Abstract
Insects are commonly utilized in biomedical research and have become increasingly popular in museum collections and as pets. Despite this, objective evaluation of insect euthanasia is scarce. This study investigated the effectiveness of targeted injections of ivermectin or potassium chloride (KCl) for the euthanasia of anesthetized thorny devil stick insects (Eurycantha calcarata). Ten clinically healthy mature insects (six males, four females) were enrolled. Insects were weighed and anesthetized via exposure to a cotton ball soaked with 1.6 mL of liquid isoflurane in a 1 L sealed chamber until loss of righting reflex and response to stimulation (induction). Insects then received one of three treatments: ivermectin 100 mg/kg (n = 4), KCl 200 mEq/kg (n = 4), or 0.9% sodium chloride 100 mL/kg (n = 2) injected along the ventral thoracic midline between the first leg plate and the caudal adjacent plate. Following injection, insects were serially monitored for return of spontaneous movement and righting reflex. Death was defined as the absence of spontaneous movement for 48 h. Median (range) induction time and isoflurane concentration at induction was 36 (22–39) min (n = 9) and 22 (19–22)%, respectively. Euthanasia was successful in 4/4, 3/4, and 0/2 isoflurane-anesthetized insects receiving ivermectin, KCl, or 0.9% sodium chloride, respectively. Recovery was prolonged at 10.5 (sodium chloride female), 11.0 (KCl male), and 18.0 (sodium chloride male) hours. This is the first prospective investigation of euthanasia in adult E. calcarata. In this preliminary study, ivermectin 100 mg/kg via ventral midline injection was effective for euthanasia of thorny devil stick insects.
1. Introduction
Insects are commonly encountered in managed care settings as display animals, research subjects, feeder animals, or pets. One insect often maintained under human care is the thorny devil stick insect (Eurycantha calcarata), a large, phytophagous insect of the family Phasmatidae. Thorny devil stick insects breed prolifically via both sexual reproduction and parthenogenesis and can live for up to 2 years; thus, surplus populations can arise rapidly in captive settings.
Emerging evidence supporting the potential for pain perception in insects has generated heightened awareness of their need for proper welfare and veterinary care, including appropriate means of euthanasia [1,2,3,4,5,6]. Despite this awareness and need, there is limited evidence-based data and guidance surrounding euthanasia methods in insects [6,7,8,9,10,11]. The American Veterinary Medical Association (AVMA) Guidelines for the Euthanasia of Animals groups insects within the broader category of captive invertebrates. As the term “invertebrates” encompasses more than 95% of animal species on earth and includes many distantly related phyla, blanket euthanasia recommendations within this grouping could result in suboptimal outcomes. Both one- and two-step methods for the euthanasia of captive invertebrates exist and these include injectable, inhaled, immersion, and/or physical techniques [7]. In clinical practice, most methods utilize anesthesia (step one) followed by physical destruction of nervous tissue (step two) to ensure irreversible loss of consciousness while minimizing potential for pain or distress [6]. While arguably effective, physical destruction can be visually unsettling, requires knowledge of the anatomic location of nervous tissue, and may limit additional use of cadaver tissue (necropsy, tissue preservation, etc.). Injectable techniques may overcome these potential challenges, and objective investigation of injectable euthanasia methods in invertebrates, specifically insects, is warranted.
Potassium chloride (KCl) is an effective cardioplegic solution in mammals, birds, reptiles, fish, and amphibians [12,13,14,15,16,17,18] and is considered an acceptable method of euthanasia in anesthetized or unconscious vertebrates [7]. Furthermore, KCl is not a federally controlled substance, is relatively affordable, and may be suitable for use in field settings due to lack of significant environmental residues [7,13]. In vertebrates, potassium is the most abundant intracellular cation and extracellular potassium levels are strictly regulated within a low and narrow range. Administration of exogenous potassium raises extracellular potassium concentrations, inactivates sodium channels, and results in slowed electrical conduction and myocardial arrest [6]. Preliminary investigations have begun to explore the use of KCl for euthanasia of invertebrates with largely good success [8,9,19]. In both American lobsters (Homarus americanus) and numerous terrestrial arthropod species, KCl was injected near the ventral nervous system ganglia with the intent to produce neuronal depolarization and death (“targeted hyperkalosis”) [8,9]. This included carbon dioxide-anesthetized E. calcarata nymphs (~2 g) which were successfully euthanized with an injection of a 10% w/v (400 mEq/kg) saturated KCl solution [9]. In blue crabs (Callinectes sapidus), an intracardiac injection of KCl was investigated and resulted in rapid loss of Doppler (heart) sounds (<60 s) and no recovery of consciousness [19]. Given the preliminary success of KCl in invertebrate species, further investigation for insect euthanasia is needed.
Ivermectin is a widely available avermectin antiparasitic agent with a variety of applications in both human and veterinary medicine. Mechanistically, it binds to and opens glutamate-gated chloride channels to hyperpolarize nerve and muscle cells subsequently inducing paralysis and death [20]. A wide body of literature supports its use as an antiparasitic [20,21] and recently it has been investigated as a novel invertebrate euthanasia agent. In eugenol-anesthetized blue crabs, ivermectin (5 mg/kg) administered via intracardiac injection resulted in clinically rapid circulatory arrest (<30 min) and irreversible unconsciousness [19]. Given its known mechanism of action, direct targeting of the nervous system (e.g., thoracic ganglia) may also be a viable route of administration.
To the authors’ knowledge, no previous studies have assessed euthanasia methods for adult thorny devil stick insects. The aim of this study was to evaluate targeted injections of potassium chloride or ivermectin for euthanasia of isoflurane-anesthetized adult thorny devil stick insects.
2. Materials and Methods
Ten, 13-month-old, clinically healthy thorny devil stick insects (six males, four females) were enrolled. Insects were obtained from a museum collection 7 months prior to the study and designated for depopulation. Insects were group-housed by sex in 36 × 24 × 36 cm enclosures lined with organic soil and coconut fiber (Eco Earth, Zoo Med Laboratories, San Luis Obispo, CA, USA) and maintained in a climate-controlled room (20–22 °C). Enclosures were misted with dechlorinated water daily. Blackberry brambles (Rubus spp.) were fed ad libitum and replaced at least once per week. No fasting period was implemented prior to the study. Invertebrates are not under ethical jurisdiction of the North Carolina State University Institutional Animal Care and Use Committee; however, standards comparable to those for the care and use of vertebrates were applied. At baseline, a physical examination, body morphometrics (weight, length), and assessment of ambulation and righting reflex were performed, the latter by manually placing the insect in dorsal recumbency. Righting reflex was considered absent if the insect did not right itself within 15 s. A baseline heart rate was obtained for each insect using a Doppler ultrasonic flow device (Model 811-B, Parks Medical Electronics Inc, Aloha, OR, USA) primed with ultrasound transmission gel (Aquasonic 100, Parker Laboratories, Inc, Fairfield, NJ, USA) and placed over the dorsum on midline at the level of the second and third tail plates. Heart rate was assessed by counting the number of beats over a 30 s period.
Following baseline data collection, insects were individually placed in ventral recumbency in a 1 L, airtight plastic container (LockNLock, Seocho, Seoul, Republic of Korea) with a cotton ball soaked with 1.6 mL of liquid isoflurane (Isoflurane USP, Piramal Enterprises Limited, Telangana, India). At 20 °C, isoflurane has a saturated vapor pressure of 238 mmHg and, at sea level (760 mmHg), this constitutes a saturated vapor concentration of approximately 31%. As 1 mL of liquid isoflurane produces approximately 195 mL of vapor, 1.6 mL of liquid isoflurane was chosen to target the maximum concentration of isoflurane (31%) able to be generated within the 1 L test container [22]. This dose was chosen based on pilot work in the authors’ laboratory. The cotton ball was separated from the insect by netting secured to the inside corner of the container. To intermittently sample intra-container gas (isoflurane, oxygen, carbon dioxide) concentrations, containers were modified prior to testing to include two airtight ports, one for sample removal and one for sample return. An 18-g hypodermic needle was used to pierce the container wall in two locations and two 20-g catheters (BD Insyte, Becton Dickinson Infusion Therapy Systems, Inc., Sandy, UT, USA) with attached injection caps (ICU Medical, Inc., San Clemente, CA, USA) were inserted into the holes and secured with glue (Figure 1).

Figure 1.
Airtight 1 L plastic container setup used for anesthetic induction of thorny devil stick insects (Eurycantha calcarata). To intermittently sample intra-container gas (isoflurane, oxygen, carbon dioxide) concentrations, containers were modified to include two airtight ports. An 18-g hypodermic needle was used to pierce the container wall in two locations and two 20-g catheters with attached injection caps were inserted into the holes and secured with glue. For induction, insects were individually placed in ventral recumbency, and a cotton ball soaked with 1.6 mL of liquid isoflurane was placed behind netting secured to an inside corner of the container.
Following placement in containers, insects were evaluated every 5 min for loss of spontaneous movement, defined as the absence of any movement for 15 consecutive seconds. If absent, the container was flipped upside down to position the insect in dorsal recumbency and the insect was again assessed for movement for 15 consecutive seconds. If present, the container was flipped to return the insect to ventral recumbency, and the above process was repeated at the next 5 min interval. If movement was absent, the container was tilted along its long axis at a 45-degree angle in both directions in succession to gently slide the insect within the container and provide an additional means of physical stimulation. The insect was observed for 15 consecutive seconds. If spontaneous movement was observed, the container was flipped to return the insect to ventral recumbency, and the entire process described above was repeated at the next 5 min interval. If movement was absent, the insect was deemed anesthetized and isoflurane, oxygen, and carbon dioxide concentrations within the container were recorded (Passport 12, Mindray, Mahwah, NJ, USA). Once anesthetized, insects were manually removed from the container, heart rate was assessed using the technique described above, and response to manual manipulation during heart rate collection was evaluated.
On the day of testing, powdered KCl (KaliSel, Morton Salt, Chicago, IL, USA) was added to sterile water (Sterile Water for Injection, B. Braun Medical Inc., Bethlehem, PA, USA) on a tared gram scale until saturation was reached (333 mg/mL, 4.3 mEq/mL). Insects were assigned to one of three treatment groups: ivermectin (10 mg/mL, ProMectin, Sparhawk Laboratories, Inc., Lenexa, KS, USA) at 100 mg/kg (n = 4), KCl (4.3 mEq/mL) at 200 mEq/kg (n = 4), or 0.9% sodium chloride (Fresemius Kabi, Lake Zurich, IL, USA) at 100 mL/kg (n = 2), the latter used as a control to mimic the largest volume of the other treatments (KCl). In light of the small sample size, at least one male and one female were assigned to each treatment group, but the individual insects within each sex were chosen at random. For administration, insects were placed in dorsal recumbency, and a 25-g needle attached to a 1 mL syringe containing the target treatment was inserted with the bevel facing upwards along the ventral midline between the first leg plate and the caudal adjacent ventral plate (Figure 2). This location was extrapolated from a previous study of E. calcarata nymph euthanasia and was chosen to target the ventral nerve cord [9]. After needle insertion, aspiration was performed to minimize potential for injection into the gastrointestinal tract. Injections were performed over 3–5 s and response to injection was recorded. A single investigator (ACH) performed all injections.
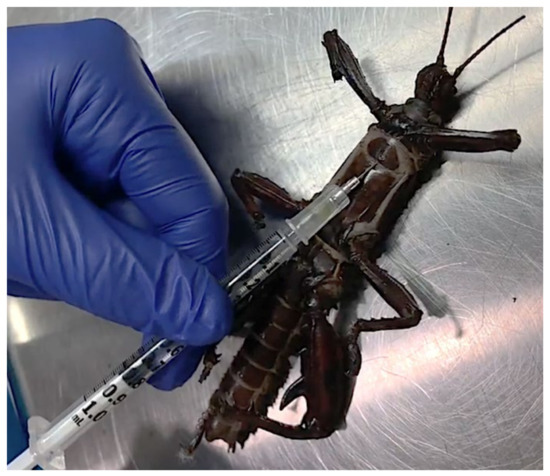
Figure 2.
Injection site used for administration of ivermectin, potassium chloride, or 0.9% sodium chloride in isoflurane-anesthetized adult thorny devil stick insects (Eurycantha calcarata). Insects were placed in dorsal recumbency, and a 25-g needle attached to a 1 mL syringe containing the assigned treatment was inserted with the bevel facing upwards along the ventral midline between the first leg plate and the caudal adjacent ventral plate. This location was extrapolated from a previous study of E. calcarata nymph euthanasia and was chosen to target the ventral nerve cord [9].
Following injection, insects were maintained in dorsal recumbency and monitored continuously for 2 h, after which, they were serially monitored at least once every hour for 10 h and then at least once every 8 h for 36 h (total 48 h) for adverse effects, resumption of spontaneous movement, and return of the righting reflex, the latter defined as recovery. Absence of spontaneous movement within the 48 h assessment period was considered synonymous with death. Any insect that recovered was monitored daily for 7 days for delayed morbidity or mortality.
3. Results
Euthanasia was successful in 4/4, 3/4, and 0/2 isoflurane-anesthetized thorny devil stick insects following injection of ivermectin, KCl, or 0.9% sodium chloride, respectively. All euthanized insects remained in dorsal recumbency throughout the 48 h assessment period. In recovered insects, time to resumption of righting reflex was prolonged at 10.5 (0.9% sodium chloride female), 11.0 (KCl male), and 18.0 (0.9% sodium chloride male) hours. All recovered insects remained clinically normal with intact righting reflexes throughout the 7 day assessment period.
Median (range) length and weight was 11 (10–11) cm and 16 (10–20) g, respectively, for males, and 13 (12–13) cm and 20 (19–22) g, respectively, for females. Median (range) baseline heart rate was 24 (18–30) bpm. Isoflurane exposure resulted in anesthetic induction in a median (range) time of 36 (22–39) min in all but one insect. In that insect (male, KCl), initial induction criteria were met at minute 18, but when manually removed from the container, the insect displayed spontaneous movement. The induction process for this insect was repeated; the previous cotton ball was removed and replaced with a fresh isoflurane-soaked cotton ball and the insect was returned to the container within 2 min of removal. At minute 19 of the second induction period (39 min from initial isoflurane exposure), the insect met induction criteria again and there was no response to manual removal from the container. Two insects in the ivermectin group regurgitated during isoflurane exposure. Median (range) isoflurane, oxygen, and carbon dioxide concentrations at induction were 22 (19–22)%, 16 (16–17)%, and 0 (0–1) mmHg, respectively. Doppler sounds were not detectable in any insect at induction and to avoid significant delay between removal from isoflurane exposure and injection, assessment time was capped at 60 s. Aspiration prior to injection was negative or yielded hemolymph in 4/10 and 6/10 insects, respectively. Median (range) injection volume was 0.18 (0.16–0.22), 0.75 (0.45–0.91), and 0.84 (0.73–0.95) mL for ivermectin, KCl, and 0.9% sodium chloride, respectively. No insect displayed any response to injection.
Regurgitation was observed in 2/4, 3/4, and 0/2 insects during or immediately following (<15 s) administration of ivermectin, KCl, and 0.9% sodium chloride, respectively; this included one insect in the ivermectin group which regurgitated during isoflurane exposure. The fluid was of similar color and clarity (clear, subjectively yellow-tinged) in all insects. While not quantified, subjective visual assessments of regurgitation volumes ranged from 0.05 to 0.5 mL per insect.
4. Discussion
Ivermectin at 100 mg/kg injected along the ventral midline between the first leg plate and caudal adjacent plate was effective for the euthanasia of isoflurane-anesthetized male and female thorny devil stick insects in the current study. To the authors’ knowledge, this is the first study to prospectively investigate ivermectin via targeted injection for euthanasia of an insect species. Although the small sample size of the current study warrants caution in overinterpretation of the findings, the positive results support its potential use for stick insect euthanasia and advocates for investigation in other terrestrial insects.
The ventral midline injection location in the current study was selected to target the thoracic ganglia, similar to a prior study investigating KCl in terrestrial arthropods [9]. The thoracic ganglia of the insect ventral nerve cord controls vital physiologic functions and, as such, it may be the most suitable target for injection of euthanasia agents [23,24]. While not definitively proven, given its known mechanism of action, the authors hypothesize that ivermectin hyperpolarized the thoracic ganglia and resulted in paralysis and irreversible unconsciousness in the stick insects in the present study [20]. As insects possess an open circulatory system in which hemolymph diffuses freely throughout body cavities, vital organ dysfunction from direct ivermectin exposure as a contributing mechanism of euthanasia cannot be ruled out.
Ivermectin is currently used as an antiparasitic in a large variety of veterinary species, making it both widely and easily accessible. It is not a controlled substance, and the commercial formulation (10 mg/mL) permits clinically practical injection volumes for insects (approximately 0.2 mL). The ivermectin dose in the current study is 20 times greater than the dose proven effective in blue crabs (5 mg/kg) [19] but was selected to optimize injection volume and maximize likelihood of proving proof of concept. The efficacy of lower doses of ivermectin in stick insects is unknown. Although not assessed in the current study, ivermectin may be sufficient as a single-step euthanasia agent in stick insects with the goal being an improvement in efficiency and a reduction in resources. To ensure animal welfare, as the potential for ivermectin-induced pain or distress in conscious invertebrates is unknown, the authors chose to use ivermectin following confirmation of anesthesia (unconsciousness).
KCl at 200 mEq/kg injected along the ventral midline between the first leg plate and caudal adjacent plate only resulted in euthanasia in a subset (3/4) of thorny devil stick insects in the current study. While not definitively proven, the authors suspect increased local concentrations of potassium hyperpolarized the thoracic ganglia, resulting in paralysis and irreversible unconsciousness in the affected individuals. In vitro studies have demonstrated that perineural potassium injections in insects can rapidly compromise nerve function, supporting this hypothesis [25,26,27]. As with ivermectin, a contribution from potassium diffusion throughout hemolymph and effects on other organs cannot be ruled out. Potential hypotheses for the lack of effectiveness in a single insect in the current study include inappropriate dose or technique failure. Of note, this insect had a comparable recovery time to the two control stick insects, no obvious morbidity, and the largest regurgitation volume (described in more detail below). A higher dose of KCl (>200 mEq/kg) might increase the reliability of this technique; however, this would translate to a larger injection volume (>0.5–1 mL) which could exceed the available space in the thoracic cavity and/or complicate tissue preservation. In thorny devil stick insect nymphs and other terrestrial arthropod species, a 400 mEq/kg dose was effective [9], whereas <15 mEq/kg was used in lobsters and blue crabs via intracardiac injection [8,19]. Thus, it is likely that there is variability in dosing effectiveness between species and injection sites. Of note, extracellular potassium concentrations in insect species, particularly those that are phytophagous, are higher than in vertebrates and extracellular potassium concentrations can vary enormously between insect orders [25,26,27,28,29,30]. As such, higher doses of KCl may be necessary for insect euthanasia compared to other taxa.
Due to anatomic and physiologic disparities between vertebrates and invertebrates, particularly regarding the nervous, circulatory, and respiratory systems, confirming death in insect species presents unique challenges [6,7,8,9,10,11]. Although Doppler (heart) sounds were detectable in all stick insects at baseline in the current study, these sounds were undetectable at the time of anesthetic induction, in both euthanized and recovered stick insects. As such, and to avoid repeated stimulation during the post-injection period, Doppler sounds were not subsequently assessed. Thus, Doppler sound presence or absence in the post-injection period was not an available indicator of the status of the circulatory system in the current study and correlation of nervous and cardiovascular system dysfunction in insects is unknown. As other advanced monitoring techniques have not been designed for use in insects in clinical settings, the authors relied on an extended post-treatment monitoring period and lack of recovery to confirm irreversible unconsciousness.
Regurgitation occurred in six stick insects during isoflurane anesthesia and/or the peri-injection period. The regurgitated fluid was of similar color and clarity in all insects at all time points, but of variable volume. While not suspected, inadvertent insertion of the needle into the gastrointestinal tract and an iatrogenic contribution to the regurgitation cannot be ruled out. Of note, the single insect in the KCl group that recovered subjectively had the largest volume of regurgitation; thus, technique failure may have played a role in this insect’s outcome. In the remaining insects, any degree of potential injection failure did not affect the ultimate outcome. While appropriate anesthetic fasting times for stick insects are unknown, fasting prior to anesthesia may reduce the incidence or degree of regurgitation in this species. To avoid potential adverse metabolic effects from fasting and to mimic a clinical situation, no pre-anesthetic fast period was implemented in the present study.
Loss of both spontaneous movement and response to stimulation, defined as anesthetic induction, was produced by isoflurane exposure in thorny devil stick insects in the current study. The technique (exposure to an isoflurane-soaked cotton ball in an airtight container) was easy to employ, repeatable between subjects, required minimal equipment, and allowed for higher concentrations of isoflurane than can be obtained with a commercial vaporizer. Time to induction was clinically practical (approximately 20–40 min) and this milestone was achieved at a similar isoflurane exposure concentration in all insects (approximately 22%). Previous investigations of isoflurane anesthesia in insects (Madagascar hissing cockroach [Gromphadorhina portentosa], Budwing praying mantis [Parasphendale affinis]) reported recovery times of less than 30 min; however, this was following exposure to isoflurane concentrations of 5% [31,32]. Pilot work in the authors’ laboratory demonstrated that exposure to 5% isoflurane did not produce anesthesia in thorny devil stick insects in a clinically practical time frame (<1 h); thus, a higher concentration was used. The prolonged recovery times (>10 h) observed in the current study supports a dose-dependent effect in insects with higher doses of isoflurane resulting in longer recovery times. As the ultimate goal of the tested techniques is irreversible unconsciousness, recovery time is arguably irrelevant, and priority should be given to doses which produce the most rapid induction times. However, as the only clinically practical marker of technique failure is return of consciousness, a prolonged time to recovery could be a complicating factor for confirming technique effectiveness and should be taken into consideration in future insect euthanasia studies.
Exposure to a liquid isoflurane-soaked cotton ball has been anecdotally used as a single-step method of euthanasia in invertebrates; however, an approximately 30–40 min exposure period using this technique was non-lethal and resulted in significantly prolonged recovery times (>10 h) in thorny devil stick insects in the current study. While it is possible that longer isoflurane exposure periods could yield different results, investigators advise caution against the use of this single-step technique for stick insect euthanasia, particularly given the challenges associated with differentiating between prolonged anesthetic recovery and irreversible unconsciousness in invertebrates [7]. Given the lack of species-specific evidence supporting use of this technique in other insects, single-step isoflurane overdose may not be appropriate in other insect species as well [7].
Limitations of this study included a dearth of clinical indicators of death in insects, a small sample size, unknown optimal dosing, and intermittent monitoring of subjects beyond the initial 2 h assessment period. As insects were not continuously monitored for the entire 48 h period, theoretically, an insect could have transiently recovered between assessment periods. Given that all successfully euthanized insects were observed in dorsal recumbency at each assessment time point, this scenario appears unlikely. The small sample size of this study did not allow for determination of optimal dosing of either drug and, as discussed above, should promote caution with interpretation of results. However, as objective assessments of euthanasia in invertebrates, particularly stick insects, are extremely limited, the value of this research remains evident. Furthermore, these preliminary results may be used to guide future euthanasia studies and help advance the standard of care for insect species.
5. Conclusions
Ivermectin (100 mg/kg) injected into the ventral thorax was effective for euthanasia of isoflurane-anesthetized thorny devil stick insects. Investigation of this technique using a larger sample size and for euthanasia of other invertebrate species is warranted. Injected potassium chloride (200 mEq/kg) demonstrates potential for euthanasia of isoflurane-anesthetized thorny devil stick insects but was not reliable in the present study.
Author Contributions
Conceptualization, A.C.H., M.A.G., G.A.L. and J.A.B.; methodology, A.C.H. and J.A.B.; formal analysis, A.C.H. and J.A.B.; investigation, A.C.H., M.A.G. and J.A.B.; resources, G.A.L. and J.A.B.; data curation, A.C.H., M.A.G. and J.A.B.; writing, A.C.H., M.A.G., G.A.L. and J.A.B.; visualization, A.C.H., M.A.G., G.A.L. and J.A.B.; supervision, G.A.L. and J.A.B.; project administration, A.C.H., M.A.G., G.A.L. and J.A.B.; funding acquisition, G.A.L. and J.A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Institutional ethical review and approval were waived for this study because invertebrates are not under ethical jurisdiction of the North Carolina State University Institutional Animal Care and Use Committee. Despite this, standards comparable to those for the care and use of vertebrates were applied and all procedures were performed under the supervision of a veterinarian (J.A.B.).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available via the corresponding author upon reasonable request.
Acknowledgments
The authors thank Taylor Gregory, Andrew Lathan, Kent Passingham, the North Carolina State University Turtle Rescue Team, and Discovery Place Science for study assistance and support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Drinkwater, E.; Robinson, E.J.; Hart, A.G. Keeping invertebrate research ethical in a landscape of shifting public opinion. Methods Ecol. Evol. 2019, 10, 1265–1273. [Google Scholar] [CrossRef]
- Elwood, R.W. Pain and suffering in invertebrates? ILAR J. 2011, 52, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, M.; Crump, A.; Barrett, M.; Sarlak, S.; Birch, J.; Chittka, L. Can insects feel pain? A review of the neural and behavioural evidence. Adv. Insect Physiol. 2022, 63, 155–229. [Google Scholar]
- Mutinelli, F. Euthanasia and welfare of managed honey bee colonies. J. Apic. Res. 2021, 2021, 2–10. [Google Scholar] [CrossRef]
- Walters, E.T. Nociceptive biology of molluscs and arthropods: Evolutionary clues about functions and mechanisms potentially related to pain. Front. Physiol. 2018, 9, 1049. [Google Scholar] [CrossRef]
- Murray, M.J. Euthanasia. In Invertebrate Medicine, 3rd ed.; Lewbart, G.A., Ed.; John Wiley & Sons: New York, NY, USA, 2022; pp. 645–650. [Google Scholar]
- American Veterinary Medical Association. AVMA Guidelines for the Euthanasia of Animals, 2020 ed.; AVMA: Schaumburg, IL, USA, 2020; pp. 90–91. [Google Scholar]
- Battison, A.; MacMillan, R.; MacKenzie, A.; Rose, P.; Cawthorn, R.; Horney, B. Use of injectable potassium chloride for euthanasia of American lobster (Homarus americanus). Comp. Med. 2000, 50, 545–550. [Google Scholar]
- Bennie, N.A.; Loaring, C.D.; Bennie, M.M.; Trim, S.A. An effective method for terrestrial arthropod euthanasia. J. Exp. Biol. 2012, 215, 4237–4241. [Google Scholar] [CrossRef]
- Cooper, J.E. Anesthesia, analgesia, and euthanasia of invertebrates. ILAR J. 2011, 52, 196–204. [Google Scholar] [CrossRef]
- Cooper, J.E.; Pellet, S.; O’Brien, M. Insects. In Invertebrate Medicine, 3rd ed.; Lewbart, G.A., Ed.; John Wiley & Sons: New York, NY, USA, 2022; pp. 413–437. [Google Scholar]
- Ascher, J.M.; Bates, W.; Ng, J.; Messing, S.; Wyatt, J. Assessment of xylazine for euthanasia of anoles (Anolis carolinensis and Anolis distichus). J. Am. Assoc. Lab. Anim. Sci. 2012, 51, 83–87. [Google Scholar]
- Harms, C.A.; McLellan, W.A.; Moore, M.J.; Barko, S.G.; Clarke, E.O., III; Thayer, V.G.; Rowles, T.K. Low-residue euthanasia of stranded mysticetes. J. Wildl. Dis. 2014, 50, 63–73. [Google Scholar] [CrossRef]
- Knutson, K.; Petritz, O.; Womble, M.; Lewbart, G.A.; Balko, J.A. Evaluation of euthanasia methods using injectable agents in leopard geckos (Eublepharis macularius). J. Herpetol. Med. Surg. 2022, 32, 35–41. [Google Scholar] [CrossRef]
- Louis, M.M.; Houck, E.L.; Lewbart, G.A.; Posner, L.P.; Balko, J.A. Evaluation of potassium chloride administered via three routes for euthanasia or anesthetized koi (Cyprinus carpio). J. Zoo Wildl. Med. 2020, 51, 485–489. [Google Scholar] [CrossRef]
- Louis, M.M.; Scott, G.; Smith, D.; Troan, B.V.; Minter, L.J.; Balko, J.A. Investigation of potassium chloride for euthanasia of anesthetized marine toads (Rhinella marina). J. Herpetol. Med. Surg. 2022, 32, 42–47. [Google Scholar] [CrossRef]
- Raghav, R.; Taylor, M.; Guincho, M.; Smith, D. Potassium chloride as a euthanasia agent in psittacine birds: Clinical aspects and consequences for histopathologic assessment. Can. Vet. J. 2011, 52, 303. [Google Scholar]
- Whitmer, E.R.; Trumbull, E.J.; Harris, H.S.; Whoriskey, S.T.; Field, C.L. Use of potassium chloride for low-residue euthanasia of anesthetized California sea lions (Zalophus californianus) and northern elephant seals (Mirounga angustirostris) with life-threatening injury or disease. J. Am. Vet. Med. Assoc. 2021, 259, 197–201. [Google Scholar] [CrossRef]
- Mones, A.B.; Heniff, A.C.; Harms, C.A.; Balko, J.A. Evaluation of intracardiac administration of potassium chloride, ivermectin, or lidocaine hydrochloride for euthanasia of anesthetized blue crabs (Callinectes sapidus). J. Zoo Wildl. Med. 2022, 53, 689–695. [Google Scholar] [CrossRef]
- Papich, M.G. Papich Handbook of Veterinary Drugs, 5th ed.; Elsevier Health Sciences: St. Louis, MO, USA, 2021; pp. 484–488. [Google Scholar]
- Õmura, S.; Crump, A. The life and times of ivermectin—A success story. Nat. Rev. Microbiol. 2004, 2, 984–989. [Google Scholar] [CrossRef]
- Biro, P. Calculation of volatile anesthetics consumption from agent concentration and fresh gas flow. Acta Anaesthesiol. Scand. 2014, 58, 968–972. [Google Scholar] [CrossRef]
- Niven, J.E.; Graham, C.M.; Burrows, M. Diversity and evolution of the insect ventral nerve cord. Annu. Rev. Entomol. 2008, 53, 253–271. [Google Scholar] [CrossRef]
- Smarandache-Wellmann, C.R. Arthropod neurons and nervous system. Curr. Biol. 2016, 26, R960–R965. [Google Scholar] [CrossRef]
- Hoyle, G. High blood potassium in insects in relation to nerve conduction. Nature 1952, 169, 281–282. [Google Scholar] [CrossRef] [PubMed]
- Hoyle, G. Potassium ions and insect nerve muscle. J. Exp. Biol. 1953, 30, 121–135. [Google Scholar] [CrossRef]
- Leiserson, W.M.; Keshishian, H. Maintenance and regulation of extracellular volume and the ion environment in Drosophila larval nerves. Glia 2011, 59, 1312–1321. [Google Scholar] [CrossRef] [PubMed]
- Dow, J.A. The essential roles of metal ions in insect homeostasis and physiology. Curr. Opin. Insect Sci. 2017, 23, 43–50. [Google Scholar] [CrossRef]
- Wyatt, G.R. The biochemistry of insect hemolymph. Annu. Rev. Entomol. 1962, 6, 75–102. [Google Scholar] [CrossRef]
- Natochin, Y.V.; Parnova, R.G. Osmolality and electrolyte concentration of hemolymph and the problem of ion and volume regulation of cells in higher insects. Comp. Biochem. Physiol. Part A Physiol. 1987, 88, 563–570. [Google Scholar] [CrossRef]
- D’Ovidio, D.; Monticelli, P.; Adami, C. Isoflurane anesthesia in budwing praying mantises (Parasphendale agrionina). J. Zoo Wildl. Med. 2021, 52, 1013–1017. [Google Scholar] [CrossRef]
- McCallion, K.; Petersen, K.; Dombrowski, D.S.; Christian, L.S.; Lewbart, G.A.; Dillard, J. Isoflurane anesthesia in the Madagascar hissing cockroach (Gromphadorhina portentosa). J. Zoo Wildl. Med. 2021, 52, 710–714. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).