Oral Cancer Disease among the Poor: A Sri Lankan Context
Abstract
1. Introduction
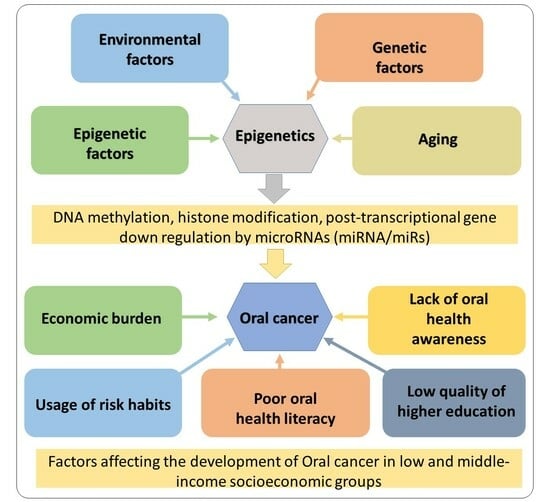
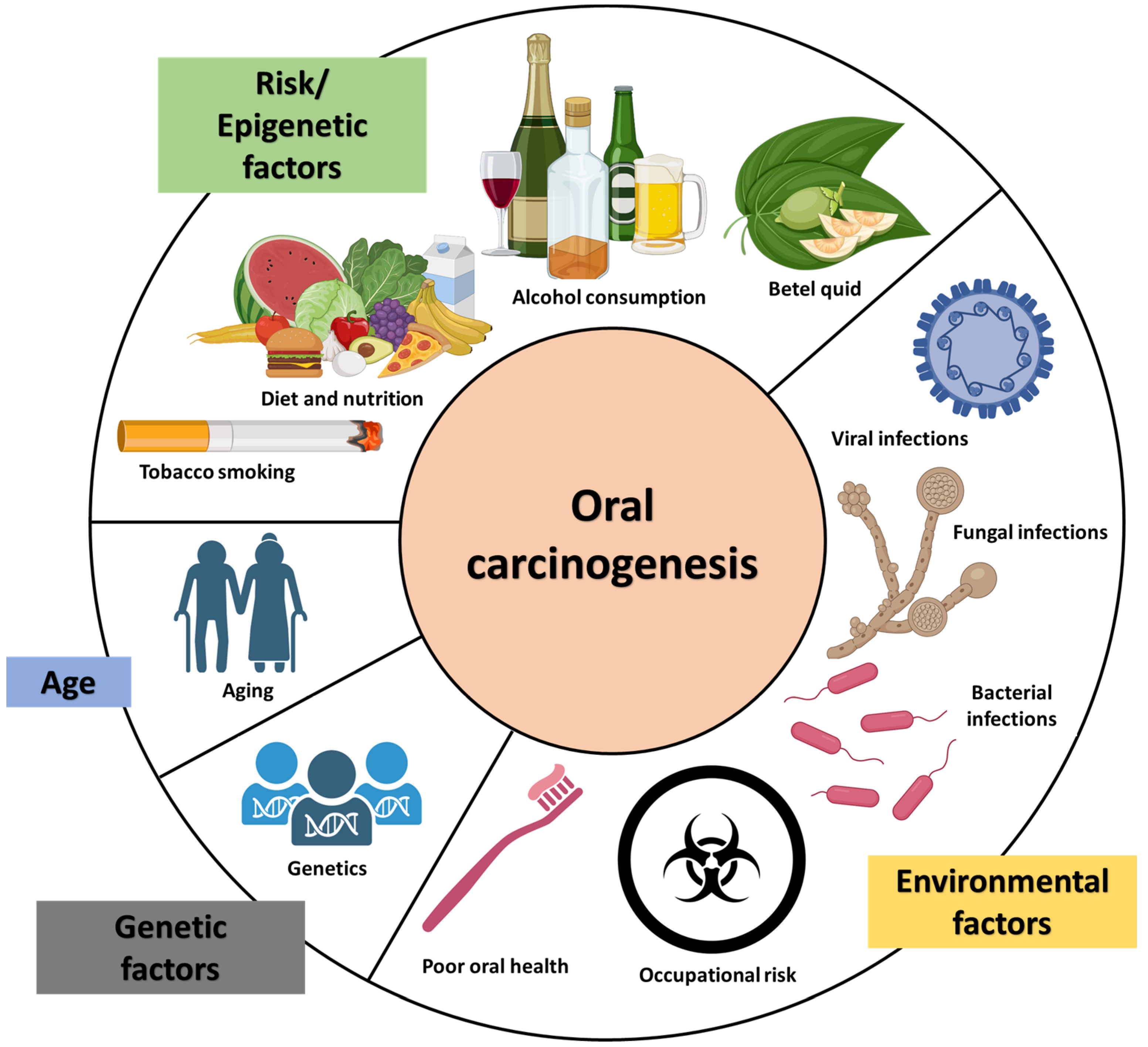
1.1. Causes of OC and OPMD in Sri Lanka
1.2. Undernourishment in the Lower/Middle-Income Group
1.3. OC as a Public Health Concern in Sri Lanka
1.4. Socioeconomic Impact of OC and Reasons for OC among Low/Middle-Income Groups
1.5. OC and OPMD Awareness among Low- and Middle-Income Groups of Sri Lanka
1.6. Future Recommendations
- Cruciferous Vegetables: Vegetables like cabbage, cauliflower, broccoli, and kale are rich in vitamins, minerals, and antioxidants that have been linked to a reduced risk of certain cancers.
- Tomatoes: Tomatoes contain lycopene, a powerful antioxidant that has been associated with a lower risk of prostate cancer. Cooking tomatoes increases the bioavailability of lycopene.
- Garlic: Garlic contains organosulfur compounds that have been shown to have cancer-fighting properties, particularly against stomach and colorectal cancers.
- Turmeric: Curcumin, the active compound in turmeric, has anti-inflammatory and antioxidant properties that may help in preventing certain types of cancer.
- Beans and Lentils: Legumes are high in fiber, which can help regulate blood sugar levels and promote a healthy digestive system. Some studies have suggested that they may also have a protective effect against certain cancers.
- Green Tea: Green tea contains catechins, which are antioxidants that have been associated with a reduced risk of certain cancers.
- Whole Grains: Whole grains like brown rice, whole wheat, and oats are rich in fiber, vitamins, and minerals, and have been linked to a decreased risk of colorectal cancer.
- Onions: Onions, like garlic, contain organosulfur compounds that may help in cancer prevention, especially against stomach and colorectal cancers.
- Citrus Fruits: Citrus fruits such as oranges, lemons, and grapefruits are rich in vitamin C and other antioxidants, which may help protect against certain types of cancer.
2. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Globocan. Cancer Incidence and Mortality Statistics Worldwide and by Region; Globocan: Lyon, France, 2020. [Google Scholar]
- National Cancer Control Programme, Minstry of Health, Nutrition & Indigeneous Medicine. Sri Lanka, Cancer Incidence Data: Sri Lanka Year 2011. In Cancer Incidence Date 2011-Book-Pages-Set 1_Print, 13th ed.; NCCP: Colombo, Sri Lanka, 2011. [Google Scholar]
- National Cancer Control Programme, Minstry of Health, Nutrition & Indigeneous Medicine. Sri Lanka—2014, 2014 Cancer Incidence Data Sri Lanka 2014; Cancer Registry: Colombo, Sri Lanka, 2014. [Google Scholar]
- National Cancer Control Programme, Minstry of Health, Nutrition & Indigeneous Medicine. Sri Lanka—2019, 2019 Cancer Incidence Data Sri Lanka 2019. In Cancer Incidence Data Book—2019; NCCP: Colombo, Sri Lanka, 2019. [Google Scholar]
- Warnakulasuriya, S.; Kujan, O.; Aguirre-Urizar, J.M.; Bagan, J.V.; González-Moles, M.Á.; Kerr, A.R.; Lodi, G.; Mello, F.W.; Monteiro, L.; Ogden, G.R.; et al. Oral potentially malignant disorders: A consensus report from an international seminar on nomenclature and classification, convened by the WHO Collaborating Centre for Oral Cancer. Oral Dis. 2021, 27, 1862–1880. [Google Scholar] [CrossRef]
- Kumari, P.; Debta, P.; Dixit, A. Oral potentially malignant disorders: Etiology, pathogenesis, and transformation into oral cancer. Front. Pharmacol. 2022, 13, 825266. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.; Ariyawardana, A. Malignant transformation of oral leukoplakia: A systematic review of observational studies. J. Oral Pathol. Med. 2016, 45, 155–166. [Google Scholar] [CrossRef]
- Idrees, M.; Farah, C.S.; Khurram, S.A.; Firth, N.; Soluk-Tekkesin, M.; Kujan, O. Observer agreement in the diagnosis of oral lichen planus using the proposed criteria of the American Academy of Oral and Maxillofacial Pathology. J. Oral Pathol. Med. 2021, 50, 520–527. [Google Scholar] [CrossRef]
- Ram, H.; Sarkar, J.; Kumar, H.; Konwar, R.; Bhatt, M.L.; Mohammad, S. Oral cancer: Risk factors and molecular pathogenesis. J. Oral Maxillofac. Surg. 2011, 10, 132–137. [Google Scholar] [CrossRef]
- Amarasinghe, A.A.; Usgodaarachchi, U.S.; Johnson, N.W.; Warnakulasuriya, S. High prevalence of lifestyle factors attributable for oral cancer, and of oral potentially malignant disorders in rural Sri Lanka. Asian Pac. J. Cancer Prev. 2018, 19, 2485. [Google Scholar]
- World Health Organization. Global Health Tobacco Survey, Sri Lanka. Fact Sheet 2015. 2015. Available online: https://www.who.int/ (accessed on 5 May 2023).
- Ministry of Health Sri Lanka. WHO: STEP Wise Approach to Chronic Disease Risk Factor Surveillance Year 2015. Available online: http://health.gov.lk/moh_final/english/ (accessed on 15 August 2023).
- GATS: Global Adult Tobacco Survey, Fact Sheet, Sri Lanka (2020). 2020. Available online: https://adicsrilanka.org/wp-content/uploads/2023/04/SLK_GATS_2019_2020-Factsheet.pdf (accessed on 5 May 2023).
- Conway, D.I.; McMahon, A.D.; Smith, K.; Black, R.; Robertson, G.; Devine, J.; McKinney, P.A. Components of socioeconomic risk associated with head and neck cancer: A population-based case–control study in Scotland. Br. J. Oral Maxillofac. Surg. 2010, 48, 11–17. [Google Scholar] [CrossRef]
- Rylands, J.; Lowe, D.; Rogers, S.N. Outcomes by area of residence deprivation in a cohort of oral cancer patients: Survival, health-related quality of life, and place of death. Oral Oncol. 2016, 52, 30–36. [Google Scholar] [CrossRef]
- Rylands, J.; Lowe, D.; Rogers, S.N. Influence of deprivation on health-related quality of life of patients with cancer of the head and neck in Merseyside and Cheshire. Br. J. Oral Maxillofac. Surg. 2016, 54, 669–676. [Google Scholar] [CrossRef]
- Aelbrecht, K.; Rimondini, M.; Bensing, J.; Moretti, F.; Willems, S.; Mazzi, M.; Fletcher, I.; Deveugele, M. Quality of doctor–patient communication through the eyes of the patient: Variation according to the patient’s educational level. Adv. Health Sci. Educ. 2015, 20, 873–884. [Google Scholar] [CrossRef]
- Network NCI. Evidence to March 2010 on Cancer Inequalities in England; National Cancer Action Team, NHS: Colombo, Sri Lanka, 2010. [Google Scholar]
- Rogers, S.N.; McNally, D.; Mahmoud, M.; Chan, M.F.; Psychol, M.; Humphris, G.M. Psychologic response of the edentulous patient after primary surgery for oral cancer: A cross-sectional study. J. Prosthet. Dent. 1999, 82, 317–321. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Control Programme Sri Lanka. Cancer Incidence Data: Sri Lanka Year 2010, 12th ed.; NCCP: Colombo, Sri Lanka, 2016; p. 8. Available online: https://www.nccp.health.gov.lk/en (accessed on 1 April 2023).
- Ariyawardana, A.; Sitheeque, M.A.; Ranasinghe, A.W.; Perera, I.; Tilakaratne, W.M.; Amaratunga, E.A.; Yang, Y.H.; Warnakulasuriya, S. Prevalence of oral cancer and pre-cancer and associated risk factors among tea estate workers in the central Sri Lanka. J. Oral Pathol. Med. 2007, 36, 581–587. [Google Scholar] [CrossRef] [PubMed]
- De Silva, V.; Samarasinghe, D.; Gunawardena, N. Alcohol and tobacco use among males in two districts in Sri Lanka. Ceylon Med. J. 2009, 54, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Evans, D.B.; Evans, D.B. (Eds.) Health Systems Performance Assessment: Debates, Methods and Empiricism; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Perera, B.; Fonseka, P.; Ekanayake, R.; Lelwala, E. Smoking in adults in Sri Lanka: Prevalence and attitudes. Asia Pac. J. Public Health 2005, 17, 40–45. [Google Scholar] [CrossRef]
- Zain, R.B. Cultural and dietary risk factors of oral cancer and precancer-a brief overview. Oral Oncol. 2001, 37, 205–210. [Google Scholar] [CrossRef]
- Amarasinghe, H.K.; Usgodaarachchi, U.; Kumaraarachchi, M.; Johnson, N.W.; Warnakulasuriya, S. Diet and risk of oral potentially malignant disorders in rural Sri Lanka. J. Oral Pathol. Med. 2013, 42, 656–662. [Google Scholar] [CrossRef]
- National Cancer Control Programme (NCCP). 2019. Available online: https://www.nccp.health.gov.lk/en (accessed on 1 April 2023).
- Amarasinghe, H.K.; Usgodaarachchi, U.S.; Johnson, N.W.; Lalloo, R.; Warnakulasuriya, S. Public awareness of oral cancer, of oral potentially malignant disorders and of their risk factors in some rural populations in Sri Lanka. Community Dent. Oral Epidemiol. 2010, 38, 540–548. [Google Scholar] [CrossRef]
- Amarasinghe, H.; Rathnapriya, M.; Abeysundara, A.; Jayaweera, S.; Jayathilake, A.; Jayasinghe, R. Assessment of the oral cancer control activities through care seeking behavior of hospital attendees and their level of awareness. J. Oral Biol. Craniofac. Res. 2021, 11, 536–540. [Google Scholar] [CrossRef]
- CEPA. The Correlation between Poverty and Inequality. 2021. Available online: https://www.cepa.lk/blog/the-correlation-between-poverty-and-inequality/ (accessed on 1 April 2023).
- Central Bank of Sri Lanka. Sri Lanka Socio Economic Data 2018. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwiX_KjzwJCBAxWWDN4KHUDKAysQFnoECBYQAQ&url=https%3A%2F%2Fwww.cbsl.gov.lk%2Fsites%2Fdefault%2Ffiles%2Fcbslweb_documents%2Fstatistics%2FSri_Lanka_%2520Socio_Economic_Data_2018_e.pdf&usg=AOvVaw1_imFUCoKz73GEFShNbhiw&opi=89978449 (accessed on 1 April 2023).
- Hagedoorn, P.; Vandenheede, H.; Vanthomme, K.; Willaert, D.; Gadeyne, S. A cohort study into head and neck cancer mortality in Belgium (2001–11): Are individual socioeconomic differences conditional on area deprivation? Oral Oncol. 2016, 61, 76–82. [Google Scholar] [CrossRef]
- Warnakulasuriya, S. Significant oral cancer risk associated with low socioeconomic status. Evid. Based Dent. 2009, 10, 4–5. [Google Scholar] [CrossRef]
- Ghorbani, Z.; Peres, K.G. Is the association between socioeconomic status and nonreplaced extracted teeth mediated by dental care behaviours in adults? Community Dent. Oral Epidemiol. 2015, 43, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, Z.; Ahmady, A.E.; Ghasemi, E.; Zwi, A. Socioeconomic inequalities in oral health among adults in Tehran, Iran. Community Dent. Health 2015, 32, 26–31. [Google Scholar] [PubMed]
- Akram, M.; Siddiqui, S.A.; Karimi, A.M. Patient related factors associated with delayed reporting in oral cavity and oropharyngeal cancer. Int. J. Prev. Med. 2014, 5, 915. [Google Scholar] [PubMed]
- Azimi, S.; Ghorbani, Z.; Ghasemi, E.; Tennant, M.; Kruger, E. Does socioeconomic status influence oral cancer awareness? The role of public education. East. Mediterr. Health J. 2020, 26, 1510–1517. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, R.; John, J.; Saravanan, S. Socio demographic profile of oral cancer patients residing in Tamil Nadu-A hospital-based study. Indian J. Cancer 2013, 50, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Al-Hakimi, H.A.; Othman, A.E.; Mohamed, O.G.; Saied, A.M.; Ahmed, W.A. Public knowledge of oral cancer and modelling of demographic background factors affecting this knowledge in Khartoum State, Sudan. Sult. Qaboos Univ. Med. J. 2016, 16, e335. [Google Scholar] [CrossRef]
- Dost, F.; Do, L.; Farah, C.S. Knowledge of oral cancer risk factors amongst high-risk Australians: Findings from the LESIONS programme. Aust. Dent. J. 2016, 61, 432–439. [Google Scholar] [CrossRef]
- Riechelmann, H. Occupational exposure and cancer of the oral cavity and pharynx. Laryngorhinootologie 2002, 81, 573–579. [Google Scholar] [CrossRef]
- Marmot, M.G.; Stansfeld, S.; Patel, C.; North, F.; Head, J.; White, I.; Brunner, E.; Feeney, A.; Smith, G.D. Health inequalities among British civil servants: The Whitehall II study. Lancet 1991, 337, 1387–1393. [Google Scholar] [CrossRef]
- Stewart, P.A.; Schairer, C.; Blair, A. Comparison of jobs, exposures, and mortality risks for short-term and long-term workers. J. Occup. Med. 1990, 1, 703–708. [Google Scholar]
- Bartley, M. Unemployment and ill health: Understanding the relationship. J. Epidemiol. Community Health 1994, 48, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Marmot, M. Smoking and inequalities. Lancet 2006, 368, 341–342. [Google Scholar] [CrossRef] [PubMed]
- Stead, M.; MacAskill, S.; MacKintosh, A.M.; Reece, J.; Eadie, D. “It’s as if you’re locked in”: Qualitative explanations for area effects on smoking in disadvantaged communities. Health Place 2001, 7, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Marmot, M. Inequality, deprivation and alcohol use. Addiction 1997, 92, S13–S20. [Google Scholar] [CrossRef] [PubMed]
- Rose, G.A.; Khaw, K.T.; Marmot, M. Rose’s Strategy of Preventive Medicine: The Complete Original Text; Oxford University Press: Oxford, UK, 2008. [Google Scholar]
- Lynch, J.W.; Smith, G.D.; Kaplan, G.A.; House, J.S. Income inequality and mortality: Importance to health of individual income, psychosocial environment, or material conditions. BMJ 2000, 320, 1200–1204. [Google Scholar] [CrossRef] [PubMed]
- Wrigley, N. ‘Food deserts’ in British cities: Policy context and research priorities. Urban Stud. 2002, 39, 2029–2040. [Google Scholar] [CrossRef]
- Macintyre, S.; Maciver, S.; Sooman, A. Area, class and health: Should we be focusing on places or people? J. Soc. Policy 1993, 22, 213–234. [Google Scholar] [CrossRef]
- Adams, J.M.; White, M. Biological ageing: A fundamental, biological link between socio-economic status and health? Eur. J. Public Health 2004, 14, 331–334. [Google Scholar] [CrossRef]
- Cawthon, R.M.; Smith, K.R.; O’Brien, E.; Sivatchenko, A.; Kerber, R.A. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet 2003, 361, 393–395. [Google Scholar] [CrossRef]
- Amarasinghe, H.; Jayasinghe, R.D.; Dharmagunawardene, D.; Attygalla, M.; Scuffham, P.A.; Johnson, N.; Kularatna, S. Economic burden of managing oral cancer patients in Sri Lanka: A cross-sectional hospital-based costing study. BMJ Open 2019, 9, e027661. [Google Scholar] [CrossRef]
- Stewart, B.W.; Kleihues, P. (Eds.) World Cancer Report; IARC Press: Lyon, France, 2003. [Google Scholar]
- Nagao, T.; Ikeda, N.; Warnakulasuriya, S.; Fukano, H.; Yuasa, H.; Yano, M.; Miyazaki, H.; Ito, Y. Serum antioxidant micronutrients and the risk of oral leukoplakia among Japanese. Oral Oncol. 2000, 36, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S. Food, nutrition and oral cancer. In Food Constituents and Oral Health; Woodhead Publishing: Cambridge, UK, 2009; pp. 273–295. [Google Scholar]
- Alahapperuma, L.S.; Fernando, E.A. Patient-linked factors associated with delayed reporting of oral and pharyngeal carcinoma among patients attending national cancer institute, Maharagama, Sri Lanka. Asian Pac. J. Cancer Prev. 2017, 18, 321. [Google Scholar] [PubMed]
- Ariyawardana, A.; Vithanaarachchi, N. Awareness of oral cancer and precancer among patients attending a hospital in Sri Lanka. Asian Pac. J. Cancer Prev. 2005, 6, 58–61. [Google Scholar]
- Ord, R.A.; Blanchaert, R.H., Jr. Current management of oral cancer: A multidisciplinary approach. J. Am. Dent. Assoc. 2001, 132, 19S–23S. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Madhulaxmi, M.; Iyer, K.; Periasamy, R.; Gajendran, P.; Lakshmi, T. Role of cisplatin in oral squamous cell carcinoma—A review. J. Adv. Pharm. Educ. Res. 2017, 7, 39–42. [Google Scholar]
- American Cancer Society. Oral Cavity (Mouth) and Oropharyngeal (Throat) Cancer; American Cancer Society: Atlanta, GA, USA, 2014; Available online: https://www.cancer.org/cancer/types/oral-cavity-and-oropharyngeal-cancer.html (accessed on 15 August 2023).
- Yeh, S.A. Radiotherapy for head and neck cancer. In Seminars in Plastic Surgery; Thieme Medical Publishers ©: New York, NY, USA, 2010; Volume 24, Number 2; pp. 127–136. [Google Scholar]
- Blanchard, P.; Baujat, B.; Holostenco, V.; Bourredjem, A.; Baey, C.; Bourhis, J.; Pignon, J.-P.; MACH-CH Collaborative Group. Meta-analysis of Chemotherapy in Head and Neck Cancer (MACH-NC): A comprehensive analysis by Tumour Site. Radiother. Oncol. 2011, 100, 33–40. [Google Scholar] [CrossRef]
- Huang, S.H.; O’Sullivan, B. Oral cancer: Current role of radiotherapy and chemotherapy. Med. Oral Patol. Oral Cir. Bucal. 2013, 18, e233–e240. [Google Scholar] [CrossRef]
- Alzahrani, R.; Obaid, A.; Al-Hakami, H.; Alshehri, A.; Al-Assaf, H.; Adas, R.; Alduhaibi, E.; Alsafadi, N.; Alghamdi, S.; Alghamdi, M.; et al. Locally advanced oral cavity cancers: What is the Optimal Care? Cancer Control 2020, 27, 1073274820920727. [Google Scholar] [CrossRef]
- Prelec, J.; Laronde, D.M. Treatment modalities of oral cancer. Can. J. Dent. Hyg. 2014, 48, 13–19. [Google Scholar]
- Devi, S.; Singh, N. Dental care during and after radiotherapy in head and neck cancer. Natl. J. Maxillofac. Surg. 2014, 5, 117–125. [Google Scholar] [CrossRef]
- Sroussi, H.Y.; Epstein, J.B.; Bensadoun, R.J.; Saunders, D.P.; Lalla, R.V.; Migliorati, C.A.; Heaivilin, N.; Zumsteg, Z.S. Common oral complications of head and neck cancer radiation therapy: Mucositis, infections, saliva change, fibrosis, sensory dysfunctions, dental caries, periodontal disease, and osteoradionecrosis. Cancer Med. 2017, 6, 2918–2931. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services. Oral Health in America: A Report of the Surgeon General; U.S. Department of Health and Human Services: Washington, DC, USA, 2000; Volume 44. [Google Scholar]
- Shavi, G.R.; Thakur, B.; Bhambal, A.; Jain, S.; Singh, V.; Shukla, A. Oral Health Related Quality of Life in Patients of Head and Neck Cancer Attending Cancer Hospital of Bhopal City, India. J. Int. Oral Health 2015, 7, 21–27. [Google Scholar] [PubMed]
- Gupta, T.; Sinha, S.; Ghosh-Laskar, S.; Budrukkar, A.; Mummudi, N.; Swain, M.; Phurailatpam, R.; Prabhash, K.; Agarwal, J.P. Intensity-modulated radiation therapy versus three-dimensional conformal radiotherapy in head and neck squamous cell carcinoma: Long-term and mature outcomes of a prospective randomized trial. Radiat. Oncol. 2020, 15, 218. [Google Scholar] [CrossRef]
- National Cancer Control Programme Sri Lanka. Cancer Incidence & Mortality Data Sri Lanka 2019; Ministry of Health Sri Lanka: Colombo, Sri Lanka, 2021. [Google Scholar]
- Joseph, N.; Gunasekera, S.; Ariyaratne, Y.; Choudhury, A. Clinical oncology in Sri Lanka: Embracing the Promise of the future. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Kosgallana, S.; Jayasekara, P.; Abeysinghe, P.; Lalloo, R. Oral health related quality of life of oral cancer patients treated with radiotherapy alone or with chemotherapy in a tertiary referral centre in Sri Lanka. BMC Oral Health 2023, 23, 162. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.W.; Warnakulasuriya, S.; Gupta, P.C.; Dimba, E.; Chindia, M.; Otoh, E.C.; Sankaranarayanan, R.; Califano, J.; Kowalski, L. Global oral health inequalities in incidence and outcomes for oral cancer: Causes and solutions. Adv. Dent. Res. 2011, 23, 237–246. [Google Scholar] [CrossRef]
- Sankaranarayanan, R.; Ramadas, K.; Amarasinghe, H.; Subramanian, S.; Johnson, N. Oral Cancer: Prevention, Early Detection, and Treatment. Cancer: Disease Control Priorities, 3rd ed.; The International Bank for Reconstruction and Development: Washington, DC, USA; The World Bank: Washington, DC, USA, 2015; Volume 3. [Google Scholar]
- Jeihooni, A.K.; Jafari, F. Oral Cancer: Epidemiology, Prevention, Early Detection, and Treatment; Intech Open: Rijeka, Croatia, 2021. [Google Scholar]
- Abati, S.; Bramati, C.; Bondi, S.; Lissoni, A.; Trimarchi, M. Oral cancer and precancer: A narrative review on the relevance of early diagnosis. Int. J. Environ. Res. Public Health 2020, 17, 9160. [Google Scholar] [CrossRef]
- Van der Waal, I.; de Bree, R.; Brakenhoff, R.; Coebegh, J.W. Early diagnosis in primary oral cancer: Is it possible? Med. Oral Patol. Oral Cir. Bucal 2011, 16, e300-5. [Google Scholar] [CrossRef]
- Lim, K.; Moles, D.R.; Downer, M.C.; Speight, P.M. Opportunistic screening for oral cancer and precancer in general dental practice: Results of a demonstration study. Br. Dent. J. 2003, 194, 497–502. [Google Scholar] [CrossRef]
- Shrestha, G.; Maharjan, L. Mouth self-examination for prevention and control of oral cavity cancer. J. Nepal Med. Assoc. 2020, 58, 360. [Google Scholar] [CrossRef]
- Hung, L.C.; Kung, P.T.; Lung, C.H.; Tsai, M.H.; Liu, S.A.; Chiu, L.T.; Huang, K.H.; Tsai, W.C. Assessment of the risk of oral cancer incidence in a high-risk population and establishment of a predictive model for oral cancer incidence using a population-based cohort in Taiwan. Int. J. Environ. Res. Public Health 2020, 17, 665. [Google Scholar] [CrossRef] [PubMed]
- Jornet, P.L.; Garcia, F.G.; Berdugo, M.L.; Perez, F.P.; Lopez, A.P. Mouth self-examination in a population at risk of oral cancer. Aust. Dent. J. 2015, 60, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Dionne, K.R.; Warnakulasuriya, S.; Binti, Z.R.; Cheong, S.C. Potentially malignant disorders of the oral cavity: Current practice and future directions in the clinic and laboratory. Int. J. Cancer 2015, 136, 503–515. [Google Scholar] [CrossRef]
- Peterson, P.E. The WHO perspective: Strengthening the Prevention of Oral Cancer. Community Dent. Oral Epidemiol. 2005, 33, 397–399. [Google Scholar]

| Author | Year of Publication | Inclusion and Exclusion Criteria | Research Objectives | Study Design | Sample Characteristics | Study Instrument | Conclusion |
|---|---|---|---|---|---|---|---|
| Perera et al. [24] | 2005 | Inclusion criteria were residents of southern district of Sri Lanka | To identify smoking prevalence rates by gender and age and respondents’ attitudes toward smoking and possible associations between alcohol use and smoking. | Descriptive cross-sectional survey | Cluster sampling method was used to select respondents. Considering the time and resources, authors chose to survey nearly 1600 adults in the population. | Self-administered anonymous questionnaire | Higher prevalence rates for tobacco were observed among less educated, middle-aged men who were from underprivileged families. Alcohol use seems to be positively associated with smoking. |
| Ariyawardana and Vithanaarachchi [59] | 2005 | Inclusion criteria were outpatients attending the Dental Teaching Hospital, Faculty of Dental Sciences, University of Peradeniya, Sri Lanka | To investigate OC and precancer awareness among patients undergoing dental treatment at a university dental hospital. | Questionnaire-type survey | Subjects (n = 410) were randomly chosen | Self-administered questionnaire | According to the results of this survey, out patients were adequately informed about OC. However, precancer awareness was relatively poor. In comparison to betel chewing, knowledge of the causal links between cigarette smoking and alcohol usage was minimal. |
| Ariyawardana et al. [21] | 2007 | Inclusion criteria were residents in or attached to a nearby estate of the central province, Sri Lanka. | To obtain demographic data on risk factors associated with OC and OPMD and to conduct and report the outcome of an oral mucosal examination on tea estate laborers in Sri Lanka. | - | 12,716 tea laborers over the age of 15 years employed by 73 tea estates in central Sri Lanka by estate medical officers | Questionnaires and oral examination | The prevalence of oral pre-cancer in tea estate laborers was higher than estimates reported in previous studies. |
| de Silva et al. [22] | 2009 | Inclusion criteria were males residents of Colombo and Polonnaruwa over 18 years of age | To investigate the prevalence and consumption of tobacco and alcohol among males in the Colombo and Polonnaruwa districts | A cross-sectional study based on multistage cluster sampling | The sample consisted of males over 18 years. There were 1318 from the Colombo District and 1366 from the Polonnaruwa District. | Interviewer-administered questionnaire | According to the mean alcohol intake and the number of people who consumed spirits on a regular basis, high-risk alcohol use was prevalent, particularly in urban regions. Smoking prevalence was substantially lower than in many Asian countries, but comparable to Western Europe. |
| Amarasinghe et al. [28] | 2010 | Inclusion criteria were residents of Sabaragamuwa province. | To investigate the level of public awareness of OC and OPMD and of risk factors | A cross-sectional community-based survey | A total of 1029 subjects were selected. | Interviewer-administered questionnaire | Knowledge of OC, OPMD and their associated risk factors was poor among this population, indicating an urgent need to implement public health education and promotion strategies. |
| Amarasinghe et al. [26] | 2013 | Inclusion criteria were residents of Sabaragamuwa province. | To investigated the association of OPMD (and leukoplakia as a subgroup) with the consumption of fruits, vegetables, chillis and tea. | A cross-sectional community survey | A total of 1029 subjects were selected by a multistage, stratified and clustered sampling technique. | Interviewer-administered questionnaire | This study reveals the prevalence of malnutrition in this rural population, with relatively low daily consumption of fruits and vegetables. Cancer-preventive characteristics in their diets are limited and overshadowed by known carcinogenic substances associated with betel quid, cigarette, and alcohol usage. |
| Alahapperuma and Fernando [58] | 2017 | Inclusion criteria were those who have been diagnosed with oral and pharyngeal cancer within 3 months of the interview date. Mentally handicapped patients and patients who were debilitated and unable to respond were excluded. | To identify patient-linked delays between the time of noticing the symptoms and definitive diagnosis and its association with the stage at diagnosis and socio-demographic factors among oral and pharyngeal carcinoma patients attending the National Cancer Institute, Maharagama. | A hospital-based descriptive cross-sectional study | 351 patients with histologically confirmed carcinoma of the oral cavity and pharynx. | Interviewer-administered questionnaire | Stage at diagnosis was associated with ‘Patient Delay-1’ (p = 0.001) but not with ‘Patient Delay-2’ (p = 0.001). ‘Patient Delay-1’ was significantly associated with education level (p = 0.001) and travel cost (p = 0.048). |
| Amarasinghe et al. [10] | 2018 | Inclusion criteria were residents of Sabaragamuwa province. | To determine the prevalence of OPMD and of lifestyle factors among the population in the Sabaragamuwa Province of Sri Lanka. | A cross-sectional community based study | A total of 1029 subjects above the age of 30 years | Interviewer-administered questionnaire | This study reveals that in these populations, OPMD and OC risk factors are highly prevalent. For the purpose of preventing OC, a comprehensive approach to manage cigarette, betel nut, and alcohol usage is urgently needed. |
| Amarasinghe et al. [54] | 2019 | The study was conducted in selected cancer treatment centers in Sri Lanka. | To estimate the costs of managing patients with OC in Sri Lanka for a 12 month period from diagnosis. | Hospital-based costing study (activity-based costing with cost apportionment and step-down costing approach was used). | Sixty-nine OC patients: 60 were males and 12 had recurrent tumors. | Interviewer-administered questionnaire | Because of the high prevalence of OC in Sri Lanka, the economic expenditures related to these diseases are significant, wreaking havoc on both the healthcare system and individual families, and severely affecting the country’s economy. |
| Amarasinghe et al. [29] | 2021 | Inclusion criteria were patients who were seeking care from the Out Patients Department attendees of the Institute of Oral Health, Maharagama, Sri Lanka. Patients who refused to provide relevant information were excluded from the study. | To assess the care seeking pattern and behavior and its associated factors for OPMD among the patients’ attendees and also to evaluate the impact of the existing early detection program for OC. | A hospital based descriptive cross-sectional study | A total number of 110 OPMD/oral cancer patients were recruited. | Interviewer-administered questionnaire | The general public was unaware about OPMD and its risk factors. The fact that incidental findings during dental screening are the primary route of identification of OPMD emphasizes the need of doing opportunistic screening in dental settings. |
| Kosgallana et al. [75] | 2023 | Inclusion criteria were OC patients awaiting radiotherapy alone or with chemotherapy at the National Cancer Institute (Apeksha Hospital), Maharagama, Sri Lanka. | To evaluate the OHRQOL and its changes from baseline through the last week of radiotherapy and three months after radiotherapy in patients with OC who underwent this treatment alone or in combination with chemotherapy. | A prospective longitudinal study. | 90 OC patients. | The modified Sinhala version of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Oral Health Module (EORTC QLQ-OH15) was used to gather data related to OHRQOL before radiotherapy. | The OHRQOL of oral cancer patients who received radiotherapy alone or in combination with chemotherapy worsened from baseline to the last week of radiotherapy, but subsequently improved three months later. However, three months after radiation, the OHRQOL did not revert to the baseline level. OHRQOL during the final week of radiation was affected by baseline OHRQOL, civil status, and metastatic sites. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senevirathna, K.; Jayasinghe, Y.A.; Jayawickrama, S.M.; Amarasinghe, H.; Jayasinghe, R.D. Oral Cancer Disease among the Poor: A Sri Lankan Context. Oral 2023, 3, 420-436. https://doi.org/10.3390/oral3030034
Senevirathna K, Jayasinghe YA, Jayawickrama SM, Amarasinghe H, Jayasinghe RD. Oral Cancer Disease among the Poor: A Sri Lankan Context. Oral. 2023; 3(3):420-436. https://doi.org/10.3390/oral3030034
Chicago/Turabian StyleSenevirathna, Kalpani, Yovanthi Anurangi Jayasinghe, Shalindu Malshan Jayawickrama, Hemantha Amarasinghe, and Ruwan Duminda Jayasinghe. 2023. "Oral Cancer Disease among the Poor: A Sri Lankan Context" Oral 3, no. 3: 420-436. https://doi.org/10.3390/oral3030034
APA StyleSenevirathna, K., Jayasinghe, Y. A., Jayawickrama, S. M., Amarasinghe, H., & Jayasinghe, R. D. (2023). Oral Cancer Disease among the Poor: A Sri Lankan Context. Oral, 3(3), 420-436. https://doi.org/10.3390/oral3030034