Artificial Intelligence Applications in Dentistry: A Systematic Review
Abstract
1. Introduction
1.1. Machine Learning (ML)
1.2. Deep Learning (DL)
1.3. Artificial Neural Networks (ANNs)
1.4. Computer Vision (CV)
2. Methods
2.1. Search Strategy and Study Selection
2.2. Search Terms
- (“artificial intelligence” [MeSH Terms] OR “artificial intelligence” [Title/Abstract] OR “machine learning” [Title/Abstract] OR “deep learning” [Title/Abstract] OR “neural network*” [Title/Abstract] OR “computer vision” [Title/Abstract] OR “convolutional neural network*” [Title/Abstract]) AND
- (“dentistry” [MeSH Terms] OR “dental” [Title/Abstract] OR “oral health” [Title/Abstract] OR “orthodontics” [Title/Abstract] OR “periodontics” [Title/Abstract] OR “endodontics” [Title/Abstract] OR “oral surgery” [Title/Abstract] OR “dental radiography” [Title/Abstract] OR “dental imaging” [Title/Abstract])
2.3. Inclusion and Exclusion Criteria
2.3.1. Inclusion Criteria
- Original research articles evaluating AI systems in dental applications
- Studies with measurable diagnostic, treatment planning, or predictive outcomes
- Both in vitro and clinical studies
- Studies published between January 2015 and December 2024
- Studies reporting sensitivity, specificity, accuracy, or other quantitative performance measures
- Studies with clear description of AI methodology
2.3.2. Exclusion Criteria
- Review articles, editorials, preprints and conference papers, and case reports
- Studies without clear AI methodology description
- Studies lacking quantitative outcome measures
- Duplicate publications
- Studies focusing solely on dental materials or laboratory techniques without clinical relevance
- Studies with insufficient data for quality assessment
2.4. Study Selection Process
2.5. Data Extraction and Quality Assessment
2.6. Data Synthesis and Analysis
- Diagnostic applications (e.g., caries, periodontal disease, oral lesions)
- Treatment planning (e.g., orthodontics, implant positioning)
- Outcome prediction (e.g., treatment duration, implant success)
3. Results
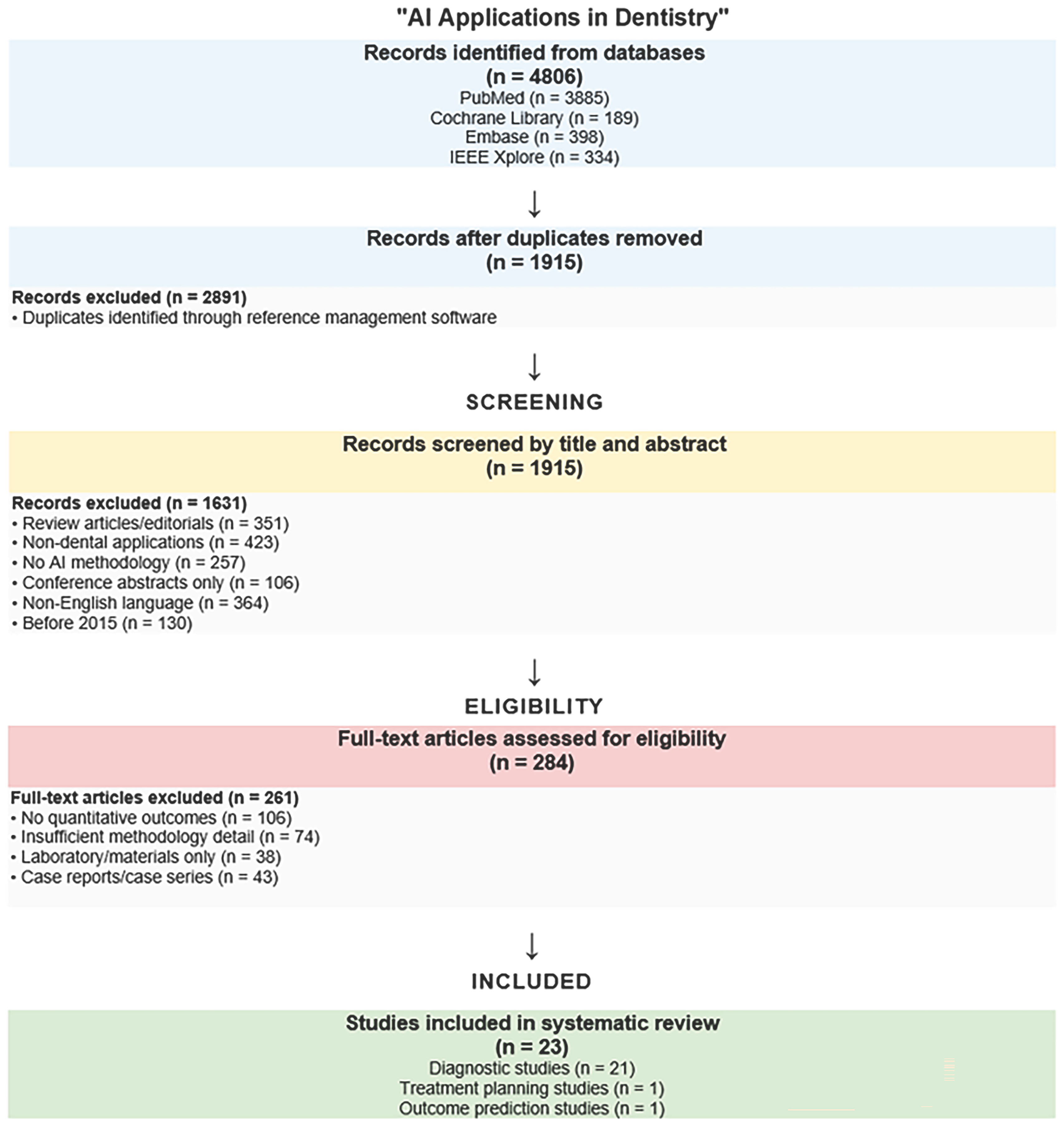
3.1. Study Selection and Characteristics
3.2. AI Applications in Diagnostic Dentistry
3.2.1. Caries Detection
Performance Summary by Imaging Modality
- Bitewing radiographs (n = 6): sensitivity 79–91%, specificity 85–96%, accuracy 82–94%
- Clinical photographs (n = 5): sensitivity 76–88%, specificity 82–93%, accuracy 85–92%
- Near-infrared imaging (n = 2): sensitivity 82–89%, specificity 89–94%, accuracy 86–91%
- Panoramic radiographs (n = 2): sensitivity 87–89%, specificity 94–96%, accuracy 92–94%
3.2.2. Periodontal Disease Assessment
Performance Summary
- Radiographic bone loss detection: sensitivity 85–92%, specificity 88–95%, accuracy 87–93%
- Clinical inflammation assessment: sensitivity 78–86%, specificity 82–91%, accuracy 80–89%
3.2.3. Oral Lesion and Cancer Detection
Performance Summary
- Oral cancer detection: sensitivity 88–96%, specificity 85–93%, accuracy 87–94%
- Benign lesion classification: sensitivity 76–84%, specificity 82–89%, accuracy 79–86%
3.3. AI in Treatment Planning
3.3.1. Orthodontic Applications
3.3.2. Key Findings
- Landmark identification accuracy: 95–98% within 2 mm tolerance
- Treatment planning recommendations: 78–85% agreement with expert orthodontists
- Treatment duration prediction: mean absolute error 3–6 months
3.3.3. Implant Planning
3.3.4. Applications and Performance
- Optimal implant positioning: 92–96% accuracy compared to expert planning
- Bone density assessment: correlation coefficient 0.85–0.92 with histological analysis
- Success prediction: 82–89% accuracy for 2-year outcomes
3.4. Outcome Prediction Applications
4. Discussion
4.1. Current State of Evidence
4.1.1. Caries Detection and Preventive Dentistry
4.1.2. Endodontics
4.1.3. Periodontology and Oral Medicine
4.1.4. Radiology, Imaging, and Diagnostics
4.1.5. Orthodontics, Prosthodontics, and Implantology
4.2. Implementation Barriers
4.2.1. Regulatory and Legal Challenges
4.2.2. Data Privacy, Security, and Bias
4.2.3. Integration into Existing Workflows
4.2.4. Cultural and Professional Resistance
4.2.5. Resource and Infrastructure Limitations
4.3. Clinical Implications for Practitioners
4.4. Future Research Priorities
- Prospective Clinical Trials: Large-scale randomized controlled trials comparing AI-assisted versus conventional diagnosis and treatment planning are urgently needed to establish clinical benefit and cost-effectiveness.
- Diverse Population Studies: Training and validation datasets must include diverse patient populations across different demographic groups, geographic regions, and clinical settings to ensure equitable performance.
- Implementation Science Research: Studies examining integration with existing workflows, practitioner acceptance, patient outcomes, and economic impact are essential for successful clinical translation.
- Longitudinal Outcome Studies: Long-term follow-up studies are needed to assess whether AI-assisted decisions lead to improved patient outcomes compared to conventional approaches.
4.5. Computer Vision Advances Relevant to Dentistry
4.5.1. Few-Shot Detection and Generalization
4.5.2. Domain Shift and Imaging Heterogeneity
4.5.3. Weakly Supervised Segmentation
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khanagar, S.B.; Alfouzan, K.; Alkadi, L.; Albalawi, F.; Iyer, K.; Awawdeh, M. Performance of Artificial Intelligence (AI) Models Designed for Application in Pediatric Dentistry—A Systematic Review. Appl. Sci. 2022, 12, 9819. [Google Scholar] [CrossRef]
- Patil, A.K.; Saha, A.; Nunna, M.; Bhumirreddy, J. Artificial Intelligence in pediatric dentistry: A Narrative review. J. Updates Pediatric. Dent. 2023, 2, 4–11. [Google Scholar]
- Mann, D.L. Artificial Intelligence Discusses the Role of Artificial Intelligence in Translational Medicine. JACC Basic Transl. Sci. 2023, 8, 221–223. [Google Scholar] [CrossRef]
- Popa, S.-L.; Ismaiel, A.; Brata, V.D.; Turtoi, D.C.; Bârsan, M.; Czako, Z.; Pop, C.; Muresan, L.; Fadgyas Stănculete, M.; Dumitrascu, D.I. Artificial Intelligence and Medical Specialties: Support or Substitution? Med. Pharm. Rep. 2024, 97, 409. [Google Scholar] [CrossRef]
- Arsiwala-Scheppach, L.; Chaurasia, A.; Müller, A.; Krois, J.; Schwendicke, F. Machine Learning in Dentistry: A Scoping Review. Stomatology 2023, 12, 937. [Google Scholar] [CrossRef] [PubMed]
- Bernauer, S.A.; Zitzmann, N.U.; Joda, T. The Use and Performance of Artificial Intelligence in Prosthodontics: A Systematic Review. Sensors 2021, 21, 6628. [Google Scholar] [CrossRef]
- Thurzo, A.; Urbanova, W.; Novák, B.; Czakó, L.; Siebert, T.; Stano, P.; Mareková, S.; Fountoulaki, G.; Kosnácová, H.; Varga, I. Where Is the Artificial Intelligence Applied in Dentistry? Systematic Review and Literature Analysis. Healthcare 2022, 10, 1269. [Google Scholar] [CrossRef]
- Al Jallad, N.; Ly-Mapes, O.; Hao, P.; Ruan, J.; Ramesh, A.; Luo, J.; Wu, T.T.; Dye, T.D.; Rashwan, N.; Ren, J.; et al. Artificial Intelligence-Powered Smartphone Application, AICaries, Improves at-Home Dental Caries Screening in Children: Moderated and Unmoderated Usability Test. PLoS Digit. Health 2022, 1, e0000046. [Google Scholar] [CrossRef] [PubMed]
- Reyes, L.T.; Knorst, J.K.; Ortiz, F.R.; Ardenghi, T.M. Scope and challenges of machine learning-based diagnosis and prognosis in clinical dentistry: A literature review. J. Clin. Transl. Res. 2021, 7, 523. [Google Scholar]
- Taleb, A.; Rohrer, C.; Bergner, B.; De Leon, G.; Rodrigues, J.A.; Schwendicke, F.; Lippert, C.; Krois, J. Self-Supervised Learning Methods for Label-Efficient Dental Caries Classification. Diagnostics 2022, 12, 1237. [Google Scholar] [CrossRef] [PubMed]
- Corbella, S.; Srinivas, S.; Cabitza, F. Applications of deep learning in dentistry. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 132, 225–238. [Google Scholar] [CrossRef]
- Rodrigues, J.A.; Krois, J.; Schwendicke, F. Demystifying artificial intelligence and deep learning in dentistry. Braz. Oral Res. 2021, 35, e094. [Google Scholar] [CrossRef]
- Aboalshamat, K.T. Perception and Utilization of Artificial Intelligence (AI) among Dental Professionals in Saudi Arabia. Open Dent. J. 2022, 16, e2208110. [Google Scholar] [CrossRef]
- Butera, A.; Maiorani, C.; Gallo, S.; Pascadopoli, M.; Buono, S.; Scribante, A. Dental Erosion Evaluation with Intact-Tooth Smartphone Application: Preliminary Clinical Results from September 2019 to March 2022. Sensors 2022, 22, 5133. [Google Scholar] [CrossRef]
- Ilhan, B.; Guneri, P.; Wilder-Smith, P. The contribution of artificial intelligence to reducing the diagnostic delay in oral cancer. Oral Oncol. 2021, 116, 105254. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.R.; Rao, S.N.; Reddy, P.R. Design of hybrid dental caries segmentation and caries detection with meta-heuristic-based ResneXt-RNN. Biomed. Signal Process. Control 2022, 78, 103961. [Google Scholar]
- Schwendicke, F.; Cejudo Grano de Oro, J.; Garcia Cantu, A.; Meyer-Lückel, H.; Chaurasia, A.; Krois, J. Artificial intelligence for caries detection: The value of data and information. J. Dent. Res. 2022, 101, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Joda, T.; Zitzmann, N.U. Personalized workflows in reconstructive dentistry—Current possibilities and future opportunities. Clin. Oral Investig. 2022, 26, 4283–4290. [Google Scholar] [CrossRef]
- Gandedkar, N.H.; Wong, M.T.; Darendeliler, M.A. Role of Virtual Reality (VR), Augmented Reality (AR) and Artificial Intelligence (AI) in tertiary education and research of orthodontics: An insight. In Seminars in Orthodontics; WB Saunders: Philadelphia, PA, USA, 2021; Volume 27, pp. 69–77. [Google Scholar]
- Hassani, H.; Amiri Andi, P.; Ghodsi, A.; Norouzi, K.; Komendantova, N.; Unger, S. Shaping the Future of Smart Dentistry: From Artificial Intelligence (AI) to Intelligence Augmentation (IA). IoT 2021, 2, 510–523. [Google Scholar] [CrossRef]
- Vishwanathaiah, S.; Fageeh, H.N.; Khanagar, S.B.; Maganur, P.C. Artificial Intelligence Its Uses and Application in Pediatric Dentistry: A Review. Biomedicines 2023, 11, 788. [Google Scholar] [CrossRef]
- Huqh, M.Z.U.; Abdullah, J.Y.; Wong, L.S.; Jamayet, N.B.; Alam, M.K.; Rashid, Q.F.; Husein, A.; Ahmad, W.M.A.W.; Eusufzai, S.Z.; Prasadh, S.; et al. Clinical Applications of Artificial Intelligence and Machine Learning in Children with Cleft Lip and Palate-A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 10860. [Google Scholar] [CrossRef]
- Kotha, S.B. Deep learning concept for early dental caries detection. J. Updates Pediatric. Dent. 2024, 3, 22–28. [Google Scholar] [CrossRef]
- Parinitha, M.S.; Doddawad, V.G.; Kalgeri, S.H.; Gowda, S.S.; Patil, S. Impact of Artificial Intelligence in Endodontics: Precision, Predictions, and Prospects. J. Med. Signals Sens. 2024, 14, 25. [Google Scholar] [CrossRef]
- Aminoshariae, A.; Kulild, J.; Nagendrababu, V. Artificial Intelligence in Endodontics: Current Applications and Future Directions. J. Endod. 2021, 47, 1352. [Google Scholar] [CrossRef]
- Paulose, A.; Jayalakshmi, M.R.; Thampy, A.M.; Kurian, C.M.; Alias, A.M.; Aluckal, E. Smartening Up with Artificial Intelligence in Dentistry: A Review. J. Orofac. Res. 2022, 11, 28–33. [Google Scholar]
- Alauddin, M.S.; Baharuddin, A.S.; Mohd Ghazali, M.I. The Modern and Digital Transformation of Oral Health Care: A Mini Review. Healthcare 2021, 9, 118. [Google Scholar] [CrossRef]
- Assaf, M.H.; Kumar, R.; Sharma, K.; Sharma, B. An optimized tongue-driven system using artificial intelligence. Comput. Methods Biomech. Biomed. Eng. Imaging Vis. 2022, 11, 696–710. [Google Scholar] [CrossRef]
- Alharbi, M.T.; Almutiq, M.M. Prediction of Dental Implants Using Machine Learning Algorithms. J. Health Eng. 2022, 2022, 7307675. [Google Scholar] [CrossRef] [PubMed]
- Dalbah, L. Digital Orthodontics. In Digitization in Dentistry; Springer: Cham, Switzerland, 2021; pp. 189–221. [Google Scholar]
- Hwang, H.-W.; Moon, J.-H.; Kim, M.-G.; Donatelli, R.E.; Lee, S.-J. Evaluation of automated cephalometric analysis based on the latest deep learning method. Angle Orthod. 2021, 91, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Chen, S.K.; Yao, J.C.C.; Chang, H.F. The Effects of Differences in Landmark Identification on the Cephalometric Measurements in Traditional versus Digitized Cephalometry. Angle Orthod. 2004, 74, 155–161. [Google Scholar] [CrossRef]
- Hwang, H.W.; Park, J.H.; Moon, J.H.; Yu, Y.; Kim, H.; Her, S.B.; Srinivasan, G.; Aljanabi, M.N.A.; Donatelli, R.E.; Lee, S.J. Automated Identification of Cephalometric Landmarks: Part 2-Might It Be Better than Human? Angle Orthod. 2020, 90, 69–76. [Google Scholar] [CrossRef]
- Chung, E.J.; Yang, B.E.; Park, I.Y.; Yi, S.; On, S.W.; Kim, Y.H.; Kang, S.H.; Byun, S.H. Effectiveness of Cone-Beam Computed Tomography-Generated Cephalograms Using Artificial Intelligence Cephalometric Analysis. Sci. Rep. 2022, 12, 20585. [Google Scholar] [CrossRef]
- Kök, H.; Acilar, A.M.; İzgi, M.S. Usage and Comparison of Artificial Intelligence Algorithms for Determination of Growth and Development by Cervical Vertebrae Stages in Orthodontics. Prog. Orthod. 2019, 20, 41. [Google Scholar] [CrossRef]
- Ozsari, S.; Güzel, M.S.; Yılmaz, D.; Kamburoğlu, K. A Comprehensive Review of Artificial Intelligence Based Algorithms Regarding Temporomandibular Joint Related Diseases. Diagnostics 2023, 13, 2700. [Google Scholar] [CrossRef]
- Jha, N.; Lee, K.S.; Kim, Y.J. Diagnosis of Temporomandibular Disorders Using Artificial Intelligence Technologies: A Systematic Review and Meta-Analysis. PLoS ONE 2022, 17, e0272715. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Chen, J.; Qiu, K.; Yang, F.; Wu, W. Artificial Intelligence for Detecting Temporomandibular Joint Osteoarthritis Using Radiographic Image Data: A Systematic Review and Meta-Analysis of Diagnostic Test Accuracy. PLoS ONE 2023, 18, e0288631. [Google Scholar] [CrossRef]
- Kabir, T.; Lee, C.T.; Chen, L.; Jiang, X.; Shams, S. A comprehensive artificial intelligence framework for dental diagnosis and charting. BMC Oral Health 2022, 22, 480. [Google Scholar] [CrossRef]
- Song, Y.B.; Jeong, H.G.; Kim, C.; Kim, D.; Kim, J.; Kim, H.J.; Park, W. Comparison of detection performance of soft tissue calcifications using artificial intelligence in panoramic radiography. Sci. Rep. 2022, 12, 19115. [Google Scholar] [CrossRef]
- Baydar, O.; Różyło-Kalinowska, I.; Futyma-Gąbka, K.; Sağlam, H. The U-Net Approaches to Evaluation of Dental Bite-Wing Radiographs: An Artificial Intelligence Study. Diagnostics 2023, 13, 453. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.; Nikhade, P. Artificial Intelligence in Dentistry: Past, Present, and Future. Cureus 2022, 14, e27405. [Google Scholar] [CrossRef] [PubMed]
- Park, W.J.; Park, J.B. History and Application of Artificial Neural Networks in Dentistry. Eur. J. Dent. 2018, 12, 594–601. [Google Scholar] [CrossRef]
- Choudhary, A.; Malik, A.; Kaul, R.; Sharma, A.; Gupta, A. A Brief Overview of Artificial Intelligence in Dentistry: Current Scope and Future Applications. J. Dent. Spec. 2023, 11, 12–16. [Google Scholar] [CrossRef]
- Hung, K.F.; Ai, Q.Y.H.; Wong, L.M.; Yeung, A.W.K.; Li, D.T.S.; Leung, Y.Y. Current Applications of Deep Learning and Radiomics on CT and CBCT for Maxillofacial Diseases. Diagnostics 2022, 13, 110. [Google Scholar] [CrossRef] [PubMed]
- Shahnavazi, M.; Mohamadrahimi, H. The application of artificial neural networks in the detection of mandibular fractures using panoramic radiography. Dent. Res. J. 2023, 20, 27. [Google Scholar] [CrossRef]
- Mohammad, N.; Ahmad, R.; Kurniawan, A.; Mohd Yusof, M.Y.P. Applications of contemporary artificial intelligence technology in forensic odontology as primary forensic identifier: A scoping review. Front. Artif. Intell. 2022, 5, 1049584. [Google Scholar] [CrossRef]
- Alsomali, M.; Alghamdi, S.; Alotaibi, S.; Alfadda, S.; Altwaijry, N.; Alturaiki, I.; Al-Ekrish, A. Development of a deep learning model for automatic localization of radiographic markers of proposed dental implant site locations. Saudi Dent. J. 2022, 34, 220–225. [Google Scholar] [CrossRef]
- Debs, P.; Fayad, L.M. The promise and limitations of artificial intelligence in musculoskeletal imaging. Front. Radiol. 2023, 3, 1242902. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.R.; Siadari, T.S.; Kim, J.E.; Huh, K.H.; Yi, W.J.; Lee, S.S.; Heo, M.S. Automatic detection of teeth and dental treatment patterns on dental panoramic radiographs using deep neural networks. Forensic Sci. Res. 2022, 7, 456–466. [Google Scholar] [CrossRef]
- Putra, R.H.; Doi, C.; Yoda, N.; Astuti, E.R.; Sasaki, K. Current applications and development of artificial intelligence for digital dental radiography. Dentomaxillofac. Radiol. 2022, 51, 20210197. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.A.; Chow, J.C. Recent progress and applications of gold nanotechnology in medical biophysics using artificial intelligence and mathematical modeling. Nano Express 2021, 2, 022001. [Google Scholar] [CrossRef]
- Imran, E.; Adanir, N.; Khurshid, Z. Significance of haptic and virtual reality simulation (VRS) in dental education: A review of the literature. Appl. Sci. 2021, 11, 10196. [Google Scholar] [CrossRef]
- Revilla-León, M.; Gómez-Polo, M.; Barmak, A.B.; Inam, W.; Kan, J.Y.; Kois, J.C.; Akal, O. Artificial intelligence models for diagnosing gingivitis and periodontal disease: A systematic review. J. Prosthet. Dent. 2022, 130, 816–824. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S149–S161. [Google Scholar] [CrossRef]
- Eke, P.I.; Thornton-Evans, G.O.; Wei, L.; Borgnakke, W.S.; Dye, B.A.; Genco, R.J. Periodontitis in US adults: National Health and Nutrition Examination Survey 2009–2014. J. Am. Dent. Assoc. 2018, 149, 576–588.e6. [Google Scholar] [CrossRef]
- Shen, K.L.; Huang, C.L.; Lin, Y.C.; Du, J.K.; Chen, F.L.; Kabasawa, Y.; Chen, C.-C.; Huang, H.L. Effects of artificial intelligence assisted dental monitoring intervention in patients with periodontitis: A randomized controlled trial. J. Clin. Periodontol. 2022, 49, 988–998. [Google Scholar] [CrossRef]
- Savage, A.; Eaton, K.A.; Moles, D.R.; Needleman, I. A systematic review of definitions of periodontitis and methods that have been used to identify this disease. J. Clin. Periodontol. 2009, 36, 458–467. [Google Scholar] [CrossRef]
- Farook, T.H.; Jamayet, N.B.; Abdullah, J.Y.; Alam, M.K. Machine learning and intelligent diagnostics in dental and orofacial pain management: A systematic review. Pain Res. Manag. 2021, 2021, 6659133. [Google Scholar] [CrossRef]
- Shan, T.; Tay, F.R.; Gu, L. Application of artificial intelligence in dentistry. J. Dent. Res. 2021, 100, 232–244. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, D.H.; Jeong, S.N.; Choi, S.H. Diagnosis and prediction of periodontally compromised teeth using a deep learning-based convolutional neural network algorithm. J. Periodontal. Implant Sci. 2018, 48, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Suzuki, N.; Kuwata, F. Predicting oral malodour based on the microbiota in saliva samples using a deep learning approach. BMC Oral Health 2018, 18, 128. [Google Scholar] [CrossRef] [PubMed]
- Sukegawa, S.; Kanno, T. Computer-Assisted Navigation Surgery in Oral and Maxillofacial Surgery. In Oral and Maxillofacial Surgery for the Clinician; Springer: Singapore, 2021; pp. 841–862. [Google Scholar]
- Thurzo, A.; Kosnáčová, H.S.; Kurilová, V.; Kosmel’, S.; Beňuš, R.; Moravansk, N.; Kováč, P.; Kuracinová, K.M.; Palkovič, M.; Varga, I. Use of Advanced Artificial Intelligence in Forensic Medicine, Forensic Anthropology and Clinical Anatomy. Healthcare 2021, 9, 1545. [Google Scholar] [CrossRef]
- Mohaideen, K.; Negi, A.; Verma, D.K.; Kumar, N.; Sennimalai, K.; Negi, A. Applications of artifcial intelligence and machine learning in orthog—Nathic surgery: A scoping review. J. Stomatol. Oral Maxillofac. Surg. 2022, 123, 962–972. [Google Scholar] [CrossRef]
- Bichu, Y.M.; Hansa, I.; Bichu, A.Y.; Premjani, P.; Flores-Mir, C.; Vaid, N.R. Appli—Cations of artifcial intelligence and machine learning in orthodontics: A scoping review. Prog. Orthod. 2021, 22, 18. [Google Scholar] [CrossRef]
- Bouletreau, P.; Makaremi, M.; Ibrahim, B.; Louvrier, A.; Sigaux, N. Artifcial intelligence: Applications in orthognathic surgery. J. Stomatol. Oral Maxil—Lofac. Surg. 2019, 120, 347–354. [Google Scholar] [CrossRef]
- Dhillon, H.; Chaudhari, P.K.; Dhingra, K.; Kuo, R.F.; Sokhi, R.K.; Alam, M.K.; Ahmad, S. Current Applications of Artificial Intelligence in Cleft Care: A Scoping Review. Front. Med. 2021, 8, 676490. [Google Scholar] [CrossRef]
- Siddiqui, A.; Sukhia, R.H.; Ghandhi, D. Artifcial intelligence in dentistry, orthodontics and Orthognathic surgery: A literature review. J. Pak. Med. Assoc. 2022, 72, 91–96. [Google Scholar]
- Patcas, R.; Bornstein, M.M.; Schatzle, M.A.; Timofte, R. Artifcial intelligence in medico-dental diagnostics of the face: A narrative review of opportunities and challenges. Clin. Oral Investig. 2022, 26, 6871–6879. [Google Scholar] [CrossRef]
- Hong, M.; Kim, I.; Cho, J.H.; Kang, K.H.; Kim, M.; Kim, S.J.; Kim, Y.J.; Sung, S.J.; Kim, Y.H.; Lim, S.H.; et al. Accuracy of artificial intelligence-assisted landmark identifcation in serial lateral cephalograms of Class III patients who underwent orthodontic treatment and two-jaw orthognathic surgery. Korean J. Orthod. 2022, 52, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Wang, M.; Dai, N.; Ma, H.; Li, L.; Fiorenza, L.; Sun, Y.; Li, Y. DCPR-GAN: Dental crown prosthesis restoration using two-stage generative adversarial networks. IEEE J. Biomed. Health Inform. 2021, 26, 151–160. [Google Scholar] [CrossRef] [PubMed]
- HonShin, W.; Yeom, H.G.; Lee, G.H.; Yun, J.P.; Jeong, S.H.; Lee, J.H.; Kim, H.K.; Kim, B.C. Deep learning based prediction of necessity for orthognathic surgery of skeletal malocclusion using cephalogram in Korean individuals. BMC Oral Health 2021, 21, 130. [Google Scholar] [CrossRef]
- Tanikawa, C.; Yamashiro, T. Development of novel artifcial intelligence systems to predict facial morphology after orthognathic surgery and orthodontic treatment in Japanese patients. Sci. Rep. 2021, 11, 15853. [Google Scholar] [CrossRef]
- Tumbelaka, B.Y.; Oscandar, F.; Baihaki, F.N.; Sitam, S.; Rukmo, M.J.S.E.J. Identification of pulpitis at dental X-ray periapical radiography based on edge detection, texture description and artificial neural networks. Saudi Endod. J. 2014, 4, 115–121. [Google Scholar] [CrossRef]
- Schwendicke, F.; Martens, S.; Cantu, A.G.; Chaurasia, A.; Meyer-Lueckel, H.; Krois, J. Cost-effectiveness of AI for caries detection: Randomized trial. J. Dent. 2022, 119, 104080. [Google Scholar] [CrossRef]
- Qayyum, A.; Tahir, A.; Butt, M.A.; Luke, A.; Abbas, H.T.; Qadir, J.; Arshad, K.; Assaleh, K.; Imran, M.A.; Abbasi, Q.H. Dental caries detection using a semi-supervised learning approach. Sci. Rep. 2023, 13, 749. [Google Scholar] [CrossRef]
- Wei, J.; Peng, M.; Li, Q.; Wang, Y. Evaluation of a novel computer color matching system based on the improved back-propagation neural network model. J. Prosthodont. 2018, 27, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Karobari, M.I.; Adil, A.H.; Basheer, S.N.; Murugesan, S.; Savadamoorthi, K.S.; Mustafa, M.; Abdulwahed, A.; Almokhatieb, A.A. Evaluation of the Diagnostic and Prognostic Accuracy of Artificial Intelligence in Endodontic Dentistry: A Comprehensive Review of Literature. Comput. Math. Methods Med. 2023, 2023, 7049360. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, H.; Mei, L.; Chen, Q.; Zhang, Y.; Zhang, H. Artificial intelligence in digital cariology: A new tool for the diagnosis of deep caries and pulpitis using convolutional neural networks. Ann. Transl. Med. 2021, 9, 763. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Lee, C.; Karaer, O.; Ban, S.; Mine, A.; Imazato, S. Predicting the debonding of CAD/CAM composite resin crowns with AI. J. Dent. Res. 2019, 98, 1234–1238. [Google Scholar] [CrossRef]
- Mahmood, H.; Shaban, M.; Indave, B.I.; Santos-Silva, A.R.; Rajpoot, N.; Khurram, S.A. Use of artificial intelligence in diagnosis of head and neck precancerous and cancerous lesions: A systematic review. Oral Oncol. 2020, 110, 104885. [Google Scholar] [CrossRef] [PubMed]
- Vadlamani, R. Application of Machine Learning Technologies for Detection of Proximal Lesions in Intraoral Digital Images: In Vitro Study. Master’s Thesis, University of Louisville, Louisville, KY, USA, 2020. [Google Scholar] [CrossRef]
- Saghiri, M.A.; Garcia-Godoy, F.; Gutmann, J.L.; Lotfi, M.; Asgar, K. The reliability of artificial neural network in locating minor apical foramen: A cadaver study. J. Endod. 2012, 38, 1130–1134. [Google Scholar] [CrossRef]
- Setzer, F.C.; Shi, K.J.; Zhang, Z.; Yan, H.; Yoon, H.; Mupparapu, M.; Li, J. Artificial intelligence for the computer-aided detection of periapical lesions in cone-beam computed tomographic images. J. Endod. 2020, 46, 987–993. [Google Scholar] [CrossRef]
- Kim, E.H.; Kim, S.; Kim, H.J.; Jeong, H.O.; Lee, J.; Jang, J.; Joo, J.Y.; Shin, Y.; Kang, J.; Park, A.K.; et al. Prediction of chronic periodontitis severity using machine learning models based on salivary bacterial copy number. Front. Cell Infect. 2020, 10, 698. [Google Scholar] [CrossRef]
- Saghiri, M.A.; Asgar, K.; Boukani, K.K.; Lotfi, M.; Aghili, H.; Delvarani, A.; Karamifar, K.; Saghiri, A.M.; Mehrvarzfar, P.; Garcia-Godoy, F. A new approach for locating the minor apical foramen using an artificial neural network. Int. Endod. J. 2012, 45, 257–265. [Google Scholar] [CrossRef] [PubMed]
- AbuSalim, S.; Zakaria, N.; Islam, M.R.; Kumar, G.; Mokhtar, N.; Abdulkadir, S.J. Analysis of deep learning techniques for dental informatics: A systematic literature review. Healthcare 2022, 10, 1892. [Google Scholar] [CrossRef] [PubMed]
- Khanagar, S.B.; Alfadley, A.; Alfouzan, K.; Awawdeh, M.; Alaqla, A.; Jamleh, A. Developments and Performance of Artificial Intelligence Models Designed for Application in Endodontics: A Systematic Review. Diagnostics 2023, 13, 414. [Google Scholar] [CrossRef]
- Mohammad-Rahimi, H.; Motamedian, S.R.; Pirayesh, Z.; Haiat, A.; Zahedrozegar, S.; Mahmoudinia, E.; Rohban, M.H.; Krois, J.; Lee, J.; Schwendicke, F. Deep learning in periodontology and oral implantology: A scoping review. J. Periodont. Res. 2022, 57, 942–951. [Google Scholar] [CrossRef]
- Smitha, T. Artificial Intelligence in Forensic Odontology. J. Forensic Dent. Sci. 2023, 13, 1–2. [Google Scholar] [CrossRef]
- Kong, H.J.; Kim, Y.L. Application of artificial intelligence in dental crown prosthesis: A scoping review. BMC Oral Health 2024, 24, 937. [Google Scholar] [CrossRef]
- Tabatabaian, F.; Vora, S.R.; Mirabbasi, S. Applications, functions, and accuracy of artificial intelligence in restorative dentistry: A literature review. J. Esthet. Restor. Dent. 2023, 35, 842–859. [Google Scholar] [CrossRef] [PubMed]
- You, W.; Hao, A.; Li, S.; Wang, Y.; Xia, B. Deep learning-based dental plaque detection on primary teeth: A comparison with clinical assessments. BMC Oral Health 2020, 20, 141. [Google Scholar] [CrossRef]
- Mirishli, S. Ethical Implications of AI in Data Collection: Balancing Innovation with Privacy. Qədim. Diyar. 2024, 6, 40–55. [Google Scholar] [CrossRef]
- Gulia, K.; Hamdan, I.A.; Datta, N.; Gupta, Y.; Kumar, P.; Yadav, A.; Mitten, S.K.; Kumar, R. Machine Learning Models for Personalised Healthcare on Marketable Generative-AI with Ethical Implications. World J. Adv. Res. Rev. 2024, 23, 707–720. [Google Scholar] [CrossRef]
- Marques, M.; Almeida, A.M.; Pereira, H. The Medicine Revolution Through Artificial Intelligence: Ethical Challenges of Machine Learning Algorithms in Decision-Making. Cureus 2024, 16, e69405. [Google Scholar] [CrossRef]
- Almasri, I.A. The Power of Artificial Intelligence for Improved Patient Outcomes, Ethical Practices and Overcoming Challenges. IgMin. Res. 2024, 2, 585–588. [Google Scholar]
- Khatri, S. The Role of Artificial Intelligence in Healthcare: Applications, Challenges, and Ethical Considerations. Int. J. Res. Publ. Semin. 2024, 15, 195–202. [Google Scholar] [CrossRef]
- Ahmed, N.; Abbasi, M.S.; Zuberi, F.; Qamar, W.; Halim, M.S.B.; Maqsood, A.; Alam, M.K. Artificial Intelligence Techniques: Analysis, Application, and Outcome in Dentistry-A Systematic Review. Biomed. Res. Int. 2021, 2021, 9751564. [Google Scholar] [CrossRef]
- Delgado-Ruiz, R.; Kim, A.S.; Zhang, H.; Sullivan, D.; Awan, K.H.; Stathopoulou, P.G. Generative Artificial Intelligence (Gen AI) in Dental Education: Opportunities, Cautions, and Recommendations. J. Dent. Educ. 2024, 89, 130–136. [Google Scholar] [CrossRef]
- Kisvarday, S.; Yan, A.; Yarahuan, J.; Kats, D.J.; Ray, M.; Kim, E.Y.; Hong, P.; Spector, J.D.; Bickel, J.; Parsons, C.; et al. ChatGPT Use Among Pediatric Healthcare Providers. JMIR Form. Res. 2024, 8, e56797. [Google Scholar] [CrossRef] [PubMed]
- Villena, F.; V’eliz, C.; Garc’ia-Huidobro, R.; Aguayo, S. Generative Artificial Intelligence in Dentistry: Current Approaches and Future Challenges. arXiv 2024, arXiv:2407.17532. [Google Scholar] [CrossRef]
- Shetty, R. Artificial Intelligence (AI) in Pediatric Dentistry. J. Updates Pediatr. Dent. 2023, 2, 1–2. [Google Scholar] [CrossRef]
- Fehér, B.; Tussie, C.; Giannobile, W.V. Applied Artificial Intelligence in Dentistry: Emerging Data Modalities and Modeling Approaches. Front. Artif. Intell. 2024, 7, 1427517. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.M.; QUADAS-2 Group. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, D.H.; Jeong, S.N.; Choi, S.H. Detection and diagnosis of dental caries using a deep learning-based convolutional neural network algorithm. J. Dent. 2018, 77, 106–111. [Google Scholar] [CrossRef]
- Schwendicke, F.; Elhennawy, K.; Paris, S.; Friebertshäuser, P.; Krois, J. Deep learning for caries lesion detection in near-infrared light transillumination images: A pilot study. J. Dent. 2020, 92, 103260. [Google Scholar] [CrossRef]
- Kühnisch, J.A.; Meyer, O.; Hesenius, M.; Hickel, R.; Gruhn, V. Caries detection on intraoral images using artificial intelligence. J. Dent. Res. 2022, 101, 158–165. [Google Scholar] [CrossRef]
- Zhang, X.; Liang, Y.; Li, W.; Liu, C.; Gu, D.; Sun, W.; Miao, L. Development and evaluation of deep learning for screening dental caries from oral photographs. Oral Dis. 2022, 28, 173–181. [Google Scholar] [CrossRef]
- Yoon, K.; Jeong, H.M.; Kim, J.W.; Park, J.H.; Choi, J. AI-based dental caries and tooth number detection in intraoral photos: Model development and performance evaluation. J. Dent. 2024, 141, 104821. [Google Scholar] [CrossRef]
- Thanh, M.T.; Van Toan, N.; Ngoc, V.T.; Tra, N.T.; Giap, C.N.; Nguyen, D.M. Deep learning application in dental caries detection using intraoral photos taken by smartphones. Appl. Sci. 2022, 12, 5504. [Google Scholar] [CrossRef]
- Ding, B.; Zhang, Z.; Liang, Y.; Wang, W.; Hao, S.; Meng, Z.; Guan, L.; Hu, Y.; Guo, B.; Zhao, R.; et al. Detection of dental caries in oral photographs taken by mobile phones based on the YOLOv3 algorithm. Ann. Transl. Med. 2021, 9, 1622. [Google Scholar] [CrossRef]
- Geetha, V.; Aprameya, K.S.; Hinduja, D.M. Dental caries diagnosis in digital radiographs using back-propagation neural network. Health Inf. Sci. Syst. 2020, 8, 8. [Google Scholar] [CrossRef]
- Patil, S.; Rao, R.S.; Majumdar, B.; Anil, S. Artificial intelligence in the diagnosis of oral diseases: Applications and pitfalls. Diagnostics 2022, 12, 1029. [Google Scholar] [CrossRef]
- Shen, D.; Wu, G.; Suk, H.I. Deep learning in medical image analysis. Annu. Rev. Biomed. Eng. 2017, 19, 221–248. [Google Scholar] [CrossRef]
- Li, W.; Liang, Y.; Zhang, X.; Liu, C.; He, L.; Miao, L.; Sun, W. A deep learning approach to automatic gingivitis screening based on classification and localization in RGB photos. Sci. Rep. 2021, 11, 16831. [Google Scholar] [CrossRef]
- Oztekin, F.; Katar, O.; Sadak, F.; Yildirim, M.; Cakar, H.; Aydogan, M.; Ozpolat, Z.; Talo Yildirim, T.; Yildirim, O.; Faust, O.; et al. An Explainable Deep Learning Model to Prediction Dental Caries Using Panoramic Radiograph Images. Diagnostics 2023, 13, 226. [Google Scholar] [CrossRef]
- Liu, L.; Xu, J.; Huan, Y.; Zou, Z.; Yeh, S.-C.; Zheng, L.-R. A smart dental health-IoT platform based on intelligent hardware, deep learning, and mobile terminal. IEEE J. Biomed. Health Inform. 2020, 24, 898–906. [Google Scholar] [CrossRef]
- Saini, D.; Jain, R.; Thakur, A. Dental caries early detection using convolutional neural network for tele dentistry. In Proceedings of the 7th International Conference on Advanced Computing and Communication Systems (ICACCS 2021), Coimbatore, India, 19–20 March 2021; pp. 958–963. [Google Scholar]
- Sonavane, A.; Yadav, R.; Khamparia, A. Dental cavity classification using convolutional neural network. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1022, 012116. [Google Scholar] [CrossRef]
- Takahashi, T.; Nozaki, K.; Gonda, T.; Mameno, T.; Ikebe, K. Deep learning-based detection of dental prostheses and restorations. Sci. Rep. 2021, 11, 1960. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, H.; Zhou, S.; Lu, M.; Huang, J.; Huang, Q.; Huang, B.; Ding, J. Simultaneous detection of dental caries and fissure sealant in intraoral photos by deep learning: A pilot study. BMC Oral Health 2024, 24, 553. [Google Scholar] [CrossRef]
- Wang, C.; Yang, J.; Wu, B.; Liu, R.; Yu, P. Trans-VNet: Transformer-based tooth semantic segmentation in CBCT images. Biomed. Signal Process. Control 2024, 97, 106666. [Google Scholar] [CrossRef]
- Moutselos, K.; Berdouses, E.; Oulis, C.; Maglogiannis, I. Recognizing occlusal caries in dental intraoral images using deep learning. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2019, 2019, 1617–1620. [Google Scholar]
- Lee, S.; Oh, S.I.; Jo, J.; Kang, S.; Shin, Y.; Park, J.-W. Deep learning for early dental caries detection in bitewing radiographs. Sci. Rep. 2021, 11, 16807. [Google Scholar] [CrossRef]
- Jagtap, R.; Samata, Y.; Parekh, A.; Tretto, P.; Roach, M.D.; Sethumanjusha, S.; Tejaswi, C.; Jaju, P.; Friedel, A.; Garrido, M.B.; et al. Clinical validation of deep learning for segmentation of multiple dental features in periapical radiographs. Bioengineering 2024, 11, 1001. [Google Scholar] [CrossRef]
- Bayrakdar, S.K.; Orhan, K.; Bayrakdar, I.S.; Bilgir, E.; Ezhov, M.; Gusarev, M.; Shumilov, E. A deep learning approach for dental implant planning in CBCT images. BMC Med. Imaging 2021, 21, 86. [Google Scholar]
- Schwendicke, F.; Samek, W.; Krois, J. Artificial intelligence in dentistry: Chances and challenges. J. Dent. Res. 2020, 99, 769–774. [Google Scholar] [CrossRef]
- Chau, R.C.W.; Thu, K.M.; Yu, O.Y.; Hsung, R.T.C.; Lo, E.C.M.; Lam, W.Y.H. Performance of Generative Artificial Intelligence in Dental Licensing Examinations. Int. Dent. J. 2024, 74, 616–621. [Google Scholar] [CrossRef]
- Bas, B.; Ozgonenel, L.; Ozden, B.; Bekcioglu, B.; Bulut, E.; Kurt, M. Use of artificial neural network in differentiation of subgroups of temporomandibular internal derangements: A preliminary study. J. Oral Maxillofac. Surg. 2012, 70, 51–59. [Google Scholar] [CrossRef]
- Yang, S.; Kim, K.D.; Ariji, E.; Kise, Y. Generative adversarial networks in dental imaging: A systematic review. Oral Radiol. 2024, 40, 93–108. [Google Scholar] [CrossRef]
- Asiri, A.F.; Altuwalah, A.S. The role of neural artificial intelligence for diagnosis and treatment planning in endodontics: A qualitative review. Saudi Dent. J. 2022, 34, 270–281. [Google Scholar] [CrossRef]
- Boreak, N. Effectiveness of artificial intelligence applications designed for endodontic diagnosis, decision-making, and prediction of prognosis: A systematic review. J. Contemp. Dent. Pract. 2020, 21, 926–934. [Google Scholar] [CrossRef]
- Chen, Y.W.; Stanley, K.; Att, W. Artificial intelligence in dentistry: Current applications and future perspectives. Quintessence Int. 2020, 51, 248–257. [Google Scholar]
- Dashti, M.; Ghaedsharaf, S.; Ghasemi, S.; Zare, N.; Constantin, E.-F.; Fahimipour, A.; Tajbakhsh, N.; Ghadimi, N. Evaluation of deep learning and convolutional neural network algorithms for mandibular fracture detection using radiographic images: A systematic review and meta-analysis. Imaging Sci. Dent. 2024, 54, 232–239. [Google Scholar] [CrossRef]
- Hung, K.; Yeung, A.W.K.; Tanaka, R.; Bornstein, M.M. Current applications, opportunities, and limitations of AI for 3D imaging in dental research and practice. Int. J. Environ. Res. Public Health 2024, 17, 4424. [Google Scholar] [CrossRef]
- Liu, T.-Y.; Lee, K.-H.; Mukundan, A.; Karmakar, R.; Dhiman, H.; Wang, H.-C. AI in Dentistry: Innovations, Ethical Considerations, and Integration Barriers. Bioengineering 2025, 12, 928. [Google Scholar] [CrossRef]
- Müller, A.; Mertens, S.M.; Göstemeyer, G.; Krois, J.; Schwendicke, F. Barriers and Enablers for Artificial Intelligence in Dental Diagnostics: A Qualitative Study. J. Clin. Med. 2021, 10, 1612. [Google Scholar] [CrossRef]
- Hoffman, J.; Wenke, R.; Angus, R.L.; Shinners, L.; Richards, B.; Hattingh, L. Overcoming Barriers and Enabling Artificial Intelligence Adoption in Allied Health Clinical Practice: A Qualitative Study. Digit. Health 2025, 11, 20552076241311144. [Google Scholar] [CrossRef]
- Nambiar, R.; Nanjundegowda, R. A comprehensive review of AI and deep learning applications in dentistry: From image segmentation to treatment planning. J. Robot Control 2024, 5, 1744–1752. [Google Scholar]
- Polizzi, A.; Leonardi, R. Automatic cephalometric landmark identification with artificial intelligence: An umbrella review. J. Dent. 2024, 146, 105056. [Google Scholar] [CrossRef]
- Kalli, V.D.R. Artificial intelligence; mutating dentistry of the modern era. Metascience 2023, 1, 45–52. [Google Scholar]
- Khanna, S.S.; Dhaimade, P.A. Artificial intelligence: Transforming dentistry today. Indian J. Basic Appl. Med. Res. 2017, 6, 161–167. [Google Scholar]
- Joda, T.; Bornstein, M.M.; Jung, R.E. Recent trends and future direction of dental research in the digital era. Int. J. Environ. Res. Public Health 2020, 17, 1987. [Google Scholar] [CrossRef] [PubMed]
- Haidar, Z.S. Digital dentistry: Past, present, and future. Digit. Med. Healthc. Technol. 2023, 16, 143–156. [Google Scholar]
- Fatima, A.; Shafi, I.; Afzal, H.; Díez, I.D.L.T. Advancements in dentistry with artificial intelligence: Current clinical applications and future perspectives. Healthcare 2022, 10, 2188. [Google Scholar] [CrossRef]
- Shafi, I.; Fatima, A.; Afzal, H.; de la Torre Díez, I.; Lipari, V.; Breñosa, J.; Ashraf, I. Comprehensive review of recent advances in artificial intelligence for dentistry e-health. Diagnostics 2023, 13, 2196. [Google Scholar] [CrossRef]
- Kukalakunta, Y.; Thunki, P.; Yellu, R.R. Integrating artificial intelligence in dental healthcare: Opportunities and challenges. J. Deep. Learn. Genom. Data Anal. 2024, 4, 34–41. [Google Scholar]
- Revilla-León, M.; Gómez-Polo, M.; Vyas, S.; Barmak, A.B.; Özcan, M.; Att, W.; Krishnamurthy, V.R. Artificial intelligence applications in restorative dentistry: A systematic review. J. Prosthet. Dent. 2022, 128, 867–875. [Google Scholar] [CrossRef]
- Deshmukh, S.V. Artificial intelligence in dentistry. J. Int. Clin. Dent. Res. Organ. 2018, 10, 47–48. [Google Scholar] [CrossRef]
- Pethani, F. Promises and perils of artificial intelligence in dentistry. Aust. Dent. J. 2021, 66, 124–135. [Google Scholar] [CrossRef]
- Surlari, Z.; Budală, D.G.; Lupu, C.I.; Stelea, C.G.; Butnaru, O.M.; Luchian, I. Current progress and challenges of using artificial intelligence in clinical dentistry—A narrative review. J. Clin. Med. 2023, 12, 7378. [Google Scholar] [CrossRef]
- Li, P.; Tao, H.; Zhou, H.; Zhou, P.; Deng, Y. Enhanced Multiview Attention Network with Random Interpolation Resize for Few-Shot Surface Defect Detection. Multimed. Syst. 2025, 31, 36. [Google Scholar] [CrossRef]
- Wang, Z.; Tao, H.; Zhou, H.; Deng, Y.; Zhou, P. A Content-Style Control Network with Style Contrastive Learning for Underwater Image Enhancement. Multimed. Syst. 2025, 31, 60. [Google Scholar] [CrossRef]
- Apedo, Y.; Tao, H. A Weakly Supervised Pavement Crack Segmentation Based on Adversarial Learning and Transformers. Multimed. Syst. 2025, 31, 266. [Google Scholar] [CrossRef]
| ID | Authors | Year | Study Design | Sample Size | AI Method | Application | Imaging Type | Sensitivity (%) | Specificity (%) | Accuracy (%) | Key Limitations |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Lee et al. [108] | 2018 | Retrospective | 3000 images | Deep CNN | Caries detection | Periapical radiographs | 89 | 88 | 88.5 | Single center, retrospective design |
| 2 | Schwendicke et al. [109] | 2020 | Retrospective | 2848 images | Deep CNN | Caries detection | NILT images | 76 | 78 | 77 | Limited imaging modality validation |
| 3 | Kühnisch et al. [110] | 2022 | Cross-sectional | 4573 images | CNN | Caries detection | Intraoral photos | 82 | 91 | 87 | Controlled lighting conditions |
| 4 | Zhang et al. [111] | 2022 | Retrospective | 1819 photos | Deep learning | Caries screening | Oral photographs | 84 | 89 | 86.5 | Limited demographic diversity |
| 5 | Yoon et al. [112] | 2024 | Prospective | 4361 teeth | MobileNet-v3 + U-Net | Caries detection | Intraoral camera | 81 | 96 | 93.4 | Single specialty clinic |
| 6 | Thanh et al. [113] | 2022 | Cross-sectional | 2400 images | Deep CNN | Caries detection | Smartphone photos | 79 | 85 | 82 | Variable image quality |
| 7 | Ding et al. [114] | 2021 | Retrospective | 1500 photos | YOLOv3 | Caries detection | Mobile phone photos | 86 | 92 | 89 | Limited clinical validation |
| 8 | Geetha et al. [115] | 2020 | Retrospective | 800 cases | ANN | Caries diagnosis | Digital radiographs | 95 | 98 | 97.1 | Small dataset, single center |
| 9 | Patil et al. [116] | 2022 | Controlled | 68 patients | ANN | TMJ diagnosis | Clinical data | 92 | 89 | 90.5 | Small sample, no imaging |
| 10 | Shen et al. [117] | 2017 | Retrospective | 1200 images | CNN + DL | Periodontal disease | Radiographs | 88 | 91 | 89.5 | Limited disease stages |
| 11 | Li et al. [118] | 2021 | Cross-sectional | 2856 photos | Deep learning | Gingivitis screening | RGB photos | 85 | 88 | 86.5 | Subjective ground truth |
| 12 | Oztekin et al. [119] | 2023 | Retrospective | 5000 images | ResNet-50 | Caries detection | Panoramic radiographs | 87 | 94 | 92 | Single imaging modality |
| 13 | Liu et al. [120] | 2020 | Pilot study | 500 cases | Deep learning IoT | Dental health screening | Mobile platform | 78 | 82 | 80 | Proof of concept only |
| 14 | Saini et al. [121] | 2021 | Laboratory | 1000 images | CNN | Early caries detection | Digital photos | 83 | 87 | 85 | Laboratory conditions only |
| 15 | Sonavane et al. [122] | 2021 | Retrospective | 800 images | CNN | Cavity classification | X-ray images | 81 | 86 | 83.5 | Limited cavity types |
| 16 | Takahashi et al. [123] | 2021 | Cross-sectional | 2500 images | Deep learning | Prosthesis detection | Radiographs | 94 | 97 | 95.5 | Limited prosthesis types |
| 17 | Xiong et al. [124] | 2024 | Pilot study | 1200 photos | Deep learning | Caries + sealant detection | Intraoral photos | 79 | 84 | 81.5 | Pilot study limitations |
| 18 | Wang et al. [125] | 2024 | Retrospective | 3200 scans | Trans-VNet | Tooth segmentation | CBCT images | 91 | 95 | 93 | Computational complexity |
| 19 | Moutselos et al. [126] | 2019 | Retrospective | 600 images | Deep learning | Occlusal caries | Intraoral images | 86 | 90 | 88 | Specific caries type only |
| 20 | Lee et al. [127] | 2021 | Retrospective | 1935 images | U-Net | Early caries detection | Bitewing radiographs | 85 | 89 | 87 | Retrospective design |
| 21 | Jagtap et al. [128] | 2024 | Clinical validation | 2000 radiographs | Deep learning | Multiple dental features | Periapical radiographs | 87 | 92 | 89.5 | Single imaging type |
| 22 | Bayrakdar et al. [129] | 2021 | Laboratory | 150 CBCT scans | CNN | Implant planning | CBCT | 94 | 92 | 93 | Laboratory validation only |
| 23 | Schwendicke et al. [130] | 2020 | Controlled | 500 cases | NN | Outcome prediction | Clinical data | 85 | 88 | 86.5 | Limited follow-up |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araidy, S.; Batshon, G.; Mirochnik, R. Artificial Intelligence Applications in Dentistry: A Systematic Review. Oral 2025, 5, 90. https://doi.org/10.3390/oral5040090
Araidy S, Batshon G, Mirochnik R. Artificial Intelligence Applications in Dentistry: A Systematic Review. Oral. 2025; 5(4):90. https://doi.org/10.3390/oral5040090
Chicago/Turabian StyleAraidy, Shareef, George Batshon, and Roman Mirochnik. 2025. "Artificial Intelligence Applications in Dentistry: A Systematic Review" Oral 5, no. 4: 90. https://doi.org/10.3390/oral5040090
APA StyleAraidy, S., Batshon, G., & Mirochnik, R. (2025). Artificial Intelligence Applications in Dentistry: A Systematic Review. Oral, 5(4), 90. https://doi.org/10.3390/oral5040090





