Simple Summary
Children have an increased risk of developing radiation-induced secondary malignancies compared to adults, especially for leukemia, due their high radiosensitivity and long life expectancy. The aim of this study was to validate published results on immunophenotypic differences underlying the increased radiosensitivity in newborns in a South African population. The repair of radiation-induced chromosomal damage was evaluated by a cytokinesis-block micronucleus (CBMN) assay. A higher number of radiation-induced micronuclei (MN) was observed in newborn T-lymphocytes compared to adult T-lymphocytes. The major difference between newborns T-lymphocytes and adult T-lymphocytes is their immunophenotypic profiles, which were confirmed in this study by determining the fraction of naïve and memory T-lymphocytes. This could be an underlying reason for the observed difference in cytogenetic damage between T-lymphocytes of newborns and adults. This radiobiological evaluation confirms an age dependency in response to radiation exposure, where children are clearly more vulnerable to radiation-induced malignancies compared to adults.
Abstract
Children have an increased risk of developing radiation-induced secondary malignancies compared to adults, due to their high radiosensitivity and longer life expectancy. In contrast to the epidemiological evidence, there is only a handful of radiobiology studies which investigate the difference in radiosensitivity between children and adults at a cellular level. In this study, the previous results on the potential age dependency in chromosomal radiosensitivity were validated again by means of the cytokinesis-block micronucleus (CBMN) assay in T-lymphocytes isolated from the umbilical cord and adult peripheral blood of a South African population. The isolated cells were irradiated with 60Co γ-rays at doses ranging from 0.5 Gy to 4 Gy. Increased radiosensitivities of 34%, 42%, 29%, 26% and 16% were observed for newborns compared to adults at 0.5, 1, 2, 3 and 4 Gy, respectively. An immunophenotypic evaluation with flow cytometry revealed a significant change in the fraction of naïve (CD45RA+) T-lymphocytes in CD4+ and CD8+ T-lymphocytes with age. Newborns co-expressed an average of 91.05% CD45RA+ (range: 80.80–98.40%) of their CD4+ cells, while this fraction decreased to an average of 39.08% (range: 12.70–58.90%) for adults. Similar observations were made for CD8+ cells. This agrees with previous published results that the observed differences in chromosomal radiosensitivity between newborn and adult T-lymphocytes could potentially be linked to their immunophenotypic profiles.
1. Introduction
In the 2013 report of the United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR), children are identified as carrying a higher risk for radiation-induced malignancies in comparison to adults. This risk was quantified to be 2–3 times higher for solid tumors and 3–5 times higher for hematological malignancies, such as leukemia [1]. This higher radiosensitivity of children is possibly due to the fact that their tissues and organs are in a phase of active development, with more rapidly dividing cells, which are more sensitive to radiation compared to fully mature and differentiated cells [2,3,4]. Secondly, children have a longer life expectancy, resulting in a larger window of probability in which potential oncogenic effects associated with the radiation exposure can appear [5,6]. However, in contrast to the well-documented epidemiological evidence for the higher radiosensitivity of children, there is only a limited number of studies investigating the underlying biological mechanisms responsible for the higher radiosensitivity of the immature and not fully differentiated cells of children. These studies indicate an age dependency in radiation-induced DNA damage and the outcome of DNA repair [7,8,9,10].
Peripheral blood samples are commonly used for radiosensitivity and biodosimetry studies as they are relatively easy to obtain via venipuncture. Particularly, the cytokinesis-block micronucleus (CBMN) assay is a straightforward and frequently used cytogenetic assay for human peripheral blood lymphocytes (PBLs), which were identified as very sensitive indicators of cytogenic damage [11,12,13,14]. The MN frequencies in PBLs measured by the CBMN assay are valid biomarkers for potentially increased cancer risk and radiosensitivity [15]. Furthermore, PBLs are in the G0-phase and, therefore, not subject to cell-cycle-dependent differences in radiosensitivity. In order to achieve resting PBLs in mitosis, phytohemagglutinin (PHA) is used, which is a well-known selective T-lymphocyte mitogen [16,17]. Immature T-lymphocytes, also known as thymocytes, migrate to the thymus to become progressively converted into fully mature and functional T-lymphocytes [18]. They are distinguished from other lymphocytes, such as B-lymphocytes and natural killer cells (NK cells), by the presence of a T-cell receptor (TCR) on the cell surface. T-lymphocytes can also be characterized by their lineage-specific CD marker CD3, which appears in the earliest T-lymphocytes as precursors in the thymus. During thymic maturation, the T-lymphocyte precursors undergo rearrangements of the TCR, which are accompanied by the appearance of other T-lymphocyte lineage-associated markers, such as CD4 and CD8 [19]. Depending on the co-expression of the CD4 or CD8 marker, the cells are characterized as helper or cytotoxic T-lymphocytes, respectively. Initially, the thymocytes express neither CD4 nor CD8, and thus are classified as double-negative (CD4−CD8−) cells. As these cells develop, they become double-positive T-lymphocytes (CD4+CD8+, DP) and lastly mature to single-positive (CD4+CD8− or CD4−CD8+, SP) T-lymphocytes, which are liberated from the thymus to peripheral tissues [20,21]. Characteristically, these mature T-lymphocytes are still signified as either immature or naïve because they are not presented with an antigen [22,23]. The human immune system preserves both naïve and memory T-lymphocytes, which are characterized by the reciprocal expression of CD45RA or CD45RO isoforms [24].
Previous research showed that naïve T-lymphocytes are more radiosensitive than their memory counterparts [7,25]. However, the underlying mechanisms of the observed difference in radiosensitivity of human naïve and memory T-lymphocyte subsets remain unclear. Overall, lymphocytes (T, B and NK cells) are among the most radiosensitive cell types, followed by monocytes, macrophages and APCs [26]. Since the fraction of CD4+ and CD8+ cells in peripheral blood differs among individuals, it can be expected that individual radiosensitivity might be influenced by the T-lymphocyte subset fractions [27]. The latter is also important in the context of radiotherapy, where the immune depression and suppression of hematopoiesis is considered as a severe toxic side effect for cancer patients [28,29,30]. This could also impact the tumor-directed local immune response, since cytotoxic CD8+ T-lymphocytes play a crucial role in cancer immunotherapy [31].
This is a replication of a previous study by Vandevoorde et al. [7], with the aim of validating the previously observed age dependency in radiosensitivity and the potential impact of the immunophenotypic profile of T-lymphocyte subsets in a South African population to generalize the results to other populations. There is only a limited number of radiobiology studies investigating the age dependency in newborns compared to adults. These studies show an age dependency in radiation-induced DNA damage and the outcome of DNA repair [7,8,9,10]. The in vitro radiosensitivity of T-lymphocytes isolated from umbilical cord blood (UCB) is compared to that of adult T-lymphocytes in response to low-LET Cobalt-60 (60Co) gamma (γ)-rays. The radiation-induced mutagenic effects are evaluated with the CBMN assay. The age-related immunophenotypic changes of T-lymphocytes are taken into consideration by analyzing the newborn and adult T-lymphocyte subpopulations of the same donors, as used for the CBMN assay. In the immunophenotypic study of the UCB and adult peripheral blood (APB) samples, the fractions of CD4+, CD8+, naive CD45RA+ and memory CD45RO+ subsets are determined.
2. Materials and Methods
2.1. Sample Collection and Isolation of Peripheral Blood Mononuclear Cells (PBMCs)
2.1.1. Collection of Umbilical Cord Blood (UCB) Samples
Hematopoiesis and radiosensitivity studies on children remain limited due to the ethical constraints related to the collection of blood samples from children. Therefore, a good alternative is the use of UCB. The UCB samples were collected from the postpartum placenta and used as a substitute for the blood of a newborn as they are physiologically, genetically and immunologically part of the human fetus [32,33,34]. Ethics approval for the collection of the UCB was granted by the Health Research Ethics Committee of the University of Stellenbosch, South Africa (Ethics Reference number: N16/10/134), and all experiments and methods were performed in line with relevant guidelines and regulations. After written, informed consent was obtained from each mother, the UCB samples were collected after scheduled elective Caesarean surgery [35]. In total, 32 UCB samples (50–90 mL) were collected in sterile collection bags containing anti-coagulant CPDA-1 (citrate-phosphate-Dextrose-Adenine) (SSEM Mthembu Medical (Pty) Ltd., Cape Town, South Africa) from full-term newborn babies, at either Tygerberg or Karl Bremer Hospital, in Cape Town, South Africa. Samples were transported to iThemba LABS at room temperature, where human peripheral blood mononuclear cells (PBMCs) were isolated.
2.1.2. Collection of Adult Peripheral Blood (APB) Sample
Ethics approval for the collection of adult peripheral blood (APB) was granted by the Biomedical Science Research Ethics Committee of the University of the Western Cape, South Africa (Ethics Reference number: BM20/3/5). Written informed consent was obtained from each adult donor, before peripheral blood samples were collected. In total, 27 APB (aged 23–49 years) samples (10–15 mL) were collected from adult donors through a needle venepuncture at a scheduled time arranged by the clinic based at iThemba LABS. The APB collection was performed by a qualified nurse (Incon HealthTM, Cape Town, South Africa) and universal precautions were applied. The APB samples were collected through venepuncture in lithium–heparin collection tubes and transported to the laboratory at room temperature for the isolation of PBMCs.
2.1.3. Isolation of Peripheral Blood Mononuclear Cells (PBMCs) from UCB and APB
The PBMCs were isolated by density gradient centrifugation on Histopaque®-1077 (density: 1.077 g/mL; Histopaque®-1077, Sigma-Aldrich Co. LLC, St. Louis, MO, USA) at 508 RCF for 15 min at room temperature, and the lymphocytes were recovered from the buffy coat. This was transferred to a 50 mL conical tube containing 30 mL of sterile Phosphate-buffered saline (PBS), following three washes with sterile PBS. To count the mononuclear cells, 10 μL of the cell suspension was added to 90 μL Türk solution (Gibco, Dun Laoghaire, Dublin, Ireland) and counted using a hemocytometer. Approximately 1.5–2 million cells were suspended in 5.0 mL CELLSTAR® round-bottom polypropylene tubes (Greiner Bio-One GmbH, Frickenhausen, Germany), containing 5 mL of complete Roswell Park Memorial Institute (RPMI) 1640 culture media supplemented with 20% fetal bovine serum (FBS) (Gibco, Dun Laoghaire, Dublin, Ireland) and 1% penicillin-streptomycin (Pen-Strep) (Lonza, Walkersville, MD, USA).
2.2. 60Co γ-Ray Irradiation
After isolation, the PBMCs were irradiated with 60Co γ-rays using a teletherapy unit (Theratron 780, MDS Nordion Inc., Ottawa, ON, Canada). The reference dose measurements were completed using the IAEA TRS-398 protocol. The cell suspensions were irradiated in the 5.0 mL CELLSTAR® round-bottom polypropylene tubes (Greiner Bio-One GmbH, Frickenhausen, Germany) with 60Co γ-rays using a teletherapy unit (Theratron 780). The vials were placed between a 6 mm thick build-up Perspex plate to ensure dose build-up and a 49.3 mm thick backscatter plate with a dose rate of 0.468 Gy/min for a 30 × 30 cm2 field size, at a source-to-surface (SSD) of 74 cm, for the build-up plate. The lateral dimensions of the build-up plate and backscatter block are 299 × 299 mm2 and 297.5 × 297.5 mm2, respectively. The samples were exposed to radiation doses of 0.5, 1, 2, 3 and 4 Gy. Sham-irradiated control samples were included for each assay.
2.3. Cytokinesis-Block Micronucleus (CBMN) Assay
Post-irradiation, 100 μL phytohemagglutinin (PHA) (25 mg/25 mL dH2O; Sigma-Aldrich/Merck, St. Louis, MO, USA) was added to each 5 mL suspension culture, and samples were incubated at 37 °C, with 5% CO2 in 95% humidified atmosphere, until termination time point. After 23 h, 20 µL cytochalasin B (CytoB) (0.75 mg/mL) (Sigma-Aldrich Co. LLC, St. Louis, MO, USA) was added, which acted as an inhibitor of microfilament ring assembly required for the completion of cytokinesis. This allowed one to distinguish once-divided cells based on their binucleated (BN) appearance [36]. After 72 h of total culture time, the cells were resuspended gently to reduce cellular clumping and each well was rinsed with PBS. The cell suspension was transferred to 15 mL conical tubes (NEST Biotechnology Co., Ltd., Wuxi, China), which were centrifuged for 8 min at 252 RCF at 4 °C (Eppendorf 5810R centrifuge, Hamburg, Germany). In the following steps, the cells were exposed to cold 7 mL of 0.075 M Potassium chloride (KCl) and an overnight fixation in 4:1:5 (methanol/acetic acid/ringer) solution. The next day, the cells were fixed in 4:1 (methanol/acetic acid), 40 μL of the cell suspension pipetted onto a microscope slide and allowed to air dry. Thereafter, slides were stained with mounting medium, Fluoroshield™ with DAPI (4′,6-Diamidino-2-phenylindole dihydrochloride) (Sigma-Aldrich/Merck, St. Louis, MO, USA) and covered with a coverslip. The Metasystems’ Metafer 4 was used to automatically scan the slides by detecting BN T-lymphocytes and counting the number of MN in these BN cells using a classifier that was optimized in collaboration with Ghent University [37,38]. After the automated scanning of the slides, the BN cells were then displayed in a computerized gallery where the BN cells were manually checked and false positive and negative MN scores were rectified through two scorers. At least two slides per condition were semi-automatically scored to provide a total of at least 1000 scored BN cells per irradiation condition.
2.4. Immunophenotyping of Adult and Newborn PBMCs
Age-related immunophenotypic changes of T lymphocytes were assessed through flow cytometric analysis of UCB (newborns) and APB (adults) T-lymphocyte subpopulations to determine the fraction of naive CD45RA+ and memory CD45RO+ subsets. The following panel of monoclonal antibodies (mAb) was used: CD3-PerCP, CD4-PE, CD8-APC, CD45RA-BB515, CD45RO-BB515 (all: BD Bioscience, Franklin Lake, NJ, USA). The analysis was performed with a BD Accuri™ C6 flow cytometer. Firstly, 50 μL (containing approximately 50,000 cells) of the PBMC cell suspension was transferred to two separate FACS tubes and 2 μL of each mAb was added. Two separate FACS tubes were used in order to distinguish between naive CD45RA+ and memory CD45RO+ subsets per donor: tube one contained CD3-PerCP, CD4-PE, CD8-APC, CD45RA-BB515 antibodies; whereas, tube two contained CD3-PerCP, CD4-PE, CD8-APC, CD45RO-BB515 antibodies. After 20 min at room temperature in the dark, 0.5 mL PBS was added, the cells were centrifuged (252 RCF, 5 min), and the cell pellet was resuspended with 100 μL of PBS. Thereafter, 400 μL of PBS was added to the FACS tubes and the cell suspension was vortexed prior to the flow cytometric measurement (Accuri C6, BD Biosciences, Franklin Lake, NJ, USA). Approximately 200,000 total events were measured for each sample. Gating strategy can be found in the Supplementary Material.
2.5. Statistical Analysis
The results from the individual experiments were averaged and the corresponding standard error of the mean (SEM) calculated. Statistical analysis was performed using Microsoft Office Excel 2019 (Microsoft Corporation, Washington DC, USA) and GraphPad Prism Software Version 5.01 for Windows (GraphPad Software, San Diego, CA, USA). FlowJo™ v10.7 (BD Bioscience, Franklin Lake, NJ, USA) was employed to analyze flow cytometry data. The numbers of experiments (n) are indicated in each figure caption. Shapiro–Wilk tests were performed to assess normality of the data. The Shapiro–Wilk test showed that data were not normally distributed. Therefore, the non-parametric Kruskal–Wallis test was performed for statistical analysis of the CBMN, (semi-automated), and CD45RA/RO fractions. All statistical tests were 2-sided, and p < 0.05 (*) were considered statistically significant, p < 0.01 (**) highly significant and p < 0.001 (***) extremely significant.
3. Results
3.1. Differences in Chromosomal Radiosensitivity between Newborn and Adult T-lymphocytes
In order to determine the extent to which age influences the frequency of cytogenic damage in response to IR, T-lymphocytes were isolated from 27 adult donors (aged 23–49 years) and from the UCB of 32 newborns (Table 1).

Table 1.
Demographic characteristics of the participants in the study.
For both APB and UCB, a prominent dose-dependent increase in the frequency of radiation-induced MN was observed (Figure 1). A high radiation-induced MN yield of 344.91 ± 22.83 MN/1000 BN cells was observed in newborns compared to a lower yield of 297 ± 14.00 MN/1000 BN cells in adults after 4 Gy; however, this observation was not statistically significant (p > 0.05). A significantly different MN frequency was observed at 0.5 and 2 Gy 60Co γ-rays (** p < 0.01), as well as at 1 and 3 Gy 60Co γ-rays (*** p < 0.001) (Figure 1). The lowest dose of 0.5 Gy yielded an average MN frequency of 14.74 ± 0.79 MN/1000 BN cells for APB and 15.94 ± 1.39 MN/1000 BN cells for UCB after 60Co γ-rays irradiation, which was significantly higher than the average background values (0 Gy) of 4.19 ± 0.45 MN/1000 BN cells for APB (p < 0.0001) and 1.88 ± 0.28 MN/1000 BN cells for UCB (p < 0.0001), respectively. The ratio of the radiation-induced MN of newborns over adults was calculated for each radiation dose and expressed as a percentage increase in radiosensitivity, resulting in 34%, 42%, 29%, 26% and 16% at doses of 0.5, 1, 2, 3 and 4 Gy, respectively. In both age groups, there was no statistically significant difference between genders (p > 0.05); however, there was a clear trend that females were more sensitive than males in the adult group and, to a lesser extent, in newborns, particularly at the higher doses (Figure 2 and Figure 3).
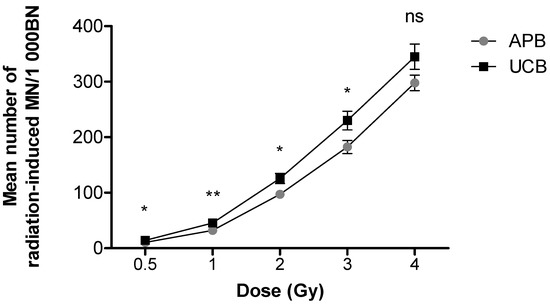
Figure 1.
Dose–response curve of the mean number of MN in peripheral T-lymphocytes of adults (n = 27) and newborns (n = 32) induced by different doses (0.5, 1, 2, 3 and 4 Gy) of 60Co γ-rays irradiation. The number of MN induced by the irradiation was obtained by subtracting the mean number of MN in the sham-irradiated controls (0 Gy) from the mean MN number scored in the irradiated samples. MN yields of UCB were significantly higher post-irradiation (0.5, 1, 2 and 3 Gy) compared to APB (** p < 0.01, * p < 0.05). However, after a 4 Gy dose, no significant (ns) difference was observed between APB and UCB (p > 0.05). Error bars represent the standard error of the mean (SEM). At least 1000 BN cells were scored for each donor per condition.
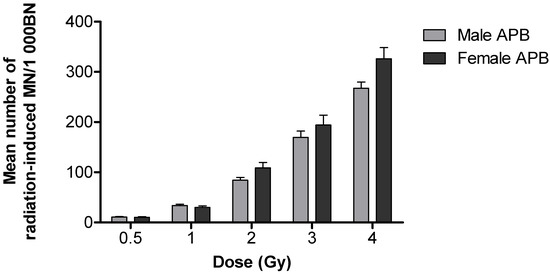
Figure 2.
The mean number of radiation-induced MN in peripheral T-lymphocytes of male (n = 13) and female adults (n = 14) induced by 60Co γ-rays irradiation. No significant difference was observed in MN yields between male and female adults post-irradiation (p > 0.05). Error bars represent the standard error of the mean (SEM). At least 1000 BN cells were scored for each donor per condition.
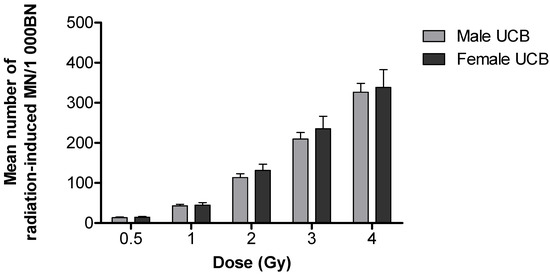
Figure 3.
The schematic representation shows the mean number of radiation-induced of MN in peripheral T-lymphocytes of male (n = 19) and female newborns (n = 11) induced by different doses (0.5, 1, 2, 3 and 4 Gy) of 60Co γ-rays irradiation (the gender of 2 newborns was not captured). No significant difference in MN yields were observed between male and female newborns post-irradiation (p > 0.05). Error bars represent the standard error of the mean (SEM). At least 1000 BN cells were scored for each donor per condition.
3.2. Expression of CD45RA+ and CD45RO+ on CD4+ and CD8+ T-lymphocytes Subsets in UCB and APB Samples
The major difference between UCB T-lymphocytes and APB T-lymphocytes, is their immunophenotypic profile, which could be an underlying reason for the observed differences in chromosomal radiosensitivity. An immunophenotypic study of the UCB and APB was performed to determine the percentage of T-lymphocyte subsets in the UCB and APB samples by using the BD Accuri™ C6 flow cytometer. The gating strategies for flow cytometry analysis were performed using FlowJo ™ v10.7 (BD Bioscience, Franklin Lake, NJ, USA) (see Figures S1–S4). Figure 4 shows a statically significant difference in CD4+ and CD8+ T-lymphocyte fractions between UCB (p < 0.001) and APB (p < 0.01). Table 2 shows the mean percentage of naïve and memory CD4+ and CD8+ T-lymphocytes fractions. The results clearly show that both the CD4+ and CD8+ T-lymphocytes of newborns are mainly naïve, illustrated by the co-expression of CD45RA+ on 91.05% (range: 80.80–98.40%) and 95.55% (range: 89.60–98.80%) of CD4+ and CD8+ cells, respectively, whereas the composition in adult T-lymphocytes is clearly different, with a more equal distribution between CD45RA+ and CD45RO+ subpopulations. The results show that at least half of the CD4+ and CD8+ T-lymphocytes of adults are memory T-lymphocytes, illustrated by the co-expression of CD45RO+ on 60.21% (range: 41.10–86.50%) and 44.41% (range: 31.40–56.90%) of CD4+ and CD8+ cells, respectively. Table 2 represents the mean percentage of expression of CD45RA+ and CD45RO+ on CD4+ and CD8+ T-lymphocyte subsets in UCB and APB samples. This observation demonstrates the differences between newborn and adult T-lymphocytes and the immunophenotypic changes of T-lymphocytes with age.
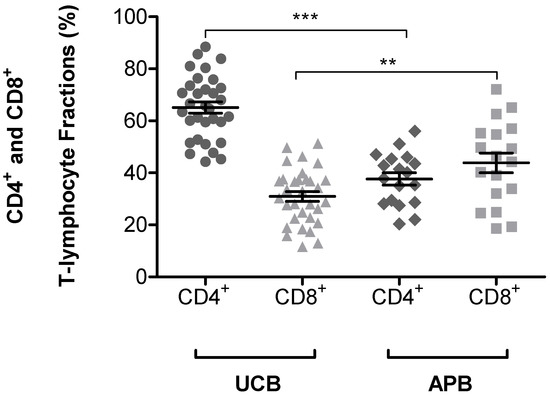
Figure 4.
This graph shows the mean percentage of CD4 and CD8 T-lymphocyte subsets in UCB (n = 32) and APB (n = 18) samples. A significant difference is noted between CD4+ (*** p < 0.001) and CD8+ (** p < 0.01) fraction when comparing UCB and APB. Error bars represent the standard error of mean (SEM) of the different donors.

Table 2.
Mean expression (%) of CD45RA and CD45RO on CD4 and CD8 T-lymphocyte subsets in UCB and APB samples validates previous results. The percentage range is given in parentheses.
4. Discussion
Worldwide, around 3.6 billion medical procedures involving IR are performed each year [39], leading to significant increases in human exposure to IR. In the case of children, increasing exposure levels to IR is of particular concern given the epidemiological proof that they are most sensitive and vulnerable to the damaging effects of IR [1]. This cytogenetic study provides a validation that newborns have a higher sensitivity to radiation-induced damage in T-lymphocytes compared to adults. Future research is needed to clarify how the immunophenotypic profile of the T-lymphocytes influences its radiosensitivity and which biological characteristics or mechanisms underly this observation.
As early as in 1994, Floyd et al. investigated the intrinsic radiosensitivity of adult and UCB lymphocytes by using the MN assay [40]. They analyzed the radiation-induced MN for 10 different APB donors and 5 UCB donors. When comparing the amount of MN induced after 2 Gy X-ray irradiation (dose rate of 2.35 Gy/min), only 2 UCB donors were more radiosensitive than the mean percentage of APB samples. However, at 4 Gy irradiation, four out of the five UCB samples showed increased radiosensitivity when compared to the average radiosensitivity percentage of the APB samples. A comparison of the slope of the dose–response curves of the five UCB donors to the mean dose–response of the APB donors revealed a significant difference (p < 0.02). These findings are in line with the results presented here, but differ in that Floyd et al. scored micronucleated cells and not the micronucleus frequency, as in the present study. A higher radiosensitivity for newborns was also reported in a study of Vandevoorde et al., where significant differences were observed between newborn and adult T-lymphocytes in radiation-induced MN frequencies. After 2 Gy X-ray irradiation, a high radiation-induced MN yield of 351 ± 23 MN/1000 BN cells was observed in newborns compared to a significantly lower yield of 275 ± 18 MN/1000 BN cells in adults (p < 0.05) [7]. In the current studies, lower numbers of MN/BN cells were scored with the semi-automatic scoring method compared to the values reported in Vandevoorde et al., but the results confirm the same trend and the difference in radiosensitivity between newborns and adults. The difference in scoring technique might explain the lower counts in the current study, since Vandevoorde et al. applied manual scoring on Giemsa-stained MN slides.
Bakhmutsky et al. investigated the influence of age on the frequency and types of chromosomal damage in response to IR, by exposing the T-lymphocytes of 20 adults (aged 22–78 years) and 10 UCB samples to 60Co γ-rays [9]. Peripheral blood lymphocytes from newborns showed statistically significant increases in the induced frequencies of translocated chromosomes, dicentrics and color junctions compared to adult cells. Individual assessment of the adults in their study displayed no significant difference with age or gender. The increased radiosensitivities of newborn to adult donors were 37%, 18%, 12% and 4%, based on the scoring of chromosomal aberrations (CA), at doses of 1, 2, 3 and 4 Gy, respectively. In the current study, increased radiosensitivities of newborn to adult donors of 42%, 29%, 26% and 16% was observed for the same radiation doses with the CBMN assay, respectively. In both studies, the increase in radiosensitivity seems to decrease with dose. When the induced CA frequencies in the irradiated samples were evaluated within each dose group, Bakhmutsky et al. observed decreases in the number of translocated chromosomes at 1, 2 and 3 Gy with age, except at 4 Gy. For each type of CA, newborns showed substantially higher levels of chromosomal aberrations at the same dose points, with the exception of acentric fragments and abnormal cells at 4 Gy [9]. These results suggest that the cells which encountered more damage at 4 Gy would have experienced a more negative selection during the 48 h (CA assay) or 72 h (MN assay) culture period, either by apoptosis or cell cycle arrest. In the current study, no significant difference was observed in the radiosensitivity of UCB and APB following 4 Gy 60Co γ-ray radiation. This confirms the observation for acentric fragments by Bakhmutsky et al. [9]. In addition, the results of semiautomated analysis in this dose range of 4 Gy might not be entirely reliable, due to the higher fraction of dead cells.
A recent publication by Gomolka et al. investigated the age-dependent radiation sensitivity of male donors (n = 43) from different age groups (newborns, children 2–5 years and adults > 20 years of age) after sham exposure (0 Gy), low-dose (0.041 Gy) and high-dose (0.978 Gy), in vitro computed tomography (CT) exposure [8]. These results showed a 1.5-fold increase in the level of dicentric aberrations in newborns and children compared to adults after 0.978 Gy. At the low dose, no significant difference was observed. Despite the fact that a significant dose–response for radiation-induced damage and dose-dependent repair was observed, the γ-H2AX foci assay did not show an age-dependent increase in DNA damage in newborns and children compared to adults. In addition, no significant difference was observed in the age-dependent sensitivity when adults were assessed individually [8]. A related study conducted by Oestreicher et al. showed that newborns and children showed a significantly increased risk for radiation-induced dicentric chromosomes after 0.41 Gy CT exposure [41].
Mei et al. used a colony formation assay to show that aging did not affect the male adult population in response to 2 or 4 Gy X-ray radiation exposure [42]. Their results suggest that the change in radiosensitivity occurs between birth and adulthood, rather than progressively over the years from birth to senescence. The lack of an age effect amongst adults is perhaps the result of the termination of growth and development in this age group. Similarly, in a group of 14 individuals (six males and eight females), aged between 27 and 48 years, no influence of age on dicentric aberrations was observed [8]. A larger age-dependent radiosensitivity study on a cohort of healthy individuals (n = 202, mean age 50.7 years) and cancer patients (n = 393, mean age 60.4 years) provided the opposite results. Here, the lymphocytes of healthy individuals were irradiated with 2 Gy of 6 MeV X-rays [43]. In the healthy individual cohort, chromosomal breaks per metaphase (B/M) clearly increased by 0.0014 B/M per year with age, while no correlation of age and B/M values was found in the cancer patient cohort. This study showed that individual radiosensitivity rises continuously with age in healthy individuals, yet with strong inter-individual variation. However, no children were included in the study, so no extrapolations could be made to newborns and children.
Even if an age effect was not apparent among adults, inter-individual variations due to intrinsic genetic factors may influence radiation responses. One of these underlying intrinsic factors could be a difference in radiosensitivity between different ethnic groups [44,45,46]. South Africa remains a complex mix of different populations and ethnic groups. According to Statistics South Africa’s 2020 mid-year estimations, the population consists of 80.8% Black, 8.8% mixed/Coloured, 2.6% Asian and 7.8% White citizens [47]. Population and ethnic differences in patients with solid and hematological malignancies are well recognized. In this study, there was a discrepancy in the ratio of the races between the adult and newborn groups. For newborns, there were 50% Black (n = 16), 44% mixed/Coloured (n = 14) and 6% White (n = 2) samples. In the adult group, there were 41% Black (n = 11), 33% mixed/Coloured (n = 9), 4% Asian (n = 1) and 22% White (n = 6) samples. No statistically significant difference was observed in radiosensitivity between the different racial groups, but only a limited number of studies investigated the effect of ethnicity on radiosensitivity [45,48], so more results are needed before conclusions can be drawn. In the field of radiotherapy-related toxicity, there are only limited data available on the effect of ethnicity on radiotherapy-related toxicity. There is some evidence, and given the fact that genetic variation differs between ethnicities, it is likely that radiosensitivity and some radiotherapy-related toxicity endpoints will be more severe in some ethnic groups due to the higher prevalence of specific mutations [49].
Phenotypic and physiological differences are known to exist between APB and UCB samples. UCB also contains a small fraction (0.33–1.98%) of hematopoietic stem and progenitor cells (HSPCs) that have a four-fold higher proliferation rate than the HSPCs (<0.01%) in peripheral blood [50,51,52,53,54,55]. Furthermore, UCB has almost three times as many total lymphocytes as adult blood [56]. The immunophenotypic study determined the percentage of T-lymphocyte subsets in the UCB and APB samples by the use of flow cytometry, and the current results are in line with both the results of D’Arena et al. [57] and Vandevoorde et al. [58]. A comparison of the values of the three studies are presented in Table 3. The high expression of the RA+ isoform of the CD45 cell surface marker on UCB T-lymphocytes is a consequence of limited antigenic experience during pregnancy [57,59]. As individuals age and encounter newer antigens, the percentage of naïve T-lymphocytes declines and that of antigen-experienced memory cells increases [57]. This shift away from a population of predominantly naive T-lymphocytes clearly reflects the influences of cumulative exposure to foreign pathogens over time. It might also represent a compensatory homeostatic response to reduced numbers of naïve cells generated in the thymus, possible intrinsic cellular differential sensitivities to apoptosis, and specific effects of the aged environment, which actually promote the appearance and dominance of memory cells [58]. Several epidemiology studies could also demonstrate that the degree of environmental antigen exposure in early life leads to changes in the immune status of individuals [24,60,61,62]. A study conducted by Payne et al. revealed a decline in the naïve/memory ratio of both CD4 and CD8 T-lymphocytes in conjunction with an increased activation markers ratio, suggesting that South African children are exposed to a wider range of environmental pathogens in early life than children in the United States (US) and Europe [62]. In the current study, a significant difference was observed in CD4+ and CD8+ T-lymphocyte subsets between UCB and APB (Figure 4). Even though some studies show that the number of CD8+ T-lymphocytes decreased with age [63], the impact of age on lymphocyte subsets is not completely expounded. The current results confirm that age influences the CD4+ and CD8+ T-lymphocyte subsets shift, which is in line with the findings of Valiathan et al., who investigated age-related (1 month to 92 years) changes within several lymphocyte subpopulations [64].

Table 3.
The percentage of naïve and memory CD4+ and CD8+ T-lymphocytes determined in UCB and APB in the study, compared to previous studies. Values are expressed as mean percentage (±SD).
Generally, naïve (CD45RA+/CD45RO−) T-lymphocytes are considered to be the most homogeneous pool of T-lymphocytes as they lack most effector functions. The different immunophenotypic profiles of newborn T-lymphocytes, as opposed to adult T-lymphocytes could be a contributing factor to the differences in sensitivity to radiation seen in this study. Overall, naïve (CD45RA+CD45RO−) T-lymphocytes represent the utmost homogeneous pool of T-lymphocytes due to their deficiency in most effector functions [65,66]. They migrate to sites that contain secondary lymphoid organs, such as the lymph nodes and tonsils, in search of antigens presented by dendritic cells. This enables the development of antigen-specific adaptive immunity. Once the T-lymphocytes encounter antigen-presenting cells (APCs) and become activated through the TCR, they proliferate and differentiate into effector T-lymphocytes that are CD45RO+ with a variety of functions and the possibility to migrate into tissues to eradicate pathogens [67,68,69]. Throughout aging, individuals are exposed to new additional antigens causing the proportion of naïve T-lymphocytes population to decline as these cells shift to memory T-lymphocytes. This is representative of the cumulative exposure to foreign pathogens over time [24,70]. There are a number of limitations involved in our study findings, and future research is required to provide evidence for the differences in radiosensitivity between different subtypes of memory T-lymphocytes.
The study conducted by Vandevoorde et al. investigated whether the immunophenotypic differences between CD4+ T-lymphocyte subsets of newborns and adults could explain the observed cellular differences in radiosensitivity by scoring residual DNA DSBs and MN after IR exposure [7]. Vandevoorde et al. revealed a difference in radiation sensitivity between human CD4+CD45RA+ and CD4+CD45RO+ cells. A statistically significant higher number of radiation-induced MN and residual γ-H2AX/53BP1 foci were observed in naïve CD4+ cells after 2 Gy X-ray exposure compared to memory CD4+ cells in both UCB and APB samples. This confirmed that their observed differences in radiosensitivity between newborn and adult T-lymphocytes are linked to the immunophenotypic changes in T-lymphocyte composition with respect to naïve and memory subsets [7]. The underlying mechanisms of the observed difference in radiation sensitivity of human naïve and memory T-lymphocyte subsets remains unknown, and further research is necessary to elucidate these mechanisms. This leads to one possible hypothesis: that these mechanisms could be the chromatin structure of the cells [7]. The chromatin structure and nucleosome organization represent an important barrier to the efficient detection and repair of DSBs [71]. Dynamic chromatin changes ensure accessibility to the damaged region by recruiting DNA repair proteins. The ability of repair factors to detect DNA lesions and DSBs is determined by histone modifications around the DSBs and involves dynamic chromatin changes that facilitate repair by promoting chromatin accessibility by contributing to a DSB repair pathway choice and coordination [72,73]. Rawlings et al. verified that during thymocyte development, a condensation of the chromatin arises, which is essential for T-lymphocyte development and the maintenance of the quiescent state [74]. This mechanism ensures that cytokine-driven proliferation can only occur when quiescent naïve T-lymphocytes encounter their TCR-specific antigen. Rawlings et al. demonstrated that TCR activation results in a decondensation of the chromatin in naïve T-lymphocytes [74]. In a study conducted by Pugh et al., the radiosensitivity in T-lymphocyte subpopulation in mice was investigated and demonstrated the critical role of an open chromatin state on radiosensitivity [25]. The survival of T-lymphocyte subsets was measured in mice 72 h after exposure to 1, 2 and 4 Gy radiation of a 137Cs Gamma Cell. Following in vivo irradiation, the memory T-lymphocytes appeared to be more resistant to IR, while naïve T-lymphocytes were more sensitive. The irradiated cells were incubated with or without valproic acid (VPA), a histone deacetylase inhibitor (HDAC) that relaxes and opens the chromatin, for 12 and 72 h. This improved the survival of naïve T-lymphocytes, whereas memory T-lymphocyte survival remained unaffected. The existence of an open, genome-wide chromatin state is a key factor of effective DNA damage repair in T-lymphocytes and a clarification for the observed variances in naïve and memory T-lymphocyte radiosensitivity. In summary, differences in radiation-induced mutagenic effects and residual DNA DSBs in human naïve and memory T-lymphocytes suggest that radiosensitivity is strongly biased by the chromatin structure [58]. However, further investigation is required to elucidate the observed age dependency of radiation effects with respect to their potential link to chromatin condensation.
5. Conclusions
This validation study showed that the IR-induced MN yields in the UCB samples were higher compared to the MN yield in APB samples. No significant difference could be observed between males and females in both age groups. This study validates previous findings that the difference in immunophenotypic profile between newborn and adult T-lymphocytes contributes to the observed difference in radiosensitivity [7]. The flow cytometry results of this study confirmed that the observed difference in radiosensitivity may be attributable to differences in the immunophenotypic profile of T-lymphocytes isolated from UCB and APB. Further studies are warranted on the impact of chromatin condensation and T-lymphocyte maturation when cytogenetic methods are used to evaluate radiosensitivity.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/radiation2010001/s1, Figure S1: The gating strategy for the expression of naïve (CD45RA) markers on peripheral blood CD4+ and CD8+ T-lymphocytes in adults (APB). The peripheral blood T-lymphocytes were gated on forward (FSC) versus side scatter (SSC) to select the lymphocyte cell population (A). The cells were gated on FSC-Height (FSC-H) vs. FSC-Area (FSC-A) to exclude all the doublets and to generate the singlets gate (B). Subsequently, the cells were gated on CD3-PerCP vs. SSC-A to include all CD3+ cells (C), then gated on the CD4+ or CD8+ population (D). All the subpopulations were analyzed on the CD4-PE-A (E) or CD8-APC-A (F) versus CD45-BB515-A. Data were analyzed using FlowJo v10.6.1 software, and population frequencies expressed as percent of the CD4+ or CD8+ parent population, Figure S2: The gating strategy for the expression of memory (CD45RO) markers on peripheral blood CD4+ and CD8+ T-lymphocytes in adults (APB). The peripheral blood T-lymphocytes were gated on forward (FSC) versus side scatter (SSC) to select the lymphocyte cell population (A). The cells were gated on FSC-Height (FSC-H) vs. FSC-Area (FSC-A) to exclude all the doublets and to generate the singlets gate (B). Subsequently, the cells were gated on CD3-PerCP vs. SSC-A to include all CD3+ cells (C), then gated on the CD4+ or CD8+ population (D). All the subpopulations were analyzed on the CD4-PE-A (E) or CD8-APC-A (F) versus CD45-BB515-A. Data were analyzed using FlowJo v10.6.1 software, and population frequencies expressed as percent of the CD4+ or CD8+ parent population, Figure S3: The gating strategy for the expression of naïve (CD45RA) markers on peripheral blood CD4+ and CD8+ T-lymphocytes in newborns (UCB). The peripheral blood T-lymphocytes were gated on forward (FSC) versus side scatter (SSC) to select the lymphocyte cell population (A). The cells were gated on FSC-Height (FSC-H) vs. FSC-Area (FSC-A) to exclude all the doublets and to generate the singlets gate (B). Subsequently, the cells were gated on CD3-PerCP vs. SSC-A to include all CD3+ cells (C), then gated on the CD4+ or CD8+ population (D). All the subpopulations were analyzed on the CD4-PE-A (E) or CD8-APC-A (F) versus CD45-BB515-A. Data were analyzed using FlowJo v10.6.1 software, and population frequencies expressed as percent of the CD4+ or CD8+ parent population, Figure S4: The gating strategy for the expression of memory (CD45RO) markers on peripheral blood CD4+ and CD8+ T-lymphocytes in newborns (UCB). The peripheral blood T-lymphocytes were gated on forward (FSC) versus side scatter (SSC) to select the lymphocyte cell population (A). The cells were gated on FSC-Height (FSC-H) vs. FSC-Area (FSC-A) to exclude all the doublets and to generate the singlets gate (B). Subsequently, the cells were gated on CD3-PerCP vs. SSC-A to include all CD3+ cells (C), then gated on the CD4+ or CD8+ population (D). All the subpopulations were analyzed on the CD4-PE-A (E) or CD8-APC-A (F) versus CD45-BB515-A. Data were analyzed using FlowJo v10.6.1 software, and population frequencies expressed as percent of the CD4+ or CD8+ parent population.
Author Contributions
Conceptualization, C.V., M.E., M.H.B. and E.Z.; methodology, C.V., M.E. and R.N.; software, C.V. and M.E.; validation, C.V., M.E. and R.N.; formal analysis, C.V., M.E. and R.N.; investigation, C.V., M.E. and R.N.; resources, C.V., M.E., M.H.B. and E.Z.; data curation, M.E.; writing—original draft preparation, M.E., C.V. and R.N.; writing—review and editing, all authors; visualization, C.V., M.E. and R.N.; supervision, C.V. and M.d.K.; project administration, C.V., M.E., R.N., X.M., S.N., M.H.B., E.Z., E.d.K. and M.d.K.; funding acquisition, C.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the International Atomic Energy Agency (IAEA CRP E35010 (22248)) and the internal running budget of the National Research Foundation (NRF) iThemba Laboratory for Accelerator Based Sciences (LABS). Furthermore, PhD funding for M.E. was provided by the Department of Science and Innovation (DSI) and the NRF of South Africa under the Professional Development Programme (PDP).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the of Health Research Ethics Committee of the University of Stellenbosch, South Africa (Ethics Reference number: N16/10/134, 9 April 2018) and Biomedical Science Research Ethics Committee of the University of the Western Cape, South Africa (Ethics Reference number: BM20/3/5, 5 May 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ethical restrictions.
Acknowledgments
We thank all the participants in the study and the staff members at Tygerberg Hospital (TBH) and Karl Bremer Hospital (KBH) for their contributions to this study. Furthermore, we also thank Yvette McDonald for the collection of adult blood samples and Rozanne Adams for help with the flow cytometry set-up and gating.
Conflicts of Interest
The authors declare no conflict of interest and the funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- UNSCEAR. United Nations Scientific Committee on the Effects of Atomic Radiation: Sources. In Effects and Risks of Ionizing Radiation; United Nations: New York, NY, USA, 2013. [Google Scholar]
- Sadetzki, S.; Mandelzweig, L. Childhood Exposure to External Ionising Radiation and Solid Cancer Risk. Br. J. Cancer 2009, 100, 1021–1025. [Google Scholar] [CrossRef]
- Kutanzi, K.R.; Lumen, A.; Koturbash, I.; Miousse, I.R. Pediatric Exposures to Ionizing Radiation: Carcinogenic Considerations. Int. J. Environ. Res. Public Health 2016, 13, 1057. [Google Scholar] [CrossRef]
- Khong, P.-L.; Ringertz, H.; Donoghue, V.; Frush, D.; Rehani, M.; Appelgate, K.; Sanchez, R. ICRP Publication 121: Radiological Protection in Paediatric Diagnostic and Interventional Radiology. Ann. ICRP 2013, 42, 11. [Google Scholar] [CrossRef] [PubMed]
- Hernanz-Schulman, M. Pediatric CT and Image Gently®; Image Wisely: Nashville, TN, USA, 2017. [Google Scholar]
- Frush, D.P. Radiation Risks to Children from Medical Imaging. Rev. Med. Clin. Condes 2013, 24, 15–20. [Google Scholar] [CrossRef][Green Version]
- Vandevoorde, C.; Vral, A.; Vandekerckhove, B.; Philippé, J.; Thierens, H. Radiation Sensitivity of Human CD34+ Cells Versus Peripheral Blood T Lymphocytes of Newborns and Adults: DNA Repair and Mutagenic Effects. Radiat. Res. 2016, 185, 580–590. [Google Scholar] [CrossRef]
- Gomolka, M.; Oestreicher, U.; Rößler, U.; Samaga, D.; Endesfelder, D.; Lang, P.; Neumaier, K.; Belka, C.; Niemeyer, M.; Kiechle, M.; et al. Age-Dependent Differences in DNA Damage after in Vitro CT Exposure. Int. J. Radiat. Biol. 2018, 94, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Bakhmutsky, M.V.; Joiner, M.C.; Jones, T.B.; Tucker, J.D. Differences in Cytogenetic Sensitivity to Ionizing Radiation in Newborns and Adults. Radiat. Res. 2014, 181, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Oestreicher, U.; Endesfelder, D.; Gomolka, M.; Kesminiene, A.; Lang, P.; Lindholm, C.; Rößler, U.; Samaga, D.; Kulka, U. Automated Scoring of Dicentric Chromosomes Differentiates Increased Radiation Sensitivity of Young Children after Low Dose CT Exposure in Vitro. Int. J. Radiat. Biol. 2018, 94, 1017–1026. [Google Scholar] [CrossRef]
- Ruiz-Ruiz, B.; Arellano-García, M.E.; Radilla-Chávez, P.; Salas-Vargas, D.S.; Toledano-Magaña, Y.; Casillas-Figueroa, F.; Luna Vazquez-Gomez, R.; Pestryakov, A.; García-Ramos, J.C.; Bogdanchikova, N. Cytokinesis-Block Micronucleus Assay Using Human Lymphocytes as a Sensitive Tool for Cytotoxicity/Genotoxicity Evaluation of AgNPs. ACS Omega 2020, 5, 12005–12015. [Google Scholar] [CrossRef] [PubMed]
- Nersesyan, A.; Fenech, M.; Bolognesi, C.; Mišík, M.; Setayesh, T.; Wultsch, G.; Bonassi, S.; Thomas, P.; Knasmüller, S. Use of the Lymphocyte Cytokinesis-Block Micronucleus Assay in Occupational Biomonitoring of Genome Damage Caused by in Vivo Exposure to Chemical Genotoxins: Past, Present and Future. Mutat. Res. Rev. Mutat. Res. 2016, 770, 1–11. [Google Scholar] [CrossRef]
- Bolognesi, C.; Fenech, M. Micronucleus Cytome Assays in Human Lymphocytes and Buccal Cells. Genotoxicity Assess. 2019, 2031, 147–163. [Google Scholar] [CrossRef]
- El-Zein, R.; Vral, A.; Etzel, C.J. Cytokinesis-Blocked Micronucleus Assay and Cancer Risk Assessment. Mutagenesis 2010, 26, 101–106. [Google Scholar] [CrossRef]
- Sommer, S.; Buraczewska, I.; Kruszewski, M. Micronucleus Assay: The State of Art, and Future Directions. Int. J. Mol. Sci. 2020, 21, 1534. [Google Scholar] [CrossRef] [PubMed]
- Movafagh, A.; Heydary, H.; Mortazavi-Tabatabaei, S.A.; Azargashb, E. The Significance Application of Indigenous Phytohemagglutinin (PHA) Mitogen on Metaphase and Cell Culture Procedure. Iran. J. Pharm. Res. IJPR 2011, 10, 895–903. [Google Scholar]
- Beinke, C.; Port, M.; Lamkowski, A.; Abend, M. Comparing Seven Mitogens with PHA-M for Improved Lymphocyte Stimulation in DiceNTRIC Chromosome Analysis for Biodosimetry. Radiat. Prot. Dosim. 2015, 168, 235. [Google Scholar] [CrossRef] [PubMed]
- Cano, R.L.E.; Lopera, H.D.E. Introduction to T and B Lymphocytes. In Autoimmunity: From Bench to Bedside [Internet]; Anaya, J.M., Shoenfeld, Y., Rojas-Villarrage, A., Eds.; El Rosario University Press: Bogota, Colombia, 2013. [Google Scholar]
- Young, N.A.; Al-Saleem, T. Lymph Nodes: Cytomorphology and Flow Cytometry. In Comprehensive Cytopathology, 3rd ed.; Elsevier Inc.: Philadelphia, PA, USA, 2008; pp. 671–711. [Google Scholar] [CrossRef]
- Chan, S.; Correia-Neves, M.; Dierich, A.; Benoist, C.; Mathis, D. Visualization of CD4/CD8 T Cell Commitment. J. Exp. Med. 1998, 188, 2321–2333. [Google Scholar] [CrossRef]
- Gegonne, A.; Chen, Q.-R.; Dey, A.; Etzensperger, R.; Tai, X.; Singer, A.; Meerzaman, D.; Ozato, K.; Singer, D.S. Immature CD8 Single-Positive Thymocytes Are a Molecularly Distinct Subpopulation, Selectively Dependent on BRD4 for Their Differentiation. Cell Rep. 2018, 24, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Merkenschlager, M.; Terry, L.; Edwards, R.; Beverley, P. Limiting Dilution Analysis of Proliferative Responses in Human Lymphocyte Populations Defined by the Monoclonal Antibody UCHL1: Implications for Differential CD45 Expression in T Cell Memory Formation. Eur. J. Immunol. 1988, 18, 1653–1662. [Google Scholar] [CrossRef]
- Merkenschlager, M.; Beverley, P.C.L. Evidence for Differential Expression of CD45 Isoforms by Precursors for Memory-Dependent and Independent Cytotoxic Responses: Human CD8 Memory CTLp Selectively Express CD45R0 (UCHL1). Int. Immunol. 1989, 1, 450–459. [Google Scholar] [CrossRef]
- Ben-Smith, A.; Gorak-Stolinska, P.; Floyd, S.; Weir, R.E.; Lalor, M.K.; Mvula, H.; Crampin, A.C.; Wallace, D.; Beverley, P.C.L.; Fine, P.E.M.; et al. Differences between Naive and Memory T Cell Phenotype in Malawian and UK Adolescents: A Role for Cytomegalovirus? BMC Infect. Dis. 2008, 8, 139. [Google Scholar] [CrossRef]
- Pugh, J.L.; Sukhina, A.S.; Seed, T.M.; Manley, N.R.; Sempowski, G.D.; Brink, M.R.M.V.D.; Smithey, M.; Nikolich-Zugich, J. Histone Deacetylation Critically Determines T Cell Subset Radiosensitivity. J. Immunol. 2014, 193, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, H.; Villar, C.C. Radiotherapy and Immune Response: The Systemic Effects of a Local Treatment. Clinics 2018, 73, 1–11. [Google Scholar]
- Nakamura, N.; Kusunoki, Y.; Akiyama, M. Radiosensitivity of CD4 or CD8 Positive Human T-lymphocytes by an in Vitro Colony Formation Assay. Radiat. Res. 1990, 123, 224. [Google Scholar] [CrossRef] [PubMed]
- Gray, W.C.; Chretien, P.B.; Suter, C.M.; Revie, D.R.; Tomazic, V.T.; Blanchard, C.L.; Aygun, C.; Amornmarn, R.; Ordonez, J.V. Effects of Radiation Therapy on T-lymphocyte Subpopulations in Patients with Head and Neck Cancer. Otolaryngol. Neck Surg. 1985, 93, 650–660. [Google Scholar] [CrossRef]
- Louagie, H.; van Eijkeren, M.; Philippe, J.; Thierens, H.; de Ridder, L. Changes in Peripheral Blood Lymphocyte Subsets in Patients Undergoing Radiotherapy. Int. J. Radiat. Biol. 1999, 75, 767–771. [Google Scholar] [CrossRef]
- Eric, A.; Juranic, Z.; Tisma, N.; Plesinac, V.; Borojevic, N.; Jovanovic, D.; Milovanovic, Z.; Gavrilovic, D.; Ilic, B. Radiotherapy-Induced Changes of Peripheral Blood Lymphocyte Subpopulations in Cervical Cancer Patients: Relationship to Clinical Response. J. BUON 2009, 14, 79–83. [Google Scholar]
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gögenur, I. Cytotoxic CD8+ T Cells in Cancer and Cancer Immunotherapy. Br. J. Cancer 2020, 124, 359–367. [Google Scholar] [CrossRef]
- Devine, K. The Umbilical Cord Blood Controversies in Medical Law. Available online: https://books.google.co.za/books?id=G_niDQAAQBAJ&pg=PT174&lpg=PT174&dq=umbilical+cord+blood+(UCB)+is+used+as+a+substitute+for+blood+of+a+new-born+as+it+is+genetically+part+of+the+foetus.&source=bl&ots=e3cRHHclYX&sig=ACfU3U0G7PMaEtxjfxekq6N31kY5VSz0iw&hl=e (accessed on 27 February 2020).
- Carroll, P.D.; Nankervis, C.A.; Iams, J.; Kelleher, K. Umbilical Cord Blood as a Replacement Source for Admission Complete Blood Count in Premature Infants. J. Perinatol. 2011, 32, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Krzyżanowski, A.; Kwiatek, M.; Gęca, T.; Stupak, A.; Kwaśniewska, A. Modern Ultrasonography of the Umbilical Cord: Prenatal Diagnosis of Umbilical Cord Abnormalities and Assessement of Fetal Wellbeing. Med. Sci. Monit. 2019, 25, 3170–3180. [Google Scholar] [CrossRef]
- Donaldson, C.; Buchanan, R.; Webster, J.; Laundy, V.; Horsley, H.; Barron, C.; Anderson, N.; Bradley, B.; Hows, J. Development of a District Cord Blood Bank: A Model for Cord Blood Banking in the National Health Service. Bone Marrow Transpl. 2000, 25, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Fenech, M. Cytokinesis-Block Micronucleus Cytome Assay. Nat. Protoc. 2007, 2, 1084–1104. [Google Scholar] [CrossRef] [PubMed]
- Willems, P.; August, L.; Slabbert, J.; Romm, H.; Oestreicher, U.; Thierens, H.; Vral, A. Automated Micronucleus (MN) Scoring for Population Triage in Case of Large Scale Radiation Events. Int. J. Radiat. Biol. 2010, 86, 2–11. [Google Scholar] [CrossRef]
- Herd, O.; Francies, F.; Kotzen, J.; Smith, T.; Nxumalo, Z.; Muller, X.; Slabbert, J.; Vral, A.; Baeyens, A. Chromosomal Radiosensitivity of Human Immunodeficiency Virus Positive/Negative Cervical Cancer Patients in South Africa. Mol. Med. Rep. 2015, 13, 130–136. [Google Scholar] [CrossRef] [PubMed][Green Version]
- United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) Sources and Effects of Ionizing Radiation. 2010, p. 1. Available online: https://www.unscear.org/unscear/en/publications/2000_1.html (accessed on 5 November 2020).
- Floyd, D.; Cassoni, A. Intrinsic Radiosensitivity of Adult and Cord Blood Lymphocytes as Determined by the Micronucleus Assay. Eur. J. Cancer 1994, 30, 615–620. [Google Scholar] [CrossRef]
- Ainsbury, E.A.; Al-Hafidh, J.; Bajinskis, A.; Barnard, S.; Francesc Barquinero, J.; Beinke, C.; de Gelder, V.; Gregoire, E.; Jaworska, A.; Lindholm, C.; et al. Inter- and Intra-Laboratory Comparison of a Multibiodosimetric Approach to Triage in a Simulated, Large Scale Radiation Emergency Inter- and Intra-Laboratory Comparison of a Multibiodosimetric Approach to Triage in. Int. J. Radiat. Biol. 2014, 90, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Mei, N.; Imada, H.; Nomoto, S.; Kunugita, N.; Norimura, T. Individual Variation and Age Dependency in the Radiosensitivity of Peripheral Blood T-lymphocytes from Normal Donors. J. Radiat. Res. 1996, 37, 235–245. [Google Scholar] [CrossRef]
- Schuster, B.; Ellmann, A.; Mayo, T.; Auer, J.; Haas, M.; Hecht, M.; Fietkau, R.; Distel, L.V. Rate of Individuals with Clearly Increased Radiosensitivity Rise with Age Both in Healthy Individuals and in Cancer Patients. BMC Geriatr. 2018, 18, 105. [Google Scholar] [CrossRef] [PubMed]
- Schnarr, K.; Dayes, I.; Sathya, J.; Boreham, D. Individual Radiosensitivity and Its Relevance to Health Physics. Dose-Response 2007, 5, 333–348. [Google Scholar] [CrossRef]
- Francies, F.Z.; Herd, O.; Muller, X.; Cairns, A.; Murdoch, M.; Nietz, M.; Slabbert, J.P.; Baeyens, A. Chromosomal Radiosensitivity of Lymphocytes in South African Breast Cancer Patients of Different Ethnicity: An Indirect Measure of Cancer Susceptibility. S. Afr. Med. J. 2015, 105, 675–678. [Google Scholar] [CrossRef]
- López, M.C.; Palmer, B.E.; Lawrence, D.A. Phenotypic Differences between Cord Blood and Adult Peripheral Blood. Cytom. Part B Clin. Cytom. 2009, 76B, 37–46. [Google Scholar] [CrossRef]
- Statistics South Africa 2020 Mid-Year Population Estimates. Available online: http://www.statssa.gov.za/publications/P0302/P03022020.pdf (accessed on 5 November 2020).
- Cucinotta, F.A.; Saganti, P.B. Race and Ethnic Group Dependent Space Radiation Cancer Risk Predictions. medRxiv 2021, 1–20. [Google Scholar] [CrossRef]
- Taylor, A.M.R.; Wakeford, R. Human Radiosensitivity: Report of the Independent Advisory Group on Ionising Radiation; Health Protection Agency: London, UK, 2013; ISBN 9780859517409. [Google Scholar]
- Jaing, T.-H. Umbilical Cord Blood: A Trustworthy Source of Multipotent Stem Cells for Regenerative Medicine. Cell Transplant. 2014, 23, 493–496. [Google Scholar] [CrossRef]
- Hordyjewska, A.; Popiołek, Ł.; Horecka, A. Characteristics of Hematopoietic Stem Cells of Umbilical Cord Blood. Cytotechnology 2015, 67, 387–396. [Google Scholar] [CrossRef]
- Yan, H.; Hale, J.; Jaffray, J.; Li, J.; Wang, Y.; Huang, Y.; An, X.; Hillyer, C.; Wang, N.; Kinet, S.; et al. Developmental Differences between Neonatal and Adult Human Erythropoiesis. Am. J. Hematol. 2017, 93, 494–503. [Google Scholar] [CrossRef]
- He, L.-N.; Cheng, Y.; Chen, W.; Yang, Y.; Liu, N.-Q.; Chen, D.-Y.; Lu, D.; Sun, X.-F. Construction and Evaluation of a Highly Effective Culture System for Erythroid Differentiation of Umbilical Cord Blood-Derived Hematopoietic Stem and Progenitor Cells. Int. J. Clin. Exp. Med. 2020, 13, 6635–6643. [Google Scholar]
- Sutherland, D.R.; Keating, A.; Nayar, R.; Anania, S.; Stewart, A.K. Sensitive Detection and Enumeration of CD34+ Cells in Peripheral and Cord Blood by Flow Cytometry. Exp. Hematol. 1994, 22, 1003–1010. [Google Scholar] [PubMed]
- Stolarek, M.; Mysliwski, A. Stem Cells of Cord Blood. Post Biol. Kom. 2005, 32, 375–390. [Google Scholar]
- Beck, R.; Lam-Po-Tang, P.R.L. Comparison of Cord Blood and Adult Blood Lymphocyte Normal Ranges: A Possible Explanation for Decreased Severity of Graft versus Host Disease after Cord Blood Transplantation. Immunol. Cell Biol. 1994, 72, 440–444. [Google Scholar] [CrossRef] [PubMed]
- D’Arena, G.; Musto, P.; Cascavilla, N.; Di Giorgio, G.; Fusilli, S.; Zendoli, F.; Carotenuto, M. Flow Cytometric Characterization of Human Umbilical Cord Blood Lymphocytes: Immunophenotypic Features. Haematologica 1998, 83, 197–203. [Google Scholar]
- Vandevoorde, C. Biomarker Investigation of the Health Effects of ct X-ray Exposure in Children; Ghent University, Faculty of Medicine and Health Sciences: Ghent, Belgium, 2015. [Google Scholar]
- Dalal, I.; Roifman, C.M. Immunity of the Newborn. Available online: https://www.uptodate.com/contents/immunity-of-the-newborn (accessed on 6 November 2020).
- Messele, T.; Abdulkadir, M.; Fontanet, A.L.; Petros, B.; Hamann, D.; Koot, M.; Roos, M.T.; Schellekens, P.T.; Miedema, F.; de Wit, R. Reduced Naive and Increased Activated CD4 and CD8 Cells in Healthy Adult Ethiopians Compared with Their Dutch Counterparts. Clin. Exp. Immunol. 1999, 115, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Miles, D.J.C.; Shumba, F.; Pachnio, A.; Begum, J.; Corbett, E.L.; Heyderman, R.S.; Moss, P. Early T Cell Differentiation with Well-Maintained Function across the Adult Life Course in Sub-Saharan Africa. J. Immunol. 2019, 203, 1160–1171. [Google Scholar] [CrossRef]
- Payne, H.; Lawrie, D.; Nieuwoudt, M.; Cotton, M.F.; Gibb, D.M.; Babiker, A.; Glencross, D.; Klein, N. Comparison of Lymphocyte Subset Populations in Children from South Africa, US and Europe. Front. Pediatr. 2020, 8, 406. [Google Scholar] [CrossRef] [PubMed]
- Klose, N.; Coulibaly, B.; Tebit, D.M.; Nauwelaers, F.; Spengler, H.P.; Kynast-Wolf, G.; Kouyaté, B.; Kräusslich, H.-G.; Böhler, T. Immunohematological Reference Values for Healthy Adults in Burkina Faso. Clin. Vaccine Immunol. 2007, 14, 782–784. [Google Scholar] [CrossRef] [PubMed]
- Valiathan, R.; Ashman, M.; Asthana, D. Effects of Ageing on the Immune System: Infants to Elderly. Scand. J. Immunol. 2016, 83, 255–266. [Google Scholar] [CrossRef]
- Martin, C.E.; Spasova, D.S.; Frimpong-Boateng, K.; Kim, H.-O.; Lee, M.; Kim, K.S.; Surh, C.D. Interleukin-7 Availability Is Maintained by a Hematopoietic Cytokine Sink Comprising Innate Lymphoid Cells and T Cells. Immunity 2017, 47, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Surh, C.D.; Sprent, J. Homeostasis of Naive and Memory T Cells. Immunity 2008, 29, 848–862. [Google Scholar] [CrossRef] [PubMed]
- Mackay, C.R. Homing of Naive, Memory and Effector Lymphocytes. Curr. Opin. Immunol. 1993, 5, 423–427. [Google Scholar] [CrossRef]
- Pennock, N.; White, J.T.; Cross, E.W.; Cheney, E.E.; Tamburini, B.A.; Kedl, R.M. T Cell Responses: Naïve to Memory and Everything in between. Adv. Physiol. Educ. 2013, 37, 273–283. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Helper T Cells and Lymphocyte Activation. In Molecular Biology of the Cell; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Li, M.; Yao, D.; Zeng, X.; Kasakovski, D.; Zhang, Y.; Chen, S.; Zha, X.; Li, Y.; Xu, L. Age Related Human T Cell Subset Evolution and Senescence. Immun. Ageing 2019, 16, 24. [Google Scholar] [CrossRef]
- Price, B.D.; D’Andrea, A.D. Chromatin Remodeling at DNA Double-Strand Breaks. Cell 2013, 152, 1344–1354. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrov, R.; Hristova, R.; Stoynov, S.; Gospodinov, A. The Chromatin Response to Double-Strand DNA Breaks and Their Repair. Cells 2020, 9, 1853. [Google Scholar] [CrossRef] [PubMed]
- Schuler, N.; Rübe, C.E. Accumulation of DNA Damage-Induced Chromatin Alterations in Tissue-Specific Stem Cells: The Driving Force of Aging? PLoS ONE 2013, 8, e63932. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, J.S.; Gatzka, M.; Thomas, P.G.; Ihle, J.N. Chromatin Condensation via the Condensin II Complex Is Required for Peripheral T-Cell Quiescence. EMBO J. 2011, 30, 263–276. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).