Contemporary Approaches to Obstructive Sleep Apnea: A Review of Orthodontic and Non-Orthodontic Interventions in Children and Adults
Abstract
1. Introduction
2. Materials and Methods
Literature Search Strategy
3. Literature Review
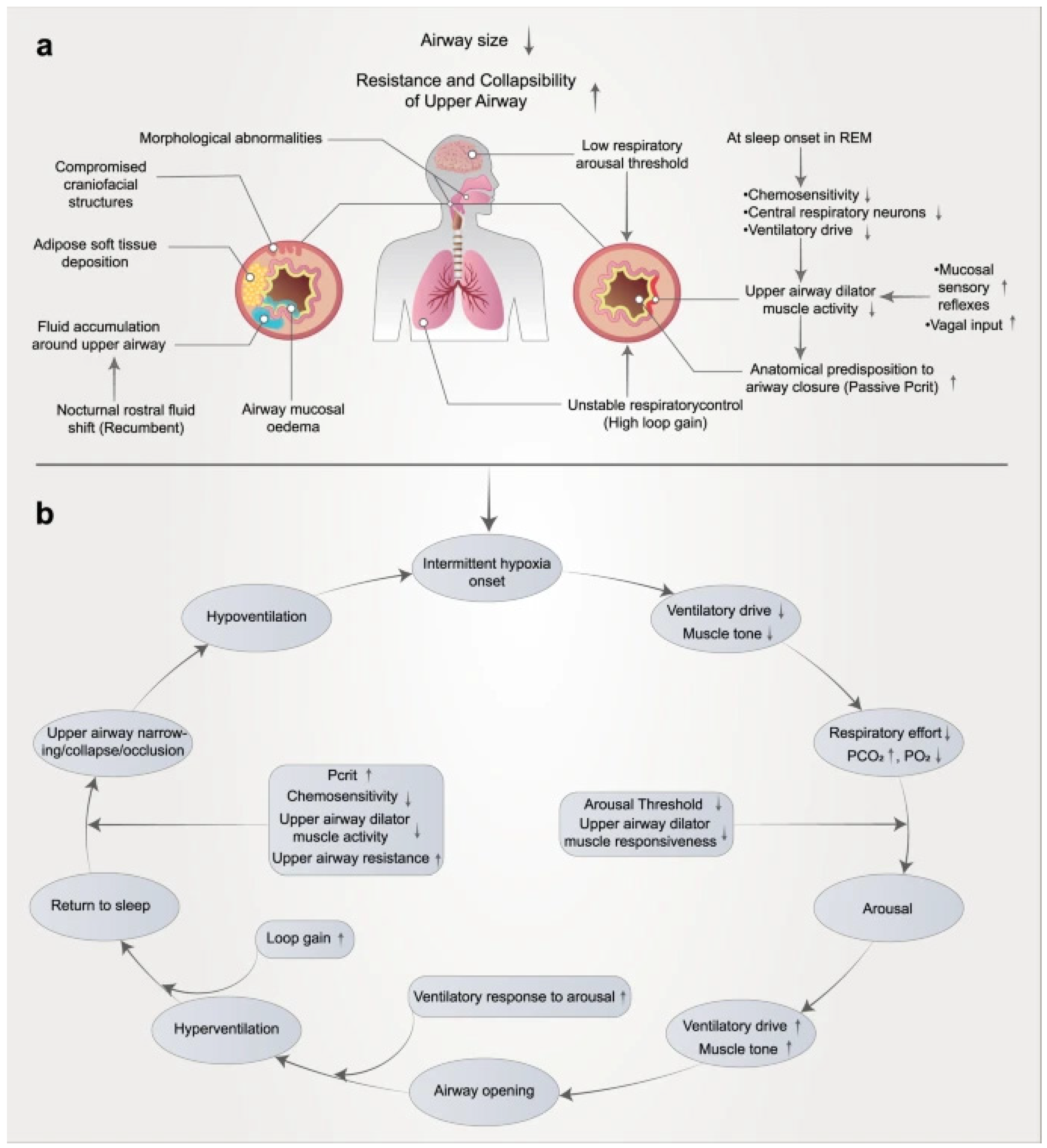
3.1. Pathophysiology of OSA
3.2. Diagnostic Considerations
3.3. Orthodontic Treatment Outcomes and Evidence in Pediatric OSA
3.4. Treatment of Pediatric OSA
3.4.1. Adenotonsillectomy Effect
3.4.2. Mandibular Advancement Appliances in Pediatric Obstructive Sleep Apnea
3.4.3. Rapid Maxillary Expansion
3.4.4. Myofunctional Therapy and Orofacial Exercises
3.5. Management of Adult OSA
3.5.1. Maxillomandibular Advancement (MMA) Surgery
3.5.2. Oral Appliance Therapy (Mandibular Advancement Devices)
3.5.3. Positive Airway Pressure (PAP) Therapy
3.5.4. Hypoglossal Nerve Stimulation (HGNS)
4. Discussion
4.1. Summary of Evidence and Future Directions
4.2. Limitations and Recommendations
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OSA | Obstructive Sleep Apnea |
| AHI | Apnea–Hypopnea Index |
| CPAP | Continuous Positive Airway Pressure |
| PAP | Positive Airway Pressure |
| MAD | Mandibular Advancement Device |
| RME | Rapid Maxillary Expansion |
| MA | Maxillomandibular Advancement |
| HNS | Hypoglossal Nerve Stimulation |
| ESS | Epworth Sleepiness Scale |
| FOSQ | Functional Outcomes of Sleep Questionnaire |
| SAQLI | Sleep Apnea Quality of Life Index |
| BDI | Beck Depression Inventory |
| PSQ | Pediatric Sleep Questionnaire |
| CHF | Congestive Heart Failure |
| CT | Computed Tomography |
| LCF | Labial Closure Force |
| MM | Modified Monobloc |
| MTBA | Modified Twin Block Appliance |
References
- Tolbert, T.M.; Ayappa, I.; Rapoport, D.M. OSA pathophysiology: A contemporary update. Aust. Dent. J. 2024, 69, S68–S83. [Google Scholar] [CrossRef]
- Cacho, V. Beyond the mouth: An overview of obstructive sleep apnea in adults for dentists. J. Prosthodont. 2025, 34, 4–9. [Google Scholar] [CrossRef]
- Hasuneh, M.M.; Toubasi, A.A.; Khraisat, B.; Aldabbas, H.; AL-Iede, M. Risk Factors of Obstructive Sleep Apnea (OSA) in Pediatric Patients: A Systematic Review and Meta-analysis. J. Pediatr. Health Care 2024, 38, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Nosetti, L.; Zaffanello, M.; De Bernardi di Valserra, F.; Simoncini, D.; Beretta, G.; Guacci, P.; Piacentini, G.; Agosti, M. Exploring the Intricate Links between Adenotonsillar Hypertrophy, Mouth Breathing, and Craniofacial Development in Children with Sleep-Disordered Breathing: Unraveling the Vicious Cycle. Children 2023, 10, 1462. [Google Scholar] [CrossRef]
- Messineo, L.; Bakker, J.P.; Cronin, J.; Yee, J.; White, D.P. Obstructive sleep apnea and obesity: A review of epidemiology, pathophysiology and the effect of weight-loss treatments. Sleep Med. Rev. 2024, 78, 101996. [Google Scholar] [CrossRef] [PubMed]
- Salman, L.A.; Shulman, R.; Cohen, J.B. Obstructive Sleep Apnea, Hypertension, and Cardiovascular Risk: Epidemiology, Pathophysiology, and Management. Curr. Cardiol. Rep. 2020, 22, 6. [Google Scholar] [CrossRef]
- Kazmierski, R.H. Obstructive sleep apnea: What is an orthodontist’s role? Prog. Orthod. 2024, 25, 21. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Zhang, L.; Lu, Y. The role of rapid maxillary expansion in pediatric obstructive sleep apnea: Efficacy, mechanism and multidisciplinary collaboration. Sleep Med. Rev. 2023, 67, 101733. [Google Scholar] [CrossRef]
- Koka, V.; De Vito, A.; Roisman, G.; Petitjean, M.; Pignatelli, G.R.F.; Padovani, D.; Randerath, W. Orofacial myofunctional therapy in obstructive sleep apnea syndrome: A pathophysiological perspective. Medicina 2021, 57, 323. [Google Scholar] [CrossRef]
- Trzepizur, W.; Cistulli, P.A.; Glos, M.; Vielle, B.; Sutherland, K.; Wijkstra, P.J.; Hoekema, A.; Gagnadoux, F. Health outcomes of continuous positive airway pressure versus mandibular advancement device for the treatment of severe obstructive sleep apnea: An individual participant data meta-analysis. Sleep 2021, 44, zsab015. [Google Scholar] [CrossRef]
- Heiser, C.; Steffen, A.; Strollo, P.J.; Giaie-Miniet, C.; Vanderveken, O.M.; Hofauer, B. Hypoglossal nerve stimulation versus positive airway pressure therapy for obstructive sleep apnea. Sleep Breath. 2023, 27, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Machado-Júnior, A.J.; Zancanella, E.; Crespo, A.N. Rapid maxillary expansion and obstructive sleep apnea: A review and meta-analysis. Med. Oral Patol. Oral Y Cir. Bucal 2016, 21, e465–e469. [Google Scholar] [CrossRef]
- Duan, J.; Xia, W.; Yang, K.; Li, X.; Zhang, F.; Xu, J.; Jiang, Y.; Liang, J.; Li, B. The Efficacy of Twin-Block Appliances for the Treatment of Obstructive Sleep Apnea in Children: A Systematic Review and Meta-Analysis. Biomed Res. Int. 2022, 2022, 3594162. [Google Scholar] [CrossRef] [PubMed]
- Pham, L.V.; Schwartz, A.R. The pathogenesis of obstructive sleep apnea. J. Thorac. Dis. 2015, 7, 1358–1372. [Google Scholar] [PubMed]
- Lv, R.; Liu, X.; Zhang, Y.; Dong, N.; Wang, X.; He, Y.; Yue, H.; Yin, Q. Pathophysiological mechanisms and therapeutic approaches in obstructive sleep apnea syndrome. Signal Transduct. Target. Ther. 2023, 8, 218. [Google Scholar] [CrossRef]
- McNicholas, W.T.; Korkalainen, H. Translation of obstructive sleep apnea pathophysiology and phenotypes to personalized treatment: A narrative review. Front. Neurol. 2023, 14, 1239016. [Google Scholar] [CrossRef]
- Sutherland, K.; Cistulli, P.A. Recent advances in obstructive sleep apnea pathophysiology and treatment. Sleep Biol. Rhythm. 2015, 13, 26–40. [Google Scholar] [CrossRef]
- Mohamed, A.S.; Habumugisha, J.; Cheng, B.; Zhao, M.; Bu, W.; Liu, L.; Guo, Y.; Zou, R.; Wang, F. A cone-beam computed tomography study of hyoid bone position and airway volume in subjects with obstructive and nonobstructive adenotonsillar hypertrophy. Angle Orthod. 2023, 93, 467–475. [Google Scholar] [CrossRef]
- Chen, H.; Aarab, G.; de Ruiter, M.H.T.; de Lange, J.; Lobbezoo, F.; van der Stelt, P.F. Three-dimensional imaging of the upper airway anatomy in obstructive sleep apnea: A systematic review. Sleep Med. 2016, 21, 19–27. [Google Scholar] [CrossRef]
- Shigeta, Y.; Ogawa, T.; Tomoko, I.; Clark, G.T.; Enciso, R. Soft palate length and upper airway relationship in OSA and non-OSA subjects. Sleep Breath. 2010, 14, 353–358. [Google Scholar] [CrossRef]
- Kim, K.A.; Kim, S.J.; Yoon, A. Craniofacial anatomical determinants of pediatric sleep-disordered breathing: A comprehensive review. J. Prosthodont. 2024, 34, 26–34. [Google Scholar] [CrossRef]
- Habumugisha, J.; Ma, S.Y.; Mohamed, A.S.; Cheng, B.; Zhao, M.Y.; Bu, W.Q. Three-dimensional evaluation of pharyngeal airway and maxillary arch in mouth and nasal breathing children with skeletal Class I and II. BMC Oral Health 2022, 22, 320. [Google Scholar] [CrossRef]
- Habumugisha, J.; Mohamed, A.S.; Cheng, B.; Liu, L.; Zou, R.; Wang, F. Analysis of maxillary arch morphology and its relationship with upper airway in mouth breathing subjects with different sagittal growth patterns. J. Stomatol. Oral Maxillofac. Surg. 2023, 124, 101386. [Google Scholar] [CrossRef]
- Umano, G.R.; Rondinelli, G.; Luciano, M.; Pennarella, A.; Aiello, F.; Mangoni di Santo Stefano, G.S.R.C.; Sessa, A.D.; Marzuillo, P.; Papparella, A.; del Giudice, E.M. Pediatric Sleep Questionnaire Predicts Moderate-to-Severe Obstructive Sleep Apnea in Children and Adolescents with Obesity. Children 2022, 9, 1303. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Sundar, K.M. Evaluation and Management of Adults with Obstructive Sleep Apnea Syndrome. Lung 2021, 199, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Huynh, N.T.; Desplats, E.; Almeida, F.R. Orthodontics treatments for managing obstructive sleep apnea syndrome in children: A systematic review and meta-analysis. Sleep Med. Rev. 2016, 25, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Blechner, M.; Williamson, A.A. Consequences of Obstructive Sleep Apnea in Children. Curr. Probl. Pediatr. Adolesc. Health Care 2016, 46, 19–26. [Google Scholar] [CrossRef]
- Mitchell, R.B.; Kelly, J. Outcome of adenotonsillectomy for obstructive sleep apnea in obese and normal-weight children. Otolaryngol. Head Neck Surg. 2007, 137, 43–48. [Google Scholar] [CrossRef]
- Suzuki, H.; Watanabe, A.; Akihiro, Y.; Takao, M.; Ikematsu, T.; Kimoto, S.; Asano, T.; Kawara, M. Pilot study to assess the potential of oral myofunctional therapy for improving respiration during sleep. J. Prosthodont. Res. 2013, 57, 195–199. [Google Scholar] [CrossRef]
- Arab, M.; El Ansari, Y.S.; Pelayo, R.; Yoon, A. Management of Paediatric Obstructive Sleep Apnoea: From a Multidisciplinary to an Interdisciplinary Care Model. In Orthodontics & Craniofacial Research; WILEY Online Library: Hoboken, NJ, USA, 2025. [Google Scholar]
- Garetz, S.L.; Mitchell, R.B.; Parker, P.D.; Moore, R.H.; Rosen, C.L.; Giordani, B.; Muzumdar, H.; Paruthi, S.; Elden, L.; Willging, P.; et al. Quality of life and obstructive sleep apnea symptoms after pediatric adenotonsillectomy. Pediatrics 2015, 135, e477–e486. [Google Scholar] [CrossRef]
- Mitchell, R.B. Adenotonsillectomy for obstructive sleep apnea in children: Outcome evaluated by pre- and postoperative polysomnography. Laryngoscope 2007, 117, 1844–1854. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, R.; Kheirandish-Gozal, L.; Spruyt, K.; Mitchell, R.B.; Promchiarak, J.; Simakajornboon, N.; Kaditis, A.G.; Splaingard, D.; Splaingard, M.; Brookd, L.J.; et al. Adenotonsillectomy outcomes in treatment of obstructive sleep apnea in children: A multicenter retrospective study. Am. J. Respir. Crit. Care Med. 2010, 182, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Redline, S.; Cook, K.; Chervin, R.D.; Ishman, S.; Baldassari, C.M.; Mitchell, R.B.; Tapia, I.E.; Amin, R.; Hassan, F.; Lbrahim, S.; et al. Adenotonsillectomy for Snoring and Mild Sleep Apnea in Children: A Randomized Clinical Trial. JAMA 2023, 330, 2084–2095. [Google Scholar] [CrossRef] [PubMed]
- Nazarali, N.; Altalibi, M.; Nazarali, S.; Major, M.P.; Flores-Mir, C.; Major, P.W. Mandibular advancement appliances for the treatment of paediatric obstructive sleep apnea: A systematic review. Eur. J. Orthod. 2015, 37, 618–626. [Google Scholar] [CrossRef]
- Villa, M.P.; Bernkopf, E.; Pagani, J.; Broia, V.; Montesano, M.; Ronchetti, R. Randomized controlled study of an oral jaw-positioning appliance for the treatment of obstructive sleep apnea in children with malocclusion. Am. J. Respir. Crit. Care Med. 2002, 165, 123–127. [Google Scholar] [CrossRef]
- Schütz, T.C.B.; Dominguez, G.C.; Hallinan, M.P.; Cunha, T.C.A.; Tufik, S. Class II correction improves nocturnal breathing in adolescents. Angle Orthod. 2011, 81, 222–228. [Google Scholar] [CrossRef]
- Zhang, C.; He, H.; Ngan, P. Effects of twin block appliance on obstructive sleep apnea in children: A preliminary study. Sleep Breath. 2013, 17, 1309–1314. [Google Scholar] [CrossRef]
- Duan, J.; Xia, W.; Li, X.; Zhang, F.; Wang, F.; Chen, M.; Chen, Q.; Wang, B.; Li, B. Airway morphology, hyoid position, and serum inflammatory markers of obstructive sleep apnea in children treated with modified twin-block appliances. BMC Oral Health 2025, 25, 162. [Google Scholar] [CrossRef]
- Zreaqat, M.; Alforaidi, S.; Hassan, R. Effects of twin-block appliance on quality of life in OSA children with class 2 malocclusion and mandibular retrognathia. J. Oral Biol. Craniofac. Res. 2025, 15, 864–868. [Google Scholar] [CrossRef]
- Modesti-Vedolin, G.; Chies, C.; Chaves-Fagondes, S.; Piza-Pelizzer, E.; Lima-Grossi, M. Efficacy of a mandibular advancement intraoral appliance (MOA) for the treatment of obstructive sleep apnea syndrome (OSAS) in pediatric patients: A pilot-study. Med. Oral Patol. Oral Y Cir. Bucal 2018, 23, e656–e663. [Google Scholar] [CrossRef]
- Zreaqat, M.; Hassan, R.; Samsudin, A.R.; Alforaidi, S. Effects of twin-block appliance on upper airway parameters in OSA children with class II malocclusion and mandibular retrognathia: A CBCT study. Eur. J. Pediatr. 2023, 182, 5501–5510. [Google Scholar] [CrossRef] [PubMed]
- Cozza, P.; Polimeni, A.; Ballanti, F. A modified monobloc for the treatment of obstructive sleep apnoea in paediatric patients. Eur. J. Orthod. 2004, 26, 523–5230. [Google Scholar] [CrossRef] [PubMed]
- Villa, M.P.; Malagola, C.; Pagani, J.; Montesano, M.; Rizzoli, A.; Guilleminault, C.; Ronchetti, R. Rapid maxillary expansion in children with obstructive sleep apnea syndrome: 12-month follow-up. Sleep Med. 2007, 8, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Pirelli, P.; Saponara, M.; Guilleminault, C. Rapid maxillary expansion (RME) for pediatric obstructive sleep apnea: A 12-year follow-up. Sleep Med. 2015, 16, 933–935. [Google Scholar] [CrossRef]
- Fernández-Barriales, M.; Lafuente-Ibáñez de Mendoza, I.; Alonso-Fernández Pacheco, J.J.; Aguirre-Urizar, J.M. Rapid maxillary expansion versus watchful waiting in pediatric OSA: A systematic review. Sleep Med. Rev. 2022, 62, 101609. [Google Scholar] [CrossRef]
- Habumugisha, J.; Cheng, B.; Ma, S.Y.; Zhao, M.Y.; Bu, W.Q.; Wang, G.L.; Liu, Q.; Zou, R.; Wang, F. A non-randomized concurrent controlled trial of myofunctional treatment in the mixed dentition children with functional mouth breathing assessed by cephalometric radiographs and study models. BMC Pediatr. 2022, 22, 506. [Google Scholar] [CrossRef]
- Guilleminault, C.; Huang, Y.S.; Monteyrol, P.J.; Sato, R.; Quo, S.; Lin, C.H. Critical role of myofascial reeducation in pediatric sleep-disordered breathing. Sleep Med. 2013, 14, 518–525. [Google Scholar] [CrossRef]
- Zhang, F.; Tian, Z.; Shu, Y.; Zou, B.; Yao, H.; Li, S.; Li, Q. Efficiency of oro-facial myofunctional therapy in treating obstructive sleep apnoea: A meta-analysis of observational studies. J. Oral Rehabil. 2022, 49, 734–745. [Google Scholar] [CrossRef]
- Camacho, M.; Certal, V.; Abdullatif, J.; Zaghi, S.; Ruoff, C.M.; Capasso, R.; Kushida, C.A. Myofunctional therapy to treat OSA: Review and meta-analysis. Sleep 2015, 38, 669–675. [Google Scholar] [CrossRef]
- Patil, S.P.; Schneider, H.; Schwartz, A.R.; Smith, P.L. Adult obstructive sleep apnea: Pathophysiology and diagnosis. Chest 2007, 132, 325–337. [Google Scholar] [CrossRef]
- Holty, J.E.C.; Guilleminault, C. Maxillomandibular advancement for the treatment of obstructive sleep apnea: A systematic review and meta-analysis. Sleep Med. Rev. 2010, 14, 287–297. [Google Scholar] [CrossRef]
- Boyd, S.B.; Walters, A.S.; Waite, P.; Harding, S.M.; Song, Y. Long-term effectiveness and safety of maxillomandibular advancement for treatment of obstructive sleep apnea. J. Clin. Sleep Med. 2015, 11, 699–708. [Google Scholar] [CrossRef]
- Jaspers, G.W.; Booij, A.; De Graaf, J.; De Lange, J. Long-term results of maxillomandibular advancement surgery in patients with obstructive sleep apnoea syndrome. Br. J. Oral Maxillofac. Surg. 2013, 51, e37–e39. [Google Scholar] [CrossRef]
- Garreau, E.; Wojcik, T.; Bouscaillou, J.; Ferri, J.; Raoul, G. Comparative effectiveness of maxillomandibular advancement surgery versus mandibular advancement device for patients with moderate or severe obstructive sleep area. Orthod. Fr. 2014, 85, 163–173. [Google Scholar] [CrossRef]
- Zhou, N.; Ho, J.P.T.F.; Spijker, R.; Aarab, G.; de Vries, N.; Ravesloot, M.J.L.; de Lange, J. Maxillomandibular Advancement and Upper Airway Stimulation for Treatment of Obstructive Sleep Apnea: A Systematic Review. J. Clin. Med. 2022, 11, 6782. [Google Scholar] [CrossRef] [PubMed]
- Pahkala, R.; Seppä, J.; Myllykangas, R.; Tervaniemi, J.; Vartiainen, V.M.; Suominen, A.L.; Muraja-Murro, A. The impact of oral appliance therapy with moderate mandibular advancement on obstructive sleep apnea and upper airway volume. Sleep Breath. 2020, 24, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Vecchierini, M.F.; Attali, V.; Collet, J.M.; d’Ortho, M.P.; Goutorbe, F.; Kerbrat, J.B.; Leger, D.; Lavergne, F.; Monaca, C.; Monteyrol, P.J.; et al. Mandibular advancement device use in obstructive sleep apnea: ORCADES study 5-year follow-up data. J. Clin. Sleep Med. 2021, 17, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.L.; Grunstein, R.R.; Darendeliler, M.A.; Mihailidou, A.S.; Srinivasan, V.K.; Yee, B.J.; Marks, G.B.; Cistulli, P.A. Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: A randomized controlled trial. Am. J. Respir. Crit. Care Med. 2013, 187, 879–887. [Google Scholar] [CrossRef]
- Yu, M.; Ma, Y.; Han, F.; Gao, X. Long-term efficacy of mandibular advancement devices in the treatment of adult obstructive sleep apnea: A systematic review and meta-analysis. PLoS ONE 2023, 18, e0292832. [Google Scholar] [CrossRef]
- Mansfield, D.R.; Gollogly, N.C.; Kaye, D.M.; Richardson, M.; Bergin, P.; Naughton, M.T. Controlled Trial of Continuous Positive Airway Pressure in Obstructive Sleep Apnea and Heart Failure. Am. J. Respir. Crit. Care Med. 2004, 169, 361–366. [Google Scholar] [CrossRef]
- Stuck, B.A.; Leitzbach, S.; Maurer, J.T. Effects of continuous positive airway pressure on apnea-hypopnea index in obstructive sleep apnea based on long-term compliance. Sleep Breath. 2012, 16, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.P.; Ayappa, I.A.; Caples, S.M.; John Kimoff, R.; Patel, S.R.; Harrod, C.G. Treatment of adult obstructive sleep apnea with positive airway pressure: An American academy of sleep medicine systematic review, meta-analysis, and GRADE assessment. J. Clin. Sleep Med. 2019, 15, 301–334. [Google Scholar] [CrossRef] [PubMed]
- Kezirian, E.J.; Goding, G.S.; Malhotra, A.; O’Donoghue, F.J.; Zammit, G.; Wheatley, J.R.; Catcheside, P.G.; Smith, P.L.; Schwartz, A.R.; Walsh, J.H.; et al. Hypoglossal nerve stimulation improves obstructive sleep apnea: 12-month outcomes. J. Sleep Res. 2014, 23, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Eastwood, P.R.; Barnes, M.; Walsh, J.H.; Maddison, K.J.; Hee, G.; Schwartz, A.R.; Smith, P.L.; Malhotra, A.; McEvoy, R.D.; Wheatley, J.R.; et al. Treating obstructive sleep apnea with hypoglossal nerve stimulation. Sleep 2011, 34, 1479–1486. [Google Scholar] [CrossRef]
- Alrubasy, W.A.; Abuawwad, M.T.; Taha, M.J.J.; Khurais, M.; Sayed, M.S.; Dahik, A.M.; Keshk, N.; Abdelhadi, S.; Serhan, H.A. Hypoglossal nerve stimulation for obstructive sleep apnea in adults: An updated systematic review and meta-analysis. Respir. Med. 2024, 234, 107826. [Google Scholar] [CrossRef]
- Tahmasbi, S.; Seifi, M.; Soleymani, A.A.; Mohamadian, F.; Alam, M. Comparative study of changes in the airway dimensions following the treatment of Class II malocclusion patients with the twin block and Seifi appliances. Dent. Med. Probl. 2023, 60, 247–254. [Google Scholar] [CrossRef]
- Chuang, L.C.; Hwang, Y.J.; Lian, Y.C.; Hervy-Auboiron, M.; Pirelli, P.; Huang, Y.S.; Guilleminault, C. Changes in craniofacial and airway morphology as well as quality of life after passive myofunctional therapy in children with obstructive sleep apnea: A comparative cohort study. Sleep Breath. 2019, 23, 1359–1369. [Google Scholar] [CrossRef]
- Baz, H.; Elshafey, M.; Elmorsy, S.; Abu-Samra, M. The role of oral myofunctional therapy in managing patients with mild to moderate obstructive sleep apnea. PAN Arab J. Rhinol. 2012, 2, 17–22. [Google Scholar]
- Faber, J.; Mota, A.; Ho, L.I.; Darendeliler, M.A. The role of orthodontists in the multidisciplinary management of obstructive sleep apnea. Prog. Orthod. 2024, 25, 40. [Google Scholar] [CrossRef]
- Alansari, R.A. The role of orthodontics in management of obstructive sleep apnea. Saudi Dent. J. 2022, 34, 194–201. [Google Scholar] [CrossRef]
- Pelteret, J.P.V.; Reddy, B.D. Development of a computational biomechanical model of the human upper-airway soft-tissues toward simulating obstructive sleep apnea. Clin. Anat. 2014, 27, 182–200. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habumugisha, J. Contemporary Approaches to Obstructive Sleep Apnea: A Review of Orthodontic and Non-Orthodontic Interventions in Children and Adults. Oral 2025, 5, 55. https://doi.org/10.3390/oral5030055
Habumugisha J. Contemporary Approaches to Obstructive Sleep Apnea: A Review of Orthodontic and Non-Orthodontic Interventions in Children and Adults. Oral. 2025; 5(3):55. https://doi.org/10.3390/oral5030055
Chicago/Turabian StyleHabumugisha, Janvier. 2025. "Contemporary Approaches to Obstructive Sleep Apnea: A Review of Orthodontic and Non-Orthodontic Interventions in Children and Adults" Oral 5, no. 3: 55. https://doi.org/10.3390/oral5030055
APA StyleHabumugisha, J. (2025). Contemporary Approaches to Obstructive Sleep Apnea: A Review of Orthodontic and Non-Orthodontic Interventions in Children and Adults. Oral, 5(3), 55. https://doi.org/10.3390/oral5030055






