rippon, E.J.; Morgan, J.C.; Mawejje, H.D.; et al. Candidate-Gene Based GWAS Identifies Reproducible DNA Markers for Metabolic Pyrethroid Resistance from Standing Genetic Variation in East African Anopheles Gambiae. Sci. Rep. 2018, 8, 2920. [Google Scholar] [CrossRef] [PubMed]
- Zhong, D.; Chang, X.; Zhou, G.; He, Z.; Fu, F.; Yan, Z.; Zhu, G.; Xu, T.; Bonizzoni, M.; Wang, M.-H.; et al. Relationship between Knockdown Resistance, Metabolic Detoxification and Organismal Resistance to Pyrethroids in Anopheles sinensis. PLoS ONE 2013, 8, e55475. [Google Scholar] [CrossRef]
- Dada, N.; Sheth, M.; Liebman, K.; Pinto, J.; Lenhart, A. Whole Metagenome Sequencing Reveals Links between Mosquito Microbiota and Insecticide Resistance in Malaria Vectors. Sci. Rep. 2018, 8, 2084. [Google Scholar] [CrossRef]
- Hughes, G.L.; Koga, R.; Xue, P.; Fukatsu, T.; Rasgon, J.L. Wolbachia Infections Are Virulent and Inhibit the Human Malaria Parasite Plasmodium Falciparum in Anopheles gambiae. PLoS Pathog. 2011, 7, e1002043. [Google Scholar] [CrossRef]
- Gomes, F.M.; Barillas-Mury, C. Infection of Anopheline Mosquitoes with Wolbachia: Implications for Malaria Control. PLoS Pathog. 2018, 14, e1007333. [Google Scholar] [CrossRef]
- Walker, T.; Quek, S.; Jeffries, C.L.; Bandibabone, J.; Dhokiya, V.; Bamou, R.; Kristan, M.; Messenger, L.A.; Gidley, A.; Hornett, E.A.; et al. Stable High-Density and Maternally Inherited Wolbachia Infections in Anopheles moucheti and Anopheles demeilloni Mosquitoes. Curr. Biol. CB 2021, 31, 2310–2320.e5. [Google Scholar] [CrossRef]
- Almeida, J.; Mohanty, A.; Kerkar, S.; Hoti, S.; Kumar, A. Current Status and Future Prospects of Bacilli-Based Vector Control. Asian Pac. J. Trop. Med. 2020, 13, 525. [Google Scholar] [CrossRef]
- Boyce, R.; Lenhart, A.; Kroeger, A.; Velayudhan, R.; Roberts, B.; Horstick, O. Bacillus thuringiensis israelensis (Bti) for the Control of Dengue Vectors: Systematic Literature Review. Trop. Med. Int. Health 2013, 18, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Herren, J.K.; Mbaisi, L.; Mararo, E.; Makhulu, E.E.; Mobegi, V.A.; Butungi, H.; Mancini, M.V.; Oundo, J.W.; Teal, E.T.; Pinaud, S.; et al. A Microsporidian Impairs Plasmodium falciparum Transmission in Anopheles arabiensis Mosquitoes. Nat. Commun. 2020, 11, 2187. [Google Scholar] [CrossRef]
- Makhulu, E.E.; Onchuru, T.O.; Gichuhi, J.; Otieno, F.G.; Wairimu, A.W.; Muthoni, J.N.; Koekemoer, L.; Herren, J.K. Localization and Tissue Tropism of the Symbiont Microsporidia MB in the Germ Line and Somatic Tissues of Anopheles Arabiensis. mBio 2024, 15, e02192-23. [Google Scholar] [CrossRef]
- Nattoh, G.; Maina, T.; Makhulu, E.E.; Mbaisi, L.; Mararo, E.; Otieno, F.G.; Bukhari, T.; Onchuru, T.O.; Teal, E.; Paredes, J.; et al. Horizontal Transmission of the Symbiont Microsporidia MB in Anopheles arabiensis. Front. Microbiol. 2021, 12, 647183. [Google Scholar] [CrossRef]
- Ahouandjinou, M.J.; Sovi, A.; Sidick, A.; Sewadé, W.; Koukpo, C.Z.; Chitou, S.; Towakinou, L.; Adjottin, B.; Hougbe, S.; Tokponnon, F.; et al. First Report of Natural Infection of Anopheles gambiae s.s. and Anopheles coluzzii by Wolbachia and Microsporidia in Benin: A Cross-Sectional Study. Malar. J. 2024, 23, 72. [Google Scholar] [CrossRef]
- Akorli, J.; Akorli, E.A.; Tetteh, S.N.A.; Amlalo, G.K.; Opoku, M.; Pwalia, R.; Adimazoya, M.; Atibilla, D.; Pi-Bansa, S.; Chabi, J.; et al. Microsporidia MB Is Found Predominantly Associated with Anopheles gambiae s.s and Anopheles coluzzii in Ghana. Sci. Rep. 2021, 11, 18658. [Google Scholar] [CrossRef]
- Tchigossou, G.; Lontsi-Demano, M.; Tossou, E.; Sovegnon, P.-M.; Akoton, R.; Adanzounon, D.; Dossou, C.; Koto, M.; Ogbon, A.; Gouété, M.; et al. Seasonal Variation of Microsporidia MB Infection in Anopheles gambiae and Anopheles coluzzii in Two Different Geographical Localities in Benin. Malar. J. 2025, 24, 95. [Google Scholar] [CrossRef]
- Bukhari, T.; Pevsner, R.; Herren, J.K. Microsporidia: A Promising Vector Control Tool for Residual Malaria Transmission. Front. Trop. Dis. 2022, 3, 957109. [Google Scholar] [CrossRef]
- Coetzee, M. Key to the Females of Afrotropical Anopheles Mosquitoes (Diptera: Culicidae). Malar. J. 2020, 19, 70. [Google Scholar] [CrossRef]
- Santolamazza, F.; Mancini, E.; Simard, F.; Qi, Y.; Tu, Z.; della Torre, A. Insertion Polymorphisms of SINE200 Retrotransposons within Speciation Islands of Anopheles gambiae Molecular Forms. Malar. J. 2008, 7, 163. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J. Organization and Mapping of a Sequence on the DROSOPHILA MELANOGASTER X and Y Chromosomes That Is Transcribed during Spermatogenesis. Genetics 1984, 107, 611–634. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Test Procedures for Insecticide Resistance Monitoring in Malaria Vector Mosquitoes, 2nd ed.; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- WHO. Standard Operating Procedure for Testing Insecticide Susceptibility of Adult Mosquitoes in WHO Tube Tests; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Lumivero. XLSTAT|Logiciel Statistique Pour Excel. Available online: https://www.xlstat.com (accessed on 16 October 2024).
- Nattoh, G.; Onyango, B.; Makhulu, E.E.; Omoke, D.; Ang’ang’o, L.M.; Kamau, L.; Gesuge, M.M.; Ochomo, E.; Herren, J.K. Microsporidia MB in the Primary Malaria Vector Anopheles gambiae Sensu Stricto Is Avirulent and Undergoes Maternal and Horizontal Transmission. Parasit. Vectors 2023, 16, 335. [Google Scholar] [CrossRef] [PubMed]
- Agyekum, T.P.; Botwe, P.K.; Arko-Mensah, J.; Issah, I.; Acquah, A.A.; Hogarh, J.N.; Dwomoh, D.; Robins, T.G.; Fobil, J.N. A Systematic Review of the Effects of Temperature on Anopheles Mosquito Development and Survival: Implications for Malaria Control in a Future Warmer Climate. Int. J. Environ. Res. Public Health 2021, 18, 7255. [Google Scholar] [CrossRef]
- Saab, S.A.; Dohna, H.Z.; Nilsson, L.K.J.; Onorati, P.; Nakhleh, J.; Terenius, O.; Osta, M.A. The Environment and Species Affect Gut Bacteria Composition in Laboratory Co-Cultured Anopheles gambiae and Aedes albopictus Mosquitoes. Sci. Rep. 2020, 10, 3352. [Google Scholar] [CrossRef]
- Cuesta, E.B.; Coulibaly, B.; Bukhari, T.; Eiglmeier, K.; Kone, R.; Coulibaly, M.B.; Zongo, S.; Barry, M.; Gneme, A.; Guelbeogo, W.M.; et al. Comprehensive Ecological and Geographic Characterization of Eukaryotic and Prokaryotic Microbiomes in African Anopheles. Front. Microbiol. 2021, 12, 635772. [Google Scholar] [CrossRef]
- Machani, M.G.; Nzioki, I.; Onyango, S.A.; Onyango, B.; Githure, J.; Atieli, H.; Wang, C.; Lee, M.-C.; Githeko, A.K.; Afrane, Y.A.; et al. Insecticide Resistance and Its Intensity in Urban Anopheles arabiensis in Kisumu City, Western Kenya: Implications for Malaria Control in Urban Areas. PLoS ONE 2024, 19, e0303921. [Google Scholar] [CrossRef]
- Akorli, E.A.; Efua, A.N.; Egyirifa, R.K.; Dorcoo, C.; Otoo, S.; Tetteh, S.N.A.; Pul, R.M.; Derrick, B.S.; Oware, S.K.D.; Samuel, K.D.; et al. Mosquito breeding water parameters are important determinants for Microsporidia MB in the aquatic stages of Anopheles species. Parasites Vectors 2024, 17, 509. [Google Scholar] [CrossRef]
- Omoke, D.; Kipsum, M.; Otieno, S.; Esalimba, E.; Sheth, M.; Lenhart, A.; Njeru, E.M.; Ochomo, E.; Dada, N. Western Kenyan Anopheles gambiae Showing Intense Permethrin Resistance Harbour Distinct Microbiota. Malar. J. 2021, 20, 77. [Google Scholar] [CrossRef]
- Pelloquin, B.; Kristan, M.; Edi, C.; Meiwald, A.; Clark, E.; Jeffries, C.L.; Walker, T.; Dada, N.; Messenger, L.A. Overabundance of Asaia and Serratia Bacteria Is Associated with Deltamethrin Insecticide Susceptibility in Anopheles coluzzii from Agboville, Côte d’Ivoire. Microbiol. Spectr. 2021, 9, e00157-21. [Google Scholar] [CrossRef]
- Boanyah, G.Y.; Koekemoer, L.L.; Herren, J.K.; Bukhari, T. Effect of Microsporidia MB Infection on the Development and Fitness of Anopheles arabiensis under Different Diet Regimes. Parasit. Vectors 2024, 17, 294. [Google Scholar] [CrossRef]
- Otieno, F.G.; Barreaux, P.; Belvinos, A.S.; Makhulu, E.E.; Onchuru, T.O.; Wairimu, A.W.; Omboye, S.M.; King’ori, C.N.; Mawuko, S.B.; Kebira, A.N.; et al. Temperature Modulates the Dissemination Potential of Microsporidia MB, a Malaria-Blocking Endosymbiont of Anopheles Mosquitoes. bioRxiv 2024. bioRxiv: 2024.11.15.623820. [Google Scholar] [CrossRef]




| Locality | n | Species | Bioassay | Ecological Setting |
|---|---|---|---|---|
| F0 parents caught indoor using Prokopack aspirators | ||||
| Gayi Niger, Magaria | 32 | An. coluzzii | - | Sahel |
| Gajerar Giwa, Katsina State | 32 | An. coluzzii | Sahel | |
| Gaa-Bolorunduro, Kwara State | 15 | An. gambiae s.s. | - | Guinea Savanna |
| Zariagi, Lokoja, Kogi State | 32 | An. gambiae s.s. | - | Guinea Savanna |
| Gotomo, Kebbi State | 15 | An. coluzzii | - | Sudan Savanna |
| Tsunami, Gusau, Zamfara State | 15 | An. coluzzii | - | Sudan Savanna |
| Ugalaba, Ezza North, Ebonyi State | 15 | An. coluzzii | - | Tropical Rainforest |
| Obio/Akpor LGA, Rivers State | 24 | An. coluzzii | - | Mangrove Swamp |
| Badagry, Lagos State | 24 | An. gambiae s.s. | - | Mangrove Swamp |
| F1 progenies used for bioassays | ||||
| Gajerar Giwa, Katsina State | 16 | An. coluzzii | Permethrin | Sahel |
| Dadin Kowa, Gombe State | 16 | An. coluzzii | Deltamethrin | Guinea Savanna |
| Zariagi, Lokoja, Kogi State | 24 | An. gambiae s.s. | Deltamethrin | Guinea Savana |
| Gamjin Bappa, Karaye, Kano State | 16 | An. coluzzii | Deltamethrin | Guinea Savana |
| Gadau, Bauchi State | 16 | An. coluzzii | Deltamethrin | Sudan Savanna |
| Obio/Akpor LGA, Rivers State | 24 | An. coluzzii | Permethrin | Mangrove Swamp |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moustapha, L.M.; Mukhtar, M.M.; Sanda, A.-N.H.; Adamu, S.; Aliyu, Y.Y.; Einoi, H.K.; Maigari, M.U.; Okeke, P.C.; Nwele, D.E.; Obembe, A.; et al. Spatial Distribution of Microsporidia MB Along Clinal Gradient and the Impact of Its Infection on Pyrethroid Resistance in Anopheles gambiae s.l. Mosquitoes from Nigeria and Niger Republic. Parasitologia 2025, 5, 31. https://doi.org/10.3390/parasitologia5030031
Moustapha LM, Mukhtar MM, Sanda A-NH, Adamu S, Aliyu YY, Einoi HK, Maigari MU, Okeke PC, Nwele DE, Obembe A, et al. Spatial Distribution of Microsporidia MB Along Clinal Gradient and the Impact of Its Infection on Pyrethroid Resistance in Anopheles gambiae s.l. Mosquitoes from Nigeria and Niger Republic. Parasitologia. 2025; 5(3):31. https://doi.org/10.3390/parasitologia5030031
Chicago/Turabian StyleMoustapha, Lamine M., Muhammad M. Mukhtar, Abdoul-Nasser H. Sanda, Shuaibu Adamu, Yusuf Y. Aliyu, Hadizat K. Einoi, Maryam U. Maigari, Peter C. Okeke, David E. Nwele, Abiodun Obembe, and et al. 2025. "Spatial Distribution of Microsporidia MB Along Clinal Gradient and the Impact of Its Infection on Pyrethroid Resistance in Anopheles gambiae s.l. Mosquitoes from Nigeria and Niger Republic" Parasitologia 5, no. 3: 31. https://doi.org/10.3390/parasitologia5030031
APA StyleMoustapha, L. M., Mukhtar, M. M., Sanda, A.-N. H., Adamu, S., Aliyu, Y. Y., Einoi, H. K., Maigari, M. U., Okeke, P. C., Nwele, D. E., Obembe, A., Nwangwu, U. C., Herren, J. K., & Ibrahim, S. S. (2025). Spatial Distribution of Microsporidia MB Along Clinal Gradient and the Impact of Its Infection on Pyrethroid Resistance in Anopheles gambiae s.l. Mosquitoes from Nigeria and Niger Republic. Parasitologia, 5(3), 31. https://doi.org/10.3390/parasitologia5030031













