Abstract
The studied introduction population of the alien North American species Amorpha fruticosa L. was formed in the Main Botanical Garden of the Russian Academy of Sciences (Moscow) 80 years ago from seeds of different geographical origin. Currently, this population consists of individuals of the second and third generations, which arose as a result of the spontaneous pollination of parental plants. It is the northernmost site of A. fruticosa growth in the secondary distribution range; in southern regions, it usually behaves like an aggressive invasive species and invades natural plant communities. A. fruticosa is known to contain a number of bioactive compounds with valuable pharmacological properties, and the aim of this study was to evaluate the biochemical composition of leaves and fruits at the northern limit of the species’ growth, since stress conditions promote active accumulation of secondary metabolites. The data on the composition of secondary metabolites, on the mineral composition, phenolic compounds, and flavonoids in the leaves and fruits of A. fruticosa, and on the amount and composition of essential oil in the extract from fruits are presented. High levels of adonitol, which is used as a sweetener in products for diabetic patients, have been reported in the fruits of A. fruticosa. α-Copaene, α-pinene, δ-cadinene, α-muurolene, and β- and α-caryophyllene predominate in the essential oil of the fruit, providing its antimicrobial activity. The phytochemical analysis of A. fruticosa from the secondary distribution range confirms the potential of this species as a valuable resource for the pharmacopoeia industry.
1. Introduction
Botanical gardens, due to their basic activities of collecting, cultivating, exchanging, and selling plant materials, pose a risk both of spreading alien invasive species and their establishment in new habitats [1]. At the same time, botanical gardens provide a unique opportunity as controlled facilities for studying invasive alien plant species from various aspects, particularly for assessing their potential practical use as ornamental, fodder, and food resources, as well as raw materials for the pharmaceutical industry. Through studies conducted in botanical gardens, invasive alien plants are starting to be used as biofuels, biopesticides, and for phytoremediation of industrial sites. Research on the biochemical composition of plants and the outflux of biologically active compounds allows for the successful testing of these compounds in the treatment of various diseases.
During intentional introductions in botanical gardens, initial populations were created from genotypes of different geographical and ecological origins. This approach leads to a rich gene pool in the subsequently formed introduced population, contributing to the identification of diverse breeding material for the artificial selection of the most valuable specimens for further cultivation.
Recently, researchers have focused on a group of alien plants that are actively invading the natural plant communities of their new homeland—also known as invasive species. Many of them are used in their native range as economically valuable species; however, in the secondary distribution range, they have not yet found application as plant resources. Amorpha fruticosa L. belongs to such a group of promising medicinal plants.
Taxonomically, A. fruticosa is rather strongly clumped from other Fabaceae taxa. The species is included in the monophyletic tribe Amorpheae of the subfamily Faboideae, distributed mainly in subtropical and tropical regions of the New World. No species closely related to A. fruticosa is cultivated in the open ground in Russian botanical gardens.
A. fruticosa is one of the little-known invasive plants in Russia. The native range of A. fruticosa includes the central regions of North America, where this species grows along forest edges and in open habitats [2,3]. A. fruticosa has been cultivated since 1724, when it was introduced to Great Britain as an ornamental plant [4]. By 1907, A. fruticosa had spread across continental Europe. The high amount of nectar produced by the flowers of amorpha made this species highly valued by beekeepers as a honey and pollen source, and an important food source for bees. Therefore, in its secondary distribution range, A. fruticosa is widely cultivated in steppe regions of Central Europe. As a result of extensive cultivation and high tolerance to various environmental conditions, as well as its strong reproductive ability, A. fruticosa has become an aggressive invasive species and is listed among invasive species threatening livelihoods and the environment worldwide [5].
In Russia, in the St. Petersburg Botanical Garden, A. fruticosa first appeared in 1796, again in 1861, and was tested extensively from 1948 to 2005 [6]. In Moscow nurseries, A. fruticosa has been growing since 1938. Starting from 1945—the year the Main Botanical Garden of the Russian Academy of Sciences (MBG RAS) was established—14 specimens grown from seed obtained from other botanical gardens were planted in the Arboretum. Currently, the introduced population is of the second and third generations, resulting from the free pollination of those specimens of various geographical origins. The plants bloom and bear fruit abundantly every year [7], with fruits ripening by mid-October. Winter hardiness is rated IV, but the shrubs regrow each year to their previous height and crown diameter.
Guided by the “Code of Conduct of invasive alien species in Russian botanical gardens” adopted in 2011 and in accordance with the “Code of Conduct of invasive alien species in botanical gardens of CIS countries” [8], the staff of the MBG RAS do not allow self-seeding of A. fruticosa at the collections and do not allow its high invasive potential to be realized. A.fruticosa is not included in the list of wood species naturalized in the Main Botanical Garden [9].
A. fruticosa is a shrub, 2–3 m tall. Young branches have short, pressed trichomes. The leaves are compound, unequally pinnate, 25–30 cm long, consisting of 11–25 elliptical or obovate leaflets (1.5–4 × 0.6–1.8 cm). The surfaces of the leaflets have dark glands, and there is a pointed short spine at the apex. The leaflets are almost glabrous, only pubescent along the edges. The flowers are usually in three narrow racemes, 7–15 cm long. The stipules are small and scale-like. The calyx is bell-shaped, with five lobes (Figure 1). The morphological features of the species show significant variability. Studies on the variability of the generative organs of A. fruticosa in the secondary distribution range have found [10] that in all samples, the lobes of the calyx have numerous cilia along the edges, but the calyx tube can be either glabrous with glands or tomentous. The axis of the inflorescence can be (a) glabrous with single short trichomes; (b) sparsely pubescent; and (c) densely pubescent. Three variants of the arrangement of the calyx teeth have been noted: (a) lobes of equal size; (b) one long narrow lobe and four short ones; (c) two long lobes and three short ones. The morphological variability of amorpha is reflected in the presence of 36 synonyms and the description of 17 varieties and 8 forms [3].
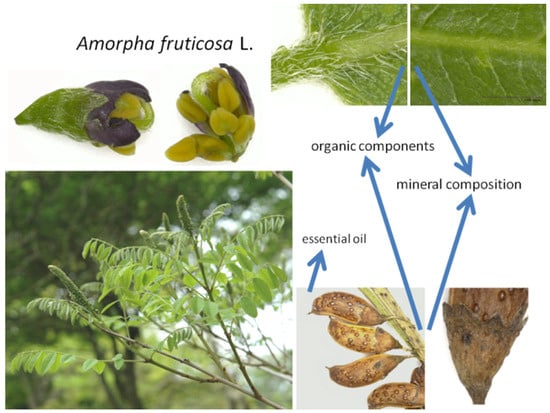
Figure 1.
Different parts of Amorpha fruticosa. The arrows indicate the organs from which the substances under study were extracted.
A. fruticosa contains a number of bioactive compounds with valuable pharmacological properties, such as antioxidant, antimicrobial, wound healing, hepatoprotective, antispasmodic, and anticancer properties, and its potential against diabetes and metabolic diseases is quite high [11,12,13]. Based on extracts from amorpha, preparations such as “Amorfin” and “Frucitin” are produced for the treatment of neuroses and tachycardia [14,15]. The fruits contain up to 3.5% essential oil [16], while the seeds consist of fatty acids, including linoleic, oleic, palmitic, and stearic acids.
A. fruticosa is a good candidate as a source of bioactive substances for new diabetes medications. The plant contains isoflavonoids (the glycoside amorphin) and phenolic compounds, namely, prenyloxy stilbenoids (amorfrutins) [13,17].
Considering the great interest in the use of bioactive substances from A. fruticosa in medical practice, the aim of this work was to assess the biochemical composition of the leaves and fruits of plants growing in the Arboretum of the Main Botanical Garden of the Russian Academy of Sciences. This is currently the northernmost point of the secondary distribution range of A. fruticosa. It is known that stress conditions in cultivation, including cold, lead to an increase in secondary metabolite content, which cannot help but take a toll on the results of the phytochemical analysis of the plants forming the northernmost introduced population.
2. Materials and Methods
2.1. Place of Research and Plant Material
The population of A. fruticosa in the territory of the Main Botanical Garden of the Russian Academy of Sciences in Moscow (55.8459° N; 37.610639° E) was the object of this study (Figure 2). Leaves were harvested in early September 2024; fruit was harvested in the first few days of October 2024.
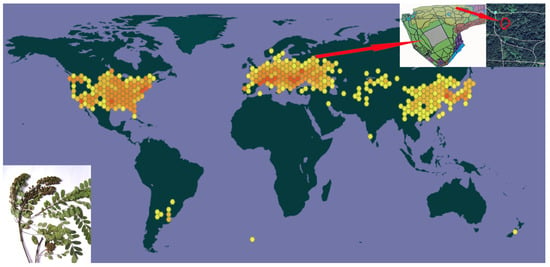
Figure 2.
Map of population’s location in the arboretum of the Main Botanical Garden of the Russian Academy of Sciences (MBG RAS).
Part of the leaves and fruits was immediately frozen after collection to preserve the state of the metabolites. The remaining leaves and fruits were dried at room temperature. All determinations were performed in triplicate.
2.2. Metabolome Analysis of Primary Metabolites by LC-MS
Lyophilized samples were ground into a fine powder, and then the analysis of secondary metabolites was conducted using LC-MS on the JMS-Q1050GC GC-MS chromatograph (JEOL Ltd., Tokyo, Japan), as previously described [18]. All data were processed using Xcalibur 2.1 software (Thermo Fisher Scientific, Waltham, MA, USA). The resulting peak area data matrix was normalized using the internal standard isovitexin (CAS: 29702-25-8). Identification and annotation of metabolites were performed using metabolite databases [19].
2.3. Analysis of Chemical Elements
The dried samples of leaves (2 g) were mineralized in a muffle furnace (Naberterm, Germany) at 400 °C. The obtained ash was dispersed by ultrasonication at 18 kHz for 15 min. An even layer of the dispersed sample was applied to the analyzer’s stage covered with carbonic scotch.
The mineral (ash) composition was determined by means of an energy dispersive spectroscopy (EDS) analyzer combined with a JEOL JSM 6090 LA scanning electron microscope (Japan) in accordance with the methodology of Motyleva et al. (2017) [20]. Spectra and element distribution data were obtained together with images by means of a raster electron microscope. Elemental composition was evaluated on the basis of the weight percentage of 13 elements (O, C, P, S, K, Ca, Mg, Fe, Si, Al, Mn, Mo, and Zn), which were reliably identified. EDS was used for qualitative and quantitative analyses of elements in the samples with the help of the X-ray spectra acquired through electronic beam scanning of the observed image. Six measurements were taken for each ash sample. The analyzed area was 3 mm2, and the scanned area was at least 12 µm2. The average quadratic deviation did not exceed 1.2–6.9%.
2.4. Extraction of A. fruticosa Fruits Essential Oil
An amount of 100 g of air-dried fruits was ground by passing through a 100-mesh sieve and then subjected for 4 h to water distillation using a Clevenger-type apparatus. The essential oil (EO) concentration has an expression of mL EO/100 g fruits. The obtained EO was stored at +4 °C until tested and analyzed.
2.5. GC-MS Analysis of Essential Oil
The identification of EOs components was obtained via GC/MS. Analyses were carried out on a Shimadzu GS 2010 gas chromatograph with a GCMS-QP 2010 mass detector. The gas chromatography column was a SPB-1 nonpolar column (solid-stage-bound methyl silicone) (Supelco, Sigma-Aldrich, St. Louis, MO, USA) (30 m × 0.25 mm ID, 0.25 μm film thickness) [21]. The carrier gas was helium. The column oven temperature was set at 60 °C for 3 min, increased to 100 °C at a rate of 1.5 °C for 1 min, increased to 180 °C at a rate of 4 °C for 1 min, and held at 180 °C for 1 min. Next, it was increased to 200 °C at a rate of 10 °C for 1 min, increased to 250 °C at a rate of 2.5 °C for 1 min, and held at 250 °C for 5 min. Injector, interface, and detector temperatures were 180 °C, 205 °C, and 250 °C, respectively.
The gas chromatography mass analysis was carried out with the same characteristics as used in gas chromatography. A 1.0 μL sample was injected in the split mode, with a split ratio of 1:150. The detector operated in positive electron impact mode (70 eV) in the range of m/z 35–300, full scan mode. The column oven temperature program was the same as in GC analysis. Helium was used as a carrier gas at a flow rate of 1.5 mL min−1. The mass range was 30–400 m z−1, while the injector and MS transfer line temperatures were set at 220 and 290 °C, respectively. All peaks of the chromatograms were analyzed using the Xcalibur® 4.1. software and NIST 11 Mass Spectral Library, the identification being carried out with a minimum similarity level of 80%. Alkane standard solution for GC (C8-C20 in hexane) was used for retention indexes (RI) calculation. The relative percentage of individual components was calculated based on GC peak areas [22].
2.6. Determination of Phenolic Compounds and Flavonoids
Total polyphenol content was measured spectrophotometrically on a Spekol 1300 spectrophotometer (Analitik Jena AG, Jena, Germany) using the Folin–Ciocalteu reagent according to the method described in detail in [23]. Gallic acid (25–300 mg/L; R2 = 0.998) was used as a standard. The results were expressed as mg/g gallic acid equivalent DW (dry weight) [23].
Total flavonoid content was determined using a modified method described in [24]. A 1 mL aliquot of each sample was mixed with 2 mL of a 2% (w/v) ethanol solution of aluminum chloride, 0.5 mL of 1 M hydrochloric acid, and 6.5 mL of ethanol (96%). After 40 min in the dark, the absorbance at 415 nm was measured using a Spekol 1300 spectrophotometer (Analitik Jena AG, Jena, Germany). Rutin (1–400 mg/L; R2 = 0.9977) was used as standard. The results were expressed as mg/g rutin equivalent on a DW (dry weight) basis [24].
3. Results
3.1. Metabolic Analysis
A total of 114 metabolites were identified in the major substances in the methanolic extract of A. fruticosa by GC-MS—103 metabolites in the fruits and only 19 metabolites in the leaves of A. fruticosa. The peak heights were at least 0.01% of the instrument scale. The metabolites were divided into four main groups: amino acids (4 compounds), sugars and their derivatives (19 compounds), organic acids (7 compounds), and polyphenolic compounds (10 compounds). The metabolite profiles of the leaves and fruits of A. fruticosa differed significantly both at the group level and at the individual metabolite level (PerMANOVA p < 0.01 in each case). Significant differences were also observed at the paired level (p < 0.05). Table 1 presents the main metabolites whose relative content exceeded 2%.

Table 1.
Composition of organic components in the leaves and fruits of A. fruticosa (% mass).
In the leaves, only one amino acid—alanine—was identified, while four amino acids were found in the fruits, with ornithine being the dominant one. The proportions of all other amino acids did not differ significantly.
Carbohydrates and their derivatives predominated in both the leaves and fruits of A. fruticosa. Eleven compounds were recorded only in the fruits, and two compounds were found exclusively in the leaves. Methyl-β-D-arabinofuranoside and D-erythro-2-pentulose dominated in the leaves, while D-allofuranose and arabinopyranose were prevalent in the fruits.
Among the secondary metabolites, the proportion of organic acids was 47.68% in the leaves and 78.33% in the fruits of A. fruticosa. Notably, specific types of compounds from this class accumulated in very high quantities; acetic acid constituted 37.33% in the leaves, while D-(+)-galacturonic acid made up 54.33% in the fruits. Among the polyphenolic compounds, three compounds—D-fucitol, erythritol, and glycerol—were found in significant amounts in the leaves. In the fruits of A. fruticosa, adonitol and glycerol were dominant.
3.2. Analysis of Elemental Composition
The leaves and fruits of A. fruticosa are rich in macro- and microelements: O, C, K, Ca, Mg, Fe, P, Si, Mn, Mo, S, Al, and Zn. However, the fruits of A. fruticosa are the undisputed leaders in the accumulation of individual elements. The species profiles for the content of chemical elements in the leaves and fruits of A. fruticosa differed significantly (p < 0.05) (Table 2).

Table 2.
Mineral composition of the leaves and fruits of A. fruticosa (% mass).
In the ash of the leaves and fruits, two elements associated with organic compounds—O and C—dominated in the overall elemental composition; the highest relative content of these elements was noted in the leaves (41.1% and 30.0%, respectively), while in the fruits it was 30.0% and 27.2%. The relative content of Mg, Si, Mn, and Mo in both organs did not differ significantly. However, the Ca content in the leaves was 1.9 times higher than in the fruits, while Na and Fe were 8.2 and 1.3 times, respectively, higher in the fruits than in the leaves.
The concentrations of P, Mn, Mo, S, and Si in the leaves did not exceed 1%, while Al and Zn were less than 0.1%. In the fruits of A. fruticosa, the content of P, Zn, and S was significantly higher, i.e., by 9.7, 7.2, and 3.4 times, respectively. The content of each chemical element, except for C, Si, Mn, and Mo, differed significantly between the leaves and fruits of A. fruticosa (p < 0.01 for each element).
3.3. Analysis of the Composition and Content of Essential Oil in the Fruits of A. fruticosa
The average content of essential oil in mature air-dried fruits of A. fruticosa was 0.39 ± 0.09%. The essential oil was colorless with a specific, persistent, light citrus scent, making it suitable for use in perfumery.
In the phytochemical profile of the essential oil from air-dried mature fruits of A. fruticosa, 34 volatile components were identified, accounting for 95.88% of the total area. The main components of the essential oil included α-pinene (9.26%), δ-cadinene (9.08%), α-muurolene (8.25%), β-caryophyllene (8.01%), α-copaene (6.98%), α-caryophyllene (6.95%), and β-myrcene (6.17%) (Table 3). The most common chemical groups were sesquiterpene hydrocarbons (48.83%), oxygenated sesquiterpenes (20.64%), monoterpene hydrocarbons (17.95%), and oxygenated monoterpenes (8.77%).

Table 3.
Composition of the essential oil of mature fruits of A. fruticosa.
3.4. Analysis of Total Phenolic and Flavonoid Content in the Organs of A. fruticosa
The total content of phenolic compounds was highest in the mature fruits: 59.5 mg GAE/g DW expressed as gallic acid; this is 1.3 to 1.6 times higher than in other plant organs (Figure 3). Significant differences were recorded between the various plant organs for this parameter: R2 = 0.96.
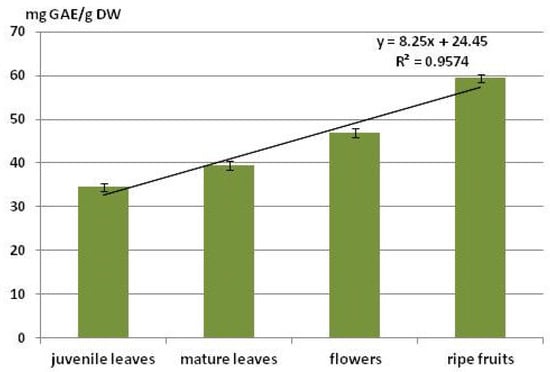
Figure 3.
Content of phenolic compounds in different organs of A. fruticosa.
The total flavonoid content was also highest in the mature fruits of A. fruticose, i.e., 3.02 mg/g DW of rutin, which is 1.3 to 1.5 times higher than in the leaves (Figure 4). Notably, the total flavonoid content in the flowers was similar to that in the fruits, unlike the total phenolic content. Significant differences were observed between the various plant organs for this parameter: R2 = 0.93. The flavonoid content in individual organs constitutes 5.1–6.2% of the total polyphenols.
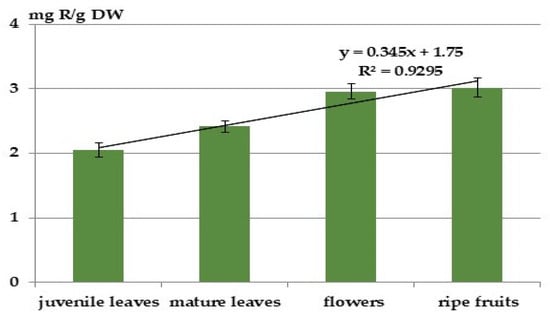
Figure 4.
Content of flavonoids in different organs of A. fruticosa.
4. Discussion
The practical use of invasive plant species as ornamental, forage, honey-producing plants, biofuels, and biopesticides, as well as for the phytoremediation of industrial waste sites, is becoming increasingly common. However, a particularly valuable direction is their use as raw materials for medical purposes and the pharmaceutical industry. The main issue with the use of invasive species is the almost complete lack of information about their biological characteristics in the secondary distribution range. Even when a body of literature exists regarding the biochemistry of a species in the native area range, it cannot always be applied to the same taxon growing in the secondary distribution range due to significant microevolutionary changes in plants under new soil–climatic conditions [15]. A. fruticosa is one of the promising invasive species with a wide potential for use in the pharmaceutical industry.
This study presents a comprehensive dataset based on biochemical and compositional analyses of the leaves and fruits of A. fruticosa for the first time. The metabolic analysis of the leaves and fruits revealed a large number of metabolites in the fruits of A. fruticosa, which has also been noted by Kozuharova et al. (2017) [25]. In the fruits of A. fruticosa, a high content of adonitol, a five-carbon alcohol used as a sweetener in diabetic products since the early 1960s, was recorded. The previously discovered elevated level of adonitol in Adonis microcarpa DC has led to recommending this species for the treatment of type 2 diabetes [26]. This fact suggests that extracts from the fruits of A. fruticosa may also have antidiabetic effects. Additionally, hydroxycarboxylic acids, particularly galacturonic acid, predominated among the organic compounds in the fruits, indicating potential antioxidant activity.
The mineral profile of the fruits of A. fruticosa, with a predominance of potassium, is generally similar to the mineral composition of pollen and A. fruticosa honey [27,28]. This composition of trace elements has led Zhu et al. (2020) [28] to consider this type of honey a good source of trace elements related to bone health, immune function, adrenaline, and glucose metabolism.
The content and composition of the essential oil from the flowers and fruits of A. fruticosa have been studied by several European researchers [16,29,30,31]. High variability in the content of essential oil from the fruits of A. fruticosa has been noted, depending on the harvest time, physiological maturity of the fruits, and ecological conditions of growth. Our results regarding the content of essential oil in the fruits of A. fruticosa are consistent with the findings of Romanian researchers [16]. However, our data on the chemical composition of the essential oil from the fruits of A. fruticosa align more closely with the results of Polish researchers [29], further demonstrating that ecological conditions significantly influence the phytochemical characteristics of plants. The α-pinene, β-myrcene δ-cadinene, and β-caryophyllene were the main compounds, as in the work of Polish researchers. Compared to data published by Marinas et al. (2021) [16], we obtained lower concentrations of δ-cadinene, α-muurolene, and γ-cadinene. Our samples also showed a high concentration of α-pinene, which was present in trace amounts in the essential oil according to Romanian researchers.
The presence of δ-cadinene, α-muurolene, β-caryophyllene, germacrene D, citronellol, and linalool in the essential oil ensures its antimicrobial activity, as demonstrated by Romanian researchers [16]. The total polyphenol content in the ethanol extract of the leaves determined in our study was at the level of values published in Hovanet et al. (2015) [32], but significantly lower than the results obtained by Ivanescu et al. (2019) [33]. Among the phenolic compounds present in A. fruticosa, a particularly important group is the diverse amorphutins and isoflavonoid derivatives. The total flavonoid content in the fruits of A. fruticosa in our samples was in line with the data from Simeonova et al. (2022) [13]. This suggests that amorphutins A and B may also be present in the fruits, with their quantity falling within the levels reported by Simeonova et al. (2022) [13], i.e., approximately 1.9%. Since the application of in viro amorphutins tests led to a significant decrease in insulin resistance, the potential of A. fruticosa as a treatment for diabetes and metabolic diseases is promising.
5. Conclusions
For the first time, a comprehensive biochemical and compositional assessment of A. fruticosa grown under controlled conditions in a botanical garden has been provided. The metabolomic and mineral composition of the leaves and fruits has been determined, and the composition of the essential oil from the fruits has been analyzed. A detailed analysis of A. fruticosa from the secondary distribution range confirms the potential of this plant as a valuable resource for the pharmacopoeia industry. The wide use of A. fruticosa will provide an ecological solution to the problem associated with this aggressive invasive species.
Author Contributions
Conceptualization, O.V.S. and Y.K.V.; methodology, Y.K.V.; formal analysis, M.A.G.; resources, O.V.S. and M.A.G.; writing—original draft preparation/review and editing, O.V.S. and Y.K.V. All authors have read and agreed to the published version of the manuscript.
Funding
The reported study was supported by assignments 122042600141-3 of the Ministry of Science and Higher Education of the Russian Federation. The APC was funded by the authors.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wondafrash, M.; Wingfield, M.J.; Wilson, J.R.; Hurley, B.P.; Slippers, B.; Paap, T. Botanical gardens as key resources and hazards for biosecurity. Biodivers. Conserv. 2021, 30, 1929–1946. [Google Scholar] [CrossRef]
- Scoggan, H.J.; Amorpha, L. The Flora of Canada; National Museums of Canada: Ottawa, Canada, 1978; Part 3; pp. 973–974. [Google Scholar]
- POWO. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:11421-2 (accessed on 7 October 2024).
- Rehder, A. Manual of Cultivated Trees and Shrubs Hardy in North America; MacMillan Company: New York, NY, USA, 1949; p. 996. [Google Scholar]
- CABI Compendium Invasive Species. Available online: https://www.cabidigitallibrary.org/product/QI (accessed on 27 October 2024).
- Svyazeva, O.A. Trees, Shrubs and Lianas of the Park of the Botanical Garden of The V.L.; [Derev’ya, Kustarniki I Liany Parka Botanicheskogo Sada Botanicheskogo Instituta Im. V.L. Komarova]; Komarov Botanical Institute: Sankt Petersburg, Russia, 2005; pp. 1–384. (In Russian) [Google Scholar]
- Demidov, A.S. (Ed.) Woody Plants of the N.V. Tsitsin Main Botanical Garden of the Russian Academy of Sciences: 60 Years of Introduction; [Drevesnye rasteniya Glavnogo botanicheskogo sada im. N.V. Tsitsina RAN: 60 let introdukcii]; Nauka: Moscow, Russia, 2005; pp. 1–586. (In Russian) [Google Scholar]
- Yatsenko, I.O.; Vinogradova, Y.K. Invasive Activity of Woody Plants in Tsytsyn Main Botanical Garden, Russian Academy of Sciences. Russ. J. Biol. Invasions 2019, 10, 92–103. [Google Scholar] [CrossRef]
- Vinogradova, Y.K. Code of Condact of Invasive Alien Species in Botanical Gardens of CIS Countries; [Kodeks upravleniya nvazionnymi chuzherodnymi vidami v botanicheskih sadah stran SNG]; MBG RAS: Moscow, Russia, 2015; pp. 1–68. (In Russian) [Google Scholar]
- Vinogradova, Y.K.; Kuklina, A.G.; Tkacheva, E.V. Fruiting of some Amorpha species in the secondary range (Plodonoshenie nekotoryh vidov roda Amorpha L. vo vtorichnom areale). Belgorod State Univ. Sci. Bull. Nat. Sci. 2013, 24, 42–50. (In Russian) [Google Scholar]
- Jakovljević, T.; Halambek, J.; Radošević, K.; Hanousek, K.; Gradečki-Poštenjak, M.; Gaurina Srček, V.; Radojčić Redovniković, I.; De Marco, A. The potential use of indigobush (Amorpha fruticosa L.) as natural resource of biologically active compounds. South-East Eur. For. 2015, 6, 171–178. [Google Scholar] [CrossRef]
- Kozuharova, E.; Benbassat, N.; Ionkova, I. The invasive alien species Amorpha fruticosa in Bulgaria and its potential as economically prospective source of valuable essential oil. Pharmacia 2020, 67, 357–362. [Google Scholar] [CrossRef]
- Simeonova, R.; Shkondrov, A.; Kozuharova, E.; Ionkova, I.; Krasteva, I. A study on the safety and effects of Amorpha fruticosa fruit extract on spontaneously hypertensive rats with induced type 2 diabetes. Curr. Issues Mol. Biol. 2022, 44, 2583–2592. [Google Scholar] [CrossRef]
- Golovkin, B.N. Biologically Active Substances of Plant Origin [Biologicheski aktivnye veshchestva rastitel’nogo proiskhozhdeniya]; Nauka: Moscow, Russia, 2001. (In Russian) [Google Scholar]
- Grabić, J.; Ljevnaić-Mašić, B.; Zhan, A.; Benka, P.; Heilmeier, H. A review on invasive false indigo bush (Amorpha fruticosa L.): Nuisance plant with multiple benefits. Ecol. Evol. 2022, 12, e9290. [Google Scholar] [CrossRef]
- Marinas, I.C.; Oprea, E.; Buleandra, M.; Badea, I.A.; Tihauan, B.M.; Marutescu, L.; Chifiriuc, M.C. Chemical composition, antipathogenic and cytotoxic activity of the essential oil extracted from Amorpha fruticosa fruits. Molecules 2021, 26, 3146. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Du, L. Qualitative and quantitative analysis of amorfrutins, novel antidiabetic dietary natural products, by HPLC. Pharm. Biol. 2015, 54, 488–493. [Google Scholar] [CrossRef]
- Motyleva, S.; Upadysheva, G.; Tumaeva, T. Influence of rootstocks on the productivity and chemical composition of Prunus domestica L. fruits. Slovak J. Food Sci. 2021, 15, 1029–1038. [Google Scholar] [CrossRef]
- Tohge, T.; Fernie, A.R. Web-based resources for massspectrometry-based metabolomics: A user’s guide. Phytochemistry 2009, 70, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Motyleva, S.M.; Kulikov, I.M.; Marchenko, L.A. EDS analysis for fruit Prunus elemental composition determination. Mater. Sci. Forum 2017, 888, 314–318. [Google Scholar] [CrossRef]
- Shelepova, O.V.; Olekhnovich, L.S.; Konovalova, L.N.; Khusnetdinova, T.I.; Gulevich, A.A.; Baranova, E.N. Assessment of essential oil yield in three mint species in the climatic conditions of Central Russia. Agron. Res. 2021, 19, 1551–1562. [Google Scholar] [CrossRef]
- Koo, I.; Kim, S.; Zhang, X. Comparative analysis of mass spectral matching-based compound identification in gas chromatography–mass spectrometry. J. Chromatogr. 2013, 1298, 132–138. [Google Scholar] [CrossRef]
- Nikolaeva, T.N.; Lapshin, P.V.; Zagoskina, N.V. Method for determining the total content of phenolic compounds in plant extracts with Folin-Denis reagent and Folin-Ciocalteu reagent: Modification and comparison. Russ. J. Bioorg. Chem. 2022, 48, 1519–1525. [Google Scholar] [CrossRef]
- Krasnyuk, I.I. Modifikatsiya metodiki kolichestvennogo opredeleniya flavonoidov v trave zolotarnika kanadskogo (Solidago canadensis). Vestn. Mosk. Univ. Ser. 2 Khimiya 2019, 60, 49–54. [Google Scholar]
- Kozuharova, E.; Matkowski, A.; Woźniak, D.; Simeonova, R.; Naychov, Z.; Malainer, C.; Atanasov, A.G. Amorpha fruticose—A noxious invasive alien plant in Europe or a medicinal plant against metabolic disease? Front. Pharmacol. 2017, 8, 333. [Google Scholar] [CrossRef]
- Abd-Alla, H.I.; Hassan, A.Z.; Soltan, M.M.; Abdelwahab, A.B.; Hanna, A.G. Potential protein antiglycation, antiproliferation, and in silico study on the antidiabetic enzymes of bioactive metabolites from Adonis microcarpa DC and their ADMET properties. J. Appl. Pharm. Sci. 2021, 12, 106–119. [Google Scholar]
- Hong, I.P.; Woo, S.O.; Han, S.M.; Kin, S.G.; Jang, H.R.; Lee, M.Y.; Choi, Y.S.; Kim, H.K.; Lee, M.L. Evaluation of nutritional potential of Amorpha fruticosa pollen collected by honey bees. J. Apic. 2016, 31, 73–77. [Google Scholar] [CrossRef]
- Zhu, M.; Zhao, H.; Wang, Q.; Wu, F.; Cao, W. A novel chinese honey from Amorpha fruticosa L.: Nutritional composition and antioxidant capacity in vitro. Molecules 2020, 25, 5211. [Google Scholar] [CrossRef]
- Lis, A.; Góra, J. Essential oil of Amorpha fruticosa L. J. Essent. Oil Res. 2001, 13, 340–342. [Google Scholar] [CrossRef]
- Ivanescu, B.; Lungu, C.; Spac, A.; Tuchilus, C. Essential oils from Amorpha fruticosa L. fruits—Chemical characterization and antimicrobial activity. Biol. Veg. 2014, 60, 33–39. [Google Scholar]
- Kozuharova, E.; Benbassat, N.; Berkov, S.; Ionkova, I. Ailanthus altissima and Amorpha fruticose—Invasive arboreal alien plants as cheap sources of valuable essential oils. Pharmacia 2020, 67, 71–81. [Google Scholar] [CrossRef]
- Hovanet, M.V.; Marinas, I.C.; Dinu, M.; Oprea, E.; Chifiriuc, M.C.; Stavropoulou, E.; Lazăr, V. The phytotoxicity and antimicrobial activity of Amorpha fruticosa L. leaves extract. Rom. Biotechnol. Lett. 2015, 20, 10670–10678. [Google Scholar]
- Ivanescu, B.; Lungu, C.; Vlase, L.; Gradinaru, A.C.; Tuchilus, C. HPLC analysis of phenolic compounds, antioxidant and antimicrobial activity of Amorpha fruticosa L. extracts. J. Plant Dev. 2019, 26, 77. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).