Abstract
Zoological institutions aim to continually improve the lives of the animals under their stewardship. To this end, bull elephants are now increasingly maintained in all-male groups to mimic social conditions observed in the wild. While cortisol is the most frequently used “stress” biomarker, secretory immunoglobulin A (sIgA) as a measure of health and positive affect, and the social hormone, oxytocin, are increasingly viewed as additional markers of welfare. The introduction of a pair of bull elephants to an existing group of three bull elephants at Denver Zoo presented an opportunity to assess sIgA, oxytocin and cortisol in response to the socialization process. In this study, sIgA varied greatly between individuals and did not correlate with cortisol but did correlate with salivary oxytocin. sIgA and oxytocin concentrations differed the most between social and solo situations during the introduction period compared to before bulls were introduced, and after a stable group had been formed. In contrast to findings in some species, sIgA and oxytocin were higher when housed alone than socially. Nonetheless, these results suggest that sIgA and oxytocin may be involved in social engagement and establishment of new social dynamics, and thus provide more insight into overall welfare states.
Keywords:
bull; cortisol; Elephas maximus; glucocorticoids; musth; oxytocin; secretory IgA; social; stress; welfare 1. Introduction
As advocates for ex situ animals, we aim to continually improve their wellbeing, which necessitates the continuous assessment of outcomes of the provided care. Everyone agrees that positive wellbeing is a great concept; however, ways to accurately measure it lack consensus. Historically, glucocorticoids (GC) from the adrenal cortex, and cortisol in particular, have been considered “stress hormones,” with measurable increases equated with poor welfare [1,2]. However, from a physiological standpoint, the primary role of GCs is energy regulation and mobilization. The hypothalamic-pituitary-adrenal (HPA) axis is activated in response to both physiological and psychological challenges [3] but does not have an intrinsic valence of a negative or positive experience [4]. That is, elevated GCs do not always equate to “stress” in the sense of poor wellbeing [1], but rather can be associated with normal life events like seasonal and reproductive changes, including rut/musth, pregnancy, lactation, parturition, migration, and spawning [5,6,7,8,9,10,11,12].
The adaptive stress response is associated with a variety of physiologic processes that affects cardiovascular, inflammatory, and immune function, and enables a shift in energy balance to allow the body to respond appropriately to imminent danger, followed by a return to homeostasis [13]. Thus, normal adrenal cortisol responses reflect transient situations. By contrast, chronic elevations are maladaptive and can result in poor health outcomes over an individual’s life, referred to as the allostatic load [14,15]. Because changes in cortisol simply reflect responses to a stimulus, assessing additional systems can provide a more integrative context within which to interpret results [1]. For example, combining cortisol with behavior data showed that fecal GC metabolite concentrations were paradoxically lower in elephants exhibiting stereotypy, leading to the new interpretation that stereotypy may be a coping mechanism that aims to reduce physiological stress [16].
Social bonds are a cornerstone of life for elephants, without which wellbeing is compromised [17]. In the wild, these bonds are foundational, especially for young elephants that must develop necessary social skills and learn how to interact with other members of the herd. One study found that wild adolescent African elephant (Loxodonta africana) males (10–20 years of age) were the most social age group compared to juveniles and adult males [18]. It has been further shown that all-male groups are an important and natural part of elephant social life [18,19,20,21,22].
In the U.S., the number of Association of Zoos and Aquariums-accredited facilities holding elephants has decreased [23]. However, the number of male elephants in the population has increased as a result of more breeding success and a 1:1 birth sex ratio [24]. This has led to a pressing need to house multiple mature bulls together. In 2016, Prado-Oviedo et al. [25] found that 43% (29/68) of zoos housed bulls, with 38% (11/29) holding more than one. In 2020, 57% (17/30) of institutions holding Asian elephants housed bulls, with 65% (11/17) holding more than one. For institutions holding African elephant bulls, 72% (26/36) housed one, while 46% (12/26) housed more than one [26,27].
Socializing all-male elephant groups in zoos is challenging because they regularly experience musth, a period of time of heightened aggression that makes agonistic interactions between elephants, as well as with staff, more likely [28,29,30]. Such behavior combined with the large size of adult bulls are just two reasons why few zoos choose to maintain all-male groups. Allowing social opportunities is associated with positive wellbeing, but introductions are not necessarily free from social conflict as evidenced by behavioral data [31,32,33]. Every time an elephant is transferred, individuals need to establish new social networks. Resolving conflicts that arise from social interactions is often associated with increased GCs [33,34], but there is a need for a suite of biomarkers to better understand the implications of socializing bulls and to evaluate how socialization efforts affect individual bull wellbeing.
Understanding individual physiological responses to these important management events can help mitigate any long-term stress for these animals. One such measure is secretory immunoglobulin A (sIgA), a primary immune protein on mucosal surfaces that plays an important role in humoral immunity, neutralizing pathogens on mucosal surfaces and providing the body with a first line of defense. More recently, an active role in triggering inflammation has also been documented for sIgA in humans [35]. As a measure of wellness, sIgA decreases during prolonged periods of stress, and is suggested to be a measure of long-term positive affect [4]. A negative relationship between sIgA and cortisol has been observed in dogs [36]. However, this relationship is not always clear. These biomarkers were studied in female elephants used for tourist activities. The concentrations of sIgA varied significantly with respect to camp size, activities, and seasonality, but not always in the predicted direction, and there was no clear association between IgA values from feces and saliva [37]. Additionally, while the physiologic effect of social change on sIgA concentrations has been studied in female elephants [37,38], similar studies in males are lacking.
Another potential measure of stress is oxytocin. Initially known for its role in parturition and lactation, today it is recognized for its involvement in complex behaviors, such as social recognition, pair bonding, anxiety, and maternal behaviors [39,40]. Thus, oxytocin concentrations may increase in elephants as a means to manage social stressors within a group [41,42]. Denver Zoo managed a group of three male elephants that were socially integrated when two younger half-brothers arrived in the fall of 2018 [31,32]. The aim of this study was to better understand how sIgA relates to cortisol and oxytocin within the context of social introductions and establishment of new relationships within this group of five male Asian elephants.
2. Materials and Methods
2.1. Study Animals
Denver Zoo managed a well-integrated group (integrated since 2016) of three unrelated Asian elephant bulls (Bulls 1–3), to which two younger bulls (half-brothers) were introduced [31,32]. The half-brothers (Bulls 4 and 5) arrived together in September 2018. Information on the study animals is given in Table 1 and additional details on the integration can be found in previous publications [31,32]. Bulls were managed in a 2.7-acre (1.1 hectare) area divided into five main yards and indoor stalls. The study was divided into three 5-month periods: (a) before introductions 6 August 2018–11 February 2019; (b) during introductions 12 February 2019–15 July 2019; (c) after introductions 6 August 2019–21 December 2019. Bulls were housed socially in varying combinations (social) or alone (solo) during the introduction period [31,32]. The quarantine and “howdy” (visual but not physical contact) periods were not included because the animals did not have physical contact, but could hear, smell and intermittently see each other.

Table 1.
Demographic data and serum and saliva samples collected in bull Asian elephants during socialization of two all-male groups.
2.2. Musth
Musth was defined by keepers based on presence of temporal gland secretions, urine dribbling or an increase in aggressive behaviors [43] and noted daily in the animal log as being in musth or not in musth.
2.3. Sample Collection
Using training and protected contact, keepers collected voluntary weekly blood samples from the bulls. The samples were centrifuged after clotting to collect serum that was stored at −80 °C until analysis. Weekly saliva samples were also collected voluntarily, but due to logistics and musth status saliva collection did not always occur on the same day as serum collection. Saliva samples were collected with the SalivaBio® system (Salimetrics LLC, Carslbad, CA, USA) by placing the swab between the cheek and the teeth with long tongs. When insufficient saliva was collected, a second session on the same day was used to collect additional saliva, which was combined with the earlier sample. Saliva and serum samples were stored at −80 °C until analysis. Samples were collected between 9am and 3pm depending on staffing and other animal needs.
2.4. Hormone Immunoassays
Salivary immunoglobulin A was quantified by an enzyme immunoassay (EIA) previously validated for elephants [44]. The substrate procedures were slightly modified as follows to improve assay consistency. Briefly, one phosphate-citrate capsule (Sigma P-4922) was broken, and contents were allowed to dissolve in 100 mL of distilled water for 10 min in a dark cabinet. Two high kinetic 3,3′,5,5′-tetramethylbenzidine (TMB) peroxidase (Sigma T-3405) tablets were dissolved in 20 mL of this phosphate-citrate buffer per plate in the dark. 200 µL of the substrate solution was added to all the wells and covered and allowed to incubate in the dark at room temperature for 10–12 min. A polyclonal rabbit anti-human IgA antibody (A0262, Dako, Glostrup, Denmark) was diluted to a working concentration of 10 mg/L in phosphate-buffered saline (0.01 M phosphate buffer, 0.15 M NaCl, pH 7.2) and 100 μL added per well to a 96-well microtiter plate (Costar, Corning Life Sciences, Tewkesbury, MA, USA). After incubation overnight at 4 °C, plates were aspirated and washed three times with PBS-T. Standards (0.39–100 µg/L), high and low concentration controls made using sIgA from human colostrum (I2636, Sigma Aldrich, St. Louis, MO, USA), and biological samples diluted as (add dilutions) were added in duplicate (50 µL). Following incubation at room temperature (RT) for 2 h on a plate shaker set to 500 RPM, plates were aspirated and washed three times with PBS-T. A polyclonal rabbit anti-human IgA antibody conjugated to horseradish peroxidase (HRP; P0216, Dako, Glostrup, Denmark) was diluted 1:2000 in PBS-T and 100 µL added per well before incubation at RT for 1 h on a plate shaker set to 500 RPM. After a final (3 times) wash step, 200 μL of the TMB substrate was added per well and incubated in the dark for 10 min at RT. Finally, the reaction was stopped with 50 μL stop solution (1N HCl) and the absorbance measured at 450 nm with a reference of 570 nm using a microplate reader (Filtermax F5, Molecular Devices, Sunnyvale, CA, USA). Samples were run in duplicated and diluted 1:200 to run on the assay. The assay sensitivity was 3.12 µg/mL, and the inter-assay coefficients of variation were <10% (12 assays).
A double antibody cortisol EIA was validated for ether extracted serum for this study. The assay utilizes anti-cortisol antisera (R4866 antisera, Coralie Munro, University of California, Davis), secondary anti-rabbit IgG from Arbor Assays, cortisol horseradish peroxidase label (C. Munro) and cortisol standards. The assay was validated demonstrating that serial dilutions of ether extracted serum pools were parallel to the standard curve (y = 1.06x + 1.67, R2 = 0.996, F1,5 = 1624.37, p < 0.001). This indicates that the antibody recognizes cortisol from ether extracted serum proportionally to the synthetic standard curve. Significant recovery (>85% and <115%) of standard hormone added to saliva pools was also demonstrated (y = 1.0557x + 0.3383, R² = 0.9994), showing there was no matrix interference. Samples were ether extracted in a 1:5 ether dilution, vortexed for 60 s and layers were allowed to separate for 10 min in a −80 °C freezer. The ether was decanted from frozen serum and evaporated to dryness. Extracted steroids were reconstituted in volume of assay buffer equal to volume of serum extracted (i.e., neat). Extracted samples were run in duplicate at varying dilutions from neat to 1:160 in the assay buffer. Assay sensitivity was 3.90 ng/mL, and the inter-assay variation was <10% (12 assays).
The salivary oxytocin EIA was measured using a commercial kit (Oxytocin ELISA, ADI-901-153, Enzo Life Sciences, Farmingdale, NY, USA), previously validated for elephant serum and urine [41]. The assay was validated for saliva by demonstrating that serial dilutions of saliva pools were parallel to the standard curve (y = 0.84x + 21.87, R2 = 0.99, F1,2 = 184.74, p= 0.005). This indicates that the antibody recognizes salivary oxytocin proportionally to the synthetic standard curve. Significant recovery (>85% and <115%) of standard hormone added to saliva pools was also demonstrated (y = 0.6443x + 0.5847, R² = 0.9979), showing there was no matrix interference. Samples were run neat in duplicate following kit instructions. The assay sensitivity was 15.6 pg/mL, and the inter-assay coefficients of variation were <11% (16 assays).
2.5. Statistical Methods
Analysis was performed with commercially available software (PRISM, GraphPad Software, San Diego, CA 92108, USA). Data did not pass normality tests (D’Agostino-Pearson), so non-parametric analyses were used, and summary statistics report median and interquartile ranges. Comparisons of analyte concentrations across different periods were performed with one-way ANOVA with Kruskal-Wallis multiple comparisons; comparisons between two groups were performed with Mann-Whitney test. For some of the correlations serum concentration of testosterone was used as a proxy for musth.
3. Results
Demographic data for the five bulls and the number of samples obtained at the end of the 17-month study can be found in Table 1.
3.1. Descriptive Statistics
From the collected saliva and serum, data points for sIgA (n = 355), salivary oxytocin (n = 356), cortisol (n = 283), and testosterone (n = 283) were available for comparison from the five bulls (Table 1). Bulls 1–3 underwent musth during the study period and it was not always possible to obtain samples during this period, while Bulls 4 and 5 were too young to experience musth. Bulls 4 and 5 had limited saliva samples (see Table 1) from the period before introductions as they were not trained for saliva collection until after arrival in Denver. Summary statistics of the complete dataset are listed in Table 2. Using a correlation matrix of all data points combined, a significant correlation between sIgA and salivary oxytocin, but not sIgA and serum cortisol was observed. Both sIgA and serum cortisol were negatively correlated with social situations compared to when they were housed alone (solo) (Table 3).

Table 2.
Summary of descriptive statistics for secretory IgA, salivary oxytocin, and serum cortisol in bull Asian elephants during socialization of two all-male groups.

Table 3.
Correlation matrix of secretory immunoglobulin A, salivary oxytocin, and serum cortisol in bull Asian elephants during socialization of two all-male groups.
3.2. Secretory IgA Concentration
There were significant differences in overall sIgA concentrations among the bulls (p < 0.0001; Figure 1), with the oldest bull having a significantly higher median concentration of sIgA over the study period compared to the other four bulls.
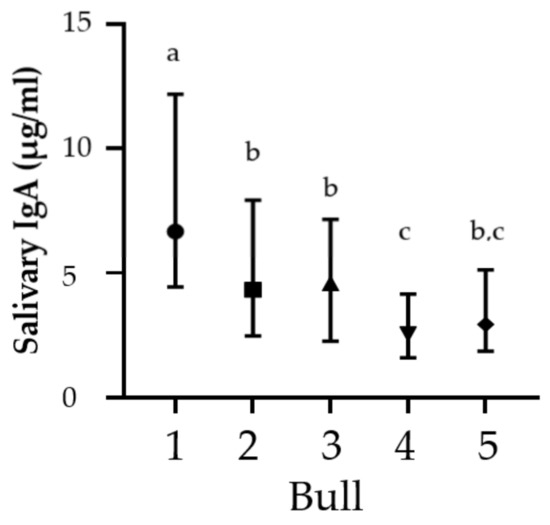
Figure 1.
Median (and interquartile ranges) of secretory IgA concentrations for five bulls over the full study period. Superscript letters indicate significant differences Kruskal–Wallis test (p < 0.05).
All bulls varied in sIgA concentrations as evidenced by a wide range of sIgA concentrations. No differences in sIgA concentrations were detected across the three periods (before, during and after introductions) for Bulls 1, 2 and 3 (p = 0.4673, p = 0.2531, p = 0.1228, respectively). However, there were significant differences for Bulls 4 and 5 (p < 0.0005, and p = 0.0005, respectively) (Figure 2). Bull 4 had significantly higher sIgA concentrations after introductions than before or during introductions (p = 0.0054 and 0.0045, respectively). Bull 5 had higher sIgA concentrations after introductions than during introductions (p = 0.0003). Figure 3 illustrates a progressive increase in sIgA concentration over time for bulls 4 and 5.
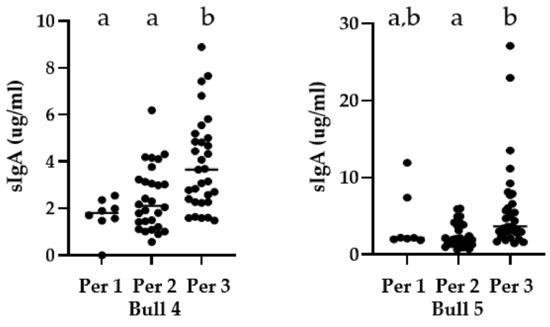
Figure 2.
Bull Asian elephant median secretory IgA concentrations for Bulls 4 and 5 over three periods (before, during and after introductions). Different superscripts (a,b) indicate statistically significant differences (p < 0.05). Note the difference in y-axis scale.
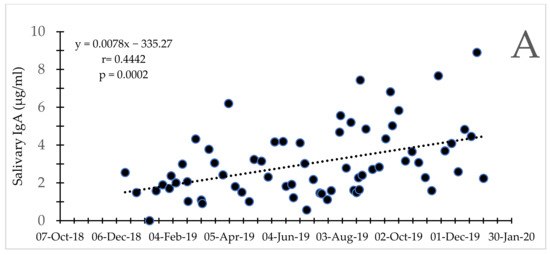
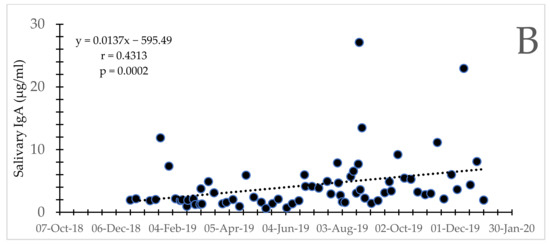
Figure 3.
Bull Asian elephants during social integration. Secretory IgA concentrations for Bull 4 (A) and 5 (B) over time. Note the difference in scale for the y-axis between the two bulls.
3.3. SIgA and Salivary Oxytocin
Although salivary oxytocin also strongly varied by individual, sIgA strongly (positively) correlated with salivary oxytocin (r = 0.3202; p < 0.0001; Figure 4). However, there was no correlation between same day salivary and serum oxytocin concentrations (Spearman r = 0.036; p = 0.670). When data were pooled for all elephants, median salivary oxytocin concentration were lower (p = 0.0183) in social situations (median= 57.01 pg/mL) compared to solo situations (median = 65.52 pg/mL). When only non-musth samples were considered there were no significant differences in salivary oxytocin (p = 0.0860; Mann–Whitney), and there were no significant differences in salivary oxytocin between social and solo situations when each bull was considered individually (Figure 5).
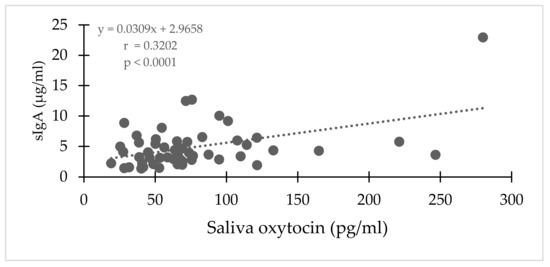
Figure 4.
Correlation between secretory IgA and salivary oxytocin in bull Asian elephants during integration of two all-male groups.
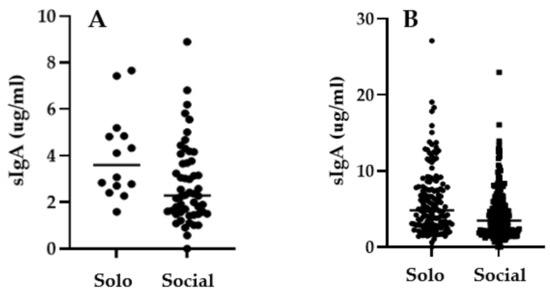
Figure 5.
Secretory IgA concentrations in (A) Asian elephant Bull 4 in solo and social situations, and (B) in all five bulls in solo and social situations, excluding musth periods during socialization of two all-male groups.
3.4. SIgA, Cortisol and Musth
Saliva and serum samples were not always collected on the same day, therefore correlations for sIgA and serum cortisol concentrations were calculated in two ways: between samples collected on the same day (from the same bull) and between averages for the same week. The present study did not find a correlation between sIgA and serum cortisol collected on the same day (n = 187 paired values; p = 0.394), or for samples collected in the same week (n = 218 paired values; p = 0.270). When all samples were analyzed together, sIgA correlated strongly with salivary oxytocin, but not with cortisol or testosterone (as a proxy for musth; Table 4). Testosterone strongly correlated with cortisol, in agreement with previous studies [10,45,46].

Table 4.
Correlation matrix of analytes in bull Asian elephants during socialization of two all-male groups.
Musth and non-musth sIgA concentrations were analyzed for the three older bulls (Bulls 1–3) only, as the younger two had not yet experienced musth before or during the study. Secretory IgA concentrations did not differ between periods of musth and non-musth when the three bulls were considered together (Mann–Whitney p = 0.0821) or individually (Mann–Whitney p > 0.9999, 0.5529, and 0.3622, for Bulls 1, 2, and 3, respectively).
3.5. SigA during Social and Solo Situations
There were no differences in sIgA concentration for Bulls 1, 2, 3, and 5 (Mann–Whitney p = 0.3311, 0.5952, 0.8295, and 0.2221, respectively). In contrast, Bull 4 had a higher concentration of sIgA when housed solo compared to socially (solo 3.6 vs. social 2.3 μg/mL; p = 0.0136) (Figure 5). When all bulls were taken together but musth samples were excluded, median sIgA concentration during solo situations (4.33 μ/mL) were higher than during social situations (3.44 μg/mL; p = 0.0008).
Evaluated over the three periods of socialization, median concentrations of sIgA and oxytocin were similar for social and solo situations before and after introductions but were higher in solo situations during introductions (p < 0.0001 for sIgA and p = 0.0011 for oxytocin). Cortisol was significantly lower in solo situations compared to social ones before and during introductions (p = 0.0008 and p = 0.0102, respectively), while the difference in cortisol concentrations after introductions was not significant (p = 0.1807).
4. Discussion
The aim of the present study was to evaluate sIgA as a biomarker of wellbeing by assessing correlations with other biomarkers of wellbeing such as cortisol and oxytocin. The study was carried out taking advantage of the integration of two new Asian elephant bulls into an established group of three bulls. Results showed no correlation of sIgA with cortisol or musth periods, but there was a strong correlation with salivary oxytocin. The median values of sIgA, cortisol and oxytocin varied across the three integration periods, indicating that new social situations can affect these biomarkers. Although a correlation between sIgA and cortisol has been documented in dogs, rats and humans [37,47], the lack of correlation between sIgA and cortisol in this study is in line with previous studies in elephants [37,38,44]. Concentrations of sIgA varied widely between elephants, something that has also been reported in other studies [37,48,49], and highlights individual adaptive processes.
Secretory IgA strongly correlated with salivary oxytocin, a known measure of social bond formation [50,51]. The biggest difference in sIgA and oxytocin concentrations between solo and social situations occurred during the introductions, a period where presumably social skills are actively used to establish new relationships. Thus, positive correlations between oxytocin and sIgA may be indicative of an affiliative social environment [51]. During the present study, bulls in musth were housed alone (solo) and so it was not possible to assess the effects of socialization during musth. Additional variation may have been introduced by the range in times of collection.
The large individual variation speaks to the concept of individual perceptions and that an individual’s point of view can dictate whether a situation is perceived as stressful or not [52,53]. Among air traffic controllers, work sessions presumed to be stressful were associated with increases in sIgA [54], and although cortisol was also elevated, there was no correlation between the two (as in this study); the authors of that study suggest that emotional engagement is the driver of sIgA increases. Similarly, establishing new social dynamics is arguably one of the most engaging social activities of any herd-living species, as the newly introduced animals approach each other and through interactions start establishing relationships and hierarchies. Zeir et al. [54] make the case for using sIgA as a measure to differentiate between positive and negative effects of stress. They also suggest it could be used as a way to assess the individual’s ability to cope with situational demands. If true, it is possible our results were confounded by an overly simplistic analysis of social vs. solo situations, as some social situations may be negative, while others may be positive depending on the relationship of the individuals involved. Other factors that may influence the perception of a stressor include early life experiences, with one individual experiencing a stimulus as acute vs. chronic and vice versa, age, health status, and genetic and temperament differences, among others [53]. Further investigation into the effect of specific dyads on sIgA, oxytocin and cortisol is needed, and overlaying behavioral observations to assign a valence to various social interactions will help classify the valence of the interaction.
Additional support for sIgA reflecting a level of social engagement is provided by the fact that the oldest male had the highest concentrations of sIgA. This male was engaged not only in his own social interactions but was regularly observed intervening in aggressive interactions between the younger bulls; that is, he was engaged in moderating social interactions between other elephants.
5. Conclusions
Overall, our results suggest that sIgA could provide a measure of social engagement and resilience rather than only reflecting stress, but additional research is needed. Use of these biomarkers to assess the progress and consequences of socialization efforts could advance bull management in zoo settings, resulting in more multiple-bull groups that better reflect in situ social situations and lead to improved welfare ex situ.
Author Contributions
Conceptualization, A.M., J.L.B. and S.J.; methodology, A.M., J.L.B. and N.P.; software, A.M.; validation, N.P.; formal analysis, A.M. and N.P.; data curation, A.M. and N.P.; writing—original draft preparation, A.M.; writing—review and editing, AM., J.L.B., N.P., A.L.S., T.S.R., M.D., S.J. and C.G.; visualization, A.M.; sample acquisition, M.D. and C.G., project administration, A.M., S.J. and J.L.B.; funding acquisition, A.M., J.L.B., S.J., A.L.S. and C.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Association of Zoos and Aquariums Conservation Grant Fund (Grant #18-1523), Denver Zoo, Smithsonian Conservation Biology Institute, and African Lion Safari.
Institutional Review Board Statement
The study protocol was reviewed and approved by Denver Zoo’s Research and Animal Welfare Committees (DZ#2018-008, July 2018).
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to institutional policy.
Acknowledgments
We thank Steve Paris for additional laboratory analysis, Denver Zoo’s elephant team for training elephants and obtaining samples: Lauren Cahill, Sarah Cesler, Rachel Chappell, Barb Junkermeier, Gabe Kibe, Danielle Lints, Jeff Stanton, and Victoria Wickens for their participation throughout this study. We thank Brian Aucone, Emily Insalaco, Dale Leeds, and Courtney Peterson for their support.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- MacDougall, S.A.; Bonier, F.; Romero, L.M.; Moore, I.T. Glucocorticoids and “Stress” are not synonymous. Integr. Org. Biol. 2019, 1, obz017. [Google Scholar] [CrossRef]
- Dathe, H.H.; Kuckelkorn, B.; Minnemann, D. Salivary cortisol assessment for stress detection in the Asian elephant (Elephas maximus): A pilot study. Zoo Biol. 1992, 11, 285–289. [Google Scholar] [CrossRef]
- Palme, R.; Touma, C.; Arias, N.; Dominchin, M.F.; Lepschy, M. Steroid extraction: Get the best out of faecal samples. Wien Tierarztl Mon. 2013, 100, 238–246. [Google Scholar]
- Staley, M.; Conners, M.G.; Hall, K.; Miller, L.J. Linking stress and immunity: Immunoglobulin A as a non-invasive physiological biomarker in animal welfare studies. Horm. Behav. 2018, 102, 55–68. [Google Scholar] [CrossRef]
- Glaeser, S.S.; Edwards, K.L.; Wielebnowski, N.; Brown, J.L. Effects of physiological changes and social life events on adrenal glucocorticoid activity in female zoo-housed Asian elephants (Elephas maximus). PLoS ONE 2020, 15, e0241910. [Google Scholar] [CrossRef]
- Dalmau, A.; Ferret, A.; Chacon, G.; Manteca, X. Seasonal changes in fecal cortisol metabolites in Pyrenean chamois. J. Wildl. Manage. 2007, 71, 190–194. [Google Scholar] [CrossRef]
- Brown, J.L.; Lehnhardt, J. Serum and urinary hormones during pregnancy and the peri-and postpartum period in an Asian elephant (Elephas maximus). Zoo Biol. 1995, 14, 555–564. [Google Scholar] [CrossRef]
- Concannon, P.; Butler, W.; Hansel, W.; Knight, P.; Hamilton, J. Parturition and lactation in the bitch: Serum progesterone, cortisol and prolactin. Biol. Reprod. 1978, 19, 1113–1118. [Google Scholar] [CrossRef]
- Weingrill, T.; Gray, D.A.; Barrett, L.; Henzi, S.P. Fecal cortisol levels in free-ranging female chacma baboons: Relationship to dominance, reproductive state and environmental factors. Horm. Behav. 2004, 45, 259–269. [Google Scholar] [CrossRef]
- Brown, J.L.; Somerville, M.; Riddle, H.S.; Keele, M.; Duer, C.K.; Freeman, E.W. Comparative endocrinology of testicular, adrenal and thyroid function in captive Asian and African elephant bulls. Gen. Comp. Endocrinol. 2007, 151, 153–162. [Google Scholar] [CrossRef]
- Birnie-Gauvin, K.; Flávio, H.; Kristensen, M.L.; Walton-Rabideau, S.; Cooke, S.J.; Willmore, W.G.; Koed, A.; Aarestrup, K. Cortisol predicts migration timing and success in both Atlantic salmon and sea trout kelts. Sci. Rep. 2019, 9, 2422. [Google Scholar] [CrossRef]
- Edwards, K.L.; Miller, M.A.; Carlstead, K.; Brown, J.L. Relationships between housing and management factors and clinical health events in elephants in North American zoos. PLoS ONE 2019, 14, e0217774. [Google Scholar]
- Chrousos, G.P. Stressors, stress, and neuroendocrine integration of the adaptive response: The 1997 Hans Selye Memorial Lecture. Ann. N. Y. Acad. Sci. 1998, 851, 311–335. [Google Scholar]
- Edes, A.N.; Edwards, K.L.; Wolfe, B.A.; Brown, J.L.; Crews, D.E. Allostatic load indices with cholesterol and triglycerides predict disease and mortality risk in zoo-housed Western Lowland Gorillas (Gorilla gorilla gorilla). Biomark. Insights 2020, 15, 1177271920914585. [Google Scholar] [CrossRef]
- Edes, A.N.; Wolfe, B.A.; Crews, D.E. Evaluating allostatic load: A new approach to measuring long-term stress in wildlife. J. Zoo Wildl. Med. 2018, 49, 272–282. [Google Scholar] [CrossRef]
- Bansiddhi, P.; Brown, J.L.; Khonmee, J.; Norkaew, T.; Nganvongpanit, K.; Punyapornwithaya, V.; Angkawanish, T.; Somgird, C.; Thitaram, C. Management factors affecting adrenal glucocorticoid activity of tourist camp elephants in Thailand and implications for elephant welfare. PLoS ONE 2019, 14, e0221537. [Google Scholar] [CrossRef]
- Vanitha, V.; Thiyagesan, K.; Baskaran, N. Social life of captive Asian elephants (Elephas maximus) in Southern India: Implications for elephant welfare. J. Appl. Anim. Welf. Sci. 2011, 14, 42–58. [Google Scholar] [CrossRef]
- Evans, K.E.; Harris, S. Adolescence in male African elephants, Loxodonta africana, and the importance of sociality. Anim. Behav. 2008, 76, 779–787. [Google Scholar] [CrossRef]
- Lee, P.C.; Moss, C.J. African elephant play, competence and social complexity. Anim. Behav. Cogn. 2014, 1, 144–156. [Google Scholar] [CrossRef]
- De Silva, S.; Wittemyer, G. A comparison of social organization in Asian elephants and African savannah elephants. Int. J. Primatol. 2012, 33, 1125–1141. [Google Scholar] [CrossRef]
- Joshi, R. Tusker’s social bonds in Rajaji. Hystrix 2015, 26, 41–45. [Google Scholar] [CrossRef]
- Srinivasaiah, N.; Kumar, V.; Vaidyanathan, S.; Sukumar, R.; Sinha, A. All-male groups in Asian elephants: A novel, adaptive social strategy in increasingly anthropogenic landscapes of Southern India. Sci. Rep. 2019, 9, 8678. [Google Scholar]
- Rees, P.A. The sizes of elephant groups in zoos: Implications for elephant welfare. J. Appl. Anim. Welf. Sci. 2009, 12, 44–60. [Google Scholar] [CrossRef]
- Saragusty, J.; Hermes, R.; Göritz, F.; Schmitt, D.L.; Hildebrandt, T.B. Skewed birth sex ratio and premature mortality in elephants. Anim. Reprod. Sci. 2009, 115, 247–254. [Google Scholar] [CrossRef]
- Prado-Oviedo, N.A.; Bonaparte-Saller, M.K.; Malloy, E.J.; Meehan, C.L.; Mench, J.A.; Carlstead, K.; Brown, J.L. Evaluation of demographics and social life events of Asian (Elephas maximus) and African elephants (Loxodonta africana) in North American zoos. PLoS ONE 2016, 11, e0154750. [Google Scholar] [CrossRef]
- AZA. African Elephant Population Analysis and Breeding and Transfer Plan; Association of Zoos and Aquariums: Silver Spring, MD, 2020. [Google Scholar]
- AZA. Asian Elephant Population Analysis and Breeding and Transfer Plan; Association of Zoos and Aquariums: Silver Spring, MD, 2020. [Google Scholar]
- Ganswindt, A.; Rasmussen, H.B.; Heistermann, M.; Hodges, J.K. The sexually active states of free-ranging male African elephants (Loxodonta africana): Defining musth and non-musth using endocrinology, physical signals, and behavior. Horm. Behav. 2005, 47, 83–91. [Google Scholar] [CrossRef]
- Hartley, M.; Wood, A.; Yon, L. Facilitating the social behaviour of bull elephants in zoos. Int. Zoo Yearb. 2019, 53, 62–77. [Google Scholar] [CrossRef]
- Poole, J.H. Announcing intent: The aggressive state of musth in African elephants. Anim. Behav. 1989, 37, 140–152. [Google Scholar] [CrossRef]
- Readyhough, T.; Joseph, S.; Davis, M.; Moresco, A.; Schreier, A.L. Impacts of socialization on bull Asian elephants (Elephas maximus) stereotypical behavior. J. Zool. Bot. Gard. 2022, 3, 113–130. [Google Scholar] [CrossRef]
- Schreier, A.L.; Readyhough, T.S.; Moresco, A.; Davis, M.; Joseph, S. Social dynamics of a newly integrated bachelor group of Asian elephants (Elephas maximus). J. Appl. Anim. Welf. Sci. 2021, 1–18. [Google Scholar] [CrossRef]
- Schmid, J.; Heistermann, M.; Ganslosser, U.; Hodges, J. Introduction of foreign female Asian elephants (Elephas maximus) into an existing group: Behavioural reactions and changes in cortisol levels. Anim. Welf. 2001, 10, 357–372. [Google Scholar]
- Laws, N.; Ganswindt, A.; Heistermann, M.; Harris, M.; Harris, S.; Sherwin, C. A case study: Fecal corticosteroid and behavior as indicators of welfare during relocation of an Asian elephant. J. Appl. Anim. Welf. Sci. 2007, 10, 349–358. [Google Scholar] [CrossRef]
- Hansen, I.S.; Baeten, D.L.; den Dunnen, J. The inflammatory function of human IgA. Cell. Mol. Life Sci. 2019, 76, 1041–1055. [Google Scholar]
- Skandakumar, S.; Stodulski, G.; Hau, J. Salivary IgA: A possible stress marker in dogs. Anim. Welf. 1995, 4, 339–350. [Google Scholar] [CrossRef]
- Kosaruk, W.; Brown, J.L.; Plangsangmas, T.; Towiboon, P.; Punyapornwithaya, V.; Silva-Fletcher, A.; Thitaram, C.; Khonmee, J.; Edwards, K.L.; Somgird, C. Effect of tourist activities on fecal and salivary glucocorticoids and Immunoglobulin A in female captive Asian elephants in Thailand. Animals 2020, 10, 1928. [Google Scholar] [CrossRef]
- Plangsangmas, T.; Brown, J.L.; Thitaram, C.; Silva-Fletcher, A.; Edwards, K.L.; Punyapornwithaya, V.; Towiboon, P.; Somgird, C. Circadian Rhythm of Salivary Immunoglobulin A and Associations with Cortisol as A Stress Biomarker in Captive Asian Elephants (Elephas maximus). Animals 2020, 10, 157. [Google Scholar] [CrossRef]
- Richard, P.; Moos, F.; Freund-Mercier, M. Central effects of oxytocin. Physiol. Rev. 1991, 71, 331–370. [Google Scholar] [CrossRef]
- Tabak, J.; Gonzalez-Iglesias, A.E.; Toporikova, N.; Bertram, R.; Freeman, M.E. Variations in the response of pituitary lactotrophs to oxytocin during the rat estrous cycle. Endocrinology 2010, 151, 1806–1813. [Google Scholar] [CrossRef][Green Version]
- Prado, N.A.; Keady, M.; Oestmann, A.; Steinbeiser, C.M.; Brown, J.L. Hyperprolactinemic African elephant (Loxodonta africana) females exhibit elevated dopamine, oxytocin and serotonin concentrations compared to normal cycling and noncycling, low prolactin elephants. Biol. Reprod. 2019, 100, 1549–1560. [Google Scholar] [CrossRef]
- Brown, J.L.; Paris, S.; Prado-Oviedo, N.A.; Meehan, C.L.; Hogan, J.N.; Morfeld, K.A.; Carlstead, K. Reproductive health assessment of female elephants in North American zoos and association of husbandry practices with reproductive dysfunction in African elephants (Loxodonta africana). PLoS ONE 2016, 11, e0145673. [Google Scholar] [CrossRef]
- Ganswindt, A.; Heistermann, M.; Hodges, K. Physical, physiological, and behavioral correlates of musth in captive African elephants (Loxodonta africana). Physiol. Biochem. Zool. 2005, 78, 505–514. [Google Scholar] [CrossRef]
- Edwards, K.L.; Bansiddhi, P.; Paris, S.; Galloway, M.; Brown, J.L. The development of an immunoassay to measure immunoglobulin A in Asian elephant feces, saliva, urine and serum as a potential biomarker of well-being. Conserv. Physiol. 2019, 7, coy077. [Google Scholar] [CrossRef]
- Glaeser, S.S.; Edwards, K.L.; Paris, S.; Scarlata, C.; Lee, B.; Wielebnowski, N.; Finnell, S.; Somgird, C.; Brown, J.L. Characterization of longitudinal testosterone, cortisol, and musth in male Asian elephants (Elephas maximus), eEffects of aging, and adrenal responses to social changes and health events. Animals 2022, 12, 1332. [Google Scholar] [CrossRef]
- Bechert, U.; Hixon, S.; Schmitt, D. Diurnal variation in serum concentrations of cortisol in captive African (Loxodonta africana) and Asian (Elephas maximus) elephants. Zoo Biol. 2021, 40, 458–471. [Google Scholar]
- Guhad, F.; Hau, J. Salivary IgA as a marker of social stress in rats. Neurosci. Lett. 1996, 216, 137–140. [Google Scholar] [CrossRef]
- Lantz, E.L.; Lonsdorf, E.V.; Heintz, M.R.; Murray, C.M.; Lipende, I.; Travis, D.A.; Santymire, R.M. Non-invasive quantification of immunoglobulin A in chimpanzees (Pan troglodytes schweinfurthii) at Gombe National Park, Tanzania. Am. J. Primatol. 2018, 1, e22558. [Google Scholar] [CrossRef]
- Palm, A.-K.E.; Wattle, O.; Lundström, T.; Wattrang, E. Secretory immunoglobulin A and immunoglobulin G in horse saliva. Vet. Immunol. Immunopathol. 2016, 180, 59–65. [Google Scholar] [CrossRef]
- Bielsky, I.F.; Young, L.J. Oxytocin, vasopressin, and social recognition in mammals. Peptides 2004, 25, 1565–1574. [Google Scholar] [CrossRef]
- Smith, A.S.; Ågmo, A.; Birnie, A.K.; French, J.A. Manipulation of the oxytocin system alters social behavior and attraction in pair-bonding primates, Callithrix penicillata. Horm. Behav. 2010, 57, 255–262. [Google Scholar] [CrossRef]
- Veissier, I.; Boissy, A. Stress and welfare: Two complementary concepts that are intrinsically related to the animal’s point of view. Physiol. Behav. 2007, 92, 429–433. [Google Scholar] [CrossRef]
- Romero, L.M. Physiological stress in ecology: Lessons from biomedical research. Trends Ecol. Evol. 2004, 19, 249–255. [Google Scholar]
- Zeier, H.; Brauchli, P.; Joller-Jemelka, H.I. Effects of work demands on immunoglobulin A and cortisol in air traffic controllers. Biol. Psychol. 1996, 42, 413–423. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).