Survival and Marginal Bone Loss in Immediate Post-Extraction Implants versus Delayed Implants: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol
2.2. Eligibility Criteria
2.3. Sources of Information and Search
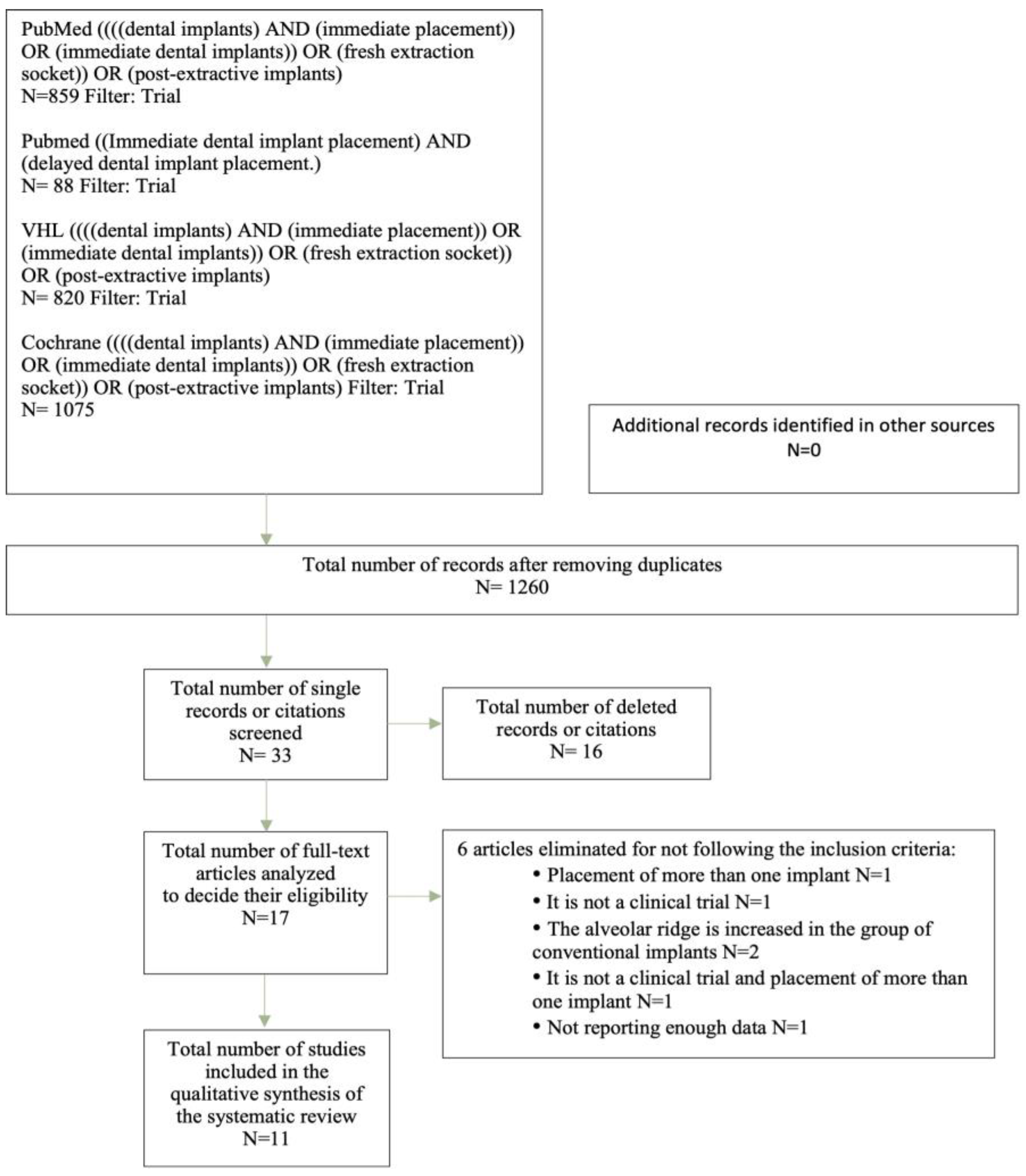
2.4. Selection of Studies
2.5. Data Collection Process and Data List
2.6. Risk of Bias in Each Article and between Studies
2.7. Synthesis of Results
2.8. Publication Bias
2.9. Quality of the Evidence
2.10. Additional Analysis
3. Results
3.1. Selection of Studies
3.2. Study Characteristics
3.3. Risk of Bias in Studies
3.4. Results of Individual Studies
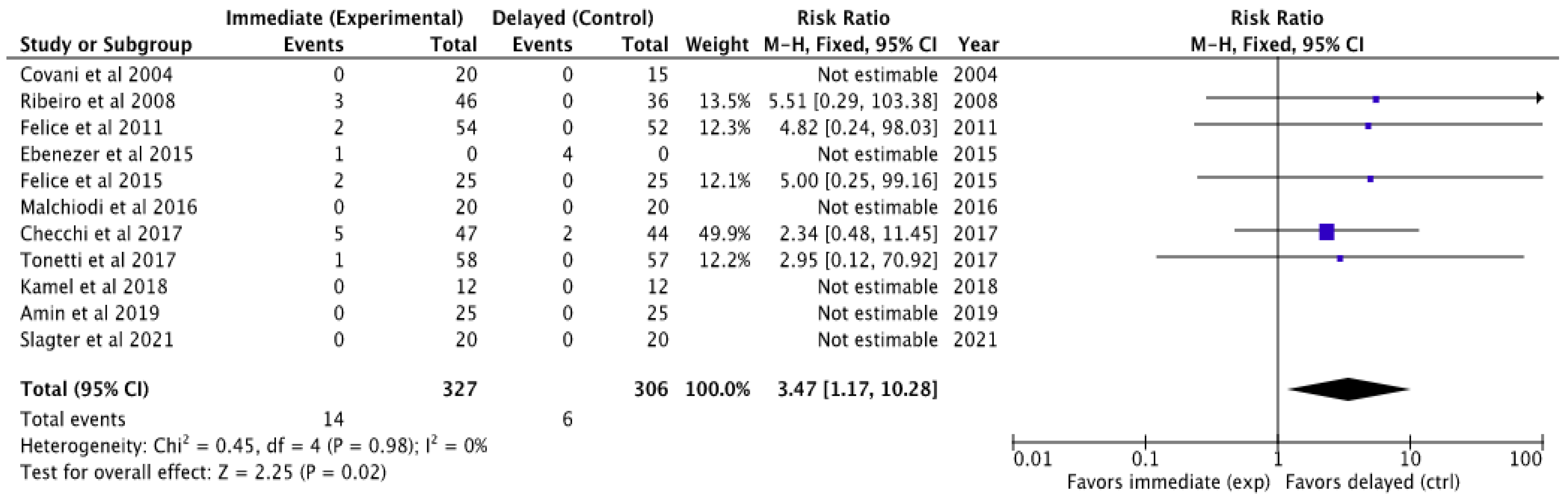
3.4.1. Success Rate of Implants Placed
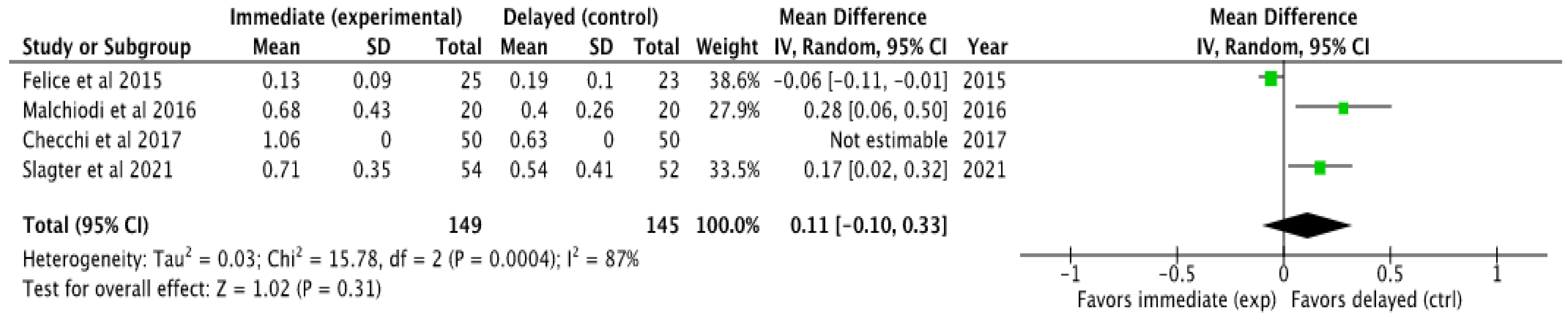
3.4.2. Marginal Bone Loss (MBL)
3.4.3. Bone Graft Placement and Alveolar Preservation
3.4.4. Immediate Non-Functional Loading
3.5. Synthesis of Results
3.5.1. Meta-Analysis of Implant Failures
3.5.2. Meta-Analysis of Marginal Bone Loss (MBL)
3.6. Additional Analysis
Sensitivity Analysis
4. Discussion
4.1. Study Design and Risk of Bias
4.2. Evaluation of Implant Success Rate and Number of Failures
4.3. Assessment of Marginal Bone Loss (MBL)
4.4. Use of Bone Grafts and Preservation of the Alveolar Ridge
4.4.1. Bone Grafts
4.4.2. Alveolar Preservation
4.5. Patient Satisfaction
4.6. Stability of Implants Placed
4.7. Immediate Non-Functional Loading
4.8. Observation Times
4.9. Summary of the Evidence
4.10. Limitations
4.11. Clinical Recommendations
5. Conclusions
- The meta-analysis of implant failures clearly indicates that implants placed following a conventional protocol after bone healing fail less frequently than those placed immediately.
- Considering the small number of clinical trials included in the meta-analysis with respect to marginal bone loss, it can be concluded that there is a tendency to favor implants placed using a delayed protocol in terms of less marginal hard tissue loss, although more studies are needed to corroborate these results.
- The aesthetic results obtained after the placement of an immediate implant are similar to those achieved with a conventional or delayed implant. It should be noted that the placement of an immediate post-extraction implant allows for the positioning of the prosthetic crown with or without occlusion immediately until definitive prosthetic rehabilitation, which considerably enhances aesthetics from the first phase of treatment.
- Overall patient satisfaction with dental implant treatments is high for both study groups: immediate and delayed implants. The immediate placement of the implants has the advantage of a shorter treatment time, which is highly valued by patients.
- There is no consensus in the literature analyzed that explicitly defines the indications for opting for immediate or conventional/deferred treatment, but there are determining factors to consider before choosing one modality over another.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jokstad, A.; Braegger, U.; Brunski, J.B.; Carr, A.B.; Naert, I.; Wennerberg, A. Quality of dental implants. Int. Dent. J. 2003, 53, 409–443. [Google Scholar] [CrossRef]
- Rodas Rivera, R. History of implantology and osseointegration, before and after Branemark. Rev. Estomatol. Hered. 2014, 23, 39. [Google Scholar] [CrossRef][Green Version]
- Bothe, R.T.; Beaton, K.E.; Davenport, H.A. Reaction of bone to multiple metallic implants. Surg. Gynecol. Obstet. 1940, 71, 598–602. [Google Scholar]
- Lemus Cruz, L.M.; Urrutia, Z.A.; Castell, A.C.L. Origin and evolution of dental implants. Rev. Habanera Cienc. Medicas 2009, 8. [Google Scholar]
- Hämmerle, C.H.F.; Chen, S.T.; Wilson, T.G. Consensus statements and recommended clinical procedures regarding the placement of implants in extraction sockets. Int. J. Oral. Maxillofac. Implant. 2004, 19, 26–28. [Google Scholar] [PubMed]
- Albrektsson, T.; Zarb, G.A.; Wormald, P.J.; Eriksson, A. The Long-Term Efficacy of Currently Used Dental Implants: A Review and Proposed Criteria of Success. Int. J. Oral. Maxillofac. Implant. 1986, 1, 11–25. [Google Scholar] [PubMed]
- Araújo, M.G.; Silva, C.O.; Misawa, M.; Sukekava, F. Alveolar socket healing: What can we learn? Periodontology 2000 2015, 68, 122–134. [Google Scholar] [CrossRef]
- Juodzbalys, G.; Stumbras, A.; Goyushov, S.; Duruel, O.; Tözüm, T.F. Morphological Classification of Extraction Sockets and Clinical Decision Tree for Socket Preservation/Augmentation after Tooth Extraction: A Systematic Review. J. Oral. Maxillofac. Res. 2019, 10, e3. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Canullo, L.; Cochran, D.; De Bruyn, H. “Peri-implantitis”: A complication of a foreign body or a man-made “disease”. Facts and Fiction. Clin. Implant. Dent. Relat. Res. 2016, 18, 840–849. [Google Scholar] [CrossRef]
- Blanco, J.; Carral, C.; Argibay, O.; Liñares, A. Implant placement in fresh extraction sockets. Periodontology 2000 2019, 79, 151–167. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; A Akl, E.; E Brennan, S.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019; pp. 1–639. [Google Scholar]
- Aguayo-Albasini, J.L.; Flores-Pastor, B.; Soria-Aledo, V. GRADE System: Classification of Quality of Evidence and Strength of Recommendation. Span. Surg. (Engl. Ed.) 2014, 92, 82–88. [Google Scholar] [CrossRef]
- GRADEpro GDT (Internet). 2021. Available online: https://gdt.gradepro.org/app/ (accessed on 10 May 2022).
- Paolantonio, M.; Dolci, M.; Scarano, A.; D’Archivio, D.; Placido, G.; Tumini, V.; Piattelli, A. Immediate Implantation in Fresh Extraction Sockets. A Controlled Clinical and Histological Study in Man. J. Periodontol. 2001, 72, 1560–1571. [Google Scholar] [CrossRef]
- Polizzi, G.; Grunder, U.; Goené, R.; Hatano, N.; Henry, P.; Jackson, W.J.; Kawamura, K.; Renouard, F.; Rosenberg, R.; Triplett, G.; et al. Immediate and delayed implant placement into extraction sockets: A 5-year report. Clin. Implant. Dent. Relat. Res. 2000, 2, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Grunder, U.; Lithner, B.; Grunder, U.; Polizzi, G.; Goené, R.; Hatano, N.; Kawamura, K.; Köhler, S.; Renouard, F.; Rosenberg, R.; et al. A 3-year prospective multicenter follow-up report on the immediate and delayed-immediate placement of implants. Int. J. Oral. Maxillofac. Implant. 1999, 14, 210–216. [Google Scholar]
- Grandi, T.; Guazzi, P.; Samarani, R.; Grandi, G. A 3-year report from a multicentre randomised controlled trial: Immediately versus early loaded implants in partially edentulous patients. Eur. J. Oral. Implantol. 2013, 6, 217–224. [Google Scholar]
- Slagter, K.W.; Meijer, H.J.A.; Hentenaar, D.F.M.; Vissink, A.; Raghoebar, G.M. Immediate single-tooth implant placement with simultaneous bone augmentation versus delayed implant placement after alveolar ridge preservation in bony defect sites in the esthetic region: A 5-year randomized controlled trial. J. Periodontol. 2021, 92, 1738–1748. [Google Scholar] [CrossRef] [PubMed]
- Schropp, L.; Isidor, F.; Kostopoulos, L.; Wenzel, A. Patient experience of, and satisfaction with, delayed-immediate vs. delayed single-tooth implant placement. Clin. Oral. Implant. Res. 2004, 15, 498–503. [Google Scholar] [CrossRef]
- Ribeiro, F.S.; Pontes, A.E.F.; Marcantonio, E.; Piattelli, A.; Neto, R.J.B.; Marcantonio, E. Success rate of immediate nonfunctional loaded single-tooth implants: Immediate versus delayed implantation. Implant. Dent. 2008, 17, 109–117. [Google Scholar] [CrossRef]
- Covani, U.; Bortolaia, C.; Barone, A.; Sbordone, L. Bucco-lingual crestal bone changes after immediate and delayed implant placement. J. Periodontol. 2004, 75, 1605–1612. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Cortellini, P.; Graziani, F.; Cairo, F.; Lang, N.P.; Abundo, R.; Conforti, G.P.; Marquardt, S.; Rasperini, G.; Silvestri, M.; et al. Immediate versus delayed implant placement after anterior single tooth extraction: The timing randomized controlled clinical trial. J. Clin. Periodontol. 2017, 44, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Ebenezer Vijay Balakrishnan, R.; Christopher, P. Comparitive Study of Immediate Vs Delayed Placement of Implant A Radiographic Evaluation of 60 Patients. BPJ 2015, 8, 513–517. [Google Scholar]
- Kamel, S.; Abd-Elwahab Radi, I.; Kamel, S.; Abd-Elwahab Radi, I. Limited Evidence Suggests Immediate Implant Placement Could be an Alternative to Delayed Implants in Molar Regions. J. Evid. Based Dent. Pract. 2018, 18, 260–262. [Google Scholar] [CrossRef] [PubMed]
- Malchiodi, L.; Balzani, L.; Cucchi, A.; Ghensi, P.; Nocini, P. Primary and Secondary Stability of Implants in Postextraction and Healed Sites: A Randomized Controlled Clinical Trial. Int. J. Oral. Maxillofac. Implant. 2016, 31, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Amin, V.; Kumar, S.; Joshi, S.; Hirani, T.; Shishoo, D. A clinical and radiographical comparison of buccolingual crestal bone changes after immediate and delayed implant placement. Med. Pharm. Rep. 2019, 92, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Felice, P.; Pistilli, R.; Barausse, C.; Trullenque-Eriksson, A.; Esposito, M. Immediate non-occlusal loading of immediate post-extractive versus delayed placement of single implants in preserved sockets of the anterior maxilla: 1-year post-loading outcome of a randomised controlled trial. Eur. J. Oral. Implant. 2015, 8, 361–372. [Google Scholar]
- Checchi, V.; Esposito, M.; Checchi, V.; Felice, P.; Zucchelli, G.; Barausse, C.; Piattelli, M.; Pistilli, R.; Grandi, G.; Esposito, M. Wide diameter immediate post-extractive implants vs delayed placement of normal-diameter implants in preserved sockets in the molar region: 1-year post-loading outcome of a randomised controlled trial. Eur. J. Oral. Implant. 2017, 10, 263–278. [Google Scholar]
- Felice, P.; Soardi, E.; Piattelli, M.; Pistilli, R.; Jacotti, M.; Esposito, M. Immediate non-occlusal loading of immediate post-extractive versus delayed placement of single implants in preserved sockets of the anterior maxilla: 4-month post-loading results from a pragmatic multicentre randomised controlled trial. Eur. J. Oral. Implant. 2011, 4, 329–344. [Google Scholar]
- Misch, C.E.; Perel, M.L.; Wang, H.L.; Sammartino, G.; Galindo-Moreno, P.; Trisi, P.; Steigmann, M.; Rebaudi, A.; Palti, A.; Pikos, M.A.; et al. Implant success, survival, and failure: The International Congress of Oral Implantologists (ICOI) pisa consensus conference. Implant. Dent. 2008, 17, 5–15. [Google Scholar] [CrossRef]
- Diago, M.P.; Dolores, M.; Adrián, G.; Mira, B.G.; Sais, M.I. Bone grafting simultaneous with implant placement. About a case Bone grafting simultaneous to implant placement. Presentation of a case. Med. Oral. Patol. Oral. Cir. Bucal. 2005, 10, 444–447. [Google Scholar]
- García-Gargallo, M.; Yassin-García, S.; Bascones-Martínez, A. Técnicas de preservación de alveolo y de aumento del reborde alveolar: Revisión de la literatura. Av. Periodoncia 2016, 28, 71–81. [Google Scholar]
- Monzón-Trujillo, D.; Martínez-Brito, I.; Rodríguez-Sarduy, R.; Piña-Rodríguez, J.J.; Pérez-Mír, E.A. Injertos óseos en implantología oral. Rev. Med. Electrón. 2014, 36, 449–461. [Google Scholar]
- Araújo, M.G.; Sukekava, F.; Wennström, J.L.; Lindhe, J. Ridge alterations following implant placement in fresh extraction sockets: An experimental study in the dog. J. Clin. Periodontol. 2005, 32, 645–652. [Google Scholar] [CrossRef]
- Araújo, M.G.; Lindhe, J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J. Clin. Periodontol. 2005, 32, 212–218. [Google Scholar] [CrossRef]
- Gallucci, G.O.; Hamilton, A.; Zhou, W.; Buser, D.; Chen, S. Implant placement and loading protocols in partially edentulous patients: A systematic review. Clin. Oral. Implant. Res. 2018, 29, 106–134. [Google Scholar] [CrossRef] [PubMed]


| Classification | Advantages | Disadvantages |
|---|---|---|
| Type 1 | Fewer interventions. Shorter time. Optimal availability of existing bone. | Placement and anchoring are dependent on the morphology of the receiving site. Fine gum biotype hinders optimal outcome. Potential lack of keratinized mucosa for flap adaptation. Possibility of complementary surgical processes. Sensitive to technique. |
| Type 2 | The increase in soft tissue dimension facilitates flap handling. The resolution of local pathology can be evaluated. | Placement and anchoring are dependent on the morphology of the receiving site. More time required. The walls of the alveolus exhibit varying amounts of resorption. Possibility of complementary surgical processes. Sensitive to technique. |
| Type 3 | The substantial bone filling of the socket facilitates implant placement. Greater ease of handling the flap. | More time is required. Possibility of complementary surgical processes. The walls of the alveolus exhibit varying amounts of resorption. |
| Type 4 | Clinically cured bone tissue. Greater ease of handling the flap. | More time is required than types 1, 2, and 3. Possibility of complementary surgical processes. Large variation in bone volume. |
| Author and Year | N Patients Home | N Final Patients | Follow-Up Time [Months] | N Total Implants | N IOII | N IOID |
|---|---|---|---|---|---|---|
| Covani et al. 2004 [22] | 33 | 33 | 12 | 35 | 20 | 15 |
| Salimon Ribeiro et al. 2008 [21] | 64 | 64 | 18–36 | 82 | 46 | 36 |
| Felice et al. 2011 [30] | 106 | 106 | 4 | 106 | 54 | 52 |
| Felice et al. 2015 [28] | 50 | 48 | 12 | 50 | 25 | 25 |
| Ebenezer et al. 2015 [24] | 30 | 30 | 6 | 33 | NS | NS |
| Malchiodi et al. 2016 [26] | 40 | 40 | 12 | 40 | 20 | 20 |
| Checchi et al. 2017 [29] | 100 | 91 | 12 | 100 | 50 | 50 |
| Tonetti et al. 2017 [23] | 124 | 115 | 36 | 124 | 62 | 62 |
| Kamel et al. 2018 [25] | 24 | 24 | 6 | 24 | 12 | 12 |
| Amin et al. 2019 [27] | 50 | 50 | 6 | 50 | 25 | 25 |
| Slagter et al. 2021 [19] | 40 | 35 | 60 | 40 | 20 | 20 |
| Total | 661 | 636 | 684 | 334 | 317 |
| Author and Year | N Total Implants | N Failures II | N Failures ID | Success Rate II [%] | Success Rate ID [%] |
|---|---|---|---|---|---|
| Covani et al. 2004 [22] | 35 | 0 | 0 | 100 | 100 |
| Salimon Ribeiro et al. 2008 [21] | 82 | 3 | 0 | 93.5 | 100 |
| Felice et al. 2011 [30] | 106 | 2 | 0 | 96 | 100 |
| Felice et al. 2015 [28] | 50 | 2 | 0 | 92 | 100 |
| Ebenezer et al. 2015 [24] | 33 | 1 | 4 | NS | NS |
| Malchiodi et al. 2016 [26] | 40 | 0 | 0 | 100 | 100 |
| Checchi et al. 2017 [29] | 100 | 5 | 2 | 89.4 | 95.4 |
| Tonetti et al. 2017 [23] | 124 | 1 | 0 | 98.4 | 100 |
| Kamel et al. 2018 [25] | 24 | 0 | 0 | 100 | 100 |
| Amin et al. 2019 [27] | 50 | 0 | 0 | 100 | 100 |
| Slagter et al. 2021 [19] | 40 | 0 | 0 | 100 | 100 |
| Total | 730 |
| Author and Year | IBL [mm] | SD [mm] | DBL [mm] | SD [mm] | Observation Time [Months] | Measurement Technique |
|---|---|---|---|---|---|---|
| Felice et al. 2015 [28] | 0.13 | 0.09 | 0.19 | 0.10 | 12 | Blinded outcome evaluator on parallelized periapical radiographs |
| Malchiod et al. 2016 [26] | 0.68 | 0.43 | 0.4 | 0.26 | 12 | Parallelized periapical radiography |
| Checchi et al. 2017 [29] | 1.06 * | 0 | 0.63* | 0 | 12 | Blinded outcome evaluator on parallelized periapical radiographs |
| Slagter et al. 2021 [19] | 0.71 | 0.35 | 0.54 | 0.41 | 60 | CBCT |
| Author and Year | Bone Frafting in Immediate Implants [Yes/No] | Type of Bone Graft in Immediate Implants | Membrane Use [Yes/No] | Preservation of the Delayed Alveolar Ridge [Yes/No] |
|---|---|---|---|---|
| Covani et al. 2004 [22] | No | No | No | NS |
| Ribeiro et al. 2008 [21] | No | No | No | No |
| Felice et al. 2011 [30] | Yes | Bio-Oss® | No | Yes |
| Felice et al. 2015 [28] | Yes | NS | No | Yes |
| Ebenezer et al. 2015 [24] | Yes | NS | No | NS |
| Malchiodi et al. 2016 [26] | Yes | Bio-Oss® | No | No |
| Checchi et al. 2017 [29] | Yes | Gen-Os® | No | Yes |
| Tonetti et al. 2017 [23] | Yes | Bio-Oss® | Yes | Yes |
| Kamel et al. 2018 [25] | Yes | Heterologous graft | Yes | Yes |
| Amin et al. 2019 [27] | Yes | NS | No | Yes |
| Slagter et al. 2021 [19] | Yes | Autologous graft from the tuberosity | No | Yes |
| Author and Year | Insertion Torque in Ii [ncm] | Sd | Insertion Torque in Di [ncm] | Sd | ISQ II | Sd | ISQ DI | Sd |
|---|---|---|---|---|---|---|---|---|
| Salimon Ribeiro et al. 2008 [21] | 40 | NS | 40 | NS | NS | NS | NS | NS |
| Felice et al. 2011 [30] | 35 | NS | 35 | NS | NS | NS | NS | NS |
| Felice et al. 2015 [28] | 35 | NS | 35 | NS | NS | NS | NS | NS |
| Malchiodi et al. 2016 [26] | 46 | 9.9 | 52 | 9.23 | 61.9 | 9.99 | 66.00 | 8.25 |
| Certainty Assessment | № of Patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| № of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Immediate Implants | Delayed Implants | Relative (95% CI) | Absolute (95% CI) | ||
| IMPLANTOLOGY FAILURE (follow-up: range 4 months to 60 months) | ||||||||||||
| 11 | randomized trials | not serious | not serious | not serious | not serious | strong association | 333/661 (50.4%) | 328/661 (49.6%) | OR 3.47 (1.17 to 10.28) | 277 more per 1000 (from 39 more to 414 more) | ⨁⨁⨁⨁ High | |
| MARGINAL BONE LOSS AT THE END OF OBSERVATION TIME (follow-up: range 12 months to 60 months; Scale from: 0.13 to 1.06) | ||||||||||||
| 4 | randomized trials | not serious | not serious | not serious | not serious | none | 115 | 115 | - | MD 0.11 SD higher (0.1 lower to 0.33 higher) | ⨁⨁⨁⨁ High | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Portal-Solera, A.; Pardal-Peláez, B. Survival and Marginal Bone Loss in Immediate Post-Extraction Implants versus Delayed Implants: A Systematic Review and Meta-Analysis. Oral 2024, 4, 325-342. https://doi.org/10.3390/oral4030027
Portal-Solera A, Pardal-Peláez B. Survival and Marginal Bone Loss in Immediate Post-Extraction Implants versus Delayed Implants: A Systematic Review and Meta-Analysis. Oral. 2024; 4(3):325-342. https://doi.org/10.3390/oral4030027
Chicago/Turabian StylePortal-Solera, Alba, and Beatriz Pardal-Peláez. 2024. "Survival and Marginal Bone Loss in Immediate Post-Extraction Implants versus Delayed Implants: A Systematic Review and Meta-Analysis" Oral 4, no. 3: 325-342. https://doi.org/10.3390/oral4030027
APA StylePortal-Solera, A., & Pardal-Peláez, B. (2024). Survival and Marginal Bone Loss in Immediate Post-Extraction Implants versus Delayed Implants: A Systematic Review and Meta-Analysis. Oral, 4(3), 325-342. https://doi.org/10.3390/oral4030027