A Validation Study on Immunophenotypic Differences in T-lymphocyte Chromosomal Radiosensitivity between Newborns and Adults in South Africa
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Isolation of Peripheral Blood Mononuclear Cells (PBMCs)
2.1.1. Collection of Umbilical Cord Blood (UCB) Samples
2.1.2. Collection of Adult Peripheral Blood (APB) Sample
2.1.3. Isolation of Peripheral Blood Mononuclear Cells (PBMCs) from UCB and APB
2.2. 60Co γ-Ray Irradiation
2.3. Cytokinesis-Block Micronucleus (CBMN) Assay
2.4. Immunophenotyping of Adult and Newborn PBMCs
2.5. Statistical Analysis
3. Results
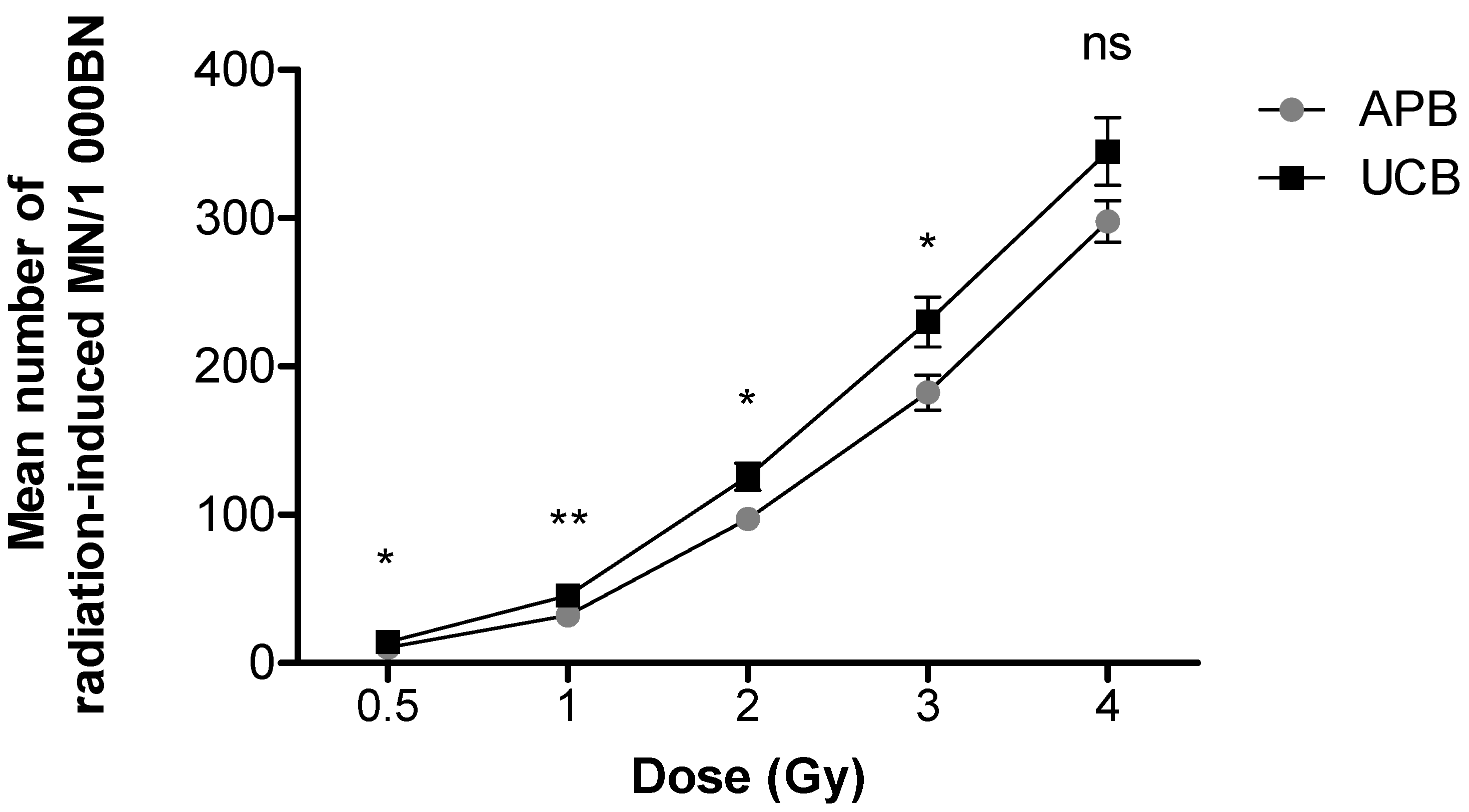
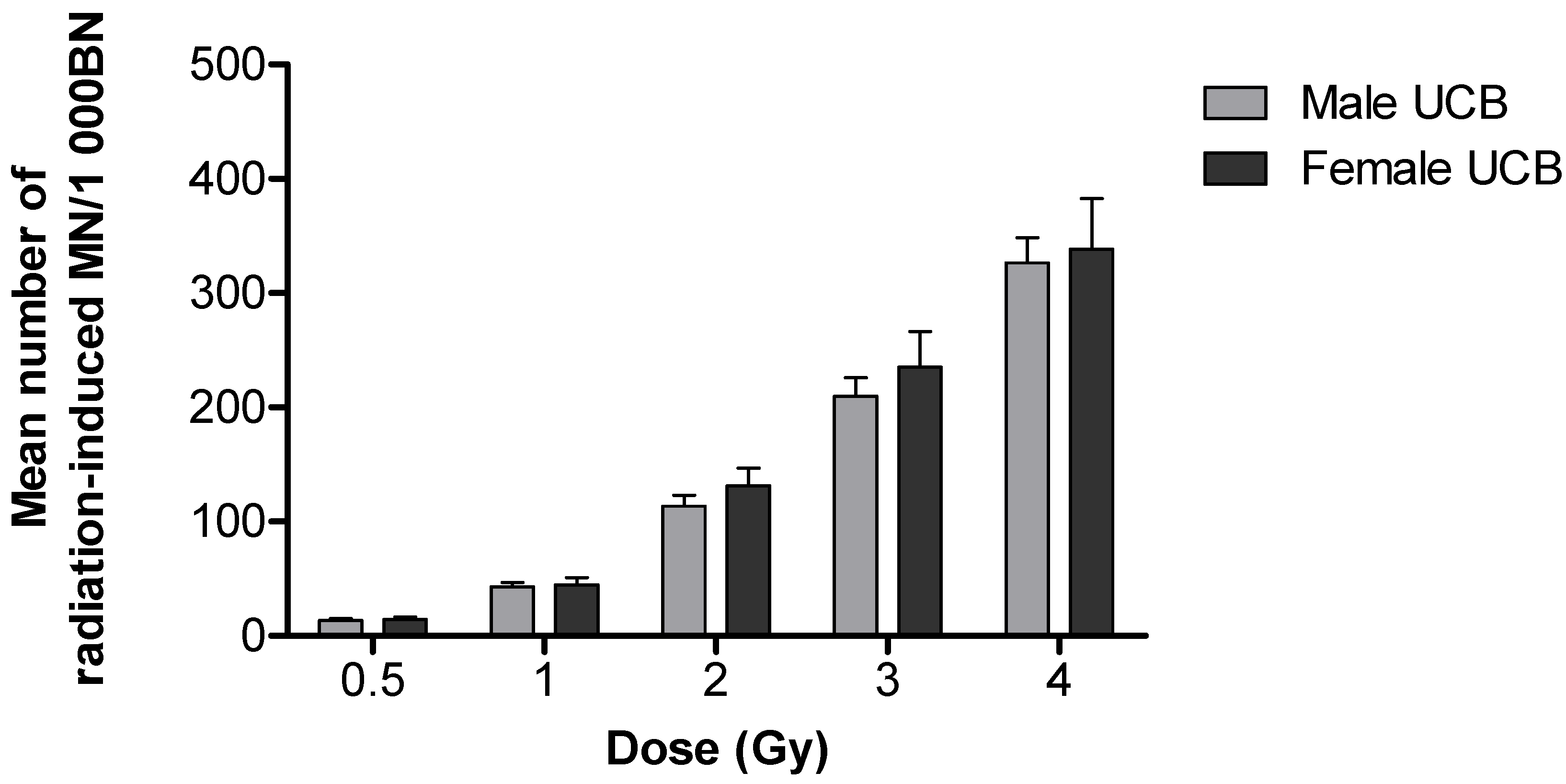
3.1. Differences in Chromosomal Radiosensitivity between Newborn and Adult T-lymphocytes
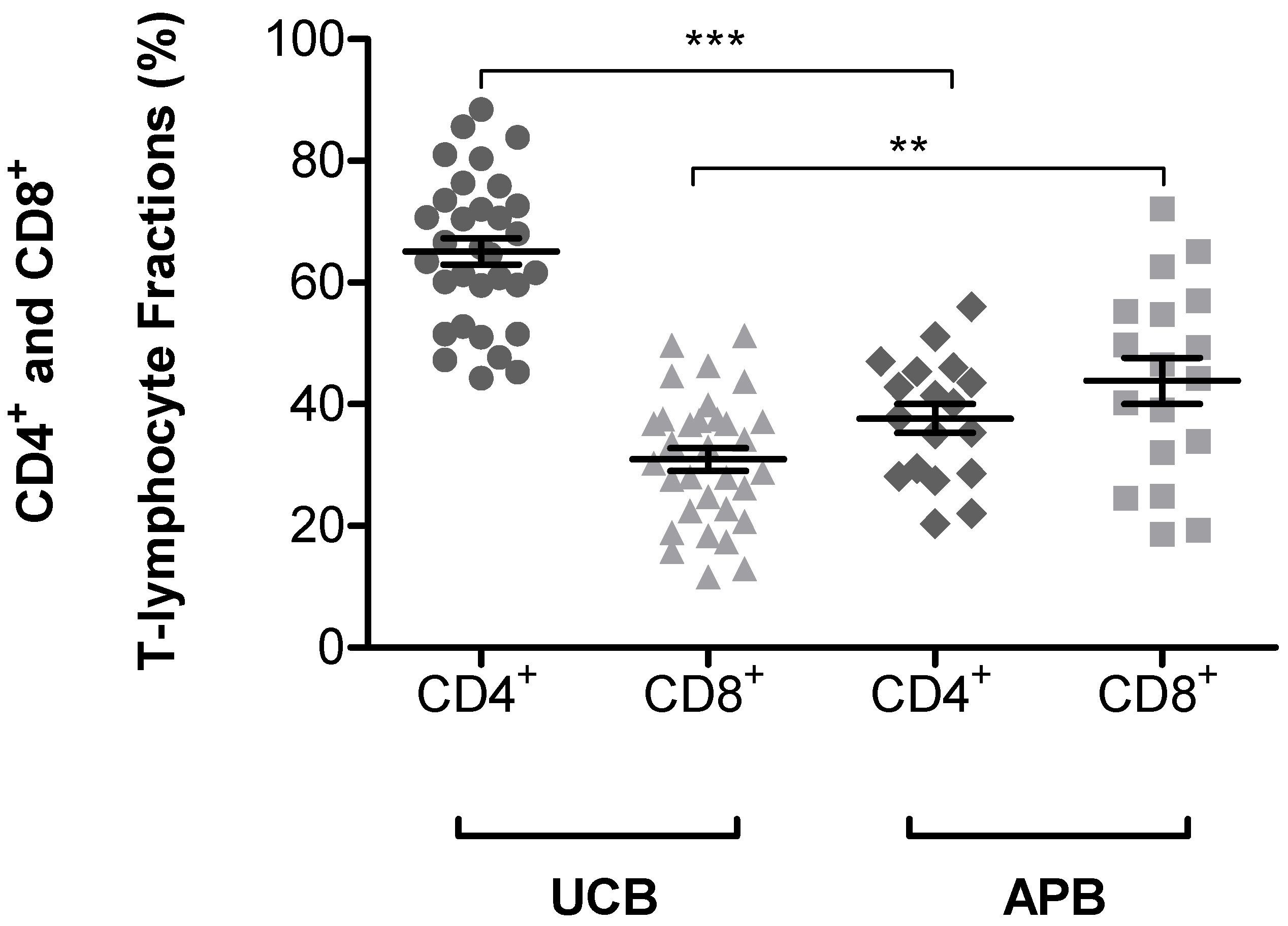
3.2. Expression of CD45RA+ and CD45RO+ on CD4+ and CD8+ T-lymphocytes Subsets in UCB and APB Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNSCEAR. United Nations Scientific Committee on the Effects of Atomic Radiation: Sources. In Effects and Risks of Ionizing Radiation; United Nations: New York, NY, USA, 2013. [Google Scholar]
- Sadetzki, S.; Mandelzweig, L. Childhood Exposure to External Ionising Radiation and Solid Cancer Risk. Br. J. Cancer 2009, 100, 1021–1025. [Google Scholar] [CrossRef]
- Kutanzi, K.R.; Lumen, A.; Koturbash, I.; Miousse, I.R. Pediatric Exposures to Ionizing Radiation: Carcinogenic Considerations. Int. J. Environ. Res. Public Health 2016, 13, 1057. [Google Scholar] [CrossRef] [Green Version]
- Khong, P.-L.; Ringertz, H.; Donoghue, V.; Frush, D.; Rehani, M.; Appelgate, K.; Sanchez, R. ICRP Publication 121: Radiological Protection in Paediatric Diagnostic and Interventional Radiology. Ann. ICRP 2013, 42, 11. [Google Scholar] [CrossRef] [PubMed]
- Hernanz-Schulman, M. Pediatric CT and Image Gently®; Image Wisely: Nashville, TN, USA, 2017. [Google Scholar]
- Frush, D.P. Radiation Risks to Children from Medical Imaging. Rev. Med. Clin. Condes 2013, 24, 15–20. [Google Scholar] [CrossRef] [Green Version]
- Vandevoorde, C.; Vral, A.; Vandekerckhove, B.; Philippé, J.; Thierens, H. Radiation Sensitivity of Human CD34+ Cells Versus Peripheral Blood T Lymphocytes of Newborns and Adults: DNA Repair and Mutagenic Effects. Radiat. Res. 2016, 185, 580–590. [Google Scholar] [CrossRef]
- Gomolka, M.; Oestreicher, U.; Rößler, U.; Samaga, D.; Endesfelder, D.; Lang, P.; Neumaier, K.; Belka, C.; Niemeyer, M.; Kiechle, M.; et al. Age-Dependent Differences in DNA Damage after in Vitro CT Exposure. Int. J. Radiat. Biol. 2018, 94, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Bakhmutsky, M.V.; Joiner, M.C.; Jones, T.B.; Tucker, J.D. Differences in Cytogenetic Sensitivity to Ionizing Radiation in Newborns and Adults. Radiat. Res. 2014, 181, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Oestreicher, U.; Endesfelder, D.; Gomolka, M.; Kesminiene, A.; Lang, P.; Lindholm, C.; Rößler, U.; Samaga, D.; Kulka, U. Automated Scoring of Dicentric Chromosomes Differentiates Increased Radiation Sensitivity of Young Children after Low Dose CT Exposure in Vitro. Int. J. Radiat. Biol. 2018, 94, 1017–1026. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Ruiz, B.; Arellano-García, M.E.; Radilla-Chávez, P.; Salas-Vargas, D.S.; Toledano-Magaña, Y.; Casillas-Figueroa, F.; Luna Vazquez-Gomez, R.; Pestryakov, A.; García-Ramos, J.C.; Bogdanchikova, N. Cytokinesis-Block Micronucleus Assay Using Human Lymphocytes as a Sensitive Tool for Cytotoxicity/Genotoxicity Evaluation of AgNPs. ACS Omega 2020, 5, 12005–12015. [Google Scholar] [CrossRef] [PubMed]
- Nersesyan, A.; Fenech, M.; Bolognesi, C.; Mišík, M.; Setayesh, T.; Wultsch, G.; Bonassi, S.; Thomas, P.; Knasmüller, S. Use of the Lymphocyte Cytokinesis-Block Micronucleus Assay in Occupational Biomonitoring of Genome Damage Caused by in Vivo Exposure to Chemical Genotoxins: Past, Present and Future. Mutat. Res. Rev. Mutat. Res. 2016, 770, 1–11. [Google Scholar] [CrossRef]
- Bolognesi, C.; Fenech, M. Micronucleus Cytome Assays in Human Lymphocytes and Buccal Cells. Genotoxicity Assess. 2019, 2031, 147–163. [Google Scholar] [CrossRef]
- El-Zein, R.; Vral, A.; Etzel, C.J. Cytokinesis-Blocked Micronucleus Assay and Cancer Risk Assessment. Mutagenesis 2010, 26, 101–106. [Google Scholar] [CrossRef]
- Sommer, S.; Buraczewska, I.; Kruszewski, M. Micronucleus Assay: The State of Art, and Future Directions. Int. J. Mol. Sci. 2020, 21, 1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Movafagh, A.; Heydary, H.; Mortazavi-Tabatabaei, S.A.; Azargashb, E. The Significance Application of Indigenous Phytohemagglutinin (PHA) Mitogen on Metaphase and Cell Culture Procedure. Iran. J. Pharm. Res. IJPR 2011, 10, 895–903. [Google Scholar]
- Beinke, C.; Port, M.; Lamkowski, A.; Abend, M. Comparing Seven Mitogens with PHA-M for Improved Lymphocyte Stimulation in DiceNTRIC Chromosome Analysis for Biodosimetry. Radiat. Prot. Dosim. 2015, 168, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cano, R.L.E.; Lopera, H.D.E. Introduction to T and B Lymphocytes. In Autoimmunity: From Bench to Bedside [Internet]; Anaya, J.M., Shoenfeld, Y., Rojas-Villarrage, A., Eds.; El Rosario University Press: Bogota, Colombia, 2013. [Google Scholar]
- Young, N.A.; Al-Saleem, T. Lymph Nodes: Cytomorphology and Flow Cytometry. In Comprehensive Cytopathology, 3rd ed.; Elsevier Inc.: Philadelphia, PA, USA, 2008; pp. 671–711. [Google Scholar] [CrossRef]
- Chan, S.; Correia-Neves, M.; Dierich, A.; Benoist, C.; Mathis, D. Visualization of CD4/CD8 T Cell Commitment. J. Exp. Med. 1998, 188, 2321–2333. [Google Scholar] [CrossRef] [Green Version]
- Gegonne, A.; Chen, Q.-R.; Dey, A.; Etzensperger, R.; Tai, X.; Singer, A.; Meerzaman, D.; Ozato, K.; Singer, D.S. Immature CD8 Single-Positive Thymocytes Are a Molecularly Distinct Subpopulation, Selectively Dependent on BRD4 for Their Differentiation. Cell Rep. 2018, 24, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Merkenschlager, M.; Terry, L.; Edwards, R.; Beverley, P. Limiting Dilution Analysis of Proliferative Responses in Human Lymphocyte Populations Defined by the Monoclonal Antibody UCHL1: Implications for Differential CD45 Expression in T Cell Memory Formation. Eur. J. Immunol. 1988, 18, 1653–1662. [Google Scholar] [CrossRef]
- Merkenschlager, M.; Beverley, P.C.L. Evidence for Differential Expression of CD45 Isoforms by Precursors for Memory-Dependent and Independent Cytotoxic Responses: Human CD8 Memory CTLp Selectively Express CD45R0 (UCHL1). Int. Immunol. 1989, 1, 450–459. [Google Scholar] [CrossRef]
- Ben-Smith, A.; Gorak-Stolinska, P.; Floyd, S.; Weir, R.E.; Lalor, M.K.; Mvula, H.; Crampin, A.C.; Wallace, D.; Beverley, P.C.L.; Fine, P.E.M.; et al. Differences between Naive and Memory T Cell Phenotype in Malawian and UK Adolescents: A Role for Cytomegalovirus? BMC Infect. Dis. 2008, 8, 139. [Google Scholar] [CrossRef] [Green Version]
- Pugh, J.L.; Sukhina, A.S.; Seed, T.M.; Manley, N.R.; Sempowski, G.D.; Brink, M.R.M.V.D.; Smithey, M.; Nikolich-Zugich, J. Histone Deacetylation Critically Determines T Cell Subset Radiosensitivity. J. Immunol. 2014, 193, 1451–1458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Andrade, H.; Villar, C.C. Radiotherapy and Immune Response: The Systemic Effects of a Local Treatment. Clinics 2018, 73, 1–11. [Google Scholar]
- Nakamura, N.; Kusunoki, Y.; Akiyama, M. Radiosensitivity of CD4 or CD8 Positive Human T-lymphocytes by an in Vitro Colony Formation Assay. Radiat. Res. 1990, 123, 224. [Google Scholar] [CrossRef] [PubMed]
- Gray, W.C.; Chretien, P.B.; Suter, C.M.; Revie, D.R.; Tomazic, V.T.; Blanchard, C.L.; Aygun, C.; Amornmarn, R.; Ordonez, J.V. Effects of Radiation Therapy on T-lymphocyte Subpopulations in Patients with Head and Neck Cancer. Otolaryngol. Neck Surg. 1985, 93, 650–660. [Google Scholar] [CrossRef]
- Louagie, H.; van Eijkeren, M.; Philippe, J.; Thierens, H.; de Ridder, L. Changes in Peripheral Blood Lymphocyte Subsets in Patients Undergoing Radiotherapy. Int. J. Radiat. Biol. 1999, 75, 767–771. [Google Scholar] [CrossRef]
- Eric, A.; Juranic, Z.; Tisma, N.; Plesinac, V.; Borojevic, N.; Jovanovic, D.; Milovanovic, Z.; Gavrilovic, D.; Ilic, B. Radiotherapy-Induced Changes of Peripheral Blood Lymphocyte Subpopulations in Cervical Cancer Patients: Relationship to Clinical Response. J. BUON 2009, 14, 79–83. [Google Scholar]
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gögenur, I. Cytotoxic CD8+ T Cells in Cancer and Cancer Immunotherapy. Br. J. Cancer 2020, 124, 359–367. [Google Scholar] [CrossRef]
- Devine, K. The Umbilical Cord Blood Controversies in Medical Law. Available online: https://books.google.co.za/books?id=G_niDQAAQBAJ&pg=PT174&lpg=PT174&dq=umbilical+cord+blood+(UCB)+is+used+as+a+substitute+for+blood+of+a+new-born+as+it+is+genetically+part+of+the+foetus.&source=bl&ots=e3cRHHclYX&sig=ACfU3U0G7PMaEtxjfxekq6N31kY5VSz0iw&hl=e (accessed on 27 February 2020).
- Carroll, P.D.; Nankervis, C.A.; Iams, J.; Kelleher, K. Umbilical Cord Blood as a Replacement Source for Admission Complete Blood Count in Premature Infants. J. Perinatol. 2011, 32, 97–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krzyżanowski, A.; Kwiatek, M.; Gęca, T.; Stupak, A.; Kwaśniewska, A. Modern Ultrasonography of the Umbilical Cord: Prenatal Diagnosis of Umbilical Cord Abnormalities and Assessement of Fetal Wellbeing. Med. Sci. Monit. 2019, 25, 3170–3180. [Google Scholar] [CrossRef]
- Donaldson, C.; Buchanan, R.; Webster, J.; Laundy, V.; Horsley, H.; Barron, C.; Anderson, N.; Bradley, B.; Hows, J. Development of a District Cord Blood Bank: A Model for Cord Blood Banking in the National Health Service. Bone Marrow Transpl. 2000, 25, 899–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenech, M. Cytokinesis-Block Micronucleus Cytome Assay. Nat. Protoc. 2007, 2, 1084–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willems, P.; August, L.; Slabbert, J.; Romm, H.; Oestreicher, U.; Thierens, H.; Vral, A. Automated Micronucleus (MN) Scoring for Population Triage in Case of Large Scale Radiation Events. Int. J. Radiat. Biol. 2010, 86, 2–11. [Google Scholar] [CrossRef]
- Herd, O.; Francies, F.; Kotzen, J.; Smith, T.; Nxumalo, Z.; Muller, X.; Slabbert, J.; Vral, A.; Baeyens, A. Chromosomal Radiosensitivity of Human Immunodeficiency Virus Positive/Negative Cervical Cancer Patients in South Africa. Mol. Med. Rep. 2015, 13, 130–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) Sources and Effects of Ionizing Radiation. 2010, p. 1. Available online: https://www.unscear.org/unscear/en/publications/2000_1.html (accessed on 5 November 2020).
- Floyd, D.; Cassoni, A. Intrinsic Radiosensitivity of Adult and Cord Blood Lymphocytes as Determined by the Micronucleus Assay. Eur. J. Cancer 1994, 30, 615–620. [Google Scholar] [CrossRef]
- Ainsbury, E.A.; Al-Hafidh, J.; Bajinskis, A.; Barnard, S.; Francesc Barquinero, J.; Beinke, C.; de Gelder, V.; Gregoire, E.; Jaworska, A.; Lindholm, C.; et al. Inter- and Intra-Laboratory Comparison of a Multibiodosimetric Approach to Triage in a Simulated, Large Scale Radiation Emergency Inter- and Intra-Laboratory Comparison of a Multibiodosimetric Approach to Triage in. Int. J. Radiat. Biol. 2014, 90, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Mei, N.; Imada, H.; Nomoto, S.; Kunugita, N.; Norimura, T. Individual Variation and Age Dependency in the Radiosensitivity of Peripheral Blood T-lymphocytes from Normal Donors. J. Radiat. Res. 1996, 37, 235–245. [Google Scholar] [CrossRef]
- Schuster, B.; Ellmann, A.; Mayo, T.; Auer, J.; Haas, M.; Hecht, M.; Fietkau, R.; Distel, L.V. Rate of Individuals with Clearly Increased Radiosensitivity Rise with Age Both in Healthy Individuals and in Cancer Patients. BMC Geriatr. 2018, 18, 105. [Google Scholar] [CrossRef] [PubMed]
- Schnarr, K.; Dayes, I.; Sathya, J.; Boreham, D. Individual Radiosensitivity and Its Relevance to Health Physics. Dose-Response 2007, 5, 333–348. [Google Scholar] [CrossRef]
- Francies, F.Z.; Herd, O.; Muller, X.; Cairns, A.; Murdoch, M.; Nietz, M.; Slabbert, J.P.; Baeyens, A. Chromosomal Radiosensitivity of Lymphocytes in South African Breast Cancer Patients of Different Ethnicity: An Indirect Measure of Cancer Susceptibility. S. Afr. Med. J. 2015, 105, 675–678. [Google Scholar] [CrossRef] [Green Version]
- López, M.C.; Palmer, B.E.; Lawrence, D.A. Phenotypic Differences between Cord Blood and Adult Peripheral Blood. Cytom. Part B Clin. Cytom. 2009, 76B, 37–46. [Google Scholar] [CrossRef]
- Statistics South Africa 2020 Mid-Year Population Estimates. Available online: http://www.statssa.gov.za/publications/P0302/P03022020.pdf (accessed on 5 November 2020).
- Cucinotta, F.A.; Saganti, P.B. Race and Ethnic Group Dependent Space Radiation Cancer Risk Predictions. medRxiv 2021, 1–20. [Google Scholar] [CrossRef]
- Taylor, A.M.R.; Wakeford, R. Human Radiosensitivity: Report of the Independent Advisory Group on Ionising Radiation; Health Protection Agency: London, UK, 2013; ISBN 9780859517409. [Google Scholar]
- Jaing, T.-H. Umbilical Cord Blood: A Trustworthy Source of Multipotent Stem Cells for Regenerative Medicine. Cell Transplant. 2014, 23, 493–496. [Google Scholar] [CrossRef]
- Hordyjewska, A.; Popiołek, Ł.; Horecka, A. Characteristics of Hematopoietic Stem Cells of Umbilical Cord Blood. Cytotechnology 2015, 67, 387–396. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.; Hale, J.; Jaffray, J.; Li, J.; Wang, Y.; Huang, Y.; An, X.; Hillyer, C.; Wang, N.; Kinet, S.; et al. Developmental Differences between Neonatal and Adult Human Erythropoiesis. Am. J. Hematol. 2017, 93, 494–503. [Google Scholar] [CrossRef]
- He, L.-N.; Cheng, Y.; Chen, W.; Yang, Y.; Liu, N.-Q.; Chen, D.-Y.; Lu, D.; Sun, X.-F. Construction and Evaluation of a Highly Effective Culture System for Erythroid Differentiation of Umbilical Cord Blood-Derived Hematopoietic Stem and Progenitor Cells. Int. J. Clin. Exp. Med. 2020, 13, 6635–6643. [Google Scholar]
- Sutherland, D.R.; Keating, A.; Nayar, R.; Anania, S.; Stewart, A.K. Sensitive Detection and Enumeration of CD34+ Cells in Peripheral and Cord Blood by Flow Cytometry. Exp. Hematol. 1994, 22, 1003–1010. [Google Scholar] [PubMed]
- Stolarek, M.; Mysliwski, A. Stem Cells of Cord Blood. Post Biol. Kom. 2005, 32, 375–390. [Google Scholar]
- Beck, R.; Lam-Po-Tang, P.R.L. Comparison of Cord Blood and Adult Blood Lymphocyte Normal Ranges: A Possible Explanation for Decreased Severity of Graft versus Host Disease after Cord Blood Transplantation. Immunol. Cell Biol. 1994, 72, 440–444. [Google Scholar] [CrossRef] [PubMed]
- D’Arena, G.; Musto, P.; Cascavilla, N.; Di Giorgio, G.; Fusilli, S.; Zendoli, F.; Carotenuto, M. Flow Cytometric Characterization of Human Umbilical Cord Blood Lymphocytes: Immunophenotypic Features. Haematologica 1998, 83, 197–203. [Google Scholar]
- Vandevoorde, C. Biomarker Investigation of the Health Effects of ct X-ray Exposure in Children; Ghent University, Faculty of Medicine and Health Sciences: Ghent, Belgium, 2015. [Google Scholar]
- Dalal, I.; Roifman, C.M. Immunity of the Newborn. Available online: https://www.uptodate.com/contents/immunity-of-the-newborn (accessed on 6 November 2020).
- Messele, T.; Abdulkadir, M.; Fontanet, A.L.; Petros, B.; Hamann, D.; Koot, M.; Roos, M.T.; Schellekens, P.T.; Miedema, F.; de Wit, R. Reduced Naive and Increased Activated CD4 and CD8 Cells in Healthy Adult Ethiopians Compared with Their Dutch Counterparts. Clin. Exp. Immunol. 1999, 115, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Miles, D.J.C.; Shumba, F.; Pachnio, A.; Begum, J.; Corbett, E.L.; Heyderman, R.S.; Moss, P. Early T Cell Differentiation with Well-Maintained Function across the Adult Life Course in Sub-Saharan Africa. J. Immunol. 2019, 203, 1160–1171. [Google Scholar] [CrossRef]
- Payne, H.; Lawrie, D.; Nieuwoudt, M.; Cotton, M.F.; Gibb, D.M.; Babiker, A.; Glencross, D.; Klein, N. Comparison of Lymphocyte Subset Populations in Children from South Africa, US and Europe. Front. Pediatr. 2020, 8, 406. [Google Scholar] [CrossRef] [PubMed]
- Klose, N.; Coulibaly, B.; Tebit, D.M.; Nauwelaers, F.; Spengler, H.P.; Kynast-Wolf, G.; Kouyaté, B.; Kräusslich, H.-G.; Böhler, T. Immunohematological Reference Values for Healthy Adults in Burkina Faso. Clin. Vaccine Immunol. 2007, 14, 782–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valiathan, R.; Ashman, M.; Asthana, D. Effects of Ageing on the Immune System: Infants to Elderly. Scand. J. Immunol. 2016, 83, 255–266. [Google Scholar] [CrossRef] [Green Version]
- Martin, C.E.; Spasova, D.S.; Frimpong-Boateng, K.; Kim, H.-O.; Lee, M.; Kim, K.S.; Surh, C.D. Interleukin-7 Availability Is Maintained by a Hematopoietic Cytokine Sink Comprising Innate Lymphoid Cells and T Cells. Immunity 2017, 47, 171–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Surh, C.D.; Sprent, J. Homeostasis of Naive and Memory T Cells. Immunity 2008, 29, 848–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mackay, C.R. Homing of Naive, Memory and Effector Lymphocytes. Curr. Opin. Immunol. 1993, 5, 423–427. [Google Scholar] [CrossRef]
- Pennock, N.; White, J.T.; Cross, E.W.; Cheney, E.E.; Tamburini, B.A.; Kedl, R.M. T Cell Responses: Naïve to Memory and Everything in between. Adv. Physiol. Educ. 2013, 37, 273–283. [Google Scholar] [CrossRef] [Green Version]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Helper T Cells and Lymphocyte Activation. In Molecular Biology of the Cell; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Li, M.; Yao, D.; Zeng, X.; Kasakovski, D.; Zhang, Y.; Chen, S.; Zha, X.; Li, Y.; Xu, L. Age Related Human T Cell Subset Evolution and Senescence. Immun. Ageing 2019, 16, 24. [Google Scholar] [CrossRef] [Green Version]
- Price, B.D.; D’Andrea, A.D. Chromatin Remodeling at DNA Double-Strand Breaks. Cell 2013, 152, 1344–1354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aleksandrov, R.; Hristova, R.; Stoynov, S.; Gospodinov, A. The Chromatin Response to Double-Strand DNA Breaks and Their Repair. Cells 2020, 9, 1853. [Google Scholar] [CrossRef] [PubMed]
- Schuler, N.; Rübe, C.E. Accumulation of DNA Damage-Induced Chromatin Alterations in Tissue-Specific Stem Cells: The Driving Force of Aging? PLoS ONE 2013, 8, e63932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rawlings, J.S.; Gatzka, M.; Thomas, P.G.; Ihle, J.N. Chromatin Condensation via the Condensin II Complex Is Required for Peripheral T-Cell Quiescence. EMBO J. 2011, 30, 263–276. [Google Scholar] [CrossRef] [PubMed]

| Group | Newborns | Adults |
|---|---|---|
| Group Size | 32 | 27 |
| Median Age (range) | 0 | 23–49 |
| Gender (%): | ||
| Male | 60 | 48 |
| Female | 34 | 52 |
| Not captured | 6 | - |
| Subpopulation | T Newborns (UCB) | T Adult (APB) | |
|---|---|---|---|
| CD4+ | 64.97 | 36.25 | |
| (44.25–88.45) | (10.94–56.00) | ||
| CD45RA+ | 91.05 | 39.08 | |
| (80.80–98.40) | (12.70–58.90) | ||
| CD45RO+ | 8.25 | 60.21 | |
| (0.87–16.70) | (41.10–86.50) | ||
| CD8+ | 30.83 | 42.21 | |
| (15.8–51.25) | (18.60–69.60) | ||
| CD45RA+ | 95.55 | 53.73 | |
| (89.60–98.80) | (41.20–76.10) | ||
| CD45RO+ | 3.79 | 44.41 | |
| (0.83–8.87) | (31.40–56.90) |
| Subpopulations | Umbilical Cord Blood (UCB) | Adult Peripheral Blood (APB) | ||||
|---|---|---|---|---|---|---|
| This Study | D’Arena | Vandevoorde | This Study | D’Arena | Vandevoorde | |
| CD4+CD45RA+ | 91.1 ± 4.6 | 87.6 ± 5.2 | 97.3 ± 2.3 | 39.1 ± 12.4 | 44.8 ± 9.6 | 38.2 ± 19.0 |
| CD4+CD45RO+ | 8.3 ± 4.1 | 12.3 ± 5.2 | 2.5 ± 2 | 60.2 ± 12.7 | 55.2 ± 9.6 | 58.6 ± 17.3 |
| CD8+CD45RA+ | 95.6 ± 2.9 | 93.5 ± 7.8 | 99.7 ± 0.3 | 53.7 ± 10.9 | 71.5 ± 8.1 | 61.9 ± 18.6 |
| CD8+CD45RO+ | 3.8 ± 2.5 | 6.4 ± 7.8 | 0.3 ± 0.3 | 44.4 ± 10.8 | 28.5 ± 8.1 | 35.6 ± 13.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Engelbrecht, M.; Ndimba, R.; Miles, X.; Nair, S.; Botha, M.H.; Zwanepoel, E.; de Kock, E.; de Kock, M.; Vandevoorde, C. A Validation Study on Immunophenotypic Differences in T-lymphocyte Chromosomal Radiosensitivity between Newborns and Adults in South Africa. Radiation 2022, 2, 1-16. https://doi.org/10.3390/radiation2010001
Engelbrecht M, Ndimba R, Miles X, Nair S, Botha MH, Zwanepoel E, de Kock E, de Kock M, Vandevoorde C. A Validation Study on Immunophenotypic Differences in T-lymphocyte Chromosomal Radiosensitivity between Newborns and Adults in South Africa. Radiation. 2022; 2(1):1-16. https://doi.org/10.3390/radiation2010001
Chicago/Turabian StyleEngelbrecht, Monique, Roya Ndimba, Xanthene Miles, Shankari Nair, Matthys Hendrik Botha, Elbie Zwanepoel, Evan de Kock, Maryna de Kock, and Charlot Vandevoorde. 2022. "A Validation Study on Immunophenotypic Differences in T-lymphocyte Chromosomal Radiosensitivity between Newborns and Adults in South Africa" Radiation 2, no. 1: 1-16. https://doi.org/10.3390/radiation2010001







