1. Introduction
Dental caries remains the most prevalent chronic disease globally, affecting a wide demographic, including both children and adults [
1]. Caries develops through a dynamic process of demineralization and remineralization. When plaque bacteria metabolizes dietary carbohydrates, producing acid that lowers the pH in the plaque biofilm, demineralization of the underlying tooth surface occurs [
2,
3]. This is followed by natural remineralization processes by saliva. Caries manifests when the demineralization outweighs the remineralization [
2,
3]. It starts as early non-cavitated lesions, but if left unmanaged, it progresses to cavitated lesions that traditionally require restorative intervention [
4]. Minimally invasive dentistry, however, has shifted focus toward preventive measures, prioritizing the remineralization of early, non-cavitated caries lesions to avoid more invasive treatments [
5,
6]. By promoting a balanced mineral exchange within the oral environment, preventive strategies offer a means of halting lesion progression and preserving natural tooth structure [
7].
Natural remineralization relies heavily on saliva, which is rich in calcium and phosphate ions that help maintain tooth integrity but requires supplemental minerals when it comes to remineralization of early caries lesions [
8]. Therapeutically, fluoride has been the standard agent for remineralization due to its multifaceted role in combating caries [
9]. It not only accelerates the formation of fluorapatite crystals, providing a stronger, more acid-resistant mineral phase, but at high concentration, it also inhibits microbial activity and acid production while reducing plaque polysaccharide synthesis [
10,
11]. Fluoride executes its antibacterial action by the acidification of the bacterial cytoplasm through the dissociation of hydrogen fluoride to produce H+ and F- ions and the disruption of the bacterial metabolism by the inhibition of vital bacterial enzymes such as proton-releasing adenosine triphosphatase and enolase, which thus reduces tooth demineralization as well as the extracellular polysaccharide production, which helps in decreasing bacterial adherence to dental hard tissues [
10]. Furthermore, fluoride contributes to caries control through the promotion of remineralization and the formation of the fluorapatite mineral phase which is, compared with hydroxyapatite, more resistant to demineralization and acid dissolution, following acid production by bacteria [
10,
11]. Daily use of high-concentration fluoride treatments, such as 5000 ppm prescription toothpaste and fluoride mouth rinses, has demonstrated substantial improvements in surface and subsurface remineralization compared with over-the-counter products containing 1000–1500 ppm fluoride [
12,
13]. For targeted professional applications, 5% sodium fluoride (NaF) varnish has shown efficacy in reversing early-stage lesions by adhering well to the tooth’s surface, enhancing fluoride uptake, and thereby helping to mitigate demineralization associated with caries [
14,
15,
16]. Furthermore, the application of fluoride, particularly at high concentrations, results in the formation of a protective calcium fluoride layer that offers prolonged protection of the tooth surfaces against acid attack. Although dental plaque is always present on the interproximal surfaces, plaque removal is not necessary before fluoride application, considering that plaque offers the benefit of serving as a reservoir for the targeted delivery of active agents to the plaque–tooth interface, to be released during an acidic challenge. Other fluoride modalities, such as 1.23% acidulated phosphate fluoride (APF), although equally effective, have limitations in tolerability and application duration compared with varnish [
17,
18,
19,
20]. For children under six years of age, topical fluoride varnish remains the only recommended topical fluoride treatment due to the potential for fluoride toxicity with systemic exposure [
21].
Although fluoride varnish seems to be the most beneficial among the fluoride modalities, its effectiveness is limited when applied to interproximal surfaces due to the difficulty in accessing these surfaces, especially when teeth are in tight contact [
16]. Dental practitioners have used different methods to get fluoride varnish into these surfaces [
22,
23,
24]. Some practitioners have used flossing techniques to drag fluoride varnish into the interproximal surfaces [
23], while others paint the varnish onto the cleavage between two contacting teeth with the aim of getting the fluoride to diffuse into the interproximal surface [
22]. In desperation to access the interproximal surface, some dentists insert an orthodontic O-ring or wedges between contacting teeth to achieve momentary teeth separation to gain access to the interproximal surfaces [
22,
23,
24]. These different application methods have been successful in remineralizing proximal lesions using fluoride; however, significant challenges persist for achieving adequate fluoride penetration in the interproximal surfaces. The need for improved delivery methods that specifically target proximal surfaces highlights a gap in the current preventive treatments and presents an opportunity for advancing caries management techniques.
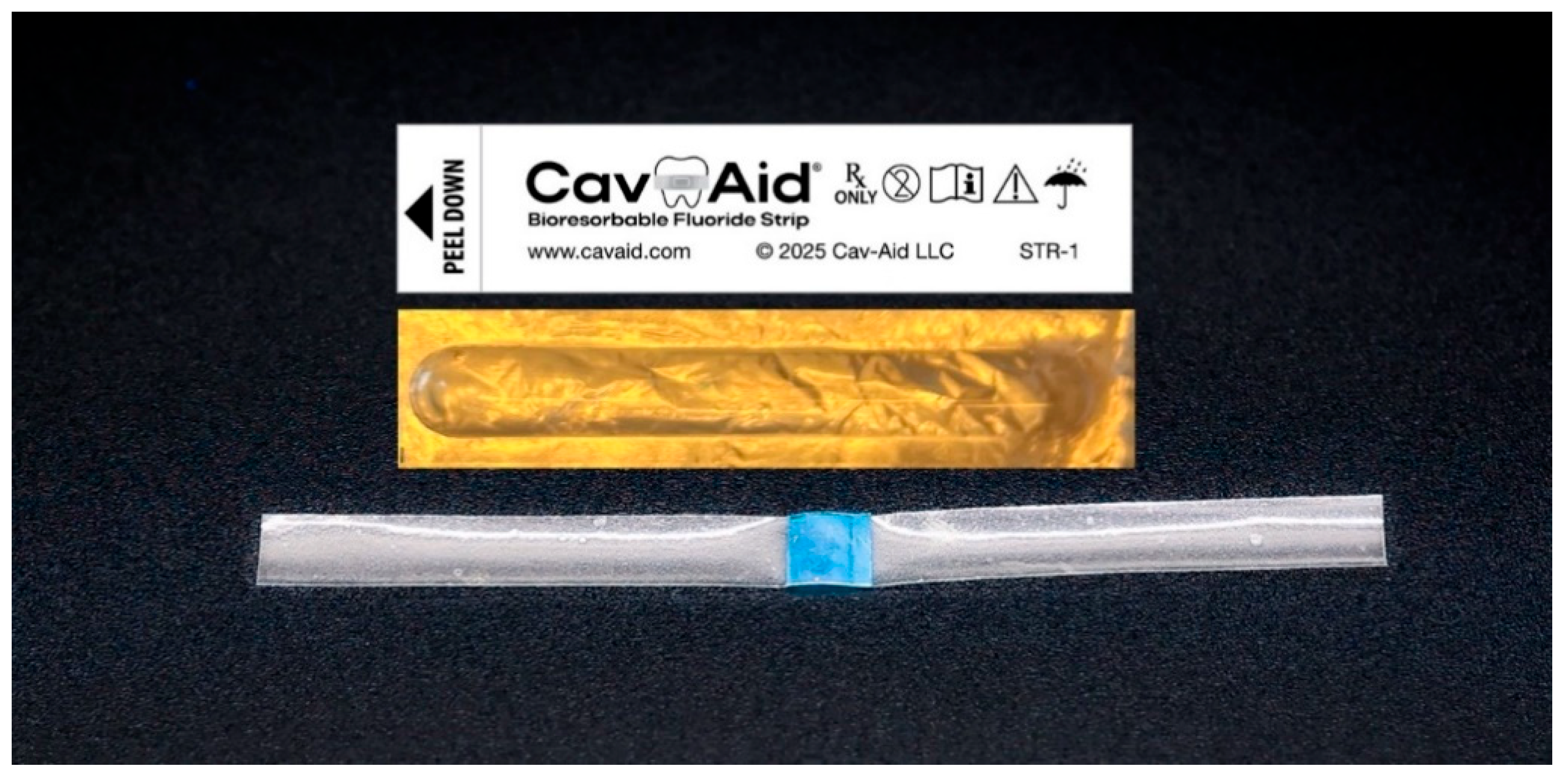
To address this gap, Cav-Aid
® (Cav-Aid LLC, San Antonio, TX, USA), a novel device designed to enhance interproximal fluoride delivery, was developed. Patented Cav-Aid
® fluoride strips (U.S. Patent No. 12,193,901 B2) are single-dose and are comprised of a rapidly-dissolving film containing sodium fluoride for insertion between the teeth. The strips have two distinct regions (
Figure 1). The quicker-dissolving “wings” are used solely for handling and placement of the active matrix over the lesion. Once the strip is positioned and exposed to moisture, the wings rapidly dissolve. As the wings dissolve, the slower-dissolving, active blue matrix softens and expands, forming an adhesive gel over the lesion and delivering fluoride directly to the caries lesion. The strips prevent and repair incipient (early) interproximal decay at the microscopic, nanoscopic, and molecular levels. Cav-Aid
® fluoride strips weigh 3 mg and contain 0.0678 mg fluoride ions. Effectiveness means any gain in mineral as measured by an increase in surface microhardness (SMH) of the tooth samples relative to the baseline SMH. By enhancing fluoride delivery in hard-to-reach areas, Cav-Aid
® may offer a patient-centered, minimally invasive approach to manage proximal caries more effectively.
Therefore, the primary aim of the present study was to evaluate the efficacy of Cav-Aid® in remineralizing initial proximal caries and to compare its efficacy with those of other conventional methods of fluoride treatment of initial proximal caries. This study hypothesized that Cav-Aid® will be more effective than the current topical fluoride delivery methods in remineralizing initial proximal caries by providing precise, targeted application to affected proximal surfaces and, as such, could significantly improve proximal remineralization outcomes.
2. Materials and Methods
2.1. Sample Preparation
Sound bovine teeth were collected and stored in 0.1% thymol solution until use. Then, the teeth were cleansed of soft tissue debris, brushed with pumice slurry using a manual toothbrush, and examined by transillumination. Teeth without cracks, hypomineralization, white spot lesions, or other malformations were selected. Using a water-cooled diamond wire saw (WELL (Walter Ebner Le Locle) Diamond Wire Saws SA, Le Locle, Switzerland), the roots of each tooth were removed, and a total of 120 tooth blocks (approximately 4 mm × 4 mm × 1.5 mm) were produced from the labial enamel surface of each tooth. The enamel surfaces and bottoms of the blocks were polished using adhesive-back lapping films of varying grit levels (30 µm to 1 µm) in a MultiPrep™ Precision Polishing Machine (Allied High Tech, Cerritos, CA, USA) to achieve the flat surfaces required for surface microhardness testing. Following polishing, the smear layer generated during polishing was removed by sonicating (Branson Sonifier 450, Cavaris, LLC, Woburn, MA, USA) the samples in a 200 mL beaker for 2 min (power setting 2) in de-ionized distilled water (pH 7.0).
2.2. Initial Caries Lesion Creation
All surfaces of each enamel block, except the buccal enamel surface, were coated with two layers of acid-resistant nail varnish. An initial caries lesion was created on each tooth block by subjecting the enamel blocks to a 3-day demineralization process using a microbial caries model. The microbial model consisted of Todd Hewitt broth inoculated with a mixed culture of
Streptococcus mutans and
Lactobacillus casei (broth-to-inoculum ratio 10:1) [
22,
23]. The culture produced a natural cariogenic biofilm, which underwent a daily cycle of feasting (10% sucrose solution for 6 min, 3 times daily, resulting in pH 4.3–4.5) and fasting (growth media for the remainder of the day) for 3 days.
2.3. Baseline Surface Microhardness Measurement and Treatment Groups
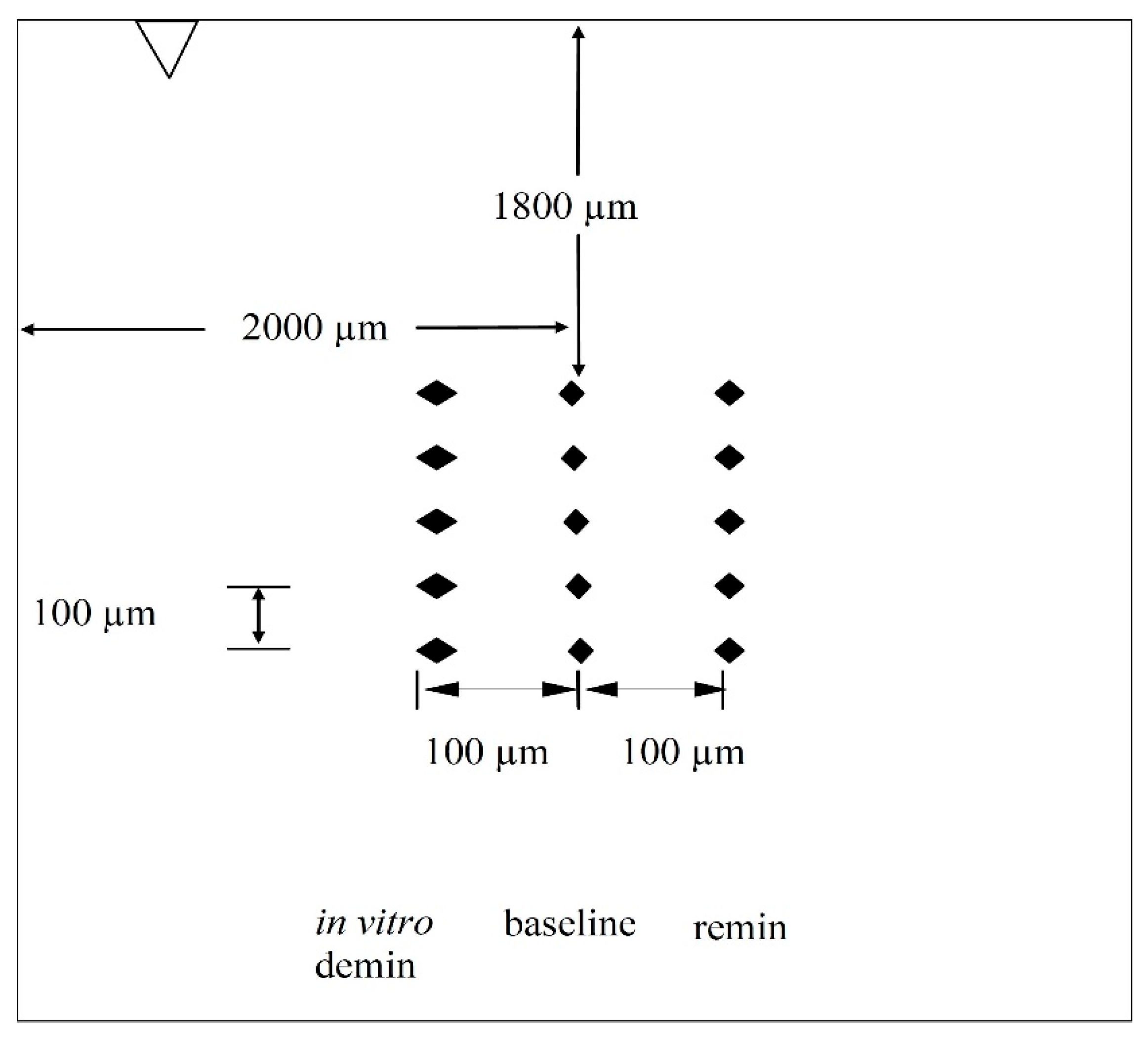
Following the initial caries lesion production, the baseline surface microhardness (SMHb) of each enamel block was measured using a microhardness tester (Tukon 2100; Wilson-Instron, Norwood, MA, USA). Each enamel block was secured onto a 1-inch square acrylic block with sticky wax and positioned on the tester. Five baseline indentations, spaced 100 µm apart (
Figure 2), were created using a Vickers diamond under a 50 g load applied for 15 s. Indentation lengths were measured using Wilson 2100–Wolpert Image Analysis Software (version 3.5.032), and the mean SMHb was calculated for each block.
Following SMHb measurement, the tooth blocks were randomly assigned to the following six treatment groups (20/group) shown in
Table 1: artificial saliva (AS), Cav-Aid
® only (CA), Cav-Aid
® application plus daily toothbrushing with fluoride toothpaste (CA+F), fluoride varnish applied via flossing (FV-Floss), fluoride varnish applied via painting on the interdental cleavage (FV-Paint), and daily fluoride mouthrinse (F-Mouthrinse). The assignment of the blocks ensured that the mean values of the SMHb did not differ significantly among the groups.
2.4. Proximal Caries Model
The 120 enamel blocks were used to construct a Proximal Caries Model, described briefly as follows. Each of the 120 bovine enamel blocks was fitted into a 4 mm × 4 mm × 2 mm cavity created on the proximal surface of an extracted human tooth and secured with heavy-duty dental putty (
Figure 3). These block-bearing teeth were mounted in pairs into typodonts (10 teeth/typodont), with the tooth blocks aligned face-to-face to simulate natural proximal contacts (
Figure 3). The tightness of the contacts was evaluated with Shim stock (Almore Shimstock, Almore International, Hickory, NC, USA) and dental floss (Oral-B Glide Pro-Health Threader floss, P&G, Cincinnati, OH, USA), and by this means, the contacts were standardized.
The teeth underwent a 14-day pH-cycling protocol, alternating between a remineralizing solution (artificial saliva) and a demineralizing solution (acidic challenge) [
22,
23]. The remineralizing solution contained 3 g/L MgCl
2·6H
2O, 0.804 g/L K
2HPO
4, 0.326 g/L KH
2PO
4, 0.385 g/L C
6H
10CaO
6, 0.625 g/L KCl, 0.4 g/L carboxymethylcellulose, and 2 g/L methyl-4-hydroxybenzoate, with the pH adjusted to 7.2. The acidic challenge solution consisted of 2.2 mM KH
2PO
4, 2.2 mM CaCl
2, and 50 mM acetic acid, with the pH adjusted to 4.5.
For the first 24 h, all groups were stored in artificial saliva. After 24 h, the experimental groups were treated as follows: Groups AS, CA, FV-floss, and FV-paint were treated as described in
Section 2.5 below and thereafter did not receive any further treatment during the pH cycling, while groups CA+F and F-mouthrinse received their respective toothpaste or daily mouthrinse treatment as described in
Section 2.5 below. All groups were immersed in the acidic challenge solution once daily for 2 h but remained in artificial saliva for the rest of the time. The artificial saliva was replaced daily. The experiment ran for 14 days in duration. It is pertinent to note that for caries remineralization, we preferred to use natural early-caries lesions, so we produced our lesions with plaque growth, but the caries remineralization procedure was performed by chemical pH cycling.
2.5. Remineralization Treatment Protocol
Artificial Saliva Only (AS): the samples remained immersed in artificial saliva, which was changed daily throughout the study period.
Cav-Aid® Only (CA): one single-dose Cav-Aid® (5% NaF) strip was inserted once between opposing tooth blocks, as directed by the manufacturer.
Cav-Aid® + Daily Fluoride Toothpaste (CA+F): one single-dose Cav-Aid® (5% NaF) strip was applied as described above, followed by twice-daily brushing with 0.24% NaF toothpaste for 2 min on each occasion throughout the study period.
Fluoride Varnish by Flossing (FV-floss): Dental floss was inserted into the interproximal space. Then, fluoride varnish (5% NaF) was coated on one side of the floss and with the aid of the floss, the varnish was dragged onto the interproximal surfaces. Then, with one upward and one downward stroke of the floss, the varnish was applied on the proximal surfaces of the opposing tooth blocks.
Fluoride Varnish by Painting (FV-paint): With one single stroke, fluoride varnish (5% NaF) was painted on the interdental cleavage between two adjacent teeth. After 24 h, the varnish was brushed off with wet pumice in accordance with our present clinical procedure.
Daily Fluoride Mouthrinse (F-Mouthrinse): teeth were immersed into fluoride mouthwash (0.05% NaF) daily for 30 s on each occasion with agitation to simulate swishing.
2.6. Post-Remineralization Surface Microhardness (SMHR)
After the remineralization protocol, the SMH of each block was measured again (SMHr), as described above for the baseline, with five indentations placed 100 µm to the right of the baseline indentations (
Figure 2). The mean values of SMHr were calculated for each block.
2.7. Statistical Analysis
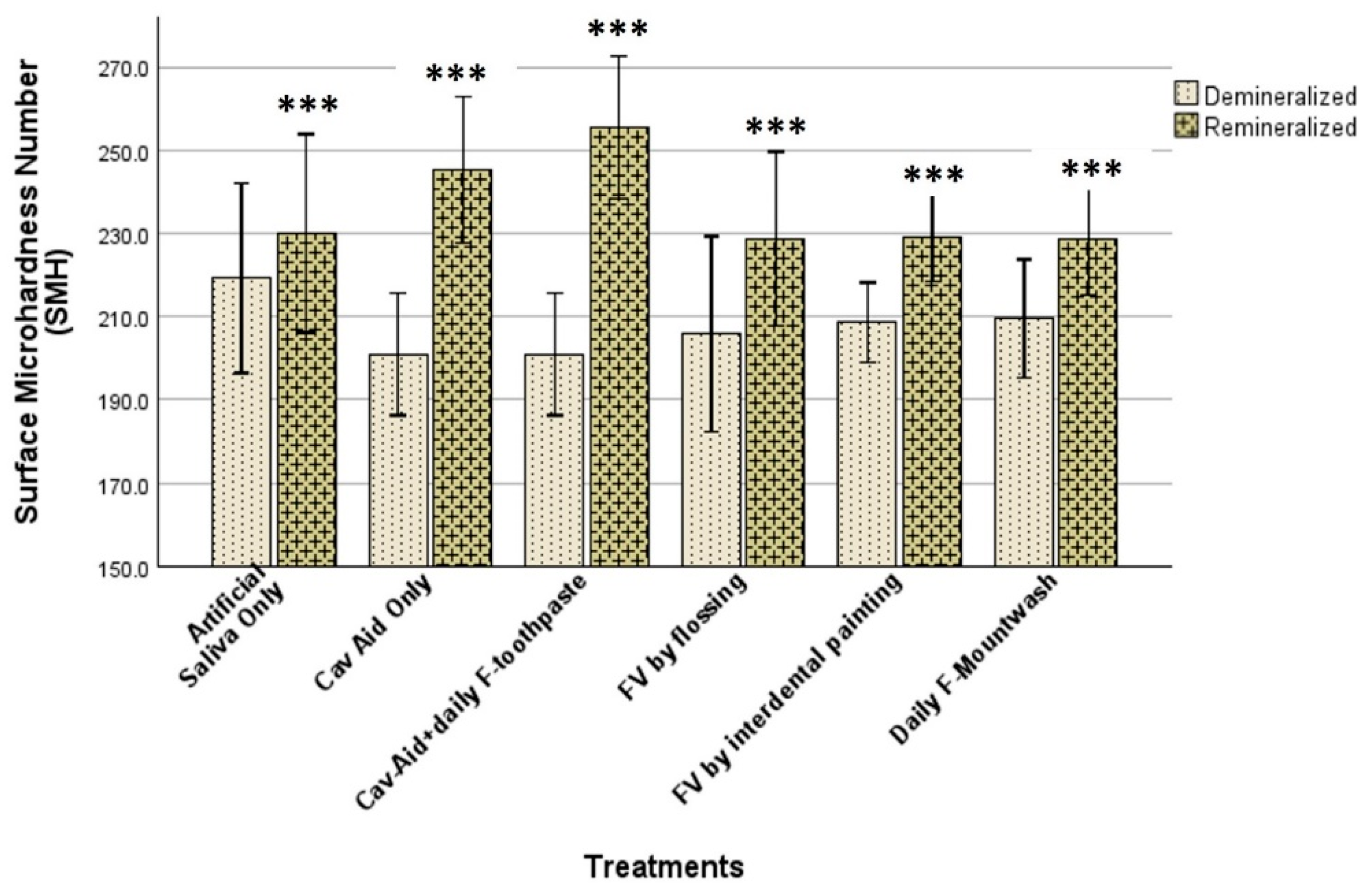
All the statistical analyses were performed using Stata 11.0 (StataCorp, College Station, TX, USA). The equality of variances was assessed using the Brown–Forsythe test, and normality was evaluated using the Shapiro–Wilk test. Intra-group comparisons of SMHb and SMHr were performed using paired t-tests. The percentage demineralization in each group, calculated relative to the baseline SMH (SMHb), were compared among the groups using one-way ANOVA, followed by Tukey’s multiple comparison test. Statistical significance was set at p < 0.05 for all statistical analysis, including t-test, ANOVA, and Tukey’s test.
4. Discussion
The challenge of achieving effective fluoride delivery in interproximal spaces has long been recognized in preventive dentistry [
22,
23,
24]. While fluoride varnish remains one of the most commonly utilized and effective approaches, its ability to penetrate tight interproximal contacts is limited, potentially reducing its efficacy in remineralizing early proximal caries lesions [
16]. To improve the delivery of fluoride into the interproximal spaces, a novel device, the Cav-Aid
® fluoride strip, was developed. Cav-Aid
® fluoride strips represent an innovative breakthrough in fluoride delivery methods in interproximal space and are a significant contribution to the clinical armamentaria for the prevention and treatment of proximal caries. Exceptionally thin, Cav-Aid
® strips are designed to fit between the teeth and, once there, to deliver a concentrated dose of fluoride to interproximal sites that were previously inaccessible to direct fluoride varnish delivery. Because Cav-Aid
® strips are inserted directly into the interproximal site, they effectively break the barrier into the interproximal space and directly deposit fluoride where it is most needed. This action prevents dental caries on intact surfaces, halts the progress of initial caries, and enhances remineralization of enamel. In clinical practice, it is expected that a single dose of Cav-Aid
® should be inserted in each interproximal space in dentition for general caries prevention, and for remineralization, a single dose should be inserted in the proximal surface that has the initial proximal caries. The present study evaluated Cav-Aid
® fluoride delivery strips, in comparison to conventional fluoride treatments, using surface microhardness testing to quantify the remineralization outcomes.
This investigation employed a pH cycling model, which alternates between demineralization and remineralization phases and closely simulates natural fluctuations in oral pH due to dietary acids and salivary buffering [
25,
26]. This provides a realistic simulation of the caries process. This model has been widely used in caries research to evaluate fluoride efficacy and mineral recovery potential [
27,
28]. Additionally, previous studies have demonstrated that in vitro pH cycling studies offer the ability to obtain sufficient data to warrant future clinical trials [
29].
To quantitatively assess remineralization, this study utilized Vickers’ surface microhardness testing, a widely accepted method for evaluating mineralization changes in dental enamel [
30,
31]. This technique is particularly suited for detecting subtle changes in enamel hardness, providing high precision at the microscopic level [
32]. Surface microhardness has been directly correlated with the degree of enamel mineralization and the effectiveness of remineralization agents in previous studies [
30,
31,
32,
33]. Furthermore, bovine enamel was selected as a substitute for human enamel due to their similar mineral compositions, microstructures, and hardness [
34,
35,
36]. Bovine teeth provide a valid model for assessing remineralization treatments, such as fluoride varnish application, as their enamel structure closely resembles that of human teeth [
37]. Additionally, bovine teeth are more ethically and logistically accessible, making them a preferred alternative in laboratory studies [
38].
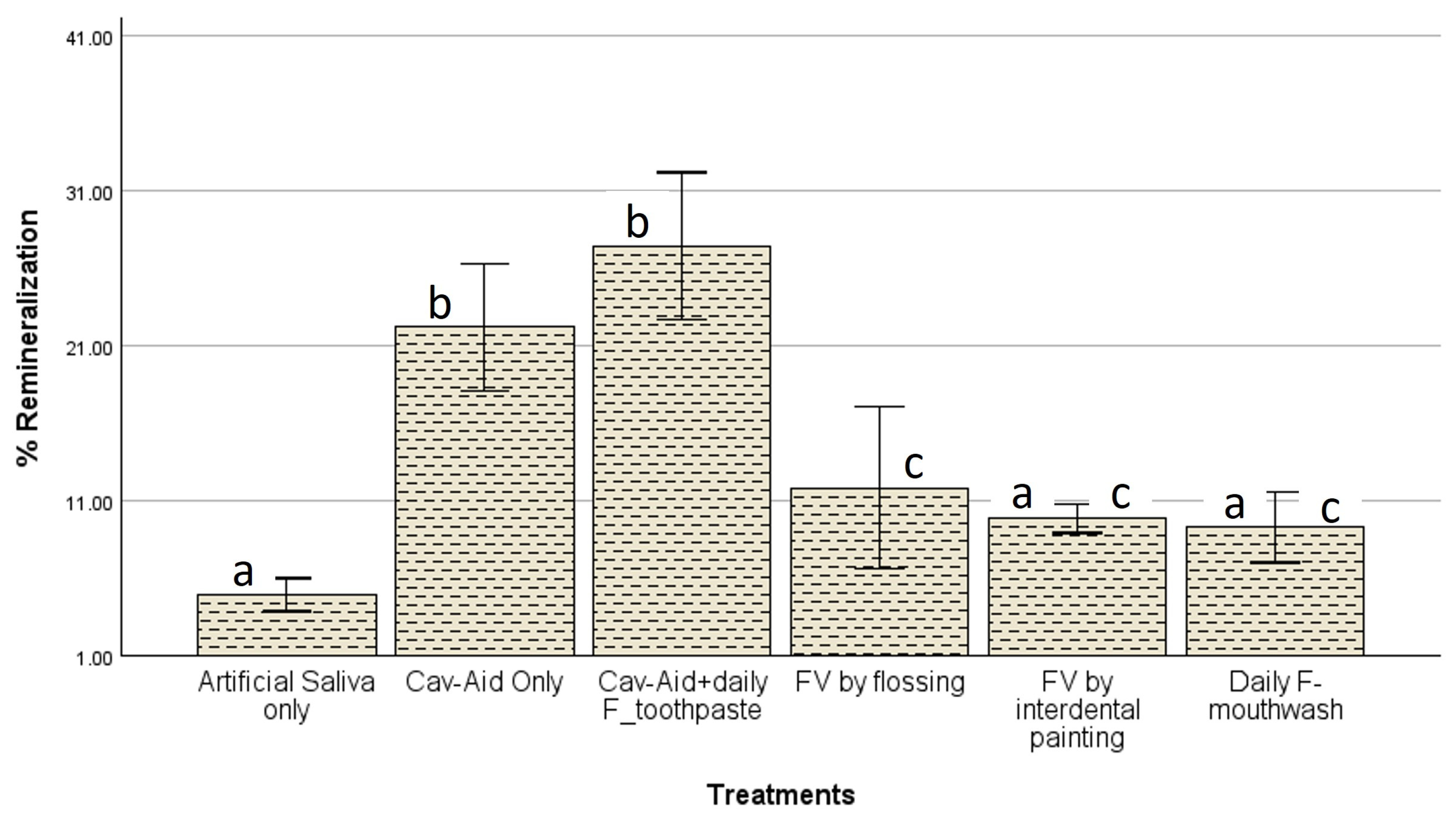
The findings of this study demonstrate that fluoride efficacy varied significantly depending on the delivery method and concentration, and these factors may have influenced the outcomes of the treatments in the present study. The highest remineralization was observed in the Cav-Aid
® combined with daily fluoride toothpaste (CA+F) group, which achieved a remineralization percentage of 27.39%, followed by Cav-Aid
® alone (CA) at 22.16% (
Figure 5). These results support previous evidence that sustained fluoride exposure, such as through daily brushing with fluoride toothpaste, enhances enamel remineralization more effectively than single-dose applications [
39]. Fluoride promotes the transformation of hydroxyapatite into fluorapatite, enhancing enamel resistance to acidic challenges. Additionally, continuous fluoride exposure has been shown to facilitate greater subsurface remineralization than isolated application [
40,
41,
42]. The improved remineralization observed in both CA and CA+F suggests that the targeted delivery mechanism of Cav-Aid
®, particularly when paired with daily fluoride exposure, offers a clinically relevant approach for enhancing proximal enamel repair.
Cav-Aid
® alone achieved a remineralization percentage of 22.16%, which is notably higher than fluoride varnish applied via flossing (11.80%) or painting (9.82%), despite the three groups having 5% NaF without additional daily fluoride application. This suggests that targeted, sustained interproximal fluoride placement, such as that provided by Cav-Aid
®, may significantly enhance fluoride retention in areas where traditional methods fall short. Prior research has shown that single-dose fluoride applications often suffer from rapid clearance due to saliva flow and plaque accumulation, which limits fluoride’s contact time [
43]. Cav-Aid
® likely offers an advantage by physically retaining fluoride within the interproximal space for a longer duration, supporting extended fluoride uptake.
Surprisingly, the current methods used in dental practice for applying fluoride varnish into the interproximal spaces, which are flossing and painting on the interdental cleavage, did not demonstrate significant differences in remineralization (
p > 0.05) when compared with daily fluoride mouthwash and artificial saliva, despite having higher fluoride concentrations (5% NaF) than the mouthwash (0.05% NaF). This finding indicates that these methods of fluoride varnish application do not substantially impact interproximal fluoride penetration. This finding is consistent with research suggesting that tight interproximal contacts reduce fluoride retention, potentially leading to inconsistent fluoride uptake [
44]. Fluoride mouthrinse exhibited a remineralization effect of 9.25%, which was not significantly different from the fluoride varnish groups (
p > 0.05). These findings align with previous research, indicating that fluoride mouthrinses can provide remineralization effects comparable to fluoride varnish [
45]. However, mouthrinses require more frequent applications to maintain efficacy and may be less reliable due to the potential for inconsistent use [
46].
As expected, the artificial saliva group exhibited the lowest remineralization effect (4.89%), substantially lower than all the fluoride-treated groups. Although saliva contains calcium and phosphate ions necessary for natural remineralization, their concentrations are insufficient to drive meaningful mineral deposition without additional therapeutic support [
47]. These findings reinforce the necessity of active fluoride interventions in interproximal caries management, as passive remineralization via saliva alone is insufficient in high-risk sites [
48].
In general, Cav-Aid
® presents a promising clinical device as a targeted interproximal fluoride delivery system. It offers a compelling alternative to traditional fluoride varnish, especially in patients at high risk for interproximal caries. Its ability to place and retain fluoride precisely within tight interproximal spaces addresses a well-known limitation of varnish-based approaches, which often struggle to achieve adequate fluoride penetration in these regions. When used in combination with daily fluoride toothpaste, Cav-Aid
® further improves fluoride retention and uptake, underscoring the value of dual-modality strategies in modern caries management. Given the variability observed among traditional fluoride treatments, Cav-Aid
® may provide a more consistent and predictable approach to delivering effective remineralization in hard-to-reach areas. No doubt, the ever-present dental plaque on interproximal surfaces plays a key role in the effectiveness of fluoride introduced into the interproximal space. The plaque on interproximal surfaces serves as a reservoir for the targeted delivery of active agents to the plaque–tooth interface, to be released during an acidic challenge [
49].
It is pertinent to mention that even though four of the five fluoride groups in the present study have the same fluoride concentration (5% NaF), the percentage of remineralization varies among the experimental groups. This observation can be attributed to the established fact that fluoride efficacy varies significantly depending on the delivery method and concentration [
10]. In the present study, the same delivery method was used for the two Cav-Aid
® groups (CA and CA+F), but CA+F received additional daily fluoride recharge from toothpaste, giving it an advantage for achieving the highest remineralization among the groups, though it was not statistically significant from CA. Despite delivery of fluoride varnish into the interproximal space via floss in FV-floss group, which enabled the direct coating of the sample surfaces with the varnish, the level of remineralization was still significantly lower when compared with the Cav-Aid
® groups with similar concentrations of fluoride (5% NaF). This finding may be attributed to the differences in the amount of the fluoride material delivered to the interproximal space, which obviously was small with FV-floss group compared with the Cav-Aid
® group. Although the FV-paint group had the same 5% NaF concentrations as the FV-floss and the Cav-Aid
® groups, it was not surprising that it achieved a lower level of remineralization, because the fluoride penetration into the interproximal space from this painting delivery method is believed to be through saliva infiltration into the interproximal space. Mouthrinse also delivers fluoride into the interproximal space through the infiltration of the rinse into the space; thus, it is not surprising that it achieved almost the same level of remineralization as the FV-paint group (
Figure 5).
Despite these promising results, this study has several limitations. As an in vitro study, it does not account for patient-specific variables such as salivary flow, oral hygiene behaviors, or dietary habits that may influence fluoride retention and overall treatment efficacy. Additionally, the short-term design did not allow for the evaluation of long-term remineralization durability or lesion arrest. Future research should include longitudinal clinical trials to assess Cav-Aid®’s performance under real-world conditions and determine optimal usage frequency. Another limitation is the use of only one assessment method (surface microhardness). Further comparative studies with other emerging remineralization technologies are also warranted to better define Cav-Aid®’s role in preventive dentistry.