Development of Ti PVD Films to Limit the Carburization of Metal Powders during SPS Process
Abstract
:1. Introduction
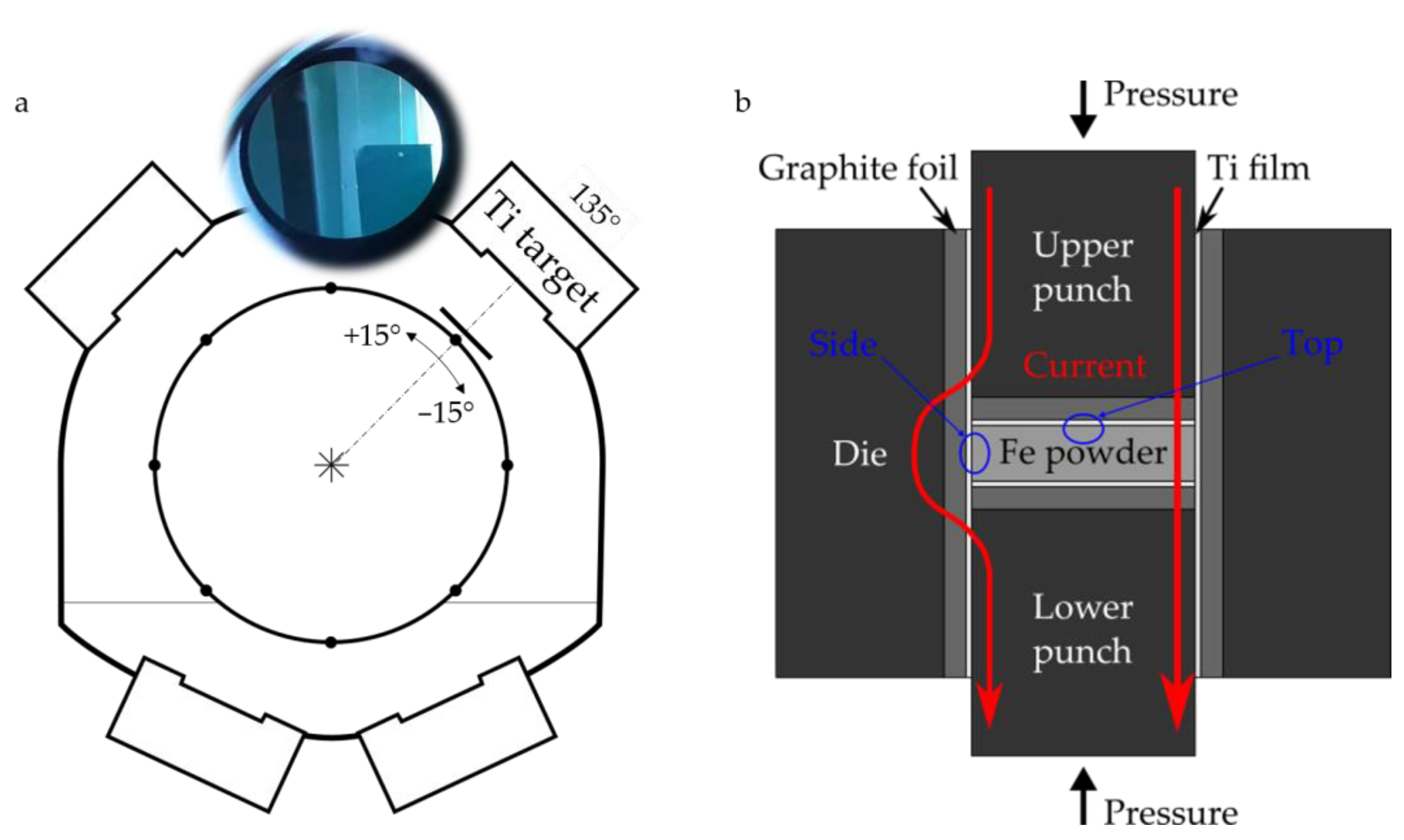
2. Materials and Methods
3. Results and Discussion
3.1. Sintering of Pure Iron Using Graphite Foils without Coating
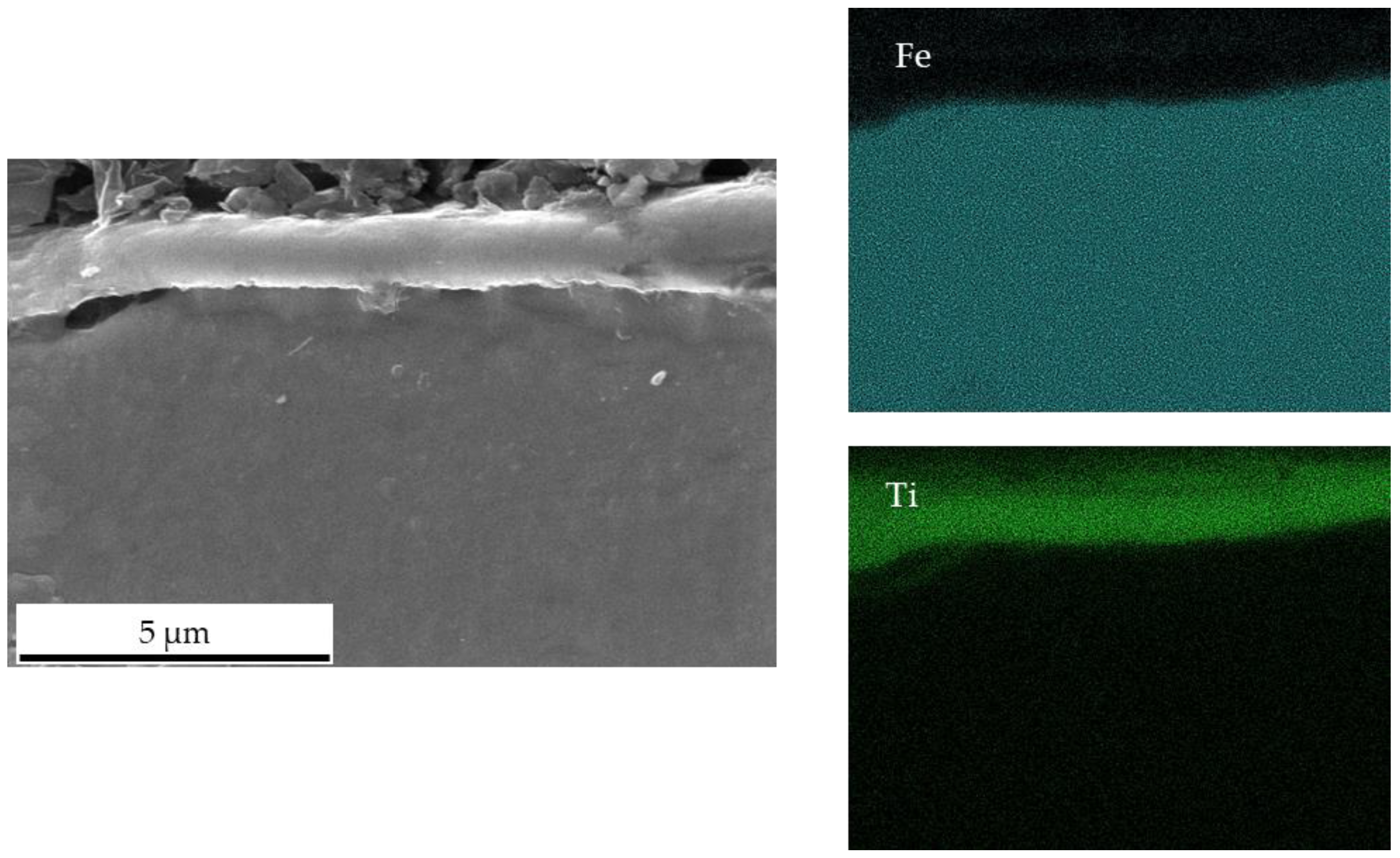
3.2. Sintering of Pure Iron Using Graphite Foils Coated with a Ti PVD Film
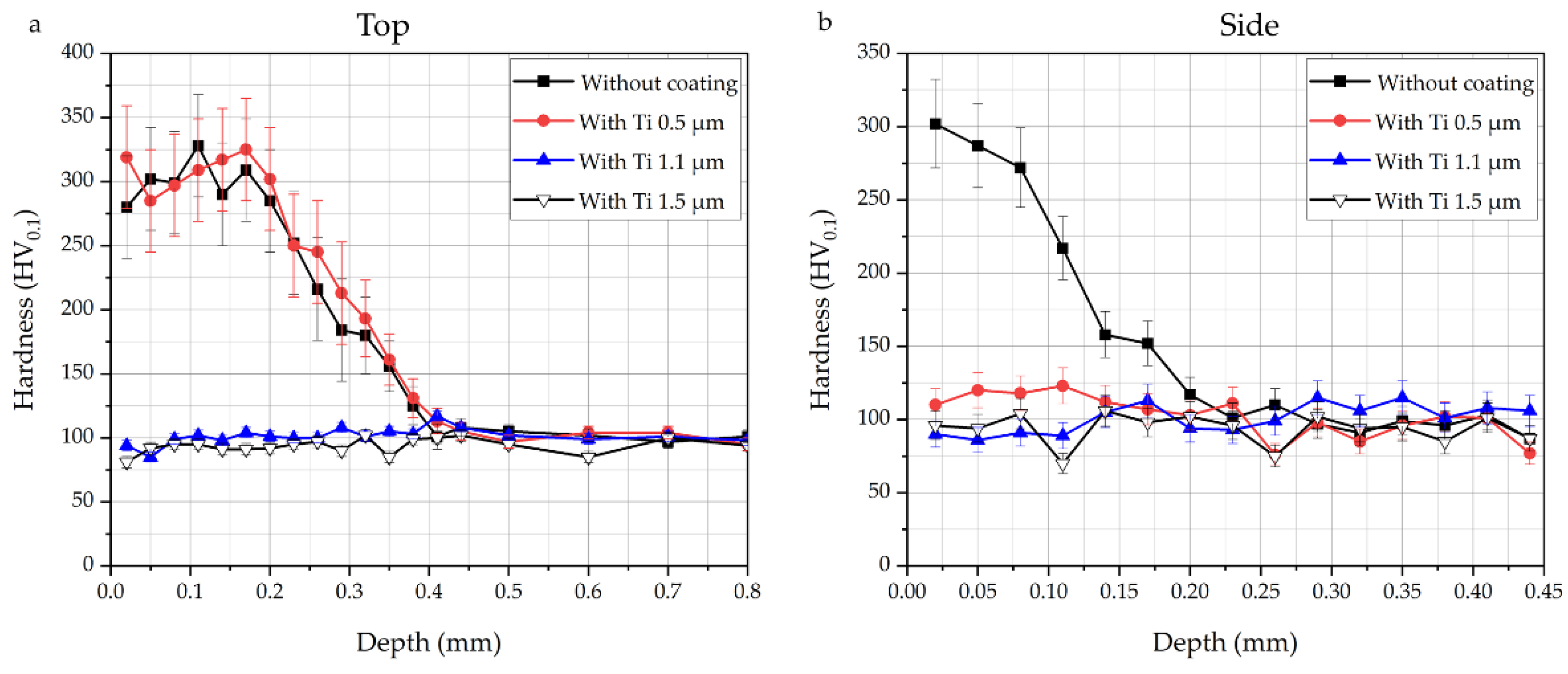
3.3. Mechanical Properties
4. Conclusions
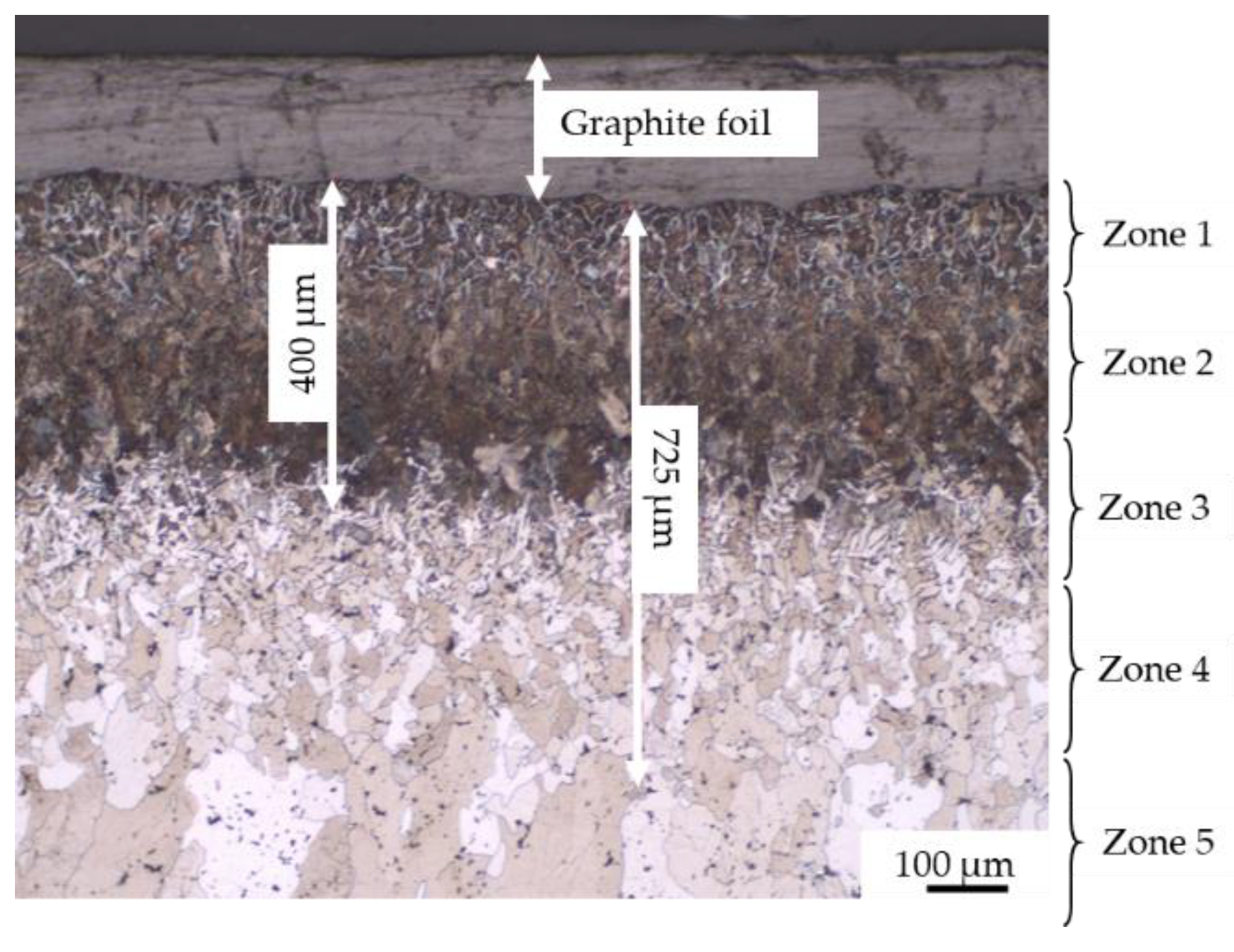
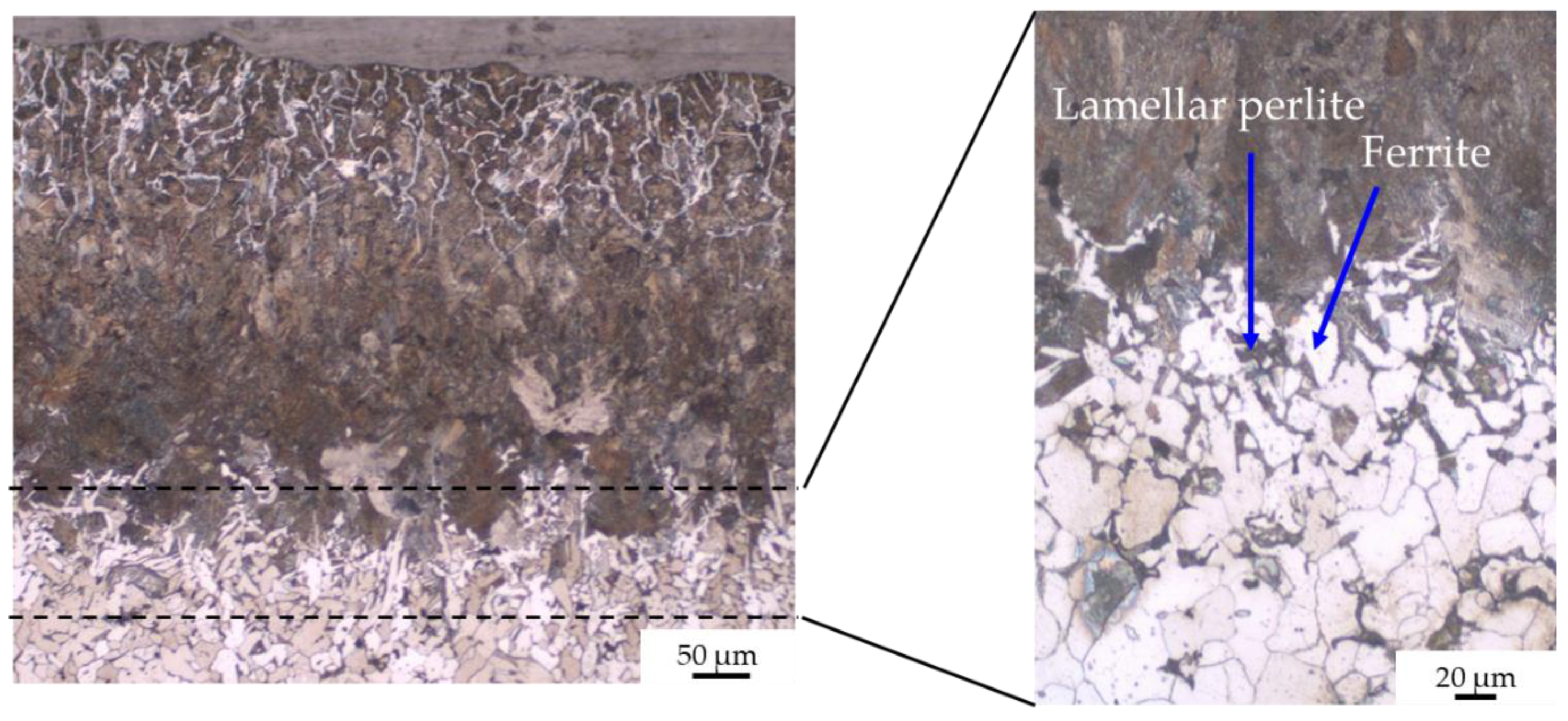
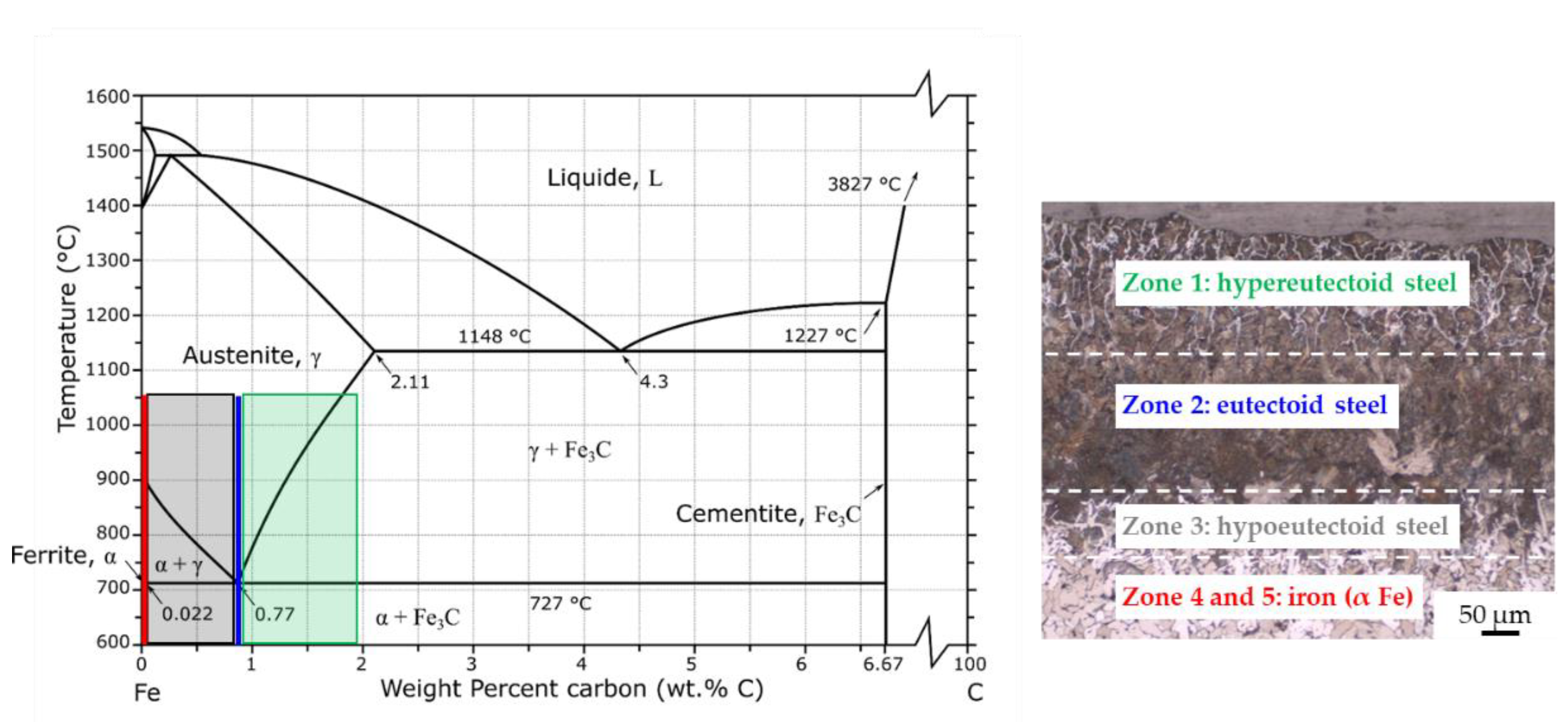
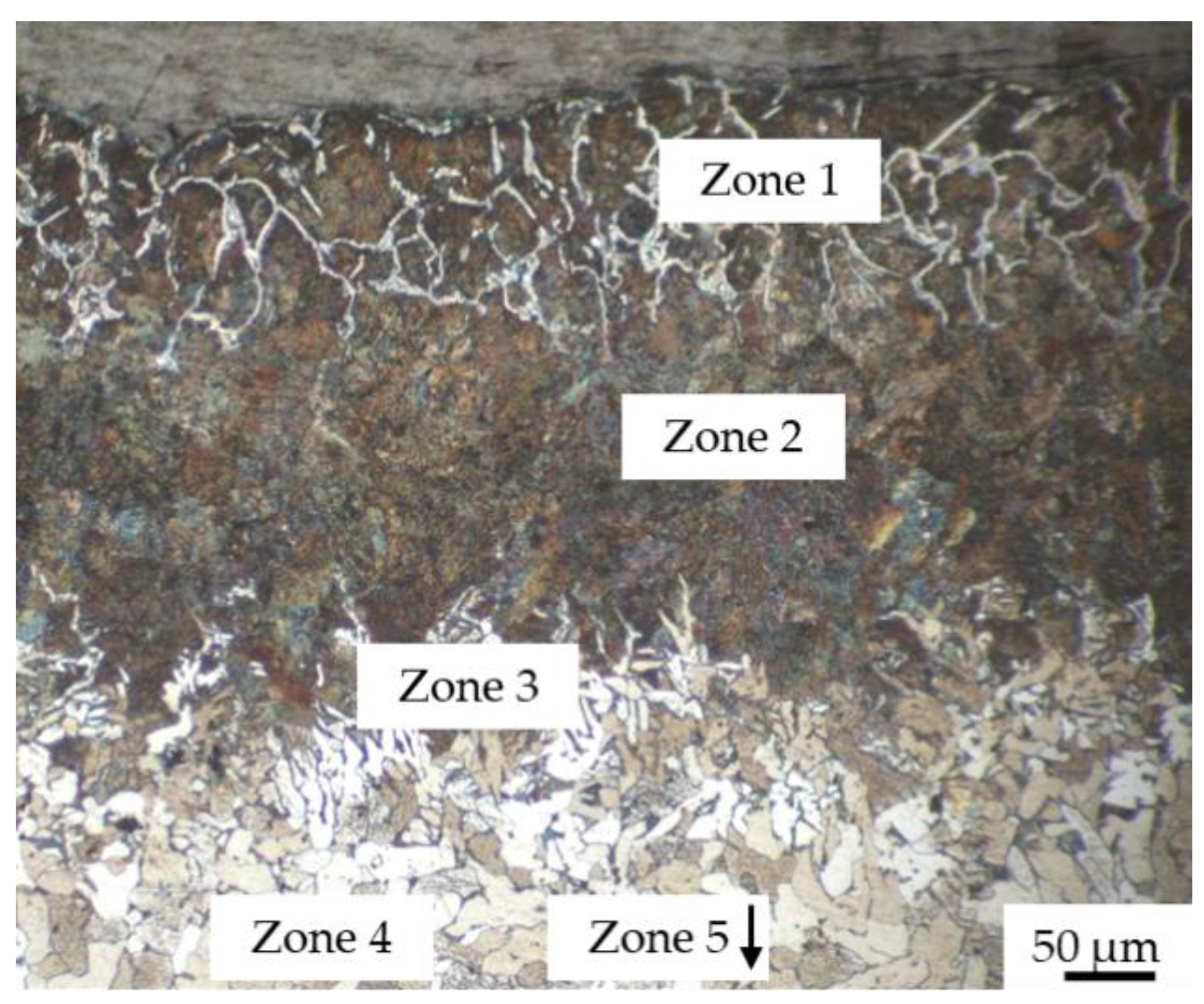
- Without coating, the carburization of iron powder took place during sintering (up to 400 µm) and five zones were detected from the top surface to the bulk:
- -
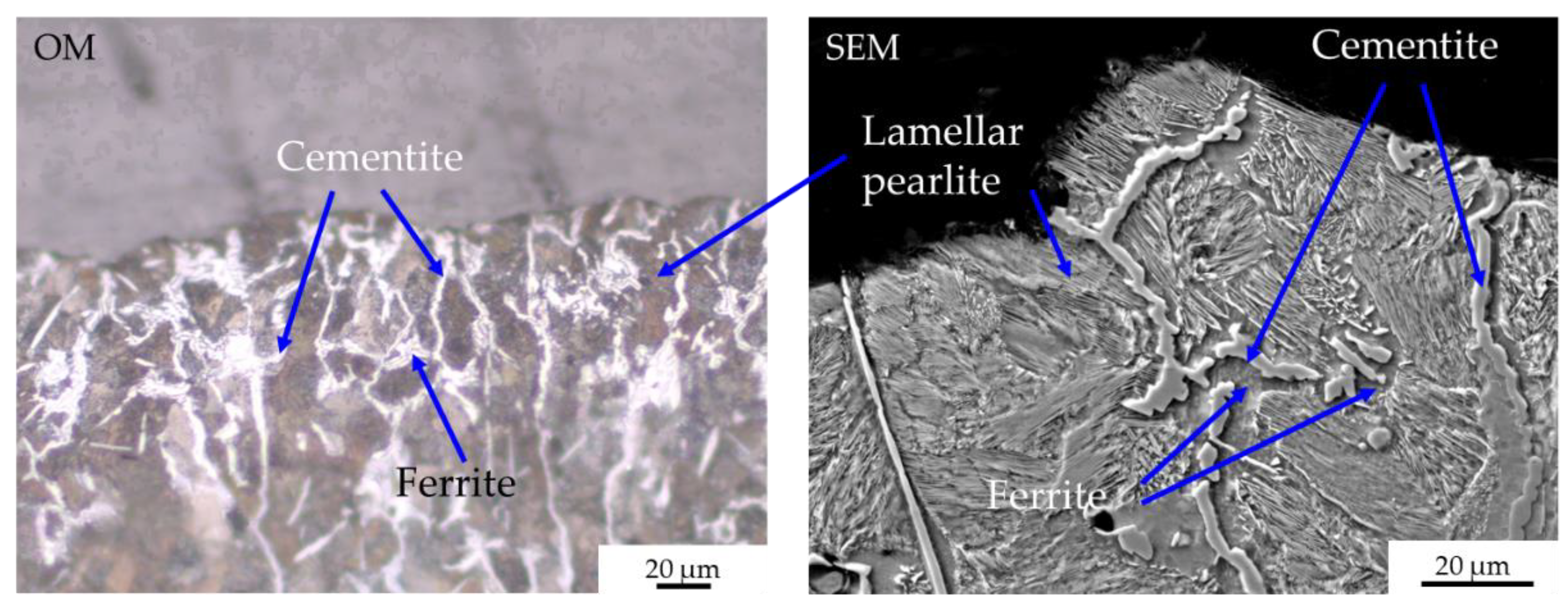
- zone 1: lamellar pearlite + cementite + abnormal ferrite (corresponding to hypereutectoid steel);
- -
- zone 2: lamellar pearlite (corresponding to eutectoid steel);
- -
- zone 3: pearlite + ferrite (corresponding to hypoeutectoid steel);
- -
- zone 4–5: recrystallized ferrite followed by grain growth (corresponding to iron).
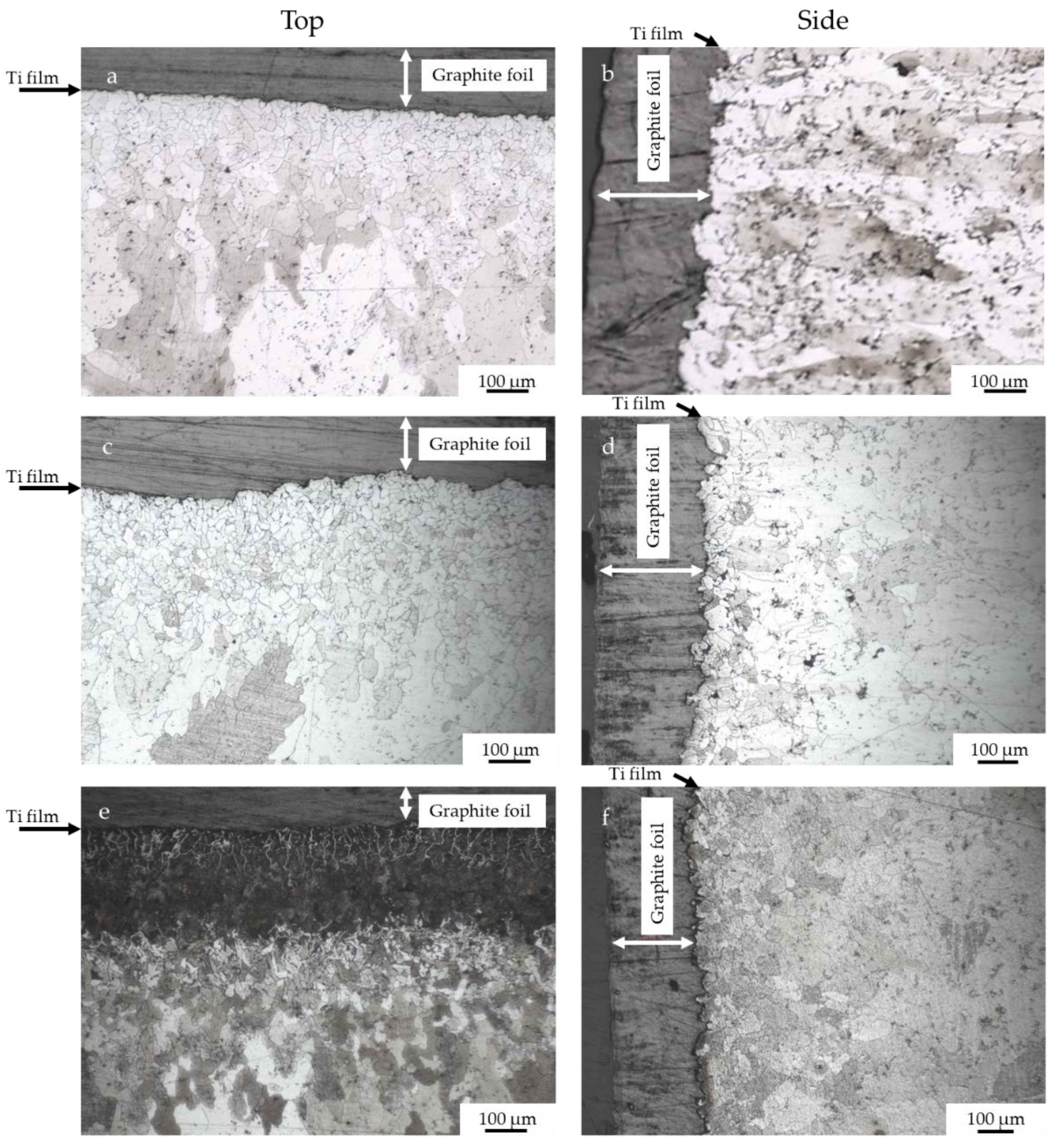
- Carburization of iron powder is faster on top (and bottom) of the sample, compared to the side in contact with the graphite foil surrounding the mold, thanks to the pressure and the current flow.
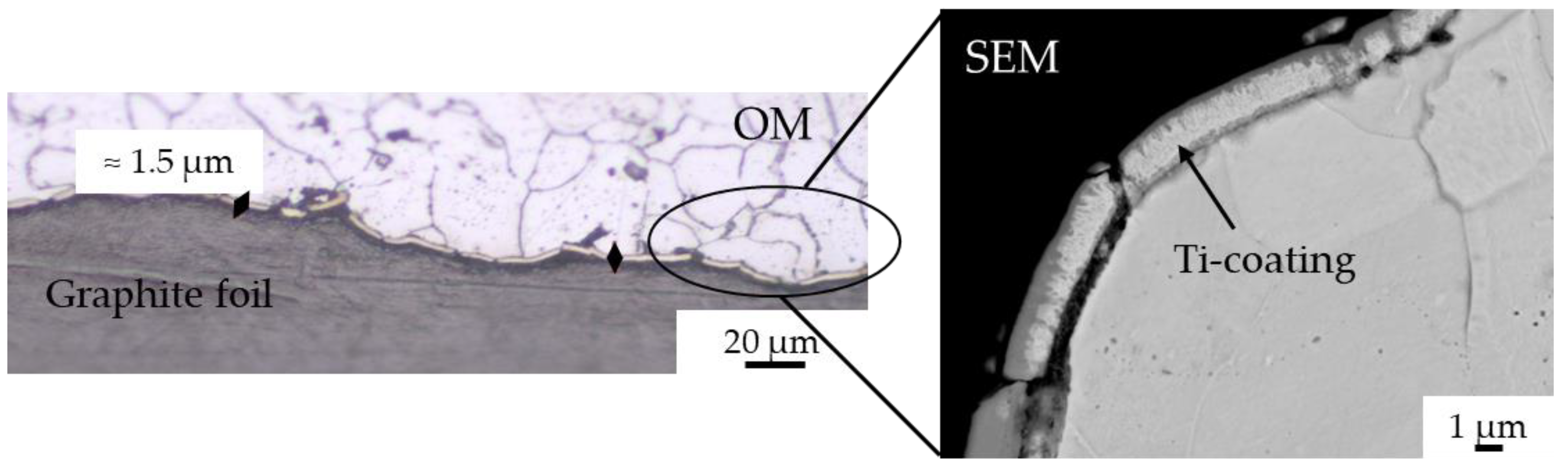
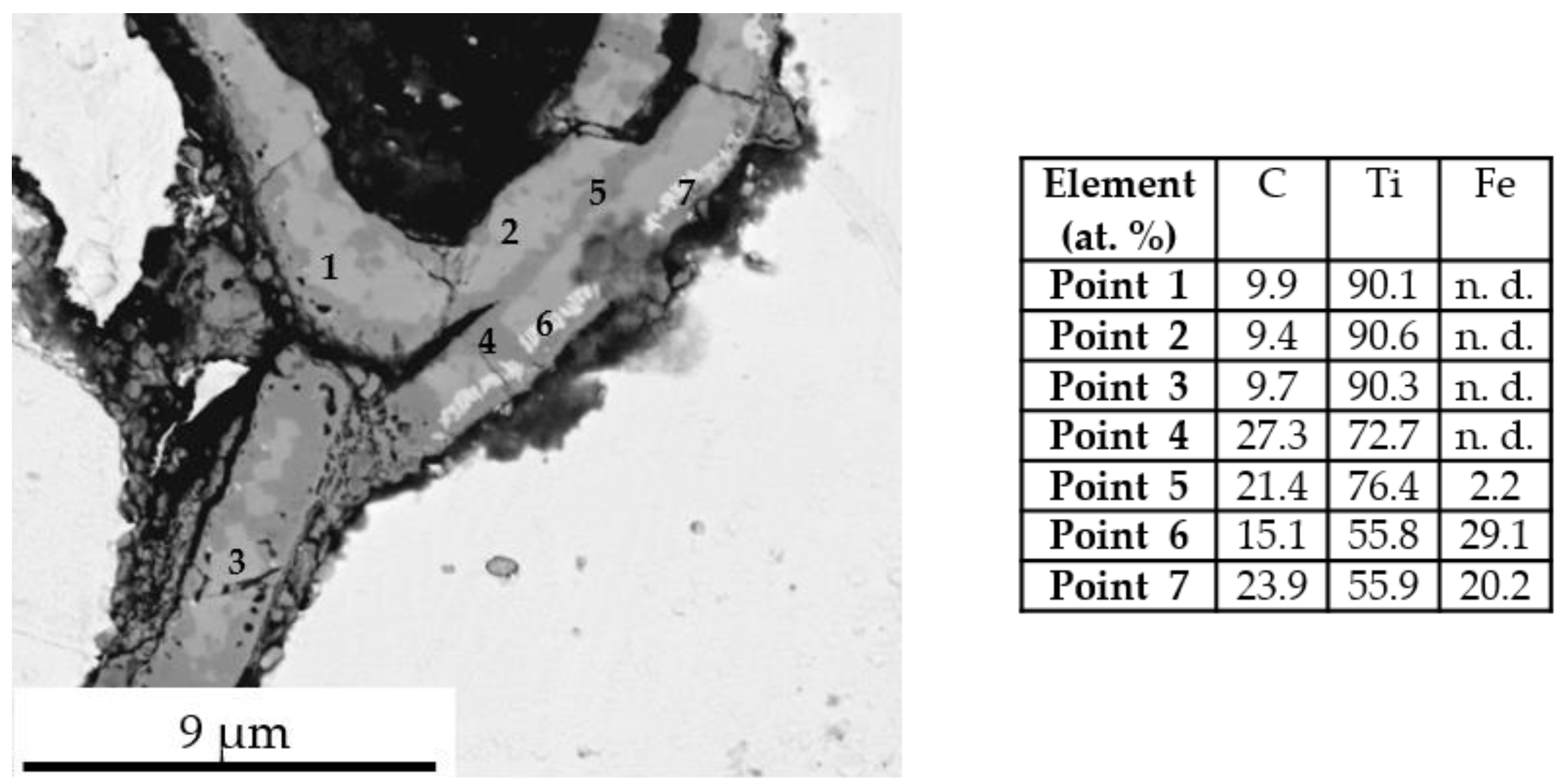
- The application of a Ti PVD film of 1.5 and 1.1 µm on graphite foils is effective to completely avoid carburization of iron powder (top, bottom and sides). Iron diffusion was revealed inside the Ti film of 1.5 µm thickness.
- With a Ti film of 0.5 µm thickness carburization of iron powder was not avoided on top and bottom of the sintered sample, but only on the side.
- Microhardness profiles confirm the OM observations.
- Ti coatings with thicknesses between 1.1 and 0.5 µm have to be tested to find the minimum coating thickness assuring protection against carbon diffusion. Anyway, the obtained results suggest that the use of graphite foils coated by a thin PVD film can represent a large-scale effective solution to avoid carbon diffusion during SPS process in the case of metal powders.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Wang, L.; Jiang, W.; Bai, G.; Chen, L. Effect of Fabrication Method on Microstructure and Properties of Al2O3–TiC Composites. Mater. Trans. 2005, 46, 2015–2019. [Google Scholar] [CrossRef] [Green Version]
- Munir, Z.A.; Anselmi-Tamburini, U.; Ohyanagi, M. The effect of electric field and pressure on the synthesis and consolidation of materials: A review of the spark plasma sintering method. J. Mater. Sci. 2006, 41, 763–777. [Google Scholar] [CrossRef]
- Mamedov, V. Spark plasma sintering as advanced PM sintering method. Powder Metall. 2002, 45, 322–328. [Google Scholar] [CrossRef]
- Hu, Z.-Y.; Zhang, Z.-H.; Cheng, X.-W.; Wang, F.-C.; Zhang, Y.-F.; Li, S.-L. A review of multi-physical fields induced phenomena and effects in spark plasma sintering: Fundamentals and applications. Mater. Des. 2020, 191, 108662. [Google Scholar] [CrossRef]
- Vanmeensel, K.; Laptev, A.; Hennicke, J.; Vleugels, J.; Van der Biest, O. Modelling of the temperature distribution during field assisted sintering. Acta Mater. 2005, 53, 4379–4388. [Google Scholar] [CrossRef]
- Nečina, V.; Pabst, W. Highly dense spinel ceramics with completely suppressed grain growth prepared via SPS with NaF as a sintering additive. J. Eur. Ceram. Soc. 2020, 40, 3354–3357. [Google Scholar] [CrossRef]
- Csáki, S.; Lukák, F.; Húlan, T.; Veverka, J.; Knapek, M. Preparation of anorthite ceramics using SPS. J. Eur. Ceram. Soc. 2021, 41, 4618–4624. [Google Scholar] [CrossRef]
- Bolsonella, A.; Naimi, F.; Heintz, O.; Tricone, T.; Couque, H.; Bernard, F. Influence of oxygen induced during high-energy ball milling process on the mechanical properties of sintered nickel by SPS. J. Alloys Compd. 2021, 856, 157869. [Google Scholar] [CrossRef]
- Hakeem, H.S.; Patel, F.; Minhas, N.; Malkawi, A.; Aleid, Z.; Ehsan, M.A.; Sharrofna, H.; Al Ghanim, A. Comparative evaluation of thermal and mechanical properties of nickel alloy 718 prepared using selective laser melting, spark plasma sintering, and casting methods. J. Mater. Res. Technol. 2021, 12, 870–881. [Google Scholar] [CrossRef]
- Nie, Q.; Chen, C.; Wang, B.; Yang, L.; Zhang, J.; Tang, W. Effect of Invar particle size on microstructures and properties of the Cu/Invar bi-metal matrix composites fabricated by SPS. J. Alloys Compd. 2022, 891, 162055. [Google Scholar] [CrossRef]
- Liu, L.; Morita, K. Fabrication of MgAl2O4/Al2O3 laminated transparent composite by spark-plasma-sintering (SPS) processing. Scr. Mater. 2021, 250, 114205. [Google Scholar] [CrossRef]
- Orru, R.; Licheri, R.; Locci, A.-M.; Cincotti, A.; Cao, G. Consolidation/synthesis of materials by electric current activated/assisted sintering. Mater. Sci. Eng. R Rep. 2009, 63, 127–287. [Google Scholar] [CrossRef]
- Koide, M.; Takei, S.; Sato, T.; Matusita, K. Preparation of silica glass by pulsed electric current method. J. Ceram. Soc. Jpn. 2002, 110, 867–869. [Google Scholar] [CrossRef] [Green Version]
- Moser, M.; Lorand, S.; Bussiere, F.; Demoisson, F.; Couque, H.; Bernard, F. Influence of carbon diffusion and the presence of oxygen on the microstructure of molybdenum powders densified by SPS. Metals 2020, 10, 948. [Google Scholar] [CrossRef]
- Macía, E.; García-Junceda, A.; Serrano, M.; Hong, S.J.; Campos, M. Effect of mechanical alloying on the microstructural evolution of a ferritic ODS steel with (Y-Ti-Al-Zr) addition processed by Spark Plasma Sintering (SPS). Nucl. Eng. Technol. 2021, 53, 2582–2590. [Google Scholar] [CrossRef]
- Ovtar, S.; Le Gallet, S.; Minier, L.; Millot, N.; Lisjak, D. Control of barium ferrite decomposition during spark plasma sintering: Towards nanostructured samples with anisotropic magnetic properties. J. Eur. Ceram. Soc. 2014, 34, 337–346. [Google Scholar] [CrossRef]
- Minier, L.; Le Gallet, S.; Grin, Y.; Bernard, F. Influence of the current flow on the SPS sintering of a Ni powder. J. Alloys Compd. 2010, 508, 412–418. [Google Scholar] [CrossRef]
- Okamoto, H. C-Ti (Carbon-Titanium). J. Phase Equilib. 1998, 19, 89. [Google Scholar] [CrossRef]
- Raghavan, V. C-Fe-Ti (Carbon-Iron-Titanium). J. Phase Equilib. 2003, 24, 62–66. [Google Scholar] [CrossRef]
- Klopotov, V.D.; Denisova, Y.A.; Teresov, A.D.; Petrikova, E.A.; Shugurov, V.V.; Seksenalina, M.A.; Ivanov, Y.F.; Klopotov, A.A. Combined treatment of steel, including electrospark doping and subsequent irradiation with a high-intensity electron beam. Mater. Sci. Eng. 2016, 124, 012125. [Google Scholar] [CrossRef]
- Evrard, M.; Besnard, A.; Lucas, S. Study of the influence of the pressure and rotational motion of 3D substrates processed by magnetron sputtering: A comparative study between Monte Carlo modelling and experiments. Surf. Coat. Technol. 2019, 378, 125070. [Google Scholar] [CrossRef]
- Nakajima, H.; Koiv, M. Diffusion in Titanium. ISIJ Int. 1991, 31, 757–766. [Google Scholar] [CrossRef] [Green Version]
- Shapovalov, V.P.; Kurasov, A.N. Diffusion of titaium in iron. Metalloved. Termich. Obrab. Metall. 1975, 9, 71–73. [Google Scholar]
- McQuaid, H.W.; Ehn, E.W. Effect of quality of steel on case-carburizing results. Trans. Am. Inst. Min. Metall. Eng. 1922, 67, 341. [Google Scholar]
- Nijhawan, B.R.; Chatterjea, A.B.; Bhatnagar, S. Micrometallurgy of abnormality in steels. J. Iron Steel Inst. 1966, 204, 821–830. [Google Scholar]
- Hillert, M. The formation of pearlite. In Decomposition of Austenite by Diffusional Processes; Zackey, V.F., Aaronson, H.I., Eds.; Interscience Publishers: New York, NY, USA, 1962; p. 197. [Google Scholar]
- Chairuangsri, T.; Edmond, D.V. Abnormal ferrite in hyper-eutectoid steel. Acta Mater. 2000, 48, 1581–1591. [Google Scholar] [CrossRef]
- Miyamoto, G.; Karube, Y.; Furuhara, T. Formation of grain boundary ferrite in eutectoid and hypereutectoid pearlitic steels. Acta Mater. 2016, 103, 370–381. [Google Scholar] [CrossRef]
- Trzaska, Z.; Couret, A.; Monchoux, J.P. Spark plasma sintering mechanisms at the necks between TiAl powder particles. Acta Mater. 2016, 118, 100–108. [Google Scholar] [CrossRef]
- Trzaska, Z.; Cours, R.; Monchoux, J.P. Densification of Ni and TiAl by SPS: Kinetics and microscopic mechanisms. Metall. Mater. Trans. A 2018, 49, 4849–4859. [Google Scholar] [CrossRef] [Green Version]
- Scotti, L.; Mottura, A. Interstitial diffusion of O, N, and C in α-Ti from first-principles: Analytical model and kinetic Monte Carlo simulations. J. Chem. Phys. 2016, 144, 084701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tisza, M. Physical Metallurgy for Engineers, 2nd ed.; ASM International: Materials Park, OH, USA; Freund Publishing House Ltd.: London, UK, 2001; p. 272. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ardigo-Besnard, M.-R.; Besnard, A.; Moser, M.; Bussière, F. Development of Ti PVD Films to Limit the Carburization of Metal Powders during SPS Process. Solids 2021, 2, 395-406. https://doi.org/10.3390/solids2040025
Ardigo-Besnard M-R, Besnard A, Moser M, Bussière F. Development of Ti PVD Films to Limit the Carburization of Metal Powders during SPS Process. Solids. 2021; 2(4):395-406. https://doi.org/10.3390/solids2040025
Chicago/Turabian StyleArdigo-Besnard, Maria-Rosa, Aurélien Besnard, Mathias Moser, and Florian Bussière. 2021. "Development of Ti PVD Films to Limit the Carburization of Metal Powders during SPS Process" Solids 2, no. 4: 395-406. https://doi.org/10.3390/solids2040025
APA StyleArdigo-Besnard, M.-R., Besnard, A., Moser, M., & Bussière, F. (2021). Development of Ti PVD Films to Limit the Carburization of Metal Powders during SPS Process. Solids, 2(4), 395-406. https://doi.org/10.3390/solids2040025





