Allostatic Load Index Effectively Measures Chronic Stress Status in Zoo-Housed Giraffes
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Sample Collection
2.3. Serum Analyses
2.4. Body Condition Scoring
- 1 = Emaciated condition, absent visible fat, prominent skeletal details, muscle wasting.
- 2 = Intermediate between 1 and 3.
- 3 = Lean body condition, suboptimal fat stores, noticeable skeletal details, lean muscling.
- 4 = Intermediate between 3 and 5.
- 5 = Ideal condition, adequate fat, only notable features of skeletal frame (pelvis and spine).
- 6 = Intermediate between 5 and 7.
- 7 = Overweight condition, lost definition of notable features, fat deposition on chest.
- 8 = Intermediate between 7 and 9.
- 9 = Obese condition, extensive fat, palpable loss of skeletal features, protruding abdomen.
2.5. Allostatic Load
2.6. Compilation of Stress Events
2.7. Statistical Analysis
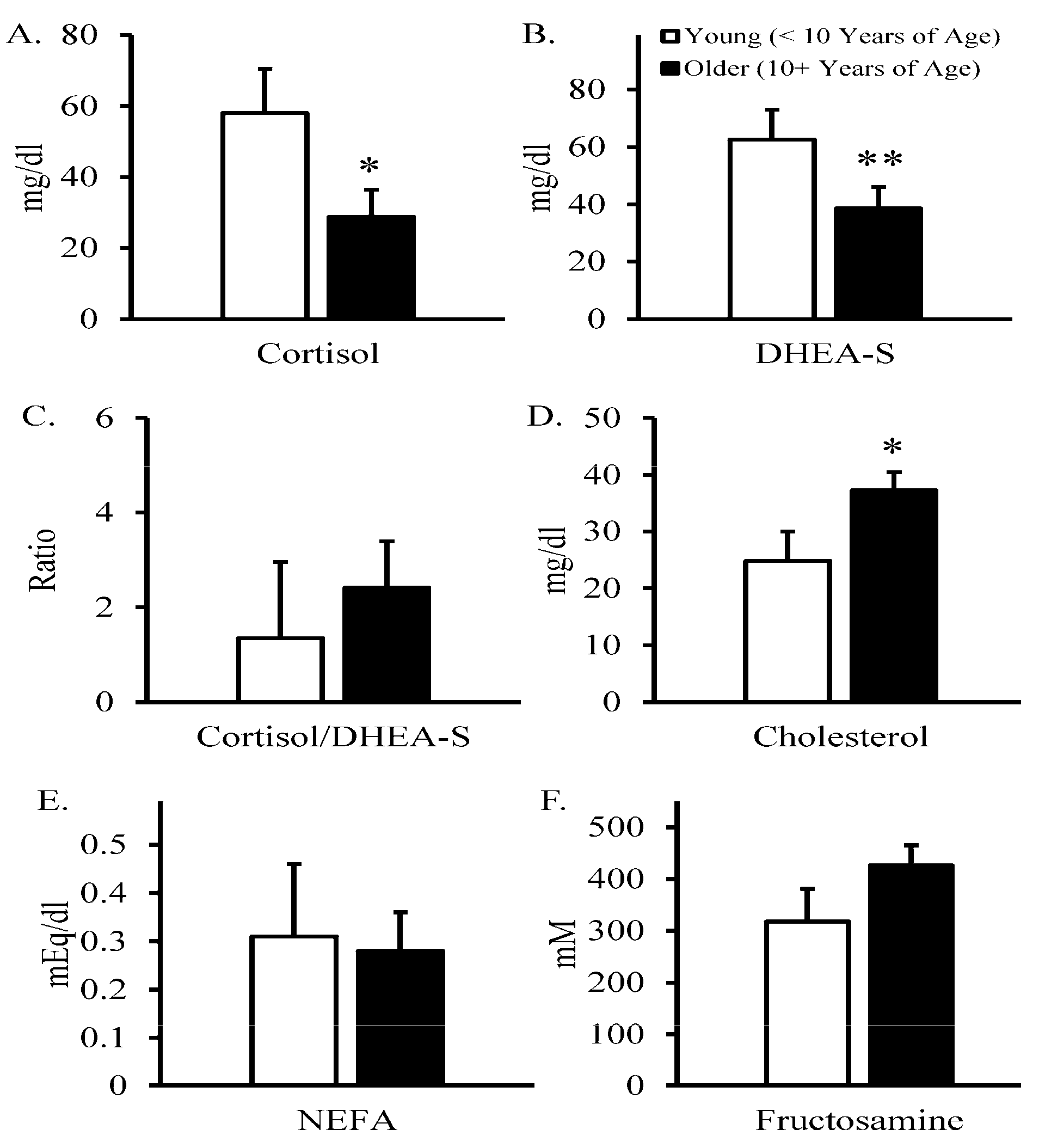
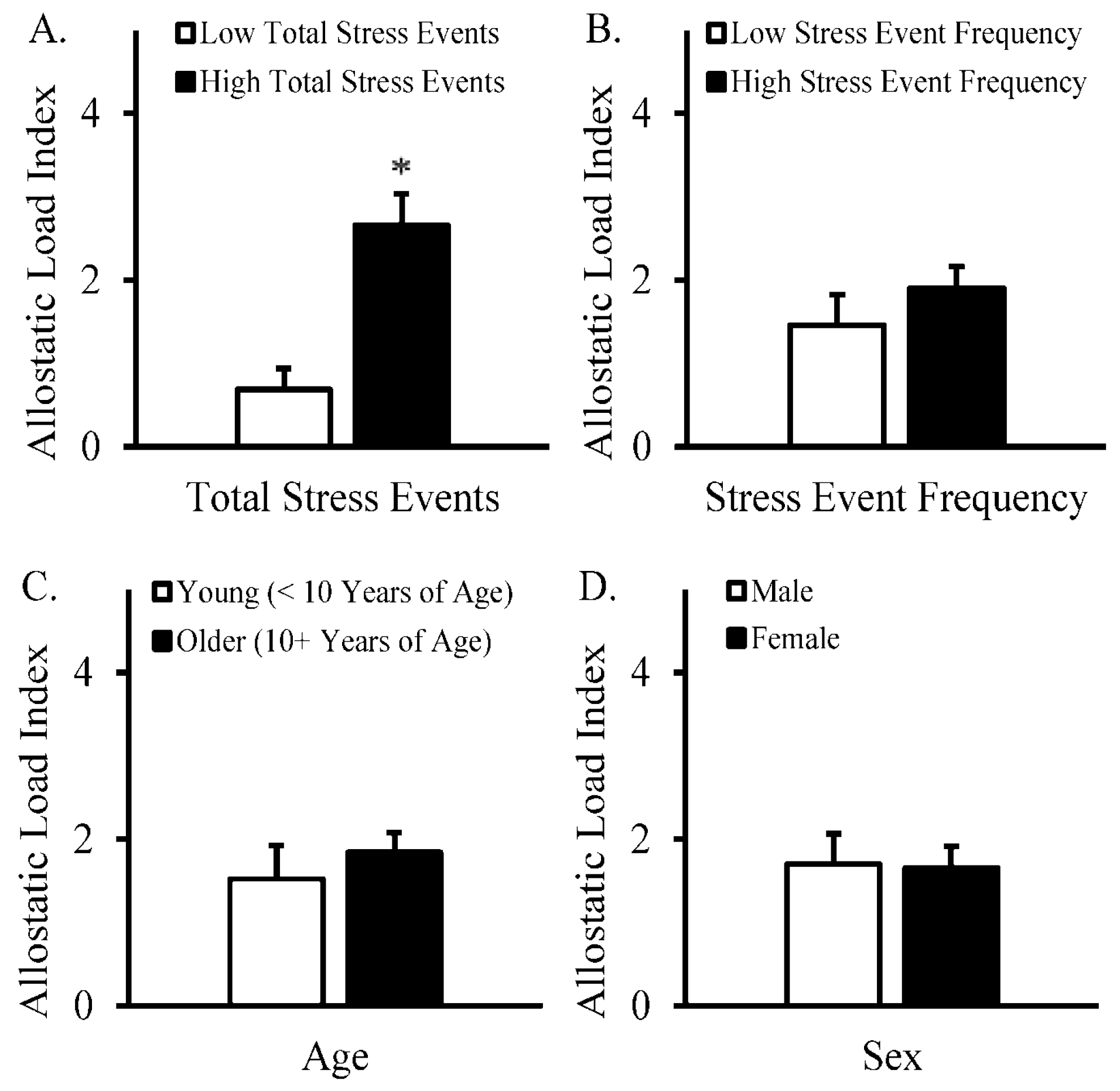
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cooper, C.L.; Dewe, P. Stress: A Brief History; Blackwell Publishing: Oxford, UK, 2008. [Google Scholar] [CrossRef]
- Dickens, M.J.; Delehanty, D.J.; Michael Romero, L. Stress: An inevitable component of animal translocation. Biol. Conserv. 2010, 143, 1329–1341. [Google Scholar] [CrossRef]
- Romero, L.M.; Gormally, B.M.G. How Truly Conserved Is the “Well-Conserved” Vertebrate Stress Response? Integr. Comp. Biol. 2019, 59, 273–281. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Stress, Adaptation, and Disease: Allostasis and Allostatic Load. Ann. N. Y. Acad. Sci. 1998, 840, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Guidi, J.; Lucente, M.; Sonino, N.; Giovanni. Allostatic Load and Its Impact on Health: A Systematic Review. Psychother. Psychosom. 2021, 90, 11–27. [Google Scholar] [CrossRef]
- Edes, A.N.; Wolfe, B.A.; Crews, D.E. Evaluating allostatic load: A new approach to measuring long-term stress in wildlife. J. Zoo Wildl. Med. 2018, 49, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.M.; Ledoux, J.E.; Sapolsky, R.M. The Influence of Stress Hormones on Fear Circuitry. Annu. Rev. Neurosci. 2009, 32, 289–313. [Google Scholar] [CrossRef]
- Seeley, K.E.; Proudfoot, K.L.; Edes, A.N. The application of allostasis and allostatic load in animal species: A scoping review. PLoS ONE 2022, 17, e0273838. [Google Scholar] [CrossRef]
- Edes, A.N.; Wolfe, B.A.; Crews, D.E. Assessing Stress in Zoo-Housed Western Lowland Gorillas (Gorilla gorilla gorilla) Using Allostatic Load. Int. J. Primatol. 2016, 37, 241–259. [Google Scholar] [CrossRef]
- Bashaw, M.J.; Tarou, L.R.; Maki, T.S.; Maple, T.L. A survey assessment of variables related to stereotypy in captive giraffe and okapi. Appl. Anim. Behav. Sci. 2001, 73, 235–247. [Google Scholar] [CrossRef]
- Benhaiem, S.; Hofer, H.; Dehnhard, M.; Helms, J.; East, M.L. Sibling competition and hunger increase allostatic load in spotted hyaenas. Biol. Lett. 2013, 9, 20130040. [Google Scholar] [CrossRef]
- Bonier, F.; Martin, P.R.; Moore, I.T.; Wingfield, J.C. Do baseline glucocorticoids predict fitness? Trends Ecol. Evol. 2009, 24, 634–642. [Google Scholar] [CrossRef]
- Ebrecht, M.; Hextall, J.; Kirtley, L.-G.; Taylor, A.; Dyson, M.; Weinman, J. Perceived stress and cortisol levels predict speed of wound healing in healthy male adults. Psychoneuroendocrinology 2004, 29, 798–809. [Google Scholar] [CrossRef]
- Hajduk, P.; Copland, M.D.; Schultz, D.A. Effects of Capture on Hematological Values and Plasma Cortisol Levels of Free-range Koalas (Phascolarctos cinereus). J. Wildl. Dis. 1992, 28, 502–506. [Google Scholar] [CrossRef]
- Lupien, S.; de Leon, M.; de Santi, S.; Convit, A.; Tarshish, C.; Nair, N.P.V.; Thakur, M.; McEwen, B.; Hauger, R.; Meaney, M. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat. Neurosci. 1998, 1, 329. [Google Scholar] [CrossRef]
- Mondelli, V.; Ciufolini, S.; Belvederi Murri, M.; Bonaccorso, S.; Di Forti, M.; Giordano, A.; Marques, T.R.; Zunszain, P.A.; Morgan, C.; Murray, R.M.; et al. Cortisol and Inflammatory Biomarkers Predict Poor Treatment Response in First Episode Psychosis. Schizophr. Bull. 2015, 41, 1162–1170. [Google Scholar] [CrossRef]
- Pride, E.R. High faecal glucocorticoid levels predict mortality in ring-tailed lemurs (Lemur catta). Biol. Lett. 2005, 1, 60–63. [Google Scholar] [CrossRef][Green Version]
- Wilkening, J.L.; Ray, C.; Varner, J. When can we measure stress noninvasively? Postdeposition effects on a fecal stress metric confound a multiregional assessment. Ecol. Evol. 2016, 6, 502–513. [Google Scholar] [CrossRef]
- Frongia, G.N.; Peric, T.; Leoni, G.; Satta, V.; Berlinguer, F.; Muzzeddu, M.; Prandi, A.; Naitana, S.; Comin, A. Assessment of Cortisol and DHEA Concentrations in Griffon Vulture (Gyps fulvus) Feathers to Evaluate its Allostatic Load. Ann. Anim. Sci. 2020, 20, 85–96. [Google Scholar] [CrossRef]
- Gundlach, N.H.; Schmicke, M.; Ludes-Wehrmeister, E.; Ulrich, S.A.; Araujo, M.G.; Siebert, U. New Approach to stress research in phocids-potential of dehydroepiandrosterone and cortisol/dehydroepiandrosterone ratio as markers for stress in harbor seals (Phoca vitulina) and gray seals (Halichoers grypus). J. Zoo Wildl. Med. 2018, 49, 556–563. [Google Scholar] [CrossRef]
- Etukudo, M.H.; Agbedana, E.O.; Akinyinka, O.O.; Osifo, B.O. Plasma electrolytes, total cholesterol, liver enzymes, and selected antioxidant status in protein energy malnutrition. Afr. J. Med. Med. Sci. 1999, 28, 81–85. [Google Scholar]
- HeliÖVaara, M.; Aho, K.; Knekt, P.; Reunamen, A.; Aromaa, A. Serum cholesterol and risk of rheumatoid arthritis in a cohort of 52 800 men and women. Rheumatology 1996, 35, 255–257. [Google Scholar] [CrossRef][Green Version]
- Kuzuya, M.; Izawa, S.; Enoki, H.; Okada, K.; Iguchi, A. Is serum albumin a good marker for malnutrition in the physically impaired elderly? Clin. Nutr. 2007, 26, 84–90. [Google Scholar] [CrossRef]
- Leardi, S.; Altilia, F.; Delmonaco, S.; Cianca, G.; Pietroletti, R.; Simi, M. Blood levels of cholesterol and postoperative septic complications. Ann. Ital. Di Chir. 2000, 71, 233–237. [Google Scholar]
- Tall, A.R.; Yvan-Charvet, L. Cholesterol, inflammation and innate immunity. Nat. Rev. Immunol. 2015, 15, 104–116. [Google Scholar] [CrossRef]
- Adewuyi, A.A.; Gruys, E.; Van Eerdenburg, F.J.C.M. Non esterified fatty acids (NEFA) in dairy cattle. A review. Vet. Q. 2005, 27, 117–126. [Google Scholar] [CrossRef]
- LeBlanc, S.J.; Leslie, K.E.; Duffield, T.F. Metabolic Predictors of Displaced Abomasum in Dairy Cattle. J. Dairy Sci. 2005, 88, 159–170. [Google Scholar] [CrossRef]
- Leroy, J.L.M.R.; Vanholder, T.; Mateusen, B.; Christophe, A.; Opsomer, G.; de Kruif, A.; Genicot, G.; Van Soom, A. Non-esterified fatty acids in follicular fluid of dairy cows and their effect on developmental capacity of bovine oocytes in vitro. Reproduction 2005, 130, 485–495. [Google Scholar] [CrossRef]
- Paolisso, G.; Tataranni, P.A.; Foley, J.E.; Bogardus, C.; Howard, B.V.; Ravussin, E. A high concentration of fasting plasma non-esterified fatty acids is a risk factor for the development of NIDDM. Diabetologia 1995, 38, 1213–1217. [Google Scholar] [CrossRef]
- Pilz, S.; Scharnagl, H.; Tiran, B.; Wellnitz, B.; Seelhorst, U.; Boehm, B.O.; März, W. Elevated plasma free fatty acids predict sudden cardiac death: A 6.85-year follow-up of 3315 patients after coronary angiography. Eur. Heart J. 2007, 28, 2763–2769. [Google Scholar] [CrossRef][Green Version]
- Valckx, S.D.M.; De Pauw, I.; De Neubourg, D.; Inion, I.; Berth, M.; Fransen, E.; Bols, P.E.J.; Leroy, J.L.M.R. BMI-related metabolic composition of the follicular fluid of women undergoing assisted reproductive treatment and the consequences for oocyte and embryo quality. Hum. Reprod. 2012, 27, 3531–3539. [Google Scholar] [CrossRef]
- Alzaga, V.; Vicente, J.; Villanua, D.; Acevedo, P.; Casas, F.; Gortazar, C. Body condition and parasite intensity correlates with escape capacity in Iberian hares (Lepus granatensis). Behav. Ecol. Sociobiol. 2008, 62, 769–775. [Google Scholar] [CrossRef]
- Cook, R.C.; Cook, J.G.; Murray, D.L.; Zager, P.; Johnson, B.K.; Gratson, M.W. Nutritional Condition Models for Elk: Which Are the Most Sensitive, Accurate, and Precise? J. Wildl. Manag. 2001, 65, 988–997. [Google Scholar] [CrossRef]
- Deacon, F.; Nel, P.J.; Bercovitch, F.B. Concurrent Pregnancy and Lactation in Wild Giraffes (Giraffa camelopardalis). Afr. Zool. 2015, 50, 331–334. [Google Scholar] [CrossRef]
- Gerhart, K.L.; White, R.G.; Cameron, R.D.; Russell, D.E. Estimating Fat Content of Caribou from Body Condition Scores. J. Wildl. Manag. 1996, 60, 713–718. [Google Scholar] [CrossRef]
- Hill, S.P.; Broom, D.M. Measuring zoo animal welfare: Theory and practice. Zoo Biol. 2009, 28, 531–544. [Google Scholar] [CrossRef]
- Parker, V.J.; Freeman, L.M. Association between Body Condition and Survival in Dogs with Acquired Chronic Kidney Disease. J. Vet. Intern. Med. 2011, 25, 1306–1311. [Google Scholar] [CrossRef]
- Sánchez, C.A.; Becker, D.J.; Teitelbaum, C.S.; Barriga, P.; Brown, L.M.; Majewska, A.A.; Hall, R.J.; Altizer, S.; Davies, J. On the relationship between body condition and parasite infection in wildlife: A review and meta-analysis. Ecol. Lett. 2018, 21, 1869–1884. [Google Scholar] [CrossRef] [PubMed]
- Schultner, J.; Kitaysky, A.S.; Welcker, J.; Hatch, S. Fat or lean: Adjustment of endogenous energy stores to predictable and unpredictable changes in allostatic load. Funct. Ecol. 2013, 27, 45–55. [Google Scholar] [CrossRef]
- Juster, R.-P.; McEwen, B.S.; Lupien, S.J. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci. Biobehav. Rev. 2010, 35, 2–16. [Google Scholar] [CrossRef]
- McEwen, B.S. Early life influences on life-long patterns of behavior and health. Ment. Retard. Dev. Disabil. Res. Rev. 2003, 9, 149–154. [Google Scholar] [CrossRef]
- Möstl, E.; Maggs, J.L.; Schrötter, G.; Besenfelder, U.; Palme, R. Measurement of Cortisol Metabolites in Faeces of Ruminants. Vet. Res. Commun. 2002, 26, 127–139. [Google Scholar] [CrossRef]
- Lumb, P.J.; Slavin, B. Determination of serum cholesterol concentration in the presence of ascorbate. J. Clin. Pathol. 1993, 46, 283–284. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, K.; Toyosato, M.; Yabumoto, S.; Tanimizu, I.; Hirakawa, H. A new enzymatic method for colorimetric determination of free fatty acids. Anal. Biochem. 1980, 108, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.F.; Lees, H.; Gutman, S.I. Effect of nitroblue tetrazolium concentration on the fructosamine assay for quantifying glycated protein. Clin. Chem. 1988, 34, 2106–2111. [Google Scholar] [CrossRef] [PubMed]
- Stringer, E.M.; Stoskopf, M.K.; Simons, T.R.; Waldstein, A.H. Ultrasonic Measurement of Body Fat as a Means of Assessing Body Condition in Free-Ranging Raccoons (Procyon lotor). Int. J. Zool. 2010, 2010, 972380. [Google Scholar] [CrossRef]
- Edes, A.N.; Edwards, K.L.; Wolfe, B.A.; Brown, J.L.; Crews, D.E. Allostatic Load Indices with Cholesterol and Triglycerides Predict Disease and Mortality Risk in Zoo-Housed Western Lowland Gorillas (Gorilla gorilla gorilla). Biomark. Insights 2020, 15, 1177271920914585. [Google Scholar] [CrossRef]
- Edes, A.N.; Wolfe, B.A.; Crews, D.E. The first multi-zoo application of an allostatic load index to western lowland gorillas (Gorilla gorilla gorilla). Gen. Comp. Endocrinol. 2018, 266, 135–149. [Google Scholar] [CrossRef]
- Edes, A.N.; Wolfe, B.A.; Crews, D.E. Testing a method to improve predictions of disease and mortality risk in western lowland gorillas (gorilla gorilla gorilla) using allostatic load. Stress 2021, 24, 76–86. [Google Scholar] [CrossRef]
- Rainey, W.E.; Nakamura, Y. Regulation of the adrenal androgen biosynthesis. J. Steroid Biochem. Mol. Biol. 2008, 108, 281–286. [Google Scholar] [CrossRef]
- Lennartsson, A.K.; Kushnir, M.M.; Bergquist, J.; Jonsdottir, I.H. DHEA and DHEA-S response to acute psychosocial stress in healthy men and women. Biol. Psychol. 2012, 90, 143–149. [Google Scholar] [CrossRef]
- Whitham, J.C.; Bryant, J.L.; Miller, L.J. Beyond Glucocorticoids: Integrating Dehydroepiandrosterone (DHEA) into Animal Welfare Research. Animals 2020, 10, 1381. [Google Scholar] [CrossRef]
- Takeshita, R.S.C.; Huffman, M.A.; Mouri, K.; Shimizu, K.; Bercovitch, F.B. Dead or alive? Predicting fetal loss in Japanese macaques (Macaca fuscata) by fecal metabolites. Anim. Reprod. Sci. 2016, 175, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Selvin, E.; Rawlings, A.M.; Lutsey, P.L.; Maruthur, N.; Pankow, J.S.; Steffes, M.; Coresh, J. Fructosamine and Glycated Albumin and the Risk of Cardiovascular Outcomes and Death. Circulation 2015, 132, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Grelet, C.; Vanden Dries, V.; Leblois, J.; Wavreille, J.; Mirabito, L.; Soyeurt, H.; Franceschini, S.; Gengler, N.; Brostaux, Y.; HappyMoo, C.; et al. Identification of chronic stress biomarkers in dairy cows. Animals 2022, 16, 100502. [Google Scholar] [CrossRef] [PubMed]
- Crenshaw, K.L.; Peterson, M.E.; Heeb, L.A.; Moroff, S.D.; Nichols, R. Serum fructosamine concentration as an index of glycemia in cats with diabetes mellitus and stress hyperglycemia. J. Vet. Intern. Med. 1996, 10, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Quiroz-Rocha, G.F.; LeBlanc, S.; Duffield, T.; Wood, D.; Leslie, K.E.; Jacobs, R.M. Evaluation of prepartum serum cholesterol and fatty acids concentrations as predictors of postpartum retention of the placenta in dairy cows. J. Am. Vet. Med. Assoc. 2009, 234, 790–793. [Google Scholar] [CrossRef]
- Dadone, L. Lameness Diagnosis and Management in Zoo Giraffe. Fower’s Zoo Wild Anim. Med. 2018, 10, 623–629. [Google Scholar]
- Boonstra, R.; Dušek, A.; Flynn, R. DHEA and territoriality during the nonbreeding season in male American martens (Martes americana). J. Mammal. 2018, 99, 826–835. [Google Scholar] [CrossRef]
- Farooqi, N.A.I.; Scotti, M.; Yu, A.; Lew, J.; Monnier, P.; Botteron, K.N.; Campbell, B.C.; Booij, L.; Herba, C.M.; Séguin, J.R.; et al. Sex-specific contribution of DHEA-cortisol ratio to prefrontal-hippocampal structural development, cognitive abilities and personality traits. J. Neuroendocr. 2019, 31, e12682. [Google Scholar] [CrossRef]
- Glei, D.A.; Goldman, N.; Chuang, Y.-L.; Weinstein, M. Do Chronic Stressors Lead to Physiological Dysregulation? Testing the Theory of Allostatic Load. Psychosom. Med. 2007, 69, 769–776. [Google Scholar] [CrossRef]
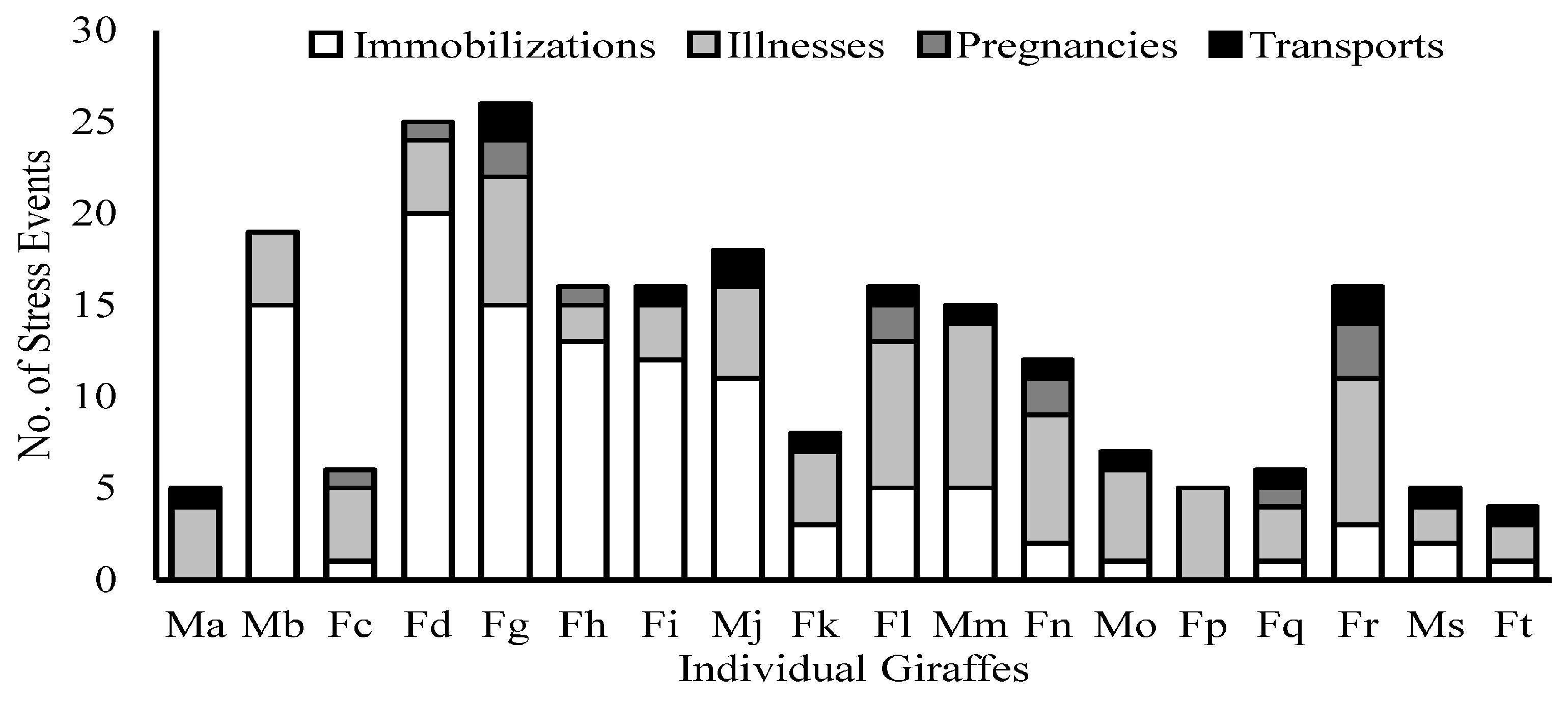
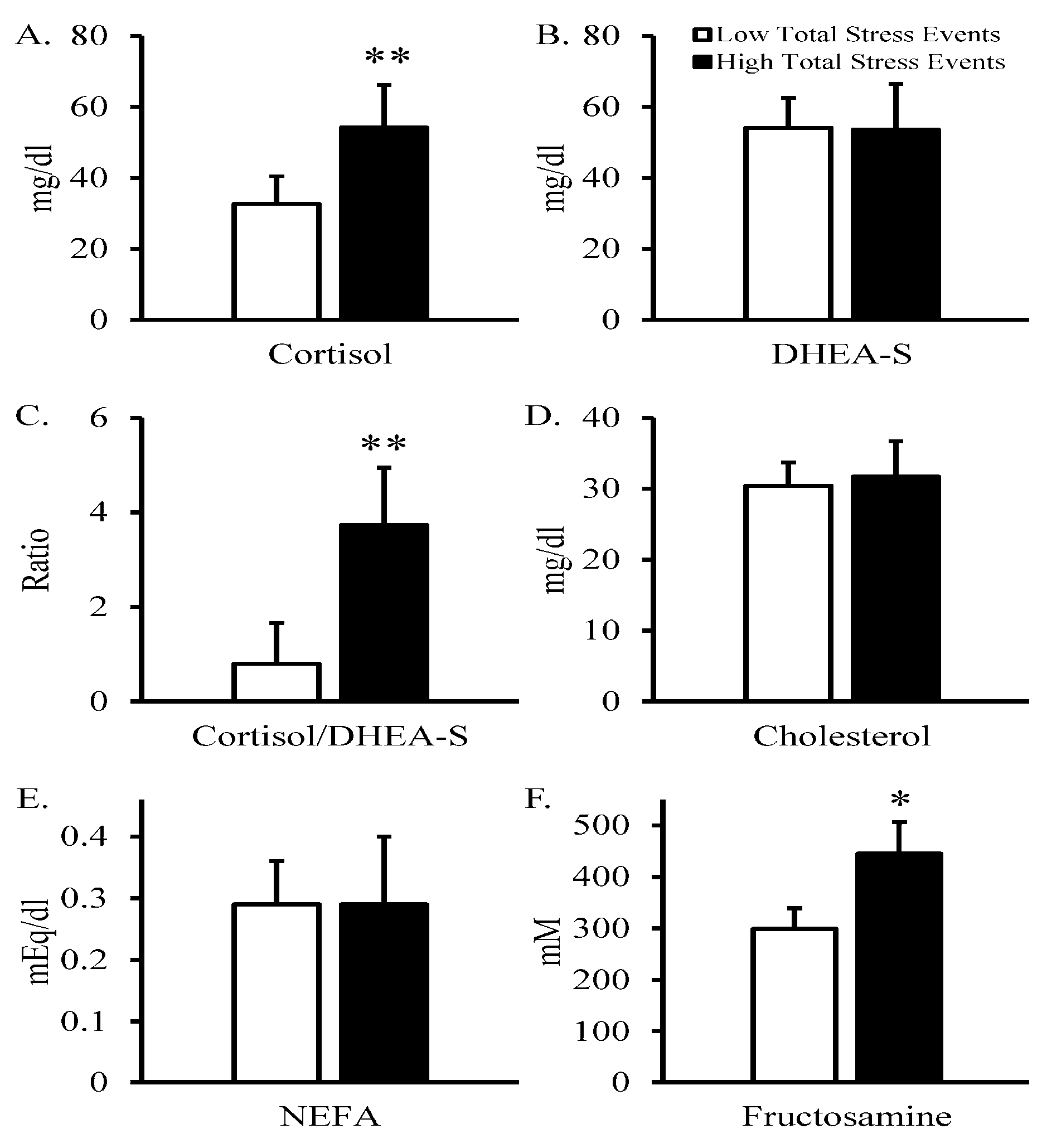
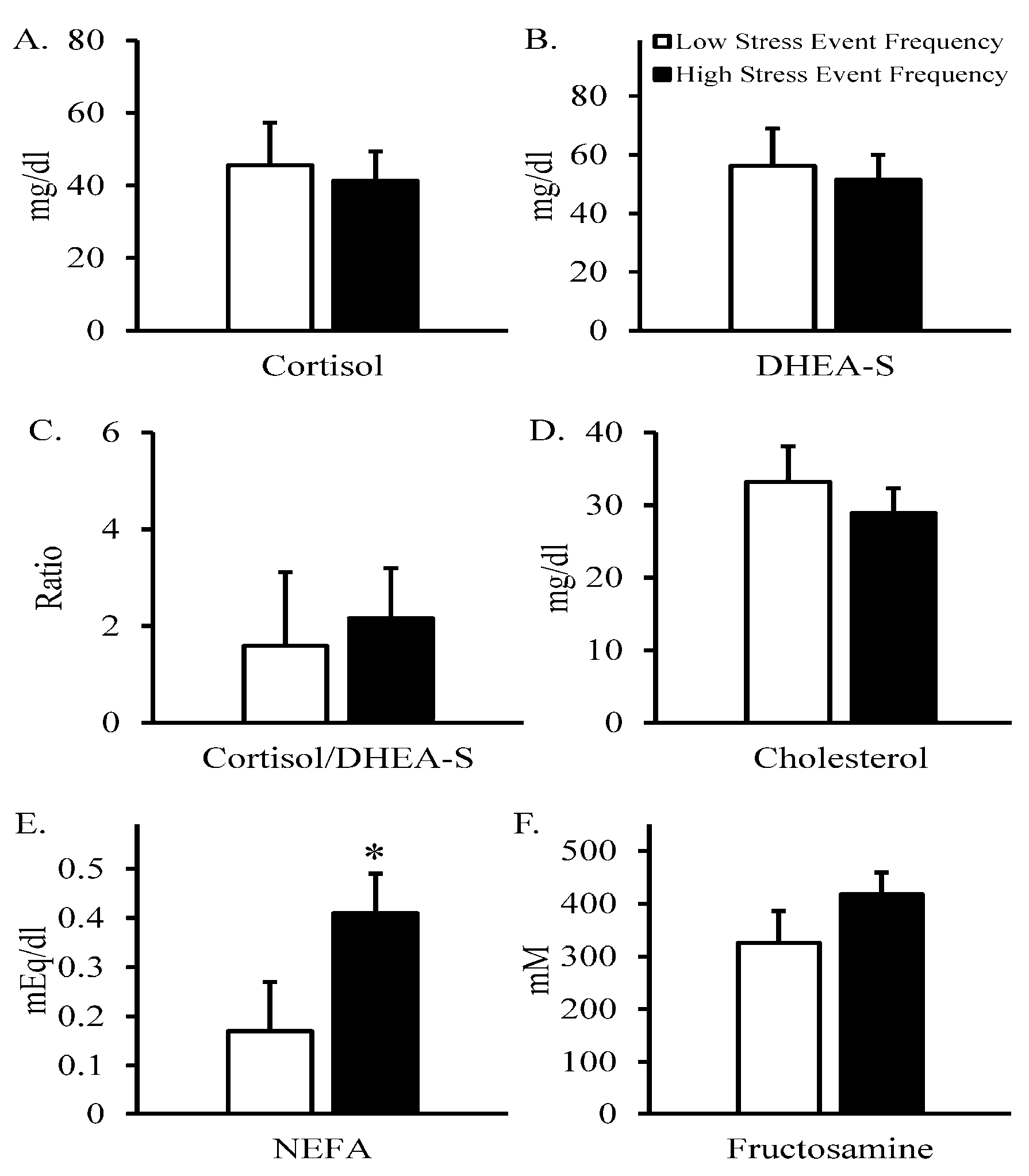


| Variable | N | Mean | Std. Error | Std. Dev. | Median | Minimum | Maximum |
|---|---|---|---|---|---|---|---|
| Age, years | 18 | 12.8 | 1.8 | 7.6 | 12.5 | 2 | 26 |
| BCS | 18 | 4.78 | 0.17 | 0.73 | 5 | 3 | 6 |
| Cortisol, mg/dL 1 | 18 | 35.8 | 6.3 | 26.7 | 25.6 | 6.8 | 99.6 |
| DHEA-S, mg/dL 1 | 18 | 46.7 | 6.4 | 27.3 | 53.3 | 5.4 | 89.4 |
| Cortisol/DHEA-S 1 | 18 | 1.77 | 0.76 | 3.21 | 0.71 | 0.1 | 13.3 |
| Cholesterol, mg/dL 1 | 18 | 29.7 | 3.3 | 14.1 | 31 | 4 | 54 |
| NEFA, mEq/L 1 | 18 | 0.31 | 0.05 | 0.20 | 0.23 | 0.07 | 0.68 |
| Fructosamine, mM 1 | 18 | 387 | 36 | 155 | 355 | 236 | 870 |
| Immobilizations 2 | 18 | 6.1 | 1.5 | 6.4 | 3 | 0 | 20 |
| Illnesses 2 | 18 | 4.8 | 0.5 | 2.2 | 4 | 2 | 9 |
| Pregnancies 2 | 18 | 0.72 | 0.23 | 0.96 | 0 | 0 | 3 |
| Transports 2 | 18 | 0.89 | 0.16 | 0.68 | 1 | 0 | 2 |
| Total Stress Events | 18 | 12.50 | 1.66 | 7.04 | 13.50 | 4 | 26 |
| Stress Events/Year | 18 | 1.07 | 0.13 | 0.57 | 0.92 | 0.3 | 2.2 |
| Allostatic Load | 18 | 1.44 | 0.29 | 1.25 | 1 | 0 | 5 |
| Variable | Variable | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Age | BCS | Cortisol | DHEA-S | Cortisol/ DHEA-S | Cholesterol | NEFA | Fructosamine | Allostatic Load | |
| Age | 0.29 | −0.26 | −0.41 | 0.28 | 0.35 | 0.00 | 0.19 | 0.16 | |
| NS | NS | 0.09 | NS | NS | NS | NS | NS | ||
| BCS | 0.29 | −0.06 | −0.23 | 0.11 | 0.06 | 0.24 | −0.09 | −0.21 | |
| NS | NS | NS | NS | NS | NS | NS | NS | ||
| Allostatic Load | 0.16 | −0.21 | 0.35 | −0.36 | 0.79 | 0.38 | 0.25 | 0.45 | |
| NS | NS | NS | NS | <0.01 | NS | NS | 0.06 | ||
| Immobilizations | 0.56 | −0.12 | 0.05 | −0.14 | 0.31 | 0.30 | 0.27 | 0.53 | 0.36 |
| 0.02 | NS | NS | NS | NS | NS | NS | 0.02 | NS | |
| Illnesses | 0.42 | 0.26 | −0.23 | −0.69 | 0.43 | 0.15 | 0.23 | 0.16 | 0.36 |
| 0.09 | NS | NS | <0.01 | 0.08 | NS | NS | NS | NS | |
| Pregnancies | 0.58 | 0.33 | −0.05 | −0.50 | 0.25 | −0.19 | 0.11 | 0.24 | 0.16 |
| 0.01 | NS | NS | 0.04 | NS | NS | NS | NS | NS | |
| Transports | 0.12 | 0.30 | 0.12 | −0.27 | 0.41 | −0.02 | 0.37 | −0.24 | 0.34 |
| NS | NS | NS | NS | 0.09 | NS | NS | NS | NS | |
| Total Stress Events | 0.73 | 0.05 | −0.02 | −0.44 | 0.49 | 0.29 | 0.37 | 0.54 | 0.49 |
| <0.01 | NS | NS | 0.07 | 0.04 | NS | NS | 0.02 | 0.04 | |
| Stress Events/Year | 0.47 | 0.34 | −0.29 | 0.09 | −0.13 | 0.23 | −0.42 | −0.15 | −0.24 |
| 0.05 | NS | NS | NS | NS | NS | 0.08 | NS | NS | |
| Variable | Variable | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Age | BCS | Cortisol | DHEA-S | Cortisol/ DHEA-S | Cholesterol | NEFA | Fructosamine | Allostatic Load | |
| Age | 0.30 | −0.26 | −0.41 | 0.04 | 0.33 | 0.10 | 0.42 | 0.10 | |
| NS | NS | 0.09 | NS | NS | NS | 0.09 | NS | ||
| BCS | 0.30 | −0.09 | −0.18 | 0.09 | 0.00 | 0.32 | −0.23 | −0.41 | |
| NS | NS | NS | NS | NS | NS | NS | 0.09 | ||
| Allostatic Load | 0.10 | −0.41 | 0.29 | −0.14 | 0.38 | 0.33 | 0.07 | 0.68 | |
| NS | 0.09 | NS | NS | NS | NS | NS | <0.01 | ||
| Immobilizations | 0.67 | −0.09 | 0.00 | −0.31 | 0.16 | 0.27 | 0.29 | 0.48 | 0.35 |
| <0.01 | NS | NS | NS | NS | NS | NS | 0.05 | NS | |
| Illnesses | 0.40 | 0.23 | −0.23 | −0.58 | 0.16 | 0.10 | 0.31 | 0.16 | 0.16 |
| 0.10 | NS | NS | 0.01 | NS | NS | NS | NS | NS | |
| Pregnancies | 0.54 | 0.27 | −0.05 | −0.39 | 0.27 | −0.16 | 0.23 | 0.31 | 0.03 |
| 0.02 | NS | NS | 0.10 | NS | NS | NS | NS | NS | |
| Transports | 0.09 | 0.31 | 0.07 | −0.18 | 0.31 | −0.03 | 0.38 | −0.19 | 0.24 |
| NS | NS | NS | NS | NS | NS | NS | NS | NS | |
| Total Stress Events | 0.77 | 0.05 | −0.12 | −0.38 | 0.09 | 0.23 | 0.39 | 0.45 | 0.26 |
| <0.01 | NS | NS | NS | NS | NS | NS | 0.06 | NS | |
| Stress Events/Year | 0.52 | 0.37 | −0.39 | 0.01 | −0.25 | 0.20 | −0.32 | 0.18 | −0.28 |
| 0.03 | NS | 0.10 | NS | NS | NS | NS | NS | NS | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beer, H.N.; Karr, L.K.; Shrader, T.C.; Yates, D.T. Allostatic Load Index Effectively Measures Chronic Stress Status in Zoo-Housed Giraffes. J. Zool. Bot. Gard. 2023, 4, 623-636. https://doi.org/10.3390/jzbg4030044
Beer HN, Karr LK, Shrader TC, Yates DT. Allostatic Load Index Effectively Measures Chronic Stress Status in Zoo-Housed Giraffes. Journal of Zoological and Botanical Gardens. 2023; 4(3):623-636. https://doi.org/10.3390/jzbg4030044
Chicago/Turabian StyleBeer, Haley N., Lisa K. Karr, Trenton C. Shrader, and Dustin T. Yates. 2023. "Allostatic Load Index Effectively Measures Chronic Stress Status in Zoo-Housed Giraffes" Journal of Zoological and Botanical Gardens 4, no. 3: 623-636. https://doi.org/10.3390/jzbg4030044
APA StyleBeer, H. N., Karr, L. K., Shrader, T. C., & Yates, D. T. (2023). Allostatic Load Index Effectively Measures Chronic Stress Status in Zoo-Housed Giraffes. Journal of Zoological and Botanical Gardens, 4(3), 623-636. https://doi.org/10.3390/jzbg4030044