In Vitro Evaluation of Dental Resin Monomers, Triethylene Glycol Dimethacrylate (TEGDMA), and 2-Hydroxyethyl Methacrylate (HEMA) in Primary Human Melanocytes: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Determination of Cytotoxicity by LDH and MTS Assays
2.4. Determination of Intracellular Melanin
2.5. Intracellular Tyrosinase Activity
2.6. Cell-Free Tyrosinase Activity Assay
2.7. Quantitation of Melanocyte Dendricity
2.8. Intracellular ROS Assay
2.9. IL-6 Cytokine Assay
2.10. Statistical Analysis
3. Results
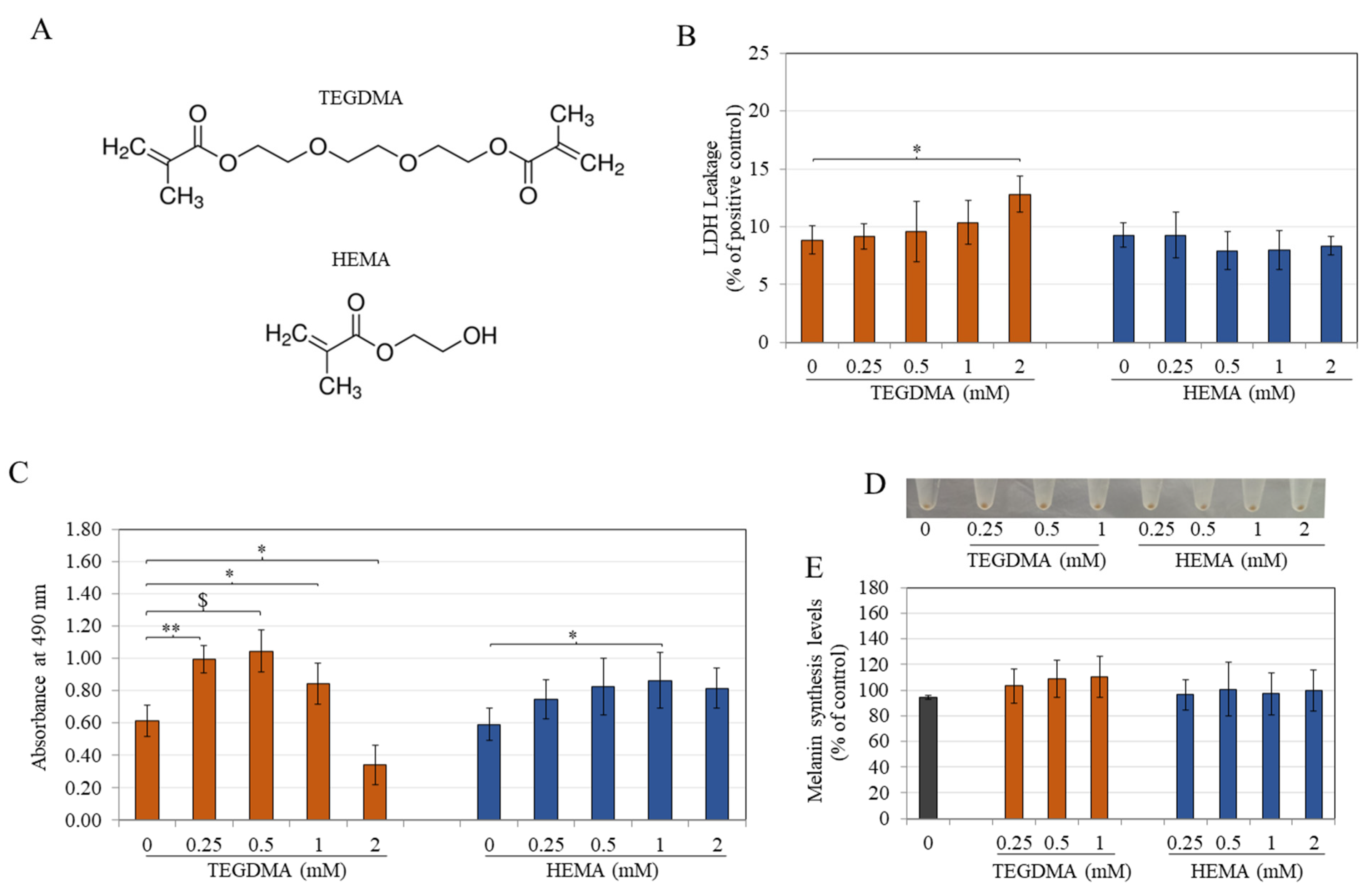
3.1. TEGDMA Is More Cytotoxic Than HEMA to Melanocytes
3.2. TEGDMA and HEMA Do Not Affect Intracellular Melanin Levels
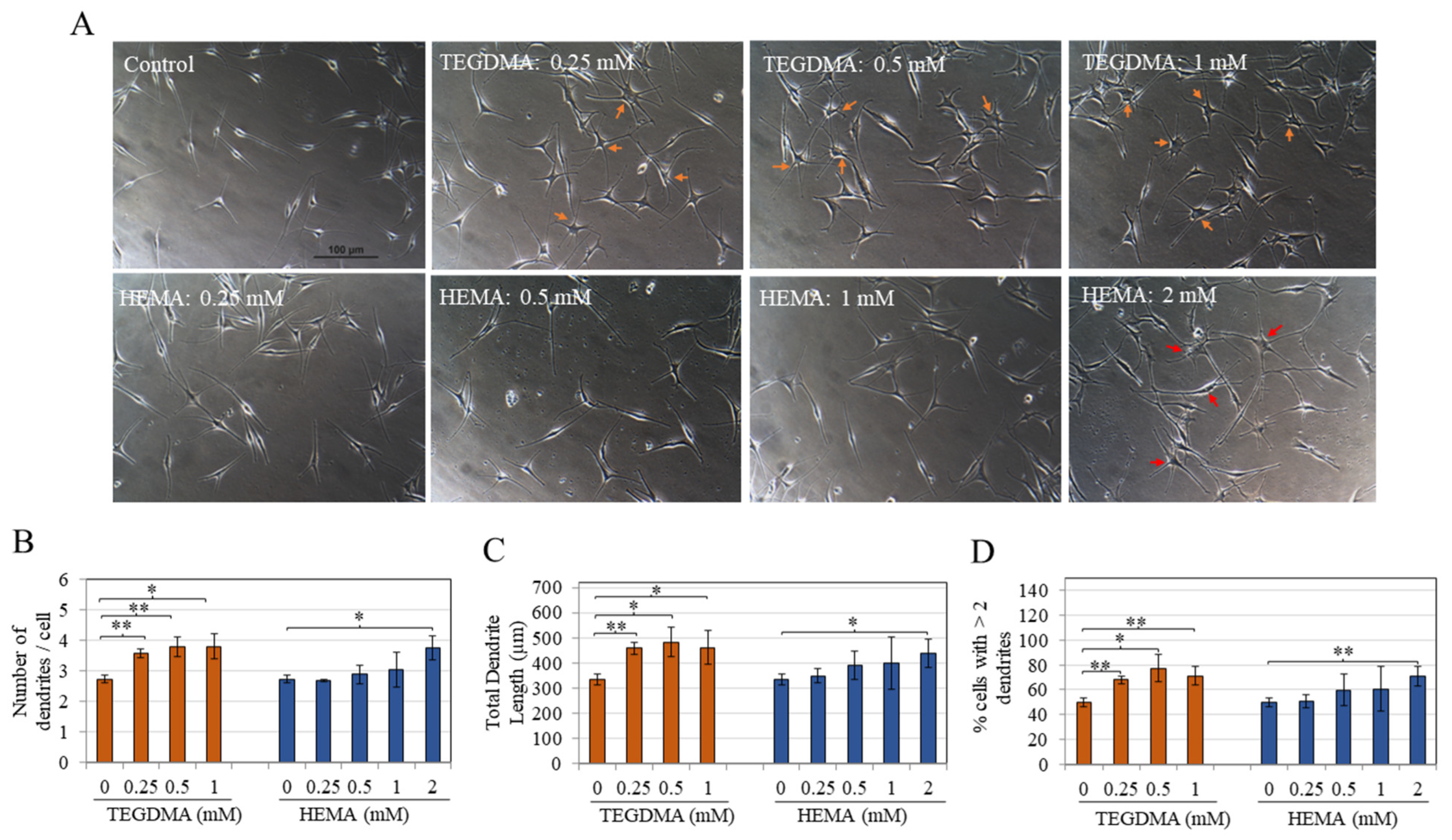
3.3. TEGDMA and HEMA Markedly Stimulate Melanocyte Dendricity
3.4. TEGDMA Has No Effect, While HEMA Inhibits Intracellular Tyrosinase Activity
3.5. TEGDMA and HEMA Do Not Affect Tyrosinase Activity in a Cell-Free System
3.6. TEGDMA and HEMA Do Not Alter Intracellular ROS Levels
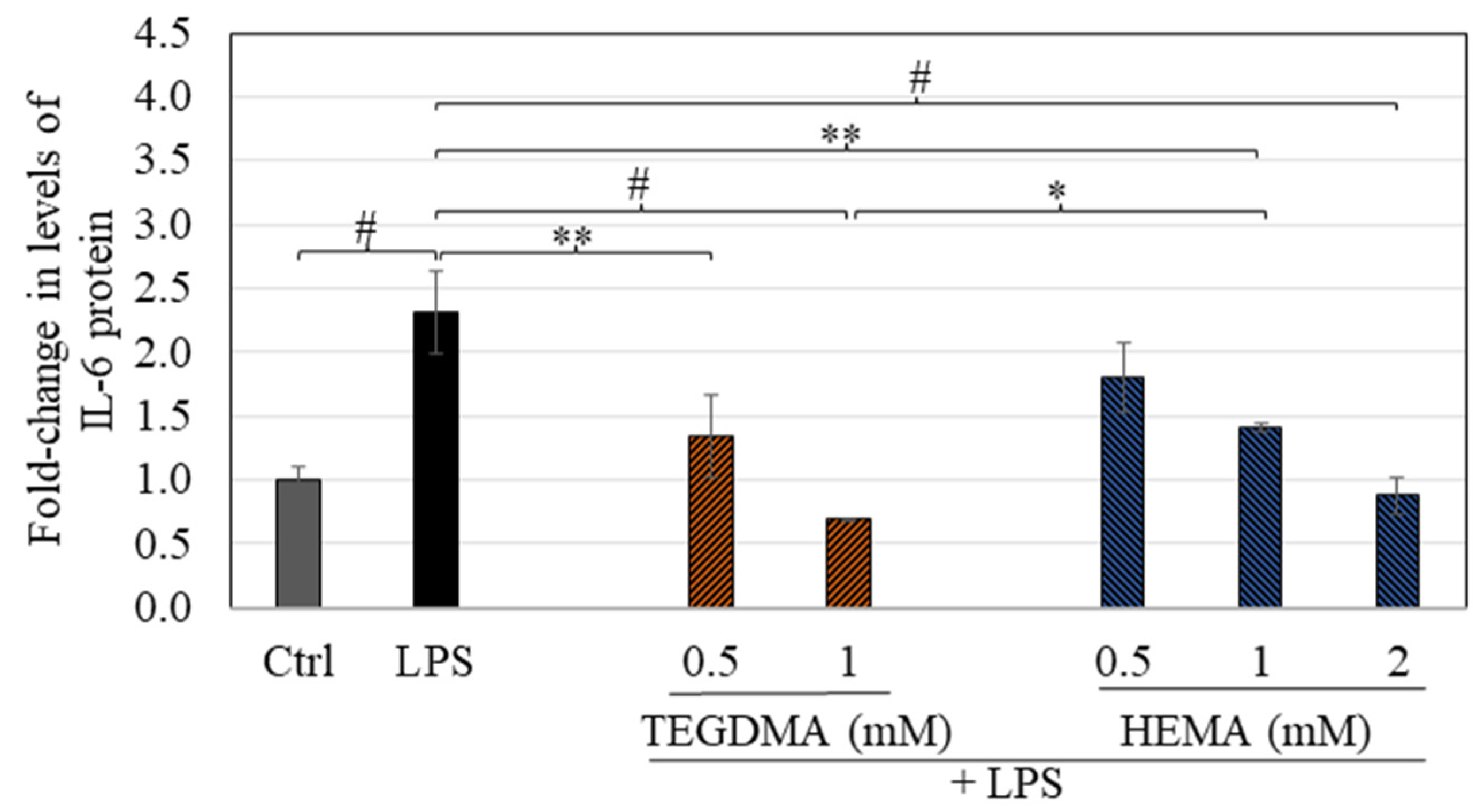
3.7. TEGDMA and HEMA Suppress LPS-Stimulated Cytokine Secretion
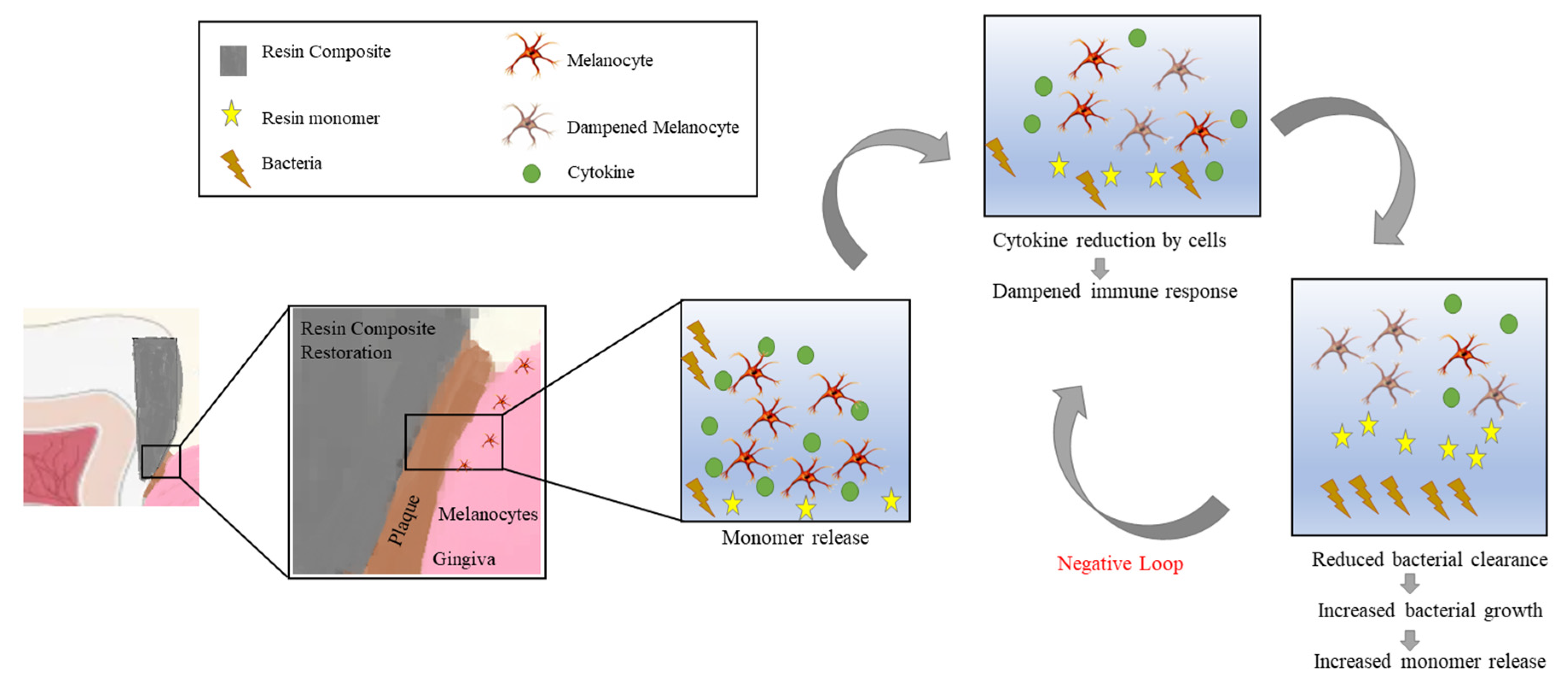
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mulligan, S.; Hatton, P.V.; Martin, N. Resin-Based Composite Materials: Elution and Pollution. Br. Dent. J. 2022, 232, 644–652. [Google Scholar] [CrossRef]
- Cho, K.; Rajan, G.; Farrar, P.; Prentice, L.; Prusty, B.G. Dental Resin Composites: A Review on Materials to Product Realizations. Compos. Part B Eng. 2022, 230, 109495. [Google Scholar]
- Yadav, R.; Lee, H.H. Fabrication, Characterization, and Selection Using Fahp-Topsis Technique of Zirconia, Titanium Oxide, and Marble Dust Powder Filled Dental Restorative Composite Materials. Polym. Adv. Technol. 2022, 33, 3286–3295. [Google Scholar] [CrossRef]
- Ali, S.; Sangi, L.; Kumar, N.; Kumar, B.; Khurshid, Z.; Zafar, M.S. Evaluating Antibacterial and Surface Mechanical Properties of Chitosan Modified Dental Resin Composites. Technol. Health Care 2020, 28, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Dahri, W.M.; Kumar, N.; Altaf, N.; Mughal, W.; Zafar, M.S. Mechanical and Biomimetic Characteristics of Bulk-Fill Resin Dental Composites Following Exposure in A Simulated Acidic Oral Environment. Biomimetics 2023, 8, 19. [Google Scholar] [CrossRef]
- Kumar, N.; Sangi, L. Water Sorption, Solubility, and Resultant Change in Strength Among Three Resin-Based Dental Composites. J. Investig. Clin. Dent. 2014, 5, 144–150. [Google Scholar] [CrossRef]
- Yadav, R.; Lee, H.; Lee, J.-H.; Singh, R.K.; Lee, H.-H. A Comprehensive Review: Physical, Mechanical, and Tribological Characterization of Dental Resin Composite Materials. Tribol. Int. 2023, 179, 108102. [Google Scholar]
- Ferracane, J. Elution of Leachable Components from Composites. J. Oral Rehabil. 1994, 21, 441–452. [Google Scholar] [CrossRef]
- Wataha, J.; Rueggeberg, F.; Lapp, C.; Lewis, J.; Lockwood, P.; Ergle, J.; Mettenburg, D.J. In Vitro Cytotoxicity of Resin-Containing Restorative Materials After Aging in Artificial Saliva. Clin. Oral Investig. 1999, 3, 144–149. [Google Scholar] [CrossRef]
- Bakkal, M.; Yılmaz, B.; Durmus, A.; Durmus, Z.; Ozalp, S. Polymerization Characteristics of Colored Compomers Cured with Different Led Units. J. Appl. Biomater. Funct. Mater. 2019, 17, 2280800019827805. [Google Scholar] [CrossRef] [Green Version]
- Bayindir, Y.Z.; Yildiz, M.; Bayindir, F. The Effect of “Soft-Start Polymerization” on Surface Hardness of Two Packable Composites. Dent. Mater. J. 2003, 22, 610–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandenbulcke, J.D.; Marks, L.A.; Martens, L.C.; Verbeeck, R.M. Comparison of Curing Depth of A Colored Polyacid-Modified Composite Resin with Different Light-Curing Units. Quintessence Int. 2010, 41, 787–794. [Google Scholar] [PubMed]
- Germain, H.S.; Swartz, M.; Phillips, R.; Moore, B.; Roberts, T. Properties of Microfilled Composite Resins As Influenced By Filler Content. J. Dent. Res. 1985, 64, 155–160. [Google Scholar] [CrossRef]
- Van Landuyt, K.; Nawrot, T.; Geebelen, B.; De Munck, J.; Snauwaert, J.; Yoshihara, K.; Scheers, H.; Godderis, L.; Hoet, P.; Van Meerbeek, B. How Much Do Resin-Based Dental Materials Release? A Meta-Analytical Approach. Dent. Mater. 2011, 27, 723–747. [Google Scholar] [CrossRef] [PubMed]
- Jaffer, F.; Finer, Y.; Santerre, J. Interactions Between Resin Monomers and Commercial Composite Resins with Human Saliva Derived Esterases. Biomaterials 2002, 23, 1707–1719. [Google Scholar] [CrossRef]
- Delima, A.J.; Van Dyke, T.E. Origin and Function of the Cellular Components in Gingival Crevice Fluid. Periodontol. 2000 2003, 31, 55–76. [Google Scholar] [CrossRef]
- Gitalis, R.; Zhou, L.; Marashdeh, M.Q.; Sun, C.; Glogauer, M.; Finer, Y. Human Neutrophils Degrade Methacrylate Resin Composites and Tooth Dentin. Acta Biomater. 2019, 88, 325–331. [Google Scholar] [CrossRef]
- Almeida, G.S.; Poskus, L.T.; Guimarães, J.G.A.; Silva, E.M. The Effect of Mouthrinses on Salivary Sorption, Solubility and Surface Degradation of A Nanofilled and A Hybrid Resin Composite. Oper. Dent. 2010, 35, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Puckett, A.D.; Fitchie, J.G.; Kirk, P.C.; Gamblin, J. Direct Composite Restorative Materials. Dent. Clin. N. Am. 2007, 51, 659–675. [Google Scholar] [CrossRef]
- Abdulmajeed, A.A.; Kokkari, A.K.; Käpylä, J.; Massera, J.; Hupa, L.; Vallittu, P.K.; Närhi, T.O. In Vitro Blood and Fibroblast Responses to Bisgma–Tegdma/Bioactive Glass Composite Implants. J. Mater. Sci. Mater. Med. 2014, 25, 151–162. [Google Scholar] [CrossRef]
- Tou, G.A.D.A.; Gomes, J.M.; Rinco, L.S.D.O.; Yamauti, M.; Diniz, I.M.A.; Pires, F.; Schmidt, M.E.P.; Menezes, H.C.; Cardeal, Z.D.L.; Bottoli, C.B.G. Release of Leachable Products from Resinous Compounds in the Saliva of Children with Anterior Open Bite Treated with Spur. J. Appl. Oral Sci. 2023, 30, e20220227. [Google Scholar] [CrossRef] [PubMed]
- Szczesio-Wlodarczyk, A.; Polikowski, A.; Krasowski, M.; Fronczek, M.; Sokolowski, J.; Bociong, K. The Influence of Low-Molecular-Weight Monomers (Tegdma, Hddma, Hema) on the Properties of Selected Matrices and Composites Based on Bis-Gma and Udma. Materials 2022, 15, 2649. [Google Scholar] [CrossRef] [PubMed]
- Tauscher, S.; Angermann, J.; Catel, Y.; Moszner, N. Evaluation of Alternative Monomers to Hema for Dental Applications. Dent. Mater. 2017, 33, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Nakabayashi, N. Effect of 2-(Methacryloxy) Ethyl Phenyl Hydrogen Phosphate on Adhesion to Dentin. J. Dent. Res. 1991, 70, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Arcís, R.W.; López-Macipe, A.; Toledano, M.; Osorio, E.; Rodríguez-Clemente, R.; Murtra, J.; Fanovich, M.A.; Pascual, C.D. Mechanical Properties of Visible Light-Cured Resins Reinforced with Hydroxyapatite for Dental Restoration. Dent. Mater. 2002, 18, 49–57. [Google Scholar] [CrossRef]
- Michelsen, V.B.; Moe, G.; Skålevik, R.; Jensen, E.; Lygre, H. Quantification of Organic Eluates from Polymerized Resin-Based Dental Restorative Materials By Use of Gc/Ms. J. Chromatogr. B 2007, 850, 83–91. [Google Scholar] [CrossRef]
- Goda, T.; Ishihara, K. Soft Contact Lens Biomaterials from Bioinspired Phospholipid Polymers. Expert Rev. Med. Devices 2006, 3, 167–174. [Google Scholar] [CrossRef]
- Lord, M.S.; Stenzel, M.H.; Simmons, A.; Milthorpe, B.K. The Effect of Charged Groups on Protein Interactions with Poly (Hema) Hydrogels. Biomaterials 2006, 27, 567–575. [Google Scholar] [CrossRef]
- Montheard, J.-P.; Chatzopoulos, M.; Chappard, D. 2-Hydroxyethyl Methacrylate (Hema): Chemical Properties and Applications in Biomedical Fields. J. Macromol. Sci. Part C Polym. Rev. 1992, 32, 1–34. [Google Scholar]
- Sideridou, I.; Tserki, V.; Papanastasiou, G. Effect of Chemical Structure on Degree of Conversion in Light-Cured Dimethacrylate-Based Dental Resins. Biomaterials 2002, 23, 1819–1829. [Google Scholar] [CrossRef]
- Geurtsen, W.; Leyhausen, G. Chemical-Biological Interactions of the Resin Monomer Triethyleneglycol-Dimethacrylate (Tegdma). J. Dent. Res. 2001, 80, 2046. [Google Scholar] [CrossRef] [PubMed]
- Cebe, M.A.; Cebe, F.; Cengiz, M.F.; Cetin, A.R.; Arpag, O.F.; Ozturk, B. Elution of Monomer from Different Bulk Fill Dental Composite Resins. Dent. Mater. 2015, 31, E141–E149. [Google Scholar] [CrossRef] [PubMed]
- Örtengren, U.; Wellendorf, H.; Karlsson, S.; Ruyter, I. Water Sorption and Solubility of Dental Composites and Identification of Monomers Released in An Aqueous Environment. J. Oral Rehabil. 2001, 28, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Venz, S.; Dickens, B. Nir-Spectroscopic Investigation of Water Sorption Characteristics of Dental Resins and Composites. J. Biomed. Mater. Res. 1991, 25, 1231–1248. [Google Scholar] [CrossRef]
- Sideridou, I.; Tserki, V.; Papanastasiou, G. Study of Water Sorption, Solubility and Modulus of Elasticity of Light-Cured Dimethacrylate-Based Dental Resins. Biomaterials 2003, 24, 655–665. [Google Scholar] [CrossRef]
- Nakamura, M.; Oshima, H.; Hashimoto, Y. Monomer Permeability of Disposable Dental Gloves. J. Prosthet. Dent. 2003, 90, 81–85. [Google Scholar] [CrossRef]
- Kaufman, G.; Skrtic, D. Morphological and Kinetic Study of Oral Keratinocytes Assembly on Reconstituted Basement Membrane: Effect of Tegdma. Arch. Oral Biol. 2019, 104, 103–111. [Google Scholar] [CrossRef]
- Mahdhaoui, K.; Fournier, B.; Derbanne, M.A. Unbound Monomers Do Diffuse Through the Dentin Barrier. Dent. Mater. 2017, 33, 743–751. [Google Scholar] [CrossRef]
- Rashid, H.; Sheikh, Z.; Vohra, F. Allergic Effects of the Residual Monomer Used in Denture Base Acrylic Resins. Eur. J. Dent. 2015, 9, 614–619. [Google Scholar]
- Kamalak, H.; Kamalak, A.; Taghizadehghalehjoughi, A.; Hacimuftuoglu, A.; Nalci, K.A. Cytotoxic and Biological Effects of Bulk Fill Composites on Rat Cortical Neuron Cells. Odontology 2018, 106, 377–388. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Khan, Q.; Schreurs, O.J.F.; Sapkota, D.; Samuelsen, J.T. Investigation of Biological Effects of Hema in 3d-Organotypic Co-Culture Models of Normal and Malignant Oral Keratinocytes. Biomater. Investig. Dent. 2023, 10, 2234400. [Google Scholar] [CrossRef]
- Jiang, E.-S.; Moon, W.; Lim, B.-S.; Chang, J.; Chung, S.H. Cytotoxicity and Reactive Oxygen Species Production Induced By Different Co-Monomer Eluted from Nanohybrid Dental Composites. BMC Oral Health 2023, 23, 55. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.J.; Erickson, C.A. The Making of A Melanocyte: The Specification of Melanoblasts from the Neural Crest. Pigment Cell Melanoma Res. 2008, 21, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Elobeid, A.S.; Kamal-Eldin, A.; Abdelhalim, M.A.K.; Haseeb, A.M. Pharmacological Properties of Melanin and Its Function in Health. Basic Clin. Pharmacol. Toxicol. 2017, 120, 515–522. [Google Scholar] [CrossRef] [Green Version]
- Nilima, S.; Vandana, K.L. Melanin: A Scavenger in Gingival Inflammation. Indian J. Dent. Res. 2011, 22, 38–43. [Google Scholar] [CrossRef]
- Del Marmol, V.; Beermann, F. Tyrosinase and Related Proteins in Mammalian Pigmentation. Febs Lett. 1996, 381, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, R.K.; Hearing, V.J.; Urabe, K.; Aroca, P.; Spritz, R.A. Mutational Mapping of the Catalytic Activities of Human Tyrosinase. J. Biol. Chem. 1992, 267, 23707–23712. [Google Scholar] [CrossRef]
- Hearing, V.J.; Tsukamoto, K. Enzymatic Control of Pigmentation in Mammals. Faseb J. 1991, 5, 2902–2909. [Google Scholar] [CrossRef] [PubMed]
- Barrett, A.; Scully, C. Human Oral Mucosal Melanocytes: A Review. J. Oral Pathol. Med. 1994, 23, 97–103. [Google Scholar] [CrossRef]
- Eisen, D. Disorders of Pigmentation in the Oral Cavity. Clin. Dermatol. 2000, 18, 579–587. [Google Scholar] [CrossRef]
- Baala Vignesh, A.; Thenmozhi, M. Effect of Smoking on Oral Pigmentation-A Survey. Drug Invent. Today 2018, 10, 2348–2350. [Google Scholar]
- Gulati, N.; Dutt, P.; Gupta, N.; Tyagi, P. Gingival Pigmentation: Revisited. J. Adv. Med. Dent. Sci. Res. 2016, 4, 48. [Google Scholar]
- Anders Hedin And, C.; Larsson, Å. The Ultrastructure of the Gingival Epithelium in Smokers’ Melanosis. J. Periodontal. Res. 1984, 19, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Fruet-Arruda, R.T.; Anselmo, G.G.; Tortamano, A.C.A.; Rossi, A.L.; Biffi, M.B.; Marco, R.L.; Kato, I.T.; Nuñez, S.C.; Prates, R.A. Melanin Pigmented Gingival Tissue Impairs Red-Light Lateral Scattering for Antimicrobial Photodynamic Therapy. Photodiagnosis Photodyn. Ther. 2021, 33, 102135. [Google Scholar] [CrossRef]
- Goenka, S. Biological Impact of the Ratio of E-Cigarette Liquid Base Constituents, Propylene Glycol and Vegetable Glycerin, on Primary Human Melanocytes. Oral 2023, 3, 40–56. [Google Scholar] [CrossRef]
- Goenka, S.; Simon, S.R. Effects of Fluoride Exposure on Primary Human Melanocytes from Dark and Light Skin. Toxics 2020, 8, 114. [Google Scholar] [CrossRef]
- Goenka, S.R. Simon, S. Asoprisnil, a Selective Progesterone Receptor Modulator (Sprm), Inhibits Melanosome Export in B16f10 Cells and Hemn-Dp Melanocytes. Molecules 2020, 25, 3581. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, J.S.; Woo, J.-T.; Lee, I.-S.; Cha, B.-Y. Hyperpigmentation Mechanism of Methyl 3, 5-Di-Caffeoylquinate Through Activation of P38 and Mitf Induction of Tyrosinase. Acta Biochim. Biophys. Sin. 2015, 47, 548–556. [Google Scholar] [CrossRef]
- Goenka, S.; Simon, S.R. Organogold Drug Auranofin Exhibits Anti-Melanogenic Activity in B16f10 and Mnt-1 Melanoma Cells. Arch. Dermatol. Res. 2020, 312, 213–221. [Google Scholar] [CrossRef]
- Jian, Q.; An, Q.; Zhu, D.; Hui, K.; Liu, Y.; Chi, S.; Li, C. Microrna 340 Is Involved in Uvb-Induced Dendrite Formation Through the Regulation of Rhoa Expression in Melanocytes. Mol. Cell. Biol. 2014, 34, 3407–3420. [Google Scholar] [CrossRef] [Green Version]
- Hempel, S.L.; Buettner, G.R.; O’malley, Y.Q.; Wessels, D.A.; Flaherty, D.M. Dihydrofluorescein Diacetate Is Superior for Detecting Intracellular Oxidants: Comparison with 2′,7′-Dichlorodihydrofluorescein Diacetate, 5 (and 6)-Carboxy-2′,7′-Dichlorodihydrofluorescein Diacetate, and Dihydrorhodamine 123. Free Radic. Biol. Med. 1999, 27, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Endo, K.; Mizutani, T.; Okano, Y.; Masaki, H. A Red Pumpkin Seed Extract Reduces Melanosome Transfer to Keratinocytes by Activation of Nrf2 Signaling. J. Cosmet. Dermatol. 2019, 18, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Schweikl, H.; Spagnuolo, G.; Schmalz, G. Genetic and Cellular Toxicology of Dental Resin Monomers. J. Dent. Res. 2006, 85, 870–877. [Google Scholar] [CrossRef]
- Noda, M.; Wataha, J.; Kaga, M.; Lockwood, P.; Volkmann, K.; Sano, H. Components of Dentinal Adhesives Modulate Heat Shock Protein 72 Expression in Heat-Stressed Thp-1 Human Monocytes At Sublethal Concentrations. J. Dent. Res. 2002, 81, 265–269. [Google Scholar] [CrossRef]
- Lee, D.H.; Lim, B.-S.; Lee, Y.-K.; Ahn, S.-J.; Yang, H.-C. Involvement of Oxidative Stress in Mutagenicity and Apoptosis Caused By Dental Resin Monomers in Cell Cultures. Dent. Mater. 2006, 22, 1086–1092. [Google Scholar] [CrossRef]
- Becher, R.; Kopperud, H.M.; Al, R.H.; Samuelsen, J.T.; Morisbak, E.; Dahlman, H.J.; Lilleaas, E.M.; Dahl, J.E. Pattern of Cell Death After in Vitro Exposure to Gdma, Tegdma, Hema and Two Compomer Extracts. Dent. Mater. 2006, 22, 630–640. [Google Scholar] [CrossRef]
- Lefebvre, C.A.; Schuster, G.; Rueggeberg, F.; Tamare-Selvy, K.; Knoernschild, K. Responses of Oral Epithelial Cells to Dental Resin Components. J. Biomater. Sci. Polym. Ed. 1996, 7, 965–976. [Google Scholar] [CrossRef]
- Inamitsu, H.; Okamoto, K.; Sakai, E.; Nishishita, K.; Murata, H.; Tsukuba, T. The Dental Resin Monomers Hema and Tegdma Have Inhibitory Effects on Osteoclast Differentiation with Low Cytotoxicity. J. Appl. Toxicol. 2017, 37, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Walters, N.J.; Xia, W.; Salih, V.; Ashley, P.F.; Young, A.M. Poly (Propylene Glycol) and Urethane Dimethacrylates Improve Conversion of Dental Composites and Reveal Complexity of Cytocompatibility Testing. Dent. Mater. 2016, 32, 264–277. [Google Scholar] [CrossRef] [Green Version]
- Moharamzadeh, K.; Van Noort, R.; Brook, I.M.; Scutt, A.M. Cytotoxicity of Resin Monomers on Human Gingival Fibroblasts and Hacat Keratinocytes. Dent. Mater. 2007, 23, 40–44. [Google Scholar] [CrossRef]
- Engelmann, J.; Leyhausen, G.; Leibfritz, D.; Geurtsen, W. Effect of Tegdma on the Intracellular Glutathione Concentration of Human Gingival Fibroblasts. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2002, 63, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.M.; Kuan, Y.H.; Lee, S.S.; Chang, Y.C. Cytotoxicity and Genotoxicity of Triethyleneglycol-Dimethacrylate in Macrophages Involved in Dna Damage and Caspases Activation. Environ. Toxicol. 2015, 30, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Batarseh, G.; Windsor, L.; Labban, N.; Liu, Y.; Gregson, K. Triethylene Glycol Dimethacrylate Induction of Apoptotic Proteins in Pulp Fibroblasts. Oper. Dent. 2014, 39, E1–E8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paranjpe, A.; Bordador, L.; Wang, M.-Y.; Hume, W.; Jewett, A. Resin Monomer 2-Hydroxyethyl Methacrylate (Hema) Is A Potent Inducer of Apoptotic Cell Death in Human and Mouse Cells. J. Dent. Res. 2005, 84, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Goenka, S.; Simon, S.R. A Novel Pro-Melanogenic Effect of Standardized Dry Olive Leaf Extract on Primary Human Melanocytes from Lightly Pigmented and Moderately Pigmented Skin. Pharmaceuticals 2021, 14, 252. [Google Scholar] [CrossRef]
- Nordenberg, J.; Wasserman, L.; Beery, E.; Aloni, D.; Malik, H.; Stenzel, K.H.; Novogrodsky, A. Growth Inhibition of Murine Melanoma By Butyric Acid and Dimethylsulfoxide. Exp. Cell Res. 1986, 162, 77–85. [Google Scholar] [CrossRef]
- Lauand, F.; Lia, R.C.; Marcantonio, E.; Acetoze, P.; Neto, C. Dendritic Melanoblasts in Pathologic Gingiva. Bull. Group. Int. Rech. Sci. Stomatol. Odontol. 1981, 24, 193–207. [Google Scholar]
- Falconi, M.; Teti, G.; Zago, M.; Pelotti, S.; Breschi, L.; Mazzotti, G. Effects of Hema on Type I Collagen Protein in Human Gingival Fibroblasts. Cell Biol. Toxicol. 2007, 23, 313–322. [Google Scholar] [CrossRef]
- Chang, H.H.; Chang, M.C.; Huang, G.F.; Wang, Y.L.; Chan, C.P.; Wang, T.M.; Lin, P.S.; Jeng, J.H. Effect of Triethylene Glycol Dimethacrylate on the Cytotoxicity, Cyclooxygenase-2 Expression and Prostanoids Production in Human Dental Pulp Cells. Int. Endod. J. 2012, 45, 848–858. [Google Scholar] [CrossRef]
- Scott, G.; Leopardi, S.; Printup, S.; Madden, B.C. Filopodia Are Conduits for Melanosome Transfer to Keratinocytes. J. Cell Sci. 2002, 115, 1441–1451. [Google Scholar] [CrossRef]
- Chiaverini, C.; Beuret, L.; Flori, E.; Abbe, P.; Bille, K.; Bahadoran, P.; Ortonne, J.-P.; Bertolotto, C.; Ballotti, R. Microphthalmia-Associated Transcription Factor Regulates Rab27a Gene Expression and Controls Melanosome Transport. J. Biol. Chem. 2008, 283, 12635–12642. [Google Scholar] [CrossRef] [Green Version]
- Bae-Harboe, Y.S.; Park, H.Y. Tyrosinase: A Central Regulatory Protein for Cutaneous Pigmentation. J. Investig. Dermatol. 2012, 132, 2678–2680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mann, T.; Gerwat, W.; Batzer, J.; Eggers, K.; Scherner, C.; Wenck, H.; Stäb, F.; Hearing, V.J.; Röhm, K.-H.; Kolbe, L. Inhibition of Human Tyrosinase Requires Molecular Motifs Distinctively Different from Mushroom Tyrosinase. J. Investig. Dermatol. 2018, 138, 1601–1608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oyama, T.; Yoshimori, A.; Ogawa, H.; Shirai, Y.; Abe, H.; Kamiya, T.; Tanuma, S.-I. The Structural Differences Between Mushroom and Human Tyrosinase Cleared By Investigating the Inhibitory Activities of Stilbenes. J. Mol. Struct. 2023, 1272, 134180. [Google Scholar] [CrossRef]
- Samuelsen, J.T.; Kopperud, H.M.; Holme, J.A.; Dragland, I.S.; Christensen, T.; Dahl, J.E. Role of Thiol-Complex Formation in 2-Hydroxyethyl- Methacrylate-Induced Toxicity in Vitro. J. Biomed. Mater. Res. A 2011, 96, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Zhang, M.; Tonks, I.; Kay, G.; Parsons, P.G.; Sturm, R.A.; Gardiner, B. Inhibition of Melanin Synthesis By Cystamine in Human Melanoma Cells. J. Investig. Dermatol. 2000, 114, 21–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, S.-Y.; Choi, M.; Jeong, D.-W.; Park, S.; Park, H.; Jang, K.-S.; Choi, K.-Y. Synthesis and Chemical Composition Analysis of Protocatechualdehyde-Based Novel Melanin Dye By 15t Ft-Icr: High Dyeing Performance on Soft Contact Lens. Dye. Pigment. 2019, 160, 546–554. [Google Scholar]
- Samuelsen, J.T.; Michelsen, V.B.; Bruun, J.A.; Dahl, J.E.; Jensen, E.; Ortengren, U. The Dental Monomer Hema Causes Proteome Changes in Human Thp-1 Monocytes. J. Biomed. Mater. Res. A 2019, 107, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Morisbak, E.; Ansteinsson, V.; Samuelsen, J.T. Cell Toxicity of 2-Hydroxyethyl Methacrylate (Hema): The Role of Oxidative Stress. Eur. J. Oral Sci. 2015, 123, 282–287. [Google Scholar] [CrossRef]
- Lefeuvre, M.; Amjaad, W.; Goldberg, M.; Stanislawski, L. Tegdma Induces Mitochondrial Damage and Oxidative Stress in Human Gingival Fibroblasts. Biomaterials 2005, 26, 5130–5137. [Google Scholar] [CrossRef]
- Yeh, C.-C.; Chang, J.Z.-C.; Yang, W.-H.; Chang, H.-H.; Lai, E.H.-H.; Kuo, M.Y.-P. Nadph Oxidase 4 Is Involved in the Triethylene Glycol Dimethacrylate-Induced Reactive Oxygen Species and Apoptosis in Human Embryonic Palatal Mesenchymal and Dental Pulp Cells. Clin. Oral Investig. 2015, 19, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Ansteinsson, V.; Kopperud, H.B.; Morisbak, E.; Samuelsen, J.T. Cell Toxicity of Methacrylate Monomers-The Role of Glutathione Adduct Formation. J. Biomed. Mater. Res. A 2013, 101, 3504–3510. [Google Scholar] [CrossRef] [PubMed]
- Stanislawski, L.; Soheili–Majd, E.; Perianin, A.; Goldberg, M. Dental Restorative Biomaterials Induce Glutathione Depletion in Cultured Human Gingival Fibroblast: Protective Effect of N-Acetyl Cysteine. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2000, 51, 469–474. [Google Scholar]
- Engelmann, J.; Leyhausen, G.; Leibfritz, D.; Geurtsen, W. Metabolic Effects of Dental Resin Components in Vitro Detected By Nmr Spectroscopy. J. Dent. Res. 2001, 80, 869–875. [Google Scholar] [CrossRef]
- Majd, E.S.; Goldberg, M.; Stanislawski, L. In Vitro Effects of Ascorbate and Trolox on the Biocompatibility of Dental Restorative Materials. Biomaterials 2003, 24, 3–9. [Google Scholar] [CrossRef]
- Stanislawski, L.; Lefeuvre, M.; Bourd, K.; Soheili-Majd, E.; Goldberg, M.; Périanin, A. Tegdma-Induced Toxicity in Human Fibroblasts Is Associated with Early and Drastic Glutathione Depletion with Subsequent Production of Oxygen Reactive Species. J. Biomed. Mater. Res. Pt. A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2003, 66, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, J.; Janke, V.; Volk, J.; Leyhausen, G.; Von Neuhoff, N.; Schlegelberger, B.; Geurtsen, W. Effects of Bisgma on Glutathione Metabolism and Apoptosis in Human Gingival Fibroblasts in Vitro. Biomaterials 2004, 25, 4573–4580. [Google Scholar] [CrossRef] [PubMed]
- Volk, J.; Engelmann, J.; Leyhausen, G.; Geurtsen, W. Effects of Three Resin Monomers on the Cellular Glutathione Concentration of Cultured Human Gingival Fibroblasts. Dent. Mater. 2006, 22, 499–505. [Google Scholar] [CrossRef]
- Bakopoulou, A.; Papadopoulos, T.; Garefis, P. Molecular Toxicology of Substances Released from Resin–Based Dental Restorative Materials. Int. J. Mol. Sci. 2009, 10, 3861–3899. [Google Scholar] [CrossRef] [Green Version]
- Schmalz, G.; Krifka, S.; Schweikl, H. Toll-Like Receptors, Lps, and Dental Monomers. Adv. Dent. Res. 2011, 23, 302–306. [Google Scholar] [CrossRef]
- Gallorini, M.; Petzel, C.; Bolay, C.; Hiller, K.-A.; Cataldi, A.; Buchalla, W.; Krifka, S.; Schweikl, H. Activation of the Nrf2-Regulated Antioxidant Cell Response Inhibits Hema-Induced Oxidative Stress and Supports Cell Viability. Biomaterials 2015, 56, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Gasque, P.; Jaffar-Bandjee, M.C. The Immunology and Inflammatory Responses of Human Melanocytes in Infectious Diseases. J. Infect. 2015, 71, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhu, W.Y.; Tan, C.; Yu, G.H.; Gu, J.X. Melanocytes Are Potential Immunocompetent Cells: Evidence from Recognition of Immunological Characteristics of Cultured Human Melanocytes. Pigment Cell Res. 2002, 15, 454–460. [Google Scholar] [CrossRef]
- Bolling, A.K.; Samuelsen, J.T.; Morisbak, E.; Ansteinsson, V.; Becher, R.; Dahl, J.E.; Mathisen, G.H. Dental Monomers Inhibit Lps-Induced Cytokine Release from the Macrophage Cell Line Raw264.7. Toxicol. Lett. 2013, 216, 130–138. [Google Scholar] [CrossRef]
- Schweikl, H.; Buchalla, W.; Krifka, S. Cell Responses to Cariogenic Microorganisms and Dental Resin Materials—Crosstalk At the Dentin-Pulp Interface? Dent. Mater. 2017, 33, 514–524. [Google Scholar] [CrossRef]
- Matalon, S.; Slutzky, H.; Weiss, E.I. Surface Antibacterial Properties of Packable Resin Composites: Part I. Quintessence Int. 2004, 35, 189–193. [Google Scholar]
- Hansel, C.; Leyhausen, G.; Mai, U.; Geurtsen, W. Effects of Various Resin Composite (Co) Monomers and Extracts on Two Caries-Associated Micro-Organisms in Vitro. J. Dent. Res. 1998, 77, 60–67. [Google Scholar] [CrossRef]
- Beyth, N.; Bahir, R.; Matalon, S.; Domb, A.J.; Weiss, E.I. Streptococcus Mutans Biofilm Changes Surface-Topography of Resin Composites. Dent. Mater. 2008, 24, 732–736. [Google Scholar] [CrossRef]
- Goenka, S. Effects of Serotype and Species Dependency of Bacterial Lipopolysaccharides in Human Melanocytes from Lightly and Darkly-Pigmented Skin. BBA Adv. 2022, 2, 100042. [Google Scholar] [CrossRef]
- Eckhardt, A.; Harorli, T.; Limtanyakul, J.; Hiller, K.-A.; Bosl, C.; Bolay, C.; Reichl, F.-X.; Schmalz, G.; Schweikl, H. Inhibition of Cytokine and Surface Antigen Expression in Lps-Stimulated Murine Macrophages By Triethylene Glycol Dimethacrylate. Biomaterials 2009, 30, 1665–1674. [Google Scholar] [CrossRef]
- Bolling, A.K.; Solhaug, A.; Morisbak, E.; Holme, J.A.; Samuelsen, J.T. The Dental Monomer Hydroxyethyl Methacrylate (Hema) Counteracts Lipopolysaccharide-Induced Il-1β Release—Possible Role of Glutathione. Toxicol. Lett. 2017, 270, 25–33. [Google Scholar] [CrossRef]
- Krifka, S.; Petzel, C.; Hiller, K.-A.; Frank, E.-M.; Bosl, C.; Spagnuolo, G.; Reichl, F.-X.; Schmalz, G.; Schweikl, H. Resin Monomer-Induced Differential Activation of Map Kinases and Apoptosis in Mouse Macrophages and Human Pulp Cells. Biomaterials 2010, 31, 2964–2975. [Google Scholar] [CrossRef] [PubMed]
- Schuster, L.; Rothmund, L.; He, X.; Van Landuyt, K.L.; Schweikl, H.; Hellwig, E.; Carell, T.; Hickel, R.; Reichl, F.-X.; Högg, C. Effect of Opalescence® Bleaching Gels on the Elution of Dental Composite Components. Dent. Mater. 2015, 31, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaee, M.H.; Arami, S.; Ghavam, M.; Rezaii, A. Monomer Release from Nanofilled and Microhybrid Dental Composites After Bleaching. J. Dent. 2014, 11, 56. [Google Scholar]
- Durner, J.; Stojanovic, M.; Urcan, E.; Spahl, W.; Haertel, U.; Hickel, R.; Reichl, F.-X. Effect of Hydrogen Peroxide on the Three-Dimensional Polymer Network in Composites. Dent. Mater. 2011, 27, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Reichl, F.-X.; Seiss, M.; Marquardt, W.; Kleinsasser, N.; Schweikl, H.; Kehe, K.; Hickel, R. Toxicity Potentiation By H2o2 with Components of Dental Restorative Materials on Human Oral Cells. Arch. Toxicol. 2008, 82, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Wisniewska-Jarosinska, M.; Poplawski, T.; Chojnacki, C.J.; Pawlowska, E.; Krupa, R.; Szczepanska, J.; Blasiak, J. Independent and Combined Cytotoxicity and Genotoxicity of Triethylene Glycol Dimethacrylate and Urethane Dimethacrylate. Mol. Biol. Rep. 2011, 38, 4603–4611. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.-J.; Hyun, H.-K.; Kim, Y.-J.; Jang, K.-T. Effect of Collagenase and Esterase on Resin-Dentin Interface: A Comparative Study Between A Total-Etch Adhesive and A Self-Etch Adhesive. Am. J. Dent. 2009, 22, 295. [Google Scholar]
- Santerre, J.; Shajii, L.; Leung, B. Relation of Dental Composite Formulations to Their Degradation and the Release of Hydrolyzed Polymeric-Resin-Derived Products. Crit. Rev. Oral Biol. Med. 2001, 12, 136–151. [Google Scholar] [CrossRef] [Green Version]
- Szczepanska, J.; Poplawski, T.; Synowiec, E.; Pawlowska, E.; Chojnacki, C.J.; Chojnacki, J.; Blasiak, J. 2-Hydroxylethyl Methacrylate (Hema), A Tooth Restoration Component, Exerts Its Genotoxic Effects in Human Gingival Fibroblasts trough Methacrylic Acid, An Immediate Product of Its Degradation. Mol. Biol. Rep. 2012, 39, 1561–1574. [Google Scholar]
- Emmler, J.; Seiss, M.; Kreppel, H.; Reichl, F.X.; Hickel, R.; Kehe, K. Cytotoxicity of the Dental Composite Component Tegdma and Selected Metabolic By-Products in Human Pulmonary Cells. Dent. Mater. 2008, 24, 1670–1675. [Google Scholar] [CrossRef]
- Pérez-Mondragón, A.A.; Cuevas-Suárez, C.E.; Castillo, O.R.S.; González-López, J.A.; Herrera-González, A.M. Evaluation of Biocompatible Monomers As Substitutes for Tegdma in Resin-Based Dental Composites. Mater. Sci. Eng. C 2018, 93, 80–87. [Google Scholar] [CrossRef]
- Axeix, T.; Hedin, C.A. Epidemiologic Study of Excessive Oral Melanin Pigmentation with Special Reference to the Influence of Tobacco Habits. Eur. J. Oral Sci. 1982, 90, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Paryag, A.; Lowe, J.; Rafeek, R. Colored Gingiva Composite Used for the Rehabilitation of Gingiva Recessions and Non-Carious Cervical Lesions. Dent. J. 2017, 5, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paryag, A.A.; Rafeek, R.N.; Mankee, M.S.; Lowe, J. Exploring the Versatility of Gingiva-Colored Composite. Clin. Cosmet. Investig. Dent. 2016, 8, 63. [Google Scholar] [PubMed] [Green Version]
- Putzeys, E.; De Nys, S.; Cokic, S.M.; Duca, R.C.; Vanoirbeek, J.; Godderis, L.; Van Meerbeek, B.; Van Landuyt, K.L. Long-Term Elution of Monomers from Resin-Based Dental Composites. Dent. Mater. 2019, 35, 477–485. [Google Scholar] [CrossRef]
- Hampe, T.; Wiessner, A.; Frauendorf, H.; Alhussein, M.; Karlovsky, P.; Bürgers, R.; Krohn, S. Monomer Release from Dental Resins: The Current Status on Study Setup, Detection and Quantification for in Vitro Testing. Polymers 2022, 14, 1790. [Google Scholar] [CrossRef]
- Ferracane, J.; Condon, J. Rate of Elution of Leachable Components from Composite. Dent. Mater. 1990, 6, 282–287. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goenka, S. In Vitro Evaluation of Dental Resin Monomers, Triethylene Glycol Dimethacrylate (TEGDMA), and 2-Hydroxyethyl Methacrylate (HEMA) in Primary Human Melanocytes: A Pilot Study. Oral 2023, 3, 353-371. https://doi.org/10.3390/oral3030029
Goenka S. In Vitro Evaluation of Dental Resin Monomers, Triethylene Glycol Dimethacrylate (TEGDMA), and 2-Hydroxyethyl Methacrylate (HEMA) in Primary Human Melanocytes: A Pilot Study. Oral. 2023; 3(3):353-371. https://doi.org/10.3390/oral3030029
Chicago/Turabian StyleGoenka, Shilpi. 2023. "In Vitro Evaluation of Dental Resin Monomers, Triethylene Glycol Dimethacrylate (TEGDMA), and 2-Hydroxyethyl Methacrylate (HEMA) in Primary Human Melanocytes: A Pilot Study" Oral 3, no. 3: 353-371. https://doi.org/10.3390/oral3030029
APA StyleGoenka, S. (2023). In Vitro Evaluation of Dental Resin Monomers, Triethylene Glycol Dimethacrylate (TEGDMA), and 2-Hydroxyethyl Methacrylate (HEMA) in Primary Human Melanocytes: A Pilot Study. Oral, 3(3), 353-371. https://doi.org/10.3390/oral3030029





