Over 25 Years of Partnering to Conserve Chiricahua Leopard Frogs (Rana chiricahuensis) in Arizona, Combining Ex Situ and In Situ Strategies
Abstract
1. The Phoenix Zoo’s Legacy of Native Species Conservation
2. Chiricahua Leopard Frogs: Background
3. History of the Phoenix Zoo’s Involvement in Chiricahua Leopard Frog Conservation
4. Evolution of Ex Situ Conservation Facilities and Strategies
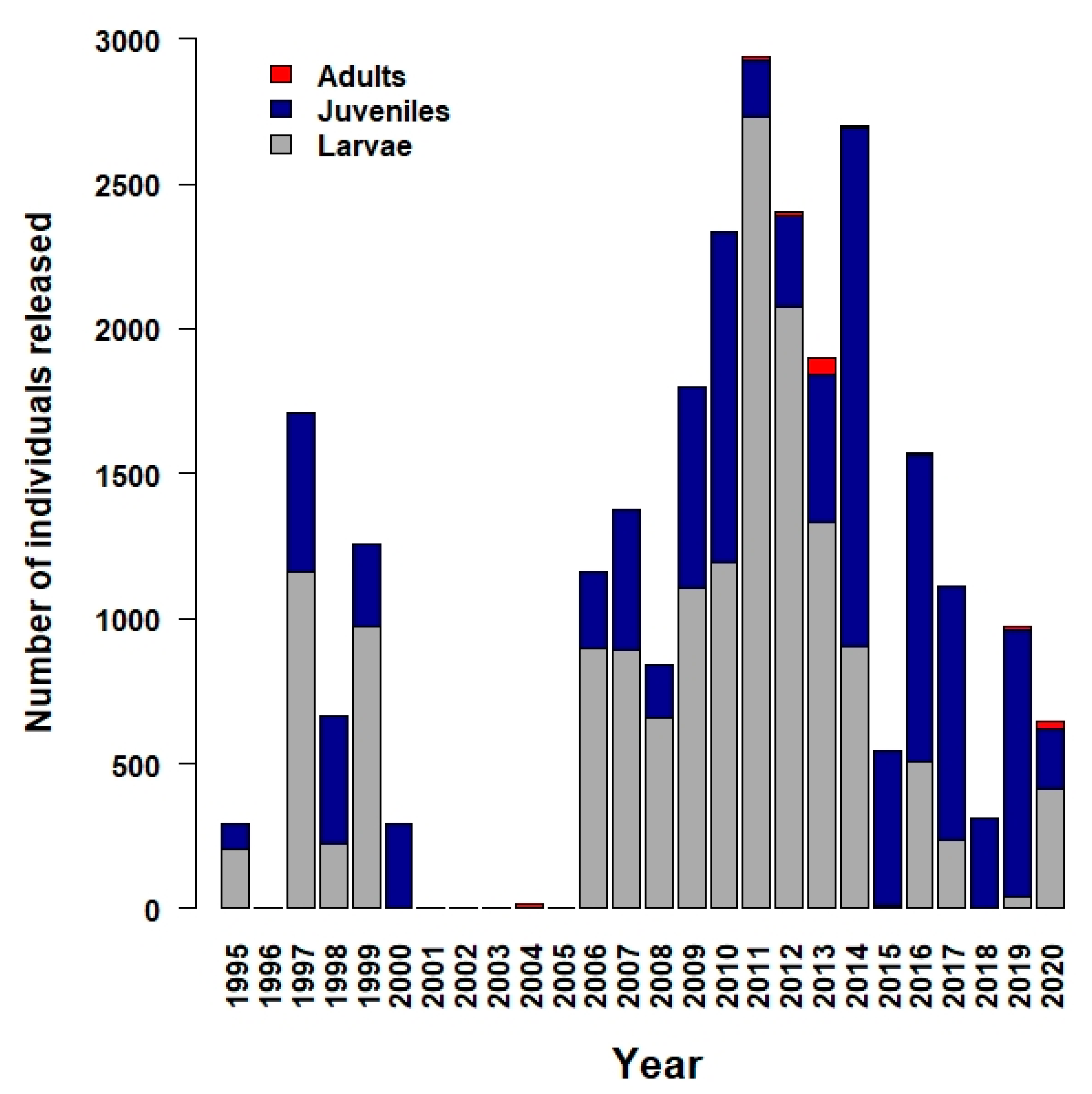
5. Conservation Successes at the Zoo and in the Field
6. Evolution and Maintenance of Partnerships
7. Challenges Faced—Example 1: Deciding to Cross Genetic Lineages
8. Challenges Faced—Example 2: Managing Disease
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allard, R.A.; Wells, S.A. The Phoenix Zoo story: Building a legacy of conservation. In The Ark and Beyond: The Evolution of Zoo and Aquarium Conservation; Minteer, B.A., Maienschein, J., Collins, J.P., Eds.; The University of Chicago Press: Chicago, IL, USA, 2018; pp. 169–175. [Google Scholar]
- Association of Zoos and Aquariums. The Accreditation Standards and Related Policies 2022; Silver Spring: Montgomery County, MD, USA, 2022. [Google Scholar]
- U.S. Fish and Wildlife Service. Endangered and threatened wildlife and plants; listing and designation of critical habitat of the Chiricahua leopard frog (Lithobates chiricahuensis). US. Fed. Reg. 2012, 77, 16234–16424. [Google Scholar]
- Secretaría de Medio Ambiente y Recursos Naturales. Protección ambiental-especies nativas de México de flora y fauna silvestrescategorías de riesgo y especificaciones para su inclusión, exclusión o cambio-Lista de especies en riesgo. (Segunda Sección). Diaro Of. 2010, 6, 158–169. [Google Scholar]
- Santos-Barrera, G.; Hammerson, G.; Sredl, M. Lithobates chiricahuensis. IUCN Red List 2004, e.T58575A11805575. [Google Scholar] [CrossRef]
- U.S. Fish and Wildlife Service. Endangered and threatened wildlife and plants; listing of the Chiricahua leopard frog (Rana chiricahuensis). US. Fed. Reg. 2002, 67, 40790–40811. [Google Scholar]
- U.S. Fish and Wildlife Service. Chiricahua Leopard Frog (Rana chiricahuensis) Recovery Plan; U.S. Fish and Wildlife Service: Albuquerque, NM, USA, 2007.
- Rorabaugh, J.C.; Hossack, B.R.; Muths, E.; Sigafus, B.H.; Lemos-Espinal, J.A. Status of the threatened Chiricahua leopard frog and conservation challenges in Sonora, Mexico, with notes on other ranid frogs and non-native predators. Herpetol. Conserv. Biol. 2018, 13, 17–32. [Google Scholar]
- Mosley, C.D.; Marsh, M.J.L.; Owens, A.K. Chiricahua leopard frog recovery in Arizona 2019. In Nongame and Endangered Wildlife Program Technical Report 330; Arizona Game and Fish Department: Phoenix, AZ, USA, 2020. [Google Scholar]
- Hossack, B.R.; Howell, P.E.; Owens, A.K.; Cobos, C.; Goldberg, C.S.; Hall, D.; Hedwall, S.; MacVean, S.K.; MacCaffery, M.; McCall, A.H.; et al. Identifying factors linked with persistence of reintroduced populations: Lessons learned from 25 years of amphibian translocations. Global Ecol. Conserv. 2022, 35, e02078. [Google Scholar] [CrossRef]
- Demlong, M.J. Head-starting Rana subaquavocalis in captivity. Reptiles 1997, 5, 24–33. [Google Scholar]
- Sprankle, T. Giving leopard frogs a head start. Endanger. Species Update 2008, 25, S15+. [Google Scholar]
- Sredl, M.J.; Field, K.J.; Peterson, A.M. Understanding and mitigating effects of chytrid fungus to amphibian populations in Arizona. In Nongame and Endangered Wildlife Program Technical Report 208; Arizona Game and Fish Department: Phoenix, AZ, USA, 2003. [Google Scholar]
- Sredl, M.J.; Jennings, R.D. Rana chiricahuensis Platz and Mecham, 1979: Chiricahua leopard frogs. In Amphibian Declines: The Conservation Status of United States Species; Lanoo, M., Ed.; University of California Press: Berkeley, CA, USA; Los Angeles, CA, USA, 2005; pp. 546–549. [Google Scholar]
- Vitt, L.J.; Caldwell, J.P. Herpetology: An Introductory Biology of Amphibians and Reptiles, 4th ed.; Academic Press: San Diego, CA, USA, 2014. [Google Scholar]
- Reading, R.P.; Miller, B.J. The black-footed ferret recovery program: Unmasking professional and organizational weaknesses. In Endangered Species Recovery: Finding the Lessons, Improving the Process; Clark, T.W., Reading, R.P., Clarke, A.L., Eds.; Island Press: Washington, DC, USA, 1994; pp. 73–100. [Google Scholar]
- Scott, J.M.; Goble, D.D.; Wiens, J.A.; Wilcove, D.S.; Bean, M.; Male, T. Recovery of imperiled species under the Endangered Species Act: The need for a new approach. Front. Ecol. Environ. 2005, 3, 383–389. [Google Scholar] [CrossRef]
- Dreitz, V. Issues in species recovery: An example based on the Wyoming toad. Bioscience 2006, 56, 765–771. [Google Scholar] [CrossRef][Green Version]
- Miller, B.; Reading, R.P. Challenges to black-footed ferret recovery: Protecting prairie dogs. West. N. Am. Nat. 2012, 72, 228–240. [Google Scholar] [CrossRef][Green Version]
- Evans, D.M.; Che-Castaldo, J.P.; Crouse, D.; Davis, F.W.; Epanchin-Niell, R.; Flather, C.H.; Frohlich, R.K.; Goble, D.D.; Li, Y.; Male, T.D.; et al. Species recovery in the United States: Increasing the effectiveness of the Endangered Species Act. Issues Ecol. 2016, 20, 1–28. [Google Scholar]
- Walls, S.C.; Ball, L.C.; Barichivich, W.J.; Dodd, C.K., Jr.; Enge, K.M.; Gorman, T.A.; O’Donnell, K.M.; Palis, J.G.; Semlitsch, R.D. Overcoming challenges to the recovery of declining amphibian populations in the United States. Bioscience 2017, 67, 156–165. [Google Scholar] [CrossRef]
- Rorabaugh, J.; Kreutzian, K.; Sredl, M.; Painter, C.; Aguilar, R.; Bravo, J.C.; Kruse, C. Chiricahua leopard frog inches toward recovery. Endanger. Species Update 2008, 25, S10+. [Google Scholar]
- Byers, O.; Lees, C.; Wilcken, J.; Schwitzer, C. The One Plan Approach: The philosophy and implementation of CBSG’s approach to integrated species conservation planning. WAZA Mag. 2013, 14, 2–5. [Google Scholar]
- Traylor-Holzer, K.; Leus, K.; Byers, O. Integrating ex situ management options as part of a One Plan Approach to species conservation. In The Ark and Beyond: The Evolution of Zoo and Aquarium Conservation; Minteer, B.A., Maienschein, J., Collins, J.P., Eds.; University of Chicago Press: Chicago, IL, USA, 2018; pp. 129–141. [Google Scholar]
- Hedrick, P.W. Gene flow and genetic restoration: The Florida panther as a case study. Conserv. Biol. 1995, 9, 996–1007. [Google Scholar] [CrossRef]
- Pimm, S.L.; Dollar, L.; Bass, O.L., Jr. The genetic rescue of the Florida panther. Anim. Conserv. 2006, 9, 115–122. [Google Scholar] [CrossRef]
- Hedrick, P.W.; Lee, R.; Hurt, C.R. Genetic evaluation of captive populations of endangered species and merging of populations: Gila topminnows as an example. J. Hered. 2012, 103, 651–660. [Google Scholar] [CrossRef][Green Version]
- Westemeier, R.L.; Brawn, J.D.; Simpson, S.A.; Esker, T.L.; Jansen, R.W.; Walk, J.W.; Kershner, E.L.; Bouzat, J.L.; Paige, K.N. Tracking the long-term decline and recovery of an isolated population. Science 1998, 282, 1695–1698. [Google Scholar] [CrossRef]
- Warnke, K. Wisconsin greater prairie-chicken management plan 2004–2014. In Wisconsin Department of Natural Resources; Madison: Wisconsin, WI, USA, 2004. [Google Scholar]
- Vrijenhoek, R.C. Genetic diversity and fitness in small populations. In Conservation Genetics EXS; Loeschcke, V., Jain, S.K., Tomiuk, J., Eds.; Birkhäuser: Basel, Switzerland, 1994; Volume 68. [Google Scholar]
- Räikkönen, J.; Vucetich, J.A.; Peterson, R.O.; Nelson, M.P. Congenital bone deformities and the inbred wolves (Canis lupus) of Isle Royale. Biol. Conserv. 2009, 142, 1025–1031. [Google Scholar] [CrossRef]
- Robinson, J.A.; Räikkönen, J.; Vucetich, L.M.; Vucetich, J.A.; Peterson, R.O.; Lohmueller, K.E.; Wayne, R.K. Genomic signatures of extensive inbreeding in Isle Royale wolves, a population on the threshold of extinction. Sci. Adv. 2019, 5, eaau0757. [Google Scholar] [CrossRef] [PubMed]
- Weeks, A.R.; Moro, D.; Thavornkanlapachai, R.; Taylor, H.R.; White, N.E.; Weiser, E.L.; Heinze, D. Conserving and enhancing genetic diversity in translocation programs. In Advances in Reintroduction Biology of Australian and New Zealand Fauna; Armstrong, D., Hayward, M., Moro, D., Seddon, P., Eds.; CSIRO Publishing: Melbourne, Australia, 2015. [Google Scholar]
- Frankham, R. Genetic rescue of small inbred populations: Meta-analysis reveals large and consistent benefits of gene flow. Molec. Ecol. 2015, 24, 2610–2618. [Google Scholar] [CrossRef] [PubMed]
- Ralls, K.; Ballou, J.D.; Dudash, M.R.; Eldridge, M.D.B.; Fenster, C.B.; Lacy, R.C.; Sunnucks, P.; Frankham, R. Call for a paradigm shift in the genetic management of fragmented populations. Conserv. Lett. 2018, 11, 1–6. [Google Scholar] [CrossRef]
- Whiteley, A.R.; Fitzpatrick, S.W.; Funk, W.C.; Tallmon, D.A. Genetic rescue to the rescue. Trends Ecol. Evol. 2015, 30, 42–49. [Google Scholar] [CrossRef]
- Frankham, R. Genetic rescue benefits persist to at least the F3 generation, based on a meta-analysis. Biol. Conserv. 2016, 195, 33–36. [Google Scholar] [CrossRef]
- Longcore, J.E.; Pessier, A.P.; Nichols, D.K. Batrachochytrium dendrobatidis gen. et sp. nov., a chytrid pathogenic to amphibians. Mycologia 1999, 91, 219–227. [Google Scholar] [CrossRef]
- Skerratt, L.F.; Berger, L.; Speare, R.; Cashins, S.; McDonald, K.R.; Phillott, A.D.; Hines, H.B.; Kenyon, N. Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. EcoHealth 2007, 4, 125. [Google Scholar] [CrossRef]
- Voyles, J.; Young, S.; Berger, L.; Campbell, C.; Voyles, W.F.; Dinudom, A.; Cook, D.; Webb, R.; Alford, R.A.; Skerratt, L.F.; et al. Pathogenesis of chytridiomycosis, a cause of catastrophic amphibian declines. Science 2009, 326, 582–585. [Google Scholar] [CrossRef]
- Forrest, M.J.; Schlaepfer, M.A. Nothing a hot bath won’t cure: Infection rates of amphibian chytrid fungus correlate negatively with water temperature under natural field settings. PLoS ONE 2011, 6, e28444. [Google Scholar] [CrossRef]
- Pessier, A.P.; Mendelson, J.R., III. A Manual for Control of Infectious Diseases in Amphibian Survival Assurance Colonies and Reintroduction Programs; Version 2.0; IUCN/SSC Conservation Breeding Specialist Group: Apple Valley, MN, USA, 2017. [Google Scholar]
- Heuring, W.L.; Poynter, B.M.; Wells, S.; Pessier, A.P. Successful clearance of chytrid fungal infection in threatened Chiricahua leopard frog (Rana chiricahuensis) larvae and frogs using an elevated temperature treatment protocol. Salamandra 2021, 57, 171–173. [Google Scholar]
- Savage, A.E.; Zamudio, K.R. MHC genotypes associate with resistance to a frog-killing fungus. Proc. Natl. Acad. Sci. USA 2011, 108, 16705–16710. [Google Scholar] [CrossRef] [PubMed]
- Savage, A.E.; Becker, C.G.; Zamudio, K.R. Linking genetic and environmental factors in amphibian disease risk. Evol. Appl. 2015, 8, 560–572. [Google Scholar] [CrossRef]
- Savage, A.E.; Zamudio, K.R. Adaptive tolerance to a pathogenic fungus drives major histocompatibility complex evolution in natural amphibian populations. Proc. R. Soc. B 2016, 283, 20153115. [Google Scholar] [CrossRef] [PubMed]
- Savage, A.E.; Mulder, K.P.; Torres, T.; Wells, S. Lost but not forgotten: MHC genotypes predict overwinter survival despite depauperate MHC diversity in a declining frog. Conserv. Genet. 2018, 19, 309–322. [Google Scholar] [CrossRef]
- Miller, D.; Gray, M.; Storfer, A. Ecopathology of ranaviruses infecting amphibians. Viruses 2011, 3, 2351–2373. [Google Scholar] [CrossRef]
- Jancovich, J.K.; Davidson, E.W.; Morado, J.F.; Jacobs, B.L.; Collins, J.P. Isolation of a lethal virus from the endangered tiger salamander Ambystoma tigrinum stebbinsi. Dis. Aquat. Organ. 1997, 31, 161–167. [Google Scholar] [CrossRef]
- Jancovich, J.K.; Davidson, E.W.; Seiler, A.; Jacobs, B.L.; Collins, J.P. Transmission of the Ambystoma tigrinum virus to alternative hosts. Dis. Aquat. Organ. 2001, 46, 159–163. [Google Scholar] [CrossRef]
- Browne, R.K.; Wolfram, K.; García, G.; Bagaturov, M.F.; Pereboom, Z.J.J.M. Zoo-based amphibian research and conservation breeding programs. Amphib. Reptile Conserv. 2011, 5, 1–14. [Google Scholar]
- Zippel, K.; Johnson, K.; Gagliardo, R.; Gibson, R.; McFadden, M.; Browne, R.; Martinez, C.; Townsend, E. The Amphibian Ark: A global community for ex situ conservation of amphibians. Herpetol. Conserv. Biol. 2011, 6, 340–352. [Google Scholar]
- Harding, G.; Griffiths, R.A.; Pavajeau, L. Developments in amphibian captive breeding and reintroduction programs. Conserv. Biol. 2016, 30, 340–349. [Google Scholar] [CrossRef]
- Murphy, J.B.; Gratwicke, B. History of captive management and conservation amphibian programs mostly in zoos and aquariums. Herp. Rev. 2017, 48, 241–260. [Google Scholar]
- Mendelson, J.R., III. Frogs in glass boxes: Responses of zoos to global amphibian extinctions. In The Ark and Beyond: The Evolution of Zoo and Aquarium Conservation; Minteer, B.A., Maienschein, J., Collins, J.P., Eds.; University of Chicago Press: Chicago, IL, USA, 2018; pp. 298–310. [Google Scholar]
- Gagliardo, R.; Crump, P.S.; Griffith, E.J.; Mendelson, J.; Ross, H.; Zippel, K. The principles of rapid response for amphibian conservation, using the programmes in Panama as an example. Int. Zoo Yearb. 2008, 42, 125–135. [Google Scholar] [CrossRef]
- Mendelson, J.R., III; Lips, K.R.; Gagliardo, R.W.; Rabb, G.B.; Collins, J.P.; Diffendorfer, J.E.; Daszak, P.; Ibáñez, D.; Zippel, K.C.; Lawson, D.P.; et al. Confronting amphibian declines and extinctions. Science 2006, 313, 48. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, L.; Johnson, K.; Mendelson, J., III. Principles of program development and management for amphibian conservation captive breeding programs. Int. Zoo News 2015, 62, 96–107. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harris, T.R.; Heuring, W.L.; Allard, R.A.; Owens, A.K.; Hedwall, S.; Crawford, C.; Akins, C. Over 25 Years of Partnering to Conserve Chiricahua Leopard Frogs (Rana chiricahuensis) in Arizona, Combining Ex Situ and In Situ Strategies. J. Zool. Bot. Gard. 2022, 3, 532-544. https://doi.org/10.3390/jzbg3040039
Harris TR, Heuring WL, Allard RA, Owens AK, Hedwall S, Crawford C, Akins C. Over 25 Years of Partnering to Conserve Chiricahua Leopard Frogs (Rana chiricahuensis) in Arizona, Combining Ex Situ and In Situ Strategies. Journal of Zoological and Botanical Gardens. 2022; 3(4):532-544. https://doi.org/10.3390/jzbg3040039
Chicago/Turabian StyleHarris, Tara R., Whitney L. Heuring, Ruth A. Allard, Audrey K. Owens, Shaula Hedwall, Cat Crawford, and Christina Akins. 2022. "Over 25 Years of Partnering to Conserve Chiricahua Leopard Frogs (Rana chiricahuensis) in Arizona, Combining Ex Situ and In Situ Strategies" Journal of Zoological and Botanical Gardens 3, no. 4: 532-544. https://doi.org/10.3390/jzbg3040039
APA StyleHarris, T. R., Heuring, W. L., Allard, R. A., Owens, A. K., Hedwall, S., Crawford, C., & Akins, C. (2022). Over 25 Years of Partnering to Conserve Chiricahua Leopard Frogs (Rana chiricahuensis) in Arizona, Combining Ex Situ and In Situ Strategies. Journal of Zoological and Botanical Gardens, 3(4), 532-544. https://doi.org/10.3390/jzbg3040039




