Behavior and Habitat Use Remain Diverse and Variable in Modern Zoological Exhibits over the Long-Term: Case Studies in 5 Species of Ursidae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Saint Louis Zoo Bear Habitats
2.3. Smithsonian National Zoo Giant Panda Habitat
2.4. Saint Louis Zoo Behavioral Observations
2.5. National Zoo Behavioral Observations
2.6. Analysis-Levels of Behavior over Time
2.7. Analysis-Activity Budget Variation over Time
2.8. Analysis-Habitat Use over Time
3. Results
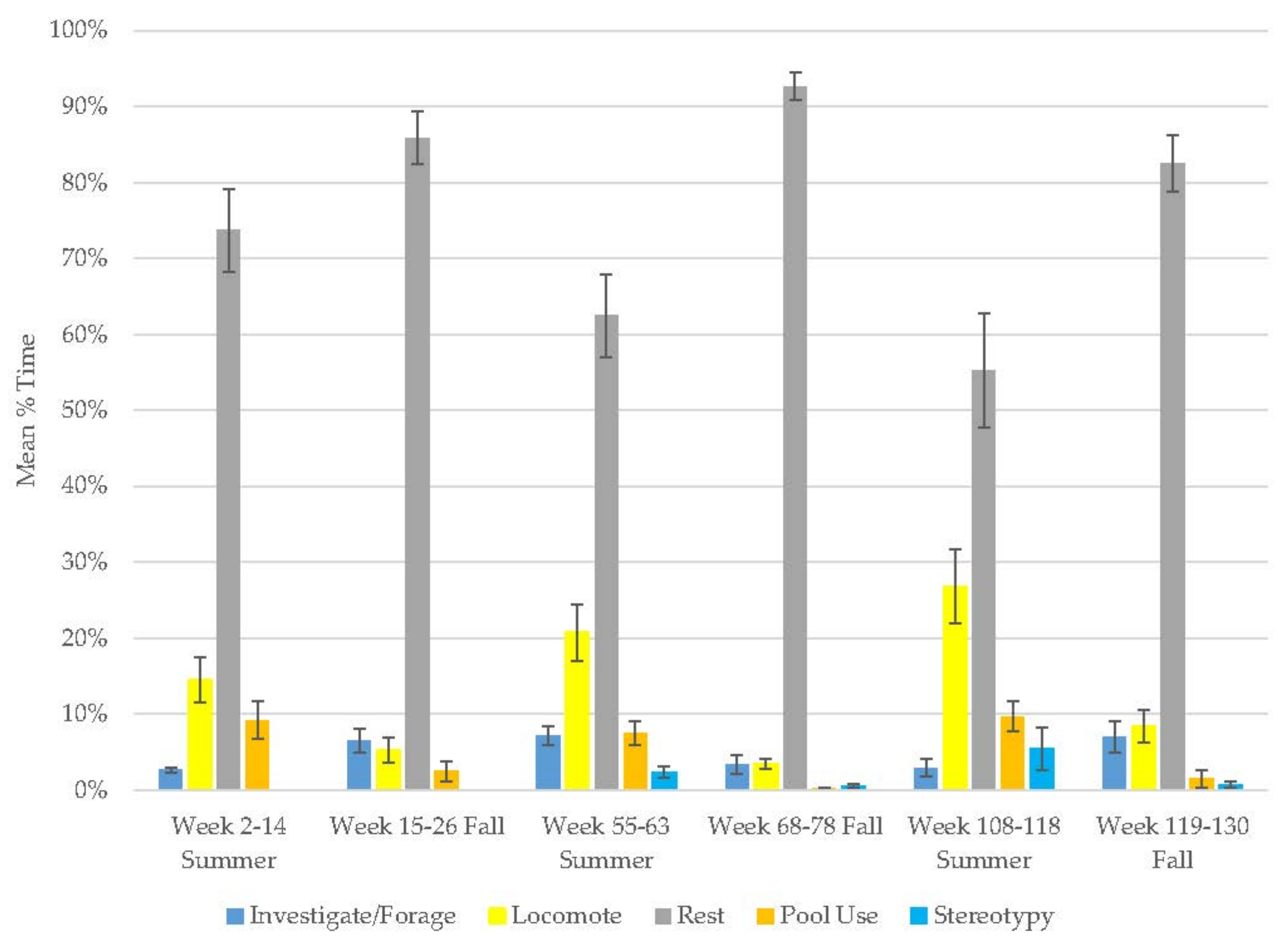
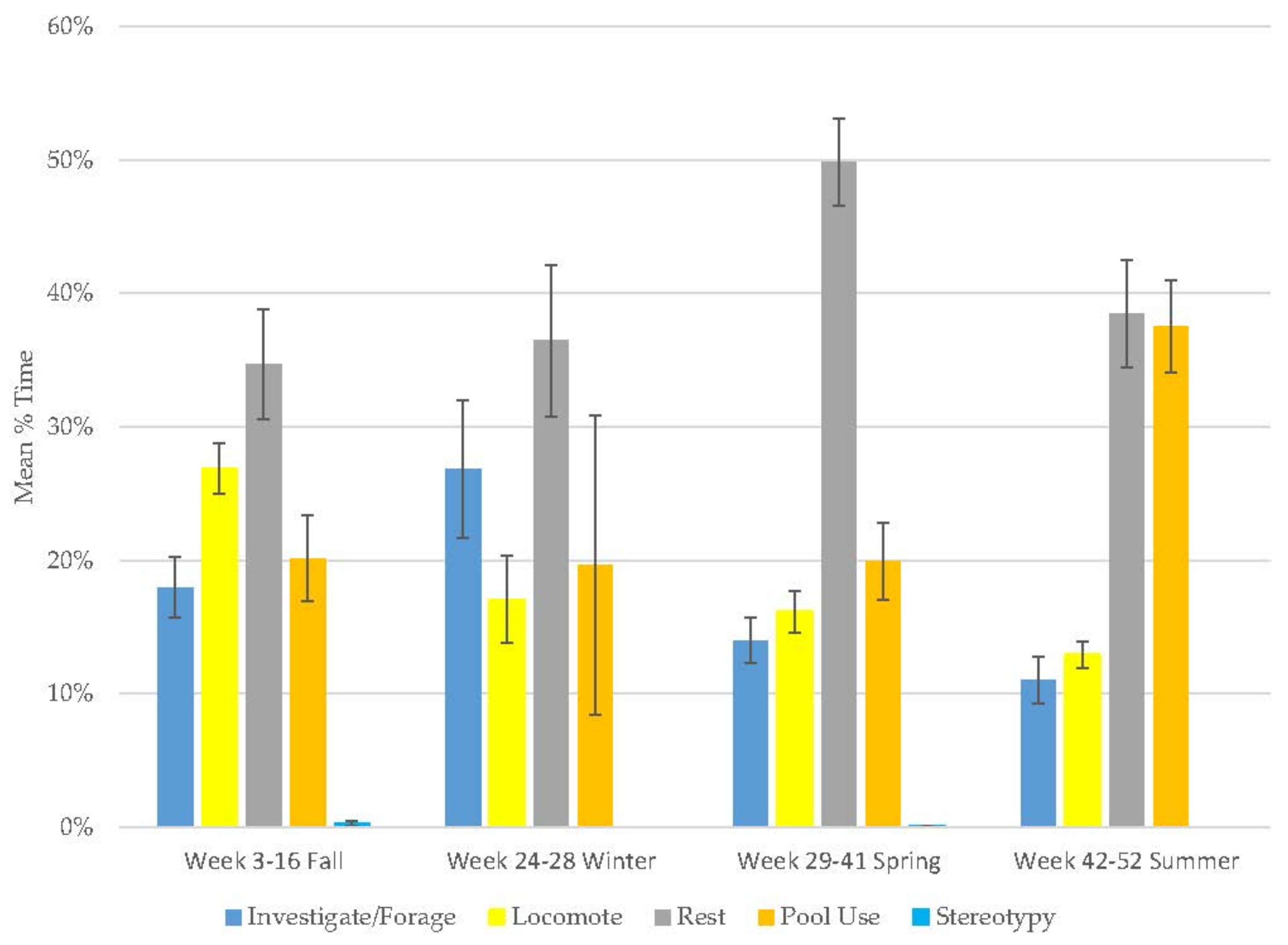
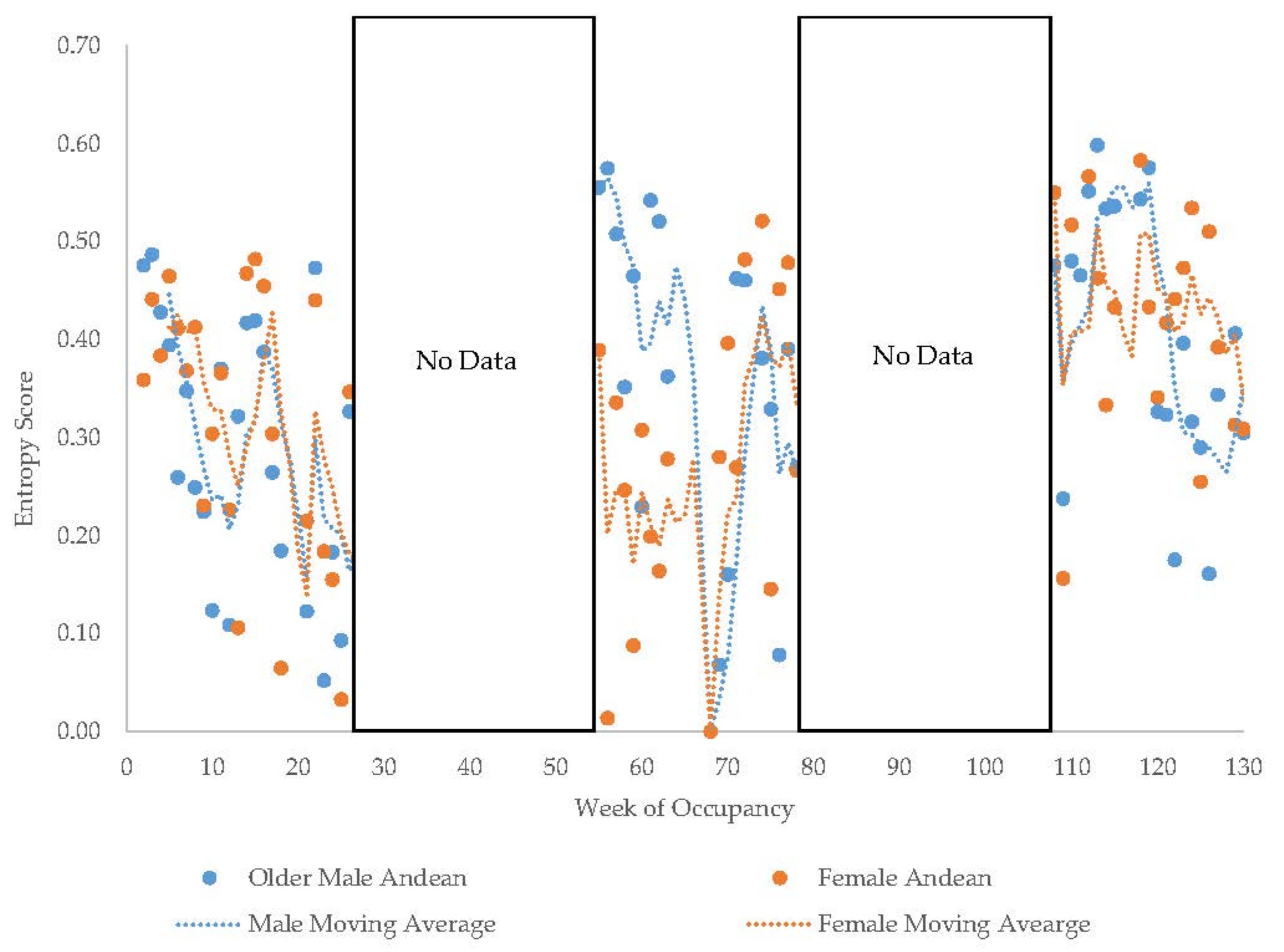
3.1. Old Male Andean Bear
3.2. Old Female Andean Bear
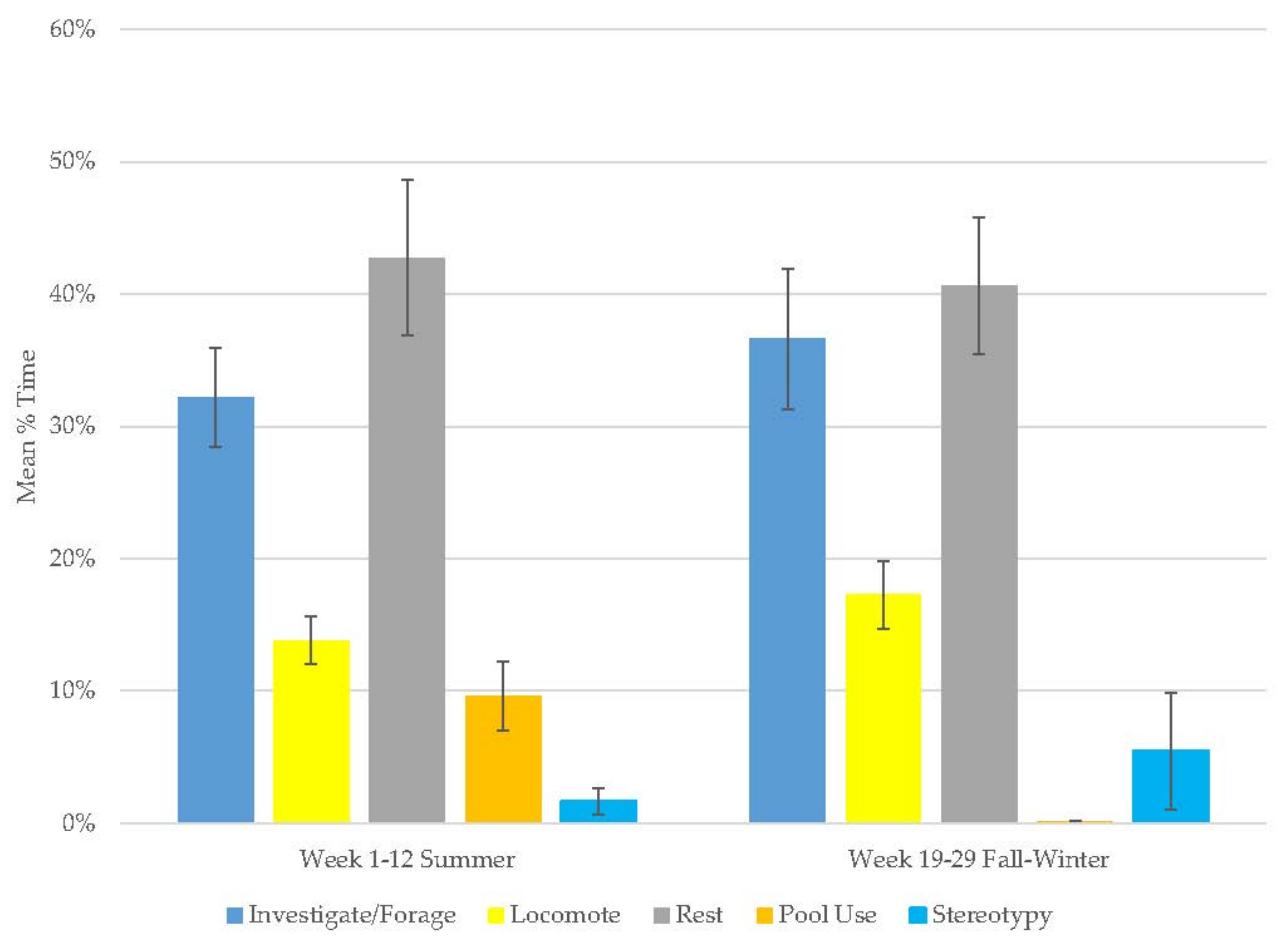
3.3. Young Male Andean Bear
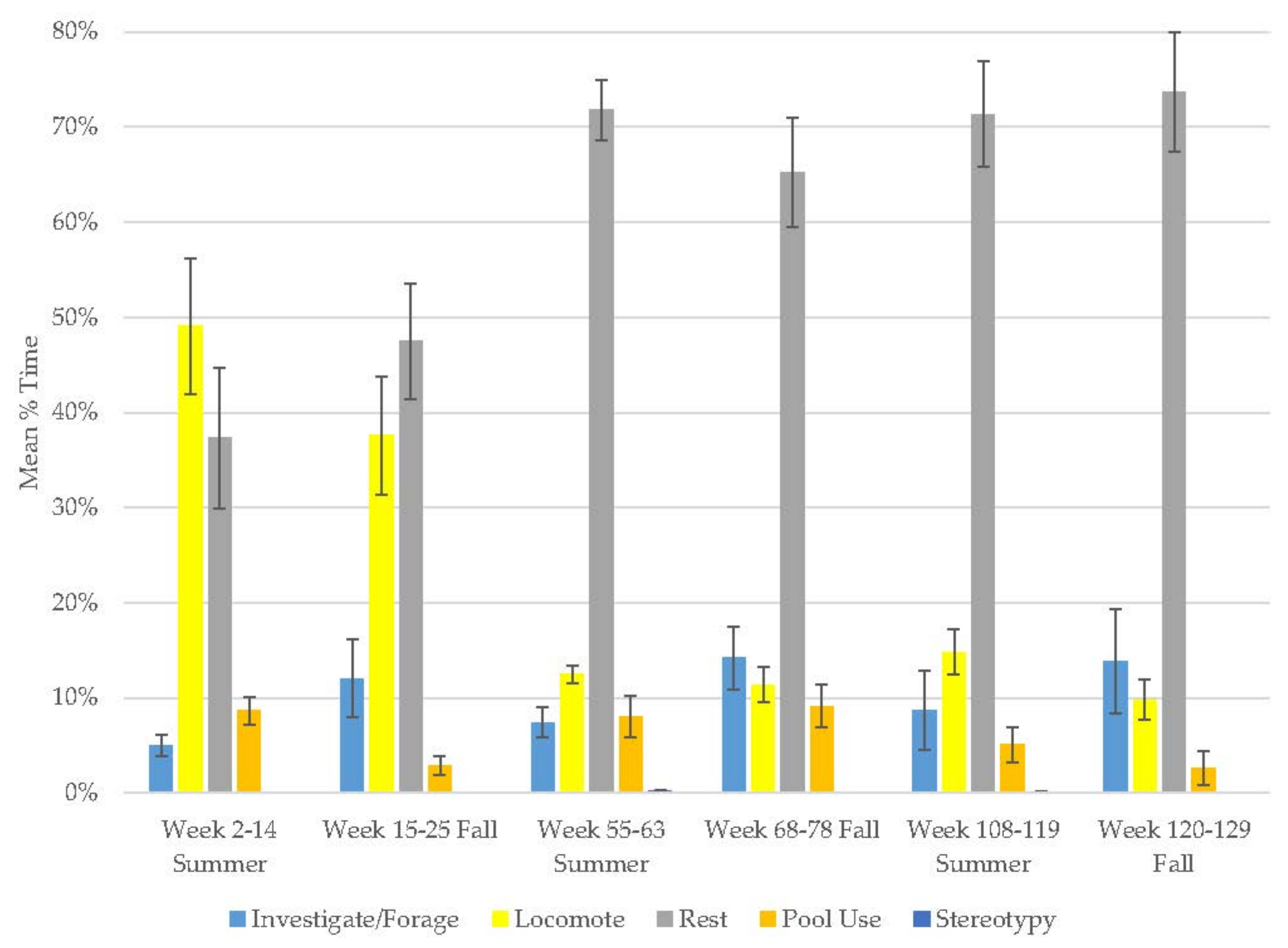
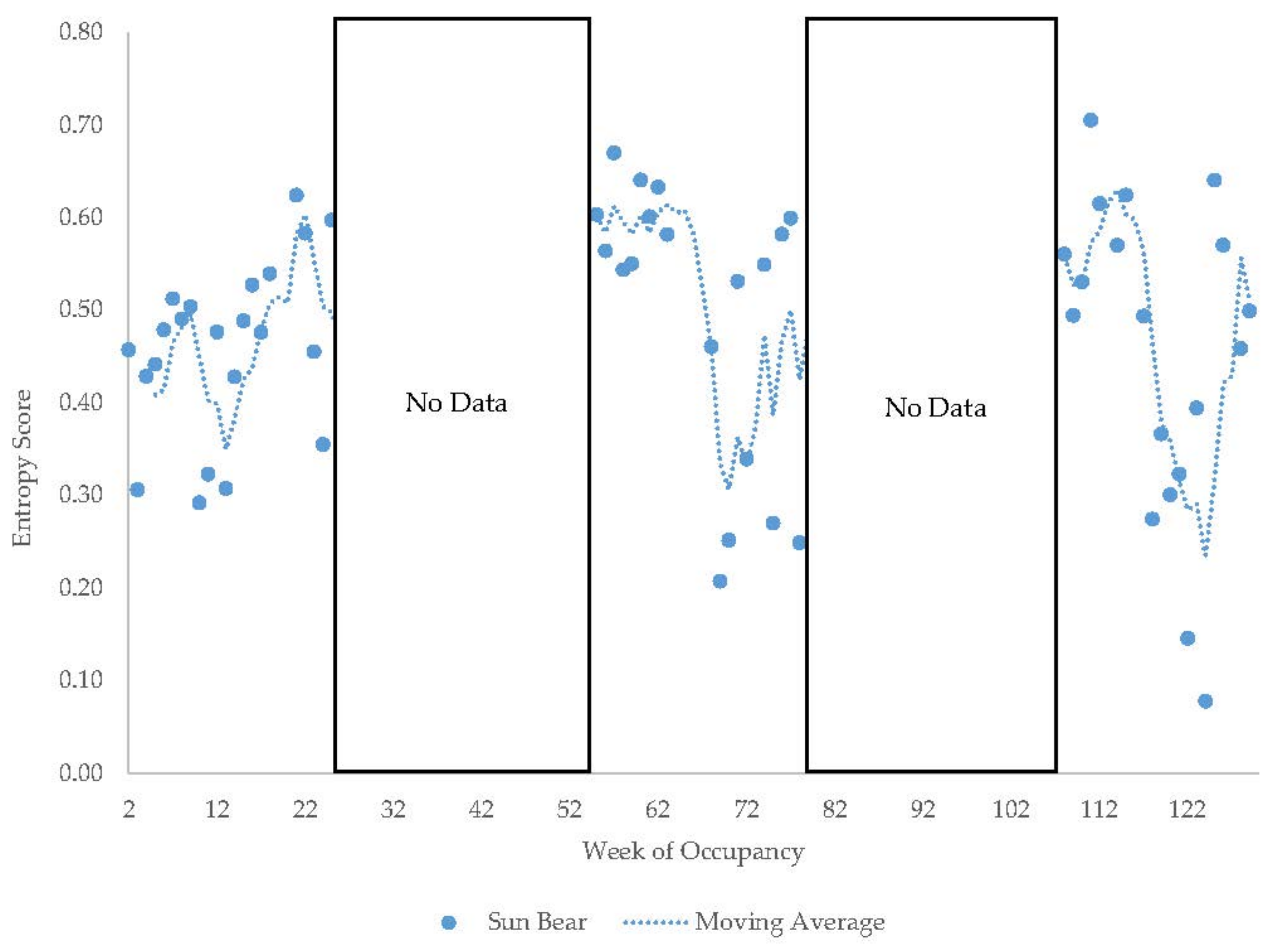
3.4. Male Sun Bear
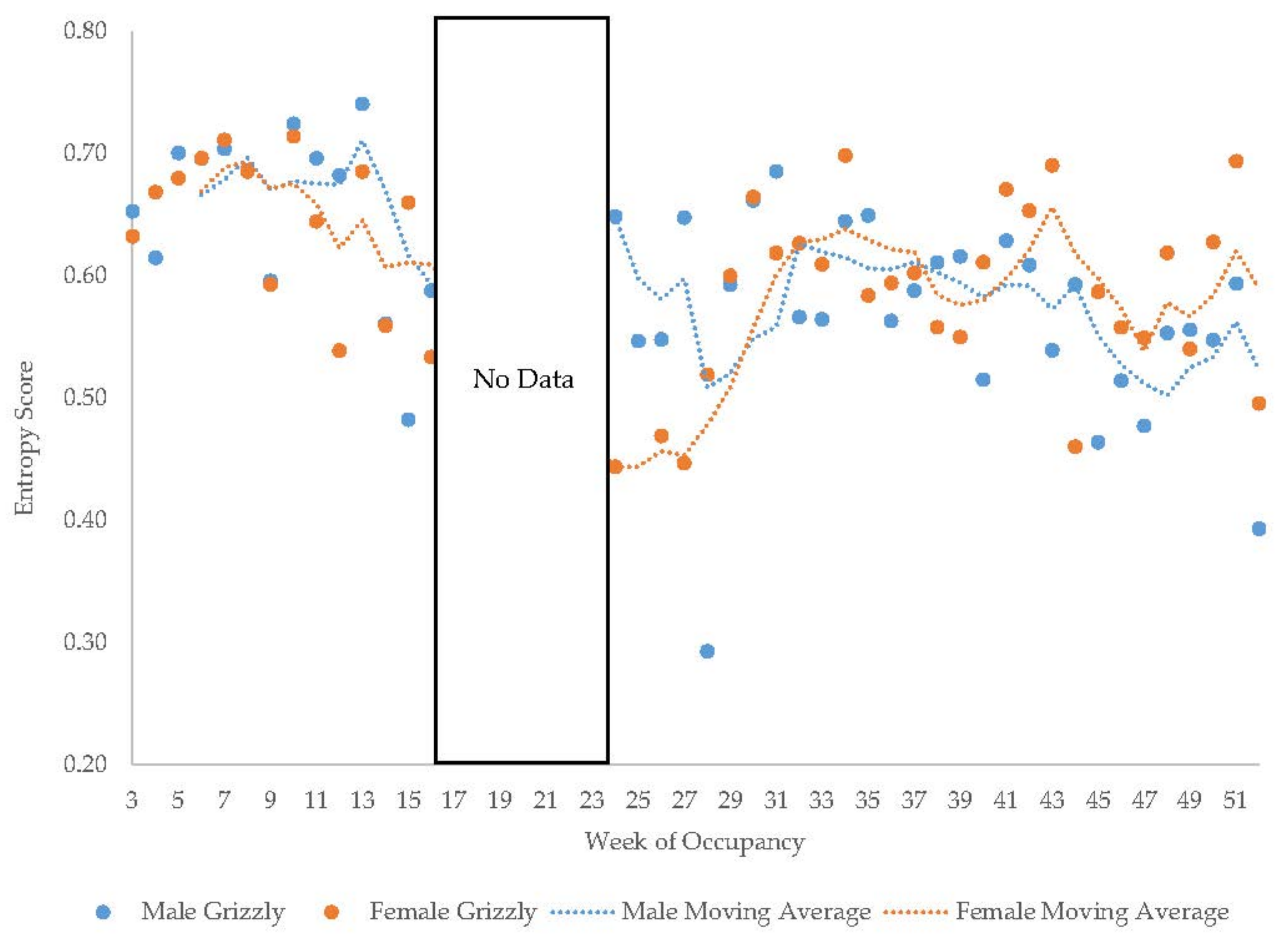
3.5. Male Grizzly Bear
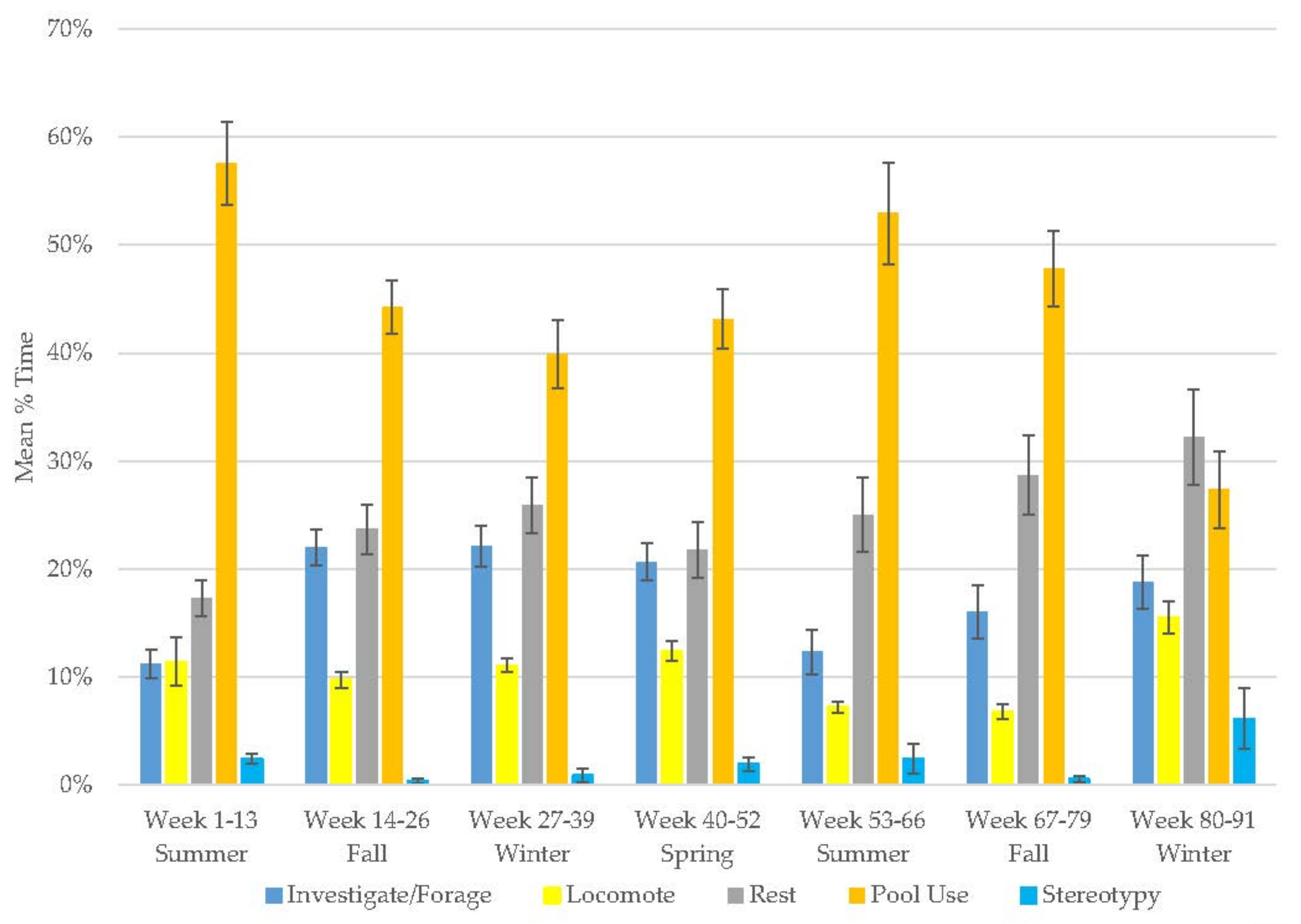
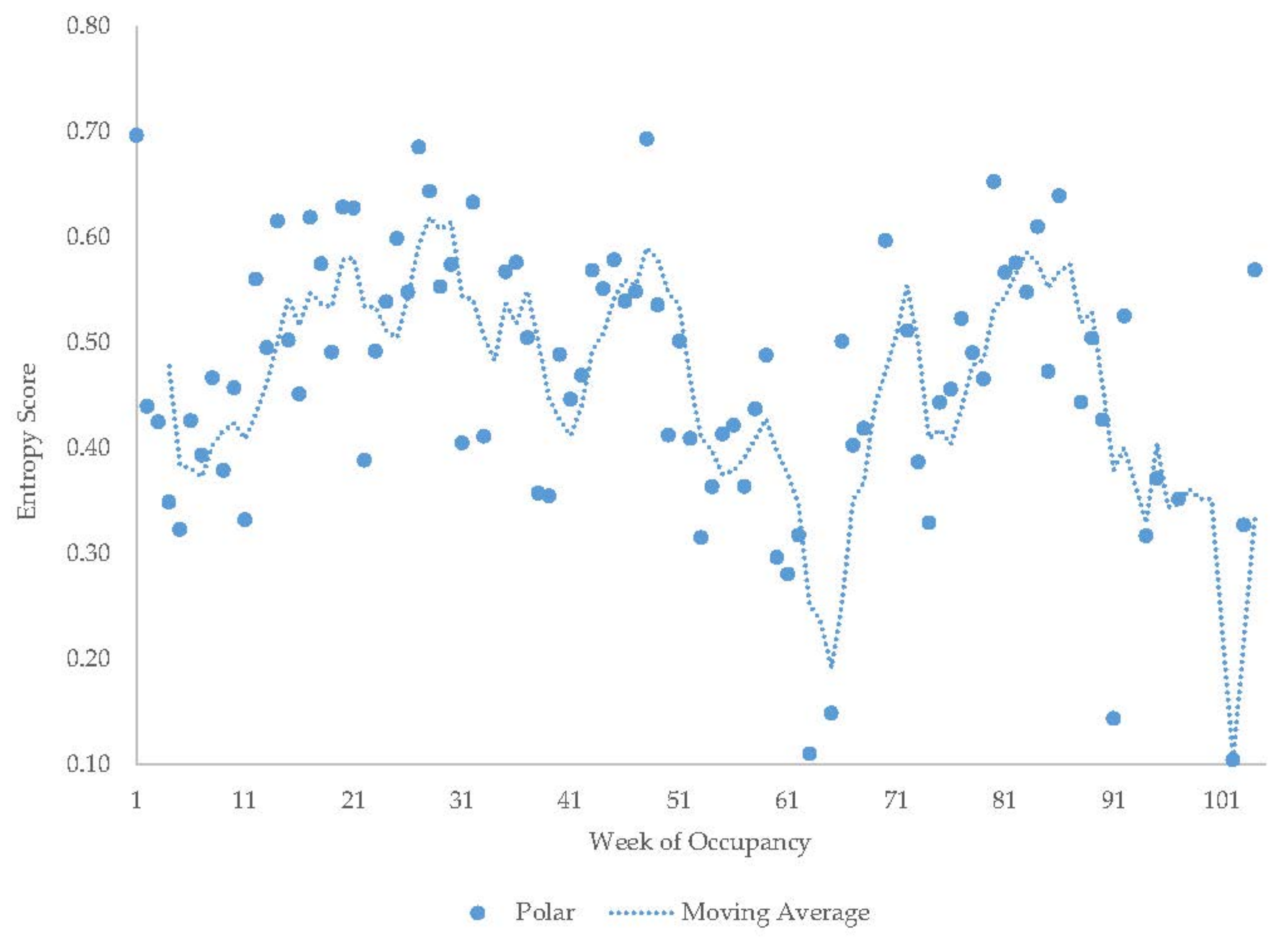
3.6. Male Polar Bear
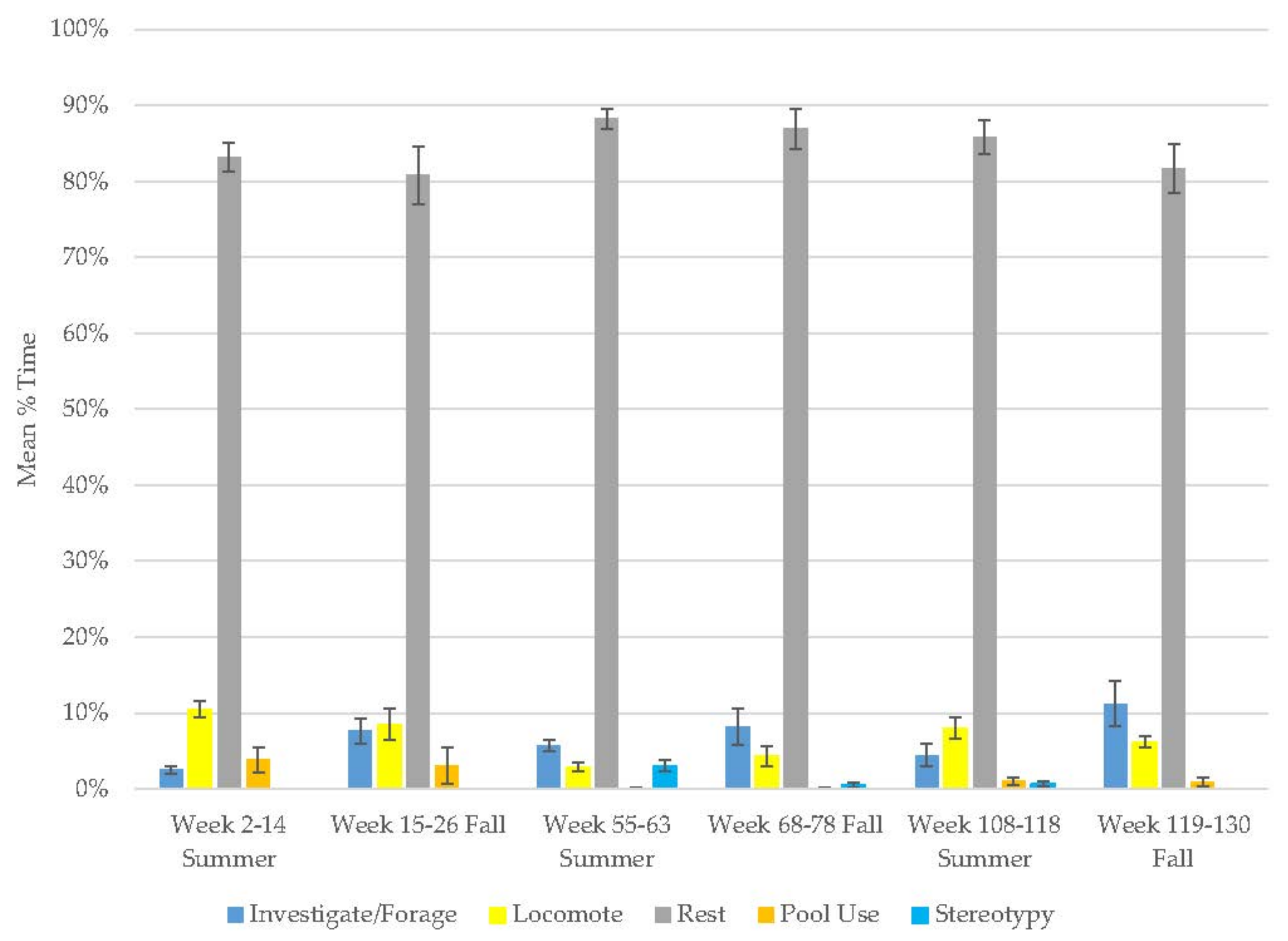
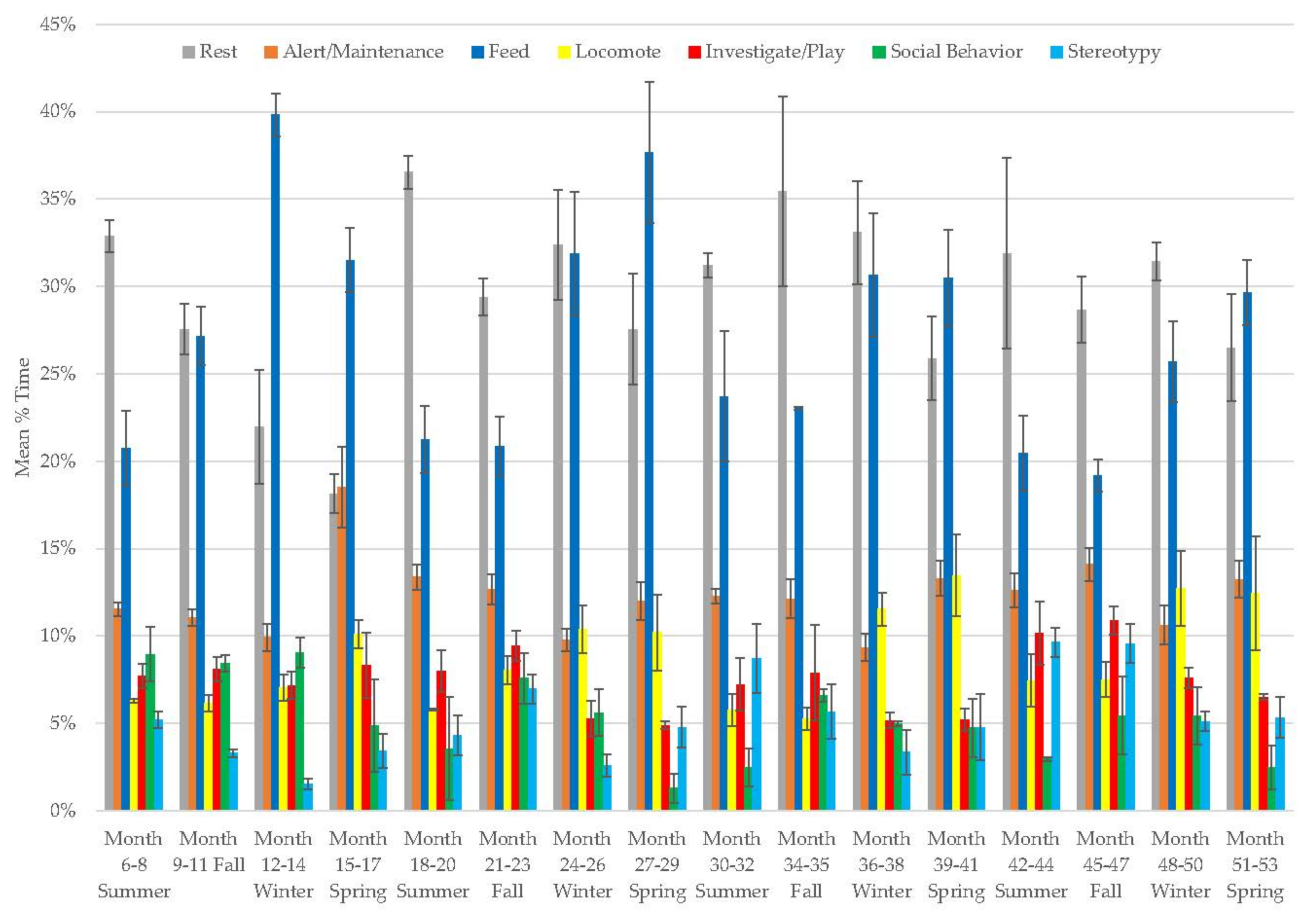
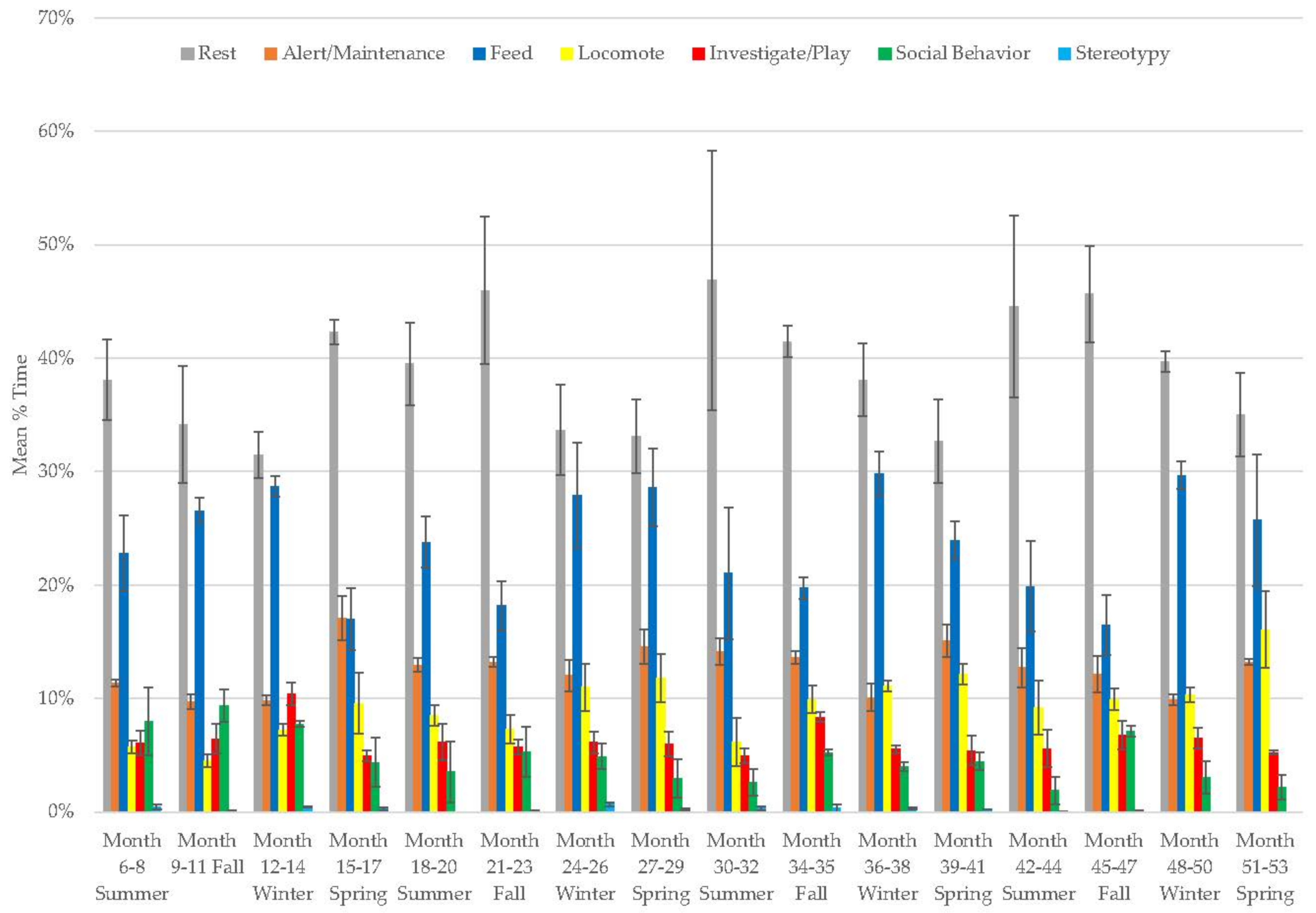
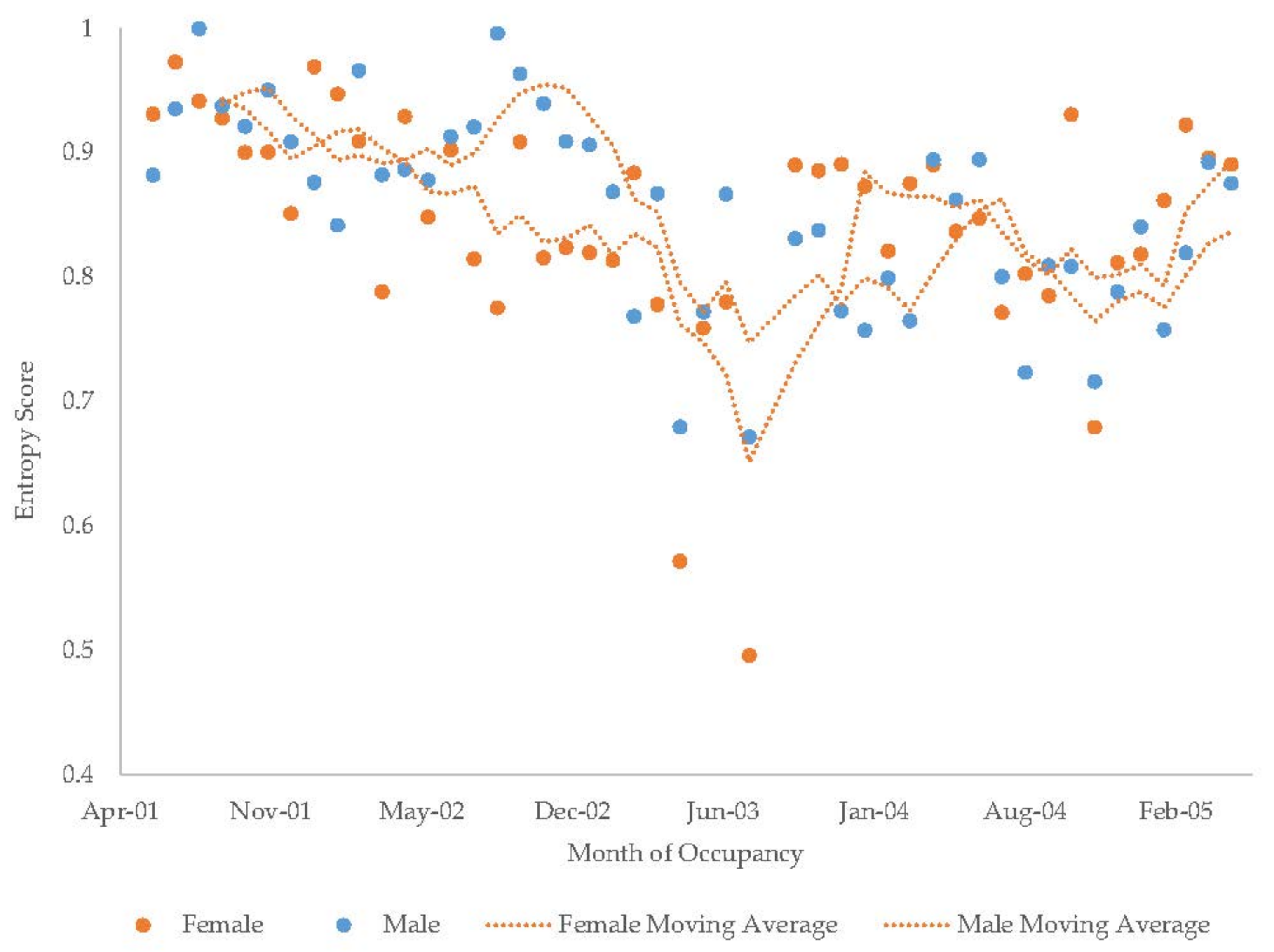
3.7. Male Giant Panda
3.8. Female Giant Panda
3.9. All Bears
3.10. Assessing Activity Budget Variation over Time
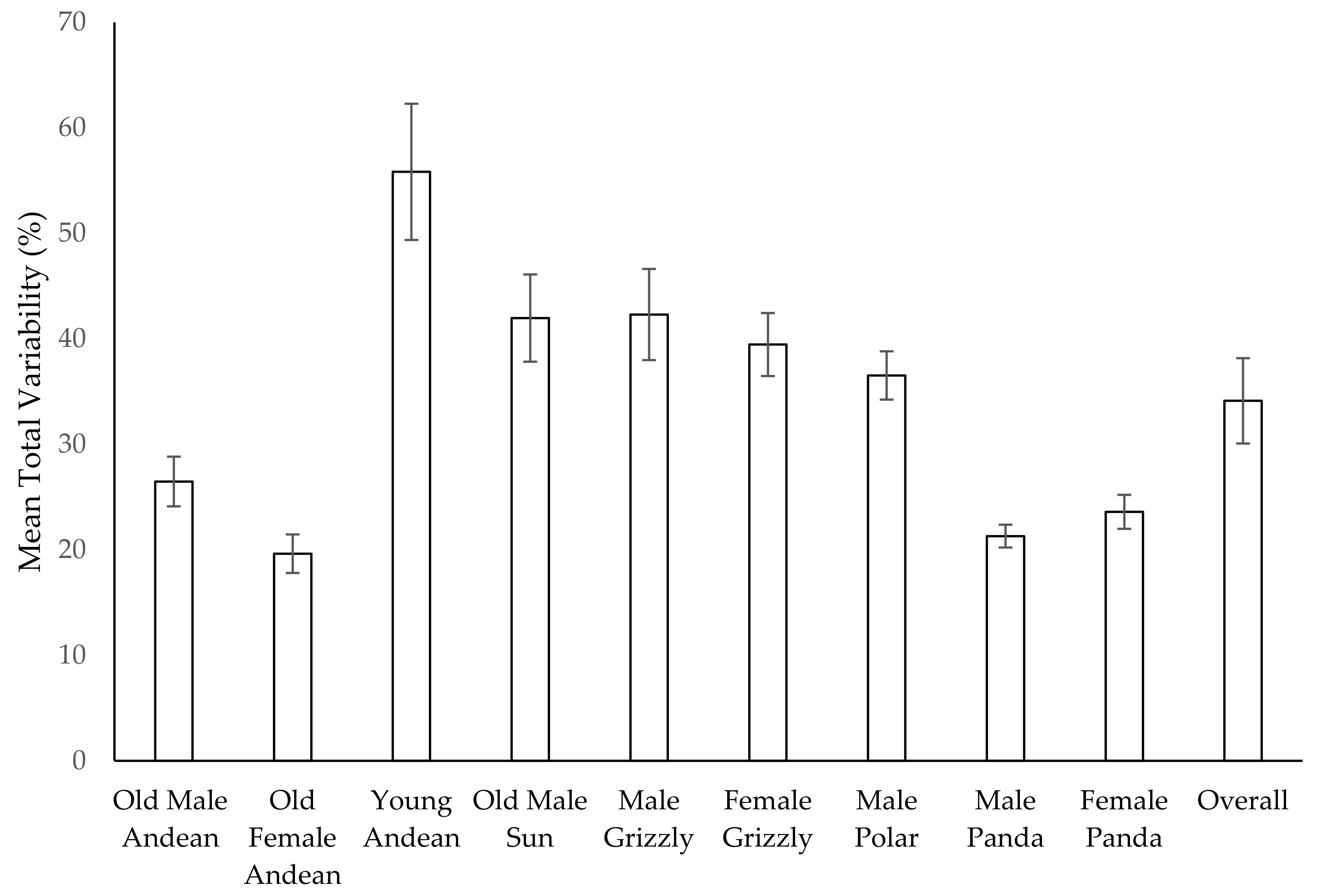
3.11. Habitat Use over Time
4. Discussion
4.1. Changes in Behavior over Time
4.2. Activity Budget Variation
4.3. Use of Space
5. Conclusions
- ➢
- Activity budgets in a variety of bear species were generally diverse and showed dynamics in the form of increasing or decreasing levels over long periods of time as well as seasonal fluctuation. Positive changes over time were documented, while changes that might suggest a decline in welfare could be explained by age or other alternative hypotheses related to boredom. Stereotypic behavior was rare in all bears studied.
- ➢
- Observation of bears’ weekly or monthly activity budgets revealed an equivalent of one to four hours of variation on a daily basis.
- ➢
- Diversity in habitat use tended to decrease over time as bears settled into patterns of use reflecting preferences for certain areas that may be associated with key resources (e.g., pools). Older bears used less of the available space than younger bears.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maple, T.L.; Finlay, T.W. Post-occupancy evaluation in the zoo. Appl. Anim. Beh. Sci. 1987, 18, 5–18. [Google Scholar] [CrossRef]
- Kelling, A.S.; Gaalema, D.E. Post-occupancy evaluations in zoological settings. Zoo Biol. 2011, 30, 597–610. [Google Scholar] [CrossRef]
- Soriano, A.I.; Ensenyat, C.; Serrat, S.; Maté, C. Introducing a semi-naturalistic exhibit as structural enrichment for two brown bears (Ursus arctos). Does this ensure their captive well-being? J. Appl. Anim. Welf. Sci. 2006, 9, 299–314. [Google Scholar] [CrossRef]
- Ogden, J.J.; Finlay, T.W.; Maple, T.L. Gorilla adaptations to naturalistic environments. Zoo Biol. 1990, 9, 107–121. [Google Scholar] [CrossRef]
- Cowan, P.E. Neophobia and neophilia: New-object and new-place reactions of three Rattus species. J. Comp. Physiol. Psych. 1977, 91, 63–71. [Google Scholar] [CrossRef]
- Glickman, S.W.; Sroges, R.W. Curiosity in zoo animals. Behaviour 1966, 26, 151–188. [Google Scholar] [CrossRef]
- Nowak, R.M. Walker’s Mammals of the World; Johns Hopkins University Press: Baltimore, MD, USA, 1999. [Google Scholar]
- Carlstead, K.; Seidensticker, J.; Baldwin, R. Environmental enrichment for zoo bears. Zoo Biol. 1991, 10, 3–16. [Google Scholar] [CrossRef]
- Forthman, D.L.; Elder, S.D.; Bakeman, R.; Kurkowski, T.W.; Noble, C.C.; Winslow, S.W. Effects of feeding enrichment on behavior of three species of captive bears. Zoo Biol. 1992, 11, 187–196. [Google Scholar] [CrossRef]
- Canino, W.; Powell, D.M. Formal behavioral evaluation of enrichment programs on a zookeeper’s schedule: A case study with a polar bear (Ursus maritimus) at the Bronx Zoo. Zoo Biol. 2010, 29, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Shepherdson, D.; Lewis, K.D.; Carlstead, K.; Bauman, J.; Perrin, N. Individual and environmental factors associated with stereotypic behavior and fecal glucocorticoid metabolite levels in zoo housed polar bears. Appl. Anim. Beh. Sci. 2013, 147, 268–277. [Google Scholar] [CrossRef]
- Soriano, A.I.; Vinyoles, D.; Maté, C. Long-term macroevaluation of environmental enrichment in three Brown bears (Ursus arctos) at Barcelona Zoo. J. Appl. Anim. Welf. Sci. 2016, 19, 49–61. [Google Scholar] [CrossRef] [Green Version]
- Bernstein-Kurtycz, L.M.; Koester, D.C.; Snyder, R.J.; Vonk, J.; Willis, M.A.; Lukas, K.E. ‘Bearly’ changing with the seasons: Bears of five species show few behavioral changes across seasons at varying visitor densities. Anim.Behav.Cogn. 2021, 8, 538–557. [Google Scholar] [CrossRef]
- Seaman, D.; Powell, R. Identifying Patterns and Intensity of Home Range Use. In Bears: Their Biology and Management; International Association for Bear Research and Management: Bozeman, MT, USA, 1990; Volume 8, pp. 243–249. [Google Scholar] [CrossRef]
- Panksepp, J. The basic emotional circuits of mammalian brains: Do animals have affective lives? Neurosci. Biobehav. R. 2011, 35, 1791–1804. [Google Scholar] [CrossRef]
- Fernandez, E.J.; Myers, M.; Hawkes, N.C. The effects of live feeding on swimming activity and exhibit use in zoo Humboldt penguins (Spheniscus humboldti). J. Zool. Bot. Gard. 2021, 2, 88–100. [Google Scholar] [CrossRef]
- Fernandez, E.J.; Harvey, E. Enclosure use as a measure of behavioural welfare in three zoo-housed African wild dogs (Lycaon pictus). J. Zoo Aquar. Res. 2021, 9, 88–93. [Google Scholar]
- Plowman, A.B. A note on a modification of the spread of participation index allowing for unequal zones. Appl. Anim. Behav. Sci. 2003, 83, 331–336. [Google Scholar] [CrossRef]
- Brereton, J.E.; Fernandez, E.J. Which index should I use? A comparison of indices for enclosure use studies. Anim. Behav. Cogn. (in press).
- Garshelis, D.L.; Pelton, M.R. Activity of black bears in the great smoky mountains national park. J. Mammal. 1980, 61, 8–19. [Google Scholar] [CrossRef]
- Kaczensky, P.; Huber, D.; Knauer, F.; Roth, H.; Wagner, A.; Kusak, J. Activity patterns of brown bears (Ursus arctos) in Slovenia and Croatia. J. Zool. 2006, 269, 474–485. [Google Scholar] [CrossRef]
- Vickery, S.; Mason, G. Stereotypic behavior in Asiatic black and Malayan sun bears. Zoo Biol. 2004, 23, 409–430. [Google Scholar] [CrossRef]
- Burn, C.C. Bestial boredom: A biological perspective on animal boredom and suggestions for its scientific investigation. Anim. Behav. 2017, 130, 141–151. [Google Scholar] [CrossRef] [Green Version]
- Wemelsfelder, F. Animal boredom: Understanding the tedium of confined lives. In Mental Health and Well-Being in Animals; McMillan, F.D., Ed.; Blackwell Publishing: Hoboken, NJ, USA, 2005; pp. 77–91. [Google Scholar]
- Assefa, S.Z.; Diaz-Abad, M.; Wickwire, E.M.; Scharf, S.M. The functions of sleep. Neuroscience 2015, 2, 155–171. [Google Scholar] [CrossRef]
- Everson, C.A.; Bergmann, B.M.; Rechtschaffen, A. Sleep deprivation in the rat: III. Total sleep deprivation. Sleep 1989, 12, 13–21. [Google Scholar] [CrossRef]
- Caro, T.M. Predatory behaviour and social play in kittens. Behaviour 1981, 76, 1–24. [Google Scholar] [CrossRef]
- Caro, T.M. Sex differences in the termination of social play in cats. Anim. Behav. 1981, 29, 271–279. [Google Scholar] [CrossRef]
- Pellis, S.M.; Pellis, V.C. Differential rates of attack, defense, and counterattack during the developmental decrease in play fighting by male and female rats. Dev. Psych. 1990, 23, 215–231. [Google Scholar] [CrossRef] [PubMed]
- Mason, G. Stereotypies: A critical review. Anim. Beh. 1991, 41, 1015–1037. [Google Scholar] [CrossRef] [Green Version]
- Mason, G.J.; Latham, N.R. Can’t stop, won’t stop; is stereotypy a reliable animal welfare indicator? Anim. Welf. 2004, 13, 557–569. [Google Scholar]
- Dierenfeld, E.S.; Hintz, H.F.; Robertson, J.B.; Van Soest, P.J.; Oftedal, O.T. Utilization of bamboo by the giant panda. J. Nutr. 1982, 112, 636–641. [Google Scholar] [CrossRef]
- Fraser, D.; Duncan, I.J.H. ‘Pleasures’, ‘Pains’ and animal welfare: Towards a natural history of affect. Anim. Welf. 1998, 7, 383–396. [Google Scholar]
- Held, S.D.E.; Špinka, M. Animal play and animal welfare. Anim. Behav. 2011, 81, 891–899. [Google Scholar] [CrossRef]
- Trezza, V.; Baarendse, P.J.J.; Vanderschuren, L.J.M.J. The pleasures of play: Pharmacological insights into social reward mechanisms. Trends Pharmacol. Sci. 2010, 31, 463–469. [Google Scholar] [CrossRef] [Green Version]
- Yamanashi, Y.; Nogami, E.; Teramoto, M.; Morimura, N.; Hirata, S. Adult-adult social play in captive chimpanzees: Is it indicative of positive welfare? Appl. Anim. Beh. Sci. 2018, 199, 75–83. [Google Scholar] [CrossRef]
- Schaller, G.B.; Jinchu, H.; Wenshi, P.; Jing, Z. The giant pandas of Wolong. University of Chicago Press: Chicago, IL, USA, 1985. [Google Scholar]
- Akinyi, M.Y.; Tung, J.; Jeneby, M.; Patel, N.B.; Altmann, J.; Alberts, S.C. Role of grooming in reducing tick load in wild baboons (Papio cynocephalus). Anim. Behav. 2013, 85, 559–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troisi, A. Displacement activities as a behavioral measure of stress in nonhuman primates and human subjects. Stress 2009, 5, 47–54. [Google Scholar] [CrossRef]
- Watters, J.V.; Krebs, B.L.; Eschmann, C.L. Assessing Animal Welfare with Behavior: Onward with Caution. J. Zool. Bot. Gard. 2021, 2, 75–87. [Google Scholar] [CrossRef]
- Fernandez, E.J.; Yoakum, E.; Andrews, N. Seasonal and daily activity of two zoo-housed grizzly bears (Ursus arctos horribilis). J. Zool. Bot. Gard. 2020, 1, 1–12. [Google Scholar] [CrossRef]
- Liu, H.; Duan, H.; Wang, C. Effects of ambient environmental factors on the stereotypic behaviors of giant pandas (Ailuropoda melanoleuca). PLoS ONE 2017, 12, e0170167. [Google Scholar] [CrossRef]
- Powell, D.M.; Stevens, L.M. Guidelines for the housing and husbandry of the giant panda (Ailuropoda melanoleuca). In Giant Panda Animal Care Manual; Lindburg, D., Wong, S., Eds.; Zoological Society of San Diego: San Diego, CA, USA, 2009; pp. 1–14. [Google Scholar]
- Hunter, S.C.; Gusset, M.; Miller, L.J.; Somers, M.J. Space use as an indicator of enclosure appropriateness in African wild dogs (Lycaon pictus). J. Appl. Anim. Welf. Sci. 2014, 17, 98–110. [Google Scholar] [CrossRef]
- Mench, J.A.; Kreeger, M.D. Ethical and welfare issues associated with keeping wild mammals in captivity. In Wild Mammals in Captivity: Principles and Techniques; Kleiman, D.G., Allen, M.E., Thompson, K.V., Lumpkin, S., Eds.; University of Chicago Press: Chicago, IL, USA, 1996; pp. 5–15. [Google Scholar]
- Perkins, D.R.; Debbage, K.G. Weather and Tourism: Thermal Comfort and Zoological Park Visitor Attendance. Atmosphere 2016, 7, 44. [Google Scholar] [CrossRef] [Green Version]
- Hancocks, D. The history and principles of zoo exhibition. In Wild Mammals in Captivity: Principles & Techniques for Zoo Management, 2nd ed.; Kleiman, D.G., Thompson, K.V., Baer, C.K., Eds.; University of Chicago Press: Chicago, IL, USA, 2010; pp. 121–136. [Google Scholar]
- Kessel, A.; Brent, L. Space utilization by captive-born baboons (Papio sp.) before and after provision of structural enrichment. Anim. Welf. 1996, 5, 37–44. [Google Scholar]
- Little, K.A.; Sommer, V. Change of enclosure in langur monkeys: Implications for the evaluation of environmental enrichment. Zoo Biol. 2002, 21, 549–559. [Google Scholar] [CrossRef]


| Subject | Sex | Birth Origin & Year | Introduced to Habitat | Age during Study (Years) | Housing | Weeks of Occupancy Observed | # Datapoints in Analysis |
|---|---|---|---|---|---|---|---|
| Sun Bear | Male | Wild ~1997 | Summer 2014 | 17–20 | Social | 2–129 | 60 weeks |
| Andean Bear | Male | Zoo 1990 | Summer 2014 | 24–27 | Social | 2–130 | 62 weeks |
| Andean Bear | Female | Zoo 1993 | Summer 2014 | 25–27 | Social | 2–130 | 61 weeks |
| Andean Bear | Male | Zoo 2016 | Spring 2019 | 3 | Single | 1–29 | 23 weeks |
| Grizzly Bear | Male | Wild 2015 | Fall 2017 | 2–3 | Social | 3–52 | 43 weeks |
| Grizzly Bear | Female | Wild 2015 | Fall 2017 | 2–3 | Social | 3–52 | 42 weeks |
| Polar Bear | Male | Wild 2013 | Summer 2015 | 5–7 | Single | 1–104 | 93 weeks |
| Giant Panda | Male | Zoo 1997 | December 2000 | 4–8 | Social | 23–201 | 47 months |
| Giant Panda | Female | Zoo 1998 | December 2000 | 3–7 | Social | 23–201 | 47 months |
| Subject | Rest | Locomotion | Investigate/ Forage | Use Pool | Stereotypy | Alert/ Maintenance | Feed | Investigate/ Play | Social |
|---|---|---|---|---|---|---|---|---|---|
| Old Male Andean Bear | Decrease | Increase | Increase | Decrease | Increase | - | - | - | - |
| Old Female Andean Bear | Increase | Decrease | Increase | Decrease | Decrease | - | - | - | - |
| Young Male Andean Bear | Decrease | Increase | Increase | Decrease | Increase | - | - | - | - |
| Male Sun Bear | Increase | Decrease | Increase | Decrease | Decrease | - | - | - | - |
| Male Grizzly Bear | Increase | Decrease | Decrease | Increase | Decrease | - | - | - | - |
| Female Grizzly Bear | Increase | Decrease | Decrease | Increase | Increase | - | - | - | - |
| Male Polar Bear | Increase | Decrease | Increase | Decrease | Increase | - | - | - | - |
| Male Giant Panda | Increase | Decrease | - | - | Increase | Increase | Decrease | Decrease | Decrease |
| Female Giant Panda | Increase | Increase | - | - | Decrease | Increase | Increase | Decrease | Decrease |
| Rest | Locomotion | Invest./ Forage | Use Pool | Stereotypy | Alert/ Maintenance | Invest./ Feed | Play | Social | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject | Mean ± SE | SD | Mean ± SE | SD | Mean ± SE | SD | Mean ± SE | SD | Mean ± SE | SD | Mean ± SE | SD | Mean ± SE | SD | Mean ± SE | SD | Mean ± SE | SD |
| Old Male Andean Bear | 76 ± 2.5 | 20 | 13 ± 1.5 | 12 | 5 ± 0.6 | 5 | 5 ± 0.8 | 7 | 2 ± 0.7 | 5 | ||||||||
| Old Female Andean Bear | 84 ± 1.1 | 9 | 7 ± 0.6 | 5 | 7 ± .8 | 6 | 2 ± 0.6 | 4 | 1 ± 0.3 | 2 | ||||||||
| Young Male Andean Bear | 42 ± 3.8 | 18 | 15 ± 1.6 | 7 | 34 ± .3.2 | 15 | 3 ± 2.2 | 10 | 5 ± 1.7 | 8 | ||||||||
| Male Sun Bear | 60 ± 3.1 | 24 | 24 ± 2.8 | 21 | 10 ± 1.4 | 11 | 6 ± 0.8 | 6 | 0 ± 0.0 | 0 | ||||||||
| Male Grizzly Bear | 40 ± 2.2 | 15 | 19 ± 1.2 | 8 | 16 ± 1.3 | 9 | 24 ± 2.3 | 15 | 0 ± 0.1 | 0 | ||||||||
| Female Grizzly Bear | 45 ± 2.2 | 14 | 20 ± 1.0 | 7 | 17 ± 1.5 | 9 | 17 ± 2.0 | 13 | 0 ± 0.1 | 0 | ||||||||
| Male Polar Bear | 25 ± 1.2 | 11 | 10 ± 0.5 | 5 | 18 ± 0.8 | 8 | 44 ± 0.6 | 15 | 3 ± 1.6 | 5 | ||||||||
| Male Giant Panda | 29 ± 0.9 | 6 | 9 ± 0.5 | 3 | 5 ± 0.4 | 3 | 12 ± 0.4 | 3 | 27 ± 1.0 | 7 | 7 ± 0.3 | 2 | 5 ± 0.5 | 3 | ||||
| Female Giant Panda | 39 ± 1.3 | 9 | 9 ± 0.5 | 4 | 0 ± 0.0 | 0 | 13 ± 0.4 | 3 | 24 ± 0.9 | 6 | 6 ± 0.3 | 2 | 5 ± 0.5 | 3 | ||||
| Season | Behavior | Old Male Andean Bear | Old Female Andean Bear | Young Male Andean Bear | Male Sun Bear | Male Grizzly Bear | Female Grizzly Bear | Male Polar Bear | Male Giant Panda | Female Giant Panda |
|---|---|---|---|---|---|---|---|---|---|---|
| Rest | 65 ± 0.0 | 85 ± 0.0 | 42 ± 0.1 | 58 ± 0.1 | 39 ± 0.0 | 48 ± 0.0 | 21 ± 0.0 | 33 ± 0.0 | 42 ± 0.0 | |
| Inv./Forage | 4 ± 0.0 | 4 ± 0.0 | 33 ± 0.0 | 7 ± 0.0 | 11 ± 0.0 | 10 ± 0.0 | 12 ± 0.0 | |||
| Locomotion | 20 ± 0.0 | 8 ± 0.0 | 14 ± 0.0 | 28 ± 0.0 | 13 ± 0.0 | 20 ± 0.0 | 9 ± 0.0 | 6 ± 0.0 | 7 ± 0.0 | |
| Pool | 9 ± 0.0 | 2 ± 0.0 | 2 ± 0.0 | 7 ± 0.0 | 38 ± 0.0 | 21 ± 0.0 | 55 ± 0.0 | |||
| Summer | Stereotypy | 10 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 2 ± 0.0 | 7 ± 0.0 | 0 ± 0.0 | ||
| Alert/Maint | 12 ± 0.0 | 13 ± 0.0 | ||||||||
| Feed | 22 ± 0.0 | 22 ± 0.0 | ||||||||
| Inv./Play | 8 ± 0.0 | 6 ± 0.0 | ||||||||
| Social | 7 ± 0.0 | 0 ± 0.0 | ||||||||
| Rest | 87 ± 0.0 | 83 ± 0.0 | 44 ± 0.1 | 62 ± 0.0 | 33 ± 0.0 | 37 ± 0.0 | 26 ± 0.0 | 30 ± 0.0 | 42 ± 0.0 | |
| Inv./Forage | 6 ± 0.0 | 9 ± 0.0 | 38 ± 0.1 | 13 ± 0.0 | 18 ± 0.0 | 20 ± 0.0 | 19 ± 0.0 | |||
| Locomotion | 6 ± 0.0 | 6 ± 0.0 | 17 ± 0.0 | 19 ± 0.0 | 27 ± 0.0 | 26 ± 0.0 | 8 ± 0.0 | 7 ± 0.0 | 8 ± 0.0 | |
| Pool | 1 ± 0.0 | 1 ± 0.0 | 0 ± 0.0 | 5 ± 0.0 | 21 ± 0.0 | 17 ± 0.0 | 46 ± 0.0 | |||
| Fall | Stereotypy | 1 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 6 ± 0.0 | 0 ± 0.0 |
| Alert/Maint | 13 ± 0.0 | 12 ± 0.0 | ||||||||
| Feed | 23 ± 0.0 | 20 ± 0.0 | ||||||||
| Inv./Play | 9 ± 0.0 | 7 ± 0.0 | ||||||||
| Social | 6 ± 0.0 | 0 ± 0.0 | ||||||||
| Rest | 40 ± 0.0 | 48 ± 0.1 | 29 ± 0.0 | 30 ± 0.0 | 36 ± 0.0 | |||||
| Inv./Forage | 25 ± 0.0 | 24 ± 0.1 | 21 ± 0.0 | |||||||
| Locomotion | 18 ± 0.0 | 15 ± 0.0 | 13 ± 0.0 | 10 ± 0.0 | 10 ± 0.0 | |||||
| Pool | 17 ± 0.1 | 13 ± 0.1 | 34 ± 0.0 | |||||||
| Winter | Stereotypy | 0 ± 0.0 | 0 ± 0.0 | 3 ± 0.0 | 3 ± 0.0 | 0 ± 0.0 | ||||
| Alert/Maint | 10 ± 0.0 | 10 ± 0.0 | ||||||||
| Feed | 32 ± 0.0 | 29 ± 0.0 | ||||||||
| Inv./Play | 6 ± 0.0 | 7 ± 0.0 | ||||||||
| Social | 3 ± 0.0 | 0 ± 0.0 | ||||||||
| Rest | 50 ± 0.0 | 49 ± 0.0 | 23 ± 0.0 | 25 ± 0.0 | 36 ± 0.0 | |||||
| Inv./Forage | 14 ± 0.0 | 19 ± 0.0 | 20 ± 0.0 | |||||||
| Locomotion | 16 ± 0.0 | 16 ± 0.0 | 11 ± 0.0 | 12 ± 0.0 | 12 ± 0.0 | |||||
| Pool | 20 ± 0.0 | 16 ± 0.0 | 40 ± 0.0 | |||||||
| Spring | Stereotypy | 0 ± 0.0 | 0 ± 0.0 | 5 ± 0.0 | 5 ± 0.0 | 0 ± 0.0 | ||||
| Alert/Maint | 14 ± 0.0 | 15 ± 0.0 | ||||||||
| Feed | 32 ± 0.0 | 24 ± 0.0 | ||||||||
| Inv./Play | 6 ± 0.0 | 5 ± 0.0 | ||||||||
| Social | 3 ± 0.0 | 0 ± 0.0 |
| Subject | # Hours in a 7-h Day that Fluctuate in Terms of Activity |
|---|---|
| Old Male Andean Bear | 1.9 |
| Old Female Andean Bear | 1.4 |
| Young Male Andean Bear | 3.9 |
| Male Sun Bear | 2.9 |
| Male Grizzly Bear | 3.0 |
| Female Grizzly Bear | 2.8 |
| Male Polar Bear | 2.6 |
| Male Giant Panda | 1.5 |
| Female Giant Panda | 1.6 |
| All Bears | 2.4 |
| Species (and Year) | # of Zones | Min H | Max H | Range | Average H | SE | Slope |
|---|---|---|---|---|---|---|---|
| Female Andean Bear 2014 | 4 | 0.03 | 0.48 | 0.45 | 0.31 | 0.028 | −0.009 |
| Female Andean Bear 2015 | 6 | 0 | 0.58 | 0.58 | 0.35 | 0.025 | 0.003 |
| Male Andean Bear 2014 | 4 | 0.05 | 0.49 | 0.44 | 0.29 | 0.028 | −0.008 |
| Male Andean Bear 2015 | 6 | 0 | 0.6 | 0.6 | 0.38 | 0.025 | 0.001 × 10−1 |
| Male Sun Bear 2014 | 5 | 0.29 | 0.62 | 0.33 | 0.46 | 0.02 | 0.005 |
| Male Sun Bear 2015 | 6 | 0.08 | 0.71 | 0.63 | 0.48 | 0.026 | −0.001 |
| Male Polar Bear | 6 | 0.1 | 0.7 | 0.59 | 0.47 | 0.013 | −0.001 |
| Female Grizzly Bear | 6 | 0.44 | 0.71 | 0.27 | 0.61 | 0.011 | −0.001 |
| Male Grizzly Bear | 6 | 0.29 | 0.74 | 0.45 | 0.6 | 0.014 | −0.003 |
| Young Male Andean Bear | 6 | 0.14 | 0.71 | 0.57 | 0.51 | 0.025 | −0.004 |
| Male Giant Panda | 14 | 0.67 | 1 | 0.33 | 0.85 | 0.01 | −0.003 |
| Female Giant Panda | 14 | 0.5 | 0.97 | 0.47 | 0.85 | 0.01 | −0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Powell, D.M.; Baskir, E. Behavior and Habitat Use Remain Diverse and Variable in Modern Zoological Exhibits over the Long-Term: Case Studies in 5 Species of Ursidae. J. Zool. Bot. Gard. 2021, 2, 677-704. https://doi.org/10.3390/jzbg2040049
Powell DM, Baskir E. Behavior and Habitat Use Remain Diverse and Variable in Modern Zoological Exhibits over the Long-Term: Case Studies in 5 Species of Ursidae. Journal of Zoological and Botanical Gardens. 2021; 2(4):677-704. https://doi.org/10.3390/jzbg2040049
Chicago/Turabian StylePowell, David M., and Eli Baskir. 2021. "Behavior and Habitat Use Remain Diverse and Variable in Modern Zoological Exhibits over the Long-Term: Case Studies in 5 Species of Ursidae" Journal of Zoological and Botanical Gardens 2, no. 4: 677-704. https://doi.org/10.3390/jzbg2040049
APA StylePowell, D. M., & Baskir, E. (2021). Behavior and Habitat Use Remain Diverse and Variable in Modern Zoological Exhibits over the Long-Term: Case Studies in 5 Species of Ursidae. Journal of Zoological and Botanical Gardens, 2(4), 677-704. https://doi.org/10.3390/jzbg2040049







