The Richness of Sarcocystis Species in the Common Gull (Larus canus) and Black-Headed Gull (Larus ridibundus) from Lithuania
Abstract
1. Introduction
2. Results
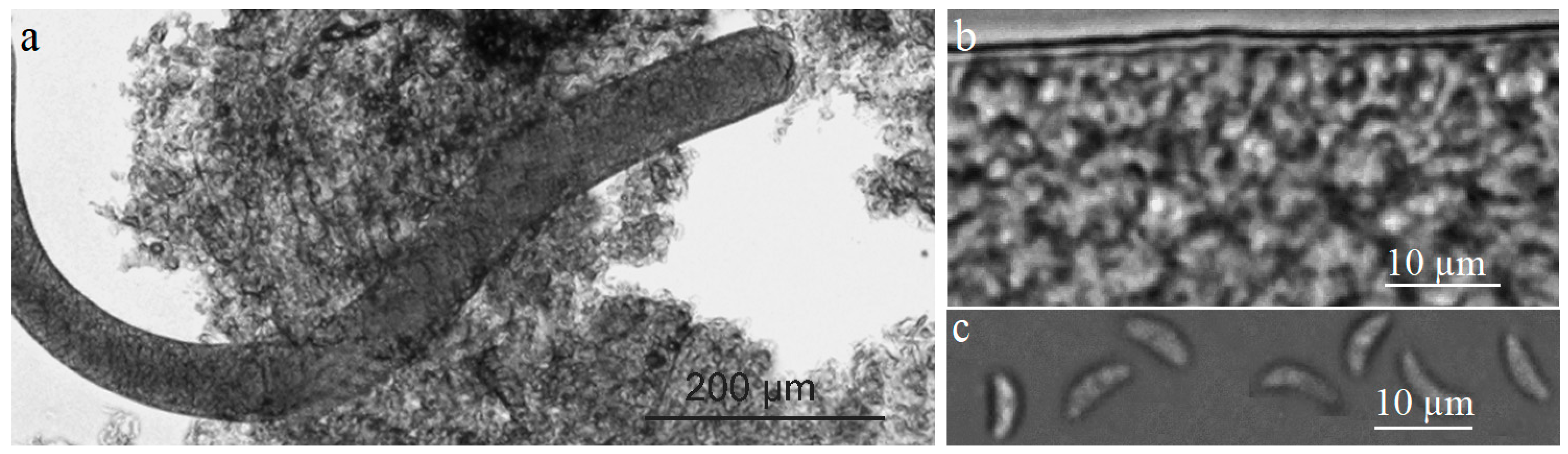
2.1. Morphological Examination of Sarcocystis spp. Detected in Common Gull and Black-Headed Gull
2.2. Genetic Identification and Intraspecific Variation of Sarcocystis spp.
3. Discussion
3.1. Similar Morphology of Saroccystis spp. in Gulls
3.2. The Richness and Possible Pathogenicity of Sarcocystis Species in Gulls
4. Materials and Methods
4.1. Collection of Samples
4.2. Morphological Examination of Sarcocysts
4.3. Molecular Analysis of Sarcocystis spp.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dubey, J.P.; Calero-Bernal, R.; Rosenthal, B.M.; Speer, C.A.; Fayer, R. Sarcocystosis of Animals and Humans, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Fayer, R. Sarcocystis spp. in human infections. Clin. Microbiol. Rev. 2004, 17, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Černá, Z. The role of birds as definitive hosts and intermediate hosts of heteroxenous coccidians. J. Protozool. 1984, 31, 579–581. [Google Scholar] [CrossRef]
- Odening, K. The present state of species-systematics in Sarcocystis Lankester, 1882 (Protista, Sporozoa, Coccidia). Syst. Parasitol. 1998, 41, 209–233. [Google Scholar] [CrossRef]
- Máca, O.; González-Solís, D. Sarcocystis cristata sp. nov. (Apicomplexa, Sarcocystidae) in the imported great blue turaco Corythaeola cristata (Aves, Musophagidae). Parasit. Vectors 2021, 14, 56. [Google Scholar] [CrossRef]
- Tenter, A.M. Current research on Sarcocystis species of domestic animals. Int. J. Parasitol. 1995, 25, 1311–1330. [Google Scholar] [CrossRef] [PubMed]
- Box, E.D.; Meier, J.L.; Smith, J.H. Description of Sarcocystis falcatula Stiles, 1983, a parasite of birds and opossum. J. Protozool. 1984, 31, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Olias, P.; Gruber, A.D.; Hafez, H.M.; Heydorn, A.O.; Mehlhorn, H.; Lierz, M. Sarcocystis calchasi sp. nov. of the domestic pigeon (Columba livia f. domestica) and the Northern goshawk (Accipiter gentilis): Light and electron microscopical characteristics. Parasitol. Res. 2010, 106, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Bamac, O.E.; Rogers, K.H.; Arranz-Solís, D.; Saeij, J.P.J.; Lewis, S.; Duerr, R.; Skoglund, J.; Peronne, L.; Mete, A. Protozoal encephalitis associated with Sarcocystis calchasi and S. falcatula during an epizootic involving Brandt’s cormorants (Phalacrocorax penicillatus) in coastal Southern California, USA. Int. J. Parasitol. Parasites Wildl. 2020, 12, 185–191. [Google Scholar] [CrossRef]
- Sibley, C.G.; Ahlquist, J.E. Phylogeny and Classification of Birds; Yale University Press: New Haven, CT, USA; London, UK, 1990. [Google Scholar]
- Burger, J.; Gochfeld, M.; Bonan, A. Gulls, Terns, Skimmers (Laridae). In Handbook of the Birds of the World Alive; del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A., de Juana, E., Eds.; Lynx Edicions: Barcelona, Spain, 2020. [Google Scholar] [CrossRef]
- Logminas, V.; Nedzinskas, V.; Drobelis, E.; Petraitis, A.; Patapavičius, R.; Žalakevičius, M.; Valius, M.; Šablevičius, B.; Gražulevičius, G.; Raudonikis, L.; et al. Lithuanian Fauna. In Birds; Mokslas Press: Vilnius, Lithuania, 1990; Volume 2. [Google Scholar]
- Kurlavičius, P.; Preikša, Ž.; Skuja, S.; Kirstukas, M.; Brazaitis, G.; Stanevičius, V.; Mačiulis, M.; Jusys, V.; Butleris, A.; Raudonikis, L.; et al. Lithuanian Breeding Bird Atlas; Lututė Press: Kaunas, Lithuania, 2006. [Google Scholar]
- Méndez, A.; Montalvo, T.; Aymí, R.; Carmona, M.; Figuerola, J.; Navarro, J. Adapting to urban ecosystems: Unravelling the foraging ecology of an opportunistic predator living in cities. Urban Ecosyst. 2020, 23, 1117–1126. [Google Scholar] [CrossRef]
- Martín-Vélez, V.; Montalvo, T.; Afán, I.; Sánchez-Márquez, A.; Aymí, R.; Figuerola, J.; Lovas-Kiss, Á.; Navarro, J. Gulls living in cities as overlooked seed dispersers within and outside urban environments. Sci. Total Environ. 2022, 823, 153535. [Google Scholar] [CrossRef]
- Vernon, R.D.J. Feeding Habitats and Food of the Black-headed and Common Gulls. Part 2—Food. Bird Study 1972, 19, 173–186. [Google Scholar] [CrossRef]
- Kubetzki, U.; Garthe, S. Distribution, diet and habitat selection by four sympatrically breeding gull species in the south-eastern North Sea. Mar. Biol. 2003, 143, 199–207. [Google Scholar] [CrossRef]
- Prakas, P.; Butkauskas, D.; Juozaitytė-Ngugu, E. Molecular identification of four Sarcocystis species in the herring gull, Larus argentatus, from Lithuania. Parasit. Vectors 2020, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Prakas, P.; Kutkienė, L.; Sruoga, A.; Butkauskas, D. Sarcocystis sp. from the herring gull (Larus argentatus) identity to Sarcocystis wobeseri based on cyst morphology and DNA results. Parasitol. Res. 2011, 109, 1603–1608. [Google Scholar] [CrossRef]
- Prakas, P.; Kutkienė, L.; Butkauskas, D.; Sruoga, A.; Žalakevičius, M. Description of Sarcocystis lari sp. n. (Apicomplexa: Sarcocystidae) from the great black-backed gull, Larus marinus (Charadriiformes: Laridae), on the basis of cyst morphology and molecular data. Folia Parasitol. 2014, 61, 11–17. [Google Scholar] [CrossRef]
- Acosta, I.C.L.; Soares, R.M.; Mayorga, L.F.S.P.; Alves, B.F.; Soares, H.S.; Gennari, S.M. Correction: Occurrence of tissue cyst forming coccidia in Magellanic penguins (Spheniscus magellanicus) rescued on the coast of Brazil. PLoS ONE 2019, 14, e0212467. [Google Scholar] [CrossRef]
- Sato, A.P.; da Silva, T.C.E.; de Pontes, T.P.; Sanches, A.W.D.; Prakas, P.; Locatelli-Dittrich, R. Molecular characterization of Sarcocystis spp. in seabirds from southern Brazil. Parasitol. Int. 2022, 90, 102595. [Google Scholar] [CrossRef]
- Rudaitytė-Lukošienė, E.; Prakas, P.; Butkauskas, D.; Kutkienė, L.; Vepštaitė-Monstavičė, I.; Servienė, E. Morphological and molecular identification of Sarcocystis spp. from the sika deer (Cervus nippon), including two new species Sarcocystis frondea and Sarcocystis nipponi. Parasitol. Res. 2018, 117, 1305–1315. [Google Scholar] [CrossRef] [PubMed]
- Rudaitytė-Lukošienė, E.; Delgado de Las Cuevas, G.E.; Prakas, P.; Calero-Bernal, R.; Martínez-González, M.; Strazdaitė-Žielienė, Ž.; Servienė, E.; Habela, M.A.; Butkauskas, D. Sarcocystis spp. diversity in the roe deer (Capreolus capreolus) from Lithuania and Spain. Parasitol. Res. 2020, 119, 1363–1370. [Google Scholar] [CrossRef]
- Juozaitytė-Ngugu, E.; Butkauskas, D.; Švažas, S.; Prakas, P. Investigations on Sarcocystis species in the leg muscles of the bird family Corvidae in Lithuania. Parasitol. Res. 2022, 121, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Rubiola, S.; Civera, T.; Ferroglio, E.; Zanet, S.; Zaccaria, T.; Brossa, S.; Cipriani, R.; Chiesa, F. Molecular differentiation of cattle Sarcocystis spp. by multiplex PCR targeting 18S and COI genes following identification of Sarcocystis hominis in human stool samples. Food Waterborne Parasitol. 2020, 21, e00074. [Google Scholar] [CrossRef] [PubMed]
- Drouin, T.E.; Mahrt, J.L. The prevalence of Sarcocystis Lankester, 1882, in some bird species in western Canada, with notes on its life cycle. Can. J. Zool. 1979, 57, 1915–1921. [Google Scholar] [CrossRef] [PubMed]
- Pak, S.M.; Eshtokina, N.V. Sarcosporidians of birds. In Sarcosporidians of Animals in Kazakhstan; Almaty: Nauka, Kazakhstan, 1984. [Google Scholar]
- Kutkienė, L.; Prakas, P.; Sruoga, A.; Butkauskas, D. The mallard duck (Anas platyrhynchos) as intermediate host for Sarcocystis wobeseri sp. nov. from the barnacle goose (Branta leucopsis). Parasitol. Res. 2010, 107, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Kutkienė, L.; Prakas, P.; Sruoga, A.; Butkauskas, D. Description of Sarcocystis anasi sp. nov. and Sarcocystis albifronsi sp. nov. in birds of the order Anseriformes. Parasitol. Res. 2012, 110, 1043–1046. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, M.; Wu, Z.; Zeng, H.; Tao, J. Description of Sarcocystis platyrhynchosi n. sp. (Apicomplexa: Sarcocystidae) from domestic ducks Anas platyrhynchos (Anseriformes: Anatidae) in China. Parasit. Vectors 2023, 16, 50. [Google Scholar] [CrossRef] [PubMed]
- Olias, P.; Olias, L.; Lierz, M.; Mehlhorn, H.; Gruber, A.D. Sarcocystis calchasi is distinct to Sarcocystis columbae sp. nov. from the wood pigeon (Columba palumbus) and Sarcocystis sp. from the sparrowhawk (Accipiter nisus). Vet. Parasitol. 2010, 171, 7–14. [Google Scholar] [CrossRef]
- Prakas, P.; Butkauskas, D.; Sruoga, A.; Švažas, S.; Kutkienė, L. Identification of Sarcocystis columbae in wood pigeons (Columba palumbus) in Lithuania. Vet. Zootech. 2011, 55, 33–39. [Google Scholar]
- Prakas, P.; Butkauskas, D.; Švažas, S.; Stanevičius, V. Morphological and genetic characterisation of Sarcocystis halieti from the great cormorant (Phalacrocorax carbo). Parasitol. Res. 2018, 117, 3663–3667. [Google Scholar] [CrossRef] [PubMed]
- Gjerde, B.; Vikøren, T.; Hamnes, I.S. Molecular Identification of Sarcocystis halieti n. sp., Sarcocystis lari and Sarcocystis truncata in the Intestine of a White-tailed Sea Eagle (Haliaeetus albicilla) in Norway. Int. J. Parasitol. Parasites Wildl. 2018, 7, 1–11. [Google Scholar] [CrossRef]
- Maier-Sam, K.; Kaiponen, T.; Schmitz, A.; Schulze, C.; Bock, S.; Hlinak, A.; Olias, P. Encephalitis Associated with Sarcocystis halieti Infection in a Free-Ranging Little Owl (Athene noctua). J. Wildl. Dis. 2021, 57, 712–714. [Google Scholar] [CrossRef] [PubMed]
- Prakas, P.; Bea, A.; Juozaitytė-Ngugu, E.; Olano, I.; Villanúa, D.; Švažas, S.; Butkauskas, D. Molecular identification of Sarcocystis halieti in the muscles of two species of birds of prey from Spain. Parasit. Vectors 2021, 14, 414. [Google Scholar] [CrossRef]
- Rogers, K.H.; Arranz-Solís, D.; Saeij, J.P.J.; Lewis, S.; Mete, A. Sarcocystis calchasi and other Sarcocystidae detected in predatory birds in California, USA. Int. J. Parasitol. Parasites Wildl. 2021, 17, 91–99. [Google Scholar] [CrossRef]
- Llano, H.A.B.; Zavatieri Polato, H.; Borges Keid, L.; Ferreira de Souza Oliveira, T.M.; Zwarg, T.; de Oliveira, A.S.; Sanches, T.C.; Joppert, A.M.; Gondim, L.F.P.; Martins Soares, R. Molecular screening for Sarcocystidae in muscles of wild birds from Brazil suggests a plethora of intermediate hosts for Sarcocystis falcatula. Int. J. Parasitol. Parasites Wildl. 2022, 17, 230–238. [Google Scholar] [CrossRef]
- Máca, O.; González-Solís, D. Role of three bird species in the life cycle of two Sarcocystis spp. (Apicomplexa, Sarcocystidae) in the Czech Republic. Int. J. Parasitol. Parasites Wildl. 2022, 17, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Hodo, C.L.; Whitley, D.B.; Hamer, S.A.; Corapi, W.V.; Snowden, K.; Heatley, J.J.; Hoffmann, A.R. Histopathologic and Molecular Characterization of Sarcocystis calchasi Encephalitis in White-winged Doves (Zenaida asiatica) and Eurasian Collared Doves (Streptopelia decaocto), East-central Texas, USA, 2010–2013. J. Wildl. Dis. 2016, 52, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, L.; Parmentier, S.L.; Fischer, D.; Heckmann, J.; Klopfleisch, R.; Kershaw, O.; Ziegler, U.; Neurath, H.; Schmidt, V.; Lierz, M. Investigations into causes of neurologic signs and mortality and the first identification of Sarcocystis calchasi in free-ranging woodpeckers in Germany. J. Zoo Wildl. Med. 2018, 49, 247–251. [Google Scholar] [CrossRef]
- Mete, A.; Rogers, K.H.; Wolking, R.; Bradway, D.S.; Kelly, T.; Piazza, M.; Crossley, B. Sarcocystis calchasi Outbreak in Feral Rock Pigeons (Columba livia) in California. Vet. Pathol. 2019, 56, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Parmentier, S.L.; Maier-Sam, K.; Failing, K.; Gruber, A.D.; Lierz, M. High prevalence of Sarcocystis calchasi in racing pigeon flocks in Germany. PLoS ONE 2019, 14, e0215241. [Google Scholar] [CrossRef] [PubMed]
- Söderström, M.; Malkamäki, S.; Sukura, A.; Sainmaa, S.; Airas, N. Sarcocystis calchasi in a captive Patagonian conure (Cyanoliseus patagonus) in Finland. J. Comp. Pathol. 2021, 189, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Gadsby, S.; Garner, M.M.; Bolin, S.R.; Sanchez, C.R.; Flaminio, K.P.; Sim, R.R. Fatal Sarcocystis calchasi-associated meningoencephalitis in 2 captive vulturine guineafowl. J. Vet. Diagn. Investig. 2022, 34, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Gonzales-Viera, O.; Arranz-Solís, D.; Smith, J.; Saeij, J.P.J.; Mete, A. Fatal Sarcocystis calchasi hepatitis in a captive Indian ringneck parakeet (Psittacula krameri manillensis). Vet. Parasitol. Reg. Stud. Reports 2023, 39, 100841. [Google Scholar] [CrossRef] [PubMed]
- Prakas, P.; Butkauskas, D.; Juozaitytė-Ngugu, E. Molecular and morphological description of Sarcocystis kutkienae sp. nov. from the common raven (Corvus corax). Parasitol. Res. 2020, 119, 4205–4210. [Google Scholar] [CrossRef] [PubMed]
- Leveau, L.M. Urbanization, environmental stabilization and temporal persistence of bird species: A view from Latin America. PeerJ 2018, 6, e6056. [Google Scholar] [CrossRef] [PubMed]
- Gjerde, B. Molecular characterisation of Sarcocystis rileyi from a common eider (Somateria mollissima) in Norway. Parasitol. Res. 2014, 113, 3501–3509. [Google Scholar] [CrossRef] [PubMed]
- Prakas, P.; Butkauskas, D.; Švažas, S.; Juozaitytė-Ngugu, E.; Stanevičius, V. Morphologic and genetic identification of Sarcocystis fulicae n. sp. (Apicomplexa: Sarcocystidae) from the Eurasian coot (Fulica atra). J. Wildl. Dis. 2018, 54, 765–771. [Google Scholar] [CrossRef]

| Bird Species | Isolate Numbers | Sarcocystis Species and GenBank Accession Numbers | ||
|---|---|---|---|---|
| S. columbae | S. halieti | S. wobeseri | ||
| Common gull | LcLt17.1–17.4 | OP419605–OP419608 | ||
| Common gull | LcLt20.1–20.4 | OP419609–OP419612 | ||
| Common gull | LcLt34.1–34.4 | OP419613 | OP419614–OP419616 | |
| Common gull | LcLt38.1–38.4 | OP419617–OP419620 | ||
| Common gull | LcLt41.1–41.4 | OP419621–OP419624 | ||
| Black-headed gull | LrLt7.1–7.4 | OP419625–OP419629 | ||
| Black-headed gull | LrLt13.1–13.4 | OP419630–OP419633 | ||
| Black-headed gull | LrLt49.1–49.4 | OP419634–OP419637 | ||
| Black-headed gull | LrLt53.1–53.4 | OP419640–OP419641 | OP419638–OP419639 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juozaitytė-Ngugu, E.; Prakas, P. The Richness of Sarcocystis Species in the Common Gull (Larus canus) and Black-Headed Gull (Larus ridibundus) from Lithuania. Parasitologia 2023, 3, 172-180. https://doi.org/10.3390/parasitologia3020018
Juozaitytė-Ngugu E, Prakas P. The Richness of Sarcocystis Species in the Common Gull (Larus canus) and Black-Headed Gull (Larus ridibundus) from Lithuania. Parasitologia. 2023; 3(2):172-180. https://doi.org/10.3390/parasitologia3020018
Chicago/Turabian StyleJuozaitytė-Ngugu, Evelina, and Petras Prakas. 2023. "The Richness of Sarcocystis Species in the Common Gull (Larus canus) and Black-Headed Gull (Larus ridibundus) from Lithuania" Parasitologia 3, no. 2: 172-180. https://doi.org/10.3390/parasitologia3020018
APA StyleJuozaitytė-Ngugu, E., & Prakas, P. (2023). The Richness of Sarcocystis Species in the Common Gull (Larus canus) and Black-Headed Gull (Larus ridibundus) from Lithuania. Parasitologia, 3(2), 172-180. https://doi.org/10.3390/parasitologia3020018







