Titanium Particle Impact on Immune Cells, Cytokines, and Inflammasomes: Helping to Profile Peri-Implantitis—A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Review Question
2.2. Screening Process
2.3. Eligibility Criteria
2.4. Data Extraction
2.5. Quality Assessment
3. Results
4. Discussion
4.1. Future Perspectives
4.2. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ting, M.; Suzuki, J.B. Peri-Implantitis. Dent. J. 2024, 12, 251. [Google Scholar] [CrossRef]
- Galarraga-Vinueza, M.E.; Pagni, S.; Finkelman, M.; Schoenbaum, T.; Chambrone, L. Prevalence, incidence, systemic, behavioral, and patient-related risk factors and indicators for peri-implant diseases: An AO/AAP systematic review and meta-analysis. J. Periodontol. 2025, 96, 587–633. [Google Scholar] [CrossRef] [PubMed]
- Lindhe, J.; Meyle, J. Peri-implant diseases: Consensus Report of the Sixth European Workshop on Periodontology. J. Clin. Periodontol. 2008, 35 (Suppl. S8), 282–285. [Google Scholar] [CrossRef] [PubMed]
- Lafaurie, G.I.; Sabogal, M.A.; Castillo, D.M.; Rincón, M.V.; Gómez, L.A.; Lesmes, Y.A.; Chambrone, L. Microbiome and Microbial Biofilm Profiles of Peri-Implantitis: A Systematic Review. J. Periodontol. 2017, 88, 1066–1089. [Google Scholar] [CrossRef]
- Zitzmann, N.U.; Berglundh, T.; Marinello, C.P.; Lindhe, J. Experimental peri-implant mucositis in man. J. Clin. Periodontol. 2001, 28, 517–523. [Google Scholar] [CrossRef]
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H.-L. Peri-implantitis. J. Periodontol. 2018, 89 (Suppl. S1), S267–S290. [Google Scholar] [CrossRef]
- Anitua, E.; Alkhraisat, M.H.; Eguia, A. On Peri-Implant Bone Loss Theories: Trying to Piece Together the Jigsaw. Cureus 2023, 15, e33237. [Google Scholar] [CrossRef]
- Wachi, T.; Shuto, T.; Shinohara, Y.; Matono, Y.; Makihira, S. Release of titanium ions from an implant surface and their effect on cytokine production related to alveolar bone resorption. Toxicology 2015, 327, 1–9. [Google Scholar] [CrossRef]
- Quabius, E.S.; Ossenkop, L.; Harder, S.; Kern, M. Dental implants stimulate expression of Interleukin-8 and its receptor in human blood—An in vitro approach. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 1283–1288. [Google Scholar] [CrossRef]
- Messous, R.; Henriques, B.; Bousbaa, H.; Silva, F.S.; Teughels, W.; Souza, J.C.M. Cytotoxic effects of submicron- and nano-scale titanium debris released from dental implants: An integrative review. Clin. Oral Investig. 2021, 25, 1627–1640. [Google Scholar] [CrossRef] [PubMed]
- Bressan, E.; Ferroni, L.; Gardin, C.; Bellin, G.; Sbricoli, L.; Sivolella, S.; Brunello, G.; Schwartz-Arad, D.; Mijiritsky, E.; Penarrocha, M.; et al. Metal Nanoparticles Released from Dental Implant Surfaces: Potential Contribution to Chronic Inflammation and Peri-Implant Bone Loss. Materials 2019, 12, 2036. [Google Scholar] [CrossRef]
- Faggion, C.M.J. Guidelines for reporting pre-clinical in vitro studies on dental materials. J. Evid. -Based Dent. Pract. 2012, 12, 182–189. [Google Scholar] [CrossRef]
- Holliday, R.S.; Campbell, J.; Preshaw, P.M. Effect of nicotine on human gingival, periodontal ligament and oral epithelial cells. A systematic review of the literature. J. Dent. 2019, 86, 81–88. [Google Scholar] [CrossRef]
- Taira, M.; Sasaki, K.; Saitoh, S.; Nezu, T.; Sasaki, M.; Kimura, S.; Terasaki, K.; Sera, K.; Narushima, T.; Araki, Y. Accumulation of element Ti in macrophage-like RAW264 cells cultured in medium with 1 ppm Ti and effects on cell viability, SOD production and TNF-α secretion. Dent. Mater. J. 2006, 25, 726–732. [Google Scholar] [CrossRef]
- Chan, E.P.; Mhawi, A.; Clode, P.; Saunders, M.; Filgueira, L. Effects of titanium(iv) ions on human monocyte-derived dendritic cells. Met. Integr. Biometal. Sci. 2009, 1, 166–174. [Google Scholar] [CrossRef]
- Makihira, S.; Mine, Y.; Nikawa, H.; Shuto, T.; Iwata, S.; Hosokawa, R.; Kamoi, K.; Okazaki, S.; Yamaguchi, Y. Titanium ion induces necrosis and sensitivity to lipopolysaccharide in gingival epithelial-like cells. Toxicol. Vitr. 2010, 24, 1905–1910. [Google Scholar] [CrossRef] [PubMed]
- Meng, B.; Yang, X.; Chen, Y.; Zhai, J.; Liang, X. Effect of titanium particles on osteoclast activity in vitro. Mol. Med. Rep. 2010, 3, 1065–1069. [Google Scholar] [CrossRef] [PubMed]
- Mine, Y.; Makihira, S.; Nikawa, H.; Murata, H.; Hosokawa, R.; Hiyama, A.; Mimura, S. Impact of titanium ions on osteoblast-, osteoclast- and gingival epithelial-like cells. J. Prosthodont. Res. 2010, 54, 1–6. [Google Scholar] [CrossRef]
- Irshad, M.; Scheres, N.; Crielaard, W.; Loos, B.G.; Wismeijer, D.; Laine, M.L. Influence of titanium on in vitro fibroblast-Porphyromonas gingivalis interaction in peri-implantitis. J. Clin. Periodontol. 2013, 40, 841–849. [Google Scholar] [CrossRef]
- Mano, S.S.; Kanehira, K.; Taniguchi, A. Comparison of cellular uptake and inflammatory response via toll-like receptor 4 to lipopolysaccharide and titanium dioxide nanoparticles. Int. J. Mol. Sci. 2013, 14, 13154–13170. [Google Scholar] [CrossRef] [PubMed]
- Dodo, C.G.; Meirelles, L.; Aviles-Reyes, A.; Ruiz, K.G.S.; Abranches, J.; Cury, A.A.D.B. Pro-inflammatory Analysis of Macrophages in Contact with Titanium Particles and Porphyromonas gingivalis. Braz. Dent. J. 2017, 28, 428–434. [Google Scholar] [CrossRef]
- Pan, Y.; Jiang, L.; Lin, H.; Cheng, H. Cell death affected by dental alloys: Modes and mechanisms. Dent. Mater. J. 2017, 36, 82–87. [Google Scholar] [CrossRef]
- Pettersson, M.; Kelk, P.; Belibasakis, G.N.; Bylund, D.; Molin Thorén, M.; Johansson, A. Titanium ions form particles that activate and execute interleukin-1β release from lipopolysaccharide-primed macrophages. J. Periodontal Res. 2017, 52, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Happe, A.; Sielker, S.; Hanisch, M.; Jung, S. The Biologic Effect of Particulate Titanium Contaminants of Dental Implants on Human Osteoblasts and Gingival Fibroblasts. Int. J. Oral Maxillofac. Implant. 2019, 34, 673–680. [Google Scholar] [CrossRef]
- Schwarz, F.; Langer, M.; Hagena, T.; Hartig, B.; Sader, R.; Becker, J. Cytotoxicity and proinflammatory effects of titanium and zirconia particles. Int. J. Implant. Dent. 2019, 5, 25. [Google Scholar] [CrossRef]
- Toledano-Serrabona, J.; Bosch, B.M.; Díez-Tercero, L.; Gil, F.J.; Camps-Font, O.; Valmaseda-Castellón, E.; Gay-Escoda, C.; Sánchez-Garcés, M.Á. Evaluation of the inflammatory and osteogenic response induced by titanium particles released during implantoplasty of dental implants. Sci. Rep. 2022, 12, 15790. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, J.; Lin, T.; Huang, S.; Ma, J.; Xu, X. Excessive production of mitochondrion-derived reactive oxygen species induced by titanium ions leads to autophagic cell death of osteoblasts via the SIRT3/SOD2 pathway. Mol. Med. Rep. 2020, 22, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Callejas, J.A.; Gil, J.; Brizuela, A.; Pérez, R.A.; Bosch, B.M. Effect of the Size of Titanium Particles Released from Dental Implants on Immunological Response. Int. J. Mol. Sci. 2022, 23, 7333. [Google Scholar] [CrossRef]
- Li, L.; Sun, W.; Yu, J.; Lei, W.; Zeng, H.; Shi, B. Effects of titanium dioxide microparticles and nanoparticles on cytoskeletal organization, cell adhesion, migration, and proliferation in human gingival fibroblasts in the presence of lipopolysaccharide. J. Periodontal Res. 2022, 57, 644–659. [Google Scholar] [CrossRef]
- Nemec, M.; Behm, C.; Maierhofer, V.; Gau, J.; Kolba, A.; Jonke, E.; Rausch-Fan, X.; Andrukhov, O. Effect of Titanium and Zirconia Nanoparticles on Human Gingival Mesenchymal Stromal Cells. Int. J. Mol. Sci. 2022, 23, 10022. [Google Scholar] [CrossRef]
- Papamanoli, E.; Kyriakidou, K.; Philippou, A.; Koutsilieris, M.; Karoussis, I.K. Free titanium particles and P. gingivalis lipopolysaccharide create a potentially synergistical effect in a periimplantitis model. Arch. Oral Biol. 2023, 153, 105739. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Gálvez, A.B.; Zurita, F.; Guerra-Valverde, J.A.; Aguilar-González, A.; Abril-García, D.; Padial-Molina, M.; Olaechea, A.; Martín-Morales, N.; Martín, F.; O’Valle, F.; et al. NLRP3 and AIM2 inflammasomes expression is modified by LPS and titanium ions increasing the release of active IL-1β in alveolar bone-derived MSCs. Stem Cells Transl. Med. 2024, 13, 826–841. [Google Scholar] [CrossRef]
- Wakuda, S.; Hasuike, A.; Fujiwara, K.; Sakai, R.; Chaurasia, A.; Uchiyama, T.; Sato, S. Titanium particle-induced inflammasome in human gingival epithelial cells. J. Dent. Sci. 2025, 20, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.G.J.; Valderrama, P.; Burbano, M.; Blansett, J.; Levine, R.; Kessler, H.; Rodrigues, D.C. Foreign bodies associated with peri-implantitis human biopsies. J. Periodontol. 2015, 86, 9–15. [Google Scholar] [CrossRef]
- Daubert, D.M.; Pozhitkov, A.E.; Safioti, L.M.; Kotsakis, G.A. Association of Global DNA Methylation to Titanium and Peri-Implantitis: A Case-Control Study. JDR Clin. Transl. Res. 2019, 4, 284–291. [Google Scholar] [CrossRef]
- Rasul, J.; Thakur, M.K.; Maheshwari, B.; Aga, N.; Kumar, H.; Mahajani, M. Assessment of Titanium Level in Submucosal Plaque Around Healthy Implants and Implants with Peri-implantitis: A Clinical Study. J. Pharm. Bioallied Sci. 2021, 13, 383–386. [Google Scholar] [CrossRef]
- Berryman, Z.; Bridger, L.; Hussaini, H.M.; Rich, A.M.; Atieh, M.; Tawse-Smith, A. Titanium particles: An emerging risk factor for peri-implant bone loss. Saudi Dent. J. 2020, 32, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Rakic, M.; Radunovic, M.; Petkovic-Curcin, A.; Tatic, Z.; Basta-Jovanovic, G.; Sanz, M. Study on the immunopathological effect of titanium particles in peri-implantitis granulation tissue: A case–control study. Clin. Oral Implant. Res. 2022, 33, 656–666. [Google Scholar] [CrossRef]
- Stolzer, C.; Müller, M.; Gosau, M.; Henningsen, A.; Fuest, S.; Aavani, F.; Smeets, R. Do Titanium Dioxide Particles Stimulate Macrophages to Release Proinflammatory Cytokines and Increase the Risk for Peri-implantitis? J. Oral Maxillofac. Surg. 2023, 81, 308–317. [Google Scholar] [CrossRef]
- Rakic, M.; Canullo, L.; Radovanovic, S.; Tatic, Z.; Radunovic, M.; Souedain, A.; Weiss, P.; Struillou, X.; Vojvodic, D. Diagnostic value of VEGF in peri-implantitis and its correlation with titanium particles: A controlled clinical study. Dent. Mater. 2024, 40, 28–36. [Google Scholar] [CrossRef]
- Noronha Oliveira, M.; Schunemann, W.V.H.; Mathew, M.T.; Henriques, B.; Magini, R.S.; Teughels, W.; Souza, J.C.M. Can degradation products released from dental implants affect peri-implant tissues? J. Periodontal Res. 2018, 53, 1–11. [Google Scholar] [CrossRef]
- Vallés, G.; González-Melendi, P.; González-Carrasco, J.L.; Saldaña, L.; Sánchez-Sabaté, E.; Munuera, L.; Vilaboa, N. Differential inflammatory macrophage response to rutile and titanium particles. Biomaterials 2006, 27, 5199–5211. [Google Scholar] [CrossRef]
- Alarcón-Sánchez, M.A.; Romero-Castro, N.S.; Reyes-Fernández, S.; Sánchez-Tecolapa, E.U.; Heboyan, A. Expression of IL-33 in subjects with periodontitis: A systematic review and meta-analysis. Eur. J. Med. Res. 2024, 29, 440. [Google Scholar] [CrossRef]
- Cayrol, C.; Girard, J.-P. Interleukin-33 (IL-33): A critical review of its biology and the mechanisms involved in its release as a potent extracellular cytokine. Cytokine 2022, 156, 155891. [Google Scholar] [CrossRef]
- Severino, V.O.; Beghini, M.; de Araújo, M.F.; de Melo, M.L.R.; Miguel, C.B.; Rodrigues, W.F.; de Lima Pereira, S.A. Expression of IL-6, IL-10, IL-17 and IL-33 in the peri-implant crevicular fluid of patients with peri-implant mucositis and peri-implantitis. Arch. Oral Biol. 2016, 72, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Bijukumar, D.R.; Salunkhe, S.; Zheng, G.; Barba, M.; Hall, D.J.; Pourzal, R.; Mathew, M.T. Wear particles induce a new macrophage phenotype with the potential to accelerate material corrosion within total hip replacement interfaces. Acta Biomater. 2020, 101, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Kheder, W.; Bouzid, A.; Venkatachalam, T.; Talaat, I.M.; Elemam, N.M.; Raju, T.K.; Sheela, S.; Jayakumar, M.N.; Maghazachi, A.A.; Samsudin, A.R.; et al. Titanium Particles Modulate Lymphocyte and Macrophage Polarization in Peri-Implant Gingival Tissues. Int. J. Mol. Sci. 2023, 24, 11644. [Google Scholar] [CrossRef]
- Bertoldo, B.B.; Paulo, G.O.; Furtado, T.C.d.S.; Pereira, T.L.; Rodrigues, V.; Rodrigues, D.B.R.; de Faria, J.B.; Rosa, R.C.; Pereira, S.A.d.L. New immunological aspects of peri-implantitis. Einstein 2024, 22, eAO0396. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Moreno, P.; Montalvo-Acosta, S.; Martín-Morales, N.; Carrillo-Gálvez, A.B.; González-Rey, E.; O’VAlle, F.; Padial-Molina, M. Inflammasomes NLRP3 and AIM2 in peri-implantitis: A cross-sectional study. Clin. Oral Implant. Res. 2023, 34, 1342–1353. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, L. New Insights into the Interplay Among Autophagy, the NLRP3 Inflammasome and Inflammation in Adipose Tissue. Front. Endocrinol. 2022, 13, 739882. [Google Scholar] [CrossRef]
- Chato-Astrain, J.; Toledano-Osorio, M.; Alaminos, M.; Toledano, M.; Sanz, M.; Osorio, R. Effect of functionalized titanium particles with dexamethasone-loaded nanospheres on macrophage polarization and activity. Dent. Mater. 2024, 40, 66–79. [Google Scholar] [CrossRef]
- Niemczyk, W.; Żurek, J.; Niemczyk, S.; Kępa, M.; Zięba, N.; Misiołek, M.; Wiench, R. Antibiotic-Loaded Platelet-Rich Fibrin (AL-PRF) as a New Carrier for Antimicrobials: A Systematic Review of In Vitro Studies. Int. J. Mol. Sci. 2025, 26, 2140. [Google Scholar] [CrossRef] [PubMed]
- Freitag, L.; Spinell, T.; Kröger, A.; Würfl, G.; Lauseker, M.; Hickel, R.; Kebschull, M. Dental implant material related changes in molecular signatures in peri-implantitis—A systematic review and integrative analysis of omics in-vitro studies. Dent. Mater. 2023, 39, 101–113. [Google Scholar] [CrossRef]
- Ivanovski, S.; Bartold, P.M.; Huang, Y.-S. The role of foreign body response in peri-implantitis: What is the evidence? Periodontology 2000 2022, 90, 176–185. [Google Scholar] [CrossRef]
- Insua, A.; Galindo-Moreno, P.; Miron, R.J.; Wang, H.-L.; Monje, A. Emerging factors affecting peri-implant bone metabolism. Periodontology 2000 2024, 94, 27–78. [Google Scholar] [CrossRef]
- Oh, J.M.; Kim, Y.; Son, H.; Kim, Y.H.; Kim, H.J. Comparative transcriptome analysis of periodontitis and pe-ri-implantitis in human subjects. J. Periodontol. 2024, 95, 337–349. [Google Scholar] [CrossRef]
- Martin, A.; Zhou, P.; Singh, B.B.; Kotsakis, G.A. Transcriptome-wide Gene Expression Analysis in Peri-implantitis Reveals Candidate Cellular Pathways. JDR Clin. Transl. Res. 2022, 7, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.J.; Lai, Y.R.; Huang, Q.R.; Li, Y.R.; Zhang, Y.J.; Chen, R.Y.; Qian, S.J. Single-cell sequencing identifies in-flammation-promoting fibroblast-neutrophil interaction in peri-implantitis. J. Clin. Periodontol. 2024, 51, 196–208. [Google Scholar] [CrossRef] [PubMed]
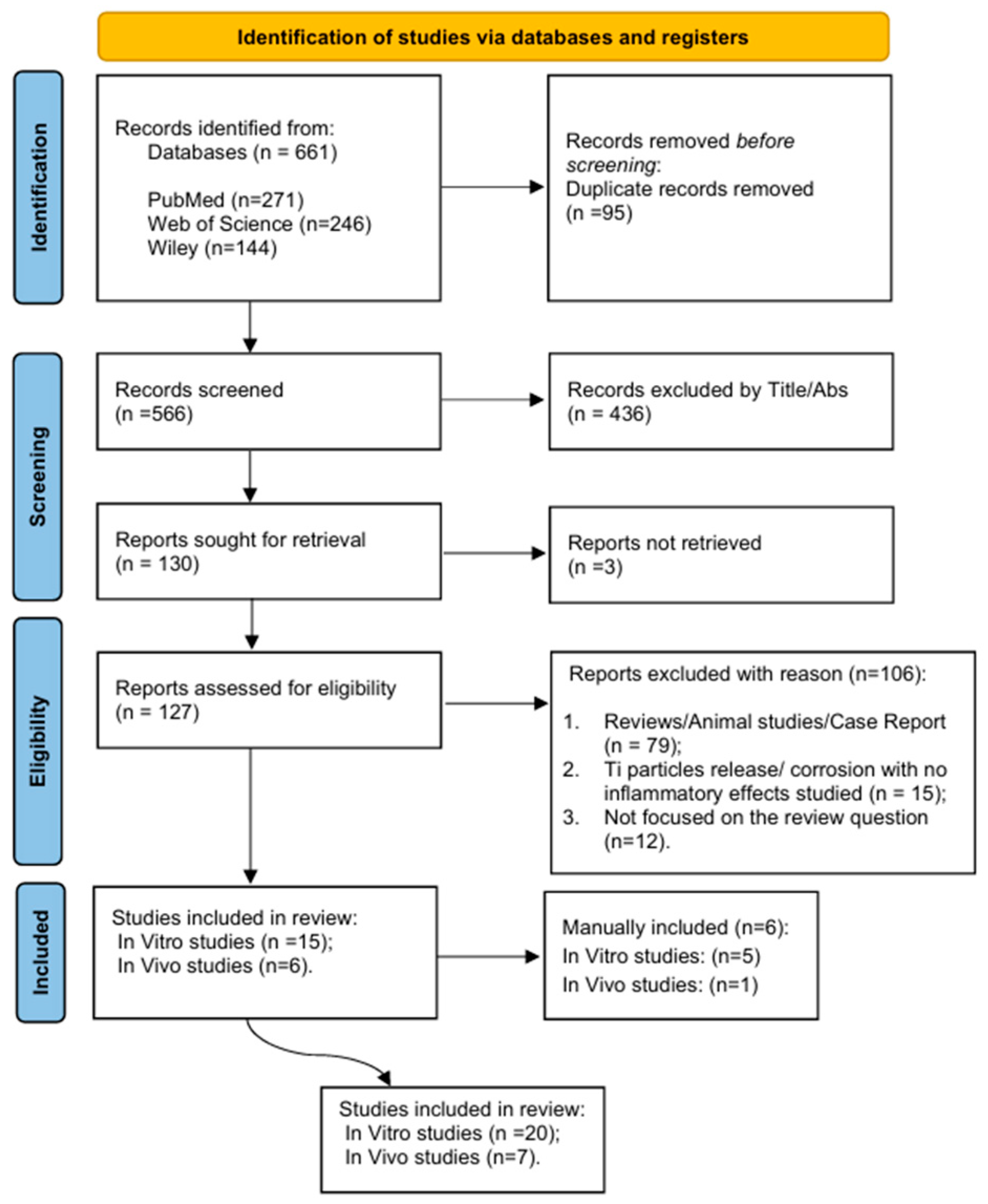

| P (Population) | Human observational studies and experimental assays (in vitro) |
| E (Exposure) | Effect of titanium particles resulting from bio-tribocorrosion in peri-implantitis inflammatory reaction |
| C (Comparison) | Healthy titanium dental implants |
| O (Outcomes) | Inflammatory response and cytokine production; cell activation and viability |
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| In vivo studies in humans, such as cohort studies, cross-sectional investigations, case–control analyses, and randomised trials | In vivo animal studies |
| In vitro studies | Studies concerning the wear of titanium particles in organs other than the oral cavity |
| Studies in which titanium particles resulted from debridement treatments (implantoplasty and implant scaling) | |
| Studies with a focus on evaluating the effects of implant–abutment connection compatibility and cover screw design on peri-implant disease | |
| Review papers, correspondence, personal viewpoints, book sections, conference proceedings, and summaries |
| In Vitro Studies | |||||
|---|---|---|---|---|---|
| Author, Year | Ti Particles/Size | Cell Line/Model | Inflammatory Biomarker | Assay | Summary |
| Taira et al., 2006 [14] | Ti ions 1 ppm | Macroph.RAW264 (mice) | TNF-α | ELISA | Cultures with Ti show increased SOD and TNF-α secretion: uptake of Ti-containing complex by macrophages induced oxidative stress and triggered inflammatory response. |
| Chan et al., 2009 [15] | Ti ions (0–100 µM) | Dendritic cells, blood derived (human) | MHCII, CD80, CD86, CD40, CD54, CD25, CCR4, CCR6 and CCR 7, IL-1β, IL-6, IL-12, TNF-α, IL-4, IL-10, TGF-β1, TGF-β2, TGF-β3, CCL17, CCL22 | Flow cytometry, ELISA | Uptake of Ti ions by DCs (membranes, cytoplasm, and nucleus). DCs decreased expression of MHCII, CD80, CD86, CD40, CD54, CD25, CCR6, and CCR7, showing a weaker immunological synapse with T lymphocytes (only CCR4 slightly increased); Th1 cytokine IL-12 improved substantially as lymphocyte stimulatory capacity increased. Titanium modifies DC functions, leading to increased T lymphocyte activation and a shift towards a Th1-type immune response. |
| Makihira et al., 2010 [16] | Ti ions (1–19 ppm) | Gingival epithelial (mice) | CCL-2, TLR-4, ICAM-1 | RT-PCR | Ti ions increased CCL2 regulation in GE subjected to LPS originating from P. gingivalis synergistically; they also increased TLR-4 and ICAM-1 expression. This suggests that Ti ions partially contribute to monocyte infiltration in the oral cavity by increasing the responsiveness of gingival epithelial cells to microbial stimuli. |
| Meng et al., 2010 [17] | Ti part 1 µm | Osteoclasts (mice) | TRAP, CAT K, CAII | RT-PCR | Ti particles were phagocytosed; osteoclast activity was enhanced (TRAP and CAT K) at a lower concentration of Ti particles but inhibited at high. |
| Mine et al., 2010 [18] | Ti ions (1–9 ppm) | Gingival epithelial cells (GE), osteoclasts RAW264.7, and osteoblasts MC3T3-E1 (all mice) | TRAP, CAT K, RANKL, OPG, Runx2, Osterix, COL-1 | RT-PCR | Ti ions at 9 ppm suppressed the expression of Runx2, Osterix, and COL-1 in osteoblast-like cells and increased RANKL and OPG expression. In osteoclasts and in GE, no effect on TRAP and CAT K (reported previously for this cell line development with RANKL). Ti ions impair osteoblast differentiation and modify the RANKL/OPG gene expression ratio, which is associated with osteoclast differentiation. |
| Irshad et al., 2013 [19] | Ti par (<5 µm) | Fibroblasts (human) biopsy | TNF-α, IL-6, IL-1β, IL-8, MCP-1 | RT-PCR, ELISA | Ti particles and P. gingivalis, independently, are capable of eliciting pro-inflammatory responses in PIGFs: TNF-α, IL-6, and IL-8 (alone) or TNFα and MCP-1 (combined); Il-1 β induced only by P. gingivalis. |
| Mano, 2013 [20] | Ti par (25 nm) | Pulmonary epithelial (NCI-H292) human | LPS binding protein, CD14, TLR-4, IL-6 | RT-PCR, Flow-cytometry | TLR 4, but not LBP or CD 14, plays a role in the internalisation of Ti particles to cells and in the inflammatory signal transduction mediated by Ti particles. The latter association was seen by the increased IL-6 expression. |
| Dodo et al., 2017 [21] | Ti par (<20 µm and 21 nm) | Macro.THP-1 (human) | TNF-α, IL-1β, IL-6 | RT-PCR, ELISA | Ti nano or microparticles and LPS (P. gingivalis) and macrophages impacted viability and inflammation–gene expression and cytokine release were significantly greater for TNF-α and IL1-β after 12 h, and for IL-6 but only 24 h later. There is robust pro-inflammatory response in nanoparticles independent of LPS presence. |
| Pan et al., 2017 [22] | Ti ions | Fibroblasts L929 (mice) | CASP-9, CASP-3 | RT-PCR | Ti particles have a cytotoxic effect on fibroblasts (time-dependent): upregulation of CASP-9 and CASP-3 and apoptosis by the intrinsic pathway. Apoptosis might not be a result of the ions released but of compromised cell adhesion and subsequent cell apoptosis. |
| Pettersson et al., 2017 [23] | Ti ions | Macro.THP-1 (human) | NLRP3, ASC, CASP-1, IL-1β, IL-1a, b, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, IL-17, IFN-γ, TNF-α, GM-CSF | RT-PCR, ELISA | Ti ions stimulated inflammasome activation: IL-1β release. LPS exposure enhances this effect. Nevertheless, Ti ions alone did not promote transcription of the inflammasome components. Ti ions establish particles that represent a secondary stimulus for a pro-inflammatory response. |
| Happe et al., 2019 [24] | Ti part < 20 µm | Gingival fibroblasts and osteoblasts (human) | TNF-α, IL-6 | ELISA | Ti particle concentration affects cell growth and proliferation and has cytotoxic effects, especially in GF; osteoblasts produce IL-6 only 24 h after contact, but GF produce IL-6 continuously for 21 days. Ti particles were identified inside the cells. |
| Schwarz et al., 2019 [25] | Ti part (60–100 nm) | Gingival fibroblasts and osteoblasts (human), macrophage THP-1 (human) | IL-1β, IL-6 | ELISA | Increasing Ti particle concentration correlates negatively with cell viability both in osteoblasts and GF. No significant improvement in IL-1β and IL-6 concentrations was observed in THP-1. Thus, no pro-inflammatory effects. |
| Toledano-Serrabona et al., 2020 [26] | Ti part. (30–70 nm) | Macrophage THP-1 (human) and bone marrow-derived mesenchymal stem cells-BM-MSCs (human) | CCR7, TNF-α, IL-1β, CD206, TGF-β, IL-10 | RT-PCR, ELISA | Macrophages stimulated by Ti particles developed an amplified pro-inflammatory expression of TNF-α and a reduced expression of TGF-β and CD206. Regarding cytokine release, there was an increase in IL-1β, whereas IL-10 was reduced. Ti particle concentration negatively correlates with BM-MSCs cell viability, and the cells showed a significant decrease in Runx2 and OC expression—osteogenic response markers. Ti particles elicit a pro-inflammatory response and suppress osteogenic gene expression. |
| Wang et al., 2020 [27] | Ti ions 10–50 µm | Osteoblasts (human) | mROS, SOD, SIRT | Western blot | Ti ions reduced osteoblast capability. With improved Ti ion concentration, the expression levels of LC3 gradually increased, P62 reduced, autophagic flow amplified, and mROS levels improved. In addition, activity of SOD2/SIRT 3 decreased. |
| Callejas et al., 2022 [28] | Ti part (5–30 µm) | Macrophage THP-1 (human) | TNFα, IL-1β, CCR7, IL-10, TGF-β, CD206, TNFα, IL-1 β, IL-10 | RT-PCR, ELISA | Smaller Ti particle sizes show less cytotoxicity. At non cytotoxic concentrations, TNFα and IL-1β expression, inflammatory indicators, were higher related to bigger Ti particles: particles of 15 µm showed a minor pro-inflammatory and higher anti-inflammatory reaction as categorised by gene expression and cytokine release: biocompatible and reveal a lower immune response. |
| Li et al., 2022 [29] | Ti part (<5 µm and <100 nm) | Gingival fibroblasts (human) | FAK, fibronectin, COL-1 | RT-PCR, Western blot | Ti nanoparticles significantly inhibited GF cell viability, proliferation, and migration compared with microparticles. Additionally, they caused cytoskeleton disruption as measured by protein expression. This effect was enhanced with LPS. |
| Nemec, 2022 [30] | Ti part (<100 nm) nano | Gingival mesenchymal stromal cells (human) | IL-6, IL-8, MCP-1 | RT-PCR, ELISA | Cell proliferation and viability were inhibited by Ti nanoparticles (<100 nm). They also elicited strong expression of IL-8, and this response was enhanced by LPS. |
| Papamanoli, 2023 [31] | Ti part (10.4 ± 6.4 μm) | Gingival fibroblasts (human) | IL-6, IL-8, Col-1a | RT-PCR | Association of Ti particles and LPS substantially increased expression of IL-6, IL-8, and Col-1a. It seems that particles may stimulate comparable reactions to the endotoxin, whereas synergistically amplifying it. |
| Carrillo-Galvèz et al., 2024 [32] | Ti ions | Bone derived mesenchymal stromal cells (human) | NLRP3, AIM2, IL-1β | RT-PCR, ELISA | There is induction of NLRP3 and absence of AIM2 inflammasome pathways facilitated by bacterial factors, with increased release of IL-1β. Bacterial agents, in combination with Ti ions, enhance NLRP3 expression while AIM2 is reduced. The progression of inflammation in peri-implantitis may be more critical due to the mutual effect of organic and inorganic elements that enhance NLRP3 inflammasome activation. |
| Wakuda et al., 2025 [33] | Ti par 25 µm (30–100 µg/mL) | Gingival epithelial (Ca9–22) (human) | COX2, ROS, TGF-β1, NLRP1, NLPR3, CASP1, IL-1β | RT-PCR, ELISA | Cells treated with Ti particles showed an increase of 75% in cell capability through all dilutions. Inflammation-related genes (COX2 and TGF-β1) substantially intensified in a dose-dependent way. NLRP3 and CASP-1 expression increased, as well as the secretion of IL-1β. Moreover, there were improved ROS levels after testing with Ti particles. |
| In Vivo Human Studies | |||||
| Author, Year | Ti Particles/size | Cell Line/model | Inflammatory Biomarker | Assay | Summary |
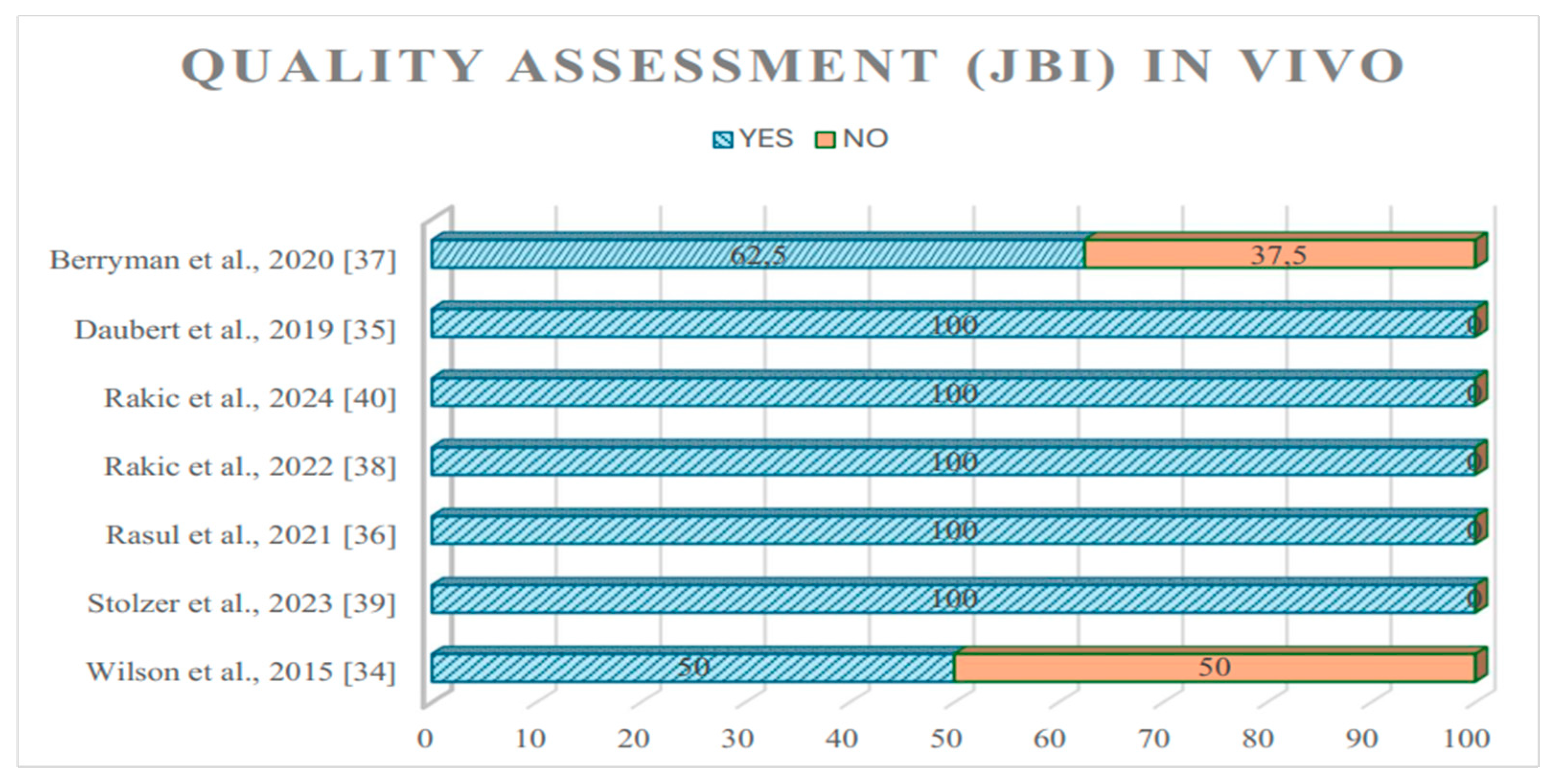
| Wilson et al., 2015 [34] | Ti particles | 36 peri-implantitis (biopsies) | Chronic inflammatory infiltrates–plasma cells; MGNCs | Histological analysis | Chronic inflammatory infiltrates–plasma cells; Multinucleated Giant Cells (MGNCs) were observed. |
| Daubert et al., 2019 [35] | Ti particles | 21 peri-implantitis, 24 healthy implants (PICF and biopsies) | DNA methylation | ELISA | Increased levels of methylated DNA in peri-implantitis; association of Ti levels and global methylation, independent of peri-implantitis, suggesting methylation may be affected by Ti dissolution products. |
| Rasul et al., 2021 [36] | Ti particles | 30 peri-implantitis, 20 healthy implants (biopsies) | Peri-implantitis parameters (probing depth, plaque index, gingival index) | Mass spectrometry | Notably higher Ti level in submucosal plaque surrounding dental implants with symptoms of peri-implantitis compared with healthy dental implants. |
| Berryman et al., 2020 [37] | Ti particles | 10 peri-implantitis (biopsies) | TGF-β1, RANKL, IL-33, and CD68 | IHC, ELISA | Tissue samples showed a mixed chronic inflammatory infiltration. Substantial upregulation of cytokine RANKL detected, with a tendency toward overexpression of IL-33 and TGF-β1 in areas with Ti. |
| Rakic et al., 2022 [38] | Ti particles | 39 peri-implantitis, 35 periodontitis (biopsies) | CD68, IL-6, NF-kβ, and VEGF | IHC and histological analysis | Neutrophil infiltration inside the granulation matrix, absence of MGNCs or specific inflammatory patterns, severe neovascularization, and persistent immune cell infiltration mainly composed of plasma cells, neutrophils, and macrophages—high CD68 and VEGF. |
| Stolzer et al., 2023 [39] | Ti particles | 20 peri-implantitis, 20 healthy implants, 20 no implants (peripheral blood) | TNF-α, IL-1β | ELISA | Significant relationship of positive Ti stimulation and clinical and radiological peri-implantitis; macrophages in 28.3% of individuals across all groups emitted pro-inflammatory cytokines beyond normal biological levels. |
| Rakic et al., 2024 [40] | Ti particles | 36 peri-implantitis, 36 peri-implant mucositis, 39 healthy implants, and 37 periodontitis (PICF and biopsies) | Chronic inflammatory infiltrates, MGNCs, and VEGF | IHC and histological analysis, ELISA | TPs were detected as unbound material enclosed within granulation tissue, but no MGNCs or impaired phagocytes were observed—no evidence of foreign body response or distinct pathological impact caused by TPs in peri-implantitis. VEGF is markedly upregulated in peri-implantitis relative to periodontitis and shows a positive association with its soluble levels in PICF. |
| In Vitro Studies | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Author, Year | Ti Particles/Ions Size | Cell Type | TNF-α | IL-6 | IL1-β | IL-8 | IL-12 | IL-10 | IL-33 |
| Taira, 2006 [14] | Ions | Mac | ↑ | ||||||
| Chan, 2009 [15] | Ions | DC | ↑ | ↓ | |||||
| Irshad, 2013 [19] | part.: <5 µm | Fib | ↑ | ↑ | ↑ | ||||
| Mano, 2013 [20] | part.: <25 µm | Epi | ↑ | ||||||
| Dodo, 2017 [21] | part.: <21 nm | Mac | ↑ | ↑ | ↑ | ||||
| Pettersson, 2017 [23] | Ions | Mac | ↑ | ↑ | |||||
| Happe, 2019 [24] | part.: <20 µm | Fib | ↑ | ||||||
| Toledano-Serrabona, 2022 [26] | part.: (30–70 nm) | Mac | ↑ | ↑ | ↓ | ||||
| Callejas, 2022 [28] | part.: 5–30 µm | Mac | ↑ | ↑ | ↑ * | ||||
| Nemec, 2022 [30] | part.: (100 nm) | GSC | ↑ | ||||||
| Papamanoli, 2023 [31] | part.: (15 µm) | Fib | ↑ | ↑ | |||||
| Wakuda, 2025 [33] | part (25 µm) | Epit | ↑ | ||||||
| TOTAL | 5 | 5 | 5 | 3 | 1 | 4 | 0 | ||
| In Vivo Studies | |||||||||
| Author, Year | Ti particles/Ions Size | Cell/Tissues | TNF-α | IL-6 | IL1-β | IL-8 | IL-12 | IL-10 | IL-33 |
| Berryman, 2020 [37] | - | PIT Biopsy | ↑ | ||||||
| Stolzer, 2023 [39] | - | PB Mon/Mac | ↑ | ↑ | |||||
| TOTAL | 1 | 2 | 1 | ||||||
| In Vitro Studies | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Author, Year | Ti Particle/Ions | Cell Type | TLR4 | RANKL | OPG | CASP-1,9,3 | NLRP3 | AIM2 | COX-2 |
| Mine, 2009 [18] | ions | GinE Ostb/Ostc | ↑ | ↑ | |||||
| Makihira, 2010 [16] | ions | GE-1 Epit | ↑ | ||||||
| Mano, 2013 [20] | par.: <25 nm | Epit | ↑ | ||||||
| Pan, 2017 [22] | ions | Fib | ↑ | ||||||
| Pettersson, 2017 [23] | ions | Mac | ↑ | ↑ | |||||
| Carrillo-Gàlvez et al., 2024 [32] | ions | HMSC | ↑ | ||||||
| Wakuda, 2025 [33] | par.: (25 µm) | Epit | ↑ | ↑ | ↑ | ||||
| TOTAL | 2 | 1 | 1 | 3 | 3 | 1 | |||
| In Vivo Studies | |||||||||
| Author, Year | Ti Particle/Ions | Tissues | TLR4 | RANKL | OPG | CASP-1,9,3 | NLRP3 | AIM2 | COX-2 |
| Berryman, 2020 [37] | - | PIT biopsy | ↑ | ||||||
| TOTAL | 1 | ||||||||
| In Vitro Studies | ||||||
|---|---|---|---|---|---|---|
| Author, Year | Ti Particles/Ions Size | Cell Type | CCR7 | MCP-1 | TGF-B | VEGF |
| Chan, 2009 [15] | Ti ions | DC | ↓ | ↓ | ||
| Irshad, 2013 [19] | par.: 5 µm | Fib | ↑ | |||
| Toledano-Serrabona, 2022 [26] | par.: (30–70 nm) | Mac | ↓ | ↓ | ||
| Callejas, 2022 [28] | Ti part (5–30 µm) | Mac | ↓ * | ↑ | ||
| Nemec, 2022 [30] | part.: (100 nm) | GSC | ↓ | |||
| Wakuda, 2025 [33] | par.: (25 µm) | Epit | ↑ | |||
| TOTAL | 3 | 2 | 4 | |||
| In Vivo Studies | ||||||
| Berryman, 2020 [37] | - | PIT Biopsy | ↑ | |||
| Rakic, 2022 [38] | - | PIT Biopsy | ↑ | |||
| Rakic, 2024 [40] | - | PIT Biopsy PICF | ↑ * | |||
| TOTAL | 1 | 2 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furlanetto, M.; Castro, R.; Silva, F.; Pereira, J.; Macedo, J.; Soares, S. Titanium Particle Impact on Immune Cells, Cytokines, and Inflammasomes: Helping to Profile Peri-Implantitis—A Systematic Review. Oral 2025, 5, 80. https://doi.org/10.3390/oral5040080
Furlanetto M, Castro R, Silva F, Pereira J, Macedo J, Soares S. Titanium Particle Impact on Immune Cells, Cytokines, and Inflammasomes: Helping to Profile Peri-Implantitis—A Systematic Review. Oral. 2025; 5(4):80. https://doi.org/10.3390/oral5040080
Chicago/Turabian StyleFurlanetto, Marco, Rita Castro, Fátima Silva, Jorge Pereira, José Macedo, and Sandra Soares. 2025. "Titanium Particle Impact on Immune Cells, Cytokines, and Inflammasomes: Helping to Profile Peri-Implantitis—A Systematic Review" Oral 5, no. 4: 80. https://doi.org/10.3390/oral5040080
APA StyleFurlanetto, M., Castro, R., Silva, F., Pereira, J., Macedo, J., & Soares, S. (2025). Titanium Particle Impact on Immune Cells, Cytokines, and Inflammasomes: Helping to Profile Peri-Implantitis—A Systematic Review. Oral, 5(4), 80. https://doi.org/10.3390/oral5040080





