Abstract
The resistance of small ruminants to synthetic anthelmintics and helminthosis poses considerable challenges to global livestock production. Integrating biological control with nematophagous fungi, particularly Duddingtonia flagrans, is crucial in addressing worm infestations. Although effective in experiments, the absence of a commercial product has been a limitation. The introduction of Bioverm®, the first commercial product using D. flagrans in Brazil, marks a significant advancement. This study on a Brazilian sheep farm evaluated a 167-day Bioverm® treatment, following moxidectin application, focusing on eggs per gram of feces (EPG), FAMACHA score, and sheep weight (WEIGHT). Statistical results showed marked improvements in all parameters after 80 days with Bioverm®. EPG values gradually declined, demonstrating successful biological control, while FAMACHA increased steadily, stabilizing after 130 days. Minor weight changes indicated effective nutritional management. These outcomes suggest Bioverm® significantly reduces dependence on chemical anthelmintics and addresses resistance issues. D. flagrans thus emerges as a promising tool for managing nematode infestations without negatively impacting animal weight. This research enhances the understanding of Bioverm®’s role, confirming its practicality as a viable alternative for helminth control in varied environments, thereby reinforcing its strategic importance in livestock management.
1. Introduction
The breeding of small ruminants varies significantly across different regions worldwide. In countries like Australia and New Zealand, it is a large-scale practice and constitutes a primary economic activity on many farms. Conversely, in regions such as northeastern Brazil and countries across Africa and Asia, small ruminant breeding holds crucial social and economic importance, often serving as a subsistence activity [1,2,3,4,5,6,7].
Regardless of the breeding model or production purpose, helminthosis, particularly due to Haemonchus contortus, poses a major challenge globally [8]. This parasite contributes to economic losses amounting to millions of dollars as it develops resistance to anthelmintics worldwide. Numerous studies indicate that these parasites exhibit resistance to commercially available anthelmintics, highlighting the urgent need for effective alternatives for helminthosis control in goats and sheep [9,10,11,12,13,14,15,16].
One promising alternative is biological control using the nematophagous fungus Duddingtonia flagrans [17]. Although various experimental studies have demonstrated its effectiveness [18], the absence of a commercial product has been due to high production costs, difficulties in achieving a formulation with a long shelf life, and challenges in large-scale production at viable costs. Recently, a commercial product (Bioverm®, Ghenvet Biotecnologia Ltda, 13148-153, Paulínia, SP, Brazil) was launched in Brazil. This study marks the first experiment conducted in the country based on the commercial-scale production model of D. flagrans.
This study aims to evaluate the characteristics of eggs per gram of feces (EPG), parasite infestation indicator scores (FAMACHA), and weight (WEIGHT) in sheep subjected to a biological control regimen with the nematophagous fungus D. flagrans over 167 days, following the use of the active ingredient moxidectin.
2. Materials and Methods
The experiment was conducted at Fazenda Talisman, located in Itapira, SP, Brazil (22°28′40.1″ S 46°45′38.3″ W). The study was based on data collected from a commercial sheep production operation, which complies with all animal welfare requirements. Commercial sheep production is considered a zootechnical practice and is not regulated under Brazilian Federal Law no. 11,794/2008, as it does not pertain to teaching or scientific research activities [19]. Therefore, there is no requirement for approval from an Ethics Committee on the Use of Animals. The treatment and use of experimental animals complied fully with Brazilian laws, guidelines, and policies related to animal welfare.
Data were collected from 50 female sheep of the Dorper (n = 15), Santa Inês (n = 21), and White Dorper (n = 14) breeds. All animals were adults, part of a commercial flock, and were estimated to be between 1 and 4 years old. During the experimental period, the animals were fed using three distinct systems: Pasture, Pasture+Silage, or Stall+Hay+Feed. These systems were tailored to meet the specific needs of the animals during maintenance, early gestation, and late pregnancy/newborn lamb phases. As this was a commercial flock, the treatments varied according to the phase the animals were in.
Prior to the biological control regimen, the flock was dewormed with moxidectin (0.2 mg/kg; orally). For the treatment with Bioverm®, as per product recommendations, it was mixed with mineral salt and provided continuously ad libitum at an estimated dose of 1 g of product for every 10 kg of live weight. The commercial product Bioverm®, containing the nematophagous fungus D. flagrans, was introduced to initiate the biological control system ten days post-deworming.
The variables assessed included eggs per gram of feces (EPG), FAMACHA scores indicating parasite infection (FAMACHA), and weight (WEIGHT). These were measured on days 1, 7, 14, 28, 43, 57, 78, 92, 139, and 167 following the start of Bioverm® treatment. For statistical analysis, EPG values below 50 were standardized to 50.
Fecal samples were collected directly from the rectum of each animal by the farm veterinarian, cooled to 8 °C, and transported to the laboratory. The McMaster technique was used for EPG determination [20]. Fecal sample analyses were conducted individually. The analyses involved 4 g of feces diluted in 56 mL of saturated NaCl solution, and eggs counted in both chambers of the McMaster slide were multiplied by a factor of 50 in order to obtain the EPG count. Only helminth eggs were counted, using the methodology described by Taylor et al. [21] as a reference. FAMACHA scores were assessed based on conjunctiva color [22,23]. Weights were measured using a scale designed for small ruminants (BL300Pro, Laboremus, Campina Grande, Brazil).
General mixed linear models were used to analyze the EPG, FAMACHA, and WEIGHT variables, incorporating the random effect of the animal simultaneously with the fixed effects of breed and days of initiating biological control (included as covariate). We do not report the coefficient of determination (R²) because, in methodologies based on mixed models, the emphasis is placed on the significance of the regression coefficients (individually and jointly) associated with the model adjusted with fixed and random effects. The quality of the model adjustment is assessed using the Akaike Information Criterion [24] and complemented by the graphical evaluation of the observed means (with error bars representing the standard errors of the mean) and the function adjusted by the mixed model. The adjusted model had a fixed breed and monitoring day included as a covariate, in addition to random components of animal and error. The animal effect was included as a random effect to account for repeated measures on the same experimental units over monitoring periods. The covariance between measurements of the same animals was considered, allowing the evaluation of different covariance structures using the AIC criteria [24]. Statistical significance was set at p < 0.05. All analyses were conducted using the SAS Program, version 9.2 (SAS Institute Inc., Cary, NC, USA).
3. Results
The number of observations (N) and estimates of means (MED), standard deviations (SD), coefficients of variation (CV), minimum (MIN), and maximum (MAX) for EPG, FAMACHA, and WEIGHT are presented in Table 1. Significant effects (p < 0.05) were detected in the analyses of variance regarding the “start days” of biological control with D. flagrans on EPG, FAMACHA, and WEIGHT. Regression analyses of these variables in relation to the introduction day of D. flagrans are shown in Figure 1, Figure 2 and Figure 3. Each variable demonstrated a statistically significant quadratic model (p < 0.05). Table 2 provides the regression equations for predicting variable behavior based on the product’s introduction day.

Table 1.
Descriptive statistics for the EPG, FAMACHA, and WEIGHT variables.
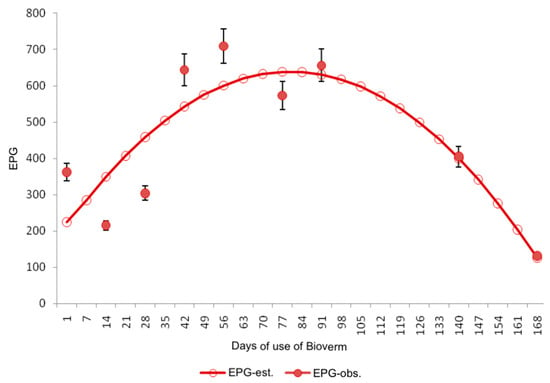
Figure 1.
Behavior of the EPG variable in relation to the start days of biological control with Bioverme® containing the nematophagous fungus D. flagrans.
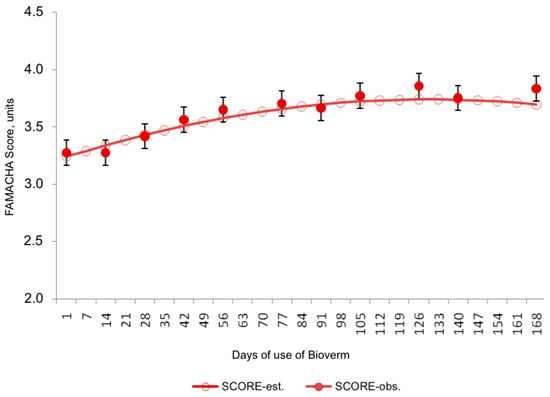
Figure 2.
Behavior of the FAMACHA variable in relation to the start days of biological control with Bioverme® containing the nematophagous fungus D. flagrans.
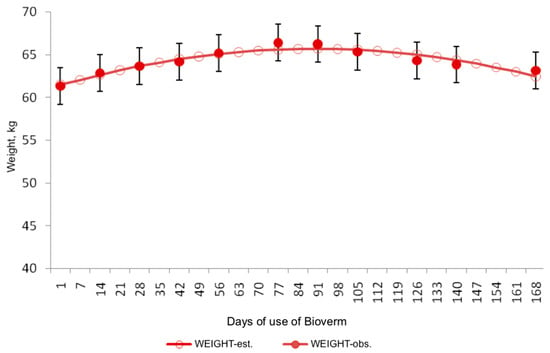
Figure 3.
Behavior of the WEIGHT variable in relation to the days of initiation of biological control with Bioverme® containing the nematophagous fungus D. flagrans.

Table 2.
Regression equations, points of.
4. Discussion
Haemonchus contortus is globally recognized as the most significant parasite affecting sheep farming, primarily due to its notable resistance to synthetic anthelmintics [8]. The development of resistant populations has been exacerbated by the indiscriminate use of these chemical agents.
Biological control presents an effective alternative for reducing parasite populations, utilizing natural antagonists such as nematode-predatory fungi [15,17]. These fungi produce structures like constricting and non-constricting rings, hyphae, buds, and three-dimensional adhesive networks along their mycelium. The process involves trapping the nematode, after which hyphae penetrate the cuticle, leading to hyphal development and digestion of the nematode’s internal contents [25]. Among these fungi, Duddingtonia flagrans is notable for its ability to pass through the ruminant digestive tract intact, significantly reducing the population of infective larvae in pastures and thereby lowering the risk of animal recontamination.
In this study, the efficacy of D. flagrans in its commercial form was assessed by analyzing EPG, FAMACHA, and WEIGHT variables. Our results show that the biological control effect on the EPG variable becomes apparent after approximately 80 days, attributed to the time required for fungal dissemination across pastures. This effect is characterized by a gradual decline in EPG values post the 80th day of D. flagrans application, as demonstrated in Figure 1, alongside a reduction in the variation of observed EPG, evidenced by decreased standard error estimates.
For the FAMACHA variable, the data reveal a steady increase in scores until they stabilize. Initial mean scores were 3.24 following deworming, peaking at 3.74 around 130 days later. These findings suggest a stronger correlation between the FAMACHA and other hemoparasite infestation indicators compared to EPG.
Regarding the WEIGHT variable, minimal fluctuations were observed throughout the experiment. The average animal weight started at 61.50 kg, peaked at 65.70 kg approximately 90 days after commencing product administration, and then slightly decreased to 62.46 kg. This minor fluctuation suggests that the nutritional management implemented was suitable for reproductive animals, where weight gain is not a primary objective.
The commercial product Bioverm® demonstrated encouraging outcomes, validating its legal registration in Brazil. The application of Bioverm® not only reduced the need for chemical anthelmintics but also decreased resistance to these agents, enabling the re-use of previously ineffective treatments. The farm continued using Bioverm® for several years post-experiment, deworming solely animals with clinical symptoms. Notably, the farm maintained a high stocking density, with over 500 animals on 15 hectares of pasture. Further studies corroborated the product’s efficacy across various species, including small ruminants [26,27], domestic buffalos [28], cattle [29,30], pigs [31], and horses [32,33]. Also, with cage-free layers (unpublished data).
Concerns about H. contortus extend beyond livestock production to include zoos and natural reserves, underscoring the resistance to synthetic anthelmintics [34]. This resistance poses a threat to a broader range of animals, especially those involved in One Conservation programs [35]. The long-term viability of species within these programs can be compromised by the introduction of resistant parasites, as seen in European bison (Bison bonasus) [34].
Research has shown D. flagrans’ efficacy in inhibiting larvae growth and viability in feces of various captive wild animals, including wapitis (Cervus canadensis) [36], Reticulated and Masai giraffes (Giraffa camelopardalis reticulata and G. c. tippelskirchi), scimitar-horned oryx (Oryx dammah), roan antelope (Hippotragus equinus), sable antelope (H. niger), gerenuk (Litocranius walleri) [37,38], grivet monkey (Chlorocebus aethiops) [39], plains zebra (Equus guagga) [39,40], African wild ass (Equus africanus) [40], blackbuck (Antilope cervicapra), Cuvier’s gazelle (Gazella cuvieri), American bison (Bison bison), sitatunga (Tragelaphus spekii), European mouflon (Ovis orientalis musimon) [41], and ostriches (Struthio camelus) [42]. The use of chemical parasiticides, common in livestock, appears to be the sole solution for parasites affecting ex situ wild animals [43]. However, integrating nematophagous fungi into the feed offers a promising, sustainable approach for reducing infective stages of roundworms in wildlife, contributing to sustainable parasitic infection management in zoos [44]. The use of nematophagous fungi in wild animal helminth management is safe, as the fungi act exclusively in the fecal matter without systemic effects. Therefore, the use of Bioverm® in captive wild animals, although off-label, should be considered a viable management strategy.
This study holds significant implications for animal production and health. By integrating D. flagrans, biological control reduces reliance on chemical treatments, cuts costs, and mitigates parasite resistance. This benefits breeders preserves herd health and positively impacts the environment by reducing the dependency on chemical agents. Its successful application may promote similar practices globally, advocating for sustainable methods in various animal breeding sectors.
5. Conclusions
The implementation of biological control using the nematophagous fungus Duddingtonia flagrans resulted in a gradual and significant reduction in EPG values after 80 days, alongside decreased variation in EPG among animals. Additionally, FAMACHA was inversely related to observed EPG values, indicating a more reliable correlation with hemoparasite infestation levels. Despite minimal variations in animal weight throughout the study, there was no indication that the dietary administration of D. flagrans negatively impacted the animals’ weight, supporting its efficacy as a biological control agent without adverse effects on animal growth. In conclusion, the biological control of nematodes using D. flagrans emerges as a strategic tool for managing parasitic worm infestations, allowing for the secure application of the product to prevent losses due to these parasites. Bioverm® shows potential for effective nematode management in small ruminants and beyond.
Author Contributions
P.N.J.-N.: Conceptualization, P.N.J.-N.; Data curation, P.N.J.-N.; Investigation, P.N.J.-N. and L.A.R.; Methodology, P.N.J.-N.; Project administration, P.N.J.-N.; Visualization, P.N.J.-N. and C.S.P.; Writing—original draft, P.N.J.-N., L.A.R., and J.C.d.C.B.; Writing—review and editing, P.N.J.-N. and C.S.P.; Formal analysis, J.C.d.C.B. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received no specific funding for this research.
Institutional Review Board Statement
Commercial sheep production is considered to be a zootechnical practice and falls outside the scope of regulation by Brazilian Federal Law no. 11,794/2008, as they do not pertain to teaching or scientific research activities [19]. Consequently, there is no requirement for approval from an ethics committee on the use of animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
This work utilized descriptive statistics and included all relevant data within the manuscript.
Conflicts of Interest
None of the authors have any conflicts of interest to declare.
Abbreviations
The following abbreviations are used in this manuscript:
| EPG | Eggs per gram of feces |
| FAMACHA | parasite infestation indicator scores |
References
- Devendra, C. Potential of Sheep and Goats in Less Developed Countries. J. Anim. Sci. 1980, 51, 461–473. [Google Scholar] [CrossRef]
- Timon, V.M.; Hanrahan, J.P. Small Ruminant Production in the Developing Countries; Fao Animal Production and Health Paper; FAO: Rome, Italy, 1985; ISBN 92-5-102343-3. [Google Scholar]
- Nygaard, D.F.; Amir, P. Research Strategies for Development: Improving Sheep and Goat Production in Developing Countries. In Increasing Small Ruminant Productivity in Semi-arid Areas; Thomson, E.F., Thomson, F.S., Eds.; Springer: Dordrecht, The Netherlands, 1988; pp. 37–50. ISBN 978-94-010-7086-7. [Google Scholar]
- Raineri, C.; Nunes, B.C.P.; Gameiro, A.H. Technological Characterization of Sheep Production Systems in B Razil. Anim. Sci. J. 2015, 86, 476–485. [Google Scholar] [CrossRef]
- Ali, S.; Zhao, Z.; Zhen, G.; Kang, J.Z.; Yi, P.Z. Reproductive Problems in Small Ruminants (Sheep and Goats): A Substantial Economic Loss in the World. Large Anim. Rev. 2019, 25, 215–223. [Google Scholar]
- Shivakumara, C.; Kiran, S. Economics of Sheep and Goat Rearing under Extensive, Semi-Intensive and Intensive Methods of Rearing. Econ. Aff. 2019, 64, 553–561. [Google Scholar] [CrossRef]
- Mazinani, M.; Rude, B. Population, World Production and Quality of Sheep and Goat Products. Am. J. Anim. Vet. Sci. 2020, 15, 291–299. [Google Scholar] [CrossRef]
- Wallera, P.J.; Chandrawathani, P. Haemonchus Contortus: Parasite Problem No. 1 from Tropics—Polar Circle. Problems and Prospects for Control Based on Epidemiology. Trop. Biomed. 2005, 22, 131–137. [Google Scholar]
- Vlassoff, A.; McKenna, P.B. Nematode Parasites of Economic Importance in Sheep in New Zealand. N. Z. J. Zool. 1994, 21, 1–8. [Google Scholar] [CrossRef]
- Veríssimo, C.J.; Niciura, S.C.M.; Alberti, A.L.L.; Rodrigues, C.F.C.; Barbosa, C.M.P.; Chiebao, D.P.; Cardoso, D.; Da Silva, G.S.; Pereira, J.R.; Margatho, L.F.F.; et al. Multidrug and Multispecies Resistance in Sheep Flocks from São Paulo State, Brazil. Vet. Parasitol. 2012, 187, 209–216. [Google Scholar] [CrossRef]
- Torres-Acosta, J.F.J.; Mendoza-de-Gives, P.; Aguilar-Caballero, A.J.; Cuéllar-Ordaz, J.A. Anthelmintic Resistance in Sheep Farms: Update of the Situation in the American Continent. Vet. Parasitol. 2012, 189, 89–96. [Google Scholar] [CrossRef]
- Besier, R.B.; Kahn, L.P.; Sargison, N.D.; Van Wyk, J.A. Diagnosis, Treatment and Management of Haemonchus Contortus in Small Ruminants. In Advances in Parasitology; Elsevier: Amsterdam, The Netherlands, 2016; Volume 93, pp. 181–238. ISBN 978-0-12-810395-1. [Google Scholar]
- Araújo, J.V.; Braga, F.R.; Mendoza-de-Gives, P.; Paz-Silva, A.; Vilela, V.L.R. Recent Advances in the Control of Helminths of Domestic Animals by Helminthophagous Fungi. Parasitologia 2021, 1, 168–176. [Google Scholar] [CrossRef]
- Bull, K.; Glover, M.J.; Rose Vineer, H.; Morgan, E.R. Increasing Resistance to Multiple Anthelmintic Classes in Gastrointestinal Nematodes on Sheep Farms in Southwest England. Vet. Rec. 2022, 190, e1531. [Google Scholar] [CrossRef]
- Mendoza-de Gives, P.; Braga, F.R.; Araújo, J.V.D. Nematophagous Fungi, an Extraordinary Tool for Controlling Ruminant Parasitic Nematodes and Other Biotechnological Applications. Biocontrol Sci. Technol. 2022, 32, 777–793. [Google Scholar] [CrossRef]
- Vilela, V.L.R.; Feitosa, T.F.; Braga, F.R.; de Araújo, J.V.; dos Santos, A.; De Morais, D.F.; De Oliveira Souto, D.V.; Athayde, A.C.R. Coadministration of Nematophagous Fungi for Biological Control over Gastrointestinal Helminths in Sheep in the Semiarid Region of Northeastern Brazil. Vet. Parasitol. 2016, 221, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Minguetto, J.G.M.; Bogado, A.L.G.; Okano, W.; Filho, L.F.C.D.C.; Da Silva, L.C.; Zanol, D.; Ferraz, C.M.; Moreira, T.F.; Tobias, F.L.; Braga, F.R.; et al. Biological Control of Gastrointestinal Nematodes in Young Ewes Treated with Fungi. Biocontrol Sci. Technol. 2021, 31, 499–511. [Google Scholar] [CrossRef]
- Braga, F.R.; De Araújo, J.V. Nematophagous Fungi for Biological Control of Gastrointestinal Nematodes in Domestic Animals. Appl. Microbiol. Biotechnol. 2014, 98, 71–82. [Google Scholar] [CrossRef]
- MCTIC Perguntas Frequentes Ao Concea. Available online: https://antigo.mctic.gov.br/mctic/opencms/institucional/concea/paginas/FAQs.html (accessed on 26 September 2023).
- Minho, A.P.; Gaspar, E.B.; Yoshihara, E. Manual de Técnicas Laboratoriais e de Campo Para a Realização de Ensaios Experimentais em Parasitologia Veterinária: Foco em Helmintos Gastrintestinais de Ruminantes; Documentos; Embrapa Pecuária Sul: Bagé, Brazil, 2015; ISBN 1982-5390. [Google Scholar]
- Laboratory Diagnosis of Parasitism. In Veterinary Parasitology; Taylor, M.A., Coop, R.L., Wall, R.L., Eds.; Wiley: Hoboken, NJ, USA, 2015; pp. 259–312. ISBN 978-0-470-67162-7. [Google Scholar]
- Van Wyk, J.A.; Bath, G.F. The FAMACHA System for Managing Haemonchosisin Sheep and Goats by Clinically Identifying Individual Animals for Treatment. Vet. Res. 2002, 33, 509–529. [Google Scholar] [CrossRef] [PubMed]
- Molento, M.B.; Tasca, C.; Gallo, A.; Ferreira, M.; Bononi, R.; Stecca, E. Método Famacha Como Parâmetro Clínico Individual de Infecção Por Haemonchus Contortus Em Pequenos Ruminantes. Ciênc. Rural 2004, 34, 1139–1145. [Google Scholar] [CrossRef]
- Akaike, H. Information Theory and an Extension of the Maximum Likelihood Principle. In Selected Papers of Hirotugu Akaike; Parzen, E., Tanabe, K., Kitagawa, G., Eds.; Springer Series in Statistics; Springer: New York, NY, USA, 1998; pp. 199–213. ISBN 978-1-4612-7248-9. [Google Scholar]
- Mota, M.D.A.; Campos, A.K.; Araújo, J.V.D. Controle Biológico de Helmintos Parasitos de Animais: Estágio Atual e Perspectivas Futuras. Pesqui. Veter. Bras. 2003, 23, 93–100. [Google Scholar] [CrossRef]
- Braga, F.R.; Ferraz, C.M.; Da Silva, E.N.; De Araújo, J.V. Efficiency of the Bioverm® (Duddingtonia flagrans) Fungal Formulation to Control in Vivo and in Vitro of Haemonchus Contortus and Strongyloides Papillosus in Sheep. 3 Biotech 2020, 10, 62. [Google Scholar] [CrossRef]
- Rodrigues, J.A.; Roque, F.L.; Lima, B.A.; Silva Filho, G.M.; Oliveira, C.S.M.; Sousa, L.C.; Silva, A.L.P.; Lima, E.F.; Feitosa, T.F.; Braga, F.R.; et al. Control of Sheep Gastrointestinal Nematodes on Pasture in the Tropical Semiarid Region of Brazil, Using Bioverm® (Duddingtonia flagrans). Trop. Anim. Health Prod. 2022, 54, 179. [Google Scholar] [CrossRef]
- Mendes, L.Q.; Ferraz, C.M.; Ribeiro, N.R.C.; Ulfeldt, K.B.; Ribeiro, J.C.C.; Merizio, M.F.; Rossi, G.A.M.; Aguiar, A.A.R.M.; Araújo, J.V.D.; Soares, F.E.D.F.; et al. Efficacy of Duddingtonia flagrans (Bioverm®) on the Biological Control of Buffalo Gastrointestinal Nematodes. Exp. Parasitol. 2023, 253, 108592. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.A.; Roque, F.L.; Álvares, F.B.V.; Silva, A.L.P.D.; Lima, E.F.D.; Silva Filho, G.M.D.; Feitosa, T.F.; Araújo, J.V.D.; Braga, F.R.; Vilela, V.L.R. Efficacy of a Commercial Fungal Formulation Containing Duddingtonia flagrans (Bioverm®) for Controlling Bovine Gastrointestinal Nematodes. Rev. Bras. Parasitol. Veter. 2021, 30, e026620. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, L.D.S.S.C.B.; Dias, F.G.S.; Melo, A.L.T.; De Carvalho, L.M.; Silva, E.N.; Araújo, J.V.D. Bioverm® in the Control of Nematodes in Beef Cattle Raised in the Central-West Region of Brazil. Pathogens 2021, 10, 548. [Google Scholar] [CrossRef] [PubMed]
- Roque, F.L.; Silva Filho, G.M.; Oliveira, C.S.M.; Rodrigues, J.A.; Feitosa, T.F.; Braga, F.R.; Araújo, J.V.; Vilela, V.L.R. Avaliação Do Fungo Duddingtonia flagrans (Bioverm®) Sobre Ovos de Ascaris Suum e Larvas Infectantes de Oesophagostomum spp. e Hyostrongylus Rubidus de Suínos. Semin. Ciênc. Agrár. 2023, 44, 1587–1596. [Google Scholar] [CrossRef]
- Fausto, G.C.; Fausto, M.C.; Vieira, Í.S.; Freitas, S.G.D.; Carvalho, L.M.D.; Oliveira, I.D.C.; Silva, E.N.; Campos, A.K.; Araújo, J.V.D. Formulation of the Nematophagous Fungus Duddingtonia flagrans in the Control of Equine Gastrointestinal Parasitic Nematodes. Vet. Parasitol. 2021, 295, 109458. [Google Scholar] [CrossRef]
- Nunes, G.T.; Corrêa, D.C.; Chitolina, M.B.; Da Rosa, G.; Pereira, R.C.D.F.; Cargnelutti, J.F.; Vogel, F.S.F. Efficacy Evaluation of a Commercial Formulation with Duddingtonia flagrans in Equine Gastrointestinal Nematodes. J. Equine Vet. Sci. 2023, 131, 104930. [Google Scholar] [CrossRef]
- Springer, A.; Kloene, P.; Strube, C. Benzimidazole Resistant Haemonchus Contortus in a Wildlife Park. Schweiz Arch Tierheilkd 2022, 164, 51–59. [Google Scholar] [CrossRef]
- Pizzutto, C.S.; Colbachini, H.; Jorge-Neto, P.N. One Conservation: The Integrated View of Biodiversity Conservation. Anim. Reprod. 2021, 18, e20210024. [Google Scholar] [CrossRef]
- Palomero, A.M.; Cazapal-Monteiro, C.F.; Viña, C.; Hernández, J.Á.; Voinot, M.; Vilá, M.; Silva, M.I.; Paz-Silva, A.; Sánchez-Andrade, R.; Sol Arias, M. Formulating Fungal Spores to Prevent Infection by Trichostrongylids in a Zoological Park: Practical Approaches to a Persisting Problem. Biol. Control 2021, 152, 104466. [Google Scholar] [CrossRef]
- Terry, J. The Use of Duddingtonia flagrans for Gastrointestinal Parasitic Nematode Control in Feces of Exotic Artiodactylids at Disney’s Animal Kingdom®. Master’s Thesis, Louisiana State University and Agricultural and Mechanical College, Baton Rouge, LA, USA, 2013. [Google Scholar]
- Young, K. The Effect of the Nematode Trapping Fungus Duddingtonia flagrans against Gastronintestinal Nematodes of Exotic Ruminant Hoofstock at Disney’s® Animal Kingdom Lodge. Master’s Thesis, Louisiana State University and Agricultural and Mechanical College, Baton Rouge, LA, USA, 2018. [Google Scholar]
- Reis, R.F.D. Diagnóstico e controlo biológico de nematodes gastrointestinais nos mamíferos selvagens mantidos em cativeiro no “Monte Selvagem—Reserva Animal”. Master’s Thesis, Faculdade de Medicina Veterinária da Universidade de Lisboa, Lisboa, Portugal, 2019. [Google Scholar]
- Arias, M.; Cazapal-Monteiro, C.; Valderrábano, E.; Miguélez, S.; Rois, J.L.; López-Arellano, M.E.; Madeira De Carvalho, L.; Mendoza De Gives, P.; Sánchez-Andrade, R.; Paz-Silva, A. A Preliminary Study of the Biological Control of Strongyles Affecting Equids in a Zoological Park. J. Equine Vet. Sci. 2013, 33, 1115–1120. [Google Scholar] [CrossRef]
- Palomero, A.M.; Cazapal-Monteiro, C.F.; Valderrábano, E.; Paz-Silva, A.; Sánchez-Andrade, R.; Arias, M.S. Soil Fungi Enable the Control of Gastrointestinal Nematodes in Wild Bovidae Captive in a Zoological Park: A 4-Year Trial. Parasitology 2020, 147, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Braga, F.R.; Araújo, J.V.; Tavela, A.D.O.; Vilela, V.L.R.; Soares, F.E.D.F.; Araujo, J.M.; Magalhães, L.Q.; Silveira, W.F.D.; Feitosa, T.F.; Dantas, E.S.; et al. First Report of Interaction of Nematophagous Fungi on Libyostrongylus Douglassii (Nematoda: Trichostrongylidae). Rev. Bras. Parasitol. Veter. 2013, 22, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Salmo, R.; Viña, C.; Lozano, J.; Palomero, A.M.; Hernández, J.Á.; Bonilla, R.; Sánchez-Andrade, R.; Paz-Silva, A.; Madeira De Carvalho, L.M.; Arias, M.S.; et al. Saprophytic Filamentous Fungi against Helminths Affecting Captive Wild Animals. Encyclopedia 2024, 4, 91–100. [Google Scholar] [CrossRef]
- Panayotova-Pencheva, M.S. Control of Helminth Infections in Captive Herbivores: An Overview of Experience. J. Zool. Bot. Gard. 2024, 5, 641–667. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).