Toxicity of Iron Mining Tailings and Potential for Revegetation Using Schinus terebinthifolia Raddi Based on the Emergence, Growth, and Anatomy of the Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Iron Mining Tailings
2.3. Experimental Design
2.4. Emergence and Biometrics of the Seedlings
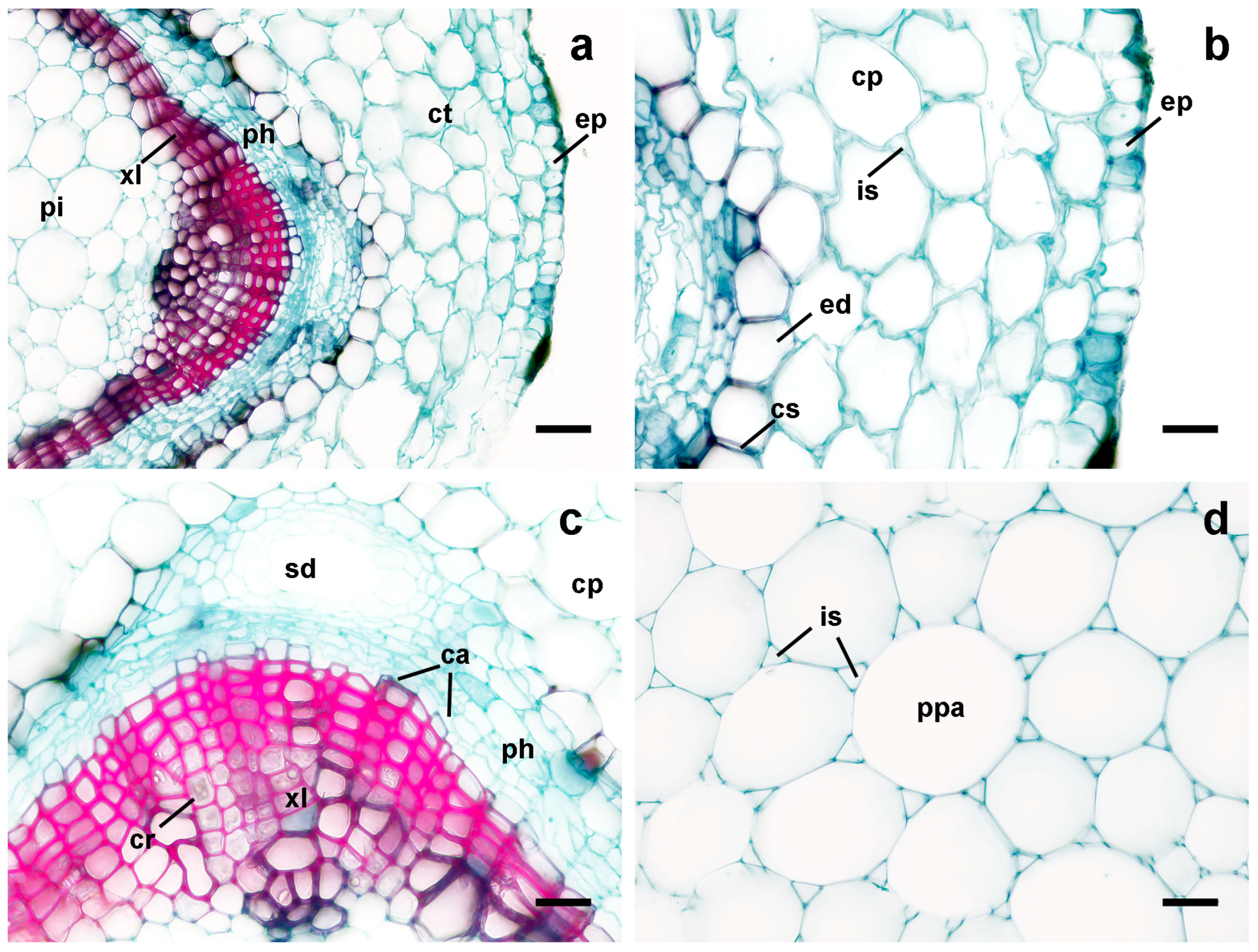
2.5. Anatomical Analysis
2.6. Statistical Analysis
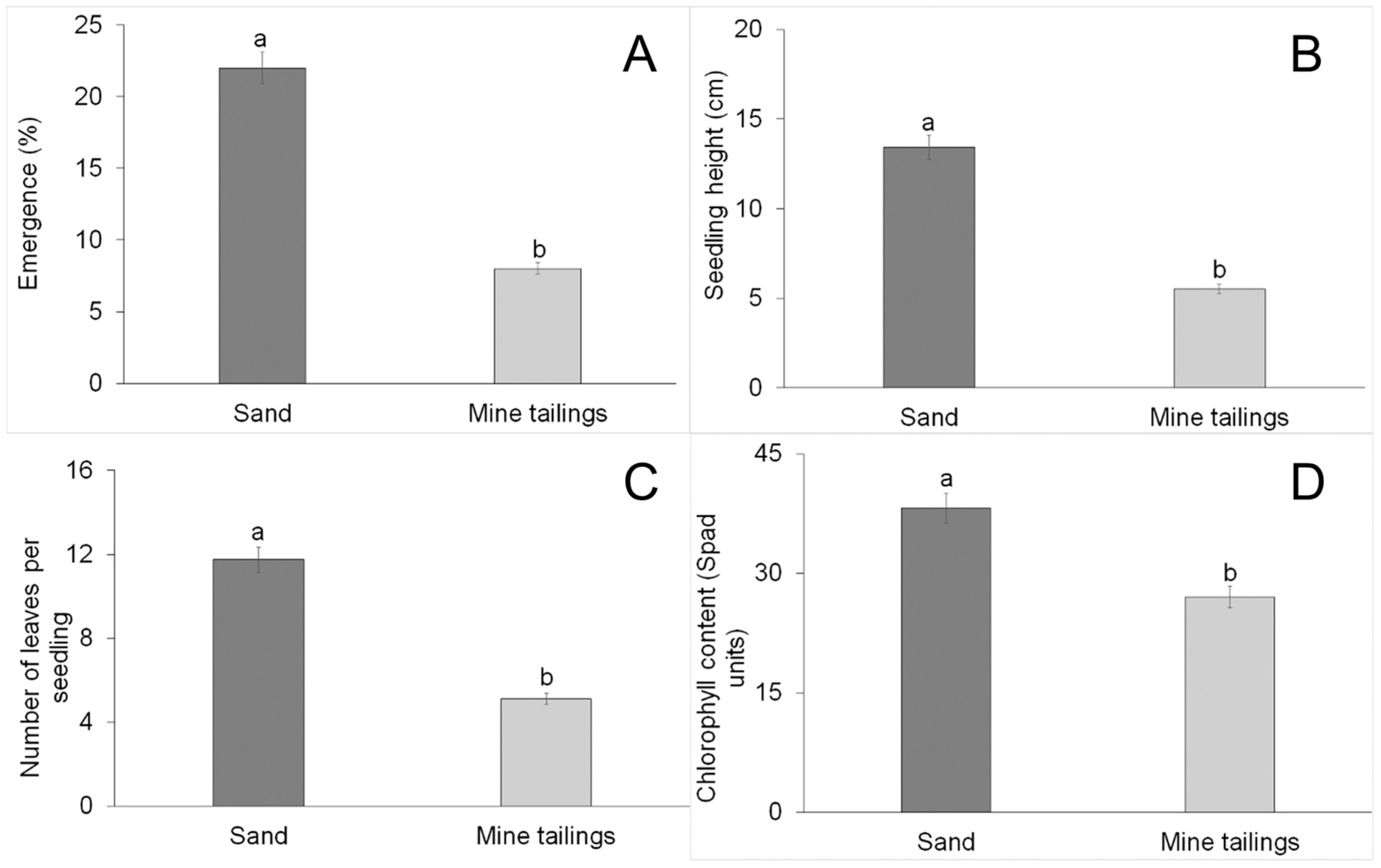
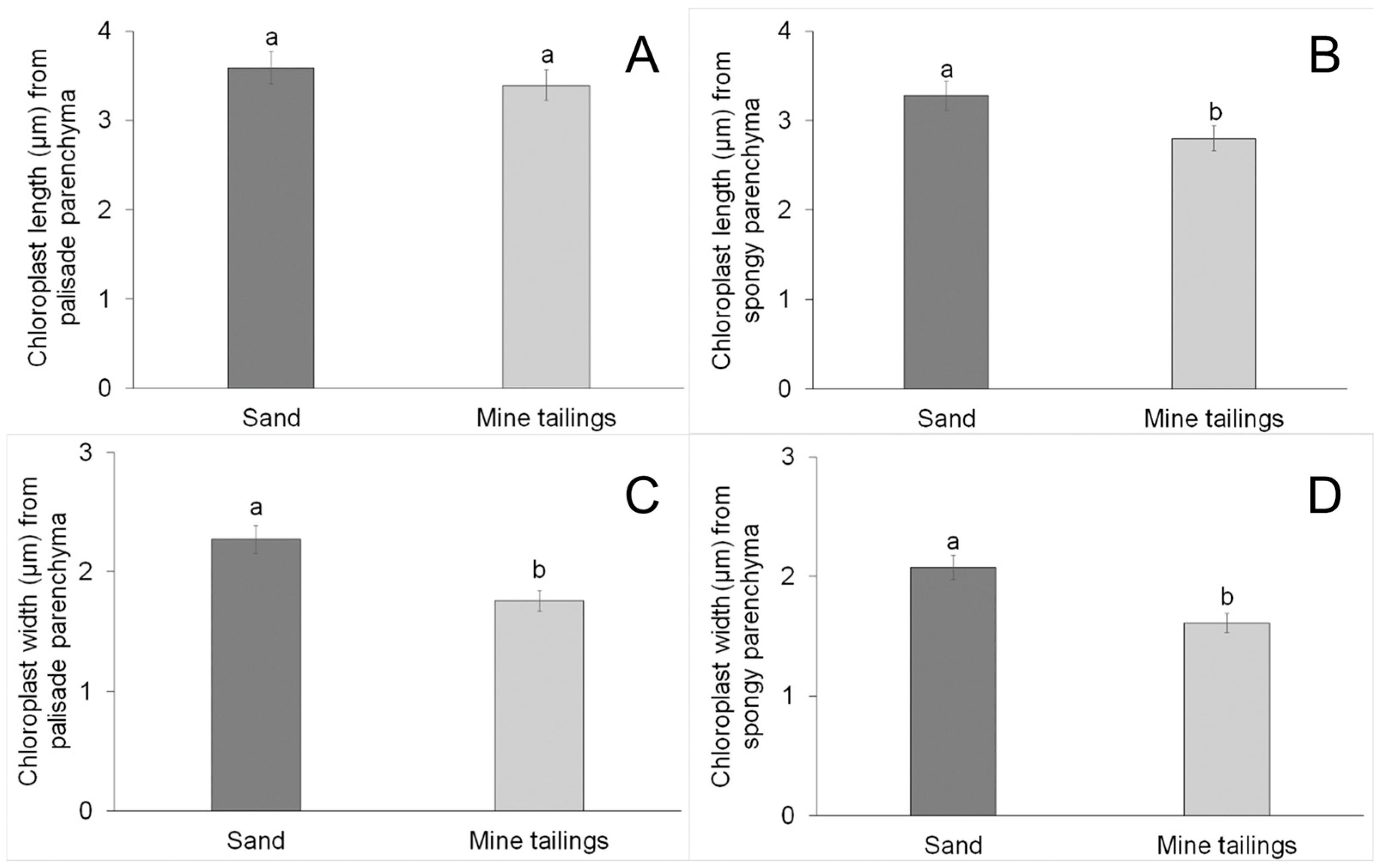
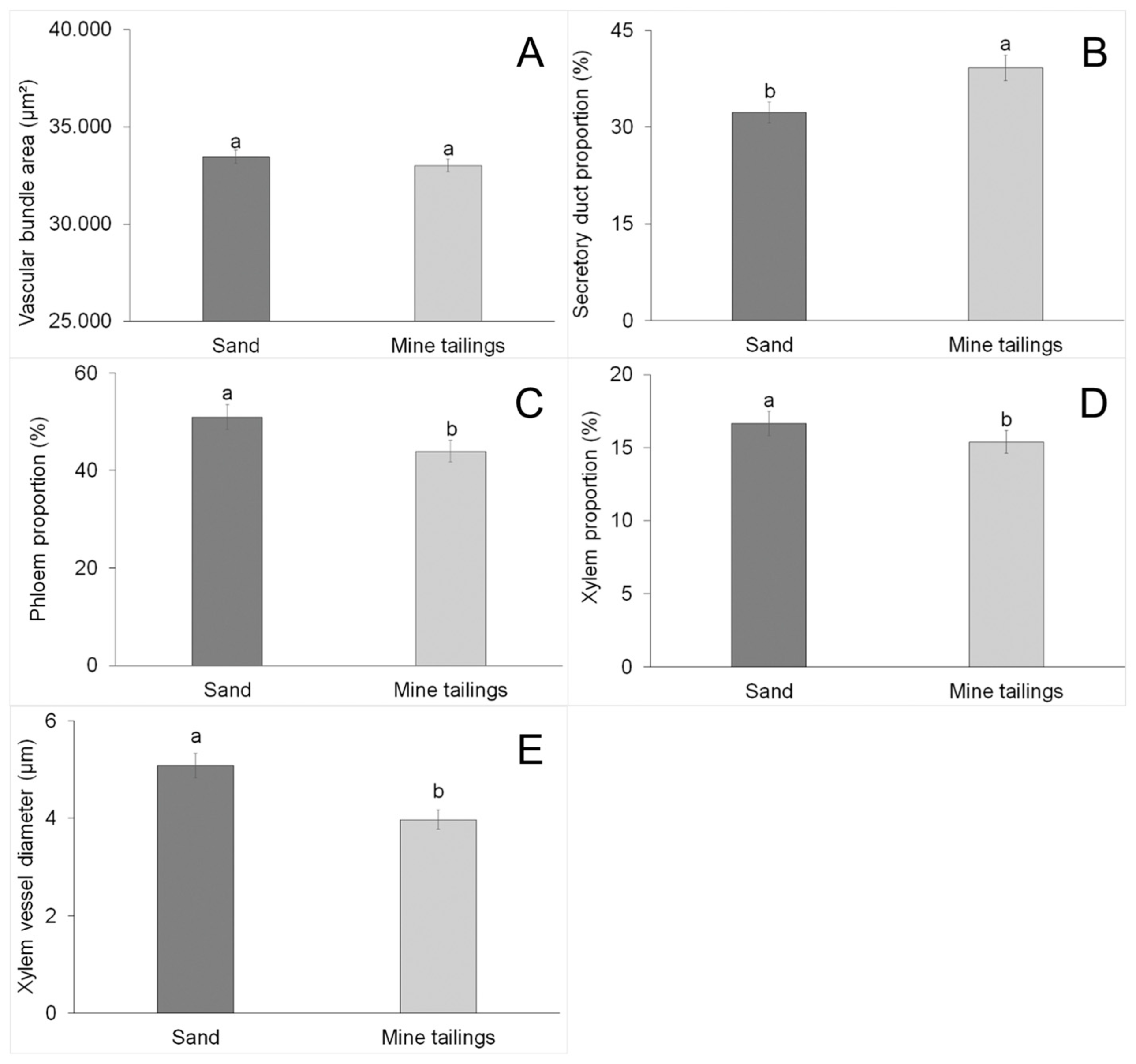
3. Results
3.1. Growth Parameters
3.2. Qualitative Effects
3.3. Leaf Anatomy
3.4. Root Anatomy
4. Discussion
4.1. Toxicity of Iron Mining Tailings to Schinus terebinthifolia
4.2. Potential of S. terebinthifolia for Reforestation in Areas Impacted by Fe Mining Tailings
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IBRAM—Instituto Brasileiro de Mineração. Gestão E Manejo de Rejeitos de Mineração; IBRAM: Brasilia, Brazil, 2016; ISBN 978-85-61993-10-8. [Google Scholar]
- Lacaz, F.A.C.; Porto, M.F.S.; Pinheiro, T.M.M. Tragédias brasileiras contemporâneas: O caso do rompimento da barragem de rejeitos de Fundão/Samarco. Rev. Bras. Saúde Ocup. 2017, 42, e9. [Google Scholar] [CrossRef]
- Andrade, G.F.; Paniz, F.P.; Martins, A.C.J.R.; Rocha, B.A.; Lobato, A.K.S.; Rodrigues, J.L.; Gustavson, P.C.; Masuda, H.P.; Batista, L.B. Agricultural use of Samarco’s spilled mud assessed by rice cultivation: A promising residue use? Chemosphere 2018, 193, 892–902. [Google Scholar] [CrossRef] [PubMed]
- Adiansyah, J.S.; Rosano, M.; Vink, S.; Keir, G. A framework for a sustainable approach to mine tailings management: Disposal strategies. Clean. Prod. 2015, 108, 1050–1062. [Google Scholar] [CrossRef]
- Carmo, F.F.; Kamino, L.H.Y.; Tobias Junior, R.; Campos, I.C.; Carmo, F.F.; Silvino, G.; Castro, K.J.S.X.; Mauro, M.L.; Rodrigues, N.U.A.; Miranda, M.P.S.; et al. Fundão tailings dam failures: The environment tragedy of the technological disaster of Brazilian mining in global. Perspect. Ecol. Conserv. 2017, 15, 145–151. [Google Scholar] [CrossRef]
- Silva, C.A.; Oliveira-Júnior, J.F.; Teodoro, P.E.; Lima, M.; Shakir, M.; Gois, G.; Johann, J.A. Analysis of the impact on vegetation caused by abrupt deforestation via orbital sensor in the environmental disaster of Mariana, Brazil. Land Use Policy 2018, 76, 10–20. [Google Scholar]
- Guimarães, L.A.O.P.; Assis, I.R.; Dias, L.E.; Cordeiro, A.L.; Freire, A.S. Espécies arbóreas potenciais para a revegetação de rejeito salino contaminado com arsênio. Ciênc. Florest. 2017, 27, 871–881. [Google Scholar] [CrossRef]
- Pádua, M.P.; Caetano, A.L.; Polo, M.; Pasqual, M.; Pereira, F.J. Ecophysiological responses of Copaifera langsdorfi grown in mining tailing under lower water availability. Water Air Soil Pollut. 2021, 232, 57. [Google Scholar] [CrossRef]
- Caetano, A.L.; Pádua, M.P.; Polo, M.; Pasqual, M.; Pereira, F.J. Growth, anatomy, and gas Exchange of Cenostigma pluviosum cultivated under reduced water levels in iron mining tailing. J. Soils Sediments 2021, 22, 381–391. [Google Scholar] [CrossRef]
- Benavides, M.P.; Gallego, S.M.; Tomaro, M.L. Cadmium toxicity in plants. Braz. J. Plant Phisiol. 2005, 17, 21–34. [Google Scholar] [CrossRef]
- Quintal, E.B.; Magana, C.E.; Machado, L.E.; Estevez, M.M. Aluminum, a friend or foe of higher plants in acid soils. Front. Plant Sci. 2017, 8, 1767. [Google Scholar]
- Sharma, P.; Dubey, R.S. Lead toxicity plants. Braz. J. Plant Physiol. 2005, 17, 35–52. [Google Scholar] [CrossRef]
- Pereira, M.P.; Pereira, F.J.; Corrêa, F.F.; Oliveira, C.; Castro, E.M.; Barbosa, S. Lead tolerance during germination and early growth of the Brazilian Peppertree and the morpho-physiological modifications. Rev. Ciênc. Agrár. 2013, 56, 72–79. [Google Scholar] [CrossRef]
- Pereira, M.P.; Rodrigues, L.C.A.; Correa, F.P.; Castro, E.M.; Ribeiro, V.E.; Pereira, F.J. Cadmium tolerance in Schinus molle trees is modulated by enhanced leaf anatomy and photosynthesis. Trees 2016, 22, 163–170. [Google Scholar] [CrossRef]
- Pereira, M.P.; Corrêa, F.F.; Castro, E.M.; Oliveira, J.P.V.; Pereira, F.J. Leaf ontogeny of Schinus molle L. plants under cadmium contamination: The meristematic origin of leaf structural changes. Protoplasma 2017, 254, 2117–2126. [Google Scholar] [CrossRef] [PubMed]
- Lorenzi, H. Árvores Brasileiras: Manual de Identificação e Cultivo de Plantas Arbóreas Nativas do Brasil; Editora Plantarum: Nova Odessa, Brazil, 1992. [Google Scholar]
- Scarpa, A.L.M.; Rodrigues, F.A.; Cruz, Y.C.; Duarte, V.P.; Castro, E.M.; Pasqual, M.; Pereira, F.J. Seed germination, initial growth and leaf anatomy of seedlings of four tree species grown in mine tailings in Brazil. Seed Sci. Res. 2022, 32, 104–114. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association Analytical Chemists; AOAC: Rockville, MD, USA, 2005. [Google Scholar]
- Scarpa, A.L.M.; Cruz, Y.C.; Duarte, V.P.; Castro, E.M.; Pasqual, M.; Oliveira, J.P.V.; Pereira, F.J. Growth response, gas exchange, and leaf anatomy of Handroanthus spp. seedling in mine tailings enriched with nutrient solution. J. Soil Sci. Plant Nutr. 2022, 22, 3774–3787. [Google Scholar] [CrossRef]
- Pereira, M.P.; Correa, F.F.; Polo, M.; Castro, E.M.; Cardoso, A.A.; Pereira, F.J. Seed germination of Schinus molle L. [Anacardiaceae] as related to its anatomy and dormancy alleviation. Seed Sci. Res. 2016, 26, 351–361. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants without Soil; California Agricultural Experiment Station: Salinas, CA, USA, 1950; Volume 347. [Google Scholar]
- Johansen, D.A. Plant Microtechnique; McGraw-Hill: New York, NY, USA, 1940. [Google Scholar]
- Kraus, J.E.; Arduin, M. Manual Básico de Métodos em Morfologia Vegetal; EDUR: Seropédica, Brazil, 1997. [Google Scholar]
- Ferreira, D.F. Sisvar: A computer statistical analysis system. Ciênc. Agrotecnol. 2011, 35, 1039–1042. [Google Scholar] [CrossRef]
- CONAMA. Conselho Nacional do Meio Ambiente. Resolução n° 420, de 28 de dezembro de 2009. Dispõe sobre critérios e valores orientadores de qualidade do solo quanto à presença de substâncias químicas e estabelece diretrizes para o gerenciamento ambiental de áreas contaminadas por essas substâncias em decorrência de atividades antrópicas. Diário União 2009, 249, 81–88. [Google Scholar]
- Guerra, M.B.B.; Teaney, B.T.; Mout, B.J.; Asunskis, D.J.; Jordan, B.T.; Barker, R.J.; Santos, E.E.; Schaefer, C.E.G.R. Post-catastrophe analyses of the Fundão tailing dan failure in the doce river system, southeast Brazil: Potentially toxic elements in affectes soils. Water Air Soil Pollut. 2017, 228, 252. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Moller, I.M.; Jensen, P.E.; Hansson, A. Oxidative Modifications to cellular components in Plants. Annu. Rev. Plant Biol. 2007, 58, 459–481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Fran, J.; Jinghua, W.; Zhang, L.; Wang, J.; Zhand, B.; Pruski, G.W. Alleviating effectof silicon on melon seed germinationunder autotoxicity stress. Ecotoxicol. Environ. Saf. 2020, 188, 109901. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.F.; Becker, A.G.; Cargnelutti, D.; Tabaldi, L.A.; Pereira, L.B.; Battisti, V.; Spanevello, R.M.; Morsch, V.M.; Nicoloso, F.T.; Schetinger, M.R.C. Cadmium toxicity causes oxidative stress and induces response of the antioxidant system in cucumber seedlings. Braz. J. Plant Physiol. 2007, 19, 223–232. [Google Scholar] [CrossRef]
- Vestena, S.; Cambraia, J.; Ribeiro, C.; Oliveira, J.A.; Oliva, M.A. Cadmium-induced oxidative stress andantioxidative enzyme response in water Hyacinth and Salvinia. Braz. J. Plant Physiol. 2011, 23, 131–139. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Chaves, L.H.G.; Souza, R.S. Crescimento, distribuição e acumulação de cádmio em plantas de Jatropha curcas. Rev. Ciênc. Agrár. 2014, 37, 286–291. [Google Scholar]
- Romeiro, S.; Lagoa, A.M.M.A.; Furlani, P.R.; Abreu, C.A.; Pereira, P.F.F. Absorção de chumbo e potencial de fitorremediação de Canavalia ensiformes L. Solos Nutr. Plantas 2007, 66, 327–334. [Google Scholar] [CrossRef]
- Oliveira, N.P.; Bastos, A.R.R.; Costa, A.L.; Cipriani, H.N. Eucalyptus urophylla cuttings under different concentrations of Cr: Toxicity symptoms and effects on Growth. Braz. J. Dev. 2022, 8, 33990–34001. [Google Scholar] [CrossRef]
- Steiner, F.; Zoz, T.; Pinto, A.S.; Castagnara, D.D.; Dranski, J.A.L. Effects of aluminum on plant growth and nutrient uptake in young physic nut plants. Semin. Ciênc. Agrár. 2012, 33, 1779–1788. [Google Scholar] [CrossRef]
- Ferreira, M.M.A.A.S.; Santos, J.A.G.; Moura, S.C.; Abreu, C.B.; Bomfim, M.R.; Azevedo Neto, A.D.A. Cadmium effects on sunflower growth and mineral nutrition. Afr. J. Agric. Res. 2016, 11, 3488–3496. [Google Scholar]
- Cunha, K.P.V.; Nascimento, C.W.A.; Pimentel, R.M.M.; Accioly, A.M.A.; Silva, A.J. Disponibilidade, acúmulo e toxidez de cádmio e zinco em milho cultivado em solo contaminado. Rev. Bras. Ciênc. Solo 2008, 32, 1319–1328. [Google Scholar] [CrossRef]
- Baroni, G.R.; Pereira, M.P.; Corrêa, F.F.; Castro, E.M.; Pereira, F.J. Cadmium tolerance during seed germination and seedling growth of Schinus molle (Anacardiaceae). Floresta Ambiente 2020, 27, e20170502. [Google Scholar] [CrossRef]
- Sridhar, B.B.M.; Han, F.X.; Diehl, S.V.; Monts, D.L.; Su, Y. Effect of phytoaccumulation of arsenic and chromium on structural and ultrastructural changes of brake fern (Pteris vittata). Braz. Soc. Plant Physiol. 2011, 23, 288–293. [Google Scholar] [CrossRef]
- Ribeiro, L.P.; Leite, A.T.; Silva, L.B.J.; Silva, V.F.; Borges, E.E.L. Physiological and biochemical changes in Brazilian Pepper (Schinus terebinthifolia Raddi) seeds during storage. Rev. Árvore 2018, 42, E420105. [Google Scholar] [CrossRef]
- Almeida, V.G.; Moura, E.N.; Vieira, G.T. Espécies vegetais utilizadas em áreas degradadas pela mineração. Res. Soc. Dev. 2019, 8, e3583710. [Google Scholar] [CrossRef]
- Silva, E.S.; Guilherme, M.F.S.; Oliveira, H.M.; Araújo, L.N.C.; Viana, Z.C.V.; Santos, C.S. Ecotoxicological effects of cadmium on the germination and initial development of Schinus terebinthifolius. Rev. Ciênc. Agrár. 2017, 40, 311–318. [Google Scholar] [CrossRef]
- José, A.C.; Davide, A.C.; Oliveira, S.L. Produção de mudas de aroeira (Schinus terebinthifolia Raddi) para áreas degradadas pela mineração de bauxita. Cerne 2005, 11, 187–196. [Google Scholar]
- Ribeiro, V.E.; Pereira, M.P.; Castro, E.M.; Correa, F.F.; Cardoso, M.G.; Pereira, F.J. Enhanced essential oil and leaf anatomy of Schinus molle plants under lead contamination. Ind. Crops Prod. 2019, 132, 92–98. [Google Scholar] [CrossRef]



| Macronutrients | mg kg−1 Dry Mass | PTE Maximum Concentrations [mg kg−1] * |
|---|---|---|
| P | 142.7 | - |
| Mg | 84.6 | - |
| K | 106.5 | - |
| Ca | 3274.4 | - |
| Micronutrients | µg kg−1 | |
| Mn | 440.9 | - |
| Fe | 31,509.1 | - |
| Zn | 5.3 | 300.0 |
| Cu | 6.1 | 63.0 |
| Na | 49.6 | - |
| Potentially toxic elements | µg kg−1 | |
| Al | 2472.1 | - |
| Cr | 11.3 | 75.0 |
| Cd | 2.3 | 1.4 |
| Pb | 5.3 | 72.0 |
| Other characteristics | ||
| pH | 6.2 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, P.N.; dos Reis, C.H.G.; Duarte, V.P.; de Castro, E.M.; de Pádua, M.P.; Pereira, F.J. Toxicity of Iron Mining Tailings and Potential for Revegetation Using Schinus terebinthifolia Raddi Based on the Emergence, Growth, and Anatomy of the Species. Mining 2024, 4, 719-732. https://doi.org/10.3390/mining4030040
da Silva PN, dos Reis CHG, Duarte VP, de Castro EM, de Pádua MP, Pereira FJ. Toxicity of Iron Mining Tailings and Potential for Revegetation Using Schinus terebinthifolia Raddi Based on the Emergence, Growth, and Anatomy of the Species. Mining. 2024; 4(3):719-732. https://doi.org/10.3390/mining4030040
Chicago/Turabian Styleda Silva, Poliana Noemia, Carlos Henrique Goulart dos Reis, Vinícius Politi Duarte, Evaristo Mauro de Castro, Maxwell Pereira de Pádua, and Fabricio José Pereira. 2024. "Toxicity of Iron Mining Tailings and Potential for Revegetation Using Schinus terebinthifolia Raddi Based on the Emergence, Growth, and Anatomy of the Species" Mining 4, no. 3: 719-732. https://doi.org/10.3390/mining4030040
APA Styleda Silva, P. N., dos Reis, C. H. G., Duarte, V. P., de Castro, E. M., de Pádua, M. P., & Pereira, F. J. (2024). Toxicity of Iron Mining Tailings and Potential for Revegetation Using Schinus terebinthifolia Raddi Based on the Emergence, Growth, and Anatomy of the Species. Mining, 4(3), 719-732. https://doi.org/10.3390/mining4030040







