Abstract
Background: Commercial oral care products commonly incorporate synthetic antimicrobials such as cetylpyridinium chloride (Cetyl Cl.), L-Arginine (L-arg.), and stannous fluoride (SnF2). Although effective against oral pathogens, these agents are often associated with adverse effects including mucosal irritation, taste alteration, and disruption of the oral microbiome. These limitations have spurred growing interest in safer, plant-based alternatives. In this study, we present a two-pronged in vitro oral care testing model that integrates cell assays with machine-guided quantitative microscopy analyses to assess both antibacterial efficacy and host biocompatibility of botanical extracts. Methods: Using Miswak (Salvadora persica) and Neem (Azadirachta indica) as representative natural products, we conducted antibacterial and antibiofilm testing including the evaluation of the minimum inhibitory concentration (MIC), minimum biofilm inhibitory concentration (MBIC), and minimum biofilm eradication concentration (MBEC), alongside biocompatibility assessments via MTT cell viability assays on probiotic bacteria and mammalian oral cells. To evaluate biofilm structure and disruption, we employed scanning electron microscopy (SEM) and confocal laser scanning microscopy (CLSM), augmented with machine-guided Weka segmentation and automated image analysis. Results: Our findings show that Miswak and Neem extracts exhibited 75–100% antibacterial and antibiofilm efficacy against all tested bacteria, as demonstrated by cell assays and microscopy analyses, comparable to synthetic oral care agents. They also maintained ~100% viability toward commensal microbes and mammalian oral cells, whereas Cetyl Cl. and SnF2 showed dose-dependent cytotoxicity. Conclusions: This dual-assessment oral care testing model provides a comprehensive and biologically relevant framework for the discovery and screening of safe and effective natural herbal extracts in oral care applications.
1. Introduction
The oral microbiota, the second most abundant microbial community after the gastrointestinal microbiota, plays a critical role in our overall health. Beyond its role in the initial stages of digestion, the complexity of the oral microbiome influences various organs throughout the body. Disturbances in the oral microbiome, known as dysbiosis, often begin with localized symptoms such as dental plaque and cavities, but if left untreated, can progress to more severe conditions like periodontitis, which affects nearly half the global population [1]. This oral pathogenic cascade can extend beyond the mouth, as pathogenic bacteria may enter the bloodstream and contribute to disorders in distant organs. Emerging research highlights a strong association between poor oral health and the development of serious conditions such as atherosclerosis, Alzheimer’s disease, and rheumatoid arthritis [2,3,4].
Efforts have been made to unravel the complexities of pathogen invasion and proliferation within the oral cavity. Pathogens can exist in two primary states: a planktonic form suspended in saliva, or an attached form on hard tooth surfaces as organized biofilms, which are more resistant to environmental stress. Biofilms represent a preferred growth mode for many bacterial cell types, enabling their persistence and posing significant challenges due to their resistance to mechanical removal and antimicrobial treatments. The formation of biofilms begins with the critical first step of irreversible bacterial adhesion to the tooth surface. This initial layer subsequently facilitates the aggregation of secondary or late-colonizing bacteria. Over time, this process leads to accumulation and maturation, which ultimately develops into dental plaque and its hardened, calcified form known as tartar [4,5]. During this progression, diverse bacterial species invade the oral environment at different stages, often interacting synergistically or antagonistically to establish plaques or trigger gingival inflammation [6]. This pathogenic sequence is illustrated in Figure 1. While pathogens such as Streptococcus mutans (S. mutans), Fusobacterium nucleatum (F. nucleatum), and Porphyromonas gingivalis (P. gingivalis) play key roles in biofilm formation and development of periodontitis, commensal species such as Streptococcus gordonii (S. gordonii) and Lactobacillus rhamnosus GG (L. rhamnosus GG) are instrumental in maintaining a healthy oral environment [6,7,8].
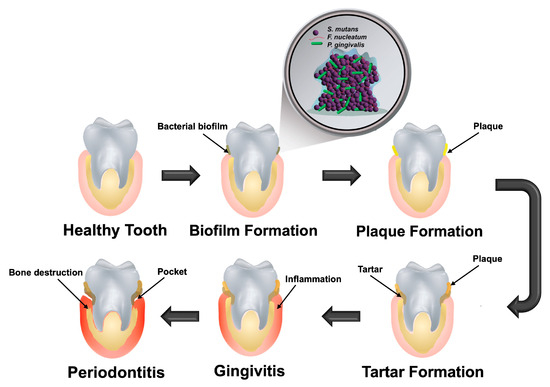
Figure 1.
Pathogenesis of periodontitis: progression begins with the formation of a bacterial biofilm on a healthy tooth surface, followed by accumulation of plaque and tartar. This buildup triggers gingival inflammation (gingivitis), which, if left untreated, can advance to periodontitis.
In the oral cavity, S. mutans contributes significantly to the formation of mature biofilms, disrupting the balance of bacterial species [9]. It creates an acidic environment by lowering the pH at the tooth surface to as low as 4.0, conditions that are not only corrosive but also detrimental to beneficial bacteria that thrive in a neutral pH range. This acidification, particularly when the pH drops below 5.5, leads to enamel demineralization and the onset of dental caries [5,10]. F. nucleatum, on the other hand, plays a central role in oral biofilm development by serving as a physical and metabolic bridge between early and late microbial colonizers. It enhances biofilm integrity through the production of polyamines [11]. Furthermore, F. nucleatum coexists and synergizes with P. gingivalis, which releases diffusible signaling molecules that alter gene expression and metabolism in F. nucleatum, promoting cooperative colonization and biofilm maturation within the oral microbiome [12]. In addition to its structural role in biofilm formation, F. nucleatum has been increasingly implicated in the progression of oral diseases, including periodontitis and oral squamous cell carcinoma. These effects are largely mediated through its interactions with P. gingivalis and modulation of host and microbial gene expression via interspecies signaling pathways [12,13]. The pathogenic potential of P. gingivalis further amplifies this effect, as it can evade the host immune response and contribute to carcinogenic processes [14].
In contrast, S. gordonii, a common inhabitant of the oral cavity, is generally regarded as a beneficial microbe that rarely causes disease under normal conditions [8]. However, it can become opportunistic and contribute to infection in certain pathological environments, despite its protective role in inhibiting the colonization of more virulent species [15,16]. Studies have shown that S. gordonii antagonizes the cariogenic bacterium S. mutans through the production of hydrogen peroxide (H2O2), an effect that is enhanced in the presence of magnesium [17]. Similarly, L. rhamnosus GG exhibits antagonistic activity against pathogenic bacteria by producing H2O2, suppressing S. mutans, and generating alkaline ammonia, which helps neutralize acidic conditions in the oral environment [8].
Beyond microbial communities, other components such as saliva, teeth, and oral soft tissues play essential roles in maintaining oral health. These soft tissues, both protective and supportive, should be preserved during therapeutic interventions. Oral keratinocytes, located in the epithelial layer of the oral mucosa, produce structural proteins that uphold cellular integrity and contribute to the formation of a protective barrier [18]. Meanwhile, human gingival fibroblasts (HGF-1) help modulate the local cellular microbiome, particularly in response to lipopolysaccharides released by periodontal pathogens [19].
Over the past decades, numerous chemical compounds have been developed and commercialized in the ongoing battle against oral bacteria. Among the most widely used standards are cetylpyridinium chloride (Cetyl Cl.), L-Arginine (L-arg.), and stannous fluoride (SnF2). While these agents are effective in reducing dental plaque and caries, they are not without disadvantages. For example, SnF2 may cause mild tooth discoloration and alter taste perception, particularly when consuming acidic beverages like orange juice [20]. Although Cetyl Cl. is effective against planktonic bacteria, its efficacy against biofilms is limited [21]. L-arg., a more recent oral antibacterial additive, promotes nitric oxide production but suffers from drawbacks such as short biological half-life, poor storage stability, and suboptimal delivery efficacy [22]. In contrast, botanical antibacterial agents are increasingly being explored as promising alternatives to traditional chemicals. These naturally derived compounds offer advantages including lower toxicity, anti-inflammatory properties, and broad-spectrum antimicrobial activity [23,24]. Some have demonstrated effectiveness against a wide range of pathogens, including certain viruses, while also exhibiting anti-inflammatory properties [25,26]. Consequently, the investigation of botanical-based products for oral health maintenance is gaining momentum, highlighting their potential as safer and more sustainable alternatives in the prevention and management of oral diseases.
Miswak (Salvadora persica) and Neem (Azadirachta indica) are plants native to India and surrounding regions. Miswak twigs have traditionally been used as natural chewing sticks for oral hygiene, while Neem leaves have been utilized for their medicinal and antibacterial properties [27,28]. Numerous studies have demonstrated the efficacy of Miswak in reducing dental plaque, combating gingivitis and dental caries, promoting gingival wound healing, whitening teeth, preserving orthodontic chains, and maintaining biocompatibility with oral tissues [27]. Additionally, both aqueous and organic extracts of Miswak have exhibited antibacterial activity against S. mutans [29]. Neem extracts, on the other hand, displayed notable dose-dependent bacteriostatic activity against P. gingivalis [28]. Both Miswak and Neem are rich in polyphenols, which contribute to their antibacterial and anti-inflammatory properties [28,29]. Figure 2A outlines the experimental workflow used to evaluate these botanical extracts as test compounds, including antibacterial screening, biofilm inhibition and disruption assays, as well as biocompatibility assessments.
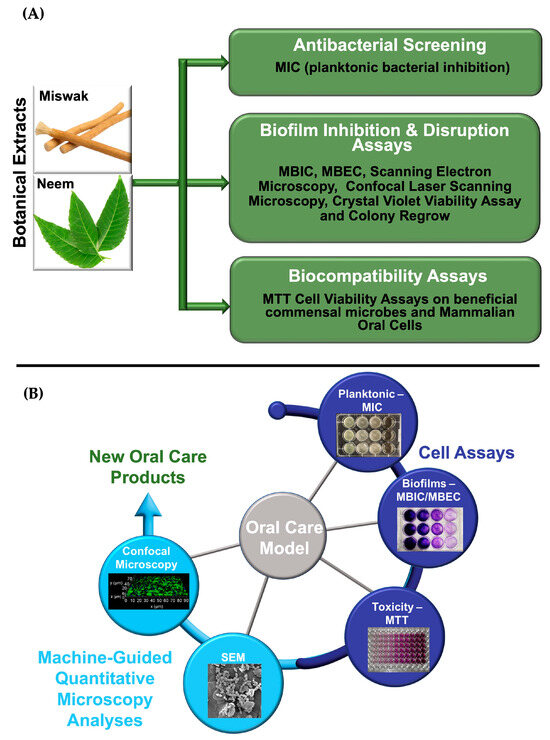
Figure 2.
(A) Schematic representation of the experimental workflow for evaluating botanical extracts for oral care. (B) Diagram of an integrated oral care testing model combining cell-based assays and machine-guided quantitative microscopy analyses to assess the efficacy and biocompatibility of botanical extracts for oral care applications.
Botanical extracts were prepared using a subcritical water extraction (SCWE) method [30]. SCWE is an environmentally friendly technique that utilizes water at elevated temperatures and pressures to extract bioactive compounds. Under high pressure, water remains in a liquid state even at high temperatures. As temperature increases, the polarity of water decreases significantly, enabling it to extract a wider range of non-polar and semi-polar compounds [31]. SCWE offers several advantages over conventional methods, including rapid processing, reduced thermal degradation, and the ability to extract diverse phytoactive substances without the use of harmful organic solvents [32]. This makes it a safer and more sustainable alternative for obtaining plant-derived bioactives.
To comprehensively evaluate the potential of botanical extracts in oral care, we developed a two-pronged oral care testing model that concurrently assesses antibacterial efficacy and host biocompatibility. This integrated approach enables a systematic analysis of (1) the botanical extracts’ capacity as test compounds to inhibit or eradicate bacteria and biofilms, and (2) their biocompatibility with beneficial commensal microbes and mammalian oral cells. Specifically, we conducted a sequence of antibacterial and antibiofilm assays to determine the minimum inhibitory concentration (MIC), minimum biofilm inhibitory concentration (MBIC), and minimum biofilm eradication concentration (MBEC). These were followed by biocompatibility assessments using MTT cell viability assays on both beneficial commensal microbes (e.g., S. gordonii, L. rhamnosus GG) and mammalian oral cells (keratinocytes and HGF-1). Quantitative findings were supported by advanced imaging techniques, including scanning electron microscopy (SEM) and confocal laser scanning microscopy (CLSM) to visualize biofilm architecture and quantify disruption at the microscale. To contextualize performance, these test compounds were tested alongside commonly used synthetic antimicrobial agents as standards found in commercial oral care products, such as Cetyl Cl., L-arg., and SnF2. This comparative analysis enabled a direct assessment of antibacterial potency, biofilm inhibition, and biocompatibility profiles across both natural and conventional synthetic treatment options. As illustrated in Figure 2B, this dual-assessment model provides a holistic framework for evaluating candidate natural product therapeutics, ensuring both pathogen-specific efficacy and preservation of host microbiota and tissue integrity, which are critical benchmarks for the development of safe and effective oral care solutions.
2. Materials and Methods
Five bacterial strains were selected for this study: S. mutans (ATCC 25175), P. gingivalis (ATCC 33277), F. nucleatum (ATCC 25586), S. gordonii (ATCC 35105), and L. rhamnosus GG (ATCC 53103). Human cell lines employed in biocompatibility tests were gingival fibroblasts (HGF-1, ATCC CRL2014) and primary human keratinocytes (ATCC PCS 200-014). All bacterial strains and mammalian cell lines along with the cell media Dermal Cell Basal Medium, Dulbecco’s Modified Eagle’s Medium (DMEM), and Keratinocytes Growth Kits, were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). Bacterial growth media and supplements, including Brain heart infusion (BHI) broth and agar, tryptic soy broth, yeast extract, hemin, de Man, Rogosa, and Sharpe (MRS) broth and agar, L-arginine, Sodium Hydroxide (NaOH), glycerin and menadione were sourced from Sigma-Aldrich (Burlington, MA, USA) and used as received. Glycerin was also obtained from Sigma-Aldrich (Burlington, MA, USA) and used as received. To support the anaerobic culture of P. gingivalis, Vitamin K1, modified chopped meat medium, and fetal bovine serum (FBS) were purchased from Fisher Scientific (Pittsburgh, PA, USA). The Invitrogen™ CyQUANT™ MTT Cell Viability Assay Kit and the Promega BacTiter-Glo™ Microbial Cell Viability Assay Kit, along with dimethyl sulfoxide (DMSO), anaerobic chambers, anaerobic gas generators, anaerobic indicators, tissue culture plates, 12-well plates and the positive control agents such as Cetyl Cl. and SnF2 crystal were purchased from Fisher Scientific (Pittsburgh, PA, USA). For the crystal violet assay and SEM sample preparation, the crystal violet, 4% paraformaldehyde, ethanol (200 proof and 70%), acetic acid (99%), and phosphate-buffered saline (PBS, 10×) were also obtained from Fisher Scientific (Pittsburgh, PA, USA). Components for artificial saliva preparation including sodium bicarbonate (99.9%), sodium chloride (99.9%), potassium chloride (99.0%), sodium dihydrogen phosphate dihydrate (99.0%), calcium chloride anhydrous (96.0%) and Hydrochloric Acid (HCl) were similarly obtained from Fisher Scientific (Pittsburgh, PA, USA). Brucella agar with 5% horse blood and 96-well plates were purchased from VWR (Radnor, PA, USA). Fluorescent staining agents used for biofilm analysis; Concanavalin A Alexa Fluor™ 647 Conjugate, SYTO™ 9 Green Fluorescent Nucleic Acid Stain, FilmTracer™ SYPRO® Ruby Biofilm Matrix Stain, were all purchased from Thermo Fisher (Waltham, MA, USA). For CLSM analyses, 35 mm glass bottom dishes with 14 mm microwells were obtained from MatTek Corporation (Ashland, MA, USA). For SEM analyses, sterile hydroxyapatite (HA) discs (9.7 mm diameter by 1.5 mm thickness) were obtained from Clarkson Chromatography Products (Williamsport, PA, USA). Dehydrated miswak twigs and dried neem leaves were obtained from sources in South Asia.
2.1. Preparation and Characterization of Botanical Extracts
Prior to extraction, each botanical raw material was chopped to an average particle size of approximately 3 mm and stored at room temperature until further processing. Extractions were conducted using a laboratory scale subcritical water extractor. For Miswak and Neem, the extraction was carried out at a raw material-to-water ratio of 1:34 and 1:33 w/w, respectively. Each material was extracted at 160 °C under pressures up to 1.3 MPa for 15 min. The resulting aqueous extracts were mixed with pure glycerin to prepare 80% w/v homogeneous solutions of bioactives that leverage glycerin–water mixture’s effective polyphenol co-solvation properties [33].
The total polyphenol content (TPC) of each extract was quantified using a modified Folin–Ciocalteu method and expressed in gallic acid equivalents (GAE). Absorbance measurements were performed using a UV-NIR spectrophotometer to help monitor the polyphenol levels in each extract at wavelengths of 760 and 850 nm. Based on this analysis, the TPC was determined to be 100 ppm for the Miswak extract and 112 ppm for the Neem extract. To further identify and estimate the individual phenolic compounds, liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis was performed. Relative peak areas were used to approximate the concentration of each compound as a proportion of the TPC.
2.2. Bacterial Strains and Culture Conditions
S. mutans (ATCC 25175) and S. gordonii (ATCC 35105) were cultured aerobically overnight at 37 °C in BHI broth. For S. mutans, the overnight cultures were adjusted to optical densities of 0.5 at 600 nm (OD600), then further diluted with a 1:300 ratio in fresh BHI broth to achieve a final concentration of 2 × 105 CFU/mL for use in planktonic and biofilm assays. For S. gordonii, the overnight cultures were diluted to OD600 = 0.65, corresponding to approximately 2 × 108 CFU/mL for use in subsequent biocompatibility tests.
P. gingivalis (ATCC 33277) and F. nucleatum (ATCC 25586) were cultured anaerobically at 37 °C overnight in anaerobic chambers using AnaeroPack gas generators. P. gingivalis was grown in αBHI broth (BHI supplemented with 5 mg/L hemin and 1 mg/L menadione), while F. nucleatum was cultured in modified chopped meat media. P. gingivalis cultures were diluted to OD600 = 1.4 in αBHI broth, yielding a concentration of 1 × 1012 CFU/mL. F. nucleatum cultures were diluted to OD600 = 1.0, corresponding to 1 × 109 CFU/mL for subsequent use in planktonic and biofilm assays.
L. rhamnosus GG (ATCC 53103) was cultured overnight in MRS broth at 37 °C in 5% CO2. The cultures were diluted to OD600 = 2.55 to yield a final concentration of 2 × 1010 CFU/mL for use in subsequent biocompatibility tests.
2.3. Antibacterial Cell Assays
2.3.1. Preparation of Artificial Saliva and Determination of MIC and MBIC with Colony Regrowth Verification
Artificial saliva was prepared by dissolving sodium bicarbonate (0.21 g), sodium chloride (0.215 g), potassium chloride (0.745 g), sodium dihydrogen phosphate dihydrate (0.515 g), and calcium chloride (0.11 g) in 80 mL of deionized (DI) water for 10 min. The resulting solution was then transferred to a 500 mL volumetric flask and adjusted to the final volume using DI water. The solution was transferred into a large beaker for pH adjustment. Initially, 1 M HCl was added dropwise to adjust the pH to 4.0, allowing the previously cloudy solution to become clear. Subsequently, the pH was carefully adjusted to pH 6.5 using 0.5 M NaOH.
The MIC against each bacterial strain was determined using a microdilution method performed in triplicate (n = 3). Bacterial inocula were diluted to the appropriate cell densities using their respective growth media. Synthetic antimicrobials were dissolved in the prepared artificial saliva to achieve final concentrations after mixing with the bacterial suspensions: Cetyl Cl. (0.05, 0.10, 0.20% w/v), L-arg. (0.75, 1.50, 3.00% w/v), and SnF2 (0.20, 0.40, 0.80% w/v). These concentrations were selected to reflect typical ranges used in commercial oral care products. Miswak and Neem botanical extracts as test compounds were similarly diluted in artificial saliva to achieve final concentrations in % w/v (GAE) as follows: Miswak, 2.5 × 10−3%, 5.0 × 10−3%, and 1.0 × 10−2%; Neem, 2.8 × 10−3%, 5.6 × 10−3%, and 1.12 × 10−2%, respectively, upon combination with the inocula. The concentrations of the synthetic antimicrobials were likewise expressed as % w/v. In each case, 500 μL of the bacterial suspension was mixed with 500 μL of the antimicrobial stock solution in 12-well plates. The plates were incubated overnight at 37 °C under conditions appropriate for each bacterial strain (aerobic, anaerobic, or CO2 enriched environments). Following incubation, OD600 was measured to assess bacterial growth and determine MIC values. To confirm growth inhibition, 100 μL aliquots from each well were plated on agar prepared with each strain’s respective growth media and incubated overnight under specific culture conditions. Colony formation was visually assessed to validate the MIC results and detect potential regrowth. MIC is defined as the lowest concentration of antimicrobial agent that significantly inhibits visible bacterial growth compared to untreated controls.
For MBIC evaluation, following MIC determination, the bacterial suspensions and antibacterial treatments were aspirated out, and the wells were washed twice with 1 mL aliquots of DI water to remove planktonic cells. To stain residual biofilms, 500 μL of 0.1% w/v aqueous crystal violet solution was added to each well and incubated for 20 min at room temperature. After staining, excess crystal violet was aspirated and the wells were rinsed 3× with DI water to remove unbound dye. Photographs of the stained wells were taken to visually assess biofilm formation. For quantification, bound crystal violet was solubilized by adding 1 mL of 30% acetic acid to each well. The resulting solutions were then diluted in a 1:8 ratio with DI water and absorbances were measured at 595 nm (OD595) using a microplate reader. All measurements were performed in triplicate (n = 3). MBIC is defined as the lowest concentration of antimicrobial agent that significantly inhibits biofilm formation compared to untreated controls. The detailed methodology for both MIC and MBIC is illustrated in Figure 3A.
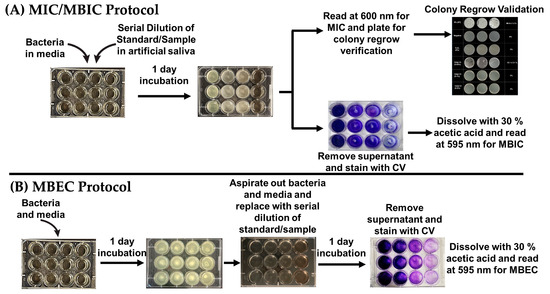
Figure 3.
(A) General workflow for determining minimum inhibitory concentration (MIC) and minimum biofilm inhibitory concentration (MBIC). MIC is assessed through optical density measurements and validated via colony regrowth assays. (B) Protocol for the minimum biofilm eradication concentration (MBEC) assay, showing treatment of pre-formed biofilms and subsequent quantification using crystal violet staining.
2.3.2. Determination of MBEC
To assess the MBEC, biofilms were grown in 12-well plates for 1–2 days using bacterial cultures at appropriate cell densities in their respective media. After complete biofilm coverage, the media were gently aspirated and replaced with treatment suspensions prepared in artificial saliva. This included Cetyl Cl. (0.05, 0.10, 0.20% w/v), L-arg. (0.75, 1.50, 3.00% w/v), SnF2 (0.20, 0.40, 0.80% w/v), Miswak (2.5 × 10−3, 5.0 × 10−3, and 1.0 × 10−2% w/v), and Neem (2.8 × 10−3, 5.6 × 10−3, and 1.12 × 10−2% w/v). Plates were incubated overnight under conditions appropriate for each bacterial strain. Following treatment, the solutions were aspirated, and the wells were washed twice with 1 mL DI water to remove residual compounds and planktonic cells. Remaining biofilms were then stained with 500 μL of 0.1% w/v aqueous crystal violet solution and incubated for 20 min at room temperature. Excess stains were aspirated, and the wells were rinsed 3× with DI water. Plates were photographed to document biofilm coverage following treatment. The bound crystal violet was dissolved using 1 mL of 30% acetic acid, then diluted 1:8 in DI water before being measured at OD595 using a microplate reader. All assays were performed in triplicate (n = 3). MBEC is defined as the lowest concentration of each treatment that results in significant reduction in biofilm mass compared to the untreated controls. The detailed methodology is illustrated in Figure 3B.
2.4. Cell Biocompatibility Assays
2.4.1. Viability Assays for Probiotic Cells
Biofilms of S. gordonii (2 × 108 CFU/mL) and L. rhamnosus GG (2 × 1010 CFU/mL) were grown overnight in 96-well plates under their respective incubation conditions. Following incubation, the bacterial suspensions were carefully aspirated and replaced with 90 µL DI water and 10 µL of antibacterial or botanical treatment solution. The plates were then incubated for 24 h at 37 °C in a 5% CO2 atmosphere. After treatment, the supernatant in each well was aspirated and replaced with 100 µL of fresh DI water containing 10 µL of MTT reagent. Subsequently, the supernatant and reagent were removed, and the wells were treated with a 1:2 mixture of DI water and DMSO to solubilize the formazan crystals. The plates were placed on a shaker for 10 min at 37 °C to ensure complete dissolution. The optical densities were measured at 540 nm using a microplate reader to assess metabolic activity and viability of the probiotic cells. Each bacterial strain was tested in 12 replicates (n = 12). This assay provided an estimate of the viability of each treatment with beneficial oral bacteria.
2.4.2. Cytotoxicity Assays for Oral Mammalian Cells
HGF-1 fibroblasts and primary human keratinocytes were seeded in 96-well plates at densities of 10,000 and 15,000 cells/well, respectively. The plates were incubated for 48 h at 37 °C in a 5% CO2 atmosphere until cell confluence exceeded 80%. The culture media were then aspirated and replaced with 100 μL of the treatment solutions (antibacterial agents or botanical extracts), with n = 12 per treatment group. The plates were further incubated for 24 h under the same conditions. After treatment, the supernatant in each well was removed and the wells were gently rinsed 3× with artificial saliva to eliminate residual treatment compounds. Fresh culture media (100 µL) supplemented with 10 μL of MTT reagent were then added to each well. The plates were incubated for 4 h at 37 °C in a 5% CO2 atmosphere to allow for formazan crystal formation. Following incubation, 85 µL of the supernatant was carefully removed from each well, and 50 µL of DMSO was added to solubilize the formazan. The plates were incubated for an additional 10 min at 37 °C in a 5% CO2 atmosphere with gentle mixing to ensure complete dissolution. The optical densities were measured at 540 nm using a microplate reader to quantify cell viability.
2.5. Machine-Guided Quantitative Microscopy Analyses
2.5.1. Scanning Electron Microscopy (SEM) Analyses of Biofilms on Hydroxyapatite Discs
Bacterial biofilms of each strain were cultured on sterile hydroxyapatite (HA) discs to simulate the tooth surface, using the same CFU/mL concentrations as described in previous assays. Each disc was treated with the corresponding antibacterial agent or botanical extract solution. For SEM analyses, the HA discs were first fixed in 4% paraformaldehyde prepared in PBS for 1 h at room temperature. After fixation, the samples were rinsed 3× with 0.1 M PBS to remove residual fixative. A graded ethanol dehydration series was then performed, with the discs subsequently immersed in 50%, 70%, 80%, and 100% ethanol for 10 min at each concentration. Following the dehydration, the samples were vacuum dried overnight at room temperature. Finally, the dried discs were sputter coated with a thin layer of gold to enable surface imaging via SEM.
SEM analyses were performed using an FEI Quanta 450 FEG environmental SEM (FEI Company, Hillsboro, OR). The measurements were carried out at an accelerating voltage of 5 kV. For each HA disc, five images were acquired at 5000× magnification from positions arranged in an “X” pattern across the disc surface to ensure a representative sample. Quantitative analyses of biofilm coverage were performed using a machine-learning based approach with the trainable Weka Segmentation plugin in Fiji version 3.3.4 (an NIH supported ImageJ version 1.54h distribution). The classifier was trained using a data set of 30 representative images, and a total of 10 regions of interest (ROIs) were selected per image for segmentation. The percent area covered by biofilm was then calculated using Fiji’s built-in area coverage calculator. To efficiently process the large data set and ensure consistency across images, a custom Python script (version 3.11.5) was developed to automate batch segmentation and analysis using the trained classifier model.
2.5.2. Confocal Laser Scanning Microscopy (CLSM) and Biofilm Fluorescence Analyses
Bacterial biofilms were cultivated under their respective incubation conditions and concentrations in 35 mm glass bottom dishes with 14 mm microwells (MatTek Corporation, Ashland, MA, USA). After biofilm formation, the samples were treated with the appropriate standard or botanical extract test compounds. Following treatment, the wells were rinsed 3× with 1 mL of PBS to remove residual agents. For biofilm visualization, a modified staining protocol based on Khajotia et al. [34] was employed. Briefly, 50 μL of Concanavalin A Alexa Fluor 647 (250 μg/mL) in PBS was added to each well and incubated in the dark for 30 min to label extracellular polysaccharides. After incubation, the wells were rinsed 3× with 1 mL aliquots of PBS. Next, 4 μL of SYTO 9 dye (1.67 mM) was added to stain bacterial nucleic acids and incubated for 30 min in the dark. The wells were again washed 3× with 1 mL PBS. To visualize biofilm matrix proteins, 1 mL of FilmTracer™ SYPRO® Ruby Biofilm Matrix Stain was added and incubated in darkness for an additional 30 min, followed by a final triple PBS rinse. Subsequently, fluorescence imaging was performed using a Leica HyVolution SP8 confocal microscope (Leica Camera, Wetzlar, Germany), equipped with a 63×, NA 1.4, oil immersion objective. Z-stack images were acquired for 3D visualization of biofilm architecture, along with top-view projections of individual components. Quantitative analyses of fluorescence intensity for each stained biofilm components were performed using LASX Software version 3.7.3 (Leica Camera, Wetzlar, Germany).
3. Results
3.1. Antibacterial Activity
S. mutans is a highly cariogenic bacterium commonly found in the oral cavity. To evaluate the efficacy of various antibacterial agents in inhibiting its growth, we employed a series of antimicrobial assays, including MIC, MBIC, and MBEC tests (Supplementary Figures S1 and S2 online). These assays involve exposing S. mutans to a range of test reagents concentrations and assessing their effects on both bacterial growth and biofilm formation or eradication. Cetyl Cl., L-arg., and SnF2, which are commonly used in commercial oral care formulations such as toothpaste and mouthwash, were adapted as standards for the cell assays. All three antibacterial agents demonstrated effective inhibition of S. mutans’ growth and biofilm development. Notably, the botanical extracts from Miswak twigs and Neem leaves exhibited comparable, and in some cases superior, antibacterial and antibiofilm activity relative to these standard synthetic agents.
These assays were also adapted to evaluate F. nucleatum and P. gingivalis, as shown in Supplementary Figures S3–S6 (online). F. nucleatum is recognized as a key contributor to the pathogenesis of periodontitis, where it promotes chronic inflammation and tissue destruction [6]. In addition, it plays a central role in oral microbial ecology by co-aggregating with other bacterial species and modulating host immune responses, further accelerating disease progression [4]. Supplementary Figure S3 (online) presents the MIC and MBIC assay results for F. nucleatum, demonstrating that Cetyl Cl., SnF2, and Miswak extract at 1.0 × 10−2% w/v were the most effective in inhibiting both bacterial growth and early biofilm formation. Supplementary Figure S4A (online) shows the MBEC assay results in which the same treatments (i.e., Cetyl Cl., SnF2, and Miswak extract at 1.0 × 10−2% w/v) were also most successful in eradicating pre-formed biofilms. These findings were further validated through colony regrowth assessments on blood agar plates as shown in Supplementary Figure S4B (online). Blood agar is a nutrient rich culture medium that contains red blood cells and hemoglobin, providing essential growth factors for oral bacteria. F. nucleatum requires these components for optimal growth, as it thrives in environments that closely resemble the human oral cavity. The red blood cells serve as a critical iron source, supporting F. nucleatum’s metabolic needs, while the hemoglobin helps scavenge residual oxygen, promoting the anaerobic conditions necessary for its survival.
P. gingivalis is a key pathogen associated with periodontitis, a chronic inflammatory disease that affects the gums and supporting structures of the teeth [6]. Due to its central role in disease progression, evaluating antibacterial agents against P. gingivalis is critical for identifying potential therapies for periodontitis and related oral diseases. Supplementary Figure S5 (online) shows the results of MIC and MBIC assays for P. gingivalis. Among the treatments tested, Cetyl Cl., L-arg., and 20% Miswak extract were most effective in inhibiting planktonic bacterial growth. Moreover, Cetyl Cl. and SnF2 demonstrated the strongest inhibition of biofilm formation. Supplementary Figure S6 (online) shows the results from the MBEC assay, along with validation through colony regrowth analyses, confirming the efficacy of selected treatments in eradicating established P. gingivalis biofilms.
Figure 4 summarizes the results of the MIC, MBIC, and MBEC assays for the pathogenic bacteria, using the relevant concentrations of each treatment: Cetyl Cl. (0.05, 0.10, 0.20% w/v), L-arg. (0.75, 1.50, 3.00% w/v), SnF2 (0.20, 0.40, 0.80% w/v), Miswak (2.5 × 10−3, 5.0 × 10−3, and 1.0 × 10−2% w/v), and Neem (2.8 × 10−3, 5.6 × 10−3, and 1.1 × 10−2% w/v). While neither of the botanical extracts outperformed Cetyl Cl., Miswak demonstrated notable efficacy in both inhibiting bacterial growth and eradicating pre-formed biofilms, highlighting its potential as a promising natural antibacterial agent.
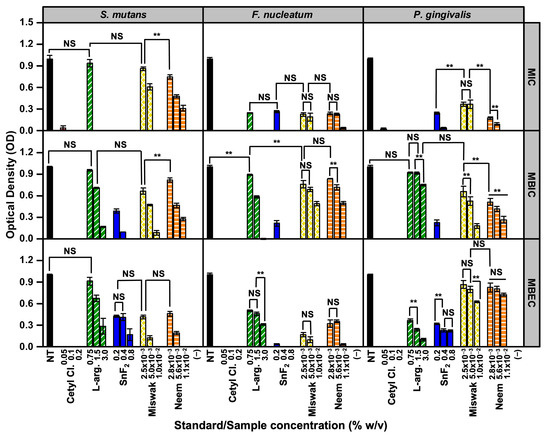
Figure 4.
Summary of the antibacterial assay results for pathogenic bacteria. All assays were performed with n = 3. Statistical significance was assessed at α = 0.01 (two-sided). Comparisons are indicated as ** (significant, p < 0.01) or NS (not significant, p ≥ 0.01), with brackets shown only for bars of similar height where clarification was needed.
3.2. Biocompatibility Evaluation
3.2.1. Effects on Commensal Bacteria
S. gordonii and L. rhamnosus GG are beneficial commensal bacteria commonly found in the oral cavity and are known to contribute to the maintenance of oral health. S. gordonii plays a key role in promoting beneficial bacterial biofilm stability and suppressing the growth of cariogenic pathogens such as S. mutans, while L. rhamnosus GG exhibits anti-inflammatory and immunomodulatory properties that support the prevention of dental caries and periodontal diseases [35,36]. Given their protective functions, preserving the viability of these probiotic species is essential when developing oral care formulations. It is therefore critical to ensure that new antibacterial agents do not negatively affect these beneficial microbes, as their disruption could lead to imbalances in the oral microbiome and compromise overall oral health.
In addition, S. gordonii and L. rhamnosus GG are both Gram-positive bacteria and share physiological characteristics with other oral microbial species involved in biofilm formation and the pathogenesis of dental plaque and oral diseases. As such, if a new oral care product exhibits toxicity toward these commensals, it is likely to affect other beneficial and potentially pathogenic bacteria within the oral microbiome. This highlights the importance of evaluating the effects of oral treatments on these representative species to ensure product safety and minimize unintended disruption of the beneficial microbiome. Figure 5A shows the biocompatibility results for S. gordonii and L. rhamnosus GG, alongside representative SEM images. A reference line at 70% viability was included to indicate the generally acceptable threshold for biocompatibility in oral care product development [37].
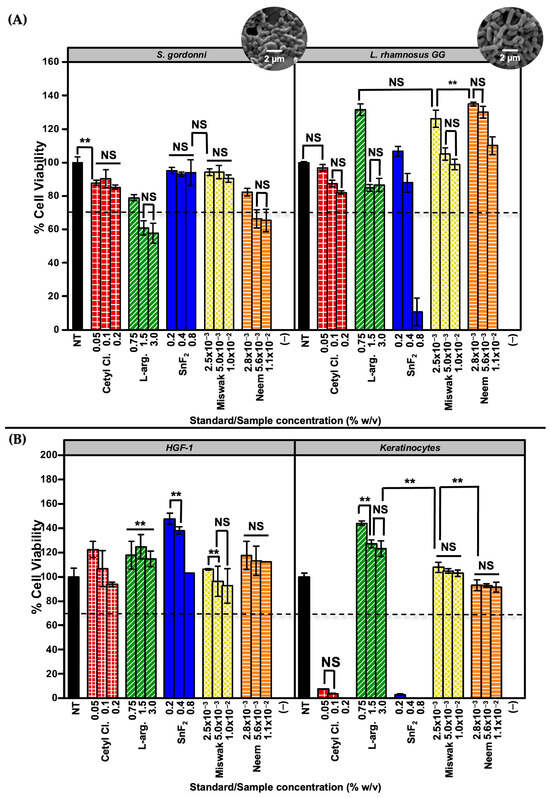
Figure 5.
MTT cell viability assay results for standards and botanical test compounds. (A) Viability of commensal bacteria (S. gordonii and L. rhamnosus GG) following treatment, assessed via MTT assay. Representative scanning electron microscopy (SEM) images are included to visualize bacterial morphology. (B) Viability of mammalian oral cells (HGF-1 and keratinocytes) treated under the same conditions. A dashed reference line at 70% viability indicates the commonly accepted cytotoxicity threshold. All assays were performed with n = 12. Statistical significance was assessed at α = 0.01 (two-sided). Comparisons are indicated as ** (significant, p < 0.01) or NS (not significant, p ≥ 0.01), with brackets shown only for bars of similar height where clarification was needed.
3.2.2. Cytotoxicity Toward Oral Cell Lines
HGF-1 fibroblasts and keratinocytes are key human cell types found within the oral cavity, including the gingival connective tissue and the epithelial lining of the mouth. These cells play vital roles in maintaining the structural integrity of oral tissues and protecting against harmful substances, including chemical irritants. When developing oral care products, it is essential to evaluate their cytotoxicity on these cell types, as this provides critical insight into product safety and potential adverse effects. If a formulation is found to be toxic to HGF-1 and keratinocytes, it may compromise oral tissue health, potentially leading to inflammation, mucosal damage, or other pathological responses. Therefore, assessing biocompatibility with these cells is a fundamental step in oral care safety evaluation.
Additionally, cytotoxicity testing with HGF-1 fibroblasts and keratinocytes helps identify potential irritants within oral care formulations, which may cause adverse reactions in sensitive individuals. Overall, evaluating the biocompatibility of antibacterial agents on these cell types is an important step in ensuring both the safety and efficacy of oral care products. Figure 5B shows the cytotoxicity results for HGF-1 and keratinocytes treated with both standards and botanical test compounds. Notably, Miswak and Neem extracts exhibited no detectable toxicity toward either cell line across all tested concentrations, underscoring their excellent biocompatibility. In contrast, commercially used standards such as Cetyl Cl. and SnF2 demonstrated significant cytotoxicity, particularly in keratinocytes, suggesting potential limitations in their long-term use.
3.3. Quantitative Microscopy Analyses
3.3.1. SEM Imaging and Quantification of Biofilms
SEM is an invaluable tool for analyzing biofilms, as it provides detailed visualization of their overall structure, including the spatial arrangement of microbial cells and their surrounding extracellular matrix. Beyond qualitative imaging, SEM can also be used for quantitative analysis, such as calculating the percentage of surface area covered by biofilms [38,39]. In this study, biofilms were grown on HA discs to closely mimic the natural tooth surface.
SEM images were collected at pre-defined locations across the HA disc surface, as illustrated in Figure 6A to provide a representative overview of total biofilm coverage after each treatment. Following image acquisition, all images were processed using the trainable Weka Segmentation plug-in in ImageJ for automated quantification, as shown in Figure 6B. Figure 6C shows representative SEM images of the untreated control samples and the bar graphs of the remaining pre-formed biofilms after treatment with the respective standards and botanical agents. The data include results from only the most relevant concentrations: Cetyl Cl. 0.1%, L-arg. 1.5%, SnF2 0.4%, Miswak extract 1.0 × 10−2%, and Neem extract 1.1 × 10−2%. Overall, the SEM analysis confirmed that botanical agents exhibited significant antibiofilm activity, particularly against S. mutans and F. nucleatum, with reductions comparable to conventional oral care standards. Additional SEM images and biofilm coverage analyses for each bacterial strain are provided in Supplementary Figures S7–S11 (online).
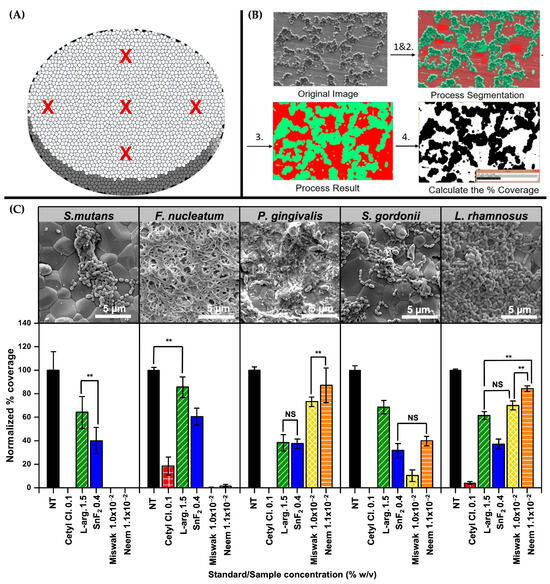
Figure 6.
SEM analyses of biofilm coverage and treatment effects on HA discs. (A) Schematic showing pre-defined imaging locations (indicated as red X marks) on HA discs used for consistent SEM sampling across the surface (n = 5). (B) Workflow of image processing using the trainable Weka segmentation plug-in in ImageJ for quantifying biofilm coverage. (C) Representative SEM images of bacterial biofilms under untreated control conditions, along with commensal bacteria, and corresponding bar graphs showing the effects of standard and botanical test compounds on pre-formed biofilms. Statistical significance was assessed at α = 0.01 (two-sided). Comparisons are indicated as ** (significant, p < 0.01) or NS (not significant, p ≥ 0.01), with brackets shown only for bars of similar height where clarification was needed.
3.3.2. CLSM Imaging and Fluorescence Analyses of Biofilm Architecture
CLSM is a powerful tool for analyzing bacterial biofilms as it enables high resolution, three-dimensional visualization without the need for physical sectioning or sample disruption [40,41]. CLSM employs laser excitation to scan the specimen at multiple focal depths, capturing fluorescent signals from each optical plane. These signals are then compiled to reconstruct a detailed three-dimensional image of the biofilms’ architecture, allowing for precise analysis of spatial organization and component distribution.
Shown in Figure 7A is a schematic representation of the biofilm architecture with each component labeled, accompanied by a table summarizing the fluorescent dyes used for component specific staining. Concanavalin A Alexa Fluor 647 was used to visualize the extracellular polysaccharides, FilmTracer™ SYPRO® Ruby Biofilm Matrix Stain was used to stain the proteins, and SYTO 9 was used to stain the bacterial nucleic acids. Figure 7B summarizes the results of the CLSM analyses, highlighting differences in biofilm structure and density across treatments. The data include results from only the most relevant concentrations: Cetyl Cl. 0.1%, L-arg. 1.5%, SnF2 0.4%, Miswak extract 1.0 × 10−2%, and Neem extract 1.1 × 10−2%. Among the agents tested, Cetyl Cl. and Miswak extract demonstrated the most significant disruption of biofilm architecture, indicating strong antibiofilm activity. Detailed CLSM results for each bacterial species are provided in Supplementary Figures S12–S16 (online).
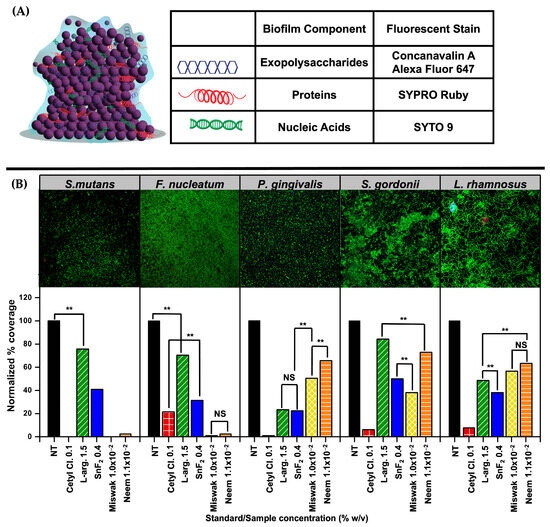
Figure 7.
Confocal Laser Scanning Microscopy (CLSM) visualization and analyses of biofilm structure and composition. (A) Schematic representation of a bacterial biofilm with labeled structural components, alongside a table identifying the fluorescent dyes used. (B) Representative CLSM images of bacterial biofilms under untreated control conditions, along with commensal bacteria, and corresponding bar graphs showing the effects of standard and botanical test compounds on pre-formed biofilms. Statistical significance was assessed at α = 0.01 (two-sided). Comparisons are indicated as ** (significant, p < 0.01) or NS (not significant, p ≥ 0.01), with brackets shown only for bars of similar height where clarification was needed.
4. Discussion
Phytomedicine refers to the use of bioactive compounds derived from plants, including flavonoids, alkaloids, and phenolic acids, for the prevention and treatment of various diseases. These naturally sourced formulations are gaining renewed interest due to their documented antimicrobial and anti-inflammatory properties, as well as their perceived safety, cost-effectiveness, and potential as alternatives to synthetic drugs, particularly in the management of infectious diseases [42]. To optimize the extraction of these therapeutic compounds and enhance their antibacterial efficacy, a range of extraction techniques have been developed, varying in solvent choice, temperature, and processing conditions. In this study, we employed Subcritical Water Extraction (SCWE), a green and sustainable method that uses water under elevated temperature and pressure as the extraction medium. Compared to conventional atmospheric methods, SCWE enables faster extraction, improved yield, greater operator safety, and better potential for industrial scalability [31]. Previous studies have demonstrated the strong antibacterial activity of plant derived extracts, and it is well established that differences in extraction techniques can significantly influence the phytochemical profile, and thus the bioactivity, of the final product [32].
Since plant extracts are inherently complex mixtures, their bioactivity is often evaluated using total phenolic content (TPC) as a standard indicator of quality and therapeutic potential [43]. Among these compounds, polyphenols, a major subclass of phenolic compounds, are particularly well recognized for their potent antimicrobial activity. These molecules exert antibacterial effects through multiple mechanisms, including disruption of microbial membranes, inhibition of key enzymes, and interference with quorum sensing pathways. Due to the robustness and reproducibility of established methods for quantifying TPC, this study employed polyphenol content as a key metric for evaluating the antibacterial potential of Miswak and Neem extracts [42,43]. Our findings support this approach, demonstrating that higher TPC values were generally associated with increased bacterial inhibition and biofilm eradication within a defined effective concentration range.
Liquid chromatography tandem mass spectrometry (LC-MS/MS) profiling revealed that Miswak extract is particularly rich in 5-hydroxyconiferaldehyde and benzoic acid, as detailed in Table 1. The compound 5-hydroxyconiferaldehyde has been shown to possess anti-inflammatory properties by downregulating pro-inflammatory mediators such as nitric oxide [44]. Benzoic acid, meanwhile, is a documented antimicrobial agent that inhibits the growth of a wide range of pathogens, including Escherichia coli (E. coli) and Staphylococcus aureus. Additionally, its strong inhibitory activity has been observed against Candida albicans and certain Lactobacillus species at elevated concentrations [45].

Table 1.
Polyphenolic compounds identified in Miswak (Salvadora persica) extract via liquid chromatography tandem mass spectrometry (LC-MS/MS).
Neem, on the other hand, was found to be rich in rutin and isoquercitrin, as shown in Table 2. Rutin has demonstrated potent antibacterial activity, particularly against multidrug-resistant Pseudomonas aeruginosa, and has also been reported to exhibit antibiofilm properties by inhibiting biofilm formation in a dose-dependent manner [46]. Isoquercitrin exerts its antibacterial effects by suppressing the growth of E. coli and inducing apoptosis-like cell death. This mechanism is associated with elevated oxidative stress, including the generation of reactive oxygen species (ROS), depletion of glutathione, disruption of bacterial membrane integrity, and activation of caspase-like enzymes that lead to DNA fragmentation [47].

Table 2.
Polyphenolic compounds identified in Neem (Azadirachta indica) extract via liquid chromatography tandem mass spectrometry (LC-MS/MS).
In addition to these major constituents, several minor phenolic compounds identified in both extracts, such as myricetin, ellagic acid, quercetin, quercitrin, vanillin, gallic acid, apigenin, and syringic acid, have also been reported to exhibit notable antibacterial properties. Myricetin, for instance, was shown to have the highest antibacterial activity among phenolics in berry extracts. Quercetin and quercitrin inhibited ATP synthase and ATPase activity in E. coli, while vanillin and gallic acid demonstrated antibiofilm effects by interfering with bacterial quorum sensing and biofilm structure. Although these compounds are present in relatively low concentrations, their strong individual bioactivities and potential synergistic interactions, may contribute significantly to the overall antibacterial efficacy of the botanical extracts [48].
The results from the antibacterial assays provide valuable insights into the therapeutic potential of botanical extracts in comparison with commercial oral care agents in inhibiting planktonic bacteria and disrupting mature biofilms, which represent the most resistant stage of microbial colonization. These assays assessed not only total bacterial burden but also targeted biofilms at different stages of development, closely simulating the dynamic and complex environment of the oral cavity. To enhance the robustness of our findings, we incorporated machine-guided quantitative microscopy analysis of the SEM and CLSM datasets, enabling accurate quantification of biofilm biomass and structural disruption following treatment. This integrated strategy reinforced and validated the antibacterial activity observed in vitro.
Notably, Miswak and Neem extracts showed comparable or, in some cases, superior efficacy in inhibiting and eradicating biofilms when compared to L-arg., a widely used agent in commercial oral care formulations. Furthermore, both extracts exhibited selective antibacterial activity, effectively reducing pathogenic biofilms while preserving the viability of beneficial probiotic strains and oral fibroblasts and keratinocytes, as evidenced by MTT assays. Although some differences were observed when comparing crystal violet assays (i.e., MBEC) with SEM analyses, we infer that quantitative measurement of biofilm coverage from SEM images offers a more accurate evaluation of biofilm eradication across all treatments. This dual functionality highlights their potential as biocompatible and targeted alternatives for managing oral biofilms, while safeguarding the native oral microbiome and minimizing cytotoxicity to host tissues.
5. Conclusions
In this study, we established a comprehensive oral testing platform that integrates microbiological and cytotoxicity assays with advanced microscopy methods to evaluate the therapeutic potential and safety of botanical extracts. By incorporating both pathogenic bacteria (S. mutans, P. gingivalis, F. nucleatum) and beneficial commensals (S. gordonii, L. rhamnosus GG), along with mammalian oral cells (keratinocytes and HGF-1), the model enables a multifaceted assessment of antibacterial efficacy, bacterial selectivity, and host cytocompatibility.
Using this platform, we evaluated the oral health potential of Miswak and Neem extracts. Across MIC, MBIC, and MBEC assays, both botanical extracts demonstrated robust antibacterial and antibiofilm activities. Importantly, they preserved the viability of probiotic bacteria and mammalian oral cells, indicating their biocompatibility. These findings were further corroborated through machine-guided Weka segmentation of SEM and CLSM images, which confirmed significant biofilm disruption. Collectively, our results highlight Miswak and Neem as promising plant-based agents for safe, selective, and effective oral healthcare applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/oral5040089/s1, Figure S1: (A) Minimum inhibitory concentration (MIC) and (B) minimum biofilm inhibitory concentration (MBIC) assays against S. mutans. All assays were performed in triplicates.; Figure S2: (A) Minimum biofilm eradication concentration (MBEC) assay against S. mutans; (B) validation of MBEC results in S. mutans using a colony regrowth assay on BHI agar. All assays were performed in triplicates.; Figure S3: (A) Minimum inhibitory concentration (MIC) assay and (B) minimum biofilm inhibitory concentration (MBIC) assay against F. nucleatum. All assays were performed in triplicates.; Figure S4: (A) Minimum biofilm eradication concentration (MBEC) assay against F. nucleatum; (B) validation of assay results in F. nucleatum using a colony regrowth assay on blood agar. All assays were performed in triplicates.; Figure S5: (A) Minimum inhibitory concentration (MIC) assay and (B) minimum biofilm inhibitory concentration (MBIC) assay against P. gingivalis. All assays were performed in triplicates.; Figure S6: (A) Minimum biofilm eradication concentration (MBEC) assay against P. gingivalis; (B) validation of the MBEC results in P. gingivalis using a colony regrowth assay on blood agar. All assays were performed in triplicates.; Figure S7: Scanning electron microscopy (SEM) analyses of S. mutans biofilms with no treatment, and after treatment with selected agents: Cetyl Cl. 0.1%, L-arg. 1.5%, SnF2 0.4%, Miswak extract 1.0 × 10−2%, and Neem extract 1.1 × 10−2% w/v. All analyses were performed with n = 5.; Figure S8: Scanning electron microscopy (SEM) analyses of F. nucleatum biofilms with no treatment, and after treatment with selected agents: Cetyl Cl. 0.1%, L-arg. 1.5%, SnF2 0.4%, Miswak extract 1.0 × 10−2%, and Neem extract 1.1 × 10−2% w/v. All analyses were performed with n = 5.; Figure S9: Scanning electron microscopy (SEM) analyses of P. gingivalis biofilms with no treatment, and after treatment with selected agents: Cetyl Cl. 0.1%, L-arg. 1.5%, SnF2 0.4%, Miswak extract 1.0 × 10−2%, and Neem extract 1.1 × 10−2% w/v. All analyses were performed with n = 5.; Figure S10: Scanning electron microscopy (SEM) analyses of S. gordonii biofilms with no treatment, and after treatment with selected agents: Cetyl Cl. 0.1%, L-arg. 1.5%, SnF2 0.4%, Miswak extract 1.0 × 10−2%, and Neem extract 1.1 × 10−2% w/v. All analyses were performed with n = 5.; Figure S11: Scanning electron microscopy (SEM) analyses of L. rhamnosus GG biofilms with no treatment, and after treatment with selected agents: Cetyl Cl. 0.1%, L-arg. 1.5%, SnF2 0.4%, Miswak extract 1.0 × 10−2%, and Neem extract 1.1 × 10−2% w/v. All analyses were performed with n = 5.; Figure S12: (A) Confocal laser scanning microscopy (CLSM) images of S. mutans biofilms with no treatment, and after treatment with selected agents: Cetyl Cl. 0.1%, L-arg. 1.5%, SnF2 0.4%, Miswak extract 1.0 × 10−2%, and Neem extract 1.1 × 10−2% w/v. (B) Quantitative analysis of S. mutans biofilm components.; Figure S13: (A) Confocal laser scanning microscopy (CLSM) images of F. nucleatum biofilms with no treatment, and after treatment with selected agents: Cetyl Cl. 0.1%, L-arg. 1.5%, SnF2 0.4%, Miswak extract 1.0 × 10−2%, and Neem extract 1.1 × 10−2% w/v. (B) Quantitative analysis of F. nucleatum biofilm components.; Figure S14: (A) Confocal laser scanning microscopy (CLSM) images of P. gingivalis biofilms with no treatment, and after treatment with selected agents: Cetyl Cl. 0.1%, L-arg. 1.5%, SnF2 0.4%, Miswak extract 1.0 × 10−2%, and Neem extract 1.1 × 10−2% w/v. (B) Quantitative analysis of P. gingivalis biofilm components.; Figure S15: (A) Confocal laser scanning microscopy (CLSM) images of S. gordonii biofilms with no treatment, and after treatment with selected agents: Cetyl Cl. 0.1%, L-arg. 1.5%, SnF2 0.4%, Miswak extract 1.0 × 10−2%, and Neem extract 1.1 × 10−2% w/v. (B) Quantitative analysis of S. gordonii biofilm components.; Figure S16: (A) Confocal laser scanning microscopy (CLSM) images of L. rhamnosus GG biofilms with no treatment, and after treatment with selected agents: Cetyl Cl. 0.1%, L-arg. 1.5%, SnF2 0.4%, Miswak extract 1.0 × 10−2%, and Neem extract 1.1 × 10−2% w/v. (B) Quantitative analysis of L. rhamnosus GG biofilm components.
Author Contributions
A.C.S.S., S.V., E.D., L.M. and Y.L., conceived and designed the experiments. N.B.M. and M.J. performed experiments. A.C.S.S., N.B.M., N.B.P., M.M.A. and C.R.M.L. analyzed data and interpreted the results. A.C.S.S. and N.B.P. drafted and prepared the manuscript while everyone was involved in editing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by The Lubrizol Corporation (RES515117 and RES601032) and the Department of Chemistry at Case Western Reserve University (INS101800).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All datasets presented in the study are included in the article/Supplementary Material.
Acknowledgments
The SEM data were obtained at the ESEM facility at the Liquid Crystal Institute at Kent State University supported by the Ohio Research Scholars Program Cluster on Surfaces in Advanced Materials.
Conflicts of Interest
Authors S.V., E.D., L.M., and Y.L. were employed by The Lubrizol Corporation. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Hascoët, E.; Blanchard, F.; Blin-Wakkach, C.; Guicheux, J.; Lesclous, P.; Cloitre, A. New insights into inflammatory osteoclast precursors as therapeutic targets for rheumatoid arthritis and periodontitis. Bone Res. 2023, 11, 26. [Google Scholar] [CrossRef]
- Scannapieco, F.A.; Cantos, A. Oral inflammation and infection, and chronic medical diseases: Implications for the elderly. Periodontol. 2000 2016, 72, 153–175. [Google Scholar] [CrossRef]
- Tomás, I.; Diz, P.; Tobías, A.; Scully, C.; Donos, N. Periodontal health status and bacteraemia from daily oral activities: Systematic review/meta-analysis. J. Clin. Periodontol. 2012, 39, 213–228. [Google Scholar] [CrossRef]
- Thomas, C.; Minty, M.; Vinel, A.; Canceill, T.; Loubières, P.; Burcelin, R.; Kaddech, M.; Blasco-Baque, V.; Laurencin-Dalicieux, S. Oral microbiota: A major player in the diagnosis of systemic diseases. Diagnostics 2021, 11, 1376. [Google Scholar] [CrossRef]
- Velsko, I.M.; Fellows Yates, J.A.; Aron, F.; Hagan, R.W.; Frantz, L.A.F.; Loe, L.; Rodriguez Martinez, J.B.; Chaves, E.; Gosden, C.; Larson, G.; et al. Microbial differences between dental plaque and historic dental calculus are related to oral biofilm maturation stage. Microbiome 2019, 7, 102. [Google Scholar] [CrossRef]
- Jenkinson, H.F. Beyond the oral microbiome. Environ. Microbiol. 2011, 13, 3077–3087. [Google Scholar] [CrossRef]
- Luo, S.-C.; Wei, S.-M.; Luo, X.-T.; Yang, Q.-Q.; Wong, K.-H.; Cheung, P.C.; Zhang, B. How probiotics, prebiotics, synbiotics, and postbiotics prevent dental caries: An oral microbiota perspective. NPJ Biofilms Microbiomes 2024, 10, 14. [Google Scholar] [CrossRef]
- Liu, S.; Sun, Y.; Liu, Y.; Hu, F.; Xu, L.; Zheng, Q.; Wang, Q.; Zeng, G.; Zhang, K. Genomic and phenotypic characterization of Streptococcus mutans isolates suggests key gene clusters in regulating its interaction with Streptococcus gordonii. Front. Microbiol. 2022, 13, 945108. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kim, Y.-J. A comparative study of the effect of probiotics on a cariogenic biofilm model for preventing dental caries. Arch. Microbiol. 2014, 196, 601–609. [Google Scholar] [CrossRef]
- Loesche, W.J. Role of Streptococcus mutans in human dental decay. Microbiol. Rev. 1986, 50, 353–380. [Google Scholar] [CrossRef]
- Sakanaka, A.; Kuboniwa, M.; Shimma, S.; Alghamdi, S.A.; Mayumi, S.; Lamont, R.J.; Fukusaki, A.; Amano, A. Fusobacterium nucleatum metabolically integrates commensals and pathogens in oral biofilms. mSystems 2022, 7, e0017022. [Google Scholar] [CrossRef]
- Yamaguchi-Kuroda, Y.; Kikuchi, Y.; Kokubu, E.; Ishihara, K. Porphyromonas gingivalis diffusible signaling molecules enhance Fusobacterium nucleatum biofilm formation via gene expression modulation. J. Oral Microbiol. 2023, 15, 2165001. [Google Scholar] [CrossRef]
- Sun, J.; Tang, Q.; Yu, S.; Xie, M.; Zheng, W.; Chen, G.; Yin, Y.; Huang, X.; Wo, K.; Zhang, J.; et al. Fusobacterium nucleatum facilitates oral squamous cell carcinoma progression via GLUT1-driven lactate production. eBioMedicine 2023, 88, 104444. [Google Scholar] [CrossRef]
- Shahoumi, L.A.; Saleh, M.H.A.; Meghil, M.M. Virulence factors of the periodontal pathogens: Tools to evade the host immune response and promote carcinogenesis. Microorganisms 2023, 11, 115. [Google Scholar] [CrossRef] [PubMed]
- Baty, J.J.; Stoner, S.N.; Scoffield, J.A. Oral commensal streptococci: Gatekeepers of the oral cavity. J. Bacteriol. 2022, 204, e0025722. [Google Scholar] [CrossRef]
- Socransky, S.S.; Smith, C.; Haffajee, A.D. Subgingival microbial profiles in refractory periodontal disease. J. Clin. Periodontol. 2002, 29, 260–268. [Google Scholar] [CrossRef]
- Cheng, X.; Redanz, S.; Treerat, P.; Qin, H.; Choi, D.; Zhou, X.; Xu, X.; Merritt, J.; Kreth, J. Magnesium-dependent promotion of H2O2 production increases ecological competitiveness of oral commensal streptococci. J. Dent. Res. 2020, 99, 847–854. [Google Scholar] [CrossRef]
- Presland, R.B.; Jurevic, R.J. Making sense of the epithelial barrier: What molecular biology and genetics tell us about the functions of oral mucosal and epidermal tissues. J. Dent. Educ. 2002, 66, 564–574. [Google Scholar] [CrossRef]
- Hosokawa, I.; Hosokawa, Y.; Ozaki, K.; Nakae, H.; Matsuo, T. Proinflammatory effects of muramyldipeptide on human gingival fibroblasts. J. Periodont. Res. 2010, 45, 193–199. [Google Scholar] [CrossRef]
- Fiorillo, L.; Cervino, G.; Herford, A.S.; Laino, L.; Cicciù, M. Stannous fluoride effects on enamel: A systematic review. Biomimetics 2020, 5, 41. [Google Scholar] [CrossRef]
- Mao, X.; Auer, D.L.; Buchalla, W.; Hiller, K.-A.; Maisch, T.; Hellwig, E.; Al-Ahmad, A.; Cielplik, F. Cetylpyridinium chloride: Mechanism of action, antimicrobial efficacy in biofilms, and potential risks of resistance. Antimicrob. Agents Chemother. 2020, 64, e00576-20. [Google Scholar] [CrossRef]
- Paul, S.; Pan, S.; Mukherjee, A.; De, P. Nitric oxide releasing delivery platforms: Design, detection, biomedical applications, and future possibilities. Mol. Pharm. 2021, 18, 3181–3205. [Google Scholar] [CrossRef]
- Dietz, B.M.; Hajirahimkhan, A.; Dunlap, T.L.; Bolton, J.L. Botanicals and their bioactive phytochemicals for women’s health. Pharmacol. Rev. 2016, 68, 1026–1073. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Dell’agli, M.; Badea, M.; Dima, L.; Colombo, E.; Sangiovannia, E. Plant food supplements with anti-inflammatory properties: A systematic review (II). Crit. Rev. Food Sci. Nutr. 2013, 53, 507–516. [Google Scholar] [CrossRef]
- Brendler, T.; Al-Harrasi, A.; Bauer, R.; Gafner, S.; Hardy, M.L.; Heinrich, M.; Hosseinzadeh, H.; Izzo, A.A.; Michaelis, M.; Nassari-Asl, M.; et al. Botanical drugs and supplements affecting the immune response in the time of COVID-19: Implications for research and clinical practice. Phytother. Res. 2021, 35, 3013–3031. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical insecticides in the twenty-first century—Fulfilling their promise? Annu. Rev. Entomol. 2020, 65, 233–249. [Google Scholar] [CrossRef]
- Nordin, A.; Bin Saim, A.; Ramli, R.; Abdul Hamid, A.; Wahida Mohd Nasri, N.; Bt Hj Idrus, R. Miswak and oral health: An evidence-based review. Saudi J. Biol. Sci. 2020, 27, 1801–1810. [Google Scholar] [CrossRef]
- Heyman, L.; Houri-Haddad, Y.; Heyman, S.N.; Ginsburg, I.; Gleitman, Y.; Feuerstein, O. Combined antioxidant effects of Neem extract, bacteria, red blood cells and lysozyme: Possible relation to periodontal disease. BMC Complement. Altern. Med. 2017, 17, 399. [Google Scholar] [CrossRef]
- Aumeeruddy, M.Z.; Zengin, G.; Mahomoodally, M.F. A review of the traditional and modern uses of Salvadora persica L. (Miswak): Toothbrush tree of Prophet Muhammad. J. Ethnopharmacol. 2018, 213, 409–444. [Google Scholar] [CrossRef]
- Vitrac, X.; Vitrac, C.; Brunel, M.; Costa, J.; Muselli, A. Particular Extract from Perfume Plants, Aromatic Plants and Medicinal Plants, Method for Obtaining Said Extract, Compositions Containing Same and Uses Thereof. WO2017/092555A1, 9 May 2019. [Google Scholar]
- Cheng, Y.; Xue, F.; Yu, S.; Du, S.; Yang, Y. Subcritical water extraction of natural products. Molecules 2021, 26, 4004. [Google Scholar] [CrossRef]
- Pu, Y.; Wang, J.-X.; Wang, D.; Foster, N.R.; Chen, J.-F. Subcritical water processing for nanopharmaceuticals. Chem. Eng. Process. Process Intensif. 2019, 140, 36–42. [Google Scholar] [CrossRef]
- Huamán-Castilla, N.L.; Mariotti-Celis, M.S.; Martínez-Cifuentes, M.; Pérez-Correa, J.R. Glycerol as Alternative Co-Solvent for Water Extraction of Polyphenols from Carménère Pomace: Hot Pressurized Liquid Extraction and Computational Chemistry Calculations. Biomolecules 2020, 10, 474. [Google Scholar] [CrossRef]
- Khajotia, S.S.; Smart, K.H.; Pilula, M.; Thompson, D.M. Concurrent quantification of cellular and extracellular components of biofilms. J. Vis. Exp. 2013, 77, e50639. [Google Scholar] [CrossRef]
- Rostami, N.; Shields, R.C.; Serrage, H.J.; Lawler, C.; Brittan, J.L.; Yassin, S.; Ahmed, H.; Trumann, A.; Thompson, P.; Wldron, K.J.; et al. Interspecies competition in oral biofilms mediated by Streptococcus gordonii extracellular deoxyribonuclease SSNA. NPJ Biofilms Microbiomes 2022, 8, 96. [Google Scholar] [CrossRef]
- D’Agostino, S.; Valentini, G.; Iarussi, F.; Dolci, M. Effect of probiotics Lactobacillus rhamnosus and Lactobacillus plantarum on caries and periodontal diseases: A systematic review. Dent. J. 2024, 12, 102. [Google Scholar] [CrossRef]
- Tansiri, N.; Ruensukon, T.; Hatsadaloi, W.; Trachoo, V.; Lochaiwatana, Y.; Laiteerapong, A. Cytotoxicity Evaluation of Herbal Mouthwashes Containing Ginseng Extract on Human Gingival Fibroblast-like Cells: An In Vitro Study. J. Dent. Assoc. Thai. 2022, 72, 490–499. [Google Scholar] [CrossRef]
- Vyas, N.; Sammons, R.L.; Addison, O.; Dehghani, H.; Walmsley, A.D. A quantitative method to measure biofilm removal efficiency from complex biomaterial surfaces using SEM and image analysis. Sci. Rep. 2016, 6, 32694. [Google Scholar] [CrossRef]
- Visperas, A.; Santana, D.; Ju, M.; Milbrandt, N.B.; Tsai, Y.H.; Wickramasinghe, S.; Klika, A.K.; Piuzzi, N.S.; Samia, A.C.S. Standardized quantification of biofilm in a novel rabbit model of periprosthetic joint infection. J. Bone Jt. Infect. 2022, 7, 91–99. [Google Scholar] [CrossRef]
- Milbrandt, N.B.; Tsai, Y.H.; Cui, K.; Massado, C.S.N.; Jung, H.; Visperas, A.; Klika, A.; Piuzzi, N.; Higuera-Rueda, C.A.; Samia, A.C.S. Combination D-amino acid and photothermal hydrogel for the treatment of prosthetic joint infections. ACS Appl. Bio Mater. 2023, 6, 1231–1241. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Robbins, R.J. Phenolic acids in foods: An overview of analytical methodology. J. Agric. Food Chem. 2003, 51, 2866–2887. [Google Scholar] [CrossRef]
- Sarkar, B.; Chakraborty, S.; Pal, C. Phytomedicine Against Infectious Diseases. Phytopharmaceuticals 2021, 161–172. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Kim, J.-M.; Chung, K.-S.; Jang, D.S.; Lee, J.-Y.; Kim, C.; Lee, J.Y.; Lee, J.K.; Lee, K.-T. In vitro and in vivo anti-inflammatory effects of 5-hydroxyconiferaldehyde via NF-κB, MAPK/AP-1, and Nrf2 modulation. Chem. Biol. Interact. 2025, 409, 111427. [Google Scholar] [CrossRef]
- Cueva, C.; Moreno-Arribas, M.V.; Martín-Álvarez, P.J.; Bills, G.; Vicente, M.F.; Basilio, A.; López Rivas, C.; Requena, T.; Rodríguez, J.M.; Bartolomé, B. Antimicrobial activity of phenolic acids against commensal, probiotic and pathogenic bacteria. Res. Microbiol. 2010, 161, 372–382. [Google Scholar] [CrossRef]
- Almuhanna, Y.; Alshalani, A.; AlSudais, H.; Alanazi, F.; Alissa, M.; Asad, M.; Joseph, B. Antibacterial, antibiofilm, and wound healing activities of rutin and quercetin and their interaction with gentamicin on excision wounds in diabetic mice. Biology 2024, 13, 676. [Google Scholar] [CrossRef]
- Yun, J.; Woo, E.-R.; Lee, D.G. Effect of isoquercitrin on membrane dynamics and apoptosis-like death in Escherichia coli. Biochim. Biophys. Acta Biomembr. 2018, 1860, 357–363. [Google Scholar] [CrossRef]
- Efenberger-Szmechtyk, M.; Nowak, A.; Czyżowska, A. Plant extracts rich in polyphenols: Antibacterial agents and natural preservatives for meat and meat products. Crit. Rev. Food Sci. Nutr. 2021, 61, 149–178. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).