Habitat Urbanization, Circulating Glucose and Carotenoid Levels, and Body Condition Predict Variation in Blood Ketone Levels in House Finches (Haemorhous mexicanus) from the American Southwest
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Circulating Levels of Carotenoids and Vitamins
2.3. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cardinale, B.J.; Duffy, J.E.; Gonzalez, A.; Hooper, D.U.; Perrings, C.; Venail, P.; Narwani, A.; Mace, G.M.; Tilman, D.; Wardle, D.A.; et al. Biodiversity loss and its impact on humanity. Nature 2012, 486, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Lawson, B.; Neimanis, A.; Lavazza, A.; López-Olvera, J.R.; Tavernier, P.; Billinis, C.; Duff, J.P.; Mladenov, D.T.; Rijks, J.M.; Savić, S.; et al. How to Start Up a National Wildlife Health Surveillance Programme. Animals 2021, 11, 2543. [Google Scholar] [CrossRef] [PubMed]
- Preece, N.D.; Abell, S.E.; Grogan, L.; Wayne, A.; Skerratt, L.F.; van Oosterzee, P.; Shima, A.L.; Daszak, P.; Field, H.; Reiss, A.; et al. A guide for ecologists: Detecting the role of disease in faunal declines and managing population recovery. Biol. Conserv. 2017, 214, 36–46. [Google Scholar] [CrossRef]
- McGraw, K.J.; de Souza Penha, V.A. Using point-of-care devices to examine covariation among blood nutritional-physiological parameters and their relationships with poxvirus infection, habitat urbanization, and male plumage coloration in house finches (Haemorhous mexicanus). J. Exp. Zool. A Ecol. Integr. Physiol. 2024, 341, 440–449. [Google Scholar] [CrossRef]
- Morales, A.; Frei, B.; Leung, C.; Titman, R.; Whelan, S.; Benowitz-Fredericks, Z.M.; Elliott, K.H. Point-of-care blood analyzers measure the nutritional state of eighteen free-living bird species. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2020, 240, 110594. [Google Scholar] [CrossRef]
- Lieske, C.L.; Ziccardi, M.H.; Mazet, J.A.K.; Newman, S.H.; Gardner, I.A. Evaluation of 4 handheld blood glucose monitors for use in seabird rehabilitation. J. Avian Med. Surg. 2002, 16, 277–285. [Google Scholar] [CrossRef]
- Freitas, F.; Almeida, K.; Machado, R.; Machado, C. Lipid and glucose metabolism of broilers (Gallus gallus domesticus) experimentally infected with Eimeria acervulina Tyzzer, 1929 oocysts. Rev. Bras. Cienc. Avic. 2008, 10, 157–162. [Google Scholar] [CrossRef]
- McGraw, K.J.; Chou, K.; Bridge, A.; McGraw, H.C.; McGraw, P.R.; Simpson, R.K. Body condition and poxvirus infection predict circulating glucose levels in a colorful songbird that inhabits urban and rural environments. J. Exp. Zool. A Ecol. Integr. Physiol. 2020, 333, 561–568. [Google Scholar] [CrossRef]
- Plüddemann, A.; Heneghan, C.; Price, C.P.; Wolstenholme, J.; Thompson, M. Point-of-care blood test for ketones in patients with diabetes: Primary care diagnostic technology update. Br. J. Gen. Pract. 2011, 61, 530–531. [Google Scholar] [CrossRef]
- Andrews, M.T.; Russeth, K.P.; Drewes, L.R.; Henry, P.G. Adaptive mechanisms regulate preferred utilization of ketones in the heart and brain of a hibernating mammal during arousal from torpor. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R383–R393. [Google Scholar] [CrossRef]
- Riches, P.L.; Sing, K.; Berg, K. Point-of-care uric acid testing is useful in routine clinical care of gout. Arthritis Res. Ther. 2019, 21, 117. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Biswas, S.; Halder, S.; Chanda, N.; Mandal, S. Efficient Point-of-Care Detection of Uric Acid in the Human Blood Sample with an Enhanced Electrocatalytic Response Using Nanocomposites of Cobalt and Mixed-Valent Molybdenum Sulfide. ACS Appl. Bio Mater. 2022, 5, 4191–4202. [Google Scholar] [CrossRef] [PubMed]
- Stoot, L.J.; Cairns, N.A.; Cull, F.; Taylor, J.J.; Jeffrey, J.D.; Morin, F.; Mandelman, J.W.; Clark, T.D.; Cooke, S.J. Use of portable blood physiology point-of-care devices for basic and applied research on vertebrates: A review. Conserv. Physiol. 2014, 2, cou011. [Google Scholar] [CrossRef] [PubMed]
- Smyth, B.J.; Polaski, R.S.; Safer, A.; Boettcher, F.A.; Konrad-Martin, D.; Gratton, M.A. Point-of-Care Glucose and Lipid Profile Measures Using a Human Point-of-Care Device in Mouse Models of Type 2 Diabetes Mellitus, Aging, and Alzheimer Disease. J. Am. Assoc. Lab. Anim. Sci. 2021, 60, 609–615. [Google Scholar] [CrossRef]
- Azeredo, L.M.M.; Oliveira, T.C.; Lopez, L.C.S. Blood metabolites as predictors to evaluate the body condition of Neopelma pallescens (Passeriformes: Pipridae) in northeastern Brazil. Zoologia 2016, 33, e20160043. [Google Scholar] [CrossRef]
- Vágási, C.I.; Tóth, Z.; Pénzes, J.; Pap, P.L.; Ouyang, J.Q.; Lendvai, Á.Z. The Relationship between Hormones, Glucose, and Oxidative Damage Is Condition and Stress Dependent in a Free-Living Passerine Bird. Physiol. Biochem. Zool. 2020, 93, 466–476. [Google Scholar] [CrossRef]
- Oliveira, M.R.; Braghirolli, F.M.; Verrastro, L.; Oliveira, G.T. Seasonal and Sexual Variation of the Intermediate Metabolism and Body Condition Indexes in the Lizard Tropidurus catalanensis (Gudynas and Skuk, 1983) (Squamata: Tropiduridae). S. Am. J. Herpetol. 2018, 13, 85–95. [Google Scholar] [CrossRef]
- Franco-Belussi, L.; de Oliveira Júnior, J.G.; Goldberg, J.; De Oliveira, C.; Fernandes, C.E.; Provete, D.B. Multiple morphophysiological responses of a tropical frog to urbanization conform to the pace-of-life syndrome. Conserv. Physiol. 2024, 12, coad106. [Google Scholar] [CrossRef]
- Gadau, A.; Crawford, M.S.; Mayek, R.; Giraudeau, M.; McGraw, K.J.; Whisner, C.M.; Kondrat-Smith, C.; Sweazea, K.L. A comparison of the nutritional physiology and gut microbiome of urban and rural house sparrows (Passer domesticus). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2019, 237, 110332. [Google Scholar] [CrossRef]
- Harris, S.E.; Munshi-South, J. Signatures of positive selection and local adaptation to urbanization in white-footed mice (Peromyscus leucopus). Mol. Ecol. 2017, 26, 6336–6350. [Google Scholar] [CrossRef]
- Melvin, S.D.; Lanctôt, C.M.; van de Merwe, J.P.; Leusch, F.D.L. Altered bioenergetics and developmental effects in striped marsh frog (Limnodynastes peronii) tadpoles exposed to UV treated sewage. Aquat. Toxicol. 2016, 175, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Tomita, I.; Tsuruta, H.; Yasuda-Yamahara, M.; Yamahara, K.; Kuwagata, S.; Tanaka-Sasaki, Y.; Chin-Kanasaki, M.; Fujita, Y.; Nishi, E.; Katagiri, H.; et al. Ketone bodies: A double-edged sword for mammalian life span. Aging Cell 2023, 22, e13833. [Google Scholar] [CrossRef] [PubMed]
- Laffel, L. Ketone bodies: A review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab. Res. Rev. 1999, 15, 412–426. [Google Scholar] [CrossRef]
- Kaliński, A.; Glądalski, M.; Markowski, M.; Skwarska, J.; Wawrzyniak, J.; Bańbura, J. Ketone body levels in wintering great tits Parus major in sites differing in artificial food availability. Conserv. Physiol. 2022, 10, coac072. [Google Scholar] [CrossRef]
- Isaksson, C.; Andersson, M.N.; Nord, A.; von Post, M.; Wang, H.L. Species-Dependent Effects of the Urban Environment on Fatty Acid Composition and Oxidative Stress in Birds. Front. Ecol. Evol. 2017, 5, 44. [Google Scholar] [CrossRef]
- Fernández, C.; Villaseñor, N.R.; Contreras, C.; Ávila, M.; Sabat, P.; Poblete, Y. Intra-urban variation in body condition, body size and oxidative status of Rufous-collared sparrow relate to urban green space attributes in a Latin American metropolis. Urban Ecosyst. 2023, 26, 575–586. [Google Scholar] [CrossRef]
- Meillère, A.; Brischoux, F.; Parenteau, C.; Angelier, F. Influence of Urbanization on Body Size, Condition, and Physiology in an Urban Exploiter: A Multi-Component Approach. PLoS ONE 2015, 10, e0135685. [Google Scholar] [CrossRef]
- Meyrier, E.; Jenni, L.; Bötsch, Y.; Strebel, S.; Erne, B.; Tablado, Z. Happy to breed in the city? Urban food resources limit reproductive output in Western Jackdaws. Ecol. Evol. 2017, 7, 1363–1374. [Google Scholar] [CrossRef]
- Bruss, M.L. Lipids and Ketones. In Clinical Biochemistry of Domestic Animals; Elsevier: Amsterdam, The Netherlands, 1997; pp. 83–115. [Google Scholar]
- Lindholm, C.; Altimiras, J.; Lees, J. Measuring ketones in the field: Rapid and reliable measures of β -hydroxybutyrate in birds. Ibis 2019, 161, 205–210. [Google Scholar] [CrossRef]
- Ohtsu, H.; Yakabe, Y.; Yamazaki, M.; Murakami, H.; Abe, H. Plasma Lipid Profiles and Redox Status are Modulated in a Ketogenic Diet-Induced Chicken Model of Ketosis. J. Poult. Sci. 2013, 50, 212–218. [Google Scholar] [CrossRef]
- Landys, M.M.; Piersma, T.; Guglielmo, C.G.; Jukema, J.; Ramenofsky, M.; Wingfield, J.C. Metabolic profile of long–distance migratory flight and stopover in a shorebird. Proc. R. Soc. B Biol. Sci. 2005, 272, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Jenni-Eiermann, S.; Jenni, L. Metabolic Differences Between the Postbreeding, Moulting and Migratory Periods in Feeding and Fasting Passerine Birds. Funct. Ecol. 1996, 10, 62. [Google Scholar] [CrossRef]
- Jenni-Eiermann, S.; Jenni, L.; Piersma, T. Plasma metabolites reflect seasonally changing metabolic processes in a long-distance migrant shorebird (Calidris canutus). Zoology 2002, 105, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Jenni-Eiermann, S.; Jenni, L. Diurnal Variation of Metabolic Responses to Short-Term Fasting in Passerine Birds during the Postbreeding, Molting and Migratory Period. Condor 1997, 99, 113–122. [Google Scholar] [CrossRef]
- Badyaev, A.V.; Hill, G.E.; Weckworth, B.V. Species divergence in sexually selected traits: Increase in song elaboration is related to decrease in plumage ornamentation in finches. Evolution 2002, 56, 412–419. [Google Scholar]
- Sykes, B.E.; Hutton, P.; McGraw, K.J. Sex-specific relationships between urbanization, parasitism, and plumage coloration in house finches. Curr. Zool. 2021, 67, 237–244. [Google Scholar] [CrossRef]
- Giraudeau, M.; Sweazea, K.; Butler, M.W.; McGraw, K.J. Effects of carotenoid and vitamin E supplementation on oxidative stress and plumage coloration in house finches (Haemorhous mexicanus). Comp. Biochem. Physiol.—A Mol. Integr. Physiol. 2013, 166, 406–413. [Google Scholar] [CrossRef]
- DePinto, K.N.; McGraw, K.J. Back to the future: Does previously grown ornamental colouration in male House Finches reveal mate quality at the time of pair formation? J. Ornithol. 2022, 163, 977–985. [Google Scholar] [CrossRef]
- Giraudeau, M.; Mousel, M.; Earl, S.; McGraw, K. Parasites in the city: Degree of urbanization predicts poxvirus and coccidian infections in house finches (Haemorhous mexicanus). PLoS ONE 2014, 9, e86747. [Google Scholar] [CrossRef]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- McGraw, K.J.; Toomey, M.B. Carotenoid accumulation in the tissues of zebra finches: Predictors of integumentary pigmentation and implications for carotenoid allocation strategies. Physiol. Biochem. Zool. 2010, 83, 97–109. [Google Scholar] [CrossRef] [PubMed]
- McGraw, K.J.; Tourville, E.A.; Butler, M.W. A quantitative comparison of the commonly used methods for extracting carotenoids from avian plasma. Behav. Ecol. Sociobiol. 2008, 62, 1991–2002. [Google Scholar] [CrossRef]
- McGraw, K.J.; Giraudeau, M.; Hill, G.E.; Toomey, M.B.; Staley, M. Ketocarotenoid circulation, but not retinal carotenoid accumulation, is linked to eye disease status in a wild songbird. Arch. Biochem. Biophys. 2013, 539, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S; Springer: New York, NY, USA, 2002. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis (Use R); Springer: Berlin/Heidelberg, Germany, 2016; 276p. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Viena, Austria, 2019. [Google Scholar]
- Smit, B.; Zietsman, G.; Martin, R.O.; Cunningham, S.J.; McKechnie, A.E.; Hockey, P.A.R. Behavioural responses to heat in desert birds: Implications for predicting vulnerability to climate warming. Clim. Change Responses 2016, 3, 9. [Google Scholar] [CrossRef]
- Thompson, M.L.; Cunningham, S.J.; McKechnie, A.E. Interspecific variation in avian thermoregulatory patterns and heat dissipation behaviours in a subtropical desert. Physiol. Behav. 2018, 188, 311–323. [Google Scholar] [CrossRef]
- Golden, J.S.; Hartz, D.; Brazel, A.; Luber, G.; Phelan, P. A biometeorology study of climate and heat-related morbidity in Phoenix from 2001 to 2006. Int. J. Biometeorol. 2008, 52, 471–480. [Google Scholar] [CrossRef]
- Tryjanowski, P.; Skórka, P.; Sparks, T.H.; Biaduń, W.; Brauze, T.; Hetmański, T.; Martyka, R.; Indykiewicz, P.; Myczko, Ł.; Kunysz, P.; et al. Urban and rural habitats differ in number and type of bird feeders and in bird species consuming supplementary food. Environ. Sci. Pollut. Res. 2015, 22, 15097–15103. [Google Scholar] [CrossRef]
- Simons, M.J.P.; Cohen, A.A.; Verhulst, S. What does carotenoid-dependent coloration tell? Plasma carotenoid level signals immunocompetence and oxidative stress state in birds-a meta-analysis. PLoS ONE 2012, 7, e43088. [Google Scholar] [CrossRef]
- McGraw, K.J.; Ardia, D.R. Carotenoids, Immunocompetence, and the Information Content of Sexual Colors: An Experimental Test. Am. Nat. 2003, 162, 704–712. [Google Scholar] [CrossRef]
- Costantini, D.; Møller, A.P. Carotenoids are minor antioxidants for birds. Funct. Ecol. 2008, 22, 367–370. [Google Scholar] [CrossRef]
- Koch, R.E.; Kavazis, A.N.; Hasselquist, D.; Hood, W.R.; Zhang, Y.; Toomey, M.B.; Hill, G.E. No evidence that carotenoid pigments boost either immune or antioxidant defenses in a songbird. Nat. Commun. 2018, 9, 491. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Qin, D.; Wang, X.; Feng, Y.; Yang, X.; Yao, J. Effect of immune stress on growth performance and energy metabolism in broiler chickens. Food Agric. Immunol. 2015, 26, 194–203. [Google Scholar] [CrossRef]
- Done, T.; Gow, E.A.; Stutchbury, B.J.M. Corticosterone stress response and plasma metabolite levels during breeding and molt in a free-living migratory songbird, the wood thrush (Hylocichla mustelina). Gen. Comp. Endocrinol. 2011, 171, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Kern, M.; Bacon, W.; Long, D.; Cowie, R.J. Blood Metabolite and Corticosterone Levels in Breeding Adult Pied Flycatchers. Condor 2005, 107, 665–677. [Google Scholar] [CrossRef]
- Müller, C.; Jenni-Eiermann, S.; Blondel, J.; Perret, P.; Caro, S.P.; Lambrechts, M.; Jenni, L. Effect of human presence and handling on circulating corticosterone levels in breeding blue tits (Parus caeruleus). Gen. Comp. Endocrinol. 2006, 148, 163–171. [Google Scholar] [CrossRef]
- Parks, S.N.; Tully, T.N.; Settle, A.L.; Lattin, C.R. Handling and restraint induce a significant increase in plasma corticosterone in Hispaniolan Amazon parrots (Amazona ventralis). Am. J. Vet. Res. 2023, 84, ajvr.22.12.0223. [Google Scholar] [CrossRef]
- Vleck, C.; Vertalino, N.; Vleck, D.; Bucher, T. Stress, corticosterone, and heterophil to lymphocyte ratios in free-living Adélie Penguins. Condor 2000, 102, 392–400. [Google Scholar] [CrossRef]
- Drake, D.J.; McGraw, K.J. Variation in plasma protein levels in House Finches (Haemorhous mexicanus): Effects of season, disease state, and urbanization. J. Ornithol. 2023, 164, 629–638. [Google Scholar] [CrossRef]
- Jenni-Eiermann, S.; Jenni, L. Postexercise Ketosis in Night-Migrating Passerine Birds. Physiol. Biochem. Zool. 2001, 74, 90–101. [Google Scholar] [CrossRef]
- Jenni-Eiermann, S.; Jenni, L. High Plasma Triglyceride Levels in Small Birds during Migratory Flight: A New Pathway for Fuel Supply during Endurance Locomotion at Very High Mass-Specific Metabolic Rates? Physiol. Zool. 1992, 65, 112–123. [Google Scholar] [CrossRef]


| Models | Variables | Estimate | t | p-Value |
|---|---|---|---|---|
| Summer 2020 | ||||
| Best model | Intercept A | 3.97 | 6.01 | <0.01 * |
| r2 = 0.35 | Glucose | −0.00 | −3.07 | <0.01 * |
| AIC = 37.71 | Sampling location (suburban) | −1.04 | −0.94 | 0.35 |
| Sampling location (rural) | −4.99 | −3.26 | <0.01 * | |
| Sampling location (suburban) × Glucose B | 0.00 | 0.95 | 0.34 | |
| Sampling location (rural) × Glucose B | 0.01 | 3.04 | <0.01 * | |
| Winter 2020–2021 | ||||
| Best model | Intercept A | 27.31 | 4.02 | <0.01 * |
| r2 = 0.55 | Glucose | −3.90 | −3.22 | <0.01 * |
| AIC = 221.31 | Sampling location (suburban) | −2.82 | −0.27 | 0.78 |
| Sampling location (rural) | −22.19 | −2.51 | 0.01 * | |
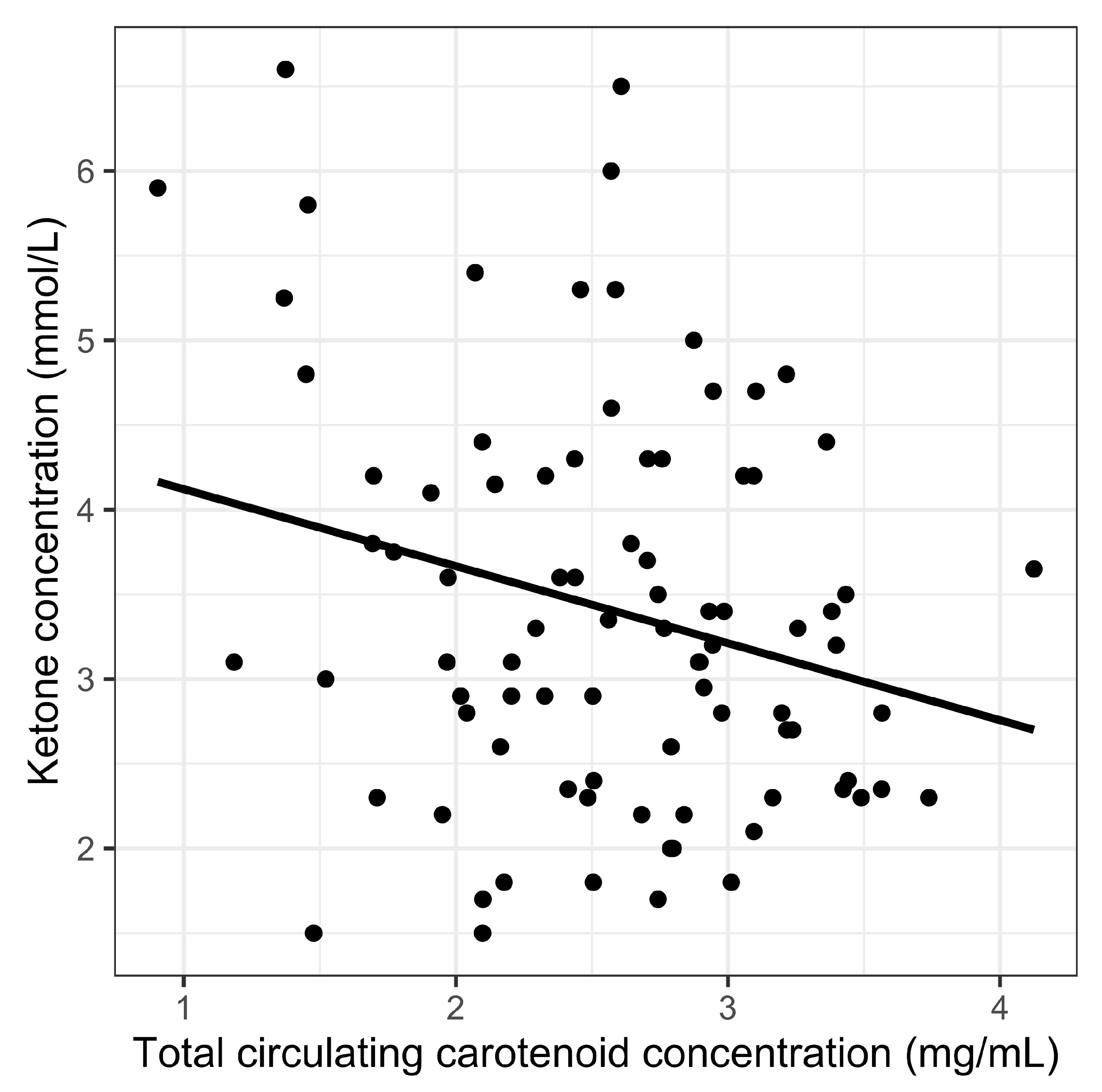
| Total circulating carotenoid concentration | −0.27 | −3.18 | <0.01 * | |
| Sampling location (suburban) × Glucose C | 0.16 | 0.08 | 0.92 | |
| Sampling location (rural) × Glucose C | 3.71 | 2.34 | 0.02 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McGraw, K.J.; de Souza Penha, V.A.; DePinto, K.N.; Drake, D.J.; Crawford-Paz Soldán, E.; Pais, D. Habitat Urbanization, Circulating Glucose and Carotenoid Levels, and Body Condition Predict Variation in Blood Ketone Levels in House Finches (Haemorhous mexicanus) from the American Southwest. Birds 2025, 6, 34. https://doi.org/10.3390/birds6030034
McGraw KJ, de Souza Penha VA, DePinto KN, Drake DJ, Crawford-Paz Soldán E, Pais D. Habitat Urbanization, Circulating Glucose and Carotenoid Levels, and Body Condition Predict Variation in Blood Ketone Levels in House Finches (Haemorhous mexicanus) from the American Southwest. Birds. 2025; 6(3):34. https://doi.org/10.3390/birds6030034
Chicago/Turabian StyleMcGraw, Kevin J., Victor Aguiar de Souza Penha, Kathryn N. DePinto, Dean J. Drake, Elise Crawford-Paz Soldán, and Danielle Pais. 2025. "Habitat Urbanization, Circulating Glucose and Carotenoid Levels, and Body Condition Predict Variation in Blood Ketone Levels in House Finches (Haemorhous mexicanus) from the American Southwest" Birds 6, no. 3: 34. https://doi.org/10.3390/birds6030034
APA StyleMcGraw, K. J., de Souza Penha, V. A., DePinto, K. N., Drake, D. J., Crawford-Paz Soldán, E., & Pais, D. (2025). Habitat Urbanization, Circulating Glucose and Carotenoid Levels, and Body Condition Predict Variation in Blood Ketone Levels in House Finches (Haemorhous mexicanus) from the American Southwest. Birds, 6(3), 34. https://doi.org/10.3390/birds6030034





