1. Introduction
Obesity is a major public health problem worldwide and is defined by the World Health Organization as “abnormal or excessive fat accumulation in the body that can affect health” [
1]. It is classified by body mass index (BMI), with overweight defined as a BMI of ≥25 kg/m
2 and obesity as a BMI of ≥30 kg/m
2 [
2]. According to the WHO, one in eight people worldwide were living with obesity in 2022, and adult obesity has more than doubled since 1990. Specifically, 43% of adults aged 18 years and over were overweight, and 16% were living with obesity. Among children, 35 million under the age of 5 were overweight, and 390 million children and adolescents between the ages of 5 and 19 were overweight in 2022, of whom 160 million were living with obesity (
https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight, accessed on 20 May 2025).
Periodontitis is a chronic inflammatory disease that attacks the tooth-supporting structures through inflammation of the gums caused by pathogenic bacteria and can lead to alveolar bone and tooth loss [
3]. It is the sixth most common chronic disease worldwide, affecting around 743 million people [
4]. The relationship between obesity and periodontitis has attracted considerable research attention, as obesity has been shown to be a risk factor for periodontitis in numerous epidemiological studies and meta-analyses [
5,
6], and there is a positive correlation between these diseases [
7,
8]. This relationship works both ways: obesity affects host susceptibility to periodontitis, while periodontitis contributes to systemic inflammation [
9]. These diseases share basic biological mechanisms, including chronic inflammation, altered immune responses and oxidative stress disorders [
10], with adipose tissue acting as an endocrine organ that releases proinflammatory cytokines and hormones (adipocytokines) that promote systemic inflammation, potentially linking obesity and periodontitis [
11]. This link has significant public health implications, as both conditions serve as risk factors for cardiovascular and other systemic diseases [
12]. The development of effective prevention and treatment strategies for both conditions requires an understanding of this complex relationship, and ongoing research into the underlying linking mechanisms could lead to more effective and targeted treatment approaches for these interrelated health problems.
This review aims to summarize and evaluate the microbial and inflammatory mechanisms driving the bidirectional relationship between obesity and periodontitis, focusing on the interactions between the oral and gut microbiomes and their clinical implications. A central argument of this review is that while associative evidence linking these conditions is abundant, significant methodological inconsistencies and a predominance of observational studies critically limit our ability to establish clear causal pathways and, consequently, to develop targeted and consistently effective clinical interventions. The aim is therefore not only to clarify how obesity alters the composition of the oral microbiota and promotes periodontal inflammation but also to critically dissect why our understanding remains incomplete despite extensive research. The current evidence is critically evaluated, and methodological limitations, conflicting results and important unresolved issues in this area are highlighted, with a particular focus on how these unresolved issues impede clinical translation. In addition, this review discusses clinical implications, identifies potential therapeutic strategies to improve oral health in obese individuals and examines recent advances in the field. The literature search was conducted in the electronic databases PubMed, MEDLINE, Ovid and Google Scholar and was limited to peer-reviewed journal articles published between January 2015 and the current year. The search strategy included keywords and MeSH terms such as “obesity”, “periodontitis”, “oral microbiome”, “gut microbiome” and “microbial dysbiosis”. As is usual in a narrative review, no rigid inclusion and exclusion criteria were defined. Instead, preference was given to the literature that was most relevant to the key themes of microbial dysbiosis, inflammatory pathways and the oral–gut axis. To support the critical perspective of this review, emphasis was placed on studies with clear methodological reporting that allowed assessment of their experimental designs and analytical approaches. Non-peer-reviewed sources, such as conference abstracts and editorials, were generally excluded to ensure the quality of the basic findings discussed.
2. The Obesity–Periodontitis Axis
The obesity–periodontitis axis constitutes a bidirectional relationship between two prevalent chronic inflammatory conditions with substantial public health implications. These conditions share common pathophysiological mechanisms despite affecting different tissues. Obesity involves excessive body fat accumulation with systemic metabolic consequences. Periodontitis involves microbial-driven inflammation targeting tooth-supporting structures [
11]. Their interrelationship affects hundreds of millions globally, with each condition potentially amplifying the other’s progression and severity [
11,
13]. Epidemiological studies have consistently demonstrated a positive association between obesity and periodontitis [
7,
14]. According to recent systematic reviews and meta-analyses, as well as cross-sectional studies, the prevalence of periodontal disease is significantly higher in people with obesity compared with normal-weight individuals, with the strength of this association increasing with the severity of obesity [
2,
15,
16]. However, it must be acknowledged that although these epidemiological studies show a strong association, the predominantly observational nature of the data limits a definitive causal conclusion. Foratori-Junior and colleagues [
17] found that overweight and obesity are established risk factors for the development and progression of periodontitis, with obese individuals being more than three times more likely to develop periodontitis compared with normal-weight individuals. In addition, a meta-analysis by Jung and colleagues [
18] confirmed the association between obesity and periodontitis in different populations and showed that central obesity (measured by waist circumference) may be even more strongly associated with periodontitis than general obesity measured by BMI.
A growing body of evidence highlights the oral–gut axis as a crucial pathway in this complex relationship [
19,
20]. This axis describes the bidirectional communication between the oral cavity and the gastrointestinal tract, primarily through two routes [
20]. First, via the enteral route, periodontal pathogens and their inflammatory byproducts, like lipopolysaccharides (LPSs), are constantly swallowed. While typically eliminated in a healthy gut, they can colonize the intestine in states of gut dysbiosis, which is common in obesity, thereby compromising the intestinal barrier and contributing to systemic inflammation [
13,
20]. Second, through the hematogenous route, bacteria from inflamed periodontal pockets can enter the bloodstream and disseminate to distant sites, including the gut and adipose tissue, further propagating inflammation [
8,
13]. Recent research has demonstrated that gut dysbiosis resulting from a high-fat diet can lead to elevated levels of certain metabolites, such as uric acid [
21]. One pivotal animal study showed that these elevated uric acid levels, driven by the altered gut microbiota of obese mice, directly aggravated alveolar bone destruction in experimental periodontitis, providing a clear mechanistic link between gut-derived metabolic changes and oral disease severity [
21].
3. The Pathophysiological Mechanisms
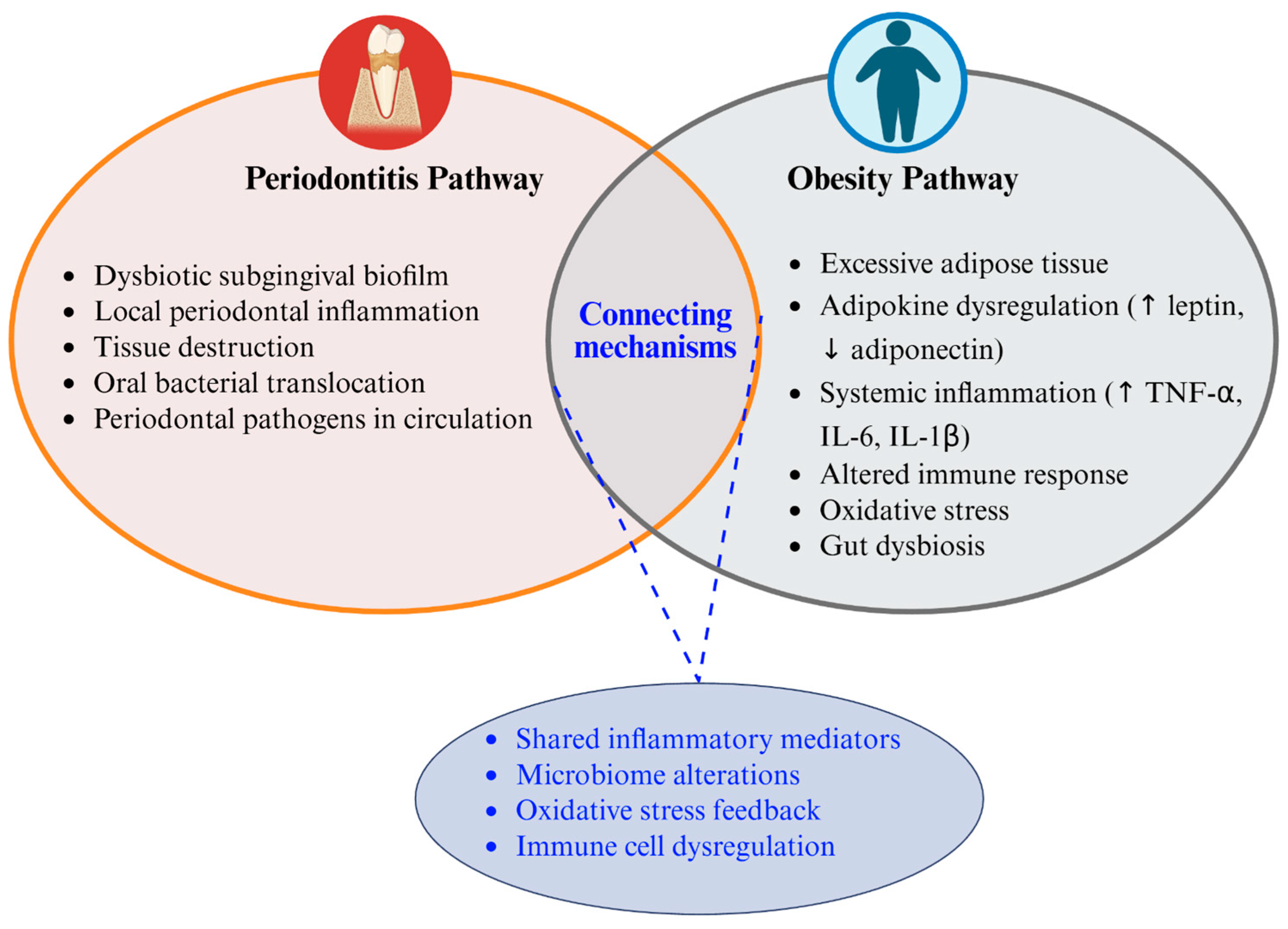
Building upon the shared inflammatory and immune-modulating mechanisms outlined earlier, this section explores the interconnected microbial and immunoinflammatory pathways that constitute the obesity–periodontitis axis, as schematically illustrated in
Figure 1. Adipose tissue functions as an endocrine organ, releasing cytokines and proinflammatory hormones that contribute to systemic inflammation and oxidative stress pathological mechanisms shared by both conditions [
11,
22]. This chronic inflammatory state induced by obesity creates a fertile environment for periodontal disease development and progression.
The “oral–gut axis” plays a crucial role in connecting these conditions. Obesity is bidirectionally associated with gut microbiome alterations, increasing the risk of inflammatory diseases like periodontitis 18. Periodontal pathogens can directly influence gut microbial communities; orally administered Porphyromonas gingivalis has been shown in murine models to disrupt gut microbiota composition, reduce gut barrier integrity and increase circulating proinflammatory cytokines, thereby linking oral dysbiosis with systemic inflammation [
23,
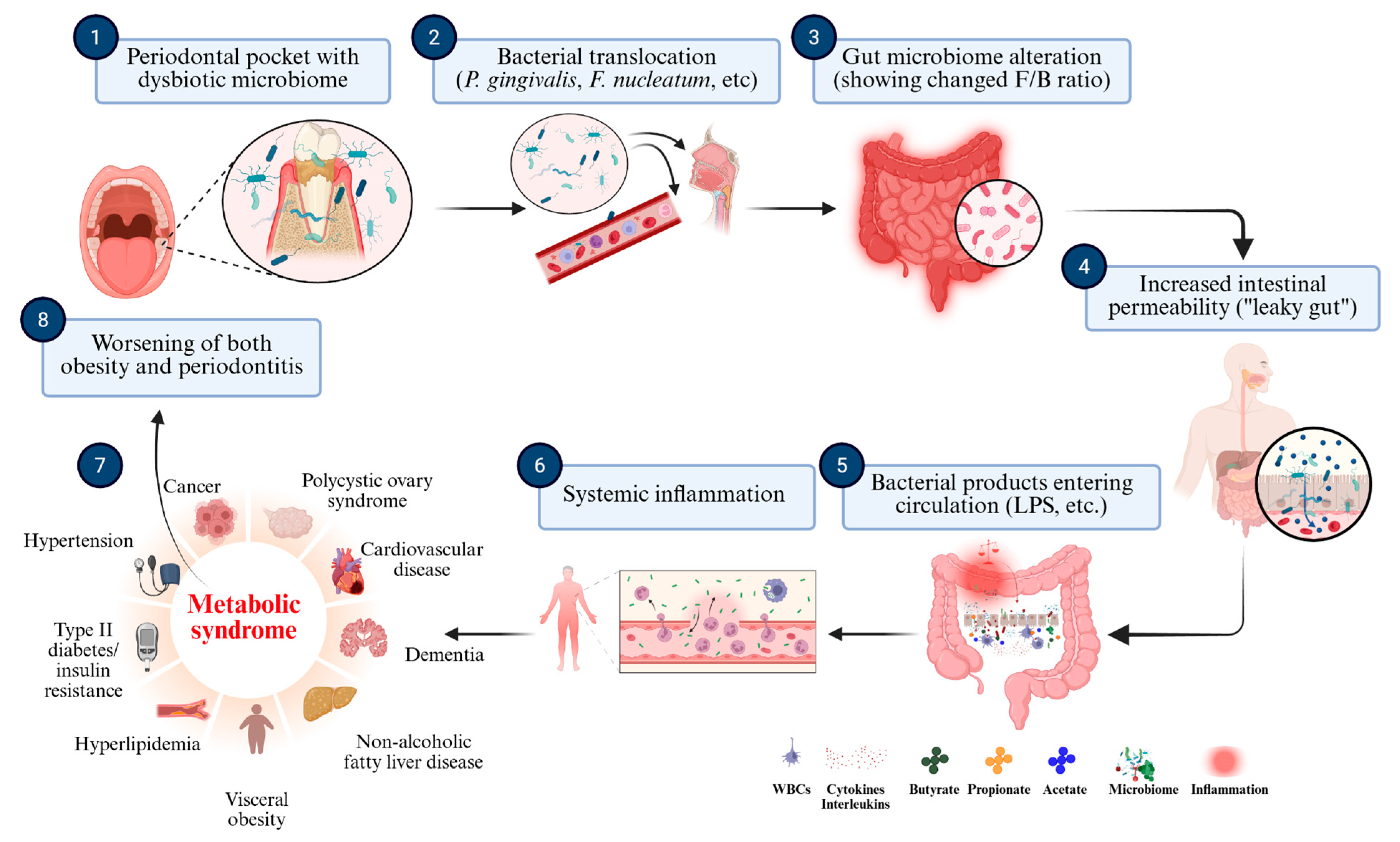
37]. These sequential microbial and immunoinflammatory interactions are synthesized in
Figure 2, which schematically illustrates the oral–gut–systemic axis linking periodontal dysbiosis to metabolic dysfunction and systemic disease.
This figure outlines the proposed mechanisms linking oral dysbiosis to systemic inflammation and metabolic dysregulation. Periodontal pathogens such as P. gingivalis and Fusobacterium nucleatum translocate to the gut, altering microbial composition and impairing intestinal barrier integrity. This process increases gut permeability, enabling microbial products like LPSs to enter the bloodstream. The resulting systemic inflammation contributes to insulin resistance, adipose tissue dysfunction and other features of metabolic syndrome, ultimately reinforcing the cycle of obesity and periodontitis.
These microbial alterations have significant metabolic consequences. Metabolite profiling revealed that several amino acids associated with diabetes and obesity risk were elevated in
P. gingivalis-administered mice, suggesting that periodontitis may increase metabolic disease risk via altered host metabolic profiles [
23]. This observation aligns with growing evidence that gut dysbiosis affects bacterial metabolites, host metabolism, intestinal barrier function and immune responses [
21].
Multiple studies document reduced microbial diversity in both the distal gut areas [
38,
39] and oral microbiomes [
40] of obese individuals. Those with lower microbiome diversity demonstrate increased adiposity, insulin resistance and dyslipidemia compared with those with high bacterial richness [
41], suggesting parallel dysbiotic processes in both environments. Immunologically, periodontitis results from microbial–host interactions destroying tooth-supporting tissues [
11,
22]. The innate immune response drives disease initiation and progression, with periodontal pathogens triggering immune responses through receptors including Toll-like receptors and nucleotide-binding oligomerization domain-containing proteins [
41,
42].
Recent Mendelian randomization studies demonstrate a bidirectional causal relationship between gut microbiota and periodontitis. Certain gut bacterial taxa, including the order
Enterobacteriales, family
Bacteroidales S24.7 group, genus
Lachnospiraceae UCG008, genus
Prevotella 7 and order
Pasteurellales, associate with higher periodontitis risk, while the genus
Ruminiclostridium 6 links to lower risk [
11,
42]. This suggests that intestinal dysbiosis may bridge extra-oral inflammatory comorbidities caused by periodontitis. The clinical significance emerges in evidence that oral microbial exposures underlying periodontitis might contribute to cardiometabolic diseases, including diabetes. Identifying early microbial signatures could help elucidate potential shared microbial etiology [
43]. This aligns with research showing that periodontal treatment may improve obesity-related systemic conditions by reducing the proinflammatory cytokine burden [
11,
44]. Despite these advances, the detailed mechanisms connecting obesity and periodontal disease susceptibility remain incompletely understood [
21]. The complex interplay between host genetics, diet, antibiotics, lifestyle and both the oral and gut microbiomes continues as an active research area [
45,
46].
3.1. Inflammatory Reaction
Chronic, low-grade systemic inflammation is widely proposed as the primary biological link between obesity and periodontitis. Adipose tissue, especially when present in excess, functions as an active endocrine organ. However, in obesity, its secretory profile is disturbed in two ways. First, there is increased production of proinflammatory cytokines such as TNF-α, IL-1β and IL-6, as well as increased leptin levels and various chemokines [
24]. Second, adipocytes often produce fewer anti-inflammatory mediators, particularly adiponectin. This inflammatory imbalance, characterized by an increase in proinflammatory signals and a decrease in anti-inflammatory mechanisms, is thought to lead to a systemic hyperinflammatory state that favors periodontal tissue destruction [
1,
47].
The evidence for this association with inflammation comes from various studies but is not entirely conclusive, mainly due to methodological inconsistencies, the prevalence of observational designs that limit causal inference and the difficulty of isolating obesity-specific effects from other comorbidities. For example, a recent study by Lê et al. [
8] showed that obesity is associated with greater periodontal inflammation, possibly mediated by a specific signature of the subgingival microbiota. Their work emphasized that imbalances in the immune system, particularly with respect to IL-17 and the Th17/Treg-cell axis, serve as key mediators in this relationship. While the strength of that study lies in the integration of microbiota profiling with immune pathway analysis, due to the cross-sectional nature of that study, the ability to infer causality is limited, and the lack of rigorous control for all systemic comorbidities may limit the ability to comprehensively isolate obesity-specific effects. Similarly, Reytor-González et al. [
11] suggested that the increase in these inflammatory cytokines in obesity may impede the clearance of periodontal pathogens while promoting the destruction of periodontal connective tissue and bone, also emphasizing IL-17-mediated pathways. This review provides a valuable synthesis of the mechanistic literature; however, as a review, it primarily summarizes existing data, and interpretation depends on the quality and potential biases of the primary studies cited.
The increased inflammatory state in obesity is also underpinned by the observation of the accumulation of macrophages in adipose tissue, which actively promote this inflammatory state and increase the production of adipocytokines [
48]. Indeed, adipose tissue-derived cytokines and hormones are intimately involved in systemic inflammatory processes, suggesting that similar mechanisms may be involved in the pathophysiologies of obesity and periodontitis [
49]. The general dysregulation of adipocytokines, with increased proinflammatory markers such as leptin and reduced anti-inflammatory markers such as adiponectin, highlighted in studies such as those by Thanakun et al. [
24] and Khan et al. [
48], supports the notion of a common inflammatory milieu. However, it is crucial to recognize that variations in adipokine measurement methods, heterogeneity in obesity classification (e.g., BMI vs. waist circumference) and, often, small sample sizes in such studies may weaken the generalizability of specific quantitative results. Despite consistent associations between these inflammatory markers and the two diseases, it is still difficult to establish a clear causal relationship, mainly due to the fact that cross-sectional data are predominantly available in this area. In addition, the lack of fully standardized protocols for the quantification of cytokines and immune-cell profiling in different research groups leads to variability that can make reproducibility and direct comparison of results difficult.
In summary, although dysregulated inflammatory pathways represent a biologically plausible and empirically supported mechanism linking obesity and periodontitis [
1,
47], the current evidence base is hampered by methodological inconsistencies, the prevalence of observational designs that limit causal inference and a relative lack of large-scale intervention studies. Although the relationship between inflammation, obesity and periodontitis is well-documented, the exact causal contribution of specific inflammatory mediators and systemic inflammatory burden and how these directly impact periodontal destruction in obese individuals have not yet been sufficiently characterized to develop specific anti-inflammatory therapeutic strategies beyond general advice. Future research should focus primarily on longitudinal and mechanistic studies that use standardized inflammatory markers and robust control as confounding factors to more accurately describe causal pathways and identify potential targets for therapeutic intervention.
3.2. Oxidative Stress
Oxidative stress is increasingly recognized as another important mechanistic link between obesity and periodontitis, often acting synergistically with chronic inflammation and exacerbating tissue damage. In obese individuals, the overproduction of reactive oxygen species (ROS) from metabolically active adipose tissue contributes to a persistent state of systemic oxidative stress [
1]. At the same time, the destructive processes in periodontitis are also characterized by localized oxidative reactions due in part to excessive inflammation with ROS release by proinflammatory cytokines’ direct cell induction. This suggests a scenario in which systemic oxidative stress due to obesity can both initiate and exacerbate gingivitis and subsequent periodontitis in susceptible individuals [
50,
51], creating a self-reinforcing cycle linking these two diseases via common inflammatory and oxidative pathways.
Evidence for this link is provided by a case–control study by Atabay et al. [
52] in which significantly elevated levels of myeloperoxidase and nitric oxide, markers directly associated with increased inflammation and tissue destruction, were found in the gingival fluid of individuals with obesity and periodontitis compared with normal-weight individuals. Remarkably, that study also showed that non-surgical periodontal therapy was able to significantly reduce those markers of oxidative stress. This suggests that targeted periodontal treatment can effectively mitigate local oxidative damage and potentially contribute to the reduction in systemic oxidative stress in obese patients. Although those results are directional, the small sample size of that study and the potentially limited control for behavioral confounders such as smoking and comprehensive oral hygiene measures may affect the external validity and generalizability of the conclusions.
The role of ROS in obesity is well-documented, with their overproduction often associated with systemic effects such as mitochondrial dysfunction and lipid peroxidation. Studies such as those by Toy et al. [
1] and Ganesan et al. [
47] have further emphasized the common oxidative environment of obesity and periodontitis. Although biologically plausible, many of those findings are predominantly associative, and there are few longitudinal or intervention studies specifically investigating whether oxidative stress causally influences the progression of disease from obesity to periodontitis. This points to a critical research gap, as direct evidence of causality between systemic oxidative stress and periodontal tissue breakdown is still largely theoretical.
Methodologically, the assessment of oxidative stress is often based on biochemical surrogate markers. These can be influenced by various coexisting systemic diseases (e.g., cardiovascular disease, diabetes) and do not always accurately reflect site-specific oxidative activity in the periodontium. In addition, heterogeneities in the selection of markers (e.g., myeloperoxidase, nitric oxide, lipid peroxidation products) and testing methods in the different studies make direct comparison and synthesis of results difficult, potentially contributing to inconsistencies in the reported results and making it difficult to establish definitive biomarkers.
3.3. Microbiome Dysbiosis
Recent studies confirm that dysbiosis of the oral microbiome is central to the obesity–periodontitis relationship, though the precise nature of this dysbiotic shift is complex and subject to debate. For instance, while some studies report increased levels of classical periodontal pathogens, such as
T. forsythia and
P. gingivalis, in obese individuals [
53], other studies present a more nuanced picture. A key study by Lê et al. [
8], for example, found a decrease in the relative abundance of those traditional pathogens (e.g.,
Porphyromonadaceae) but a concurrent increase in Gram-positive bacteria. This contradictory evidence strongly suggests that obesity does not simply amplify the growth of all known pathogens. Instead, it appears to induce a distinct “obesity-associated” dysbiotic signature, fundamentally reshaping the entire microbial community structure. The discrepancies in findings across studies are likely attributable to methodological differences in sampling techniques, sequencing platforms and patient population characteristics, which currently hinder the development of reliable microbial biomarkers. Adding another layer of complexity, the oral–gut axis provides a pathway for obesity-related gut dysbiosis to influence the oral microbiome and vice versa, further contributing to the inflammatory state [
13]. Ultimately, despite the conflicting details on specific microbial species, the unifying concept is that the proinflammatory systemic environment of obesity fosters a dysbiotic oral community, which in turn perpetuates the local and systemic inflammation that links both diseases [
54,
55].
The impact of obesity on the microbial ecosystem in the mouths of individuals with periodontitis is significant and leads to marked changes compared with individuals of normal weight. A key feature of this change is a reduction in microbial diversity in the periodontal pockets, a finding by Le et al. [
8] that correlates with increased periodontal inflammation as measured by the periodontal inflamed surface area index (PISA). The robust sample size and standardized clinical measures used in that study increased its reliability, although assessment at a single time point provides limited insight into the dynamics of the microbiome over time. The reduced diversity observed is a hallmark of the dysbiotic state in obesity-related periodontitis and reflects changes commonly observed in the gut microbiomes of obese individuals [
53].
At the phylum level, clear shifts in the relative abundances of the most important bacterial groups can be observed. In particular, the
Firmicutes/Bacteroidetes (F/B) ratio, which is often associated with obesity in both the oral and gut microbiomes, is altered [
53]. In particular,
Proteobacteria,
Chloroflexes and
Firmicutes are often over-represented in the subgingival plaque of obese individuals, whereas
Bacteroidetes may be reduced or even absent there compared with non-obese individuals [
40]. This is consistent with the broader hypothesis that the microbiota associated with obesity typically contain fewer
Bacteroidetes and more
Firmicutes [
38,
40]. However, differences in the sampling sites, e.g., saliva or subgingival plaque, are not only due to methodological reasons but may also reflect biologically relevant compartmentalization. In obese individuals, there is evidence that pathogenic bacteria originating from the gingival pocket, such as
P. gingivalis, may migrate into the saliva or even the gut, potentially contributing to systemic inflammation. Therefore, differences in microbial profiles between compartments should be interpreted not only as technical artifacts but also as indicators of microbial dispersal and host–microbe interaction dynamics.
The composition of the periodontal microbiota in obese individuals with periodontitis also shows different and sometimes complex signatures at the genus level. Thomas et al. [
56] found that obesity was associated with increased numbers of Capnocytophaga genera, and there were interesting gender differences: obese men had increased Neisseria genera, which are associated with more severe periodontal status, while obese women had increased Streptococcus genera. Rahman et al. [
53] demonstrated different taxonomic signatures for different BMI categories and periodontal health conditions, adding to the complexity. They found that healthy-weight individuals with moderate to severe periodontitis harbored more periopathogenic pathogens, including
Porphyromonas gingivalis,
F. nucleatum,
Filifactor alocis,
Parvimonas micra and
Streptococcus sobrinus, than obese individuals with similar periodontal statuses. These observations support the idea that obesity does not simply exacerbate periodontal disease through a uniform increase in periopathogens. Rather, it may reshape microbial communities in a context-specific manner. This suggests a complex, non-additive relationship between obesity, the presence of specific periodontal pathogens and overall disease severity rather than a simple increase in all pathogens with obesity.
These microbial changes have significant clinical implications. Le et al. [
8] demonstrated a lower efficacy of periodontal treatment in obese individuals, with persistent inflammation indicating unstable periodontal conditions with a risk of recurrence. This poorer outcome may be related to the different microbial profiles and increased systemic inflammation that characterize obesity. While that study is compelling, it did not examine systemic inflammatory markers and did not address the question of whether adjunctive therapies could improve outcomes, which is a starting point for future research.
Ultimately, the changes observed in the oral microbiomes of obese individuals with periodontitis mirror similar dysbiotic changes, found in the gut microbiome, associated with obesity and metabolic disease [
39,
41,
45]. Given the recognized similarity between the microbial communities of the oral cavity and the upper gastrointestinal tract [
40], these findings strongly suggest possible systemic links in the pathophysiology of periodontitis associated with obesity that extend beyond the local oral environment and emphasize a holistic, interconnected biological system.
3.4. Specific Bacterial Profiles and Signatures
Research has identified characteristic bacterial species and patterns that are clearly associated with obesity-related periodontitis. Studies consistently report higher abundances of
Tannerella forsythia in the subgingival biofilms and saliva of overweight and obese individuals compared with normal-weight individuals [
57,
58].
Aggregatibacter actinomycetemcomitans and
Fusobacterium species (especially
Fusobacterium nucleatum subsp.
vincentii) are also found in significantly higher amounts in overweight patients with chronic periodontitis [
58,
59]. Other periodontal pathogens, such as
Eubacterium nodatum,
Parvimonas micra,
Prevotella intermedia,
Prevotella melaninogenica and
Treponema socranskii, are also increased in obese individuals with periodontitis [
59,
60]. These findings are summarized in
Table 1, which presents the key bacterial taxa and microbial indicators altered in obesity-related periodontitis, along with their associations with clinical parameters and relevant studies.
In addition to the individual species, broader microbial patterns have also been found in obesity-related periodontitis. The ratio between
Firmicutes and
Bacteroidetes is altered, with an increase in
Firmicutes and a decrease in
Bacteroidetes, reflecting the changes observed in the gut microbiomes of obese individuals [
40,
53]. At the phylum level,
Proteobacteria,
Chloroflexes and
Firmicutes are over-represented in obese patients, whereas
Bacteroidetes,
Spirochaetes and
Firmicutes predominate in normal-weight individuals [
40]. Obese individuals with periodontitis show characteristic decreases in Gram-negative bacteria and increases in Gram-positive bacteria in the periodontal pockets [
8], accompanied by lower bacterial diversity, which correlates with increased periodontal inflammation. Interestingly, some studies have found that certain periopathogens, including
P. gingivalis and
Filifactor alocis, are more likely to be enriched in normal-weight subjects with moderate to severe periodontitis than in obese individuals [
53], suggesting a complex relationship between host metabolism and the composition of the oral microbiome. Recent research has also identified the logarithmic ratio of
Treponema to
Corynebacterium bacteria as a microbial indicator of periodontitis that correlates with both poor periodontal health and cardiometabolic markers [
43], providing a potential diagnostic tool for monitoring interrelated diseases. However, further validation studies are needed to establish its clinical utility, sensitivity and specificity as a reliable biomarker in different populations.
4. Clinical Implications
The proven link between obesity and periodontitis has important implications for clinical practice, as it affects diagnosis, treatment planning, therapeutic efficacy and long-term outcomes. A central theme of current research is that obesity may compromise the efficacy of conventional periodontal therapy due to systemic inflammation, impaired lipid metabolism and the different microbial profiles observed in obese individuals.
4.1. Periodontal Treatment—Impact on Obesity and Systemic Health
There is increasing evidence that obese patients suffer more severe periodontal destruction and respond less well to standard periodontal treatments. In a cross-sectional study, Jia et al. [
30] reported that obese individuals with periodontitis not only have elevated levels of proinflammatory adipokines and altered lipid metabolism but also suffer from more pronounced periodontal breakdown. Although that study supports the hypothesis that systemic inflammation plays a critical role in mediating that relationship, its design does not address whether interventions such as weight loss or metabolic control can improve periodontal outcomes: an area of great clinical importance.
Several studies have consistently reported that obese patients respond less well to periodontal therapy compared with people of normal weight [
51,
62]. For example, Le et al. [
8] demonstrated that obese patients experienced persistent inflammation after conventional therapy, indicating an unstable periodontal condition with an increased risk of disease recurrence. Their study also found a strong correlation between obesity and increased periodontal inflammation as measured by the PISA index. Specifically, they found that obese individuals exhibited a distinct microbial profile: an increased abundance of Gram-positive bacteria; a reduction in Gram-negative species, including traditional periodontal pathogens such as
Porphyromonadaceae and
Tannerellaceae; and a decrease in overall microbial diversity [
8]. The observed inverse relationship between the periodontal Simpson index (a measure of microbial diversity) and the PISA score suggests that interventions to improve microbial diversity could improve periodontal health in overweight individuals. This is consistent with the general principles of microbiome research that greater diversity is often associated with greater resilience and better health outcomes.
A prime example of this systemic impact, which is highly relevant to the often insulin-resistant obese population, is the extensively studied effect of periodontal therapy on glycemic control in patients with type 2 diabetes. A substantial body of evidence, consolidated in numerous studies, has demonstrated that non-surgical periodontal therapy can lead to a statistically and clinically significant reduction in hemoglobin A1c (HbA1c) levels [
63,
64]. The magnitude of this reduction is typically in the range of 0.3% to 0.4% over 3–4 months, an effect comparable to adding a second oral hypoglycemic agent to a patient’s drug regimen [
65,
66]. The underlying mechanism is believed to be the reduction in the systemic inflammatory burden. By treating the periodontal infection, there is a decrease in circulating proinflammatory cytokines like TNF-α and IL-6, which are known contributors to insulin resistance [
67]. This improvement in insulin sensitivity subsequently leads to better glycemic control, as reflected by the lower HbA1c. These findings underscore that periodontal treatment is not merely a localized oral health intervention but a medical necessity that can form part of the comprehensive metabolic management of at-risk patients, including those with obesity. However, these results are not consistent across studies, likely due to variability in sample populations, treatment modalities, follow-up time and underlying metabolic phenotypes. In many cases, studies are also limited by small cohort sizes and inadequate control of confounding behavioral factors, such as patient compliance and oral hygiene practices. In addition, the complex relationship between obesity and periodontitis is oversimplified when BMI is used as the primary indicator of metabolic status. This is particularly important, as visceral fat, rather than total body weight, may have a more direct impact on systemic inflammation and healing outcomes. Future research should therefore incorporate more sophisticated markers of metabolic health, such as insulin resistance, body fat percentage and visceral adiposity, in the evaluation of periodontal treatment outcomes.
Conversely, emerging evidence suggests that periodontal therapy may itself contribute to improved systemic inflammatory profiles and possibly metabolic parameters in obese individuals [
68]. For instance, Suvan et al. [
51] proposed that periodontal therapy in obese patients should be integrated with broader strategies that address systemic metabolic dysfunction. Although preliminary, these findings open the possibility that periodontal intervention could play a supportive role in managing obesity-related systemic inflammation. Nevertheless, the current evidence base remains limited, and rigorous, large-scale randomized controlled trials are necessary to validate these effects and identify optimal treatment protocols.
4.2. Obesity Treatment—Implications for Periodontal Health (Including Bariatric Surgery)
Therapeutic strategies for the treatment of obesity, in particular bariatric surgery, provide an additional opportunity to investigate the dynamic interplay between systemic metabolic control and periodontal health. De Souza et al. [
69] found that weight loss after bariatric surgery was associated with significant improvements in periodontal parameters, likely due to a reduction in systemic inflammation. This supports the concept that the treatment of obesity can positively influence periodontal health.
However, the effects of bariatric surgery on periodontal status are not universally positive. A systematic review by Fontanille et al. [
70] found a paradoxical short-term deterioration in periodontal health in the immediate postoperative period (typically within the first six months). This deterioration could be due to nutritional deficiencies, changes in immune function or other physiological stressors associated with rapid weight loss. These findings underscore the importance of a multidisciplinary, proactive approach to care that includes a comprehensive periodontal assessment and customized dental care both before and after bariatric surgery.
Given the pronounced microbial and inflammatory features associated with obesity-related periodontitis, conventional treatment paradigms need to be increasingly reconsidered. The microbial changes described by Le et al. [
8], particularly the shift toward Gram-positive dominance and the reduction in classical Gram-negative pathogens, may partly explain the lower response to treatment observed in obese patients. These changes in microbial community structure, including significant differences in beta diversity between obese and non-obese individuals, suggest that standard periodontal therapies, which often target traditional pathogens, may be less effective without modification.
4.3. Tailored Treatment Options for Periodontitis Associated with Obesity
Given that obese patients present a unique host profile characterized by systemic inflammation, metabolic dysregulation and an altered microbiome, standard periodontal therapy is often insufficient to achieve long-term stability. This necessitates a shift toward a multi-faceted treatment paradigm that addresses both the local oral disease and the underlying systemic contributors. The following specific approaches should be elaborated and integrated into clinical practice to optimize outcomes for this high-risk population.
4.3.1. Enhanced Risk Assessment and Intensified Mechanical Therapy
Treatment planning should begin with a risk assessment that goes beyond standard periodontal charting. Clinicians should recognize that a high BMI and particularly high visceral adiposity signify a proinflammatory state that predisposes the patient to more severe disease and a compromised healing response. Consequently, these patients often benefit from a more intensive initial phase of non-surgical therapy and, crucially, a more frequent periodontal maintenance schedule (e.g., every 3 months) to keep the microbial biofilm load consistently low and prevent the host’s exaggerated inflammatory response from causing rapid disease recurrence.
4.3.2. Adjunctive Host Modulation Therapy (HMT)
Since a hyper-inflammatory response is a hallmark of the obesity–periodontitis link, therapies targeting the host’s destructive enzymes are highly relevant. Subantimicrobial-dose doxycycline (SDD) is a well-established HMT that functions by inhibiting matrix MMPs: key enzymes responsible for collagen breakdown and tissue destruction [
60]. For obese patients with chronic periodontitis, prescribing a course of SDD as an adjunct to scaling and root planing can be a powerful tool to help control the downstream effects of systemic inflammation and improve treatment outcomes.
4.3.3. Microbiome-Modulating Interventions
As dysbiosis is central to the disease process, the adjunctive use of specific probiotic strains can improve periodontal health through several mechanisms. These include competitive inhibition, where probiotics compete with pathogens for adhesion sites [
71,
72], and the production of antimicrobial substances that directly inhibit pathogens like
P. gingivalis [
73,
74]. Furthermore, probiotics like
Lactobacillus reuteri can modulate the local host immune response by reducing proinflammatory cytokines in the gingival crevicular fluid [
75,
76], thereby promoting a healing environment [
77]. Meta-analyses have confirmed that probiotics can offer modest but significant improvements in clinical parameters [
78].
The benefits extend systemically. Probiotics can help restore gut eubiosis, strengthen the intestinal barrier and reduce the translocation of inflammatory endotoxins like LPSs [
79]. This can help alleviate the systemic metabolic dysregulation linked to obesity, including improvements in metabolic markers [
80], thus addressing a root cause of the heightened inflammatory state. While promising, it is crucial to recognize that the effects are strain-specific and more research is needed to define optimal protocols [
81].
4.3.4. Integrated Medical Collaboration and Patient Counseling
The dental setting provides a unique opportunity for systemic health intervention. Clinicians should proactively counsel patients on the bidirectional relationship between their periodontal disease, diet and metabolic health. The most effective approach involves interdisciplinary collaboration with the patient’s physician or endocrinologist or a registered dietitian. Coordinated care that integrates periodontal treatment with medical strategies for weight management and metabolic control is essential for managing the overall health of these complex patients and achieving long-term stability.
5. Limitations and Future Research Directions
Despite the growing body of evidence suggesting a significant interaction between obesity, microbiome alterations and periodontitis, the current research landscape is characterized by several important limitations that make definitive conclusions difficult and highlight critical areas for future investigation.
A major limitation is the heterogeneity of the obesity classification (e.g., the use of BMI without consideration of visceral fat distribution) and periodontal diagnostic criteria (e.g., different case definitions for the severity of periodontitis), which makes meta-analysis and causal conclusions difficult. To improve comparability, standardized protocols are needed that include, for example, visceral fat measurements and uniform definitions for periodontitis cases. For example, the use of BMI alone often means that important metabolic differences (e.g., visceral obesity, insulin resistance) are not captured in individuals with similar BMI values. Similarly, inconsistent periodontal diagnostic criteria, case definitions and outcome measures make robust comparability of data and meta-analysis difficult. This lack of standardization has led some researchers to consider the evidence inconclusive regarding the exact nature and strength of the relationship between these conditions [
55], making it difficult to draw definitive conclusions about specific microbial signatures and their direct clinical impact.
Another limitation is the cross-sectional nature of many existing studies. While such studies may reveal associations—for example, some obese individuals have been found to have increased levels of key periodontal pathogens such as
Aggregatibacter actinomycetemcomitans,
Eubacterium nodatum and
Fusobacterium spp. [
60]—they do not reveal clear causal relationships or temporal sequences. It remains largely unclear whether the observed microbial shifts and inflammatory profiles are responsible for the progression of periodontal disease associated with obesity or whether they are merely consequences of pre-existing pathology and the associated chronic inflammatory state. This significantly limits our understanding of whether specific microbial changes precede disease development or occur as byproducts.
The complexity of host–microbe interactions, particularly in the unique systemic environment of obesity, poses additional research challenges. While recent studies have identified correlations between specific bacterial species or community structures and disease parameters, the functional consequences of these changes are often not yet fully understood. The heterogeneity of microbiome sampling methods (e.g., saliva vs. subgingival plaque vs. tongue swabs), sequencing platforms, bioinformatic pipelines and taxonomic resolution adds further complexity, making direct comparisons between different studies problematic. In addition, many studies do not adequately account for a number of important confounding factors such as diet, smoking habits, medication use (e.g., antibiotics, anti-inflammatory drugs) and socioeconomic status, all of which can independently influence both microbial composition and inflammatory profiles.
To advance the field and overcome these limitations, future research should focus on several key areas:
Longitudinal cohort studies are crucial to go beyond mere association and decipher the temporal sequence of and possible causal links between obesity-related changes, microbial shifts and the onset or progression of periodontitis: a critical gap that has been repeatedly identified in the current literature.
Mechanistic studies using integrated multi-omic approaches (e.g., metagenomics, metabolomics, transcriptomics) to elucidate causal pathways.
Standardized clinical trials to evaluate the efficacy of tailored periodontal therapies, including probiotic, anti-inflammatory or antioxidant supplements, especially in obese populations.
Interventional studies investigating whether periodontal therapy can improve systemic metabolic parameters and vice versa.
In addition, the link between the mouth and the gut microbiome deserves greater attention, particularly through studies investigating how oral pathogens influence gut ecology and systemic inflammation in obesity. Understanding these intersystemic dynamics could open new avenues for preventive and therapeutic strategies.
In summary, although there is convincing evidence that obesity and periodontitis are linked by common biological mechanisms, further robust, multidisciplinary studies are needed to fill the existing research gaps. Of the priorities identified, longitudinal cohort studies and mechanistic research with integrated multi-omic approaches appear to be the most important. These efforts are essential to establish temporal relationships; clarify causality; and unravel the complex relationships between microbial shifts, systemic inflammation and tissue destruction. Without this fundamental understanding, attempts to develop effective personalized interventions or evaluate their clinical impact are premature.
Equally important is the collaboration between different disciplines: particularly dentistry, endocrinology, microbiology and immunology. Only through such integrated approaches can we comprehensively address the multifactorial nature of obesity-related periodontitis and advance both prevention and treatment strategies. In addition, the oral–gut microbiome axis deserves increased attention, as it holds promise for reshaping our understanding of systemic inflammation and metabolic comorbidities. Focusing on these fundamental areas through collaborative, standardized and mechanistically informed research will be critical to finding a more effective path forward.
6. Conclusions
The relationship between obesity and periodontitis is a complex, bidirectional cycle driven by systemic inflammation, microbial dysbiosis, immune dysfunction and oxidative stress, affecting both the oral and gut ecosystems. This critical review emphasizes that while there is ample evidence of a link between obesity and periodontitis, the path from observed associations to the development of highly effective, evidence-based interventions remains difficult. These difficulties include the preponderance of observational data and methodological inconsistencies in the research landscape. These factors contribute to gaps in our understanding of precise causal pathways and pose a challenge to the ultimate validation of specific mechanisms as therapeutic targets. Despite these gaps, obesity consistently correlates with adverse changes in the oral microbiome, increased inflammation and poorer response to periodontal treatment. This underscores the urgent need for personalized strategies. The complex interaction between obesity and periodontitis requires a paradigm shift toward truly integrated, multidisciplinary treatment approaches. While the integration of periodontal treatment with systemic metabolic treatment is promising, its clinical implementation still requires deeper insights into mechanisms and validation through robust, well-designed clinical trials to truly help those affected.