Tooth-Surface-Specific Effects of MI Varnish™: A 3-Year Randomized Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Interventions
2.2. Assessment of Oral Hygiene
2.3. Statistical Methods
3. Results
3.1. Primary Outcomes
3.1.1. Caries Increment
3.1.2. Oral Hygiene Habits
3.2. Secondary Outcomes
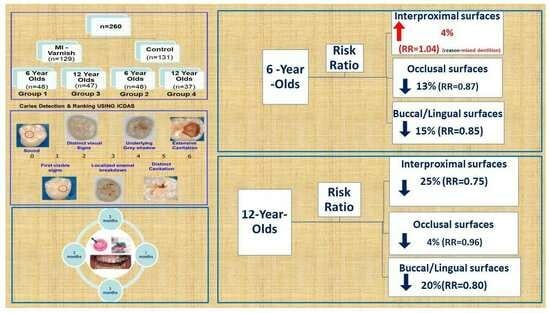
3.2.1. Ratio Risk Calculations
3.2.2. Correlation Analysis
3.3. Adverse Effects
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frencken, J.E.; Peters, M.C.; Manton, D.J.; Leal, S.C.; Gordan, V.V.; Eden, E. Minimal Intervention Dentistry (MID) for managing dental caries a review: Report of a FDI task group. Int. Dent. J. 2012, 62, 223–243. [Google Scholar] [CrossRef] [PubMed]
- Tafere, Y.; Chanie, S.; Dessie, T.; Gedamu, H. Assessment of prevalence of dental caries and the associated factors among patients attending dental clinic in Debre Tabor general hospital: A hospital-based crosssectional study. BMC Oral Health 2018, 18, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Gudkina, J.; Brinkmane, A.; Abrams, S.H.; Amaechi, B.T. Factors influencing the caries experience of 6 and 12-year-old children in Riga Latvia. Stomatologija 2016, 18, 14–20. [Google Scholar] [PubMed]
- Gudkina, J.; Amaechi, B.T.; Abrams, S.H.; Brinkmane, A.; Jelisejeva, I. Caries Increment and Oral Hygiene Changes in 6- and 12-Year-Old Children in Riga, Latvia: A 3–Year Follow–Up Report Using ICDAS II and RADKE Criteria. Eur. J. Dent. 2019, 13, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Gudkina, J.; Amaechi, B.T.; Abrams, S.H.; Brinkmane, A.; Jelisejeva, I. Inadequacy of Self-Implemented Preventive Measures to Control Caries Increment due to Poor Dietary Habits in 6 and 12 Years Old Children in Riga, Latvia. Stomatologija 2022, 24, 13–20. [Google Scholar] [PubMed]
- Gudkina, J.; Amaechi, B.T.; Abrams, S.H.; Brinkmane, A.; Petrosina, E. The Effect of MI Varnish™ on Caries Increment and Dietary Habits among 6- and 12-Year-Old Children in Riga, Latvia: A 3-Year Randomized Controlled Trial. Dent. J. 2022, 10, 96. [Google Scholar] [CrossRef] [PubMed]
- Nebu, P. State of the Art Enamel Remineralization Systems: The Next Frontier in Caries Management. Caries Res. 2019, 53, 284–295. [Google Scholar] [CrossRef]
- Nadar, B.G.; Yavagal, P.C.; Velangi, C.S.; Yavagal, C.M.; Basavaraj, S.P. Efficacy of casein phosphopeptide-amorphous calcium phosphate varnish in remineralizing white spot lesions: A systematic review and meta-analysis. Dent. Res. J. 2022, 19, 48. [Google Scholar] [CrossRef]
- Jablonski-Momeni, A.; Stachniss, V.; Ricketts, D.N.; Heinzel-Gutenbrunner, M.; Pieper, K. Reproducibility and accuracy of the ICDAS-II for detection of occlusal caries in vitro. Caries Res. 2008, 42, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Baik, A.; Alamoudi, N.; El-Housseiny, A.; Altuwirqi, A. Fluoride Varnishes for Preventing Occlusal Dental Caries: A Review. Dent. J. 2021, 9, 64. [Google Scholar] [CrossRef] [PubMed]
- Phantumvanit, P.; Makino, Y.; Ogawa, H.; Rugg-Gunn, A.; Moynihan, P.; Petersen, P.E.; Evans, W.; Feldens, C.A.; Lo, E.; Khoshnevisan, M.H.; et al. WHO Global Consultation on Public Health Intervention against Early Childhood Caries. Community. Dent. Oral. Epidemiol. 2018, 46, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Sirivichayakul, P.; Jirarattanasopha, V.; Phonghanyudh, A.; Tunlayadechanont, I.; Khumsub, P.; Duangthi, D. The effectiveness of topical fluoride agents on preventing development of approximal caries in primary teeth: A randomized clinical trial. BMC Oral Health 2023, 23, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Sionov, R.V.; Tsavdaridou, D.; Aqawi, M.; Zaks, B.; Steinberg, D.; Shalish, M. Tooth mousse containing casein phosphopeptide-amorphous calcium phosphate prevents biofilm formation of Streptococcus mutans. BMC Oral Health 2021, 21, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Meyer, F.; Enax, J.; Epple, M.; Amaechi, B.T.; Simader, B. Cariogenic Biofilms: Development, Properties, and Biomimetic Preventive Agents. Dent. J. 2021, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Nebu, P.; Suneja, B.; Walsh, L.J. Ecological Approaches to Dental Caries Prevention: Paradigm Shift or Shibboleth? Caries Res. 2018, 52, 153–165. [Google Scholar] [CrossRef]
- Cho, H.; Ren, Z.; Divaris, K.; Roach, J.; Lin, B.M.; Liu, C.; Azcarate-Peril, A.M.; Simancas-Pallares, M.A.; Shrestha, P.; Orlenko, A.; et al. Selenomonas sputigena acts as a pathobiont mediating spatial structure and biofilm virulence in early childhood caries. Nat. Commun. 2023, 14, 2919. [Google Scholar] [CrossRef] [PubMed]
- Nyvad, B. The role of oral hygiene. In Dental Caries: The Disease and Its Clinical Management, 3rd ed.; Fejerskov, O., Nyvad, B., Kidd, E., Eds.; Blackwell Munksgaard: Copenhagen, Denmark; Blackwell Publishing Company: Oxford, UK, 2015; pp. 277–285. [Google Scholar]
- Tubert-Jeannin, S.; Auclair, C.; Amsallem, E.; Tramini, P.; Gerbaud, L.; Ruffieux, C.; Schulte, A.G.; Koch, M.J.; Rège-Walther, M.; Ismail, A. Fluoride supplements (tablets, drops, lozenges or chewing gums) for preventing dental caries in children. Cochrane Database Syst. Rev. 2011, 12, CD007592. [Google Scholar] [CrossRef]
- Pine, C.M.; Curnow, M.M.T.; Burnside, G.; Nicholson, J.A.; Roberts, A.J. Caries prevalence four years after the end of randomized controlled trial. Caries Res. 2007, 41, 431–436. [Google Scholar] [CrossRef]
- Fejerskov, O.; Cury, J.A.; Tenuta, L.M.; Marinho, V.C. Fluorides in caries control. In Dental Caries: The Disease and Its Clinical Management, 3rd ed.; Fejerskov, O., Nyvad, B., Kidd, E., Eds.; Blackwell Munksgaard: Copenhagen, Denmark; Blackwell Publishing Company: Oxford, UK, 2015; pp. 245–276. [Google Scholar]


| Group 1 (n = 48) (MI Varnish) 6 Year Olds | Group 2 (n = 48) (Control) 6 Year Olds | |||||
|---|---|---|---|---|---|---|
| At Baseline (%) | At 36 Months (%) | p Values | At Baseline (%) | At 36 Months (%) | p Values | |
| Interproximal ICDAS 1 and 2 | 15.38 (5.49) 95% CI [13.8, 16.9] | 14.02 (5.67) 95% CI [12.4, 15.6] | Decrease 0.245 | 15.73 (5.18) 95% CI [14.3, 17.2] | 16.25 (6.60) 95% CI [14.4, 18.1] | Increase 0.614 |
| Interproximal ICDAS 3–6 | 3.71 (4.14) 95% CI [2.54, 4.88] | 1.35(1.64) 95% CI [0.886, 1.81] | Decrease <0.001 | 3.29(4.45) 95% CI [2.03, 4.55] | 1.46 (1.68) 95% CI [0.985, 1.94] | Decrease 0.003 |
| Interproximal F | 2.65 (2.65) 95% CI [1.9, 3.4] | 2.35 (2.32) 95% CI [1.69, 3.01] | Decrease 0.495 | 1.65 (2.23) 95% CI [1.02, 2.28] | 1.67(2.05) 95% CI [1.09, 2.25] | Increase 0.954 |
| Interproximal CAR n/c | 0.06 (0.25) 95% CI [−0.0107, 0.131] | 0 (0) | Decrease 0.083 | 0 (0) | 0.02 (0.14) 95% CI [−0.0196, 0.0596] | Increase 0.322 |
| Interproximal CAR/c | 0.44(0.77) 95% CI [0.222, 0.658] | 0.02 (0.14) 95% CI [−0.0196, 0.0596] | Decrease <0.001 | 0.27 (0.82) 95% CI [0.038, 0.502] | 0.06 (0.25) 95% CI [−0.0107, 0.131] | Decrease 0.096 |
| Interproximal decayed (ICDAS 1–6+ CARn/c + CARc) | 19.58 (6.89) 95% CI [17.6, 21.5] | 15.40 (5.86) 95% CI [13.7, 17.1] | Decrease 0.002 | 19.29 (6.36) 95% CI [17.5, 21.1] | 17.79 (6.82) 95% CI [15.9, 19.7] | Decrease 0.248 |
| Interproximal decayed and filled (ICDAS 1–6) | 22.23 (6.91) 95% CI [20.3, 24.2] | 17.75 (6.46) 95% CI [15.9, 19.6] | Decrease 0.002 | 20.94 (6.30) 95% CI [19.2, 22.7] | 19.46 (6.94) 95% CI [17.5, 21.4] | Decrease 0.291 |
| Occlusal ICDAS 1 and 2 | 0.96 (1.34) 95% CI [0.581, 1.34] | 1.31 (1.45) 95% CI [0.9, 1.72] | Increase 0.061 | 1.13 (1.28) 95% CI [0.768, 1.49] | 1.02 (1.25) 95% CI [0.666, 1.37] | Decrease 0.646 |
| Occlusal ICDAS 3–6 | 0.98 (1.38) 95% CI [0.59, 1.37] | 0.46 (0.77) 95% CI [0.242, 0.678] | Decrease 0.010 | 0.81 (1.51) 95% CI [0.383, 1.24] | 0.35 (0.57) 95% CI [0.189, 0.511] | Decrease 0.049 |
| F | 3.33 (2.41) 95% CI [2.65, 4.01] | 3.25 (2.14) 95% CI [2.65, 3.85] | Decrease 0.820 | 2.45 (2.20) 95% CI [1.83, 3.07] | 3.02 (2.56) 95% CI [2.3, 3.74] | Increase 0.121 |
| CAR n/c | 0.02 (0.14) 95% CI [−0.0196, 0.0596] | 0 (0) | Decrease 0.322 | 0.08 (0.28) 95% CI [0.0008, 0.159] | 0.02 (0.14) 95% CI [−0.0196, 0.0596] | Decrease 0.083 |
| CAR/c | 0.42 (0.65) 95% CI [0.236, 0.604] | 0.06 (0.25) 95% CI [−0.0107, 0.131] | Decrease 0.001 | 0.42 (0.96) 95% CI [0.148, 0.692] | 0.10 (0.37) 95% CI [−0.005, 0.205] | Decrease 0.046 |
| Occlusal decayed (ICDAS 1–6+ CARn/c + CARc) | 2.38 (1.78) 95% CI [1.88, 2.88] | 1.83 (1.74) 95% CI [1.34, 2.32] | Decrease 0.019 | 2.44 (2.03) 95% CI [1.87, 3.01] | 1.50 (1.61) 95% CI [1.04, 1.96] | Decrease 0.012 |
| Occlusal decayed and filled (ICDAS 1–6+) | 5.71 (2.43) 95% CI [5.02, 6.4] | 5.08 (2.37) 95% CI [4.41, 5.75] | Decrease 0.103 | 4.88 (2.66) 95% CI [4.13, 5.63] | 4.52 (2.99) 95% CI [3.67, 5.37] | Decrease 0.407 |
| Buccal/Lingual ICDAS 1 and 2 | 11.27 (6.25) 95% CI [9.5, 13] | 12.75 (5.35) 95% CI [11.2, 14.3] | Increase 0.126 | 9.19 (5.64) 95% CI [7.59, 10.8] | 9.77 (4.97) 95% CI [8.36, 11.2] | Increase 0.487 |
| Buccal/Lingual ICDAS 3–6 | 1.96 (3.07) 95% CI [1.09, 2.83] | 1.33 (1.80) 95% CI [0.821, 1.84] | Decrease 0.037 | 1.71 (2.72) 95% CI [0.941, 2.48] | 0.42 (0.92) 95% CI [0.16, 0.68] | Decrease <0.001 |
| F | 1.17 (1.79) 95% CI [0.664, 1.68] | 0.77 (1.29) 95% CI [0.405, 1.14] | Decrease 0.097 | 0.35 (1.21) 95% CI [0.008, 0.692] | 0.4 (0.89) 95% CI [0.148, 0.652] | Increase 0.808 |
| CAR n/c | 0.02 (0.14) 95% CI [−0.0196, 0.0596] | 0.04 (0.20) 95% CI [−0.0166, 0.0966] | Increase 0.569 | 0.13 (0.39) 95% CI [0.02, 0.24] | 0.10 (0.37) 95% CI [−0.005, 0.205] | Decrease 0.743 |
| CAR/c | 0.19 (0.53) 95% CI [0.04, 0.34] | 0.02 (0.14) 95% CI [−0.0196, 0.0596] | Decrease 0.031 | 0.08 (0.28) 95% CI [0.0008, 0.159] | 0.02 (0.14) 95% CI [−0.0196, 0.0596] | Decrease 0.182 |
| Buccal/Lingual decayed (ICDAS 1–6+ CARn/c + CARc) | 13.44 (7.71) 95% CI [11.3, 15.6] | 14.15 (5.97) 95% CI [12.5, 15.8] | Increase 0.458 | 11.10 (7.14) 95% CI [9.08, 13.1] | 10.31 (5.42) 95% CI [8.78, 11.8] | Decrease 0.410 |
| Buccal/Lingual decayed and filled (ICDAS 1–6) | 14.60 (8.50) 95% CI [12.2, 17] | 14.92 (6.18) 95% CI [13.2, 16.7] | Increase 0.772 | 11.46 (7.43) 95% CI [9.36, 13.6] | 10.71 (5.57) 95% CI [9.13, 12.3] | Decrease 0.464 |
| Interproximal total | 39.33 (3.77) 95% CI [38.3, 40.4] | 41.71 (4.81) 95% CI [40.4, 43.1] | Increase 0.009 | 37.79 (5.26) 95% CI [36.3, 39.3] | 43.17 (4.79) 95% CI [41.8, 44.5] | Increase <0.001 |
| Occlusal total | 10.27 (1.71) 95% CI [9.79, 10.8] | 10.21 (1.66) 95% CI [9.74, 10.7] | Decrease 0.858 | 9.52 (1.83) 95% CI [9, 10] | 10.71 (1.64) 95% CI [10.2, 11.2] | Increase 0.002 |
| Buccal/Lingual total | 39.33 (3.77) 95% CI [38.3, 40.4] | 41.71 (4.81) 95% CI [40.4, 43.1] | Increase 0.009 | 37.79(5.26) 95% CI [36.3, 39.3] | 43.17 (4.79) 95% CI [41.8, 44.5] | Increase <0.001 |
| Group 3 (n = 47) (MI Varnish) 12 Year Olds | Group 4 (n = 37) (Control) 12 Year Olds | |||||
|---|---|---|---|---|---|---|
| At Baseline (%) | At 36 Months (%) | p Values | At Baseline (%) | At 36 Months (%) | p Values | |
| Interproximal ICDAS 1 and 2 | 23.81 (8.50) 95% CI [21.4, 26.2] | 32.40 (8.34) 95% CI [30, 34.8] | Increase <0.001 | 19.05 (9.62) 95% CI [16, 22.2] | 29.51 (6.32) 95% CI [27.5, 31.6] | Increase <0.001 |
| Interproximal ICDAS 3–6 | 1.40 (2.75) 95% CI [0.614, 2.19] | 1.02 (1.92) 95% CI [0.471, 1.57] | Decrease 0.371 | 0.68 (1.18) 95% CI [0.3, 1.06] | 0.98 (1.85) 95% CI [0.384, 1.58] | Increase 0.488 |
| F | 2.06 (3.44) 95% CI [1.08, 3.04] | 3.92 (7.37) 95% CI [1.81, 6.03] | Increase 0.006 | 0.92 (1.38) 95% CI [0.475, 1.36] | 1.35 (1.86) 95% CI [0.751, 1.95] | Increase 0.069 |
| CAR n/c | 0.02 (0.15) 95% CI [−0.0229, 0.0629] | 0 (0) | Decrease 0.323 | 0 (0) | 0 (0) | NaN |
| CAR/c | 0.12 (0.38) 95% CI [0.011, 0.229] | 0.04 (0.20) 95% CI [−0.0172, 0.0972] | Decrease 0.323 | 0.03 (0.16) 95% CI [−0.0216, 0.0816] | 0.05 (0.23) 95% CI [−0.0241, 0.124] | Increase 0.571 |
| Interproximal decayed (ICDAS 1–6+ CARn/c + CARc) | 25.34 (8.66) 95% CI [22.9, 27.8] | 33.47 (8.76) 95% CI [31, 36] | Increase <0.001 | 19.76 (9.78) 95% CI [16.6, 22.9] | 30.49 (7.08) 95% CI [28.2, 32.8] | Increase <0.001 |
| Interproximal decayed and filled (ICDAS 1–6) | 27.40(10.43) 95%CI [24.4, 30.4] | 37.38 (10.22) 95% CI [34.5, 40.3] | Increase <0.001 | 20.68 (10.12) 95% CI [17.4, 23.9] | 31.84 (7.38) 95% CI [29.5, 34.2] | Increase <0.001 |
| Occlusal ICDAS 1 and 2 | 2.34 (1.72) 95%CI [1.85, 2.83] | 3.09 (2.16) 95% CI [2.47, 3.71] | Increase 0.008 | 1.78 (1.65) 95% CI [1.25, 2.31] | 2.62 (1.96) 95% CI [1.99, 3.25] | Increase 0.016 |
| Occlusal ICDAS 3–6 | 1 (1.34) 95%CI [0.617, 1.38] | 0.72 (0.99) 95% CI [0.437, 1] | Decrease 0.102 | 0.76 (1.38) 95% CI [0.315, 1.21] | 0.65 (1.23) 95% CI [0.254, 1.05] | Decrease 0.512 |
| F | 2.66 (3.15) 95%CI [1.76, 3.56] | 3.85(4.03) 95% CI [2.7, 5] | Increase <0.001 | 2.14(1.99) 95% CI [1.5, 2.78] | 3.14(2.52) 95% CI [2.33, 3.95] | Increase 0.001 |
| CAR n/c | 0 (0) | 0.02 (0.15) 95% CI [−0.0229, 0.0629] | Increase 0.323 | 0.08 (0.28) 95% CI [−0.0102, 0.17] | 0 (0) | Decrease 0.083 |
| CAR/c | 0.17 (0.52) 95% CI [0.021, 0.319] | 0.09(0.35) 95% CI [−0.01, 0.19] | Decrease 0.351 | 0.05(0.23) 95% CI [−0.0241, 0.124] | 0 (0) | Decrease 0.160 |
| Occlusal decayed (ICDAS 1–6+ CARn/c + CARc) | 3.51 (2.09) 95% CI [2.91, 4.11] | 3.91 (2.59) 95% CI [3.17, 4.65] | Increase 0.211 | 2.68 (2.45) 95% CI [1.89, 3.47] | 3.27 (2.35) 95% CI [2.51, 4.03] | Increase 0.131 |
| Occlusal decayed and filled (ICDAS 1–6) | 6.17 (3.81) 95% CI [5.08, 7.26] | 7.77 (4.28) 95% CI [6.55, 8.99] | Increase <0.001 | 4.81 (3.15) 95% CI [3.8, 5.82] | 6.41 (3.51) 95% CI [5.28, 7.54] | Increase <0.001 |
| Buccal/Lingual ICDAS 1 and 2 | 12.36 (7.19) 95% CI [10.3, 14.4] | 19.53 (8.29) 95% CI [17.2, 21.9] | Increase <0.001 | 9.89 (6.15) 95% CI [7.91, 11.9] | 14.68(6.33) 95% CI [12.6, 16.7] | Increase <0.001 |
| Buccal/Lingual ICDAS 3–6 | 1.21 (1.92) 95% CI [0.661, 1.76] | 1.02 (1.23) 95% CI [0.668, 1.37] | Decrease 0.386 | 0.49 (0.84) 95% CI [0.219, 0.761] | 0.62 (1.66) 95% CI [0.085, 1.16] | Increase 0.549 |
| F | 1.51 (2.01) 95% CI [0.935, 2.08] | 2.62 (3.72) 95% CI [1.56, 3.68] | Increase 0.003 | 0.62 (1.01) 95% CI [0.295, 0.945] | 1 (1.35) 95% CI [0.565, 1.44] | Increase 0.029 |
| CAR n/c | 0.55(1.21) 95% CI [0.204, 0.896] | 0.79(1.44) 95% CI [0.378, 1.2] | Increase 0.109 | 0.24(0.55) 95% CI [0.063, 0.417] | 0.32(0.71) 95% CI [0.091, 0.549] | Increase 0.262 |
| CAR/c | 0.06 (0.25) 95% CI [−0.0115, 0.132] | 0.09 (0.41) 95% CI [−0.027, 0.207] | Increase 0.710 | 0.08 (0.49) 95% CI [−0.078, 0.238] | 0 (0) | Decrease 0.324 |
| Buccal/Lingual decayed (ICDAS 1–6+ CARn/c + CARc) | 14.19 (8.27) 95% CI [11.8, 16.6] | 21.43 (8.74) 95% CI [18.9, 23.9] | Increase <0.001 | 10.70 (6.47) 95% CI [8.62, 12.8] | 15.62 (6.94) 95% CI [13.4, 17.9] | Increase <0.001 |
| Buccal/Lingual decayed and filled (ICDAS 1–6) | 15.70 (9.04) 95% CI [13.1, 18.3] | 24.04 (10.72) 95% CI [21, 27.1] | Increase <0.001 | 11.32 (6.49) 95% CI [9.23, 13.4] | 16.62 (7.34) 95% CI [14.3, 19] | Increase <0.001 |
| Interproximal total | 51.32 (5.23) 95% CI [49.8, 52.8] | 54.72 (3.13) 95% CI [53.8, 55.6] | Increase <0.001 | 50.0 (5.23) 95% CI [48.3, 51.7] | 55.35 (1.57) 95% CI [54.8, 55.9] | Increase <0.001 |
| Occlusal total | 14.0 (2.18) 95% CI [13.4, 14.6] | 17.6 (14.74) 95% CI [13.4, 21.8] | Increase 0.099 | 13.51 (2.18) 95% CI [12.8, 14.2] | 15.76 (0.72) 95% CI [15.5, 16] | Increase <0.001 |
| Buccal/Lingual total | 51.32 (5.23) 95% CI [49.8, 52.8] | 54.72(3.13) 95% CI [53.8, 55.6] | Increase <0.001 | 50.0 (5.23) 95% CI [48.3, 51.7] | 55.35 (1.57) 95% CI [54.8, 55.9] | Increase <0.001 |
| Group 1 (n = 48) | (MI Varnish) | Group 2 (n = 48) | (Control) | |
|---|---|---|---|---|
| At Baseline (%) | At 36 Months (%) | At Baseline (%) | At 36 Months (%) | |
| Frequency of daily toothbrushing | 70.83 (n = 34)— 2 times daily 22.92 (n = 11)—once daily 6.25 (n = 3)—>2 times daily | 60.42 (n = 29)— 2 times daily 37.5 (n = 18—once daily 2.08 (n = 1)—once a week | 64.58 (n = 31)— 2 times daily 25 (n = 12)—once daily 10.42 (n = 5)—>2 times daily | 75 (n = 36)— 2 times daily 22.92 (n = 11)—once daily 2.08 (n = 1)—once a week |
| The use of F-containing toothpaste (TP) | 47.92 (n = 23)—confirmed that they use F-containing TP 6.25 (n = 3)—stated they do not use F-containing TP 45.83(n = 22)—do not know about F in used TP | 39.58 (n = 19)— confirmed that they use F-containing TP 0 (n = 0)— stated they do not use F-containing TP 60.42 (n = 29)—do not know about F in used TP | 45.83 (n = 22)— confirmed that they use F-containing TP 10.42 (n = 5)— stated they do not use F-containing TP 43.75 (n = 21)—do not know about F in used TP | 31.25 (n = 19)— confirmed that they use F-containing TP 2.08 (n = 1)— stated they do not use F-containing TP 66.67 (n = 32)—did not know about F in used TP |
| Parental supervision | 54.17 (n = 26)—supervised 45.83 (n = 22)— not supervised | 37.5 (n = 18)—supervised 62.5 (n = 30)— not supervised | 77.08 (n = 37)—supervised 22.92 (n = 11)— not supervised | 31.25 (n = 15)—supervised 68.75 (n = 33)— not supervised |
| Name of used toothpaste | 27.08 (n = 13)—know 72.92 (n = 35)—do not know | 58.33 (n = 28)—know 41.67 (n = 20)—do not know | 39.58 (n = 19)—know 60.41 (n = 29)—do not know | 64.58 (n = 31)—know 35.42 (n = 17)—do not know |
| Group 3 (n = 47) | (MI Varnish) | Group 4 (n = 37) | (Control) | |
|---|---|---|---|---|
| At Baseline (%) | At 36 Months (%) | At Baseline (%) | At 36 Months (%) | |
| Frequency of daily toothbrushing | 53.19 (n = 25)— 2 times daily 44.68 (n = 21)—once daily 2.13 (n = 1)—> 2 times daily | 57.45 (n = 27)— 2 times daily 36.17 (n = 17)—once daily 6.38 (n = 3)—> 2 times daily | 51.35 (n = 19)— 2 times daily 40.54 (n = 15)—once daily 2.7 (n = 1)—> 2 times daily 5.41 (n = 2)—once a week | 43.24 (n = 16)— 2 times daily 48.65 (n = 18)—once daily 8.11 (n = 3)—> 2 times daily |
| The use of F-containing toothpaste (TP) | 31.92 (n = 15)— confirmed that they use F-containing TP 8.51 (n = 4)— stated they do not use using F-containing TP 59.57 (n = 28)—do not know about F in used TP | 14.89 (n = 7)— confirmed that they use F-containing TP 6.39 (n = 3)— stated they do not use F-containing TP 78.72 (n = 37)-do not know about F in used TP | 16.21 (n = 6)— confirmed that they use F-containing TP 2.7 (n = 1)— stated they do not use F-containing TP 89.91 (n = 33)—do not know about F in used TP | 10.81 (n = 4)— confirmed that they use F-containing TP 0 (n = 0)— stated they do not use F-containing TP 89.91 (n = 33)—did not know about F in used TP |
| Name of used toothpaste | 8.51 (n = 4)—know 91.49 (n = 43)—do not know | 34.04 (n = 16)—know 65.96 (n = 31)—do not know | 18.92 (n = 7)—know 81.08 (n = 30)—do not know | 43.24 (n = 16)—know 56.76 (n = 21)—do not know |
| 6-Year Olds | 12 Year Olds | |
|---|---|---|
| At 36 Months | At 36 Months | |
| Interproximal surfaces (ICDAS 1–6+ CARn/c + CARc + F) | RR = 1.04 (increase 4%) | RR = 0.75 (reduction 25%) |
| Occlusal surfaces (ICDAS 1–6+ CARn/c + CARc + F) | RR = 0.87 (reduction 13%) | RR = 0.96 (reduction 4%) |
| Buccal/Lingual surfaces (ICDAS 1–6+ CARn/c + CARc + F) | RR = 0.85 (reduction 15%) | RR = 0.80 (reduction 20%) |
| Group 3 (n = 47) | (MI Varnish) | Group 4 (n = 37) | (Control) | |
|---|---|---|---|---|
| At Baseline | At 36 Months | At Baseline | At 36 Months | |
| Interproximal Decayed to Occlusal Decayed | r = 0.329 p < 0.05 | r = 0.381 p < 0.001 | r = 0.459 p < 0.001 | r = 0.270 p > 0.05 |
| Interproximal Decayed to Buccal/Lingual Decayed | r = 0.480 p < 0.001 | r = 0.274 p > 0.05 | r = 0.573 p < 0.001 | r = 0.602 p < 0.001 |
| Occlusal Decayed to Buccal/Lingual Decayed | r = 0.368 p < 0.05 | r = 0.056 p > 0.05 | r = 0.469 p < 0.001 | r = 0.466 p < 0.001 |
| Interproximal Decayed and Filled to Occlusal Decayed and Filled | r = 0.516 p < 0.001 | r = 0.535 p < 0.001 | r = 0.528 p < 0.001 | r = 0.537 p < 0.001 |
| Interproximal Decayed and Filled to Buccal/Lingual Decayed and Filled | r = 0.619 p < 0.001 | r = 0.615 p < 0.001 | r = 0.570 p < 0.001 | r = 0.640 p < 0.001 |
| Occlusal Decayed and Filled to Buccal/Lingual Decayed and Filled | r = 0.665 p < 0.001 | r = 0.618 p < 0.001 | r = 0.612 p < 0.001 | r = 0.685 p < 0.001 |
| Group 1 (n = 48) | (MI Varnish) | Group 2 (n = 48) | (Control) | |
|---|---|---|---|---|
| At Baseline | At 36 Months | At Baseline | At 36 Months | |
| Interproximal Decayed to Occlusal Decayed | r = 0.449 p < 0.001 | r = 0.461 p < 0.001 | r = 0.140 p < 0.05 | r = 0.227 p > 0.05 |
| Interproximal Decayed to Buccal/Lingual Decayed | r = 0.527 p < 0.001 | r = 0.610 p < 0.001 | r = 0.280 p > 0.05 | r = 0.265 p > 0.05 |
| Occlusal Decayed to Buccal/Lingual Decayed | r = 0.420 p < 0.001 | r = 0.358 p < 0.05 | r = 0.336 p < 0.05 | r = 0.028 p > 0.05 |
| Interproximal Decayed and Filled To Occlusal Decayed and Filled | r = 0.439 p < 0.001 | r = 0.469 p < 0.001 | r = 0.329 p < 0.05 | r = 0.351 p < 0.05 |
| Interproximal Decayed and Filled to Buccal/Lingual Decayed and Filled | r = 0.484 p < 0.001 | r = 0.695 p < 0.001 | r = 0.398 p < 0.001 | r = 0.312 p < 0.05 |
| Occlusal Decayed and Filled to Buccal/Lingual Decayed and Filled | r = 0.406 p < 0.001 | r = 0.598 p < 0.00 | r = 0.635 p < 0.001 | r = 0.459 p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gudkina, J.; Amaechi, B.T.; Abrams, S.H.; Brinkmane, A. Tooth-Surface-Specific Effects of MI Varnish™: A 3-Year Randomized Clinical Trial. Oral 2023, 3, 372-388. https://doi.org/10.3390/oral3030030
Gudkina J, Amaechi BT, Abrams SH, Brinkmane A. Tooth-Surface-Specific Effects of MI Varnish™: A 3-Year Randomized Clinical Trial. Oral. 2023; 3(3):372-388. https://doi.org/10.3390/oral3030030
Chicago/Turabian StyleGudkina, Jekaterina, Bennett T. Amaechi, Stephen H. Abrams, and Anda Brinkmane. 2023. "Tooth-Surface-Specific Effects of MI Varnish™: A 3-Year Randomized Clinical Trial" Oral 3, no. 3: 372-388. https://doi.org/10.3390/oral3030030
APA StyleGudkina, J., Amaechi, B. T., Abrams, S. H., & Brinkmane, A. (2023). Tooth-Surface-Specific Effects of MI Varnish™: A 3-Year Randomized Clinical Trial. Oral, 3(3), 372-388. https://doi.org/10.3390/oral3030030