Abstract
Objective: The purpose of this study was to compare the flexural strength, flexural modulus, hardness, and surface roughness of one brand each of 3D-printed and heat-cured acrylic resin materials after they were immersed in various disinfection solutions. Methods: The study included 160 specimens, consisting of 80 heat-cured and 80 3D-printed specimens. Forty specimens of each resin material type were prepared for flexural testing, while an additional forty specimens were designated for hardness and surface roughness assessments. Each collection of 40 specimens was subsequently randomized into four subgroups (n = 10) for immersion in either distilled water (control), 1% sodium hypochlorite, Superdent, or Kin Oro denture cleansers. Flexural test, hardness, and surface roughness assessments were performed. Data analysis was conducted using SPSS, with a level of significance set at p < 0.05. Results: Flexural strength and surface roughness did not differ significantly between the two resin types. Flexural modulus was significantly higher in the heat-cured resin among all the disinfectants (p = 0.000). The heat-cured resin had significantly higher microhardness than the 3D-printed resin among the disinfectants except for the Kin Oro group, and both resins showed a significant reduction in hardness after immersion in disinfectants compared to distilled water (p < 0.05). Conclusions: The heat-cured resin demonstrated higher flexural modulus and surface hardness compared to the 3D-printed resin. Flexural strength and surface roughness were comparable between the two materials. Both resins had their highest mechanical properties in distilled water.
1. Introduction
Computer-aided design (CAD) and computer-aided manufacturing (CAM) technologies have brought substantial progress and innovation to dental practices. The procedure of constructing complete dentures via digital technology entails the digitization of clinical data recorded from the patient’s mouth, the digital design of complete dentures using software, and an automatic manufacturing process [1]. CAD/CAM techniques offer precise restorations while greatly minimizing production time and manual effort compared to traditional methods. Additionally, these systems can be used to quickly create replacement prostheses using stored data, which reduces the number of visits and enhances overall comfort of the patient [2,3]. 3D printing has become a valuable technology in dentistry, reducing costs and allowing for customized approaches based on the needs of patients [4]. Additive manufacturing, or 3D printing, is the process in which multiple layers of material are added one by one under computer control to create an object, and it has recently gained popularity for constructing removable dental prostheses [5,6]. Studies show that 3D-printed dentures have better retention than compression-molded heat-cured denture base resin [7,8]. Despite these benefits, the mechanical strength and clinical reliability of 3D-printed denture bases continue to be subjects of ongoing research.
The properties that are investigated in this study have a direct impact on both prosthesis durability and oral health. Therefore, evaluating these properties is essential for assessing the clinical reliability of 3D-printed denture base materials. Superior mechanical properties are essential for ensuring the durability and functional performance of appliances. A denture is subjected to masticatory forces on an ongoing basis that may lead to cracking and fracture. As a result, a high flexural strength is an essential variable in the prevention of fractures due to load [9]. A number of investigations indicate that heat-cured resins exhibit higher flexural strength than 3D-printed resins [10,11,12,13,14,15,16,17,18,19,20,21,22,23], while one study reports only insignificantly higher values for heat-cured resin [24]. Conversely, a few studies show insignificantly higher flexural strength for 3D-printed resins [25,26,27], and others demonstrate significantly higher flexural strength for 3D-printed resins [28,29]. Additionally, microhardness is a crucial property of dental materials, as it directly influences their resistance to surface abrasion and indentation during routine cleaning and functional use [30,31]. Several studies show that heat-cured resin has higher hardness than 3D-printed resin [10,12,15,16,17,20,21,23,26,32,33,34]. Meanwhile, some studies show comparable hardness between the two types of resins [19,28]. On the other hand, some studies show a significantly higher hardness for 3D-printed resin [24,35]. Moreover, surface roughness is an essential feature of denture surfaces, rougher surfaces are more likely to promote microbial adhesion and bacterial colonization and the creation of areas that lead to food retention. As a result, these factors may result in diseases of the tissues underneath. Additionally, surfaces that are rough are more susceptible to staining [31,36]. Some studies show that 3D-printed resin has higher surface roughness than heat-cured resin [19,22,33,34]. On the other hand, other studies report a higher surface roughness for heat-cured resin [23,35]. While other studies show insignificantly higher surface roughness values for heat-cured resin compared to 3D-printed resin [10,37]. These contradicting findings show variability in material performance and testing conditions, implying that the mechanical behavior of 3D-printed resins in comparison to conventional heat-cured polymethyl methacrylate (PMMA) is not yet fully known.
Adequate denture hygiene is fundamental in maintaining the health of oral mucosa. The use of denture cleansers helps to prevent infections from bacteria and fungi, which are common contributors to denture-related stomatitis [38,39]. However, numerous studies have shown that they can also degrade the mechanical and physical properties of polymer-based materials. Specifically, exposure to sodium hypochlorite and effervescent peroxide solutions has been associated with reduced flexural strength, reduced hardness, and increased surface roughness [13,16,19,34,35,40,41,42,43,44,45,46,47,48,49]. While evidence exists on the impact of disinfectants on heat-cured PMMA, there is limited data on 3D-printed resins, and direct comparisons between the two fabrication methods under similar conditions across numerous properties are scarce. This constitutes a critical gap, as disinfection is a routine procedure, and adopting 3D-printed resins as denture bases requires robust evidence whether these materials can withstand repeated chemical exposure to ensure patient safety and denture longevity and prevent surface deterioration that promotes microbial retention. Therefore, it is crucial to evaluate the performance of these new materials under such conditions. The successful transition of 3D printing into clinical applications in dentistry depends heavily on the materials used, which must not only meet the required accuracy but also exhibit the necessary biological, mechanical, and physical properties. Therefore, this study aimed to compare the flexural strength, flexural modulus, hardness, and surface roughness of heat-cured and 3D-printed acrylic resin materials after immersion in various disinfectants, as these may impact the mechanical and physical properties. The results of this study will enhance the understanding of material performance under simulated clinical conditions and will help guide the appropriate choice of materials and maintenance protocols for removable prostheses. Although only one brand per fabrication method was tested, these were selected based on widespread availability and established reputation for reliable quality; also, the SprintRay resin is FDA-approved, which further supports its clinical applicability. Nonetheless, further multi-brand studies are warranted to validate and generalize the findings of this study. The null hypothesis was that no significant differences would exist in flexural strength, flexural modulus, hardness, and surface roughness between heat-cured and 3D-printed acrylic resins after immersion in different disinfectants, and that immersion in disinfectant solutions would not significantly affect these properties in either resin type.
2. Materials and Methods
2.1. Preparation of Specimens
One hundred sixty specimens were prepared, consisting of eighty heat-cured and eighty 3D-printed specimens. Forty specimens of each resin material type were prepared for flexural testing, measuring 64 mm × 10 mm × 3.3 mm ± 0.2 mm in accordance with ISO 20795-1:2013 standards [50], while an additional forty specimens were designated for hardness and surface roughness assessments, measuring 35 mm × 35 mm × 6 mm per ASTM D2240 standards [51]. Each set of 40 specimens was subsequently randomized into four subgroups (n = 10) for immersion in various disinfectant solutions. Using G*Power (v3.1.9.4, Germany), the sample size was estimated with an effect size of 0.5, a significance level of 0.05, and a statistical power of 80% from a previous study [34]. The calculation indicated that nine specimens per group were needed. However, to enhance reliability, ten specimens per group were selected.
Metallic moulds were used to prepare the heat-cured specimens for the flexural test. The heat-cured resin (SR Triplex Hot®; Ivoclar Vivadent, Schaan, Liechtenstein) was prepared following the manufacturer’s instructions. The material was packed into the molds, with metallic plates placed between each mold. The assembly was secured using bolts, washers, and nuts and placed under hydraulic pressure. Simultaneously, screws were tightened, and the metallic sheets were fastened using four metallic clamps. Curing was performed according to the manufacturer’s recommended cycle: the assembly was immersed in cold water, the temperature was gradually raised to boiling, and maintained at boiling for 45 min. The assembly was then removed from the water and allowed to bench-cool overnight. The heat-cured acrylic resin specimens for the hardness and surface roughness tests were fabricated using the conventional flasking technique.
The 3D-printed specimens were designed using CAD software (Blender v3.6.0; Blender Foundation, Amsterdam, Netherlands), which was exported in Standard Tessellation Language (STL) format. The STL file was imported into the 3D printer software (RayWare, v2.9.1; SprintRay Inc., Los Angeles, CA, USA) for slicing and arrangement on the build platform. The print command was then sent to a Digital Light Processing (DLP) printer (SprintRay Pro 95; SprintRay Inc., Los Angeles, CA, USA) to print the specimens using SprintRay EU High Impact Denture Base, Light Pink resin (Pro3dure medical GmbH, Iserlohn, Germany) at a 0-degree print orientation without using supports. The printer uses a 405 nm LED light source. Printing was performed at the default exposure time recommended in the RayWare software for this resin. For the flexural specimens, twenty specimens were printed at a time with print parameters of 0.1 mm layer thickness, print time of 10 min, and resin consumption of 38 mL. For the hardness and roughness specimens, eight specimens were printed at a time with print parameters of 0.1 mm layer thickness, print time of 18 min, and resin consumption of 59 mL. Following printing, the specimens were detached from the build platform using a removal tool and cleaned in a washing unit (Pro Wash/Dry; SprintRay Inc., Los Angeles, CA, USA) containing 99% isopropyl alcohol for 10 min. Subsequently, they were placed in a post-curing unit (ProCure; SprintRay Inc., Los Angeles, CA, USA) equipped with 405 nm LED arrays and a 360° reflective interior. The curing was performed for 5 min at 60 °C, following the manufacturer’s recommendations.
All the specimens were finished sequentially with sandpaper grits 400, 1000, and 2000 without using water [13,14,16]. Each surface was polished with 20 uniform passes per grit to ensure consistent finishing across all specimens. After finishing, they were rinsed with tap water and then polished with brown Tripoli polishing compound using a wet cotton buffing wheel on a lathe operating at 1500 rpm for 30 s. All the specimens were prepared, finished, and polished by the same operator to minimize variability. To confirm dimensional uniformity, the specimens were measured with a digital caliper.
2.2. Immersion Solutions and Protocol
Three disinfectant solutions, along with distilled water as a control, were used. Table 1 shows the immersion solutions, their preparation, and immersion procedures as specified by the manufacturers. All specimens were immersed in the disinfectants 180 times, nine cleaning cycles were carried out daily for a period of 20 days, thereby simulating 180 days of cleansing [16,34,37,52]. Each type of resin specimen was disinfected separately, using 400 mL of solution per 20 specimens. Regarding the sodium hypochlorite (NaOCl), 400 mL of 1% NaOCl was used to immerse the specimens per cleaning cycle. For the tablets, 400 mL of water was added to the container to fully immerse the specimens, then 2 denture tablets were added. Following each cleaning cycle, the specimens were rinsed for half a minute under tap water after being removed from the disinfection solutions. A new solution was prepared for each cleaning cycle. A beaker was used to measure the solution volumes. The timing of the immersion and washing procedures was controlled using a timer. A digital liquid thermometer was used to measure the temperature of the water before use, which was aimed to be 35 ± 5 °C. When the specimens were not being disinfected, they were kept in distilled water at room temperature. In order to prevent operator variability, the disinfection procedure was executed by a single operator.

Table 1.
Immersion solutions, their preparation, and immersion procedures.
2.3. Flexural Test
A three-point bending test, conducted in accordance with ISO guidelines, was used to measure flexural properties, including strength and flexural modulus utilizing a Universal Testing Machine (Cussons Technology Co. Ltd., Manchester, UK). Compressive force was applied to the specimens at a speed of 5.0 mm/min until fracture occurred, with a distance of 50 mm between the supports [50], as shown in (Figure 1). The data was collected, calculated, and read automatically by use of computer software supported by the testing machine company. The specimens were taken out from the solution, dried with a paper towel, and immediately tested to simulate moisture in the oral cavity. The testing procedure was done in ambient conditions of 23 ± 2 °C and 50 ± 5% relative humidity. The specimens after flexural testing are shown in (Figure 2).
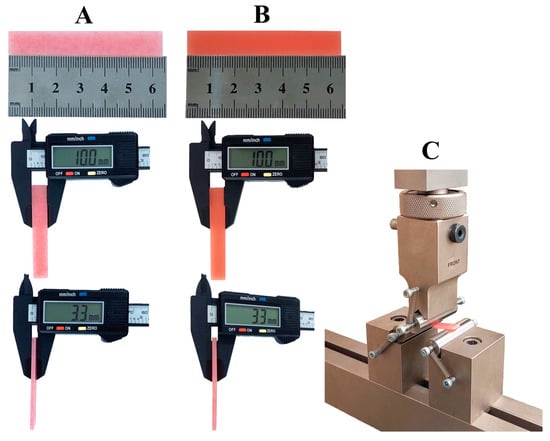
Figure 1.
Flexural testing of specimens. (A) Heat-cured specimen dimensions; (B) 3D-printed specimen dimensions; (C) universal testing machine setup.

Figure 2.
(A) Heat-cured specimens after flexural testing; (B) 3D-printed specimens after flexural testing.
2.4. Microhardness Test
The microhardness test was performed using a Shore-D durometer tester (FstDgte Co. Ltd., China), held by hand. The durometer was placed on the specimen so that its flat base made full contact with the acrylic resin surface, and the reading was recorded within 1 s. Five readings were taken in different areas of the specimen, where each point should be at least six millimeters away from each other and each of them at least 12 mm away from the specimen edges [51], as shown in (Figure 3), and the average of these five readings was calculated [53,54].
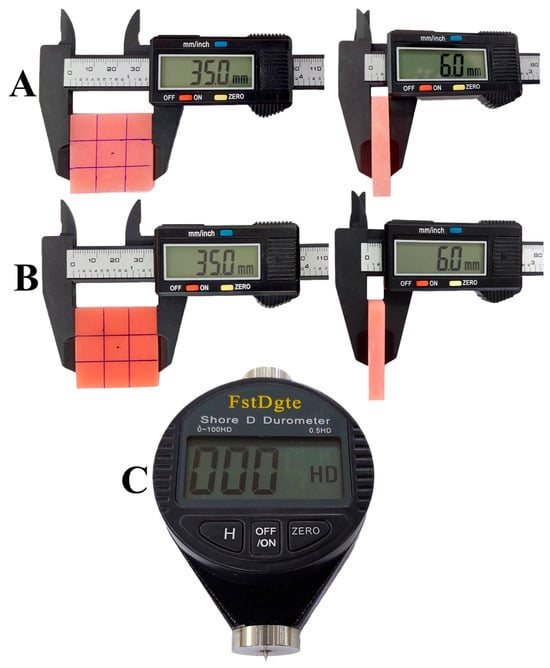
Figure 3.
Microhardness testing. (A) Heat-cured specimen ready for testing; (B) 3D-printed specimen ready for testing; (C) hand-held Shore-D durometer.
2.5. Surface Roughness Test
Surface roughness measurements were conducted using a contact profilometer (SRT 6200 Surface Roughness Tester, Guangzhou Landtek Instruments Co., Ltd., Guangzhou, China) with an accuracy of 0.001 µm. The device’s probe tip traverses along the surface of the specimens, and four readings were taken at different areas in different directions at a cutoff of 0.8 mm. The average roughness (Ra) was then calculated based on the data obtained from these four areas [37]. Prior to measurements, the profilometer was calibrated using the certified reference standard with known roughness values provided with the device.
2.6. Randomization and Blinding
Following the immersion protocol and prior to testing, each group was labeled with a letter. The tester performing all measurements was blinded to both the resin type and the immersion solution corresponding to each specimen, minimizing potential bias and ensuring objective, reliable results.
2.7. Statistical Analysis
SPSS software (v25.0, IBM Corp., Armonk, NY, USA) was used to analyze the data. Descriptive statistics were expressed as mean ± standard deviation (SD). Data distribution was assessed using the Shapiro–Wilk test. The independent samples t-test was employed to compare the two resin types within each disinfectant solution for normally distributed data, or Mann–Whitney U when the normality assumption was not observed. The assumption of homogeneity of variances was evaluated using Levene’s test. One-way analysis of variance (ANOVA) was employed to compare the mean values among disinfectant groups within each resin type for normally distributed data. The Tukey test was employed to conduct post hoc pairwise comparisons when significant differences were identified. The Kruskal–Wallis test was utilized for data that was not normally distributed. p < 0.05 was considered statistically significant.
3. Results
Table 2 summarizes the mean, SD, and significance levels for the tested properties across the groups.

Table 2.
Mean ± SD and statistical comparison of the two resins under different disinfectants for the tested properties.
3.1. Flexural Strength and Flexural Modulus
A Shapiro–Wilk test showed normal distribution for flexural test data in all groups except Kin Oro group. In the heat-cured group, flexural modulus data were non-normally distributed (p = 0.002), while in the 3D-printed group, flexural strength data violated normality (p = 0.001). The heat-cured resin exhibited higher mean flexural strength values across all immersion media in comparison to the 3D-printed resin; however, the differences were not statistically significant (p > 0.05). Effect sizes (Cohen’s d) indicated large effects for distilled water (0.91) and Superdent (0.90), a medium–large effect for sodium hypochlorite (0.73), and a medium effect for Kin Oro (0.61). Flexural strength did not differ significantly among the immersion groups for either the heat-cured (p = 0.445) or the 3D-printed resins (p = 0.204). For both resins, the distilled water group exhibited the highest flexural strength. For the heat-cured resin, the effect size was medium (partial η2 = 0.071) among the immersions, while for the 3D-printed resin, the Kruskal–Wallis effect size was small (ε2 = 0.0443).
The heat-cured resin demonstrated higher flexural modulus values than the 3D-printed resin across all immersion solutions, with significant differences in all groups (p = 0.000) shown in (Figure 4). The effect sizes (Cohen’s d) between the two resin types were extremely large across all immersion groups: distilled water (5.07), sodium hypochlorite (4.85), Superdent (4.44), and Kin Oro (4.87). Flexural modulus did not vary significantly across immersion groups for both the heat-cured (p = 0.484) and 3D-printed resins (p = 0.763). The effect size among the immersions for the heat-cured resin was negligible (Kruskal–Wallis ε2 = 0.000), while for the 3D-printed resin the effect size was small (partial η2 = 0.031).
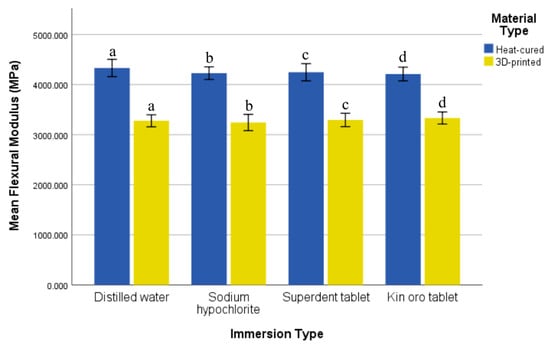
Figure 4.
Comparison of mean flexural modulus (MPa) and standard deviation of the two resin types after immersion in various solutions. Similar letters indicate statistical significance.
3.2. Microhardness
A Shapiro–Wilk test showed normally distributed data for both types of resin across all immersion groups (p > 0.05). The heat-cured resin exhibited higher hardness values than the 3D-printed resin in all immersion groups, with significant differences observed for distilled water (p = 0.000), sodium hypochlorite (p = 0.000), and Superdent tablet (p = 0.009). The effect sizes (Cohen’s d) between the two resin types were very large for distilled water (3.31) and sodium hypochlorite (3.03), large for Superdent (1.31), and large for Kin Oro (0.81). Hardness varied significantly among immersion groups for both the heat-cured (p = 0.000) and 3D-printed resins (p = 0.001). In the case of the heat-cured resin, the highest mean hardness was observed in the distilled water group (91.73 ± 1.08), and the lowest was in the Kin Oro tablet group (88.81 ± 1.13). Pairwise comparisons showed that distilled water had significantly higher hardness compared to sodium hypochlorite (p = 0.007), Superdent tablet (p = 0.001), and Kin Oro tablet (p = 0.000). Among the 3D-printed resin groups, the greatest mean hardness was observed in the distilled water group (88.84 ± 0.60), while the lowest was in the sodium hypochlorite group (86.97 ± 0.99). Pairwise comparisons showed that distilled water exhibited significantly higher hardness compared to sodium hypochlorite (p = 0.000), but hardness did not vary significantly between distilled water and Superdent tablet (p = 0.308) or Kin Oro tablet (p = 0.113). Moreover, the Superdent tablet exhibited significantly higher hardness than sodium hypochlorite (p = 0.044) shown in (Figure 5). Among immersion solutions, hardness showed large effect sizes for both resin types, with partial η2 = 0.486 for the heat-cured resin and partial η2 = 0.366 for the 3D-printed resin.
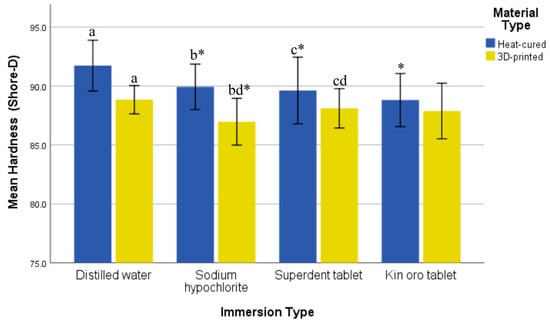
Figure 5.
Comparison of mean hardness (Shore-D) and standard deviation of the two resin types after immersion in various solutions. Similar letters indicate statistical significance. * indicates significance compared with the distilled water control group of the corresponding resin.
3.3. Surface Roughness
A Shapiro–Wilk test showed normally distributed data for both types of resin across all immersion groups (p > 0.05). Although the 3D-printed resin exhibited higher mean surface roughness than the heat-cured resin, the differences across all solutions were not significant (p > 0.05). The effect sizes (Cohen’s d) between the two resin types were small for distilled water (0.36), negligible for sodium hypochlorite (0.02), and large for Superdent (0.85) and Kin Oro (0.79). Surface roughness did not vary significantly among the immersion groups for the heat-cured resin (p = 0.677), whereas there was a significant variation among the immersion groups for the 3D-printed resin (p = 0.043). Despite this, the Tukey HSD test did not reveal significant differences between specific immersion groups (p > 0.05), although comparisons involving Superdent and Kin Oro tablets approached significance. The lowest mean surface roughness value was observed in the distilled water group for both resins. The effect size among the immersions for the heat-cured resin was small (partial η2 = 0.041), while for the 3D-printed resin the effect size was large (partial η2 = 0.201).
4. Discussion
This study measured and compared the flexural strength, flexural modulus, hardness, and surface roughness of one brand each of 3D-printed and heat-cured acrylic resin materials following immersion in various disinfection solutions for a period that simulated six months of clinical use. The findings revealed that immersion in disinfectant solutions affected the tested properties of both denture base materials to varying degrees. While the heat-cured resin exhibited significantly higher flexural modulus and surface hardness values compared to the 3D-printed resin, there was no significant variation in flexural strength or surface roughness. Therefore, the null hypothesis was partially rejected.
Heat-cured resin incorporates a cross-linking agent that forms a cross-linked polymer network, restricting the movement of polymer chains under stress and thus strengthening the mechanical behavior of the material [55]. Alternatively, 3D-printed resins are composed of a blend of oligomers and monomers that are polymerized by ultraviolet light to produce a cross-linked polymer structure. Nevertheless, the presence of residual uncured monomers necessitates a post-curing process to finalize polymerization and cross-linking [14,56,57]. Denture cleansers containing enzymes and oxidizing agents may expand intermolecular spaces, promoting leakage of degraded materials and penetration of water and chemicals into the resin matrix during immersion. Prolonged immersion may lead to surface expansion and irreversible deformation [16,34]. The mechanical properties of 3D printing resins are influenced by their internal structure and various parameters, including orientation of the layers, software and printer type, number and thickness of the layers, and post-processing protocol [12,58].
ISO regulations require a minimum flexural strength of 65 MPa [50]. Flexural strength may indicate the resin’s degree of polymerization, as a low degree of conversion will lead to mechanical properties that are inferior [59]. In the current study, the heat-cured resin exhibited higher flexural strength than the 3D-printed specimens across all immersion conditions, although the differences were not statistically significant. This trend is consistent with previous findings [10,13,14,15,17,19,21,23]. However, these earlier studies demonstrated statistically significant differences, which may be attributed to differences in resin formulations and 3D-printing parameters such as layer thickness, print orientation, and post-curing protocols [60]. Specimens utilized for this research were manufactured by 3D printing using a 0° orientation, or flat to the build platform. In the flexural test, this printing direction positions the printed layers perpendicular to the loading surface and parallel to the specimen’s length. Because tensile stresses are usually the weakest points in additively manufactured materials, the applied stress primarily acts along continuous printed layers, reducing the exposure of interlayer interfaces to these stresses. Therefore, when compared to other orientations like 45° or 90°, where interlayer bonding interfaces are more severely stressed during bending, the 0° printing orientation is linked to increased flexural strength [61]. According to a study, the flexural properties of the 3D-printed resin were influenced by varying washing periods and post-polymerization devices [62]. Similarly, increasing postcuring time, the flexural strength increases [61]. Generally, the lower strength of 3D-printed specimens in comparison to heat-cured may be due to variations in polymerization mechanisms and microstructural characteristics between the two materials. The polymerization of heat-cured acrylic resin occurs under controlled heat and pressure, resulting in more conversion of monomer to produce a homogeneous continuous polymer matrix. In contrast, 3D-printed resins are fabricated layer by layer through photopolymerization that creates weak interlayer bonds and incomplete conversion of resin double bonds, leaving residual monomer even after post-curing [14,15,23,63]. This difference in material behavior was also reflected in the fracture patterns observed during the flexural test. The heat-cured specimens typically exhibited a clean, sharp fracture line, indicative of a more homogeneous and cohesive material structure that fails uniformly under applied stress. In contrast, the 3D-printed specimens displayed irregular fracture sites with small, fragmented pieces separating from the main fracture area, as shown in (Figure 2). This fracture behavior can be attributed to the weaker interlayer bonding and the presence of microdefects within the printed material, which enable the initiation and propagation of cracks in a less predictable manner. Furthermore, the 3D-printed resins exhibit a higher water absorption rate than the heat-cured resins [64], which may contribute to their reduced flexural strength. Although the 3D-printed material used had a lower flexural strength value, it met the ISO recommendation of more than 65 MPa. Therefore, it can be considered as an option when fabricating denture bases in terms of flexural strength, especially when advantages like faster fabrication, digital workflow, and greater customization are prioritized. Additionally, no statistically significant effect of immersion medium on flexural strength was observed within either the heat-cured or 3D-printed groups. This finding contrasts with other studies [13,16,19,46]. The lack of significance in the current study could be attributed to immersion periods of shorter duration, different disinfectant types, and improvements in the formulation of both conventional and 3D-printed resins. Both resins achieved their highest flexural strength values in the distilled water group. This observation aligns with previous research by Elhagali et al. [19], who stated that mechanical properties are least affected by water immersion because of the absence of aggressive chemical substances. The effect sizes for flexural strength between resin types were medium to large across all immersion solutions, showing that the heat-cured resin was stronger than the 3D-printed resin. Clinically, this shows that dentures made of heat-cured resin may be more resistant to fracture under masticatory stresses, whereas 3D-printed dentures may be more susceptible to breaking when subjected to high functional loads. Among immersion solutions, effect sizes were negligible for the heat-cured resin and small for the 3D-printed resin, indicating that within the relatively short immersion period of this study, disinfectant type had little practical impact on flexural strength, and resin selection is the most important determinant of durability.
Flexural modulus is a material property that measures how stiff or rigid a material is, a higher modulus reflects greater material stiffness. An ideal denture material combines high rigidity to resist stress with adequate flexibility to spread the load uniformly, thereby reducing the risk of fracture [15]. In this study, the results revealed that the heat-cured resin had a significantly higher flexural modulus in comparison to the 3D-printed specimens under all immersion conditions. This finding aligns with other studies [13,19,21], since heat-cured resins are cured thermally, which produces a polymer structure that is highly cross-linked. The flexural modulus in this study exceeded ISO’s recommended value of 2000 MPa [50]. The intrinsic properties of additive manufacturing, such as incomplete polymerization, deficiencies in bonding between the layers, and anisotropic mechanical behavior brought on by the layering process, may be the cause of the lower flexural modulus of 3D-printed resins. Interestingly, the immersion medium did not significantly influence the flexural modulus within either material group, in contrast to the study conducted by Alkaltham et al. [16], this may be due to using different resin brands and shorter immersion duration of this study. The effect sizes for flexural modulus between resin types were extremely large, indicating a major difference in stiffness between the two resins. Clinically, this means that heat-cured dentures may hold their shape better under functional loads, whereas 3D-printed dentures may deform more easily, potentially impacting fit and occlusion. The effect sizes among immersion solutions were small to negligible, indicating that the type of disinfection solution had less influence on stiffness than the resin material.
Hardness refers to a material’s resistance to permanent surface deformation or indentation [65]. It is a key factor influencing prosthesis durability and resistance to surface wear. The findings demonstrated that the heat-cured resin exhibited significantly higher hardness than the 3D-printed specimens in most immersion conditions. This is in agreement with previous findings [10,15,16,17,20,21,23,32,33,34]. On the other hand, Elhagali et al. [19] and Altarazi et al. [28] reported that heat-cured resin exhibited an insignificantly higher hardness than 3D-printed resin. This may be attributed to differences in resin brands, different printing parameters, or the types of disinfectants. The reduced hardness observed in the 3D-printed specimens may be due to differences in polymerization mechanisms, degree of conversion, and the inherent layering structure of additive manufacturing processes, which can introduce weak interlayer bonding and higher susceptibility to surface degradation following chemical exposure. Conversely, El-Olimy et al. [35] and Al-Ameri et al. [24] stated that the hardness of 3D-printed resin was higher than heat-cured. Therefore, different material brands may contribute to differences in mechanical properties such as hardness. In addition, the use of different printers and printing parameters can further influence these properties. Furthermore, both resin types exhibited significant differences in hardness among the immersion groups, which aligns with previous studies [16,19,34,49]. Regarding the heat-cured resin, Kin Oro group had the least hardness. This result supports the findings of Amin et al. [66], which demonstrated that these chemical disinfectants have plasticizer leaching that diffuses between the polymer chains, causing matrix degradation and subsequently affecting the hardness. Denture tablets are alkaline peroxides and contain components that release oxygen, such as sodium perborate and bicarbonate. These substances dissolve in water and produce hydrogen peroxide solutions, releasing oxygen. For the 3D-printed resin, the lowest hardness was noted in the sodium hypochlorite group, which is in accordance with the studies conducted by Alqanas et al. [34] and Alkaltham et al. [16], a finding consistent with the known oxidizing effects of hypochlorite, which can cause surface degradation and reduce material hardness. Conversely, another study found that sodium hypochlorite had no significant effect on hardness in comparison to distilled water [67]. The inconsistencies in results might be attributed to differences in material brands and immersion protocols, particularly time and concentration. The effect sizes for hardness between resin types ranged from large to very large, demonstrating that the heat-cured resin is considerably harder than the 3D-printed resin. Clinically, increasing hardness may result in superior wear resistance and a longer service life for heat-cured dentures. In immersion solutions, both resin types had large effect sizes, implying that disinfectant type can influence surface hardness; however, the effect is more pronounced in heat-cured resin. This may help clinicians select disinfectants that maintain the mechanical integrity of denture surfaces.
Surface roughness is a key property of resin materials that promotes bacterial colonization. As surface roughness increases, denture cleansing efficacy decreases and bacterial accumulation rises [68]. The clinical threshold for microbial adhesion is 0.2 µm [23]. The surface roughness values with the polishing protocol in this study were less than the clinical threshold. In this study, surface roughness did not differ significantly between the two resin types after exposure to the various disinfectants. This aligns with the findings of Takhtdar et al. [37] and Çakmak et al. [52]. Though 3D-printed specimens generally exhibited higher surface roughness values than heat-cured ones across all immersion media. This may be explained by the inherent layering effect of additive manufacturing, which is susceptible to surface alterations upon exposure to chemical agents. This result opposes those of El-Olimy et al. [35], Al-Dwairi et al. [10], and Gad et al. [69], where the 3D-printed resin exhibited better surface smoothness compared to the heat-cured resin. This may be related to different polishing protocol or different material brands. Additionally, while the current study observed a significant difference in surface roughness within the 3D-printed group across different disinfectants, no such difference was observed within the heat-cured group. However, this result opposes a study by Fotovat et al. [70], which found significant differences among the disinfectants for the heat-cured resin but not in the 3D-printed resin. Other studies by Alqanas et al. [34], El-Olimy et al. [35], Takhtdar et al. [37] and Mahmoud et al. [13] found significant differences in surface roughness following immersion in disinfectants. On the other hand, some studies state a non-significant change in surface roughness [19,52]. These discrepancies may be due to differences in material composition and brand, printer technology, post-curing processes, or immersion durations employed. Variations in specimen preparation and roughness measuring methodology may also have influenced the results. It was observed that immersion in sodium hypochlorite resulted in higher surface roughness values in heat-cured denture base resins compared to other disinfectants. This finding can be attributed to the oxidative nature of sodium hypochlorite, which has been reported to degrade the surface integrity of PMMA by breaking polymer chains and increasing surface porosity and irregularities. The alkaline pH and strong oxidizing capacity of sodium hypochlorite contribute to hydrolysis and surface softening of acrylic resins. Conversely, for the 3D-printed denture base material, the tablet-based disinfectants produced the highest surface roughness values. This could be explained by the different chemical composition and polymer network structure of 3D-printed resins, which are typically based on photopolymerized urethane dimethacrylate (UDMA) or similar monomers with varying degrees of cross-linking. The effervescent tablet solutions may interact more aggressively with the 3D-printed resin surface, possibly causing leaching of residual monomers or softening of the polymer matrix, leading to increased surface irregularities. Moreover, previous studies have suggested that 3D-printed resins exhibit lower surface hardness and higher water sorption compared to conventional heat-cured PMMA, which may predispose them to greater surface changes upon immersion in certain disinfectant solutions [12,28,64,71,72]. Effect sizes for surface roughness between resin types ranged from negligible to large, with the 3D-printed resin being rougher than the heat-cured resin. Clinically, greater surface roughness can encourage biofilm accumulation and compromise oral hygiene. In addition, it can harbor more stains, decrease denture aesthetics, and shorten service life. In immersion solutions, heat-cured resin had a small effect, however 3D-printed resin had a large effect, showing that some disinfectants may alter surface smoothness in 3D-printed dentures. This emphasizes the importance of carefully selecting both denture material and disinfection to reduce roughness and promote oral health.
The discrepancy between the current and previous findings from the literature could be attributed to variations in resin formulations, printing parameters, post-processing protocols, PMMA polymerization, polishing procedures, and immersion durations.
The findings of this study suggest that while heat-cured resins continue to exhibit superior mechanical properties, contemporary 3D-printed resins demonstrate acceptable performance in terms of flexural strength, hardness, and surface roughness. However, their clinical use should be addressed with caution, as further research is required to fully understand their long-term mechanical and surface behavior in oral conditions and under repeated functional loading. At the moment, their use may be most suited in instances where the benefits of faster manufacturing, digital workflow, and increased customization are valued. Additionally, the selection of disinfectant can have a subtle impact on surface properties. Clinicians should avoid the routine use of sodium hypochlorite for denture disinfection, as it was associated with reduced hardness in 3D-printed resin and increased surface roughness in heat-cured resin. Effervescent tablets should also be used cautiously with 3D-printed resins, as they were found to cause greater surface alterations.
The scope of this investigation was restricted to in vitro conditions that were distinct from oral conditions, like the absence of saliva that contains different enzymes and proteins, dietary intake, temperature changes, pH fluctuations, and occlusal stress, with immersion protocols that are somewhat short-term which does not adequately represent the long-term exposure encountered by dentures worn for several years. Moreover, flat specimens lack the anatomical features of real dentures, limiting their clinical relevance. In addition, reliance on Shore-D durometer might introduce operator variability. Furthermore, only one brand of each type of resin was utilized. Further research with multiple resin brands is needed to validate and generalize the findings, as well as to account for potential variations in resin formulations, manufacturing protocols, 3D printing technologies, printing orientations, and post-curing processes. Additionally, future studies should include long-term disinfection cycles, thermal cycling, fatigue testing, and wear testing to better simulate clinical use. Furthermore, more clinically relevant polishing methods should be applied, and findings should be validated through clinical trials to better reflect in vivo conditions.
5. Conclusions
Within the limitations of this in vitro study, it can be concluded that:
- The heat-cured resin showed superior flexural modulus and hardness relative to the 3D-printed resin, with statistical significance.
- Flexural strength and surface roughness did not differ significantly between the materials, although 3D-printed specimens tended to display slightly lower flexural strength and higher surface roughness values.
- Both heat-cured and 3D-printed acrylic resins exhibited clinically acceptable mechanical properties after the immersion protocol used in this study, which simulated six months of routine disinfection.
- Both resins exhibited the highest mechanical properties in distilled water, indicating minimal degradation in the absence of chemical disinfectants.
- Sodium hypochlorite caused the greatest reduction in hardness, particularly in the 3D-printed resin, and increased surface roughness in the heat-cured resin. Its routine use should be avoided.
- Effervescent tablets produced more surface changes in the 3D-printed resin.
Author Contributions
Conceptualization, S.A.K. and J.F.A.; methodology, S.A.K. and J.F.A.; software, S.A.K.; validation, S.A.K. and J.F.A.; formal analysis, S.A.K.; investigation, S.A.K.; resources, S.A.K.; data curation, S.A.K.; writing—original draft preparation, S.A.K.; writing—review and editing, J.F.A.; visualization, S.A.K.; supervision, J.F.A.; project administration, S.A.K. and J.F.A.; funding acquisition, S.A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 3D | Three-Dimensional |
| CAD | Computer-Aided Design |
| CAM | Computer-Aided Manufacturing |
| SPSS | Statistical Package for the Social Sciences |
| PMMA | Polymethyl methacrylate |
| NaOCl | Sodium hypochlorite |
| STL | Standard Tessellation Language |
| DLP | Digital Light Processing |
| SD | Standard Deviation |
| ANOVA | One-Way Analysis of Variance |
| MPa | Megapascal |
| µm | Micrometer |
| UDMA | Urethane dimethacrylate |
References
- Dusmukhamedov, S.; Lee, C.N.; Jeong, S.M.; Choi, B.H. Digital Denture Fabrication: A Technical Note. Appl. Sci. 2021, 11, 8093. [Google Scholar] [CrossRef]
- Hada, T.; Kanazawa, M.; Iwaki, M.; Arakida, T.; Soeda, Y.; Katheng, A.; Otake, R.; Minakuchi, S. Effect of Printing Direction on the Accuracy of 3D-Printed Dentures Using Stereolithography Technology. Materials 2020, 13, 3405. [Google Scholar] [CrossRef] [PubMed]
- Janeva, N.M.; Kovacevska, G.; Elencevski, S.; Panchevska, S.; Mijoska, A.; Lazarevska, B. Advantages of Cad/Cam versus Conventional Complete Dentures-a Review. Open Access Maced. J. Med. Sci. 2018, 6, 1498–1502. [Google Scholar] [CrossRef]
- Tian, Y.; Chen, C.X.; Xu, X.; Wang, J.; Hou, X.; Li, K.; Lu, X.; Shi, H.Y.; Lee, E.S.; Jiang, H.B. A Review of 3D Printing in Dentistry: Technologies, Affecting Factors, and Applications. Scanning 2021, 2021, 9950131. [Google Scholar] [CrossRef]
- Singh, S.; Dhawan, P.; Nautiyal, M. Application of Rapid Prototyping in Prosthodontics. Front. Biomed. Technol. 2022, 9, 237–245. [Google Scholar] [CrossRef]
- Revilla-León, M.; Özcan, M. Additive Manufacturing Technologies Used for Processing Polymers: Current Status and Potential Application in Prosthetic Dentistry. J. Prosthodont. 2019, 28, 146–158. [Google Scholar] [CrossRef]
- Qadir, G.; Abdulkareem, J. An In Vitro Comparative Study of Maxillary Denture Base Retention Between Conventional Fabrication and 3D Printed Techniques. Sulaimani Dent. J. 2023, 10, 45–53. [Google Scholar] [CrossRef]
- Emera, R.M.K.; Shady, M.; Alnajih, M.A. Comparison of Retention and Denture Base Adaptation between Conventional and 3D-Printed Complete Dentures. J. Dent. Res. Dent. Clin. Dent. Prospects 2022, 16, 179–185. [Google Scholar] [CrossRef]
- Srinivasan, M.; Gjengedal, H.; Cattani-Lorente, M.; Moussa, M.; Durual, S.; Schimmel, M.; Müller, F. CAD/CAM Milled Complete Removable Dental Prostheses: An in Vitro Evaluation of Biocompatibility, Mechanical Properties, and Surface Roughness. Dent. Mater. J. 2018, 37, 526–533. [Google Scholar] [CrossRef]
- Al-Dwairi, Z.N.; Al Haj Ebrahim, A.A.; Baba, N.Z. A Comparison of the Surface and Mechanical Properties of 3D Printable Denture-Base Resin Material and Conventional Polymethylmethacrylate (PMMA). J. Prosthodont. 2023, 32, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Al-Qarni, F.D.; Gad, M.M. Printing Accuracy and Flexural Properties of Different 3D-Printed Denture Base Resins. Materials 2022, 15, 2410. [Google Scholar] [CrossRef]
- Lourinho, C.; Salgado, H.; Correia, A.; Fonseca, P. Mechanical Properties of Polymethyl Methacrylate as Denture Base Material: Heat-Polymerized vs. 3D-Printed—Systematic Review and Meta-Analysis of In Vitro Studies. Biomedicines 2022, 10, 2565. [Google Scholar] [CrossRef]
- Mahmoud, M.Y.; Alshimy, A.M.; El-Shabrawy, S.M.; Hanno, K.I. Effect of Disinfectants on Flexure Strength and Surface Roughness of 3D-printed Versus Conventional Denture Base Resins (A Comparative in-vitro Study). Alexandria Dent. J. 2025, in press. [Google Scholar] [CrossRef]
- Perea-Lowery, L.; Gibreel, M.; Vallittu, P.K.; Lassila, L.V. 3D-Printed vs. Heat-Polymerizing and Autopolymerizing Denture Base Acrylic Resins. Materials 2021, 14, 5781. [Google Scholar] [CrossRef]
- Prpić, V.; Schauperl, Z.; Ćatić, A.; Dulčić, N.; Čimić, S. Comparison of Mechanical Properties of 3D-Printed, CAD/CAM, and Conventional Denture Base Materials. J. Prosthodont. 2020, 29, 524–528. [Google Scholar] [CrossRef]
- Alkaltham, N.S.; Aldhafiri, R.A.; Al-Thobity, A.M.; Alramadan, H.; Aljubran, H.; Ateeq, I.S.; Khan, S.Q.; Akhtar, S.; Gad, M.M. Effect of Denture Disinfectants on the Mechanical Performance of 3D-Printed Denture Base Materials. Polymers 2023, 15, 1175. [Google Scholar] [CrossRef] [PubMed]
- Bento, V.A.; Gomes, J.M.; Oliveira-Limirio, J.P.; Rosa, C.D.D.R.D.; Lemos, C.A.; Dos Santos, D.M.; Pellizzer, E.P. Effect of Aging on the Mechanical Properties of CAD/CAM–Milled and 3D-Printed Acrylic Resins for Denture Bases. Int. J. Prosthodont. 2024, 37, S5–S11. [Google Scholar] [CrossRef]
- Chhabra, M.; Nanditha Kumar, M.; RaghavendraSwamy, K.N.; Thippeswamy, H.M. Flexural Strength and Impact Strength of Heat-Cured Acrylic and 3D Printed Denture Base Resins—A Comparative in Vitro Study. J. Oral Biol. Craniofacial Res. 2022, 12, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Elhagali, A.F.; Sharaf, M.Y.; Abd El-Aziz, M.E.-S.A.; Ali Bayiumy, A.S.; Refaei, M.A.A.; Al-Agamy, A.H.; Ali, A.; Elakel, A.; Altayyar, R.; Alzahrani, R.; et al. The Effects of Different Chemical Disinfectants on the Strength, Surface, and Color Properties of Conventional and 3D-Printed Fabricated Denture Base Materials. Prosthesis 2025, 7, 24. [Google Scholar] [CrossRef]
- Falahchai, M.; Ghavami-Lahiji, M.; Rasaie, V.; Amin, M.; Asli, H.N. Comparison of Mechanical Properties, Surface Roughness, and Color Stability of 3D-Printed and Conventional Heat-Polymerizing Denture Base Materials. J. Prosthet. Dent. 2023, 130, e1–e266. [Google Scholar] [CrossRef]
- Fouda, S.M.; Gad, M.M.; Abualsaud, R.; Ellakany, P.; AlRumaih, H.S.; Khan, S.Q.; Akhtar, S.; Al-Qarni, F.D.; Al-Harbi, F.A. Flexural Properties and Hardness of CAD-CAM Denture Base Materials. J Prosthodont. 2023, 32, 318–324. [Google Scholar] [CrossRef]
- Freitas, R.F.C.P.D.; Duarte, S.; Feitosa, S.; Dutra, V.; Lin, W.S.; Panariello, B.H.D.; Carreiro, A.D.F.P. Physical, Mechanical, and Anti-Biofilm Formation Properties of CAD-CAM Milled or 3D Printed Denture Base Resins: In Vitro Analysis. J. Prosthodont. 2023, 32, 38–44. [Google Scholar] [CrossRef]
- Gad, M.M.; Fouda, S.M.; Abualsaud, R.; Alshahrani, F.A.; Al-Thobity, A.M.; Khan, S.Q.; Akhtar, S.; Ateeq, I.S.; Helal, M.A.; Al-Harbi, F.A. Strength and Surface Properties of a 3D-Printed Denture Base Polymer. J. Prosthodont. 2022, 31, 412–418. [Google Scholar] [CrossRef]
- Al-Ameri, A.; Alothman, O.Y.; Alsadon, O.; Bangalore, D. An In-Vitro Evaluation of Strength, Hardness, and Color Stability of Heat-Polymerized and 3D-Printed Denture Base Polymers After Aging. Polymers 2025, 17, 288. [Google Scholar] [CrossRef]
- Di Fiore, A.; Meneghello, R.; Brun, P.; Rosso, S.; Gattazzo, A.; Stellini, E.; Yilmaz, B. Comparison of the Flexural and Surface Properties of Milled, 3D-Printed, and Heat Polymerized PMMA Resins for Denture Bases: An in Vitro Study. J. Prosthodont. Res. 2022, 66, 502–508. [Google Scholar] [CrossRef]
- Neves, C.B.; Chasqueira, A.F.; Rebelo, P.; Fonseca, M.; Portugal, J.; Bettencourt, A. Microhardness and Flexural Strength of Two 3D-Printed Denture Base Resins. Rev. Port. Estomatol. Med. Dent. Cir. Maxilofac. 2022, 63, 198–203. [Google Scholar] [CrossRef]
- Sonam, D.; Dayalan, M.; Rahath Fatima, S.; K, S. Comparative Evaluation of Impact and Flexural Strength of 3D Printed, CAD/CAM Milled and Heat Activated Poylmethyl Methacrylate Resins—An In-Vitro Study. Int. J. Sci. Res. 2021, 10, 194–202. [Google Scholar] [CrossRef]
- Altarazi, A.; Haider, J.; Alhotan, A.; Silikas, N.; Devlin, H. Impact of Artificial Aging on the Physical and Mechanical Characteristics of Denture Base Materials Fabricated via 3D Printing. Int. J. Biomater. 2024, 2024, 8060363. [Google Scholar] [CrossRef] [PubMed]
- Temizci, T.; Bozoğulları, H.N. Effect of Thermal Cycling on the Flexural Strength of 3-D Printed, CAD/CAM Milled and Heat-Polymerized Denture Base Materials. BMC Oral Health 2024, 24, 357. [Google Scholar] [CrossRef] [PubMed]
- Neppelenbroek, K.H.; Pavarina, A.C.; Vergani, C.E.; Giampaolo, E.T. Hardness of Heat-Polymerized Acrylic Resins after Disinfection and Long-Term Water Immersion. J. Prosthet. Dent. 2005, 93, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.L.; Breeding, L.C.; Vergani, C.E.; da Cruz Perez, L.E. Hardness and Surface Roughness of Reline and Denture Base Acrylic Resins after Repeated Disinfection Procedures. J. Prosthet. Dent. 2009, 102, 115–122. [Google Scholar] [CrossRef]
- Arora, O.; Ahmed, N.; Nallaswamy, D.; Ganapathy, D.; Srinivasan, M. Denture Base Materials: An in Vitro Evaluation of the Mechanical and Color Properties. J. Dent. 2024, 145, 104993. [Google Scholar] [CrossRef]
- Baciu, E.R.; Savin, C.N.; Tatarciuc, M.; Mârțu, I.; Butnaru, O.M.; Aungurencei, A.E.; Mihalache, A.M.; Diaconu-Popa, D. Experimental Study on Mechanical Properties of Different Resins Used in Oral Environments. Medicina 2023, 59, 1042. [Google Scholar] [CrossRef]
- Alqanas, S.S.; Alfuhaid, R.A.; Alghamdi, S.F.; Al-Qarni, F.D.; Gad, M.M. Effect of Denture Cleansers on the Surface Properties and Color Stability of 3D Printed Denture Base Materials. J. Dent. 2022, 120, 104089. [Google Scholar] [CrossRef]
- El-Olimy, G.; Salem, A. Effect of Two Cleansing Materials on Hardness and Surface Roughness of Conventional and Three-Dimensional Printed Denture Base Materials. Tanta Dent. J. 2022, 19, 125–131. [Google Scholar] [CrossRef]
- Berger, J.C.; Driscoll, C.F.; Romberg, E.; Luo, Q.; Thompson, G. Surface Roughness of Denture Base Acrylic Resins after Processing and after Polishing. J. Prosthodont. 2006, 15, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Takhtdar, M.; Azizimoghadam, N.; Kalantari, M.H.; Mohaghegh, M. Effect of Denture Cleansers on Color Stability and Surface Roughness of Denture Bases Fabricated from Three Different Techniques: Conventional Heat-Polymerizing, CAD/CAM Additive, and CAD/CAM Subtractive Manufacturing. Clin. Exp. Dent. Res. 2023, 9, 840–850. [Google Scholar] [CrossRef] [PubMed]
- Dhamande, M.; Pakhan, A.; Thombare, R.; Ghodpage, S. Evaluation of Efficacy of Commercial Denture Cleansing Agents to Reduce the Fungal Biofilm Activity from Heat Polymerized Denture Acrylic Resin: An in Vitro Study. Contemp. Clin. Dent. 2012, 3, 168–172. [Google Scholar] [CrossRef]
- Bae, C.-H.; Lim, Y.-K.; Kook, J.-K.; Son, M.-K.; Heo, Y.-R. Evaluation of Antibacterial Activity against Candida Albicans According to the Dosage of Various Denture Cleansers. J. Adv. Prosthodont. 2021, 13, 100–106. [Google Scholar] [CrossRef]
- Porwal, A.; Khandelwal, M.; Punia, V.; Sharma, V. Effect of Denture Cleansers on Color Stability, Surface Roughness, and Hardness of Different Denture Base Resins. J. Indian Prosthodont. Soc. 2017, 17, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Gad, M.M.; Abualsaud, R.; Fouda, S.M.; Rahoma, A.; Al-Thobity, A.M.; Khan, S.Q.; Akhtar, S.; Al-Harbi, F.A. Effects of Denture Cleansers on the Flexural Strength of PMMA Denture Base Resin Modified with ZrO2 Nanoparticles. J. Prosthodont. 2021, 30, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Garg, S.; Kalra, N.M. Effect of Denture Cleansers on Surface Roughness and Flexural Strength of Heat Cure Denture Base Resin—An in Vitro Study. J. Clin. Diagnostic Res. 2017, 11, ZC94–ZC97. [Google Scholar] [CrossRef] [PubMed]
- Savabi, O.; Attar, K.; Nejatidanesh, F.; Goroohi, H.; Badrian, H. Effect of Different Chemical Disinfectants on the Flexural Strength of Heat-Polymerized Acrylic Resins. Eur. J. Prosthodont. Restor. Dent. 2013, 21, 105–108. [Google Scholar]
- Peracini, A.; Davi, L.R.; de Queiroz Ribeiro, N.; de Souza, R.F.; da Silva, C.H.L.; Paranhos, H.D.F.O. Effect of Denture Cleansers on Physical Properties of Heat-Polymerized Acrylic Resin. J. Prosthodont. Res. 2010, 54, 78–83. [Google Scholar] [CrossRef]
- Costa, R.T.F.; Pellizzer, E.P.; Vasconcelos, B.C.D.E.; Gomes, J.M.L.; Lemos, C.A.A.; de Moraes, S.L.D. Surface Roughness of Acrylic Resins Used for Denture Base after Chemical Disinfection: A Systematic Review and Meta-Analysis. Gerodontology 2021, 38, 242–251. [Google Scholar] [CrossRef]
- Al-Thobity, A.M.; Gad, M.; ArRejaie, A.; Alnassar, T.; Al-Khalifa, K.S. Impact of Denture Cleansing Solution Immersion on Some Properties of Different Denture Base Materials: An In Vitro Study. J. Prosthodont. 2019, 28, 913–919. [Google Scholar] [CrossRef]
- Goiato, M.C.; Dos Santos, D.M.; Andreotti, A.M.; Nobrega, A.S.; Moreno, A.; Haddad, M.F.; Pesqueira, A.A. Effect of Beverages and Mouthwashes on the Hardness of Polymers Used in Intraoral Prostheses. J. Prosthodont. 2014, 23, 559–564. [Google Scholar] [CrossRef]
- Goiato, M.C.; Dos Santos, D.M.; Baptista, G.T.; Moreno, A.; Andreotti, A.M.; Dekon, S.F. de C. Effect of Thermal Cycling and Disinfection on Microhardness of Acrylic Resin Denture Base. J. Med. Eng. Technol. 2013, 37, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Ozyilmaz, O.Y.; Akin, C. Effect of Cleansers on Denture Base Resins’ Structural Properties. J. Appl. Biomater. Funct. Mater. 2019, 17, 2280800019827797. [Google Scholar] [CrossRef]
- ISO 20795-1; Dentistry—Base Polymers—Part 1, Denture Base Polymers; 2nd ed. International Organization for Standardization: Geneva, Switzerland, 2013.
- ASTM D2240-15; Standard Test Methods for Rubber Property-Durometer Hardness. American Society for Testing and Materials: West Conshohocken, PA, USA, 2015.
- Çakmak, G.; Hess, J.A.; Dönmez, M.B.; Yılmaz, D.; Alhotan, A.; Schimmel, M.; Peutzfeldt, A.; Yilmaz, B. Effect of Polishing and Denture Cleansers on the Surface Roughness of New-Generation Denture Base Materials and Their Color Change after Cleansing. J. Prosthodont. 2024, 33, 783–790. [Google Scholar] [CrossRef]
- Ali, A.M.; Raghdaa, K.J. Evaluation and Comparison of The Effect of Repeated Microwave Irradiations on Some Mechanical and Physical Properties of Heat Cure Acrylic Resin and Valplast (Nylon) Denture Base Materials. J. Bagh Coll. Dent. 2011, 23, 6–10. [Google Scholar]
- Anwar, D.; Al-Kaisy, N.; Azhdar, B. Influence of Activated Bentonite Nanoclay/TiO2 on Antibiofilm and Mechanical Properties of PMMA Denture Base Nanocomposite. Mater. Res. Express 2025, 12, 045402. [Google Scholar] [CrossRef]
- Saen-Isara, T.; Dechkunakorn, S.; Anuwongnukroh, N.; Srikhirin, T.; Tanodekaew, S.; Wichai, W. Influence of the Cross-Linking Agent on Mechanical Properties of PMMA Powder with Compromised Particle Morphology. Int. Orthod. 2017, 15, 151–164. [Google Scholar] [CrossRef]
- Revilla-León, M.; Meyers, M.J.; Zandinejad, A.; Özcan, M. A Review on Chemical Composition, Mechanical Properties, and Manufacturing Work Flow of Additively Manufactured Current Polymers for Interim Dental Restorations. J. Esthet. Restor. Dent. 2019, 31, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Bayarsaikhan, E.; Lim, J.-H.; Shin, S.-H.; Park, K.-H.; Park, Y.-B.; Lee, J.-H.; Kim, J.-E. Effects of Postcuring Temperature on the Mechanical Properties and Biocompatibility of Three-Dimensional Printed Dental Resin Material. Polymers 2021, 13, 1180. [Google Scholar] [CrossRef] [PubMed]
- Hassanpour, M.; Narongdej, P.; Alterman, N.; Moghtadernejad, S.; Barjasteh, E. Effects of Post-Processing Parameters on 3D-Printed Dental Appliances: A Review. Polymers 2024, 16, 2795. [Google Scholar] [CrossRef] [PubMed]
- Gharechahi, J.; Asadzadeh, N.; Shahabian, F.; Gharechahi, M. Flexural Strength of Acrylic Resin Denture Bases Processed by Two Different Methods. J. Dent. Res. Dent. Clin. Dent. Prospects 2014, 8, 148–152. [Google Scholar] [CrossRef]
- Gad, M.M.; Fouda, S.M. Factors Affecting Flexural Strength of 3D-Printed Resins: A Systematic Review. J. Prosthodont. 2023, 32, 96–110. [Google Scholar] [CrossRef]
- Al-Dulaijan, Y.A.; Alsulaimi, L.; Alotaibi, R.; Alboainain, A.; Akhtar, S.; Khan, S.Q.; Al-Ghamdi, M.; Gad, M.M. Effect of Printing Orientation and Postcuring Time on the Flexural Strength of 3D-Printed Resins. J. Prosthodont. 2023, 32, 45–52. [Google Scholar] [CrossRef]
- An, S.; Tazikeh, M.; Sa, S.; Wang, T.; Cameron, A. Impact of Isopropanol Post-Washing Duration and Post-Polymerization Devices on Flexural Properties of 3D-Printed Denture Base: An in-Vitro Study. Digit. Dent. J. 2025, 2, 100020. [Google Scholar] [CrossRef]
- Alifui-Segbaya, F.; Bowman, J.; White, A.R.; George, R.; Fidan, I. Characterization of the Double Bond Conversion of Acrylic Resins for 3D Printing of Dental Prostheses. Compend. Contin. Educ. Dent. 2019, 40, e7–e11. [Google Scholar]
- Gad, M.M.; Alshehri, S.Z.; Alhamid, S.A.; Albarrak, A.; Khan, S.Q.; Alshahrani, F.A.; Alqarawi, F.K. Water Sorption, Solubility, and Translucency of 3D-Printed Denture Base Resins. Dent. J. 2022, 10, 42. [Google Scholar] [CrossRef]
- Abdulwahhab, S.S. High-Impact Strength Acrylic Denture Base Material Processed by Autoclave. J. Prosthodont. Res. 2013, 57, 288–293. [Google Scholar] [CrossRef]
- Amin, F.; Akram, S.; Shaikh, A.A. Denture Cleansers Affect the Mechanical Behavior of Heat Polymerized Acrylic Resins. J. Pakistan Dent. Assoc. 2015, 24, 87–92. [Google Scholar]
- Aziz, H.K. Comparisons the Microhardness of Different Cured Acrylic Denture Base Systems after Subjected to Chemical Cleaning Solutions. Mustansiria Dent. J. 2013, 10, 77–87. [Google Scholar] [CrossRef]
- Rapone, B.; Pedone, S.; Carnevale, A.; Plantamura, P.; Scarano, A.; Demelio, A.; Demelio, G.P.; Corsalini, M. Profilometer Comparison of the Surface Roughness of Four Denture Base Resins: An In Vitro Study. Appl. Sci. 2022, 12, 1837. [Google Scholar] [CrossRef]
- Gad, M.M.; Fouda, S.M.; ArRejaie, A.S.; Al-Thobity, A.M. Comparative Effect of Different Polymerization Techniques on the Flexural and Surface Properties of Acrylic Denture Bases. J. Prosthodont. 2019, 28, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Fotovat, F.; Abassi, S.; Nikanjam, S.; Alafchi, B.; Baghiat, M. Effects of Various Disinfectants on Surface Roughness and Color Stability of Thermoset and 3D-Printed Acrylic Resin. Eur. J. Transl. Myol. 2024, 34, 11701. [Google Scholar] [CrossRef]
- Elbanna, A.A.A.; Harby, N.M.; Hamam, F.A. 3D-Printed Resin Showed Higher Water Sorption than Heat-Cured PMMA and Polyamide. J. Angiother. 2024, 8, 1–5. [Google Scholar] [CrossRef]
- Dimitrova, M.; Vlahova, A.; Hristov, I.; Kazakova, R.; Chuchulska, B.; Kazakov, S.; Forte, M.; Granberg, V.; Barile, G.; Capodiferro, S.; et al. Evaluation of Water Sorption and Solubility of 3D-Printed, CAD/CAM Milled, and PMMA Denture Base Materials Subjected to Artificial Aging. J. Compos. Sci. 2023, 7, 339. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).